Abstract
There is an increasing proportion of the general population surviving to old age with significant chronic disease, multi-morbidity, and disability. The prevalence of pre-frail state and frailty syndrome increases exponentially with advancing age and is associated with greater morbidity, disability, hospitalization, institutionalization, mortality, and health care resource use. Frailty represents a global problem, making early identification, evaluation, and treatment to prevent the cascade of events leading from functional decline to disability and death, one of the challenges of geriatric and general medicine. Cardiac arrhythmias are common in advancing age, chronic illness, and frailty and include a broad spectrum of rhythm and conduction abnormalities. However, no systematic studies or recommendations on the management of arrhythmias are available specifically for the elderly and frail population, and the uptake of many effective antiarrhythmic therapies in these patients remains the slowest. This European Heart Rhythm Association (EHRA) consensus document focuses on the biology of frailty, common comorbidities, and methods of assessing frailty, in respect to a specific issue of arrhythmias and conduction disease, provide evidence base advice on the management of arrhythmias in patients with frailty syndrome, and identifies knowledge gaps and directions for future research.
Keywords: European Heart Rhythm Association, Position paper, Consensus document, Frailty, Elderly, Frailty syndrome, Frailty assessment, Frailty domains, Pre-frailty state, Elderly, Cachexia, Arrhythmias, Atrial fibrillation, Ventricular tachycardia, Heart failure, Stroke, Cognitive impairment, Antiarrhythmic drugs, Anticoagulants, Ablation, Pacemaker, Implantable cardioverter-defibrillator, Cardiac resynchronization therapy, Cardiac resynchronization therapy-defibrillator
Introduction
Cardiac arrhythmias become more common and embrace a broad spectrum of rhythm and conduction abnormalities with advancing age and chronic illness. According to the World Health Organization, in 2019, 1 billion of people worldwide were aged 60 years and older, with this number set to double by 2050.1 The greatest rise is in persons aged 80 years or older, with expected upsurge by four-fold to 434 million worldwide. Concurrently, there is an increasing proportion of the general population surviving with significant chronic disease and disability.
Ageing is frequently characterized by the coexistence of several comorbid conditions, often reciprocally interacting to produce a greater than additive negative impact on health status. Pre-frail state and frailty, mainly in association with old age and multi-morbidity, is increasingly seen among patients with cardiovascular disease and arrhythmias. Management of patients with frailty syndrome has been deliberated in specific settings such as acute cardiac and critical care,2 heart failure (HF)3 as well as in general cardiology.4 Timely and appropriate identification and assessment of the reduction in physiological reserves and frailty, which can be done using the dedicated methods and definitions (see Section ‘Assessment of frailty and frailty scores’) or employing simplified tools based on the characterization of single domains of frailty or self-report questionnaires is useful in guiding the individual approach to patient management and ensuring safe and effective therapies.
However, no systematic studies or recommendations on the management of arrhythmias in the frail population are available, not in the least because these patients have been excluded from major clinical trials, whereas lack of the awareness about the safety and efficacy of antiarrhythmic therapies accounts for withholding effective pharmacological (e.g. anticoagulation) or non-pharmacological (e.g. ablation) interventions. Surveys have revealed a wide variation among physicians in understanding of what constitutes frailty syndrome and highlighted the lack of guidance on the use of the variety of available therapies. Whereas frailty is not synonymous with ageing and the high heterogeneity in older age populations should be acknowledged, practitioners treating arrhythmias will more and more encounter patients who are frail.
Therefore, this consensus document will explain the biology of frailty, common comorbidities, methods of assessing frailty, issues specific to various types of arrhythmias and provide advice on the management of arrhythmias in elderly and frail patients as well as identify knowledge gaps and directions for future research. The document is targeted at primary and secondary care practitioners involved in treating older pre-frail and frail patients with cardiac arrhythmias, conduction disease, and cardiac implanted electronic devices.
Review of evidence
This consensus document was prepared by the Task Force with representation from European Heart Rhythm Association (EHRA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). The document will be peer-reviewed by official external reviewers representing EHRA, HRS, APHRS, LAHRS, and CASSA.
Members of the Task Force were asked to perform a detailed literature review, weigh the strength of evidence for or against a particular treatment or procedure, and include estimates of expected health outcomes where data exist. Patient-specific modifiers, comorbidities, and issues of patient preference that might influence the choice of particular investigations or therapies were considered, as are frequency of follow-up and cost-effectiveness. In controversial areas, or regarding the issues without evidence other than usual clinical practice, a consensus was achieved by agreement of the expert panel after discussions.
Consensus statements are based upon strength of evidence and consensus as outlined in Table 1.
Table 1.
Categories of consensus statements
| Consensus statement | Definition | |
|---|---|---|
| ‘Should do this’ | Scientific evidence that a treatment or procedure is beneficial and effective, or is strongly supported by author’s consensus. |

|
| ‘May do this’ | General agreement and/or scientific evidence favour the usefulness/efficacy of a treatment or procedure |

|
| ‘Do not do this’ | Scientific evidence or general agreement not to use or recommend a treatment or procedure |

|
Relationships with industry and other conflicts of interest
It is EHRA/European Society of Cardiology (ESC) policy to sponsor position papers and guidelines without commercial support, and all members volunteered their time. Thus, all members of the writing group as well as reviewers have disclosed any potential conflict of interest in detail (see Appendix I).
Definition, epidemiology, and associations of frailty
Definition and epidemiology
Frailty identifies a syndrome characterized by high biological vulnerability, decreased physiologic reserve, and reduced capacity to resist stressors, due to multiple impairments in inter-related systems, leading to reduced homeostatic reserve.5
The prevalence of frailty has been estimated to be 12% (10–15%) in the community, rising to 45% (27–63%) in the non-community cohorts,6 with higher rates in women and the highest in individuals aged 85 years and older.7,8 Multiple comorbidities are associated with a more than two-fold increase in the prevalence of frailty (up to 63–81%). In analysis including 240 studies from 62 countries representing 1 755 497 participants, the prevalence of frailty in studies using physical frailty measures was 12% compared with 24% for those using a frailty index (FI).9 For pre-frailty, this was 46% and 49%, respectively. Physical frailty was more commonly identified in women than men (15% vs. 11%). Combination of multiple comorbidities with frailty phenotype substantially increased long-term mortality risk in septuagenarian men [hazard ratio (HR) 2.93, 95% confidence interval (CI) 2.10–4.07].10
Two different conceptual models of frailty have been proposed.11,12 According to model proposed by Fried et al.,11 a cascade of events—from molecular oxidative stress to DNA damage accelerating cellular senescence—leads to endocrine and immune system dysregulation, which results in the development of a frailty phenotype, consisting of reduced muscle strength, body weight, and gait speed, and of increased fatigue or inability to perform demanding activities. An alternative model by Mitnitski et al.12 describes the cumulative deficit model defines frailty not as a specific syndrome, but rather as an age-related state of additive medical and functional problems. Despite theoretical differences, the two models have much in common and are able to identify older individuals at higher risk of events.13
Pre-frail state
The concept of pre-frail state is less well developed and supported by epidemiological and clinical data, and the definitions are vague. Pre-frail state or intermediate frailty phenotype is identified if one or two out of five criteria based on the Fried model11 or as the number of accumulated deficits based on the FI.14 It is not uncommon to refer to pre-frailty as a clinically silent phase preceding frailty or a condition that predisposes to frailty.15
The exact rates of pre-frailty are difficult to establish. It has been estimated that the prevalence of the pre-frailty state in individuals aged 65 years and older may range between 18.8% and 50.9%. In a recent meta-analysis in the community-dwelling older adults who were robust at baseline 30.9% became pre-frail during a median follow-up of 2.5 years (the incidence rate of 150.6 per 1000 person-years), whereas among non-frail (robust and pre-frail at baseline) individuals, 13.6% progressed to frailty over a medial follow-up of 3 years (the incidence rate of 43.4 per 1000 person-years).16 The pre-frailty incidence rates were significantly higher in women than men (173.2 vs. 129.0 per 1000 person-years).
The clinical importance of the pre-frail state concept as a transitional state between robust and frail lies in the possibility of reversal from frailty with effective rehabilitation interventions. Frequent and/or long-term hospitalization associated with sarcopenia and weakness is a major risk factor for transitioning from robust to pre-frail and subsequently developing frailty.
However, the hypothesis that reducing risk factors or enhancing protective factors may prevent or delay age-associated frailty has not been formally tested in appropriately sized randomized controlled trials. There is general consensus that frailty derives from the acceleration of biological ageing and that as our understanding of the biology of ageing progresses, effective prevention and treatment strategies will be developed.
Knowledge gaps
Better definition of pre-frail state and tools (derived from the frailty assessment tools or specifically developed) for its evaluation are required.
Epidemiology of pre-frail state needs to be prospectively studied.
The driving risk factors associated with gender differences in the incidence and prevalence of pre-frailty and frailty should be explored.
It is still unknown whether a global care plan that is triggered by the diagnosis of frailty improves objective health outcomes and whether the processes which lead to frailty can be attenuated or reversed is also unknown.
Physician’s awareness of the importance of evaluation for the pre-frail state and identification of modifiable components with appropriate interventions needs to be improved.
Frailty vs. clinical complexity
Ageing is associated with progressive loss of biological homeostatic and functional reserve that puts an older individual at high risk of developing adverse health outcomes, including multiple comorbidities, mobility loss, disability, cognitive impairment and eventually becomes so severe to be incompatible with life. While part of this decline of health and functional status is directly attributable to diseases, in some specific individuals the accumulation of damage is so pervasive and multisystemic that it is impossible to recognize a single cause and, therefore, clinicians and scientists define them as frail.
Frailty may have unpredictable trajectories and coexists with other geriatric conditions, such as multiple comorbidities and disability.17,18 Ageing is associated with frailty, multiple comorbidities and disability, and these conditions are largely overlapping. Frailty and complexity are sometimes used synonymously, though the term ‘complexity’ should be reserved to indicate the presence of multiple comorbidities with its implicit burden of polypharmacy.19 In this respect, complexity may be a component or, better, may contribute to frailty but does not identify with it.
Major clinical conditions related to frailty
Anorexia and malnutrition
Anorexia (loss of appetite or inability to eat), is frequently observed in the older individuals promoting malnutrition and leading through sarcopenia and muscle loss to disability and higher morbidity and mortality. The anorexia of ageing affects approximately 20% of the older population and is higher in hospitalized elderly patients (23–62%) and long-term nursing home residents (up to 85%).20 Advanced age often contributes to this cascade through different pathways.20 Older patients are less sensitive to the action of ghrelin, the hunger hormone, because of the increased concentration of insulin and leptin, the satiety hormones.21 Many conditions that are common in older persons affecting nutritional balance: cancer, HF, chronic obstructive pulmonary disease, chronic kidney disease (CKD), gastrointestinal disorders, mal-absorption syndrome, Parkinson’s disease—all determine anorexia and/or increased energy expenditure. Psychiatric disorders such as depression, cognitive impairment, and dementia also contribute to the reduced appetite. Commonly prescribed medications, swallowing and chewing difficulties, decline of senses, living alone or in a nursing home, and other social or economic problems may negatively influence the nutritional profile. Despite their importance, anorexia and malnutrition are not routinely assessed in everyday clinical practice.
Consensus statement
|

|
Sarcopenia
Sarcopenia, the age-dependent loss of both the muscle mass and muscle strength and function, is a common condition associated with frailty and adverse health outcomes.22 After the age of 70 years, the average muscle loss amounts to 15% per each decade. The prevalence of sarcopenia in the community is 1–29% rising to 14–33% in the long-term care. Aging, anorexia, malnutrition, age-related hormonal changes, sedentary lifestyle, and limited mobility mark decreased anabolism, whereas disease state, inflammation, oxidative stress, and mitochondrial dysfunction determine increased catabolism leading to the development of sarcopenia. Sarcopenia should be considered when implementing preventative and therapeutic interventions (e.g. optimized nutrition, elimination of vitamin D deficiency, and physical exercise) aiming at reversing physical frailty at its initial stage and slowing or halting the progressive decline towards disability and dependency.22
Heart failure
Frailty is particularly common in HF: in a meta-analysis, a 47.4% prevalence of frailty in patients with HF was estimated using multidimensional assessment tools.23 The prevalence of frailty in patients with HF is independent of age, suggesting a more complex interaction between the two syndromes and the progressive decline in physiological reserve. The mechanisms linking frailty to HF are multi-factorial, with inflammatory markers, impaired skeletal muscle function due to mitochondrial dysfunction, decreased capillary density, and adipose tissue infiltration potentially involved.3 Conversely, ageing, frailty, comorbidities, and immobility due to hospitalization, may all contribute to increase the severity and accelerate the progression of HF leading to increased risk of morbidity and mortality.24
Consensus statements
|

|
|

|
Cancer
Frailty is particularly important in the management of cancer patients, who may have mortality rates as high as 80%, as cancer and related therapies may both greatly challenge patient’s physiologic reserve,25 when a pre-frail or a clearly frail status can be found in more than 50% of cancer cases.25,26 Specific oncologic adverse outcomes possibly related to frailty are chemotherapy side effects,27 disease recurrence or progression, and death.25 Registry data show that cancer prognosis is associated with frailty-related conditions such as weight loss, reduced gait speed, major depression, and institutionalization in nursing home.28 The presence of frailty should promote multidisciplinary decision-making and individually tailored therapeutic approaches aimed at preserving health-related quality of life.
Consensus statement
|

|
Falls
Frail older adults are likely to experience recurrent falls. In the meta-analysis of 102 130 community-dwelling individuals aged 65 years and older, frail patients had a 2.5-fold increased risk of falls, whereas those in the pre-frail state had 1.5-fold increased risk of fall compared with robust individuals.29 Frailty-induced falls incur a low quality of life in older adults and increase risk of bone fractures, hospitalization, and death as well as burden on their carers.
Frail patients with suspected arrhythmias should be assessed for falls risk. Modifiable risk factors for falls outwit environmental hazards (loose rugs, steps, etc.) include gait and balance impairment, cognitive impairment, depression, polypharmacy, psychotropic drugs, cardiovascular drugs, visual abnormalities (poor visual acuity and impaired contrast sensitivity), orthostatic hypotension, low blood pressure (BP), arrhythmias (most commonly bradyarrhythmias), urinary incontinence, prior falls, and fear of falling.30 All identified risk factors should be modified.
Falls are categorized as accidental, i.e. a slip or trip or non-accidental. The latter are more likely to be attributable to cardiovascular abnormalities, particularly hypotensive disorders or arrhythmias. In practice, the distinction is less clear unless a fall has been witnessed (not the case in up to 50% of events) and/or the older person has a clear recall of events.31
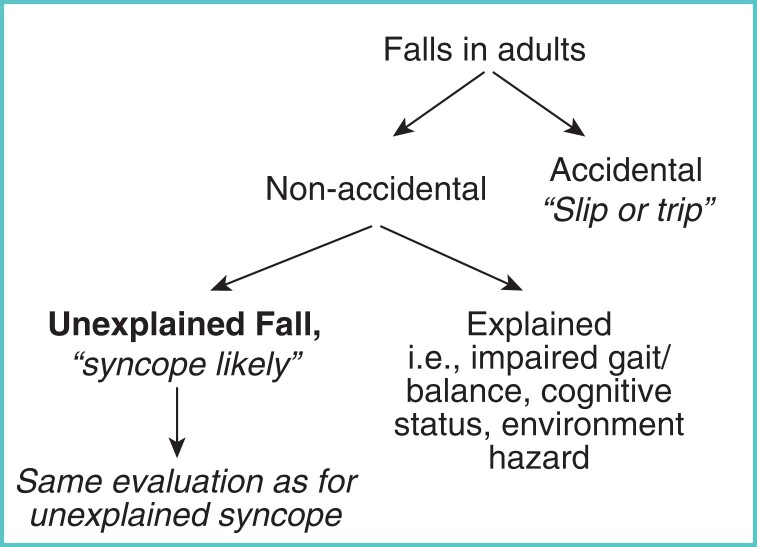
In older fallers, a detailed cognitive assessment will assist in determining whether the patient’s recall is unreliable, i.e. recall of prodrome, circumstances of fall, loss of consciousness, palpitations, chest pain, post-event characteristics. Such falls should be investigated as per syncope guidelines (Figure 1).32
Figure 1.
Flow diagram for the identification of unexplained falls. Reproduced from Brignole M, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. European Heart Journal, 2018;39(21):1883–1948, by permission of Oxford University Press and the European Society of Cardiology (ESC).32
In older frail individuals, orthostatic intolerance manifesting as orthostatic hypotension due to pharmacotherapy with antihypertensive and antianginal agents, and sedatives and deconditioning is a common cause of falls. Other causes are primary, secondary autonomic failure, hypovolemia, and anaemia.33 Sarcopenia and consequent deconditioning are contributory causes in frail persons.
In one-quarter to one-third of patients with falls, particularly when long-term continuous rhythm monitoring is employed, falls are directly linked with arrhythmias—most commonly bradyarrhythmia, asystole, and tachy-brady forms of atrial fibrillation (AF).34–36 Antiarrhythmic medications may potentiate orthostatic intolerance, increase risk of bradycardia, contribute to other falls risk factors, such as impairment of attention/concentration, sleep impairment, electrolyte disturbance, or visual impairment.
Consensus statements
|

|
|

|
|

|
|

|
|

|
|

|
Neurological conditions including cognitive impairment and dementia
Frailty and falls are more common in age-related neurological disorders such as stroke, Parkinson’s disease, dementia, or epilepsy.37 The proportion of ‘fallers’ is higher even in subgroups with least fall-associated neurological diseases such as tinnitus and headache. Medical treatment, including that for dementia, may also increase the risk of falls through different mechanisms. Psychotropic drugs are related to fall injuries, hospitalization, and death.38
Dementia and cognitive impairment play a significant role in this context and are associated with an increased risk of falls. Since prevention of future falls is a major objective of treatment, there is particular interest in the effect of cognitive training on the future risk of falls. As recently shown in meta-analyses of randomized trials, combined exercise and cognitive training improved balance in mild cognitive impairment39 and physical exercise had a significant effect in preventing falls in older adults with cognitive impairment.40
Another important aspect is the fear of falling in frail patients. Recent studies demonstrated an independent association of frailty with fear of falling, and cognitive behavioural therapies may improve fear of falling.41
Consensus statements
|

