Abstract
Aims
Pulmonary vein isolation using radiofrequency ablation is an effective treatment option for patients with symptomatic atrial fibrillation (AF). Application of high power over a short period of time (HPSD) is reported to create more efficient lesions and may prevent collateral thermal oesophageal injury. This study aims to compare efficacy and safety of two different HPSD ablation approaches using different ablation index settings.
Methods and results
Consecutive patients undergoing AF ablation with HPSD (50 W; ablation index–guided) using the ThermoCool SmartTouch SF catheter were included. Patients were grouped by ablation protocol: ablation with target ablation index (AI) of 400 on the anterior left atrial wall vs. 300 at the posterior left atrial wall (AI 400/300) or AI 450/350 was performed upon the operator’s preference and compared. Peri-procedural parameters and complications were recorded, and incidences of endoscopically detected thermal oesophageal lesions (EDEL) analysed. Recurrence rates after a mean follow-up of 25 ± 7 months and reconnection patterns in patients undergoing redo procedures were investigated. A total of 795 patients (67 ± 10 years; 58% male; 48% paroxysmal AF) underwent a first AF ablation with HPSD (211 in group AI 400/300 and 584 in group 450/350). Median procedure time was 82.9 ± 24.6 min with longer ablation times in patients with target AI 400/300 due to higher intraprocedural reconnection rates, increased box lesions, and additional right atrial isthmus ablations. EDEL rates among target AI 400/300 procedures were significantly lower (3% vs. 7%; P = 0.019). Correspondingly, AI 450/350 was the strongest independent predictor of post-ablation EDEL (OR 4.799, CI 1.427–16.138, P = 0.011). Twelve-month (76% vs. 76%; P = 0.892) and long-term ablation single procedure success (68% vs. 71%; log-rank P = 0.452) after a mean of 25 ± 7 months were comparable among both target AI groups; however, long-term success was significantly higher for paroxysmal AF compared to persistent AF (12 months: 80% vs. 72%; P = 0.010; end of follow-up: 76% vs. 65%; log-rank P = 0.001). One hundred three patients (16%) underwent a redo procedure during follow-up documented comparable pulmonary vein (PV) reconnection among groups. Multivariate predictors of AF recurrence were age, left atrium (LA) size, persistent AF, and extra-PV ablation targets.
Conclusion
High-power short-duration AF ablation with target AI of 400 for non-posterior wall and 300 for posterior wall lesions resulted in comparable long-term results compared to higher AI (450/350) ablations with significantly lower risk for thermal oesophageal lesions. Older age, larger LA size, persistent AF, and extra-PV ablation targets were identified in a multivariate analysis as independent risk factors for recurrences of atrial arrhythmias.
Keywords: Ablation index, Atrial fibrillation, Endoscopically detected oesophageal lesions, Pulmonary vein isolation, High-power short-duration ablation, Prognosis bull
Graphical Abstract
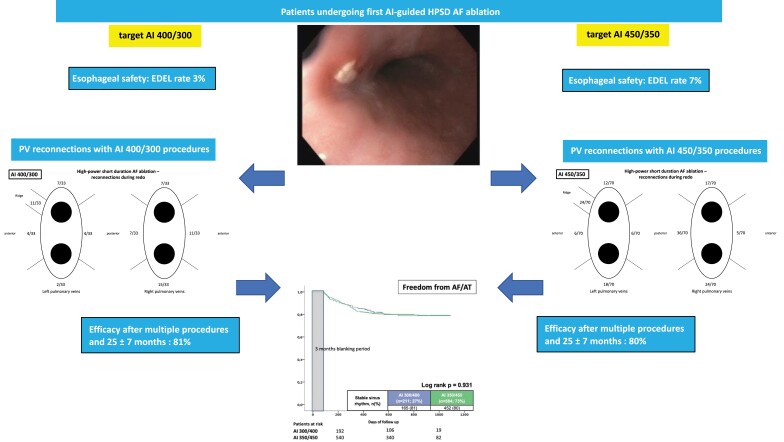
Graphical Abstract.
What’s new?
This study aims to compare efficacy and safety of two different HPSD ablation approaches using different ablation index settings.
Atrial fibrillation ablation with HPSD with target AI of 300 for posterior wall lesions results in significantly less oesophageal lesions compared to AI 350.
High-power short-duration AF ablation with target AI of 400 for non-posterior wall and 300 for posterior wall lesions resulted in comparable long-term results compared to AI 450/350.