|
|

|
Multiple comorbidities and polypharmacy
Cardiovascular Health Study demonstrated a strong association of frailty with a number of chronic diseases including cardiovascular and pulmonary diseases and diabetes: 33% of the frailty subjects had 3–4 chronic diseases, 27% two, and 8% >5 concomitant conditions.11 Multiple comorbidities in frailty subjects may not only aggravate frailty phenotypes, but result in the increased risk of polypharmacy which has repeatedly been shown to be a marker of adverse clinical outcome. The French study of a cohort aged >70 years revealed that the mean number of drugs prescribed increased with increasing frailty from 4.6 in non-frail subjects to 6.1 in pre-frail, 7.1 in frail, and 7.5 in dependent individuals.42 Importantly, frailty and excessive polypharmacy with >10 drugs were independent risk factors for mortality, and the combination multiplied the mortality risk for 2.6 years by 6.30. HF, renal failure, AF, dementia, and cancer were among the most common comorbidities recognized by physicians as associated with frailty.43
Electrolyte disorders, renal impairment, metabolic issues
Chronic hyponatremia is the most common electrolyte disorder in the elderly. It is caused mainly by drugs such as diuretics and antidepressants and by syndrome of inappropriate antidiuretic hormone secretion. Though often mild and asymptomatic, it aggravates frailty phenotypes leading to cognitive disorders and gait impairments and contributes to osteoporosis leading to bone frailness, thus predisposing the patients to falls and hip fractures.44 CKD is common in ageing. Sarcopenia increases progressively along with renal impairment in CKD, being highest in dialysis patients. Prevalence of frailty has been documented in 7% of the elderly, 14% of CKD patients not requiring dialysis, and 42% of those on haemodialysis.45 Frailty in haemodialysis patients is associated with 2.6 times greater risk of mortality and 1.4 times more hospitalization, independent of age, sex, comorbidity, and disability.46 Of the metabolic issues, insulin resistance significantly increases with age, and is considered as a major risk factor for many age-related diseases. Insulin resistance plays an important mediating role in the frailty cycle including chronic inflammation, reduced skeletal muscle metabolism, cognitive decline and sarcopenic obesity. Interventions aimed at correcting insulin resistance therefore may have a critical role in preventing or slowing the downward spiral of frail older persons.47
Assessment of frailty and frailty scores
Frailty is considered an age-related syndrome with unknown pathophysiology, operationally defined as loss of functional reserve in multiple physiologic systems, lack of resilience to everyday stressors and elevated risk for a range of adverse health outcomes. Criteria were developed to identify frail older persons in spite of the substantial heterogeneity of this population.
The most popular set of criteria has been developed using data from the Cardiovascular Health Study.11 Building on the conceptual framework that frailty derives from a mutually exacerbating cycle of negative energy balance, sarcopenia, and diminished strength and tolerance for exertion, frailty can be defined according to the following criteria: unintentional weight loss, feeling of exhaustion, muscle weakness, slowness while walking, and low levels of activity, ascertained by a combination of self-reported and performance-based measures (Table 2). Individuals who have three or more of these criteria should be considered ‘frail’, and those with two of these criteria should be considered ‘pre-frail’. The predictive validity of these criteria toward different outcomes has been overwhelmingly demonstrated in hundreds of manuscripts published in and outside the geriatric literature.
Table 2.
Diagnostic criteria used for the diagnosis of frailty (Fried criteria)
| Measure | Definition |
|---|---|
| Weight loss | Lost 4.5 kg or more unintentionally over the last year |
| Exhaustion | Self-report of either ‘felt that everything I did was an effort’ and/or ‘could not get going’ in the last week |
| Low physical activity | Self-report, equivalent to <90 kCal in women and <128 kCal in men |
| Slow walking | 4 m at usual pace: |
| speed <0.76 m/s for height <159 cm in women and <173 cm in men or speed <0.80 m/s for height >159 cm in women and >173 cm in men | |
| Weakness | Grip strength |
| Women: <17 kg for BMI <23 kg/m2; <17.3 kg for BMI 23.1–26 kg/m2; <18 kg for BMI 26.1–29 kg/m2; and <21 kg for BMI >29 kg/m2 | |
| Men: <29 kg for BMI <24 kg/m2; <30 kg for BMI 24.1–26 kg/m2; <30 kg for BMI 26.1–28 kg/m2; <32 kg for BMI >28 kg/m2 |
Please note diagnostic thresholds for different criteria were modified for different population and different studies. At least 2/5 positive criteria defines pre-frailty and >3/5 criteria defines frailty.
BMI, body mass index.
The second approach is the FI which is calculated as the ratio between the number of deficits detected and the total number of deficits considered, which may be quite variable and include diseases, physical and cognitive impairments, psychosocial risk factors, and geriatric syndromes such as falls, delirium, and urinary incontinence (Table 3).48 The FI is a strong predictor of adverse health outcomes and allows robust clinical inferences.49,50 The FI categorizes older individuals in several classes, from ‘robust’ to ‘severely frail’. Since FI can be generated from almost any set of health-related variables, this tool is highly flexible and can be adapted to the large number of situations and harmonized across research projects and clinical centres.
Table 3.
Short version of the frailty index
|
The index is calculated as (total number of variables present)/(total number of variables assessed). A score ≥0.36 indicates frailty.
There are also self-report questionnaires or instruments based on the assessment of single performances (i.e. single domains of frailty).
The most effective clinical use of frailty has been the stratification of patients to verify what medical interventions are beneficial in this special population or whether alternative approaches should be considered. Frail older persons should not be excluded a priori from any type of treatment, and decision-making should be treatment-specific and based on scientific evidence. Specifically, where treatment of different types of cardiac arrhythmias have the same beneficial effects in frail and non-frail older persons is unknown.
Overall, the definition of frailty appears to be a powerful tool to establish prognosis, the risk of complication after surgery or aggressive medical interventions as well as forecasting future health care resource utilization. Whether the identification of frailty triggers alternative approaches to care that results in better clinical outcomes remains uncertain.
Knowledge gaps
Inconsistency in assessment of frailty by the existing tools, with the physical function and mobility being the only consistent domain, needs to be addressed.
The need for disease-specific frailty assessment tools should be assessed and methodology for development and validation of such tools should be defined.
Pathophysiology overview of biological changes associated with frailty
Frailty is a syndrome characterized by decreased reserve and resistance to stressors, causing vulnerability to adverse outcomes such as morbidity, iterative hospitalizations, loss of autonomy and death. Frailty can result from decline across one or more physiologic systems. Schematically, we can distinguish four major domains of frailty:
Physical, mainly related to loss of muscle mass and function and reduced physical performance.
Cognitive, due to cognitive decline and/or dementia.
Psychological, mainly related to depressive manifestations.
Social, related to isolation and absence of social activities.
Frailty and age-related electrical and structural changes of the heart and vasculature
Cardiovascular ageing is the result of alterations of the structure and function of:
Arteries: endothelial dysfunction, intima-media thickness, arterial wall calcifications, alterations in the extracellular matrix.
Heart: wall hypertrophy and fibrosis, cavities dilation, valve calcifications and degeneration, changes in cardiac muscle cell contractility.
All these alterations concern the totality of the cardiovascular components (large and small arteries, coronary circulation, myocardium, valves, conductive system) and are the pathophysiological background of peripheral vasoconstriction, central artery stiffness, cardiac diastolic and systolic dysfunction. Acute and chronic manifestations of these alterations are very frequent in the elderly, such as systolic hypertension, ischaemic heart disease, arrhythmias, valvular heart disease, stroke, and acute and chronic HF.
Interaction between ageing cardiovascular system and frailty
Frailty and pre-frailty have been shown to be associated with any type of cardiovascular disease, with an HR of 1.70 (95% CI: 1.18–2.45) and 1.23 (95% CI: 1.07–1.36), respectively, compared with the robust individuals.51
The combination of age-related arterial and cardiac alterations and their interactions (cardiac-artery coupling) are responsible for the above-mentioned cardiovascular diseases and also contribute to the development of other age-related degenerative diseases and syndromes such as dementia, sarcopenia, renal failure, leading to frailty and loss of autonomy. Ageing, frailty, and cardiovascular disease are linked by multiple mechanisms and share several biomarkers including inflammation and oxidative stress biomarkers, natriuretic peptides and troponins, and markers of CKD.52 Some cardiovascular risk factors such as obesity are important long-term risk factors (already before the appearance of clinical cardiovascular disease) for frailty, particularly in younger individuals.52
The extent of cardiovascular ageing which is very variable among older people of the same age, will be a major modulator of functional status, and the degree of functionality and autonomy especially after the age of 80 years.
Older individuals with marked frailty will have a more pronounced evolution of cardiovascular disease. Thus, for the same level of cardiac dysfunction a frail person will have a more pronounced clinical impact than a more robust person. This is very often observed in heart failure patients in whom the presence of frailty and sarcopenia is synergistic with the consequences of heart disease and amplifies clinical signs such as fatigue, dyspnoea, and cachexia. In addition, the presence of frailty will increase the risk of medication-related adverse effects. Clinical studies have shown that the severity of frailty modifies the benefits/risk ratio of several medical and surgical cardiovascular therapies.
Consensus statements
|

|
|

|
|

|
|

|
Knowledge gaps
Further research is needed to identify biomarkers that may be used for the assessment of interaction between ageing, frailty, and cardiovascular disease and outcomes.
Clinical pharmacology
Normal ageing produces physioflogical changes which affect the pharmacokinetics (absorption, distribution, metabolism, and excretion) and pharmacodynamics of antiarrhythmic drugs (AADs) (Table 4).53–56
Table 4.
Age-associated changes in pharmacokinetics and pharmacodynamics
| Pharmacokinetic changes | |||
|---|---|---|---|
| Physiological change | Pharmacokinetic effect | Drugs affected | |
| Absorption |
|
|
|
| Distribution |
|
||
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
| Biotransformation |
|
|
|
| Excretion |
|
|
|
| Pharmacodynamic changes | |||
| Change | Consequence | ||
| Baroreceptor is blunted | Postural hypotension, falls: Class I and IV AADs | ||
| Decreased response to catecholamines | Increased sensitivity to amiodarone, β-blockers and sotalol | ||
| Increased myocardial fibrosis | Decreased conduction velocity (Class I AADs) | ||
| Sinoatrial and atrioventricular node dysfunction | Higher risk of bradycardia and atrioventricular block with Class II and IV AADs and digoxin | ||
| Decreased cardiac reserve | Higher risk of HF with disopyramide and Class IV AADs | ||
| Decreased left ventricular compliance | Decreased cardiac output with Class II AADs | ||
| Increased sensitivity to anticoagulants | Higher risk of bleeding | ||
| Comorbidities and polypharmacy |
Increased drug–drug and drug–disease, drug–diet supplements interactions
|
||
AADs, antiarrhythmic drugs; ACEIs, angiotensin converting enzyme inhibitors; GFR, glomerular filtration rate; HF, heart failure; Vd, volume of distribution.
Metabolism
The plasma concentrations of some AADs (propranolol, verapamil) increase due to a decrease in their first-pass metabolism. The increase in body fat increases the volume of distribution (Vd) and the half-life of lipophilic drugs (amiodarone) and the decrease in total body water reduces the Vd and increases the serum concentrations of hydrophilic drugs (digoxin). The decrease in albumin plasma levels increases the free-active fraction of AADs.
Most AADs are biotransformed in the liver by CYP2D6 (flecainide, metoprolol, mexiletine, propafenone, vernakalant), CYP3A4 (amiodarone, diltiazem, dronedarone, quinidine, verapamil) and CYP1A2 and CYP3A4 isoenzymes (lidocaine).53–57 Age reduces hepatic blood flow and CYP450 activity increasing the plasma levels and half-lives of AADs metabolized by the liver.54–58 Biotransformation of some ADDs (amiodarone, disopyramide, lidocaine, mexiletine, procainamide, propafenone, quinidine, verapamil) produces active metabolites with electrophysiological effects that can be different from those of the parent compound (N-acetyl-procainamide is a Class III drug; 5-hydroxypropafenone lacks β-adrenergic blocking effects). Active metabolites explain why the AADs can exert different effects when the drug is administered intravenously or orally.
Age-related reduction in renal blood flow, glomerular filtration rate and tubular secretion decreases the clearance and increases the half-lives of renally-cleared drugs (digoxin, ibutilide, sotalol, and dofetilide).59 Other AADs undergo hepatic and renal elimination (dofetilide, procainamide, and disopyramide). Thus, dose adjustment needs to be made for AADs that are directly excreted or whose active metabolites are eliminated by the kidney. Most AADs interact with other widely used drugs. Quinidine, amiodarone, and dronedarone inhibit the P-glycoprotein required for renal excretion of digoxin, thereby increasing its plasma levels. Amiodarone inhibits CYP3A4, CYP2C9, and P-glycoprotein, increasing the plasma levels of drugs widely used in the geriatric population (flecainide, Class II and IV AADs, anticoagulants).60
Adverse effects
Frail patients are more susceptible to some adverse effects of AADs, including bradycardia and atrioventricular (AV) block (Class II and IV AADs or digoxin), intracardiac conduction block (Class I AADs), HF (disopyramide, sotalol, and IV AADs), orthostatic hypotension and falls and urinary retention (Class I A).53,61,62 Conversely, older patients present a decreased sensitivity to beta-blockers. The Beers criteria recommend to avoid: (i) amiodarone as first-line therapy for AF unless the patient has HF or substantial left ventricular hypertrophy; (ii) disopyramide because of its anticholinergic properties; and (iii) digoxin as first-line therapy for AF or HF and should be prescribed at doses <0.125 mg/day for any indication.62
Furthermore, AAD treatment is complicated by coexisting comorbidities (HF, hypertension, coronary artery disease) that affect the pharmacodynamic/pharmacokinetics of AADs and lead to polypharmacy that increases the incidence of adverse effects and drug interactions. Interestingly, several non-cardiovascular QT-prolonging drugs prescribed in the elderly increases the risk of proarrhythmia and should be avoided.
Prescribers should carefully evaluate how age affects the pharmacodynamic/pharmacokinetics of AADs and their possible drug interactions with other drugs widely prescribed in older patients with coexisting comorbidities. Treatment should be started at lower-than-recommended dosages based on hepatic and renal function and gradually titrated until reaching the desired dose, assessing for adverse reactions, mainly proarrhythmic effects.
Bradyarrhythmias
Bradyarrhythmia incidence is known to increase with age and comorbidity.63–65 Therefore, with increasing frailty, more bradyarrhythmias are expected. In a murine model, some animals the same age have reduced heart rate and sinus node function that can be predicted by FI. Furthermore, electrical conduction, action potential morphology and fibrosis are correlated with, and graded by, frailty scores.63 Idiopathic degeneration of the sinus node caused by ageing is probably the most common cause of SND.64 AV block is more prevalent with advanced ageing. Comparing centenarians with a control group averaging 75 years, first- or second-degree AV block was observed in 25% of the centenarians compared with 7% of the controls.65
Drug-induced bradycardia
Patients with frailty are more often prescribed rate-slowing therapy such as calcium channel blockers, beta-blockers and antiarrhythmics for hypertension, HF, and AF. Frail patients have unpredictable pharmacokinetics due to reduced first-pass metabolism, reduction in muscle mass and worsening renal function leading to adverse effects at standard doses. Even medications within the same class may differ. In the CIBIS Elderly study, patients with HF randomized to bisoprolol or carvedilol had similar incidence of side effects (24–25%). However, bisoprolol conferred greater reduction in heart rate and more dose-limiting bradycardia (bisoprolol 16% vs. carvedilol 11%), whereas carvedilol was associated with shortness of breath (bisoprolol 4% vs. carvedilol 10%).66
It is estimated that only 15% of AV block is truly caused by drugs. Although resolution of AV block has been reported in 41% when rate-limiting drugs are discontinued, over half have recurrence of AV block in the absence of therapy.67 Patients receiving cholinesterase inhibitors for dementia are more likely to be hospitalized for syncope (HR 1.76) or symptomatic bradycardia (HR 1.69) and to undergo pacemaker implantation (HR 1.49).68
Intraventricular conduction abnormalities
The incidence and prevalence of bundle branch block (BBB) increases with age. For 855 men aged 50 years, prevalence of BBB increased from 1% to 17% over 30 years.69 Isolated right BBB is more frequent than left BBB (0.18% vs. 0.1%), increasing with age from 0.4% for 45–54 years to 1.3% for >64 years.70 Gender differences are present; BBB has been observed in 11% of men but only 5% of women older than 60 years.71 In old and frail patients with syncope and bi-fascicular block, empirical pacemaker implantation can be carried out without the preceding electrophysiological study.72
Pacing: indications, mode selection, programming, follow-up, remote monitoring
Existing guidelines do not recommend pacemaker therapy be altered for the frail patient, other than to consider frailty when considering cardiac resynchronisation therapy (CRT) and to suggest CRT-pacemaker (CRT-P) over CRT-defibrillator (CRT-D), but stress the need for a thorough review of the individual risk-benefit ratio, including the implications of living with a device, and patient preferences.72,73 The diagnosis of significant frailty along with other risk factors such as advanced age, limited mobility, and life expectancy-limiting co-morbidities may favour the decision to implant a single-chamber pacemaker.73 Untreated SND appears to confer a worse prognosis in the frail. With 17-month follow-up, 57% of patients developed syncope, HF, or AF. Multivariate analysis revealed that age of >65 years was the most important predictor of an event (HR 7.80).74 Generally, the risks of pacemaker implantation are similar in younger and older patients, but pneumothorax, lead dislodgement, and erosion due to low body weight are more common in the elderly.75 Therefore, given potentially greater benefits of pacing the frail, it is suggested that standard pacing indications are followed, even if taking into consideration the increased complication risk and costs of the procedures.72,73
Mode selection depends on the indication for pacing. The UK-PACE trial randomized 2021 patients aged >70 years with high-grade AV block to single- or dual-chamber pacemaker and found no difference in mortality or cardiovascular events.76 In very old and/or frail patients with infrequent pauses who have limited functional capacity and/or a short expected survival, the benefit of DDD(R) vs. VVIR pacing is expected to have limited or no clinical impact, and the incremental risk of complications related to the second atrial lead should also be taken into account when choosing the pacing mode.72 Conversely, maintaining AV synchrony in patients with SND reduces AF, pacemaker syndrome and HF hospitalizations.77,78 Patients with suspected deficits should be formally assessed for frailty using the approved methodology prior to pacemaker implant and selecting the mode of pacing.
For frail patients, attending follow-up may be arduous. With the advent of remote monitoring, in-person follow-ups can be minimized without compromising quality of care. In fact, there is evidence to show remote monitoring can be advantageous.79
Pacing for undocumented bradycardia
Falls are one of the common features of frailty syndrome. Older patients are likely to have multi-factorial aetiology for falls where it may be difficult to differentiate between mechanical falls and falls due to other causes, e.g. bradyarrhythmic. The differential diagnosis may often be hampered by cognitive impairment and amnesia. In this setting, conventional syncope work-up followed by an implantable loop recorder (ILR) rather than empiric pacemaker implant, is endorsed.73
Leadless pacing
Leadless pacemakers may prevent some of the complications related to implantation including pre-pectoral haematomas, extrusions, pocket infections, pneumothorax, tamponade and lead dislodgement. Vascular complications because of the large introducer sheaths and pericardial tamponade (around 1%) are significant risks in the older frail patients and need to be considered before implanting a leadless pacemaker.80
Over the long-term, the cardiac pacing lead is generally considered the system’s weakest link, with risk of the breakdown of the insulation or wire rupture and infections. The rates of these complications are increased in the presence of concomitant disorders often associated with older age. The absence of a lead decreases the rate of lead-related complications and the system is compatible with magnetic resonance imaging. These features make leadless pacing an attractive option for older and frail patients in need of a pacemaker.81,82
Compared to patients with transvenous pacemakers, patients with leadless pacing may experience fewer complications but more dramatic pericardial effusions which highlights the care needed for implantation in older and frail patients. The Micra™ device may have a safety profile similar to that of a transvenous system while providing low and stable pacing thresholds.81
The long-term status of these devices is unknown, particularly the risk of endothelialization and fibrosis, which might hamper their extraction. This may need the abandonment of the leadless electrode, which may be a less significant matter for older patients. Currently, up to 25% of patients are paced in VVIR mode, which is particularly suitable in the older and frail patients, likely to undergo few device replacements.
Consensus statements
|