Predictors for development of EDEL are target AI 300 vs. 350, increasing ablation time and larger LA size, whereas female gender and increased BMI were protective.
Predictors of AF recurrences were older age, larger LA size, and persistent AF.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia worldwide. Radiofrequency (RF) catheter ablation has become an effective treatment option in symptomatic patients.1,2 Electrical isolation of the pulmonary veins (PVI) is the cornerstone of AF ablation procedures.1,2 Conventionally, irrigated RF ablations with power ranges of 20–40 W over a 20–40 s ablation duration are used to create atrial lesions.3
Despite satisfying success rates for PVI, electrical reconnection of one or more pulmonary veins (PVs) is considered as a major mechanism of AF recurrence.4,5 Reconnection is mostly related to non-transmural lesions due to tissue oedema. Ablation settings using higher power for a short duration (HPSD) are considered to minimize conductive heating transfer and increase resistive heating in order to achieve transmural atrial lesion formation due to less oedema and more irreversible myocardial tissue injury with reduced risk of collateral tissue damage such as endoscopically detected asymptomatic oesophageal lesions (EDEL).6,7
High-power short-duration ablation is not a uniform strategy and outcome, and safety maybe influenced by multiple factors like energy, duration, and catheter–tissue contact. Several studies could show improved short- and long-term efficacy of AF ablation using the ablation index (AI).8,9 AI is a parameter defining energy application at a single ablation point and is calculated using the integration of contact force, ablation time, ablation energy, and catheter stability (10). Optimal AI cut-off values should imply a superior efficacy and safety profile, but comparative studies are missing.
Therefore, our study aimed to investigate the safety and outcome of HPSD 50 W ablation in patients undergoing AF ablation using a pre-defined AI of 400 for non-posterior ablation lesions and 300 for posterior lesions vs. a higher level AI 450/350 in a high-volume single-centre experience.
Methods
Study population
Patients undergoing de novo AF ablation in our department were consecutively included from January 2019 until May 2021. Patients with drug-refractory symptomatic paroxysmal or persistent AF scheduled for HPSD AF ablation were included. Atrial fibrillation ablation was performed according to the HPSD protocol with the standard ablation catheter (ThermoCool SmartTouch SF; Biosense Webster, Irvine, California, USA). Patients were ablated using two different AI cut-off levels either 400/300 (Group 1) or 450/350 (Group 2) and compared. Respective cut-off values were used based on the operator’s preference but were fixed throughout the study period. The AI 400/300 was used by two operators whereas AI 450/350 was used by four operators during the study. Allocation of the patients to the respective operators/groups was randomly performed via the chief of the department. The respective AI protocols were based on early ‘in-house’ experience and not changed over time until now. In a run-in phase, we evaluated AI values for each individual operator which was based on the individual ablation strategies (duration using lower-power ablation) which already included different strategies (foremost reduced energy delivery at the posterior wall) for ablation at the anterior vs. posterior wall. Therefore, these AI values were established historically and kept throughout the experience with higher-power delivery as efficacy and safety were monitored and comparable.
A second part of the study compared patients with paroxysmal to persistent AF. Atrial fibrillation was categorized as paroxysmal (lasting <1 week) or persistent (lasting >1 week and <1 year or requiring pharmacological or electrical cardioversion in <1 week).
Post-ablation endoscopy was performed in all patients according to our standard procedure at the first workday after ablation to identify possible ablation-induced thermal oesophageal injury (EDEL). Values of left ventricular ejection fraction (LVEF) and left atrium (LA) size were retrieved from standardized transthoracic or oesophageal echocardiographic examinations usually being performed before AF ablation.
Major groin complications were defined as decrease in haemoglobin > 2 g/dL or atrio–venous fistula, arterial bleeding, or slow venous haemorrhage requiring surgical intervention.
All patients gave written informed consent to the ablation procedure and all pre- and post-ablation diagnostics. The study was carried out according to the principles of the Declaration of Helsinki and was approved by the local medical ethics committee of the Heart Centre Bad Neustadt, Germany.