|
|

|
Ventricular arrhythmias
Ventricular premature beats and ventricular tachycardia
The incidence of ventricular arrhythmias increases with ageing independently of the presence of underlying heart disease,83 and the prevalence of ventricular premature beats (VPB) on Holter ECG in older individuals people has been reported to be as high as 70–80%.84,85 Frequent VPBs may occur as a result underlying electrical, structural, ischaemic abnormalities, with different underlying mechanisms such as re-entry involving post-MI scarring, enhanced automaticity in the chronically ischaemic tissue, or triggered activity due to afterdepolarizations associated with acquired QT interval prolongation or caused by digoxin and are associated with increased risk of developing new-onset cardiomyopathy or worsening of the existing disease.85,86
Some monomorphic VPBs, most commonly of the right or left ventricular outflow tract origin occur in the absence of structural heart disease, are not associated with an adverse prognosis and usually do not require any specific AAD therapy.86
Management of frail patients with frequent VPBs on the background of cardiac pathology, chiefly coronary artery and ischemic heart disease or cardiomyopathy is challenging because of the unfavourable risk/benefit ratio of AAD therapy and only limited data on VPB ablation in this population. Bundle branch re-entry is rare but needs to be identified since it is curable by ablation.
Sudden cardiac death (SCD) in older patients may often be related to malignant ventricular arrhythmias.87 The leading cause is myocardial ischaemia, and the prognosis is poor in older patients, with the survival rate <5%.88 SCD in old or frail people may also be related to electromechanical dissociation or asystole,87,88 which was associated with a nearly 100% mortality rate in most studies. Older survivors may often exhibit cognitive or mood disorders highlighting the influence of age following resuscitated cardiac arrest.89
Pharmacological management of VPBs and ventricular tachycardia
The acute management of ventricular tachycardia (VT) includes intravenous beta-blockers, amiodarone (150–300 mg iv bolus), lidocaine, and mexiletine which may also prevent immediate recurrence of VT and the occurrence of ventricular fibrillation (VF). Amiodarone remains the only AAD that could be used in critically ill patients with frailty.
Beta-blockers and non-dihydropyridine calcium antagonists
As mentioned earlier, beta-blockers are often considered first-line treatment in symptomatic patients with a high burden of ventricular ectopy, but their efficacy is modest. In some cases, a non-dihydropyridine calcium antagonist (verapamil) may be used in selected patients and verapamil-sensitive ventricular ectopy. The same therapeutical principles apply to non-sustained ventricular tachycardia (NSVT). Patients with frequent idiopathic runs of NSVT should be evaluated for the primary genetical electrical heart disease.
Mexiletine and lidocaine
Mexiletine and lidocaine are effective in suppression of ectopic ventricular automaticity and triggered activity caused by delayed afterdepolarizations, and they also may interfere with the re-entrant mechanism of arrhythmias by converting unidirectional to bidirectional block in partially depolarized myocardium observed during ischaemia. The efficacy of mexiletine as monotherapy was assessed in small studies and during programmed electrical stimulation and ranged between 20–30% in suppressing induced ventricular tachycardia and 75% in reducing the number of NSVT runs.90 Mexiletine and lidocaine are similar in both structure and function; unlike lidocaine, mexiletine is well absorbed from the gastrointestinal tract. Mexiletine and lidocaine exert an antiarrhythmic effect without significant inhibition of cardiac function.
Proarrhythmia or other serious toxicity from the drugs is uncommon. Sinus bradycardia and sinus arrest have been documented in patients with pre-existing SND which required monitoring in older frail individuals with a likely impaired sinus and/or AV nodes.
Mexiletine and lidocaine are predominantly metabolized by the liver, and the drug elimination may be delayed in HF and other causes of hepatic insufficiency. For treatment of significant ventricular arrhythmias, mexiletine is given at 200–300 mg tds.; a loading dose of 400 mg can be used followed by 200 mg tds., but the maximum dose should not exceed 1200 mg/day. The elimination half-life of mexiletine is 9–12 h.
D, L-Sotalol
Class III (HERG channel-mediated rapid potassium current blockers) D, L-sotalol is generally avoided in frail patients with multiple comorbidities, polypharmacy, and frequent electrolytes disorders, but may be used in selected patients, more commonly for ventricular arrhythmias, if certain provision is followed such as QT interval monitoring and ensuing there is no significant left ventricular hypertrophy. Sotalol also exerts a nonselective competitive beta-1-adrenoceptor antagonism (predominantly confined to the levo-isomer, I-sotalol).56 It is effective in suppressing complex forms of ventricular ectopy, displaying superior anti-ectopic activity to beta-blockers in patients with stable coronary artery disease, but is not suitable for patients with HF on the background of hypertensive heart disease and left ventricular hypertrophy and significant left ventricular systolic impairment. Sotalol 160–640 mg/day reduced ventricular ectopy, most notably higher grade ventricular arrhythmias (polymorphic and repetitive premature ventricular complexes, couplets and runs of NSVT); this action was maintained in the presence of mild left ventricular dysfunction and was sustained in the long-term (∼2–6 years).91
Amiodarone
When beta-blockers alone are ineffective, current evidence suggests that amiodarone is usually beneficial in patients with HFrEF and high VPB burden, also considering its cardiac safety. There are different loading regimens of the drug.
Other antiarrhythmic drugs
Based on current evidence, Class IA (disopyramide) and IC (flecainide and propafenone) agents, despite the efficacy and the wide use of the latter in subjects without significant structural heart disease, are not indicated in patients with underlying cardiac conditions and/or HF which are common in frail individuals, because of their negative inotropic effect and risk of ventricular proarrhythmias.58 Pure Class III dofetilide is not available worldwide, including many European countries, whereas the use of a multichannel ion blocker dronedarone is limited to AF and is not widely used in Europe.
When to intervene?
There is no established clear cut-off point for the initiation of treatment with regard to the number of VPBs. Patients with extremely frequent VPB (>10% of the total beats during 24-h monitoring) are deemed more likely to experience some degree of left ventricular dysfunction or developing arrhythmia-induced cardiomyopathy.92,93 A VPB burden of >24% or >20 000 VPBs during the 24-h period has shown a strong association with the development of cardiomyopathy.94 However, the threshold varies greatly and may be significantly lower in patients with impaired left ventricular systolic function and HF, with cardiomyopathy and worsening HF being reported in association with VPB frequency as little as 4%.
Other VPB features such as QRS duration as a measure of ventricular dyssynchrony, prematurity index, multiform VPBs, increased VBP density during exercise, repetitive forms such as ventricular couplets and triplets, intrapolated VBPs, VPBs of epicardial origin, and duration of exposure to frequent VPBs may be associated with the development of cardiomyopathy or worsening HF and should prompt further imaging such as cardiac MRI and more intensive follow-up aimed at assessing the frequency of VPBs and left ventricular systolic function (an echocardiogram if MRI did not reveal any underlying condition).
Drug-induced ventricular arrhythmias
Acquired long QT potentially resulting in polymorphic and torsade de pointes VT is the most significant drug-induced proarrhythmia. The majority of AADs may cause proarrhythmia, particularly in the presence of precipitating factors such as electrolyte abnormalities and drug interaction,56 with torsade de pointes considered a significant concern in frail patients where electrolyte disorders and polypharmacy are common. The main cause is iatrogenic QT prolongation (the full list of agents with a torsadogenic potential may be seen at https://crediblemeds.org), but it may also occur when QT prolongation is secondary to sinus bradycardia or AV block. Thus, in the study of 202 hospitalized patients aged above 75 years, 22% of VT cases were ischaemic and 50% were iatrogenic.84
Treating proarrhythmias
Effective treatment requires the accurate recognition and confirmation of drug-induced proarrhythmia and prompt discontinuation of the implicated agent. Important are also the identification modification of risk factors potentially associated with arrhythmia onset or worsening (e.g. female gender, advanced age, renal or liver dysfunction, underlying structural heart disease, hypokalaemia, hypomagnesaemia, high drug doses/concentrations, rapid intravenous administration, bradycardia, QT prolongation, and pre-existing channelopathies).56
The offending drug should be stopped, and intravenous administration of magnesium sulfate irrespective of serum magnesium levels should be administered (e.g. 2000 mg bolus followed by a second bolus and by continuous infusion if the proarrhythmia persists). Bradycardia and pauses that may trigger torsade de pointes should be reversed by either pacing at >70 bpm or isoproterenol infusion. Hypokalaemia should be corrected, aiming at replenishing serum potassium to the high-normal range (i.e. 4.5–5.0 mEq/L).
Beta-blockers may be used in some circumstances. In milder cases, arrhythmias due to digitalis toxicity can be managed by discontinuation of the drug, potassium supplementation and observation. For digitalis-induced life-threatening arrhythmias, several AADs have been proposed in the past (e.g. phenytoin, lidocaine and beta-blockade). More recently, digitalis-specific antibodies have proven effective in reversing digitalis toxicity by rapidly binding to and acutely lowering serum digitalis. Isoproterenol infusion or cardiac pacing are usually effective when symptomatic bradyarrhythmias secondary to conduction abnormalities occur.
Ablation for ventricular arrhythmias
In patients with high VPB burden and arrhythmia-induced cardiomyopathy worsening HF, ablation may be a preferred treatment option which has been reported to result in a sustained reduction in the VPB burden and associated with a lower risk of hospitalization for HF, cardiac death, or the need for heart transplant.95 However, this intervention has not been explored in prospective studies and is limited in patients with frailty syndrome. Some data available from the retrospective cohorts enrolling older patients ≥70 years with structural heart disease (but without systematic formal frailty assessment) undergoing ablation for VT suggest that older individuals were more likely to sustain peri- procedural complications and had higher in-hospital (4.4% vs. 2.3%; P = 0.01) and 1-year mortality (15% vs. 11%; P = 0.002) compared with their younger counterparts, but a similar incidence of VT recurrence at 1 year (26% vs. 25%) and time to VT recurrence (280 vs. 289 days).96 The absence of VT recurrence during follow-up was strongly associated with improved survival in patients ≥70 years. It should be acknowledged that these data concern older, but not necessarily frail individuals and that these data cannot be extrapolated on patients with frailty. On the other hand, these patients may potentially derive a great benefit from the procedure as it may eliminate the long-term risk of pharmacological rhythm control. While it would be challenging to carry out a prospective trial in this setting, collecting data on VT ablation in frail patients should constitute part of national ablation registries or an international registry should be initiated.
Knowledge gaps
Due to multi-morbidity and polypharmacy, the usual risk assessment for proarrhythmias may not be sensitive in patients with frailty syndrome; a standardized protocol specifically developed for frail patients may be required.
There is very limited data on the efficacy and safety of ablation for ventricular arrhythmias in frail patients; an international registry is needed to accumulate these data in a structured fashion.
Consensus statements
|

|
|

|
|

|
|

|
|

|
|

|
ICD: indications, selection, and outcome
Implantable cardioverter-defibrillator (ICD) therapy is beneficial in older patients for primary prevention of SCD when life expectancy is >1 year.97,98 Randomized studies have reported divergent results on the benefit of ICDs in older patients.99,100 In well-selected patients at high risk of arrhythmic death and with few comorbidity factors despite advanced age, ICD intervention may reduce mortality to nearly age-specific life expectancy. It should be acknowledged that these were highly selected populations, and the indications for a primary prevention ICD in the non-ischaemic population is not strong enough to warrant routine ICD implantation in the older and frail patients because of the competing causes for deaths (usually non-cardiac). Thus, in the recent study of octogenarian primary prevention ICD recipients, three quarters had no more than one comorbidity, resulting in similar rates of both appropriate and inappropriate device therapies compared with their younger counterparts.99 Among patients who died during 19-month follow-up (35%; of these 38% from non-cardiovascular causes), one-third received at least one appropriate ICD therapy.
In a cohort of 83 792 Medicare patients the NCDR ICD registry, who underwent first primary prevention ICD implantation, approximately 1% had dementia, whereas 10% had frailty defined using a coding algorithm of frailty markers based on 99 ICD-9 codes, selected by a group of geriatricians, and categorized into 10 clusters: malnutrition, dementia, severe vision impairment, decubitus ulcer, incontinence of urine, incontinence of faeces, weight loss, social support needs, difficulty walking, and falls.100 Patients with dementia and frailty had substantially higher mortality within the first year after ICD implantation (27% and 22%, respectively) compared with those without these conditions (12%). Several multi-morbidity patterns were associated with high 1-year mortality rates: dementia with frailty (29%), frailty with COPD (25%), and frailty with diabetes (23%).
The predictive usefulness of Charlson Comorbidity Index (CCI) has recently been explored in older (mean age: 78 years) candidates for ICD.101 At 5-year follow-up after ICD implantation, survival was 78%, 57%, and 29% in patients with a CCI score of 0–1, 2–3, and ≥4, respectively, compared with 72% in non-frail controls. There was no significant difference in appropriate ICD therapy. The median potential survival gain after an appropriate therapy was >5, 4.7, and 1.4 years, with a CCI score of 0–1, 2–3 and ≥4, respectively. The limitations of CCI include difficulties using in clinical practice, not being developed in older adults, whereas conditions that may have the most bearing on outcomes, such as frailty and dementia, are not incorporated or not appropriately weighted.
Therefore, a multivariable score (rather than chronological age per se) and individualized consideration, focusing on comorbidities, projected expectancy, the life-long risk of complications, impact of an ICD on quality of life, including psychological health, and patient preference should help in decision-making for ICD selection and its survival benefit.102
In secondary prevention ICD, a meta-analysis suggested that ICD therapy did not show any survival benefit in patients aged >75 years [HR for all-cause death 1.06 (95% CI: 0.69–1.64), HR for arrhythmic death 0.90 (95% CI: 0.42–1.95)].103 However, older age does not diminish the likelihood of receiving appropriate therapy.104 A careful evaluation of comorbidities that may increase the relative risk of non-arrhythmic mortality is again needed.
In the oldest patients, discussion regarding the effect of implantable devices on the mode of death (ICD preventing SCD but exposing to the risk of prolonged and progressive HF) takes a particular place in decision-making according to patient choice.105,106 ICD intervention among the elderly as a group may be less cost-effective, but cost-effectiveness is expected when ICD is implanted in patients expected to live sufficiently, e.g. >5–7 years after implantation.106
However, in all these studies much of information comes from carefully selected older individuals with few comorbidities, low-grade frailty, or from mixed populations using heterogeneous definitions for frailty, as this was not the primary endpoint in these studies. The presence of frailty generally was an exclusion criterion from both RCTs and even observational studies. Hence, very limited data exist regarding the risk–benefit of ICD therapy. A review of nine studies, including two RCTs, one prospective cohort, and six retrospective cohort studies, with the number of patients ranging from 77 to 98 437 and follow-up ranging from days to 6 years, has revealed that patients with elements of frailty defined according to varying validated methods, including cumulative deficit models, low weight, and walking speed, had higher all-cause or peri-operative in-hospital mortality.107 As mentioned earlier, there is no uniformly established score to assist in identification of a suitable candidate for ICD therapy in frailty, although some physical components, such as a 6-minute walking test, timed chair stands and balance, handgrip strength, as well as questions about weight loss, physical activity, and exhaustion, have been found useful in some reports.107,108
Wearable cardioverter-defibrillators may be an alternative to some patients. However, these devices require a high level of compliance, good understanding how it works, and a certain physical strength. Therefore, these devices will have a limited role for prevention of SCD in frail patients.
ICD programming in frail older patients
Recent ICD studies focused on new programming strategies in patients with little or no exclusion criteria in order to decrease the rate of inappropriate therapies and to deliver less aggressive therapy in case of sustained ventricular arrhythmia.109–113 Whether such modern strategies are as safe and efficient in elderly patients is an open question, but such conclusions can be made from these studies. Prolonged detection as well as high VF rate settings decrease the number of inappropriate therapies and the number of shocks with the use of antitachycardia pacing in fast VT. Such programming parameters are proposed in current consensus documents by scientific societies112,113 and may be safely applied in elderly or frail patients.
Subcutaneous ICDs
The subcutaneous ICD (S-ICD) is emerging as a therapy for the prevention of SCD avoiding the complications associated with transvenous leads.114 The S-ICD system is essentially promising in terms of reduction of electrode-related complications such as infection or lead failure, which may be more relevant for relatively young and active patients. This may, however, be an option in elderly or frail patients in case of limited vascular access or persistent infection. Low body weight at baseline and risk of progressive body mass and muscle loss, unless revised and corrected, may be the limitation for implanting S-ICD in patients with higher frailty scores.
Lead failure management
The need for managing CIED infection and abandoned leads increases with advanced age. There are numerous gaps in evidence regarding lead extraction tools, management of abandoned and recalled leads, prevention and treatment of infections, and risk stratification for lead extraction. Such evidence in older and frail patients is difficult to collect because the number of such patients even in high-volume centres, is too low to create statistically solid data. In very old and frail patients with limited life expectancy, leads (and the generator, if therapy is terminated) are usually abandoned.
Knowledge gaps
The decision on implantation of an ICD or CRT-D in a patient with frailty should be assisted by a dedicated simple and robust score in order to ensure equal access to therapy for all frail patients; such a score should be developed to be specifically used in people with frailty.
Consensus statements
|

|
|

|
Heart failure and cardiac resynchronization therapy
Definition and assessment of frailty in HF
Among all cardiovascular pathologies, HF has the strongest link with frailty, with up to 79% of patients with HF identified as frail.3,23 The two conditions share several pathophysiological mechanisms, primarily myocardial and metabolic incompetence, implicated in a so-called ‘dependency cascade’—a process that identifies a sequence of progressive damage involving multiple organ systems that perpetuates itself. 115,116 The rates of mortality and hospitalization are the highest in patients with HF who are also identified as frail.
Assessment of frailty is essential in the management of older patients with HF as chronological age does not automatically identifies the health status. Heart Failure Association (HFA) has proposed a definition of frailty in patients with HF as a multidimensional dynamic and partially reversible state, independent of age, that makes the individual with HF more vulnerable to the effect of stressors.3 Although several frailty assessment instruments have been used in HF patients,117 none of them has been validated in HF. HFA has called for the development of a score that is disease-specific to identify frailty in HF.
CRT: indications, optimization, follow-up
CRT with (CRT-D) or without defibrillator function is one of the most widely employed non-pharmacological managements of patients with NYHA II–IV class HF, wide QRS complex (primarily LBBB), and left ventricular ejection fraction <35%, with proven efficacy in symptom relief, improvement in exercise capacity and quality of life, hospitalization for HF, and mortality.72,118 The prevalence of frailty in patients treated with CRT has not been systematically assessed, but several small studies, using various frailty measurement tools, have reported that it may be as high as 81% in patients undergoing de novo implant and 68% in those undergoing the system upgrade.119
The benefits of CRT in frail patients are expected to be lower due to multiple comorbidities, including the higher prevalence of AF, although preliminary reports have suggested that the progression in frailty-associated symptoms, such as cognitive impairment, can be deterred by CRT.120
Frailty defined by G8 score <14 was associated with a poorer response to CRT and a higher proportion of non-responders.119,121 Mortality and hospitalization rates were also significantly higher than in non-frail individuals, with the majority of deaths occurring from HF rather than arrhythmic causes. This underscores the need for systematic screening for frailty in patients at risk who have been offered CRT and the importance of optimal medical therapy and exercise training to reverse or deter frailty-related reduced mobility and nutritional and cognitive impairment.
Knowledge gaps
Difficulty assessing frailty in the presence of HF due to the overlap of two conditions, suboptimal performance of frailty assessment tools.
No reliable tools for the prediction of life expectancy in patients with HF and frailty which is essential for the selection of appropriate therapies (e.g. CRT-D, ICD).
Lack of data on the outcome of therapies due to the exclusion of elderly frail patients and patients with multiple comorbidities from clinical trials—studies should include the full spectrum of community-dwelling and institutionalized older adults and assess such measures as health status, quality of life, functional capacity, as well as conventional outcomes.
Further research is needed focusing on the link between mental health (depression) and the clinical course and outcome of HF in association with frailty.
Need for the improved awareness of frailty and structured management with an emphasis on symptom control, exercise training, and quality of life via rehabilitation programmes.
Consensus statements
|

|
|

|
Device replacement, upgrade/downgrade, and deactivation at end of life
Patients with cardiovascular disease at risk of arrhythmic death and/or heart failure are candidates for ICD and/or CRT, yet, patients with cardiovascular disease are likely to become frail.23,122 Clinical effectiveness of ICDs in older populations may be due to “healthy candidate” bias.123 Frail patients, being at great risk of death and disability are less desirable candidates for CRT upgrade, ICD implant and/or replacement106,124 and many become frail after ICD implant, at time of pulse generator change. A median of 1.2 life-years were gained for those > 80 who came for ICD replacement (2-year mortality 38.1%; 16.7% having life-threatening arrhythmias).104 While no prospective trial proves lack of any benefit from ICD replacement or CRT upgrade in a frail population, a substantial percent of frail patients are less likely to respond to CRT (53% vs 73%),119 and are at greater risk of heart failure decompensation (55.6% vs. 16.4%),125 cardiovascular readmission and death119,126 after device implant. Frail patients also have risk of complications including infection, erosion, perforation, and, if necessary, lead extraction with poor outcomes.
Before ICD replacement, at end-of-battery life, frailty (an albeit multifaceted, non-dichotomous condition) should be re-assessed (perhaps in part via activity device monitors127) and, whether or not ‘appropriate’ antitachycardia therapies have been given, it is reasonable to forgo ICD replacement and even consider turning off the device.128 For those considered for CRT upgrade, similarly, based on the severity of the frailty and underlying comorbidities, it is reasonable to forgo CRT revision.
Because of potentially lower efficacy and higher risk of complications of device therapy, particularly ICD and CRT-D in patients with frailty syndrome, an informative and honest discussion involving legal and ethical issues, including the eventual need for device deactivation should be held with the patient and a caregiver in all individual cases. In frail patients, to improve the dying process, and, with informed, and complete, knowledge of the consequences, it is reasonable for the patient, or legal representative, to request deactivation of any, and all, ICD or CRT and to work with the doctor to ensure this occurs.128
However, as generally there is insufficient data, there is also lack of directives regarding advance care planning including device deactivation, palliative, and end-of-life care, whereas lack of training for healthcare providers (e.g. 90% are willing to discuss withdrawal of various life support therapies, but less than 50% would engage in the discussion of ICD deactivation) creates an unmet need in such as sensitive matter.
ICD recipients need education and conversation with their physicians about managing their devices in a systematic fashion. As one survey from octa- and nonagenarian Swedish ICD recipients has revealed, one-third (34%) had discussed their illness trajectory with their physician, a minority (13%) had discussed what turning off shocks would involve with their physician, and just 7% had told their family their wishes about a possible deactivation in the future. About one-fourth of the octo- and nonagenarians had insufficient knowledge regarding the ethical aspects, function of the ICD, and practical consequences of withdrawing the ICD treatment in the end-of-life.129 However, it is important that the majority of participants expressed their desire for battery replacement when one was needed, even if they had reached a very advanced age (69%), or were seriously ill with a life-threatening disease (55%).
Knowledge gaps
Need for developing a plan for the structured and sensitive discussion involving the physician, the frail patient and his/her family about the possibility of a rapid clinical deterioration, with the onset of a terminal condition and the need for device inactivation and an advance care provision.
Consensus statement
|

|
Supraventricular arrhythmias
Atrial tachycardias are the least frequent form of supraventricular tachycardias in the general population, and there are no specific data in frail patients. There is a higher proportion of macro-re-entrant atrial tachycardias with advance age.83,130 Atrial tachycardia is often drug-resistant, whereas ablation may be ineffective due to significant atrial remodelling.
Atrial flutter rates increase significantly with ageing, ranging from 5/100 000 in patients under 50 years of age to 587/100 000 in those aged 80 years and older.131 Older frail patients have higher rates of impaired heart rate response and variability characteristics that are further reduced with poor mobility. Although these patients are less likely to be treated with ablation, limited data in selected individuals who were functionally preserved, ablation of typical atrial flutter had a high success rate of 86% and was not associated with excess complications.132
Atrioventricular node re-entrant tachycardia can present later in life due to an increase in triggers from ageing and coexistent cardiovascular diseases. Age-related changes in the AV node electrophysiology may lead to prolongation of atrial refractoriness of the slow pathway. No systematic data is available on the efficacy and safety of AV node modification, some limited series reported success rates up to 98%.133
Atrial fibrillation
AF is the most prevalent sustained cardiac arrhythmia in adults, associated in a multifaceted manner with significant morbidity and mortality, hospitalization and impaired quality of life.134 Hospitalized AF patients have been shown to have a four-fold greater odds of being classified as frail in comparison to non-AF patients independently of age, sex and comorbidity.135,136
The prevalence of frailty in AF patients ranged from 4.4% to 75.4%, while AF prevalence in the frail population ranged from 48.2% to 75.4%.137 Among AF patients, frailty was significantly associated with prolonged hospitalization and increased symptom severity, incidence of stroke and all-cause mortality.136,138
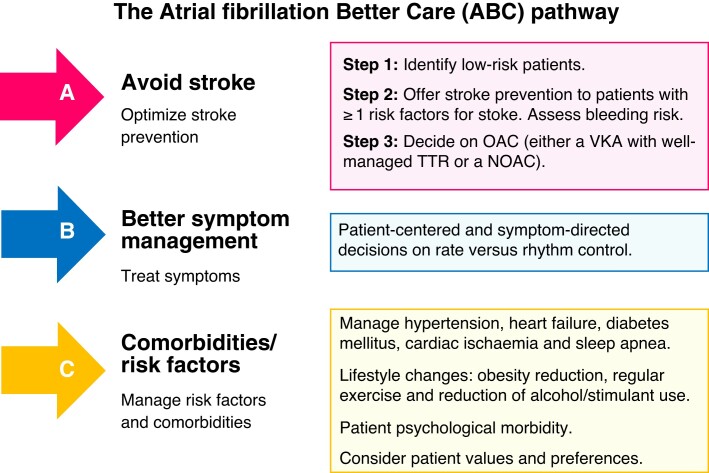
Owing to a greater prevalence of cognitive impairment, tendency to fall, polypharmacy and cardiovascular or other comorbidity among frail patients, management of AF in such setting may be challenging, since anticipated suboptimal adherence to treatment, drug–drug interactions and increased bleeding risk may influence treatment decisions. The ABC-integrated AF management pathway139 (Figure 2), that provides a holistic approach to management of AF patients and reminds clinicians of essential decision-making steps in this process, also applies to frail AF patients.
Figure 2.
The ABC-integrated AF management pathway. Adapted from Lip GYH.139 ABC, Atrial fibrillation Better Care; AF, atrial fibrillation; NOAC, non-vitamin K antagonist oral anticoagulant; OAC, oral anticoagulant; TTR, time in therapeutic range; VKA, vitamin K antagonist.
Rate vs. rhythm control management
There are two primary clinical approaches to managing the arrhythmia.
Rate control: slowing the ventricular rate to a level which is physiologically appropriate. Advantages of the rate control approach include ease simplicity avoiding the potential toxicity of AADs or the risks and discomfort associated with electrical cardioversion or invasive left atrial ablation for recurrences of AF.
Rhythm control: restoration and long-term maintenance of sinus rhythm; AADs (ion channel blockers) are predominantly used but occasionally autonomic manipulation, for example with beta-blockers may prove valuable.
Rate control remains an essential component of therapy even if the primary strategy is rhythm control (e.g. in the case of a recurrent arrhythmia).
Two targets of rate control have been debated: (i) strict rate control with a target ventricular rate response of <80 bpm at rest and <110 bpm on moderate exercise, usually achieved by the combination of two drugs with an AV blocking effect and repeatedly assessed by different means including an ECG, ambulatory Holter ECG monitoring, exercise stress test, and in some circumstances, implantable rhythm monitoring devices, and (ii) lenient rate control which allows ventricular rates of <110 bpm at rest which usually does not require extensive monitoring.134
While lenient rate control with a resting heart rate <110 bpm can be employed as the initial target, it should not lead to the assumption that lenient rate control remains an acceptable, or preferable, option to a more aggressive rate control approach in the long-term. Lenient rate control, often employing a single agent is suitable for older frail individuals with limited morbidity, in whom AF is deemed permanent and is asymptomatic.
Beta-blockers, which are part of the mainstream therapy of HF, represent the first-line choice for acute and long-term rate control, especially in patients with evidence of HF (particularly, HFrEF and HFmrEF), due to their efficacy at high sympathetic drive. Digoxin (62.5–250 µg daily), which has an antiadrenergic effect, increases the AV node refractory period, and inhibits the sodium-potassium adenosine triphosphatase pump—with the resultant improvement in ventricular contractility—can also be used as a first-line rate control drug in permanent AF patients intolerant to beta-blockers, particularly, in older, sedentary, ones. Digoxin is beneficial as a second-line remedy in those with a suboptimal response to beta- or calcium channel blockers. In a small randomized open label study in older patients the majority of whom had HFpEF, digoxin favoured better than bisoprolol in respect of symptom relief and quality of life scores and was associated with a greater adherence to treatment at 6 months.140 Digitoxin is a potential alternative to digoxin and is currently being evaluated in a randomized placebo-controlled trial (ClinicalTrials.gov Identifier: NCT03783429). As a last resort amiodarone is advocated, especially in patients with HFrEF.134
Of the two prime treatment strategies for AF rhythm control is intuitively more attractive as it offers physiological rate control, normal atrial activation and contraction, the correct sequence of AV activation, normal haemodynamic and AV valve function, and theoretically eliminates one (stasis) or more (endothelial abnormality or increased thrombogenic blood constituents) of Virchow’s triad of elements that encourage thrombosis within the atria and embolization of blood clots to potentially critical parts of the circulation. Elimination of irregular AV conduction, which adds to ventricular dysfunction, is an important component of the beneficial effects of the rhythm control approach. However, the choice of AADs in frail patients with multiple comorbidities, polypharmacy, reduced repolarization reserve, and high risk of proarrhythmia is often limited to amiodarone. The relative cardiac safety of amiodarone should be balanced against significant extra-cardiac side effects. The rate-slowing effect of amiodarone is an additional benefit, particularly in paroxysmal AF.
Knowledge gaps
The consequences of the reduction in arrhythmia burden for disability and frailty development or reversal constitute an important knowledge gap in geriatric cardiovascular medicine.
Stroke prevention
General principles
Optimal prevention of stroke or systemic thromboembolism associated with AF involves three crucial steps (Figure 2).139
Clinician first needs to identify truly low-risk patients (i.e., men with a CHA2DS2-VASc score of 0 and women with a CHA2DS2-VASc score of 1 due to female sex), who do not need any antithrombotic therapy, whereas all other AF patients benefit from a stroke prevention strategy, using oral anticoagulant (OAC) drugs [preferably a non-vitamin K antagonist OAC (NOAC), or, alternatively, a well-managed vitamin K antagonist (VKA), with ≥70% time in therapeutic range (TTR)].134,141 The preference to NOACs over VKAs largely stems from their better safety, especially concerning haemorrhagic stroke and other intracranial bleeding, and more convenient long-term use in comparison to VKAs.142
The concomitant assessment of bleeding risk is needed to control modifiable risk factors and to identify patients with non-modifiable risk factors, who will need frequent clinical follow-up evaluations. The use of the well-validated HAS-BLED bleeding score was superior to the approach concerning only modifiable bleeding risk factors in multiple cohorts,143–146 likely due to a more holistic bleeding risk management using the HAS-BLED score. Importantly, neither stroke nor bleeding risk are static, but change over time and need to be re-assessed during clinical follow-up.134,142
Antithrombotic management in old and frail
A meta-analysis of 6 studies suggested that OAC prescribing patterns in frail AF patients are influenced by a complex interplay of multiple factors including thromboembolic and bleeding risks, frailty status and AF management setting (e.g., the community, hospital or nursing care) reflective of concomitant competing risks, life expectancy, physician’s expertise in AF management and completeness of case assessment.136
Factors that may influence OAC under-prescription or discontinuation, such as advanced age, multiple comorbidities, impaired cognitive function, suboptimal adherence and increased bleeding risk,136,146 are commonly seen in the general AF population. Regarding advanced age, warfarin was associated with a positive net clinical benefit in AF patients aged ≥75 years in comparison to aspirin [a 2% absolute risk reduction in stroke or systemic embolism, with comparable major bleeding rates (1.4% vs. 1.6%)],147 as well as in those ≥90 years old in comparison to no therapy or antiplatelet drugs.148
All trials of NOAC treatment in AF included significant populations of older people (defined as ≥75 years) ranging from 31% to 43% in the individual trials, comprising over 27 000 older patients in whom NOACs were studied. In a meta-analysis of pivotal NOAC AF trials, overall efficacy and safety of NOACs were consistent in all age groups,141 but there was a significant interaction between age and extracranial major bleeding rates with both dabigatran doses in patients aged ≥80 years (in comparison to warfarin, event rates were similar with the 110 mg dose and significantly higher with the 150 mg dose).149 No such interaction was seen with rivaroxaban,150 apixaban,151 or edoxaban.152 Indeed, the higher absolute stroke risk in the elderly resulted in a larger absolute risk reduction with NOACs vs. VKAs and a lower number needed to treat in comparison to younger patients.152 Overall, prescribing aspirin instead of OAC in the elderly with AF was actually harmful—the rates of major bleeding with aspirin were similar to those with well-managed VKAs147 or NOACs,153 whereas aspirin was essentially ineffective in stroke prevention.154 Extreme frailty was linked to increased risk of bleeding associated with aspirin.134
The use of VKAs was associated with better cognitive function in comparison to aspirin among elderly AF patients, and better cognitive function was also observed among NOAC users compared with warfarin.155 In a meta-analysis of five AF studies (one RCT, four observational), OAC use was associated with a significant 21% risk reduction in dementia vs. no antithrombotic therapy, and a TTR of ≥75% was associated with less dementia among warfarin users.156 Pivotal NOAC AF trials have not specifically addressed cognitive function, but numerous trials are currently investigating the effects of NOACs on cognitive function in AF patients.
Falls are more common among frail AF patients and are a marker of increased risk of adverse events, but not an independent predictor of OAC-related bleeding, and the net clinical benefit of OAC outweighs the risk of severe bleeding among AF patients who sustained falls. Treatment effects of apixaban and edoxaban were consistent irrespective of the falling risk status, with a larger absolute risk reduction with the respective NOAC vs warfarin, owing to the greater absolute risk of events among patients at increased risk of falls.157,158 In a propensity score matched analysis of Medicare data, apixaban was associated with lower rates of adverse events across frailty levels, however residual confounding could not be fully excluded.159
Additional considerations when choosing OAC drug (and dose) for a frail AF patient include low body weight (which is a dose-reduction criterion for apixaban and edoxaban), polypharmacy (with increased potential for drug–drug interactions) and comorbidity (i.e. CKD, malignancy, epilepsy, etc.) and are discussed in detail in the EHRA Practical Guide on the use of NOACs in AF patients.160 Weight is an important factor affecting the dose selection in a frail patient. Low body weight (usually defined as BMI <18.5 kg/m2) may increase exposure to any NOAC and therefore, may increase the risk of bleeding. Body weight ≤ 60 kg requires dose reduction of apixaban [in patients with age ≥ 80 years and/or serum creatinine ≥133 mmol/L (1.5 mg/dL)] as well as for edoxaban, whereas it is in itself not a factor for dose reduction of rivaroxaban or use of lower dose dabigatran. Noteworthy, prescribing a reduced dose of OAC is less effective in preventing AF adverse outcomes.
Notwithstanding all these factors, frailty itself should not preclude the use of OAC (preferably a NOAC) in eligible AF patients. Observational data suggest that among frail AF patients OAC use is associated with lower event rates compared with OAC non-use or aspirin.136 In acutely hospitalized frail elderly AF patients, OAC non-use was associated with significantly higher adjusted rate of composite outcome of ischaemic stroke or bleeding compared with OAC use [HR 4.54 (95%CI, 1.83–11.25)],138 and in a community-dwelling cohort of older AF patients using OAC, the stroke incident rate was higher than that of major bleeding (1.73 vs. 0.9/100 person-years).161 In a retrospective, propensity score-matched analysis of a US administrative dataset of frail AF patients, stroke or systemic embolism rates were significantly lower with rivaroxaban vs. warfarin [1.78 vs. 2.61, HR 0.68 (95%CI 0.49–0.95)] and similar with apixaban (1.68 vs. 2.15) and dabigatran (2.06 vs. 2.20) vs. warfarin, while major bleeding rates were similar with all four OAC drugs and intracranial bleeding was significantly lower with all three NOACs compared with warfarin.162
The ELDERCARE-AF trial was a placebo-controlled trial investigating a NOAC (very low-dose edoxaban, 15 mg once daily) in the elderly Japanese patients with AF deemed unsuitable for standard OAC therapy. In this trial, the use of edoxaban was associated with a 4.4%/year absolute risk reduction in stroke at the cost of a non-significant absolute increase in 1.5%/year of major bleeding.163
In older patients, the prevalence of cerebral amyloid angiopathy and cerebral microbleeds on MRI is high, and indicates increased risk of intracerebral haemorrhage and is often considered a contraindication for anticoagulation.134,160 Whether this risk is lower and the net clinical benefit sufficient to initiate anticoagulation remains unknown, it is advisable to discuss this opportunity on an individual basis.132
Importantly, frail AF patients considered for OAC require a detailed assessment of their baseline risk profile and personal values and preferences, as well a frequent clinical follow-up. Patient compliance and adherence, particularly to the twice daily regime, should be assessed at the initiation of therapy and monitored.
Left atrial appendage occlusion
In general, the most common justification for left atrial (LAA) closure over systemic OAC is high bleeding risk (e.g. due to falls, liver and kidney dysfunction, polypharmacy and drug interaction) or absolute contraindications to systemic OAC, with frail patients being particularly vulnerable. The decision-making is hindered by insufficient high-quality prospective data comparing LAA occlusion devices with NOACs and the need for antithrombotic therapy after implant and by lack of systematic experience of such an intervention in frail patients, although, according to recent Medicare database analysis, nearly half the patients who underwent LAA closure were considered frail at intermediate or high levels of frailty.164 At present, the ESC Guidelines restrict their recommendations for LAA closure therapy to patients with contraindications for long-term OAC, for example, intracranial bleeding without a reversible cause and to patients undergoing cardiac surgery who could have simultaneous surgical LAA occlusion or exclusion.134
Although the peri-procedural complications and mortality associated with percutaneous LAA closure is likely to be higher in frailty, this therapy may benefit frail patients in the long-term as analysis of the Medicare database has recently demonstrated. It included 21 787 patients aged 65 years and older who underwent LAA closure, 10 740 (49.3%) of whom were considered frail based on the Hospital Frailty Risk Score (HFRS) >5; 33.5% were regarded as intermediate (HFRS 5–15) and 15.8% as high risk (HFRS >15). HFRS was calculated on the basis of 109 International Classification of Diseases, Tenth Revision, Clinical Modification secondary diagnosis codes from all hospitalizations occurring at least 1 year before the date of admission for the index hospitalization or using secondary diagnosis codes during the index admission.
HFRS >15 was associated with 8.3-fold increased risk of long hospital stay (>10 days) and 1.8 times and nearly 5.7 times higher 30-day readmission and 30-day mortality, respectively, compared with HFRS <5. One-year mortality was increased by 2.8-fold in the high risk group. The one-year mortality rates (8.2%) in frail patients were nearly three- to four-fold higher than reported in the PROTECT-AF (2.5%) and PREVAIL (3.0%) trials.164
Knowledge gaps
More high-quality data are needed to inform optimal management of stroke risk in frail AF patients.
A prospective study systematically evaluating the frail status in patients undergoing LAA closure is needed to assess the relationship between frailty and treatment outcomes and benefits.
Further research is needed for the efficacy and safety of left atrial appendage occluders in high-risk patients (with contraindications for oral anticoagulation) vs. best medical care.
Consensus statements
|