Atrial fibrillation ablation procedure
All patients underwent either contrast-enhanced cardiac computer tomography with cardiac segmentation or transoesophageal echocardiography for exclusion of intracardiac thrombus <24 h prior to ablation. All ablations were performed on uninterrupted anticoagulation using direct oral anticoagulants (half dose at the day of PVI prior to PVI in the morning and half dose at the evening of the same day) or vitamin K antagonists with an international normalized ratio between 2 and 3. Heparin boli were administered to achieve activated clotting times >300 s during the procedure. All procedures were performed under conscious sedation using continuous propofol infusion in conjunction with morphine derivates. After three-time venous access, a steerable diagnostic catheter was placed into the coronary sinus. In all patients, a single transseptal puncture with double access to the LA using a steerable 11.7-French sheath was performed (Agilis, Abbott, Minneapolis, MN, USA). Mapping was performed using a three-dimensional electroanatomic mapping system (CARTO 3, Biosense Webster, Diamond Bar, CA, USA) in conjunction with a decapolar mapping catheter (LASSO; Biosense Webster, Diamond Bar, CA, USA) or in selected patients with a multipolar high-density mapping catheter (PENTARAY; Biosense Webster, Diamond Bar, CA, USA). For PVI, the ThermoCool SmartTouch SF catheter (Biosense Webster, Irvine, California, USA) in conjunction with the SMARTABLATE generator (Biosense Webster, Irvine, California, USA) was used. According to the CLOSE-protocol, intertag distance ≤ 6 mm was aimed.10 Radiofrequency energy was applied in an ablation index-guided manner (maximum AI 300 or 350 for posterior wall ablation, AI 400 or 450 for non-posterior wall ablation). The AI values were individually titrated to not exceed indicated cut-off values by the operators. Target contact force was set between 10 and 25 g at all ablation sites for both groups. Complete wide antral en bloc PVI was performed in every procedure according to our standard approach using a point-by-point ablation technique. Confirmation of effective PVI was performed with a decapolar/multipolar mapping catheter placed in the PV ostia after elimination of all PV potentials followed by 20 min waiting period and proof of entry/exit block on the circumferential mapping catheter. In case of no first-pass isolation, additional segmental ablation was applied at the earliest PV activation time on the level of the circumferential lesion in order to achieve complete PVI. Additional ablation strategies in case of further LA substrate were to the operator’s discretion and were all performed with 50 W according to the AI protocol. Adenosine testing for dormant reconduction of PVs was not routinely performed, only in a minority of cases with recurring reconduction of PVs after multiple ablation attempts to finally prove effective isolation. In case of additional isolation of the superior vena cava, 25 W was used. All ablation procedures were performed by experienced operators each having done >1000 AF ablation procedures before. All operators were certified by the European Heart Rhythm Association (EHRA) and the German Cardiac Society (DGK) with an invasive electrophysiology diploma. No oesophageal temperature probes were used.
Post-ablation oesophageal endoscopy
Oesophageal endoscopy (EE) was performed within the next workday post-AF ablation. Presence and extend of EDEL were investigated. Endoscopically detected thermal oesophageal lesion was defined as any oesophageal lesion in the ‘typical’ area of oesophagus and LA proximity (identified during endoscopy) and was classified as either erythema/erosion/small ulcer ≤ 5 mm (Category 1) or ulceration > 5 mm (Category 2). The differentiation was performed based on the operator’s estimation and visual aspect during initial endoscopy. Based on the visual aspect during endoscopy, erythema/erosion was defined as a thermal oesophageal ablation–induced lesion with reddish discoloration or superficial disruption of the oesophageal mucosa but without disruption of the lamina muscularis mucosae. An ulcer was defined as disruption of the oesophageal mucosa extending into the submucosal layers, including fibrinous coverage.11 Description and examples of EDEL from both categories were distributed to all endoscopy operators before performing upper endoscopy in post-AF ablation patients to ensure correct and uniform classification of oesophageal findings. The differentiation was performed based on the operators’ estimation and visual aspect during initial endoscopy. All EEs were performed from highly experienced operators each having performed >2000 EE after AF ablations. Irrespective of EE findings, all patients received proton pump inhibitors (twice standard dose) for 6 weeks after AF ablation.
Study endpoint
The endpoint of this study was recurrence of any atrial tachyarrhythmia after a 3-month blanking period. Arrhythmia recurrences within the blanking period were treated conservatively with antiarrhythmic drugs and/or electrical cardioversion. Atrial arrhythmia recurrences after the blanking period were defined as an ECG-documented atrial tachycardia.