|
|

|
|

|
|

|
|

|
|

|
|

|
Ablation: indications and outcome
The frail state, with associated risk factors, can also negatively impact clinical decisions regarding use of more aggressive therapies such as non-pharmacologic interventions, AAD therapy, and anticoagulation.165,166 Ablation therapies include AV node ablation and pacing (which has been used predominantly in older patients) and left atrial ablation aiming at rhythm control. Ablation if successful in selected patients with symptomatic AF can provide a durable long-term rhythm benefit without the need for AADs therapies.167 Some insight regarding procedural benefit of ablation can be gleaned from examining catheter ablation outcomes in the very aged. Age alone is a significant risk factor for AF recurrence after ablation in older patients.168 In patients with successful ablation, the long-term rates of stroke are relatively low across all age groups and associated risk factor profiles. In this regard, patient selection who may benefit from ablation becomes critical.
In observational studies that examined ablation in patients >80 years of age, catheter ablation was safe and effective compared with younger patients. In octogenarian patients, despite more coexisting cardiovascular diseases, 1-year survival free of arrhythmias was 78% vs. 75% in younger patients.169 In another study, over a follow-up of 18 ± 6 months, 68% of octogenarians were free of AF compared with 71% of patients that were <80 years of age. In both groups additional procedures increased long-term AF free success rates to 87%.170 In both studies severe complication rates were not increased in the older groups. Other studies have addressed slightly younger populations (75 years and older) demonstrating an 86% efficacy rate at 1 year and 52–59% efficacy at 3–5 years.171–173 However, older patients are more likely to have non-paroxysmal AF and non-pulmonary vein triggers requiring more extensive left atrial ablation and/or repeated procedures.169
There is limited evidence from retrospective reports that some degree of frailty may not be uncommon in patients undergoing AF ablation and that it is associated with higher mortality and adverse outcomes after ablation. Using HFRS calculated from ICD-10 diagnostic codes, 38.6% out of 5070 in-patients treated with catheter ablation were defined as frail with HFRS >5 including 8,3% including 8.3% at high risk (HFRS >15).172 Frailty was independently associated with length of stay, post-procedure 30-day mortality, and 30-day readmission rates. The long-term mortality (up to 630 days) was 5.8% in the low-risk group, 23.4% in the intermediate-risk group (HFRS 5-15), and 42.2% in the high-risk group. In propensity score matching analysis, frail patients did not benefit from ablation with respect to HF admissions or stroke as opposed to their non-frail counterparts.173
These observational studies represent selected elderly AF patients that were felt to be candidates for the procedure, but in aggregate provide evidence of procedural benefit when ideal candidates are identified. The CABANA trial randomized 2204 AF patients age >65 years, or <65 years with ≥1 risk factor for stroke to antiarrhythmic/rate control drugs vs. catheter ablation. In the intention-to-treat analysis, the primary endpoint of all-cause mortality, disabling stroke, serious bleeding or cardiac arrest (primary endpoint) by intention-to-treat trended towards harm with ablation in those patients >75 years of age [HR 1.46 (95% CI: 0.80–2.67)], however those >75 years of age still were much more likely to remain in sinus rhythm compared with medications [HR 0.48 (95% CI: 0.33–0.68)].174
Clearly, some patients, particularly those with multiple other medical conditions, are reluctant to consider a major procedure and have a strong preference for a pharmacological approach. A shared decision-making process is critical in these patients as the disease management is navigated.
Knowledge gaps
There is insufficient data on the effect of ablation on mortality, bleeding, and stroke in older patients with multiple comorbidities/pre-frail/frail state.
Appropriate timing of ablation needs better criteria because older patients are often diagnosed late due to lack of symptoms.
Type of ablation (pulmonary vein isolation, substrate-based ablation) is not established in the presence of age-related atrial remodelling.
Consensus statements
|

|
|

|
Silent arrhythmias and screening for AF
AF may often be asymptomatic in up to 40% of cases175 or it may present atypical symptoms (in around 25% of cases).176 Asymptomatic AF has a higher prevalence in older subjects with permanent AF and is associated with more complex clinical conditions, in terms of comorbidities, that condition a higher thromboembolic risk, resulting in a higher risk of stroke, as well as of cardiovascular and all-cause mortality compared with symptomatic AF.177
Detection of asymptomatic (‘silent’) AF, and prescription of oral anticoagulants in patients at risk of thromboembolic events is the target of opportunistic screening.134 There is evidence that pharmacy-based, automated AF screening in elderly citizens identified subjects with unknown AF and an excess mortality risk over the next year.178 A large variety of devices are currently available for AF screening.179 An important aspect of screening is also detection of undertreated known AF, which may be particularly common among frail patients who often do not receive appropriate therapy because of unrecognized AF in 40–50%.180
Atrial high-rate episodes (AHREs) detected by CIEDs are usually discovered during routine device follow-up and classified in terms of duration of the single episode or time spent in atrial tachyarrhythmias during a day.181,182 These extended diagnostic capabilities have led to new terms, such as ‘AF burden’, defined as the overall time spent in AF during a specified period of time, and ‘subclinical AF’, corresponding to episodes of atrial tachyarrhythmias with duration between 5 min and 24 h, detected by a CIED in patients without clinical history or clinical symptoms of AF.181
The prevalence of AHREs or AF burden, among patients implanted with CIEDs varies, depending on underlying heart disease, periods of observation, and above all previous history of clinically overt atrial tachyarrhythmias, including AF.183 An analysis of all the data from the literature reveals that that AHREs with a duration >5–6 min are common in patients implanted with CIEDs, with an incidence between 10 and 68%.184
The association between CIED-detected atrial tachyarrhythmias of variable durations and stroke or systemic thromboembolism has been evaluated by several studies that overall collected data on >22,000 patients. It has been shown that AHRE burden with a duration ≥5–6 min is significantly associated with increased risk of stroke or systemic thromboembolism, with a hazard ratio ranging between 2 and 9.180,184 However, risk of stroke for subclinical AF is around 2.4-fold, i.e. lower than what traditionally reported for clinical AF (4.8-fold).185 Therefore, the clinical significance of CIED-detected AHREs with regard to prescription of anticoagulants in terms of risk benefit ratio is currently investigated in prospective trials. The randomized controlled LOOP study has shown that in individuals 70 years of age and older with risk factors for stroke, screening using ILR devices increased the likelihood of AF detection by three-fold accompanied by initiation of OAC therapy, but this strategy was not associated with any significant reduction in rates of stroke or systemic embolism.186
Current evidence suggests that a patient-tailored decision-making, particularly in frail patients, is useful when deciding to anticoagulate subjects with AHREs. This approach includes continued patient follow-up, also using remote monitoring of the CIED, targeted to detect the development of clinical AF, to monitor the evolution of AHRE or AF burden and specifically the transition to AHRE lasting more than 24 h, onset or worsening of HF, or any clinical change that might suggest a change in clinical conditions.187,188
Consensus statements
|

|
|

|
|

|
|

|
Stroke as a frailty component and specific characteristics in the very elderly
Ischaemic vs. haemorrhagic stroke risk
Frail patients face a heightened risk of stroke because they have a substantial burden of vascular risk factors and their clinicians hesitate to treat them with antithrombotic drugs.189 Frail patients have worse outcomes than other stroke patients,190 and stroke worsens frailty.191 Most strokes in AF patients are ischaemic, even in those with an initial haemorrhagic stroke192 or a high burden of cerebral microbleeds.193
Implications for acute management and chronic anticoagulation
There is no strong evidence of a net benefit from anticoagulation during the first 14 days after cardioembolic stroke,194 although acute anticoagulation appears relatively safe after minor cardioembolic stroke.195 A reasonable approach is to initiate anticoagulation within 14 days for most cardioembolic strokes but delay initiation until after 14 days if there is a large infarct, haemorrhagic transformation on neuroimaging, or uncontrolled hypertension.196 No good evidence exists to support a different balancing approach in frail patients.
There are few robust data specifically in frail patients regarding chronic anticoagulation for thromboembolism prevention in AF. Advancing age is more strongly associated with thromboembolism than bleeding197 and the net clinical benefit of anticoagulation increases with age.198 Although physicians hesitate to anticoagulate older patients given fears about falls and intracranial haemorrhage, anticoagulation provides a net benefit even in patients with frequent falls.199 There are anticoagulant drugs available that have a similar risk of bleeding as aspirin even in vulnerable patients such as those with prior ischaemic stroke.200 These factors support treating frail patients with anticoagulation as otherwise indicated for non-frail patients.
Consensus statement
|