Follow-up
Procedural efficacy was evaluated by multiple (at least two within the first 3 months, then every 3 months on) 24–72-h Holter ECG recordings and clinical evaluation in relation to AF episodes. If clinical symptoms potentially related to AF were noted, an in-house follow-up was scheduled and an ECG or 24–72-h Holter ECG was recorded. If symptoms were present but no ECG document at that time was available, tighter ECG controls including telemonitoring ECG devices were used.
Repeat procedures
Most redo procedures were performed by the same operator as the initial procedure (91%). All redo procedures were performed with the same AI targets as the initial procedure. During redo procedures, electrical conduction of the PVs was assessed in all cases using a decapolar/multipolar mapping catheter and segmental reisolation was performed if necessary. Afterwards, a voltage map during sinus rhythm was conducted and low voltage areas and complex fractionated atrial electrograms (CFAEs) were targeted within the LA as well as in the CS and/or the RA at the discretion of the operator. Furthermore, additional lesion sets for substrate modification were based on the patient’s respective substrate.
Statistical methods
Quantitative data are presented as mean ± standard error of mean (SEM), median and interquartile range (IQR), and ranges depending on the distribution of the data and were compared using the Student’s t-test for normally distributed data or the Mann–Whitney U test for non-parametric data. Deviations from a Gaussian distribution were tested by the Kolmogorov-Smirnov test. Spearman’s rank correlation for non-parametric data was used to test univariate correlations. Qualitative data are presented as absolute and relative frequencies and compared using the χ2 test or the Fisher’s exact test, as appropriate.
The following analyses were applied stepwise to evaluate the prognostic value of pre-defined variables on study endpoints: Kaplan-Meier curves were calculated with log-rank testing for statistical significance. Univariable hazard ratios (HR) are given together with 95% confidence intervals. Multivariable Cox regression models with stable sinus rhythm (SR) after follow-up as the dependent variables were developed using the ‘forward selection’ option. Multivariable models were adjusted by univariably statistically significant variables such as age, gender, LA size, type of AF, BMI, arterial hypertension, diabetes mellitus, CAD, LVEF, and AI 450/350 vs. 400/300. The result of a statistical test was considered significant for P < 0.05, and P values ≤ 0.1 were defined as a statistical trend. SAS release 9.4 (SAS Institute Inc., Cary, NC, USA) and SPSS (Version 25, IBM Armonk, New York, USA) were used for statistics.
Results
Study population
Between March 2019 and May 2021, 795 consecutive patients undergoing a first AF ablation procedure and were ablated using a HPSD strategy at our institution were included. In total, 903 procedures were considered (795 first ablations, 108 redo procedures). Group 1 contained 211 patients, Group 2 584 patients. AF was paroxysmal in 379 (48%) and persistent in 416 (52%) patients. The majority of all patients were males (58%), and mean age was 67 years. Mean follow-up time was 25 ± 7 months (see Table 1).
Table 1.
Clinical characteristics
| Characteristic | PAF (n = 379; 48%) | PersAF (n = 416; 52%) | All patients (n = 795) | P value | AI 400/300 (n = 211; 27%) | AI 450/350 (n = 584; 73%) | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 65 (26–83) | 69 (30–88) | 67 (26–86) | 0.001 | 68 (26–83) | 66 (26–83) | 0.157 | |||||
| Males, n (%) | 199 | (53) | 259 | (62) | 458 | (58) | 0.005 | 132 | (63) | 326 | (56) | 0.090 |
| HTN, n (%) | 311 | (82) | 378 | (91) | 689 | (87) | 0.001 | 175 | (83) | 514 | (88) | 0.063 |
| DM, n (%) | 47 | (12) | 79 | (19) | 126 | (16) | 0.011 | 25 | (12) | 101 | (17) | 0.063 |
| CAD, n (%) | 96 | (25) | 149 | (36) | 245 | (31) | 0.001 | 57 | (27) | 188 | (32) | 0.163 |
| Stroke/TIA, n (%) | 19 | (5) | 26 | (6) | 45 | (6) | 0.456 | 14 | (7) | 31 | (5) | 0.478 |
| LVEF (%) | 58 ± 9 | 51 ± 13 | 54 ± 12 | 0.001 | 55 ± 11 | 54 ± 12 | 0.625 | |||||
| LA size (qcm) | 22.9 ± 5.9 | 26.9 ± 6.7 | 25.0 ± 6.6 | 0.001 | 24.8 ± 6.5 | 25.1 ± 6.7 | 0.662 | |||||
| BMI | 29.0 ± 5.3 | 30.0 ± 6.0 | 29.5 ± 5.7 | 0.015 | 28.7 ± 5.6 | 29.8 ± 5.7 | 0.012 | |||||
| CHADS-Vasc score | 2.6 ± 1.5 | 3.1 ± 1.4 | 2.9 ± 1.5 | 0.001 | 2.7 ± 1.5 | 2.9 ± 1.5 | 0.205 | |||||
| PAF, n (%) | — | — | — | — | 106 | (50) | 273 | (47) | 0.475 | |||
Bold values indicate statistical significance.
AF, atrial fibrillation; AHT, arterial hypertension; AI, ablation index; BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; LA, left atrium; LVEF, left ventricular ejection fraction; PAF, paroxysmal AF; PersAF, persistent AF; TIA, transient ischaemic attack.
Ablation and procedural parameters of index procedure
Group 1 ablations revealed comparable procedure (81.6 ± 20.9 min vs. 83.3 ± 24.6 min; P = 0.362) and fluoroscopy times (6.4 ± 4.2 min vs. 5.9 ± 3.9 min; P = 0.151), however, longer ablation times compared to Group 2 (19.5 ± 8.4 min vs. 16.5 ± 10.0 min; P = 0.001). Overall procedure time was 82.9 ± 23.7 min with longer total procedures times among patients with persistent AF (85.0 ± 24.3 min vs. 80.6 ± 22.9 min; P = 0.009). Fluoroscopy and ablation times were comparable among AF groups (Table 2). In 100 patients of each group, real effective AI values were calculated. Group 1 showed real AI values of 432 ± 18 for non-posterior and 343 ± 29 for posterior lesions. In Group 2, the real AI values were 485 ± 24 and 384 ± 18, respectively.
Table 2.
Procedural data first PVI
| Characteristic | PAF (n = 379; 48%) | PersAF (n = 416; 52%) | All patients (n = 795) | P value | AI 400/300 (n = 211; 27%) | AI 450/350 (n = 584; 73%) | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Procedural duration (min) | 80.6 ± 22.9 | 85.0 ± 24.3 | 82.9 ± 23.7 | 0.009 | 81.6 ± 20.9 | 83.3 ± 24.6 | 0.362 | |||||
| Fluoroscopy duration (min) | 5.8 ± 3.7 | 6.1 ± 4.2 | 6.0 ± 4.0 | 0.305 | 6.4 ± 4.2 | 5.9 ± 3.9 | 0.151 | |||||
| Ablation time (min) | 17.0 ± 11.1 | 17.5 ± 8.1 | 17.3 ± 9.7 | 0.499 | 19.5 ± 8.4 | 16.5 ± 10.0 | 0.001 | |||||
| Extra-PV ablation | 77 | (20) | 138 | (33) | 215 | (27) | 0.001 | 78 | (37) | 137 | (24) | 0.001 |
| ȃRoof line | 21 | (6) | 62 | (15) | 83 | (10) | 0.001 | 20 | (10) | 63 | (11) | 0.594 |
| ȃAnterior line | 39 | (10) | 89 | (21) | 128 | (16) | 0.001 | 33 | (16) | 95 | (16) | 0.832 |
| ȃBox lesion | 13 | (3) | 34 | (8) | 47 | (6) | 0.005 | 30 | (14) | 17 | (3) | 0.001 |
| ȃSVC | 4 | (1) | 6 | (1) | 10 | (1) | 0.435 | 3 | (1) | 7 | (1) | 0.521 |
| ȃCTI | 27 | (7) | 29 | (7) | 56 | (7) | 0.933 | 24 | (11) | 32 | (6) | 0.004 |
| Intraprocedural PV reconnection | 68 | (18) | 72 | (17) | 140 | (18) | 0.815 | 48 | (23) | 92 | (16) | 0.022 |
Bold values indicate statistical significance.
AF, atrial fibrillation; AI, ablation index; CTI, cavotricuspidal isthmus; PAF, paroxysmal AF; PersAF, persistent AF; PV, pulmonary vein; SVC, superior vena cava.