|
Orthostatic hypotension and syncope syndrome
Carotid hypersensitivity syndrome
Carotid sinus syndrome (CSS) is a form of reflex syncope characterized by bradycardia and hypotension. The syndrome is almost exclusively diagnosed in older (male) patients and rare before the age of 40 years. Because a hypersensitive response is present in up to 30% of older patients with syncope, it is recommended that patients above 50 years of age who present with unexplained falls or syncope should have carotid sinus massage (CSM) performed using particular attention in those at risk of neurologic events.32 The diagnosis requires reproduction of spontaneous symptoms during asystole and/or a vasodepressor response.32 One-third of patients with a hypersensitive response (CSH) to CSM present with unexplained falls; amnesia for loss of consciousness can be reproduced during CSM in such patients.201 The finding of a hypersensitive response should not preclude further investigation for other causes of syncope. One-third of CSH patients have other reflex or cardiac abnormalities; conversely, in 80 previously asymptomatic individuals, CSH was present in 28 (35%) and accompanied by symptoms in 10.202 The 95th percentile for CSM response in a large random community cohort, mean age 75 years was 7.3 s asystole and a 77 mmHg drop in systolic BP,202 suggesting these thresholds for diagnosis and intervention.203
Orthostatic intolerance syndrome
Orthostatic intolerance syndrome is characterized by the abnormal, progressive and sustained drop of systolic and diastolic pressure ≥20 and ≥10 mmHg, respectively, or the decrease in systolic pressure to <90 mmHg.32 It can be classified into initial, classical and delayed orthostatic hypotension, according to the time of the onset of the abnormal pressure changes, as described in Table 5.
Table 5.
Syndromes of orthostatic intolerance
| Syndrome | Test | Time to abnormal BP response | Pathophysiology | Symptoms | Associated Conditions |
|---|---|---|---|---|---|
| Initial OH | Beat-to-beat BP on active standing test | 0–15 s | Transient mismatch between cardiac output and total peripheral resistance |
Light-headedness, dizziness, visual disturbances few seconds after standing. |
Old age, drug-induced (alpha-blockers) |
| Classical OH | Active standing test; TT |
<3 min | Impaired total peripheral resistance and HR increase in autonomic failure; severe volume depletion | Dizziness, light-headedness, weakness, visual and hearing disturbances | Frailty, drug-induced (vasoactive drugs, diuretics), autonomic failure, hypovolaemia |
| Delayed OH | TT | >3 min | Likely progressive fall in venous return and low cardiac output | Prolonged prodromes: dizziness, light-headedness, visual and hearing disturbances, low back pain, neck or precordial pain | Frailty, incipient autonomic failure, drug-induced (vasoactive drugs and diuretics), comorbidity |
BP, blood pressure; ESC, European Society of Cardiology; OH, orthostatic hypotension; TT, tilt testing.
Adapted from 2018 ESC Guidelines for the diagnosis and management of syncope.32
Investigations and management
The diagnostic pathway proposed by the ESC guidelines on syncope,32 was used also in older patients, enabling a reduction of unexplained syncope to around 10%.204 The initial evaluation relies on clinical history, physical examination, active standing test, and 12-lead ECG. Physicians should search for systemic diseases, physical frailty and locomotor disabilities. Details of cognitive status, social circumstances, injuries, impact of syncope on basal/instrumental activities of daily living, should be recorded. Possible witnesses should be interview, because retrograde amnesia is frequent in older and frail patients. A careful observation of gait and standing balance to assess the risk of falling is mandatory.32 Given the high prevalence of CSS in the elderly, the CSM may be performed initially. Even if very rarely (0.24%), the manoeuvre can be complicated by cerebrovascular events. For this reason, it is advised that CSM is undertaken with caution in subjects with a previous TIA or stroke, or when a carotid stenosis >70% is present.32 The clinical history has a limited value in the differential diagnosis between cardiac and neurally mediated causes of syncope in older patients,205 thus tilt testing (TT) and CSM are essential diagnostic steps.
TT was validated in older subjects206 and is well tolerated, even in the oldest old.207 TT may detect hypotensive susceptibility, initial and delayed orthostatic hypotension and guide the differential diagnosis between syncope and other clinical conditions causing unexplained falls.
Older patients are more likely to receive an ILR, because of higher rate of structural and electrical heart disease, limited value of the clinical history in determining the syncope aetiology, lack of prodromal symptoms and higher risk of trauma. ILR may be useful in distinguishing between syncope, unexplained falls and epileptic seizures.36 In the last few years, technology has allowed to introduce the use of smartwatches in automatic Fall Detection Systems. Benefits in terms of quickness of intervention derive from the widespread acceptance, low cost and networking interfaces of the devices, even if some methodologic aspects are still to be improved.
In older patients with vasodepressor reflex syncope, the recurrence of syncope/pre-syncope may be reduced by discontinuing/reducing vasoactive therapy, without higher adverse events, aimed at achieving an average systolic BP around 140 mmHg, or not <130 mmHg, as recommended in older and frail adults.208,209 Medical therapy for orthostatic intolerance includes midodrine, droxidopa, fludrocortisone and piridostigmine, considering supine hypertension.32,210 Additionally, isometric physical counter-pressure manoeuvres, and support stockings or abdominal binders to reduce venous pooling, may find an indication to prevent recurrences.32
Despite the lack of data on syncope, a comprehensive assessment on cognitive status and physical frailty, may help in the selection of patients for cardiac pacing. According to current ESC Guidelines, dual-chamber cardiac pacing should be considered in subjects with a reflex, asystolic, syncope. A similar level of recommendation can be found also when a cardioinhibitory reflex is dominant in a tilt-induced vasovagal syncope and in a CSS. However, it should be taken into account that the device can significantly reduce the burden of events, but not prevent all of them. Therapy should be also limited to subjects ≥40 years, presenting recurrent episodes with a significant impact on social and active life, when alternative treatment failed or were not feasible.213 Moreover, pacing for these specific indications can be found further limitations in older frail individuals.
Improving patient outcome: specific consideration
Quality of life
Frailty results in diminished physical, emotional, and social functioning which significantly impacts quality of life.211,212 For most frail patients, their desired goals from therapy are improvements in functional status and quality of life, rather than extending life; these patient-centred outcomes should be incorporated into the treatment decision-making process. There is currently a paucity of research specifically examining the impact of frailty per se on quality of life in patients with cardiac arrhythmias.
Frailty significantly impacts treatment choices, with frail patients with cardiac arrhythmias less likely to be aggressively treated compared with their non-frail counterparts.166 For example, frail AF patients are less likely to receive oral anticoagulation,213–215 primarily due to physicians’ fears of bleeding related to frailty-associated characteristics (risk of falls and cognitive impairment) and are also less likely to receive a rhythm-control strategy.43,216 There is also controversy regarding the use of ICDs in frail individuals given the increased risk of non-cardiac death in frail patients that may diminish the benefits of the ICD.217,218 However, some types of devices, may be important therapies in frail patients given their impact on outcomes of significance to patients. For example, CRT improves functional capacity, with consequent improvements in physical and cognitive functioning,120,219 whilst pacemaker implantation for bradyarrhythmias decreases the risk of falls and reduces frailty,220 in addition to preventing onset or worsening of HF, all of which are likely to positively impact overall quality of life.
Care models
Research suggests that there is little or no evidence for routine comprehensive screening for unmet health needs in the older population.221,222 Targeted approaches are better, to identify ‘at-risk’ groups of individuals, with care models focussing on reversing modifiable elements of frailty, particularly addressing underlying medical conditions causing and/or contributing to frailty and optimizing management and reviewing medication, to stabilize the underlying disease processes.
The fundamental issue is that healthcare professionals need to recognize frailty as they are often focussed on specific diseases/comorbidities and these may mask frailty. Education of healthcare professionals is needed to highlight the components of frailty, to acknowledge that frailty is a dynamic process, and demonstrate how to optimize management of this vulnerable patient group. Identification of frailty with an appropriate tool (see Section ‘Assessment of frailty and frailty scores’) should be the first step and if identified, further comprehensive assessment (by a geriatrician) should be undertaken. The concept of frailty requires a more holistic approach rather than a disease-based medical approach and should integrate health and social care and take into consideration the outcomes of importance to patients and their families/carers. As such, this approach requires multidisciplinary interventions and the support and input from a multidisciplinary team.
Development of a specialist team and aftercare
Respondents of a recent EP Wire,43 preferred an ‘Arrhythmia Team’ consisting of an electrophysiologists, clinical cardiologists, geriatricians, internists, palliative care specialists, nurses and family members/carers to manage frail patients. In addition, the specialist team should also include specialist nurses, occupational therapists, physiotherapists/exercise physiologists, general practitioners, pharmacists, and social workers. Composition of the team is largely dependent on the individual needs of the patient; ideally input and care would be provided by all appropriate experts. Ongoing care for frail patients with cardiac arrhythmias is probably best managed by specialist nurse-led aftercare in the community, with appropriate access and referral to a multidisciplinary Arrhythmia team, as required.
Nurses and specialist nurses have an important role in providing a person-centred holistic approach to managing patients with frailty syndrome, monitoring and timely identifying changes in components of frailty syndrome such as assessment of nutrition, pharmacotherapy, adherence to treatment, falls risk, exercise, and mood and cognitive impairment, and prompting interventions to minimize further weight loss, loss of muscle mass and strength, and reduce fall risk factors to help maintain a state of homeostasis.3
Patient education and active involvement in decision-making are important for good adherence to OAC and optimal treatment effects.223 In addition, various decision supporting tools (built in electronic medical records or mobile applications) have been shown to improve stroke risk management in AF patients.224,225
Digital technology and eHealth
Frail patients who require long-term care with frequent assessment of health state may benefit from digital health technologies (telehealth, mHealth, and wearables such as heart rate and activity tracking and biological sensors).4 These technologies allow for for detection and monitoring components of frailty syndrome such as physical activity, gait speed, postural changes and falls, heart rate and fitness, arrhythmias, and assist in early identification of subclinical deterioration in health. Cognitive, visual, or sensory impairments may potentially be assessed through telehealth or mHealth. Telemedicine may enable high-quality and cost-efficient care for an increasing number of patients with frailty. For example, mHealth has become an important component of many AF outpatient clinics in the light of the COVID-19 pandemic and decreased capacity to see patients in the outpatient clinic.226 Several validated mHealth solutions are available for remote heart rate and rhythm monitoring as well as for risk factor assessment.227–229
Supplementary Material
Acknowledgement
The authors thank the EHRA Scientific Document Committee: Dr Nikolaos Dagres, Prof. Thomas Deneke, Prof. Arthur Wilde, Prof. Frank R. Heinzel, Prof. Christian Meyer, Prof. Lucas Boersma, Prof. Radoslaw Lenarczyk, Prof. Luigi di Biase, Dr Elena Arbelo, Dr Avi Sabbag, Prof. Pierre Jais, Prof. Milos Taborsky, Asso. Prof. Markus Stühlinger.
Contributor Information
Irina Savelieva, Cardiovascular Clinical Academic Group, Molecular and Clinical Sciences Research Institute, St George's University of London, London, UK.
Stefano Fumagalli, Department of Experimental and Clinical Medicine, Geriatric Intensive Care Unit and Geriatric Arrhythmia Unit, University of Florence and AOU Careggi, Florence, Italy.
Rose Anne Kenny, Mercer’s Institute for Successful Ageing, Department of Medical Gerontology, St James’s Hospital, Dublin, Ireland.
Stefan Anker, Department of Cardiology (CVK), Germany; Berlin-Brandenburg Center for Regenerative Therapies (BCRT), Germany; German Centre for Cardiovascular Research (DZHK) partner site Berlin, Germany; Charité Universitätsmedizin Berlin, Germany.
Athanase Benetos, Department of Geriatric Medicine CHRU de Nancy and INSERM U1116, Université de Lorraine, Nancy, France.
Giuseppe Boriani, Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Policlinico di Modena, Modena, Italy.
Jared Bunch, (HRS representative): Intermountain Medical Center, Cardiology Department, Salt Lake City, Utah, USA; Stanford University, Department of Internal Medicine, Palo Alto, CA, USA.
Nikolaos Dagres, Heart Center Leipzig, Department of Electrophysiology, Leipzig, Germany.
Sergio Dubner, (LAHRS representative): Clinica Suizo Argentina, Cardiology Department, Buenos Aires Capital Federal, Argentina.
Laurent Fauchier, Centre Hospitalier Universitaire Trousseau et Université François Rabelais, Tours, France.
Luigi Ferrucci, National Institute on Aging, MD, USA.
Carsten Israel, Evangelisches Krankenhaus Bielefeld GmbH, Bielefeld, Germany.
Hooman Kamel, Department of Neurology, Weill Cornell Medical College, New York, NY, USA.
Deirdre A Lane, Liverpool Centre for Cardiovascular Science, University of Liverpool, Liverpool, United Kingdom; Department of Cardiovascular and Metabolic Medicine, Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, United Kingdom; Liverpool Heart and Chest Hospital, Liverpool, United Kingdom; Aalborg Thrombosis Research Unit, Department of Clinical Medicine, Aalborg University, Aalborg, Denmark.
Gregory Y H Lip, Liverpool Centre for Cardiovascular Science, University of Liverpool, Liverpool, United Kingdom; Department of Cardiovascular and Metabolic Medicine, Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, United Kingdom; Liverpool Heart and Chest Hospital, Liverpool, United Kingdom; Aalborg Thrombosis Research Unit, Department of Clinical Medicine, Aalborg University, Aalborg, Denmark.
Niccolò Marchionni, Department of Experimental and Clinical Medicine, General Cardiology Division, University of Florence and AOU Careggi, Florence, Italy.
Israel Obel, (CASSA representative): Milpark Hospital, Cardiology Unit, Johannesburg, South Africa.
Ken Okumura, (APHRS representative): Saiseikai Kumamoto Hospital, Kumamoto, Japan.
Brian Olshansky, University of Iowa Hospitals and Clinics, Iowa City Iowa, USA; Covenant Hospital, Waterloo, Iowa, USA; Mercy Hospital Mason City, Iowa, USA.
Tatjana Potpara, School of Medicine, Belgrade University, Serbia; Cardiology Clinic, Clinical Center of Serbia, Serbia.
Martin K Stiles, (APHRS representative): Waikato Clinical School, University of Auckland and Waikato Hospital, Hamilton, New Zealand.
Juan Tamargo, Department of Pharmacology, School of Medicine, CIBERCV, Universidad Complutense, Madrid, Spain.
Andrea Ungar, Department of Experimental and Clinical Medicine, Geriatric Intensive Care Unit and Geriatric Arrhythmia Unit, University of Florence and AOU Careggi, Florence, Italy.
Jedrzej Kosiuk, Department of Electrophysiology, Heart Center Leipzig, Leipzig, Germany.
Torben Bjerregaard Larsen, Department of Cardiology, Aalborg University Hospital, Aalborg, Denmark.
Borislav Dinov, Department of Electrophysiology, Heart Center Leipzig, Leipzig, Germany.
Heidi Estner, Klinikum d. Universität München Campus, München, Germany.
Rodrigue Garcia, Chu De Poitiers, Service De Cardiologie, Poitiers, France.
Francisco Manuel Moscoso Costa, Cardiology Department, Hospital Santa Cruz, Lisbon, Portugal.
Rachel Lampert, Yale University School of Medicine, Section of Cardiovascular Medicine, CT, USA.
Yenn-Jiang Lin, Taipei Veterans General Hospital, Cardiology Division, Taipei, Taiwan.
Ashley Chin, UCT Private Academic Hospital, Cape Town, South Africa.
Heliodoro Antonio Rodriguez, Centro Medico Docente la Trinidad, Cardiology Unit, Caracas, Miranda, Venezuela.
Timo Strandberg, Department of Health Sciences/Geriatrics, University of Oulu, Oulu, Finland.
Tomasz Grodzicki, Jagiellonian University Medical College, Cracow, Poland.
Supplementary material
Supplementary material is available at Europace online.
References
- 1.https://www.who.int/health-topics/ageing
- 2. Walker DM, Gale CP, Lip G, Martin-Sanchez FJ, McIntyre HF, Mueller C, et al. . Frailty and the management of patients with acute cardiovascular disease: a position paper from the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care 2018; 7:176–93. [DOI] [PubMed] [Google Scholar]
- 3. Vitale C, Jankowska E, Hill L, Piepoli M, Doehner W, Anker SD, et al. . Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail 2019; 21:1299–305. [DOI] [PubMed] [Google Scholar]
- 4. Richter D, Guasti L, Walker D, Lambrinou E, Lionis C, Abreu A, et al. . Frailty in cardiology: definition, assessment and clinical implications for general cardiology. A consensus document of the Council for Cardiology Practice (CCP), Acute Cardiovascular Care Association (ACCA), Association of Cardiovascular Nursing and Allied Professions (ACNAP), European Association of Preventive Cardiology (EAPC), European Heart Rhythm Association (EHRA), Council on Valvular Heart Diseases (VHD), Council on Hypertension (CHT), Council of Cardio-Oncology (CCO), Working Group (WG) Aorta and Peripheral Vascular Diseases, WG e-Cardiology, WG Thrombosis, of the European Society of Cardiology, European Primary Care Cardiology Society (EPCCS). Eur J Prev Cardiol 2022; 29:216–27. [DOI] [PubMed] [Google Scholar]
- 5. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Caoimh R, Galluzzo L, Rodríguez-Laso Á, Van der Heyden J, Ranhoff AH, Lamprini-Koula M, et al. . Prevalence of frailty at population level in European ADVANTAGE Joint Action Member States: a systematic review and meta-analysis. Ann Ist Super Sanita 2018; 54:226–38. [DOI] [PubMed] [Google Scholar]
- 7. Walston J, Robinson TN, Zieman S, et al. . Integrating frailty research into the medical specialties-Report from a U13 Conference. J Am Geriatr Soc 2017; 65:2134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012; 60:1487–92. [DOI] [PubMed] [Google Scholar]
- 9. O'Caoimh R, Sezgin D, O'Donovan MR, Molloy DW, Clegg A, Rockwood K, Liew A. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing 2021; 50:96–104. [DOI] [PubMed] [Google Scholar]
- 10. Strandberg TE, Lindström L, Jyväkorpi S, Urtamo A, Pitkälä KH, Kivimäki M. Phenotypic frailty and multimorbidity are independent 18-year mortality risk indicators in older men: The Helsinki Businessmen Study (HBS). Eur Geriatr Med 2021; 12:953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fried LP, Tangen CM, Walston Jet al. . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–56. [DOI] [PubMed] [Google Scholar]
- 12. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Scientific World Journal 2001; 1:323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rockwood K, Howlett SE. Fifteen years of progress in understanding frailty and health in aging. BMC Med 2018; 16:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet 1999; 353:205–6. [DOI] [PubMed] [Google Scholar]
- 15. Rasiah J, Cummings GG, Gruneir A, Oelke ND D, Estabrooks C, Holroyd-Leduc J. Prefrailty in older adults: a concept analysis. Int J Nurs Stud 2020; 108:103618. [DOI] [PubMed] [Google Scholar]
- 16. Ofori-Asenso R, Chin KL, Mazidi M, Zomer E, Ilomaki J, Zullo AR, et al. . Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open 2019; 2:e198398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chamberlain AM, Finney Rutten LJ, Manemann SM, et al. . Frailty trajectories in an elderly population-based cohort. J Am Geriatr Soc 2016; 64:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fried LP FL, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2005:255–63. [DOI] [PubMed] [Google Scholar]
- 19. McGeorge SJ. Unravelling the differences between complexity and frailty in old age: findings from a constructivist grounded theory study. J Psychiatr Ment Health Nurs 2011; 18:67–73. [DOI] [PubMed] [Google Scholar]
- 20. Landi F, Calvani R, Tosato M, et al. . Anorexia of aging: risk factors, consequences, and potential treatments. Nutrients 2016; 8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chapman IM. Endocrinology of anorexia of ageing. Best Pract Res Clin Endocrinol Metab 2004; 18:437–52. [DOI] [PubMed] [Google Scholar]
- 22. Marzetti E, Calvani R, Tosato M, Cesari M, Di Bari M, Cherubini A, et al. . Sarcopenia: an overview. Aging Clin Exp Res 2017; 29:11–7. [DOI] [PubMed] [Google Scholar]
- 23. Denfeld QE, Winters-Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: a systematic review and meta-analysis. Int J Cardiol 2017; 236:283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y, Yuan M, Gong M, Tse G, Li G, Liu T. Frailty and clinical outcomes in heart failure: a systematic review and meta-analysis. J Am Med Dir Assoc 2018; 19:1003–8. [DOI] [PubMed] [Google Scholar]