Complete isolation of all PVs was achieved in all patients. In 215 patients, additional left and/or right atrial RF ablation was performed. Longer ablation times among Group 1 procedures resulted from increased extra-PV ablation sites among those patients (box lesion: 14% vs. 3%; P = 0.001 and CTI: 11% vs. 6%; P = 0.004). Furthermore, in AI Group 1 procedures, intraprocedural PV reconnection was more common compared to Group 2 procedures (23% vs. 16%; P = 0.022) and resulted in additional ablations for reisolation.
In persistent AF patients, LA roof line (10%; P = 0.001) and anterior line (16%; P = 0.001) were more often ablated compared to paroxysmal AF patients. Posterior wall isolation was added in 47 patients (6%) with 3% in paroxysmal AF and 8% in persistent AF (P = 0.005). Intraprocedural PV reconnections occurred comparable often among AF types (Table 2).
Complications
Complications were comparable in between AI groups and between paroxysmal vs. persistent AF groups (see Table 3). One pericardial tamponade (0.1%) occurred most likely due to difficult transseptal puncture. Three patients (0.4%) suffered thromboembolic complications (one transitory ischaemic attack 3 days after PVI without CT or MRI imaging correlate; one patient with hemiparesis the day after PVI proven via CT and MRI, and one patient with hemiplegia after the PVI proven with MRI). All patients underwent pre-procedural exclusion of LA/left atrial appendage (LAA) thrombi and had ACT >300 during AF ablation. Four patients (0.5%) suffered from post-conversion symptomatic bradycardia (unmasked sinus node dysfunction) and required pacemaker implantations after the ablation procedure. No atrio-oesophageal fistula was identified.
Table 3.
Complications
| Characteristic | PAF (n = 379; 48%) | PersAF (n = 416; 52%) | All patients (n = 795) | P value | AI 400/300 (n = 211; 27%) | AI 450/350 (n = 584; 73%) | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Major complications | ||||||||||||
| ȃPericardial tamponade | 0 | (0) | 1 | (0) | 1 | (0) | 1.000 | 0 | (0) | 1 | (0) | 1.000 |
| ȃThromboembolic complications | 2 | (1) | 1 | (0) | 3 | (0) | 0.608 | 1 | (0) | 2 | (0) | 1.000 |
| ȃAccess site complications | 5 | (1) | 5 | (1) | 10 | (1) | 1.000 | 4 | (2) | 6 | (1) | 0.305 |
| ȃPacemaker implantation | 1 | (0) | 3 | (1) | 4 | (1) | 0.626 | 1 | (0) | 3 | (1) | 1.000 |
| ȃAspiration pneumonia | 1 | (0) | 3 | (1) | 4 | (1) | 0.626 | 0 | (0) | 4 | (1) | 0.578 |
| ȃAir embolism | 0 | (0) | 1 | (0) | 1 | (0) | 1.000 | 0 | (0) | 1 | (0) | 1.000 |
| ȃPhrenicus paresis | 1 | (0) | 0 | (0) | 1 | (0) | 1.000 | 0 | (0) | 1 | (0) | 1.000 |
| Minor complications | ||||||||||||
| ȃThermal oesophagus lesion | 24 | (6) | 25 | (6) | 49 | (6) | 0.850 | 6 | (3) | 43 | (7) | 0.019 |
| ȃEDEL I | 5 | (1) | 12 | (3) | 17 | (2) | 0.128 | 1 | (0) | 16 | (3) | 0.054 |
| ȃEDEL II | 19 | (5) | 13 | (3) | 32 | (4) | 0.176 | 5 | (2) | 27 | (5) | 0.153 |
Bold values indicate statistical significance.
AI, ablation index; EDEL, endoscopically detected oesophageal lesion; PAF, paroxysmal AF; PersAF, persistent AF.
Occurrence and predictors of endoscopically detected thermal oesophageal lesions
All patients underwent post-ablation EE and 49 out of 795 patients demonstrated EDEL (6.2%), 17 patients (2.1%) revealed Category 1 and 32 (4.0%) Category 2 lesions. Group 1 procedures showed significantly fewer EDEL rates compared to Group 2 (3% vs. 7%; P = 0.019), numerically for both EDEL categories (0.5% vs. 2.7%; P = 0.054 for Category 1 and 2.4% vs. 4.6%; P = 0.153 for Category 2). Rates and categories of EDEL were comparable between patients with paroxysmal and persistent AF (Table 3). Comparing patients with additional box lesion and those without, no significant differences among EDEL rates could be found (box lesion 4.3% vs. no box lesion 6.3%; P = 0.575; 2/47 patients with box lesions with EDEL 2; 47/748 patients without box lesion with EDEL 1/2).