- 25. Ethun CG, Bilen MA, Jani AB, Maithel SK, Ogan K, Master VA. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin 2017; 67:362–77. [DOI] [PubMed] [Google Scholar]
- 26. Orum M, Gregersen M, Jensen K, Meldgaard P, Damsgaard EMS. Frailty status but not age predicts complications in elderly cancer patients: a follow-up study. Acta Oncologica 2018; 57:1458–66. [DOI] [PubMed] [Google Scholar]
- 27. Ruiz J, Miller AA, Tooze JAet al. . Frailty assessment predicts toxicity during first cycle chemotherapy for advanced lung cancer regardless of chronologic age. J Geriatr Oncol 2019; 10:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Edwards BJ, Zhang X, Sun M, Song J, Khalil P, Karuturi MS, et al. . Overall survival in older patients with cancer. BMJ Support Palliat Care 2020; 10:25–35. [DOI] [PubMed] [Google Scholar]
- 29. Cheng MH, Chang SF. Frailty as a risk factor for falls among community dwelling people: evidence from a meta-analysis. J Nurs Scholarsh 2017; 49:529–36. [DOI] [PubMed] [Google Scholar]
- 30. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society . Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Amer Geriatr Soc 2011; 59:148–57. [DOI] [PubMed] [Google Scholar]
- 31. Bhangu J, Hall P, Devaney N, Bennett K, Carroll L, Kenny RA, McMahon CG. The prevalence of unexplained falls and syncope in older adults presenting to an Irish urban emergency department. Eur J Emerg Med 2019; 26:100–4. [DOI] [PubMed] [Google Scholar]
- 32. Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, et al. . 2018. ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 2018; 39:1883–948. [DOI] [PubMed] [Google Scholar]
- 33. Goldstein DS, Sharabi Y. Neurogenic orthostatic hypotension: a pathophysiological approach. Circulation 2009; 119:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bhangu J, McMahon CG, Hall P, Bennett K, Rice C, Crean P, et al. . Long-term cardiac monitoring in older adults with unexplained falls and syncope. Heart 2016; 102:681–6. [DOI] [PubMed] [Google Scholar]
- 35. Maggi R, Rafanelli M, Ceccofiglio A, Solari D, Brignole M, Ungar A. Additional diagnostic value of implantable loop recorder in patients with initial diagnosis of real or apparent transient loss of consciousness of uncertain origin. Europace 2014; 16:1226–30. [DOI] [PubMed] [Google Scholar]
- 36. Brignole M, Vardas P, Hoffman E, Huikuri H, Moya A, Ricci R, et al. . Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 2009; 11:671–87. [DOI] [PubMed] [Google Scholar]
- 37. Homann B, Plaschg A, Grundner M, Haubenhofer A, Griedl T, Ivanic G, et al. . The impact of neurological disorders on the risk for falls in the community dwelling elderly: a case-controlled study. BMJ Open 2013; 3:e003367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnell K, Jonasdottir Bergman G, Fastbom J, Danielsson B, Borg N, Salmi P. Psychotropic drugs and the risk of fall injuries, hospitalisations and mortality among older adults. Int J Geriatr Psychiatry 2017; 32:414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lipardo DS, Aseron AMC, Kwan MM, Tsang WW. Effect of exercise and cognitive training on falls and fall-related factors in older adults with mild cognitive impairment: a systematic review. Arch Phys Med Rehabil 2017; 98:2079–96. [DOI] [PubMed] [Google Scholar]
- 40. Chan WC, Yeung JWF, Wong CSM, Lam LCW, Chung KF, Luk JKH, et al. . Efficacy of physical exercise in preventing falls in older adults with cognitive impairment: a systematic review and meta-analysis. J Am Med Dir Assoc 2015; 16:149–54. [DOI] [PubMed] [Google Scholar]
- 41. Parry SW, Bamford C, Deary V, Finch TL, Gray J, MacDonald C, et al. . Cognitive–behavioural therapy-based intervention to reduce fear of falling in older people: therapy development and randomised controlled trial – the Strategies for Increasing Independence, Confidence and Energy (STRIDE) study. Health Technol Assess 2016; 20:1–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Herr M, Robine JM, Pinot J Arvieu JJ, Ankri J. Polypharmacy and frailty: prevalence, relationship, and impact on mortality in a French sample of 2350 old people. Pharmacoepidemiol Drug Saf 2015; 24:637–46. [DOI] [PubMed] [Google Scholar]
- 43. Fumagalli S, Potpara TS, Bjerregaard Larsen T, Haugaa KH, Dobreanu D, Proclemer A, et al. . Frailty syndrome: an emerging clinical problem in the everyday management of clinical arrhythmias. The results of the European Heart Rhythm Association survey. Europace 2017; 19:1896–902. [DOI] [PubMed] [Google Scholar]
- 44. Ayus JC, Negri AL, Kalantar-Zadeh K, Moritz ML. Is chronic hyponatremia a novel risk factor for hip fracture in the elderly? Nephrol Dial Transplant 2012; 27:3725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Musso CG, Jauregui JR, Macías Núñez JF. Frailty phenotype and chronic kidney disease: a review of the literature. Int Urol Nephrol 2015; 47:1801–7. [DOI] [PubMed] [Google Scholar]
- 46. McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG, et al. . Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 2013; 61:896–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abbatecola AM, Paolisso G. Is there a relationship between insulin resistance and frailty syndrome? Curr Pharm Des2008; 14:405–10. [DOI] [PubMed] [Google Scholar]
- 48. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. . A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buta BJ, Walston JD, Godino JG, Park M, Kalyani RR, Xue QL, et al. . Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 2016; 26:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med 2016; 31:3–10. [DOI] [PubMed] [Google Scholar]
- 51. Veronese N, Cereda E, Stubbs B, Solmi M, Luchini C, Manzato E, et al. . Risk of cardiovascular disease morbidity and mortality in frail and pre-frail older adults: results from a meta-analysis and exploratory meta-regression analysis. Ageing Res Rev 2017; 35:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stewart R. Cardiovascular disease and frailty: what are the mechanistic links? Clin Chem 2019; 65:80–6. [DOI] [PubMed] [Google Scholar]
- 53. Fleg JL, Aronow WS, Frishman WH. Cardiovascular drug therapy in the elderly: benefits and challenges. Nat Rev Cardiol 2011; 8:13–28. [DOI] [PubMed] [Google Scholar]
- 54. Lee H-C, Huang K, Shen W-K. Use of antiarrhythmic drugs in elderly patients. J Geriatric Cardiology 2011; 8:184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Deneer VH, van Hemel NM. Is antiarrhythmic treatment in the elderly different? A review of the specific changes. Drugs Aging 2011; 28:617–33. [DOI] [PubMed] [Google Scholar]
- 56. Dan GA, Martinez-Rubio A, Agewall S, Boriani G, Borggrefe M, Gaita F, et al. . Antiarrhythmic drugs-clinical use and clinical decision making: a consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP). Europace 2018; 20:731–2. [DOI] [PubMed] [Google Scholar]
- 57. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev 2009; 41:67–7. [DOI] [PubMed] [Google Scholar]
- 58. Shi S, Klotz U. Age-related changes in pharmacokinetics. Curr Drug Metab 2011; 12:601–10. [DOI] [PubMed] [Google Scholar]
- 59. Boriani G, Savelieva I, Dan GA, Deharo JC, Ferro C, Israel CW, et al. . hronic kidney disease in patients with cardiac rhythm disturbances or implantable electrical devices: clinical significance and implications for decision making-a position paper of the European Heart Rhythm Association endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace 2015; 17:1169–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zimetbaum P. Amiodarone for atrial fibrillation. N Engl J Med 2007; 356:935–41. [DOI] [PubMed] [Google Scholar]
- 61. El-Desoky ES. Pharmacokinetic-pharmacodynamic crisis in the elderly. Am J Ther 2007; 14:488–98. [DOI] [PubMed] [Google Scholar]
- 62. Society American Geriatrics 2015. Updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–46. [DOI] [PubMed] [Google Scholar]
- 63. Moghtadaei M, Jansen HJ, Mackasey M, et al. . The impacts of age and frailty on heart rate and sinoatrial node function. J Physiol 2016; 594:7105–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kumar P, Kusumoto FM, Goldschlager N. Bradyarrhythmias in the elderly. Clin Geriatr Med 2012; 28:703–15. [DOI] [PubMed] [Google Scholar]
- 65. Wakida Y, Okamoto Y, Iwa T, Yonemoto T, Kanemaki K, Shiomi T, et al. . Arrhythmias in centenarians. Pacing Clin Electrophysiol 1994; 17:2217–21. [DOI] [PubMed] [Google Scholar]
- 66. Dungen HD, Apostolovic S, Inkrot S, et al. . Titration to target dose of bisoprolol vs. carvedilol in elderly patients with heart failure: the CIBIS-ELD trial. Eur J Heart Fail 2011; 13:670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zeltser D, Justo D, Halkin A, et al. . Drug-induced atrioventricular block: prognosis after discontinuation of the culprit drug. J Am Coll Cardiol 2004; 44:105–8. [DOI] [PubMed] [Google Scholar]
- 68. Gill SS, Anderson GM, Fischer HD, et al. . Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Arch Intern Med 2009; 169:867–73. [DOI] [PubMed] [Google Scholar]
- 69. Eriksson P, Hansson PO, Eriksson H, Dellborg M. Bundle-branch block in a general male population: the study of men born 1913. Circulation 1998; 98:2494–500. [DOI] [PubMed] [Google Scholar]
- 70. Fahy GJ, Pinski SL, Miller DP, et al. . Natural history of isolated bundle branch block. Am J Cardiol 1996; 77:1185–90. [DOI] [PubMed] [Google Scholar]
- 71. Kreger BE, Anderson KM, Kannel WB. Prevalence of intraventricular block in the general population: the Framingham Study. Am Heart J 1989; 117:903–10. [DOI] [PubMed] [Google Scholar]
- 72. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. . 2021. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Europace 2022; 24:71–164. [DOI] [PubMed] [Google Scholar]
- 73. Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, et al. . 2018. ACC/AHA/HRS Guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2019; 140:e382-e482. [DOI] [PubMed] [Google Scholar]
- 74. Menozzi C, Brignole M, Alboni P, et al. . The natural course of untreated sick sinus syndrome and identification of the variables predictive of unfavorable outcome. Am J Cardiol 1998; 82:1205–9. [DOI] [PubMed] [Google Scholar]
- 75. Link MS, Estes NA, 3rd, Griffin JJ, et al. Complications of dual chamber pacemaker implantation in the elderly. Pacemaker Selection in the Elderly (PASE) Investigators. J Interv Card Electrophysiol 1998; 2:175–9. [DOI] [PubMed] [Google Scholar]
- 76. Toff WD, Camm AJ, Skehan JD, United Kingdom P, Cardiovascular Events Trial I. Single-chamber versus dual-chamber pacing for high-grade atrioventricular block. N Engl J Med 2005; 353:145–55. [DOI] [PubMed] [Google Scholar]
- 77. Kerr CR, Connolly SJ, Abdollah H, et al. . Canadian Trial of Physiological Pacing: effects of physiological pacing during long-term follow-up. Circulation 2004; 109:357–62. [DOI] [PubMed] [Google Scholar]
- 78. Lamas GA, Lee KL, Sweeney MO, et al. . Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med 2002; 346:1854–62. [DOI] [PubMed] [Google Scholar]
- 79. Slotwiner D, Varma N, Akar JG, et al. . HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm 2015; 12:e69-100. [DOI] [PubMed] [Google Scholar]
- 80. Boveda S, Marijon E, Lenarczyk R, Iliodromitis KE, Marin F, Defaye P, et al. . Factors influencing the use of leadless or transvenous pacemakers: results of the European Heart Rhythm Association Prospective Survey. Europace 2020; 22:667–73. [DOI] [PubMed] [Google Scholar]
- 81. Defaye P, Klug D, Anselme F, Gras D, Hermida JS, Piot O, et al. . Recommendations for the implantation of leadless pacemakers from the French Working Group on Cardiac Pacing and Electrophysiology of the French Society of Cardiology. Arch Cardiovasc Dis 2018; 111:53–8. [DOI] [PubMed] [Google Scholar]
- 82. Higuchi S, Okada A, Shoda M, Yagishita D, Saito S, Kanai M, et al. . Leadless cardiac pacemaker implantations after infected pacemaker system removals in octogenarians. J Geriatr Cardiol 2021; 18:505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Curtis AB, Karki R, Hattoum A, Sharma UC. Arrhythmias in patients 80 years of age: pathophysiology, management, and outcomes. J Am Coll Cardiol 2018; 71:2041–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Raybaud F, Camous JP, Tibi T, Baudouy M, Morand P. [Severe arrhythmia in the elderly: a prospective hospital study]. Arch Mal Coeur Vaiss 1995; 88:27–33. [PubMed] [Google Scholar]
- 85. Sherman H, Sandberg S, Fineberg HV. Exponential increase in age-specific prevalence of ventricular dysrhythmia among males. J Chronic Dis 1982; 35:743–50. [DOI] [PubMed] [Google Scholar]
- 86. Arnar DO, Mairesse GH, Boriani G, Calkins H, Chin A, Coats A, et al. . Management of asymptomatic arrhythmias: a European Heart Rhythm Association (EHRA) consensus document, endorsed by the Heart Failure Association (HFA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin America Heart Rhythm Society (LAHRS). Europace 2019; 21:844–5. [DOI] [PubMed] [Google Scholar]
- 87. Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest 2005; 115:2305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Murphy DJ, Murray AM, Robinson BE, Campion EW. Outcomes of cardiopulmonary resuscitation in the elderly. Ann Intern Med 1989; 111:199–205. [DOI] [PubMed] [Google Scholar]
- 89. Bayer AJ, Ang BC, Pathy MS. Cardiac arrests in a geriatric unit. Age and Ageing 1985; 14: 271–76. [DOI] [PubMed] [Google Scholar]
- 90. Manolis AS, Deering TF, Cameron J, Estes NA 3rd. Mexiletine: pharmacology and therapeutic use. Clin Cardiol 1990; 13:349–59. [DOI] [PubMed] [Google Scholar]
- 91. Fitton A, Sotalol Sorkin EM.. An updated review of its pharmacological properties and therapeutic use in cardiac arrhythmias. Drugs 1993; 46:678–719. [DOI] [PubMed] [Google Scholar]
- 92. Simantirakis EN, Koutalas EP, Vardas PE. Arrhythmia-induced cardiomyopathies: the riddle of the chicken and the egg still unanswered? Europace 2012; 14:466–73. [DOI] [PubMed] [Google Scholar]
- 93. Park KM, Im SI, Chun KJ, Hwang JK, Park SJ, Kim JS, On YK. Asymptomatic ventricular premature depolarizations are not necessarily benign. Europace 2016; 18:881–7. [DOI] [PubMed] [Google Scholar]
- 94. Marcus GM. Evaluation and management of premature ventricular complexes. Circulation 2020; 141:1404–18. [DOI] [PubMed] [Google Scholar]
- 95. Blandino A, Bianchi F, Frankel DS, Liang JJ, Mazzanti A, D'Ascenzo F, et al. . Safety and efficacy of catheter ablation for ventricular tachycardia in elderly patients with structural heart disease: a systematic review and meta-analysis. J Interv Card Electrophysiol 2021. doi: 10.1007/s10840-021-01007-w [DOI] [PubMed] [Google Scholar]
- 96. Vakil K, Garcia S, Tung R, Vaseghi M, Tedrow U, Della Bella P, et al. . Ventricular tachycardia ablation in the elderly: an International Ventricular Tachycardia Center Collaborative Group Analysis. Circ Arrhythm Electrophysiol 2017; 10:e005332. [DOI] [PubMed] [Google Scholar]
- 97. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. . 2015. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2015; 17:1601–87. [DOI] [PubMed] [Google Scholar]
- 98. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. . Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. New Engl J Med 2005; 352:225–37. [DOI] [PubMed] [Google Scholar]
- 99. Zakine C, Garcia R, Narayan K, Gandjbakhch E, Algalarrondo V, Lellouche N, et al. . Prophylactic implantable cardioverter-defibrillator in the very elderly. Europace 2019; 21:1063–9. [DOI] [PubMed] [Google Scholar]
- 100. Green AR, Leff B, Wang Y, Spartz ES, Masoudi FA, Peterson P, et al. . Geriatric conditions in patients undergoing defibrillator implantation for prevention of sudden cardiac death: prevalence and impact on mortality. Circ Cardiovasc Qual Outcomes 2016; 9:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Poupin P, Bouleti C, Degand B, et al. . Prognostic value of Charlson Comorbidity Index in the elderly with a cardioverter-defibrillator implantation. Int J Cardiol 2020; 314:64–9. [DOI] [PubMed] [Google Scholar]
- 102. Fauchier L, Marijon E, Defaye P, Piot O, Sadoul N, Perier MC, et al. . Effect of age on survival and causes of death after primary prevention implantable cardioverter-defibrillator implantation. Am J Cardiol 2015; 115:1415–22. [DOI] [PubMed] [Google Scholar]
- 103. Healey JS, Hallstrom AP, Kuck K-H, Nair G, Schron EP, Roberts RS, et al. . Role of the implantable defibrillator among elderly patients with a history of life-threatening ventricular arrhythmias. Eur Heart J 2007; 28:1746–9. [DOI] [PubMed] [Google Scholar]
- 104. Goonewardene M, Barra S, Heck P, Begley D, Fynn S, Virdee M, et al. . Cardioverter-defibrillator implantation and generator replacement in the octogenarian. Europace 2015; 17:409–46. [DOI] [PubMed] [Google Scholar]
- 105. Barra S, Providência R, Paiva L, Heck P, Agarwal S. Implantable cardioverter-defibrillators in the elderly: rationale and specific age-related considerations. Europace 2015; 17:174–86. [DOI] [PubMed] [Google Scholar]
- 106. Fauchier L, Alonso C, Anselme F, Blangy H, Bordachar P, Boveda S, et al. . Position paper for management of elderly patients with pacemakers and implantable cardiac defibrillators: Groupe de Rythmologie et Stimulation Cardiaque de la Societe Francaise de Cardiologie and Societe Francaise de Geriatrie et Gerontologie. Arch Cardiovasc Dis 2016; 109:563–85. [DOI] [PubMed] [Google Scholar]
- 107. Chen MY, Orkaby AR, Rosenberg MA, Driver JA. Frailty, implantable cardioverter-defibrillators, and mortality: a systematic review. J Gen Intern Med 2019; 34:2224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bibas L, Levi M, Touchette J, Mardigyan V, Bernier M, Essebag V, Afilalo J. Implications of frailty in elderly patients with electrophysiological conditions. JACC Clin Electrophysiol 2016; 2:288–94. [DOI] [PubMed] [Google Scholar]
- 109. Wathen MS, DeGroot PJ, Sweeney MO, Stark AJ, Otterness MF, Adkisson WO, et al. . Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation 2004; 110:2591–6. [DOI] [PubMed] [Google Scholar]
- 110. Wilkoff BL, Williamson BD, Stern RS, Moore SL, Lu F, Lee SW, et al. . Strategic programming of detection and therapy parameters in implantable cardioverter-defibrillators reduces shocks in primary prevention patients: results from the PREPARE (Primary Prevention Parameters Evaluation) study. J Am Coll Cardiol 2008; 52:541–50. [DOI] [PubMed] [Google Scholar]
- 111. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, et al. . Reduction in inappropriate therapy and mortality through ICD programming. New Engl J Med 2012; 367:2275–83. [DOI] [PubMed] [Google Scholar]
- 112. Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al-Khatib SM, Almendral J, et al. . 2015. HRS/EHRA/APHRS/SOLAECE Expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Europace 2016; 18:159–83. [DOI] [PubMed] [Google Scholar]
- 113. Stiles MK, Fauchier L, Morillo CA, Wilkoff BL. 2019HRS/EHRA/APHRS/LAHRS focused update to 2015 expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Europace 2019; 21:1442–3. [DOI] [PubMed] [Google Scholar]
- 114. McLeod CJ, Boersma L, Okamura H, Friedman PA. The subcutaneous implantable cardioverter defibrillator: state-of-the-art review. Eur Heart J 2017; 38:247–57. [DOI] [PubMed] [Google Scholar]
- 115. Joseph SM, Rich MW. Targeting frailty in heart failure. Curr Treat Options Cardiovasc Med 2017; 19:31. [DOI] [PubMed] [Google Scholar]
- 116. Walston J, Hadley EC, Ferrucci L, et al. . Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006; 54:991–1001. [DOI] [PubMed] [Google Scholar]
- 117. McDonagh J, Martin L, Ferguson C, et al. . Frailty assessment instruments in heart failure: a systematic review. Eur J Cardiovasc Nurs 2018; 17:23–35. [DOI] [PubMed] [Google Scholar]
- 118. Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, et al. . Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21:1169–86. [DOI] [PubMed] [Google Scholar]
- 119. Kubala M, Guédon-Moreau L, Anselme F, Klug D, Bertaina G, Traullé S, et al. . Utility of frailty assessment for elderly patients undergoing cardiac resynchronization therapy. JACC Clin Electrophysiol 2017; 3:1523–33. [DOI] [PubMed] [Google Scholar]
- 120. Fumagalli S, Pieragnoli P, Ricciardi G, Mascia G, Mascia F, Michelotti F, et al. . Cardiac resynchronization therapy improves functional status and cognition. Int J Cardiol 2016; 219:212–7. [DOI] [PubMed] [Google Scholar]
- 121. Milner A, Braunstein ED, Umadat G, Ahsan H, Lin J, Palma E. Utility of the modified frailty index to predict cardiac resynchronization therapy outcomes and response. Am J Cardiol 2020; 125:1077–82. [DOI] [PubMed] [Google Scholar]
- 122. Sergi G, Veronese N, Fontana L, De Rui M, Bolzetta F, Zambon S, et al. . Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015; 65:976–83. [DOI] [PubMed] [Google Scholar]
- 123. Setoguchi S, Warner Stevenson L, Stewart GC, Bhatt DL, Epstein AE, et al. . Influence of healthy candidate bias in assessing clinical effectiveness for implantable cardioverter-defibrillators: cohort study of older patients with heart failure. BMJ May 8 2014; 348:g2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bibas L, Levi M, Touchette J, Mardigyan V, Bernier M, Essebag V, Afilalo J. Implications of frailty in elderly patients with electrophysiological conditions. JACC Clin Electrophysiol 2016; 2:288–94. [DOI] [PubMed] [Google Scholar]
- 125. Dominguez-Rodriguez A, Abreu-Gonzalez P, Jimenez-Sosa A, Gonzalez J, Caballero-Estevez N, Martin-Casanas FV, et al. . The impact of frailty in older patients with non-ischaemic cardiomyopathy after implantation of cardiac resynchronization therapy defibrillator. Europace 2015; 17:598–602. [DOI] [PubMed] [Google Scholar]
- 126. Takeda T, Tsubaki A, Ikeda Y, Kato R, Hotta K, Inoue T, et al. . The impacts of preoperative frailty on readmission after cardiac implantable electrical device implantation. PLoS One 2022; 17:e0277115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kramer DB, Tsai T, Natarajan P, Tewksbury E, Mitchell SL, Travison TG. Frailty, physical activity, and mobility in patients with cardiac Implantable electrical devices. J Am Heart Assoc 2017; 6:e004659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Padeletti L, Arnar DO, Boncinelli L, Brachman J, Camm AJ, Daubert JC, et al. . EHRA Expert Consensus Statement on the management of cardiovascular implantable electronic devices in patients nearing end of life or requesting withdrawal of therapy. Europace 2010; 12:1480–9. [DOI] [PubMed] [Google Scholar]
- 129. Thylén I, Moser DK, Strömberg A. Octo- and nonagenarians’ outlook on life and death when living with an implantable cardioverter defibrillator: a cross-sectional study. BMC Geriatr 2018; 18:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Khurshid S, Choi SH, Weng LC, Wang EY, Trinquart L, Benjamin EJ, et al. . Frequency of cardiac rhythm abnormalities in a half million adults. Circ Arrhythm Electrophysiol 2018; 11:e006273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Granada J, Uribe W, Chyou PH, Maassen K, Vierkant R, Smith PN, et al. . Incidence and predictors of atrial flutter in the general population. J Am Coll Cardiol 2000; 36:2242–6. [DOI] [PubMed] [Google Scholar]
- 132. Brembilla-Perrot B, Sellal JM, Olivier A, Manenti V, Villemin T, Beurrier D, et al. . Risk and outcome after ablation of isthmus-dependent atrial flutter in elderly patients. PLoS One 2015; 10:e0127672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Epstein LM, Chiesa N, Wong MN, Lee RJ, Griffin JC, Scheinman MM, Lesh MD. Radiofrequency catheter ablation in the treatment of supraventricular tachycardia in the elderly. J Am Coll Cardiol 1994; 23:1356–62. [DOI] [PubMed] [Google Scholar]
- 134. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. . 2020. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021; 42:373–498. [DOI] [PubMed] [Google Scholar]
- 135. Polidoro A, Stefanelli F, Ciacciarelli M, Pacelli A, Di Sanzo D, Alessandri C. Frailty in patients affected by atrial fibrillation. Arch Gerontol Geriatr 2013; 57:325–7. [DOI] [PubMed] [Google Scholar]
- 136. Wilkinson C, Todd O, Clegg A, Gale CP, Hall M. Management of atrial fibrillation for older people with frailty: a systematic review and meta-analysis. Age Ageing 2019; 48:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Villani ER, Tummolo AM, Palmer P, Gravina EM, Vetrano DL, Bernabei R, et al. . Frailty and atrial fibrillation: A systematic review. Eur J Intern Med 2018; 56:33–8. [DOI] [PubMed] [Google Scholar]
- 138. Ekerstad N, Karlsson T, Soderqvist S, Karlson BW. Hospitalized frail elderly patients - atrial fibrillation, anticoagulation and 12 months’ outcomes. Clin Interv Aging 2018; 3:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol 2017; 14: 627–8. [DOI] [PubMed] [Google Scholar]
- 140. Kotecha D, Bunting KV, Gill SK, Mehta S, Stanbury M, Jones JC, et al. . Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: the RATE-AF randomized clinical trial. JAMA 2020; 324:2497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. . Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383:955–62. [DOI] [PubMed] [Google Scholar]
- 142. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. . 2016. ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016; 18:1609–78. [DOI] [PubMed] [Google Scholar]
- 143. Esteve-Pastor MA, Rivera-Caravaca JM, Shantsila A, Roldán V, Lip GYH, Marín F. Assessing bleeding risk in atrial fibrillation patients: comparing a bleeding risk score based only on modifiable bleeding risk factors against the HAS-BLED Score. The AMADEUS Trial. Throm Haemost 2017; 117: 2261–6. [DOI] [PubMed] [Google Scholar]
- 144. Chao TF, Lip GYH, Lin YJ, Chang SL, Lo LW, Hu YF, et al. . Major bleeding and intracranial hemorrhage risk prediction in patients with atrial fibrillation: Attention to modifiable bleeding risk factors or use of a bleeding risk stratification score? A nationwide cohort study. Int J Cardiol 2018; 254:157–61. [DOI] [PubMed] [Google Scholar]
- 145. Guo Y, Zhu H, Chen Y, Lip GYH. Comparing bleeding risk assessment focused on modifiable risk factors only versus validated bleeding risk scores in atrial fibrillation. Am J Med 2018; 131:185–92. [DOI] [PubMed] [Google Scholar]
- 146. Lefebvre MC, St-Onge M, Glazer-Cavanagh M, et al. . The effect of bleeding risk and frailty status on anticoagulation patterns in octogenarians with atrial fibrillation: the FRAIL-AF study. Can J Cardiol 2016; 32:169–76. [DOI] [PubMed] [Google Scholar]
- 147. Mant J, Hobbs FD, Fletcher K, et al. . Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370:493–503. [DOI] [PubMed] [Google Scholar]
- 148. Chao TF, Liu CJ, Lin YJ, Chang SL, Lo LW, Hu YF, et al. . Oral anticoagulation in very elderly patients with atrial fibrillation: a nationwide cohort study. Circulation 2018; 138:37–47. [DOI] [PubMed] [Google Scholar]
- 149. Lauw MN, Eikelboom JW, Coppens M, Wallentin L, Yusuf S, Ezekowitz M, et al. . Effects of dabigatran according to age in atrial fibrillation. Heart 2017; 103:1015–23. [DOI] [PubMed] [Google Scholar]
- 150. Halperin JL, Hankey GJ, Wojdyla DM, Piccini JP, Lokhnygina Y, Patel MR, et al. . Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation 2014; 130:138–46. [DOI] [PubMed] [Google Scholar]
- 151. Halvorsen S, Atar D, Yang H, De Caterina R, Erol C, Garcia D, et al. . Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J 2014; 35:864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kato ET, Giugliano RP, Ruff CT, Koretsune Y, Yamashita T, Kiss RG, et al. . Efficacy and safety of edoxaban in elderly patients with atrial fibrillation in the ENGAGE AF-TIMI 48 trial. J Am Heart Assoc 2016; 5:e003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. . Apixaban in patients with atrial fibrillation. New Engl J Med 2011; 364:806–17. [DOI] [PubMed] [Google Scholar]
- 154. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–67. [DOI] [PubMed] [Google Scholar]
- 155. Dagres N, Chao TF, Fenelon G, et al. . European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on arrhythmias and cognitive function: what is the best practice? Europace 2018; 20:1399–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Mongkhon P, Naser AY, Fanning L, Tse G, Lau WCY, Wong ICK, Kongkaew C. Oral anticoagulants and risk of dementia: a systematic review and meta-analysis of observational studies and randomized controlled trials. Neurosci Biobehav Rev 2018; 96:1–9. [DOI] [PubMed] [Google Scholar]
- 157. Rao MP, Vinereanu D, Wojdyla DM, Alexander JH, Atar D, Hylek EM. Clinical outcomes and history of fall in patients with atrial fibrillation treated with oral anticoagulation: insights from the ARISTOTLE Trial. Am J Med 2018; 131:269–75. [DOI] [PubMed] [Google Scholar]
- 158. Steffel J, Giugliano RP, Braunwald E, Murphy SA, Mercuri M, Choi Y, et al. . Edoxaban versus warfarin in atrial fibrillation patients at risk of falling: ENGAGE AF-TIMI 48 Analysis. J Am Col Cardiol 2016; 68:1169–78. [DOI] [PubMed] [Google Scholar]
- 159. Kim DH, Pawar A, Gagne JJ, Bessette LG, Lee H, Glynn RJ, et al. . Frailty and clinical outcomes of direct oral anticoagulants versus warfarin in older adults with atrial fibrillation: a cohort study. Ann Intern Med 2021; 174:1214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. . 2021. European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021; 23:1612–76. [DOI] [PubMed] [Google Scholar]
- 161. van Doorn S, Tavenier A, Rutten FH, Hoes AW, Moons KGM, Geersing GJ. Risk of cardiac and non-cardiac adverse events in community-dwelling older patients with atrial fibrillation: a prospective cohort study in the Netherlands. BMJ Open 2018; 8:e021681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Martinez BK, Sood NA, Bunz TJ, Coleman CI. Effectiveness and safety of apixaban, dabigatran, and rivaroxaban versus warfarin in frail patients with nonvalvular atrial fibrillation. J Am Heart Assoc 2018; 7:e008643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Okumura K, Akao M, Yoshida T, Kawata M, Okazaki O, Akashi S, et al. . Low-dose edoxaban in very elderly patients with atrial fibrillation. N Engl J Med 2020; 383:1735–45. [DOI] [PubMed] [Google Scholar]
- 164. Wang A, Ferro EG, Song Y, Xu J, Sun T, Yeh RW, Strom JB, Kramer DB. Frailty in patients undergoing percutaneous left atrial appendage closure. Heart Rhythm 2022:S1547–5271(22)00012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Nguyen TN, Cumming RG and Hilmer SN. Atrial fibrillation in older inpatients: are there any differences in clinical characteristics and pharmacological treatment between the frail and the non-frail? Intern Med J 2016; 46:86–95. [DOI] [PubMed] [Google Scholar]
- 166. Bibas L, Levi M, Touchette J, Mardigyan V, Bernier M, Essebag V, Afilalo J. Implications of frailty in elderly patients with electrophysiological conditions. JACC Clin Electrophysiol 2016; 2:288–94. [DOI] [PubMed] [Google Scholar]
- 167. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. . 2017. HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018; 20:e1–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Bunch TJ, May HT, Bair TL, Jacobs V, Crandall BG, Cutler M, et al. . The Impact of Age on 5-Year Outcomes After Atrial Fibrillation Catheter Ablation. J Cardiovasc Electrophysiol 2016; 27:141–6. [DOI] [PubMed] [Google Scholar]
- 169. Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, et al. . Long-term clinical efficacy and risk of catheter ablation for atrial fibrillation in octogenarians. Pacing Clin Electrophysiol 2010; 33:146–52. [DOI] [PubMed] [Google Scholar]
- 170. Santangeli P, Di Biase L, Mohanty P, Burkhardt JD, Horton R, Bai R, et al. . Catheter ablation of atrial fibrillation in octogenarians: safety and outcomes. J Cardiovasc Electrophysiol 2012; 23:687–93. [DOI] [PubMed] [Google Scholar]
- 171. Natale V, Mohanty S, Trivedi C, Baqai FM, Gallinghouse J, Della Rocca DG, et al. . Arrhythmia profile and ablation-outcome in elderly women with atrial fibrillation undergoing first catheter ablation. Pacing Clin Electrophysiol 2021; 44:835–42. [DOI] [PubMed] [Google Scholar]
- 172. Kundi H, Noseworthy PA, Valsdottir LR, Shen C, Yao X, Yeh RW, Kramer DB. Relation of frailty to outcomes after catheter ablation of atrial fibrillation. Am J Cardiol 2020; 125:1317–23. [DOI] [PubMed] [Google Scholar]
- 173. Yang PS, Sung JH, Kim D, Jang E, Yu HT, Kim TH, et al. . Frailty and the effect of catheter ablation in the elderly population with atrial fibrillation - a real-world analysis. Circ J 2021; 85:1305–13. [DOI] [PubMed] [Google Scholar]
- 174. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, et al. . Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019; 321:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Boriani G, Laroche C, Diemberger I, Fantecchi E, Popescu MI, Rasmussen LH, et al. . Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP-AF Pilot General Registry. Am J Med 2015; 128:509–18.e2. [DOI] [PubMed] [Google Scholar]
- 176. Siontis KC, Gersh BJ, Killian JM, Noseworthy PA, McCabe P, Weston SA, et al. . Typical, atypical, and asymptomatic presentations of new-onset atrial fibrillation in the community: Characteristics and prognostic implications. Heart Rhythm 2016; 13:1418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Mairesse GH, Moran P, Van Gelder IC, Elsner C, Rosenqvist M, Mant J, et al. . Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLAECE). Europace 2017; 19:1589–1623. [DOI] [PubMed] [Google Scholar]
- 178. Zink MD, Mischke KG, Keszei AP, Rummey C, Freedman B, Neumann G, et al. . Screen-detected atrial fibrillation predicts mortality in elderly subjects. Europace 2021; 23:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Perales CR Lopez, Van Spall HGC, Maeda S, Jimenez A, Laţcu DG, Milman A, et al. . Mobile health applications for the detection of atrial fibrillation: a systematic review. Europace 2021; 23:11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Freedman B, Camm J, Calkins H, Healey JS, Rosenqvist M, Wang J, et al. . Screening for Atrial Fibrillation: A Report of the AF-SCREEN International Collaboration. Circulation 2017; 135:1851–67. [DOI] [PubMed] [Google Scholar]
- 181. Gorenek B, Bax J, Boriani G, Chen SA, Dagres N, Glotzer TV, et al. . Device-detected subclinical atrial tachyarrhythmias: definition, implications and management-an European Heart Rhythm Association (EHRA) consensus document, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Europace 2017; 19:1556–78. [DOI] [PubMed] [Google Scholar]
- 182. Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M, et al. . Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J 2014; 35:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, et al. . Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012; 366:120–9. [DOI] [PubMed] [Google Scholar]
- 184. Freedman B, Boriani G, Glotzer TV, Healey JS, Kirchhof P, Potpara TS. Management of atrial high-rate episodes detected by cardiac implanted electronic devices. Nat Rev Cardiol 2017; 14:701–14. [DOI] [PubMed] [Google Scholar]
- 185. Mahajan R, Perera T, Elliott AD, Twomey DJ, Kumar S, Munwar DA, et al. . Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J 2018; 39:1407–15. [DOI] [PubMed] [Google Scholar]
- 186. Svendsen JH, Diederichsen SZ, Højberg S, Krieger DW, Graff C, Kronborg C, et al. . Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet 2021; 398:1507–16. [DOI] [PubMed] [Google Scholar]
- 187. Boriani G, Glotzer TV, Ziegler PD, De Melis M, Mangoni, di S Stefano L, et al. Detection of new atrial fibrillation in patients with cardiac implanted electronic devices and factors associated with transition to higher device-detected atrial fibrillation burden. Heart Rhythm 2018; 15:376–83. [DOI] [PubMed] [Google Scholar]
- 188. Camm AJ, Simantirakis E, Goette A, Lip GY, Vardas P, Calvert M, et al. . Atrial high-rate episodes and stroke prevention. Europace. 2017; 19:169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Hess PL, Kim S, Piccini JP, et al. . Use of evidence-based cardiac prevention therapy among outpatients with atrial fibrillation. Am J Med. 2013; 126:625–632.e621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Winovich DT, LongstrethWT, Jr., Arnold AM, et al. . Factors associated with ischemic stroke survival and recovery in older adults. Stroke. 2017; 48:1818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Dhamoon MS, LongstrethWT, Jr., Bartz TM, Kaplan RC, Elkind MSV. Disability trajectories before and after stroke and myocardial infarction: the Cardiovascular Health Study. JAMA Neurol 2017; 74:1439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Biffi A, Kuramatsu JB, Leasure A, et al. . Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol 2017; 82:755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Wilson D, Ambler G, Shakeshaft C, et al. . Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol 2018; 17:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Whiteley WN, AdamsHP, Jr., Bath PM, et al. . Targeted use of heparin, heparinoids, or low-molecular-weight heparin to improve outcome after acute ischaemic stroke: an individual patient data meta-analysis of randomised controlled trials. Lancet Neurol 2013; 12:539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Hong KS, Kwon SU, Lee SH, et al. . Rivaroxaban vs warfarin sodium in the ultra-early period after atrial fibrillation-related mild ischemic stroke: a randomized clinical trial. JAMA Neurol 2017; 74:1206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Kernan WN, Ovbiagele B, Black HR, et al. . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45:2160–236. [DOI] [PubMed] [Google Scholar]
- 197. McGrath ER, Kapral MK, Fang J, et al. . Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke 2012; 43:2048–54. [DOI] [PubMed] [Google Scholar]
- 198. Ng KH, Shestakovska O, Connolly SJ, et al. . Efficacy and safety of apixaban compared with aspirin in the elderly: a subgroup analysis from the AVERROES trial. Age Ageing. 2016; 45:77–83. [DOI] [PubMed] [Google Scholar]
- 199. Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–85. [DOI] [PubMed] [Google Scholar]
- 200. Diener HC, Weber R, Lip GY, Hohnloser SH. Stroke prevention in atrial fibrillation: do we still need warfarin? Curr Opin Neurol 2012; 25:27–35. [DOI] [PubMed] [Google Scholar]
- 201. Parry SW, Steen IN, Baptist M, Kenny RA. Amnesia for loss of consciousness in carotid sinus syndrome: implications for presentation with falls. J Am Coll Cardiol 2005; 45:1840–3. [DOI] [PubMed] [Google Scholar]
- 202. Kerr SR, Pearce MS, Brayne C, Davis RJ, Kenny RA. Carotid sinus hypersensitivity in asymptomatic older persons: implications for diagnosis of syncope and falls. Arch Intern Med 2006; 166:515–20. [DOI] [PubMed] [Google Scholar]
- 203. Krediet CT, Parry SW, Jardine DL, Benditt DG, Brignole M, Wieling W. The history of diagnosing carotid sinus hypersensitivity: why are the current criteria too sensitive? Europace 2010; 13:14–22. [DOI] [PubMed] [Google Scholar]
- 204. Ungar A, Mussi C, Del Rosso A, Noro G, Abete P, Ghirelli L, et al. . Diagnosis and characteristics of syncope in older patients referred to geriatric departments. J Am Geriatr Soc 2006; 54:1531–36. [DOI] [PubMed] [Google Scholar]
- 205. Galizia G, Abete P, Mussi C, Noro G, Morrione A, Langellotto A, et al. . Role of early symptoms in assessment of syncope in elderly people: results from the Italian group for the study of syncope in the elderly. J Am Geriatric Soc 2009:57:18–23. [DOI] [PubMed] [Google Scholar]
- 206. Del Rosso A, Bartoletti A, Bartoli P, Ungar A, Bonechi F, Maioli M, et al. . Methodology of head-up tilt testing with sublingual nitroglycerin in unexplained syncope. Am J Cardiol 2000; 85:1007–11. [DOI] [PubMed] [Google Scholar]
- 207. Ungar A, Rivasi G, Rafanelli M, Toffanello G, Mussi C, Ceccofiglio A, et al. . Safety and tolerability of tilt testing and carotid sinus massage in the octogenarians. Age Ageing 2016; 45:242–48. [DOI] [PubMed] [Google Scholar]
- 208. Solari D, Tesi F, Unterhuber M, Gaggioli G, Ungar A, Tomaino M, et al. . Stop vasodepressor drugs in reflex syncope: a randomised controlled trial. Heart 2017; 103:449–55. [DOI] [PubMed] [Google Scholar]
- 209. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. . 2018. ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018; 39:3021–104. [DOI] [PubMed] [Google Scholar]
- 210. Fanciulli A, Jordan J, Biaggioni I, Calandra–Buonaura G, Cheshire WP, Cortelli P, et al. . Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS). Clin Auton Res 2018; 28:355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. De Lepeleire J, Iliffe S, Mann E, Degryse JM. Frailty: an emerging concept for general practice. Br J Gen Pract 2009; 59:e177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Puts MT, Shekary N, Widdershoven G, Heldens J, Lips P, Deeg DJ. What does quality of life mean to older frail and non-frail community-dwelling adults in the Netherlands? Qual Life Res 2007; 16:263–77. [DOI] [PubMed] [Google Scholar]
- 213. Frewen J, Finucane C, Cronin H, et al. . Factors that influence awareness and treatment of atrial fibrillation in older adults. QJM 2013; 106:415–24. [DOI] [PubMed] [Google Scholar]
- 214. O'Brien EC, Holmes DN, Ansell JE, et al. . Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am J Cardiol 2014; 167:601–609.e601. [DOI] [PubMed] [Google Scholar]
- 215. Perera V, Bajorek BV, Matthews S, Hilmer SN. The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing 2009; 38:156–62. [DOI] [PubMed] [Google Scholar]
- 216. Steinberg BA, Holmes DN, Ezekowitz MD, et al. . Rate versus rhythm control for management of atrial fibrillation in clinical practice: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am Heart J 2013; 165:622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Goldfarb M, Sheppard R, Afilalo J. Prognostic and therapeutic implications of frailty in older adults with heart failure. Curr Cardiol Rep 2015; 17:92. [DOI] [PubMed] [Google Scholar]
- 218. Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev 2013; 12:719–36. [DOI] [PubMed] [Google Scholar]
- 219. Adelstein EC, Liu J, Jain S, Schwartzman D, Althouse AD, Wang NC, et al. . Clinical outcomes in cardiac resynchronization therapy-defibrillator recipients 80 years of age and older. Europace 2016; 18:420–7. [DOI] [PubMed] [Google Scholar]
- 220. Brenner R, Ammann P, Yoon SI, et al. . Reduction of falls and fractures after permanent pacemaker implantation in elderly patients with sinus node dysfunction. Europace 2017; 19:1220–6. [DOI] [PubMed] [Google Scholar]
- 221. Fletcher AE, Price GM, Ng ES, et al. . Population-based multidimensional assessment of older people in UK general practice: a cluster-randomised factorial trial. Lancet 2004; 364:1667–77. [DOI] [PubMed] [Google Scholar]
- 222. Iliffe S, Gould M, Wallace P. Assessment of older people in the community: lessons from Britain’s ‘75-and-over checks’. Rev Clin Geront 1999; 9:305–16. [Google Scholar]
- 223. Lane DA, Aguinaga L, Blomström-Lundqvist C, Boriani G, Dan GA, Hills MT, et al. . Cardiac tachyarrhythmias and patient values and preferences for their management: the European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Europace 2015; 17:1747–69. [DOI] [PubMed] [Google Scholar]
- 224. Roldan VD Torres, Brand-McCarthy SR, Ponce OJ, Belluzzo T, Urtecho M, Espinoza Suarez NR, et al. . Shared decision making tools for people facing stroke prevention strategies in atrial fibrillation: a systematic review and environmental scan. Med Decis Making 2021; 41:540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Kunneman M, Branda ME, Hargraves IG, Sivly AL, Lee AT, Gorr H, et al. . Assessment of shared decision-making for stroke prevention in patients with atrial fibrillation: a randomized clinical trial. JAMA Intern Med 2020; 180:1215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Pluymaekers NAHA, Hermans ANL, van der Velden RMJ, Gawałko M, den Uijl DW, Buskes S, et al. . Implementation of an on-demand app-based heart rate and rhythm monitoring infrastructure for the management of atrial fibrillation through teleconsultation: TeleCheck-AF. Europace 2021; 23:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Hermans ANL, van der Velden RMJ, Gawalko M, Verhaert DVM, Desteghe L, Duncker D, et al. . On-demand mobile health infrastructures to allow comprehensive remote atrial fibrillation and risk factor management through teleconsultation. Clin Cardiol 2020; 43:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Lane DA, McMahon N, Gibson J, Weldon JC, Farkowski MM, Lenarczyk R, et al. . Mobile health applications for managing atrial fibrillation for healthcare professionals and patients: a systematic review. Europace 2020; 22:1567–78. [DOI] [PubMed] [Google Scholar]
- 229. Leclercq C, Witt H, Hindricks G, Katra RP, Albert D, Belliger A, et al. . Wearables, telemedicine, and artificial intelligence in arrhythmias and heart failure: Proceedings of the European Society of Cardiology Cardiovascular Round Table. Europace 2022; 24:1372–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.