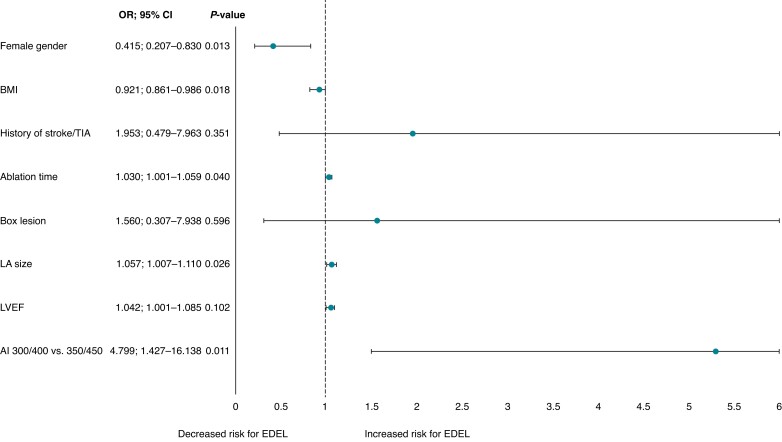
In multivariable logistic regression analysis of our cohort, increasing AI (=AI 450/350 vs. AI 400/300) was the strongest independent predictor of post-ablation EDEL (OR 4.799, CI 1.427–16.138, P = 0.011). Furthermore, total ablation time (OR 1.030, CI 1.001–1.059, P = 0.040) and larger LA size (OR 1.057, CI 1.007–1.110, P = 0.026) were associated with increased occurrence of EDEL, whereas female gender (OR 0.415, CI 0.207–0.830, P = 0.013) and increased BMI (OR 0.921, CI 0.861–0.986, P = 0.018) were associated with smaller risk for EDEL after AF ablation (Figure 1).
Figure 1.
Predictors of occurrence of EDEL. AI, ablation index; BMI, body mass index; LA, left atrium; LVEF, left ventricular ejection fraction; TIA, transitory ischaemic attack.
Clinical outcome after first ablation procedure and predictors of success
Intrahospital AF recurrence occurred in 63 patients (8%) and was comparable between both AI group ablations (7% AI 400/300 vs. 8% AI 450/350; P = 0.609). At 12 months and end of follow-up, 591 patients (76%) (76% AI 400/300 vs. 76% AI 450/350; P = 0.892) and 544 patients (70%) (68% AI 400/300 vs. 71% AI 450/350; log-rank P = 0.451) of all patients, respectively, remained free from AF recurrence after one AF ablation procedure. Comparing blanking period of 6 weeks and 12 weeks, we found no significant difference between both groups in terms of AF recurrences. Within the first 6 weeks, 22% of all patients suffered from AF recurrences (AI 400/300 27% vs. AI 450/350 21%; P = 0.071), whereas only 10% of all patients suffered from AF recurrences between weeks 7 and 12 (AI 400/300 11% vs. AI 450/350 10%; P = 0.836). In total, 7% (AI 400/300 8% vs. AI 450/350 7%; P = 0.785) of all patients suffered from recurrences between weeks 1–6 and between weeks 7–12. The remaining 3% with first recurrence between weeks 7 and 12 of blanking period both groups were comparable (AI 400/300 2.8% vs. AI 450/350 3.0%; P = 0.961).
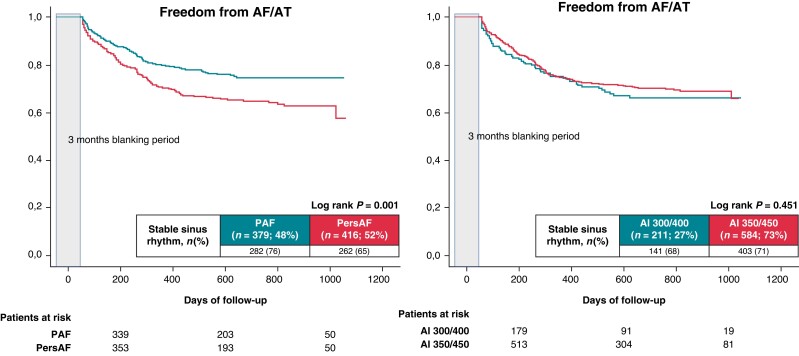
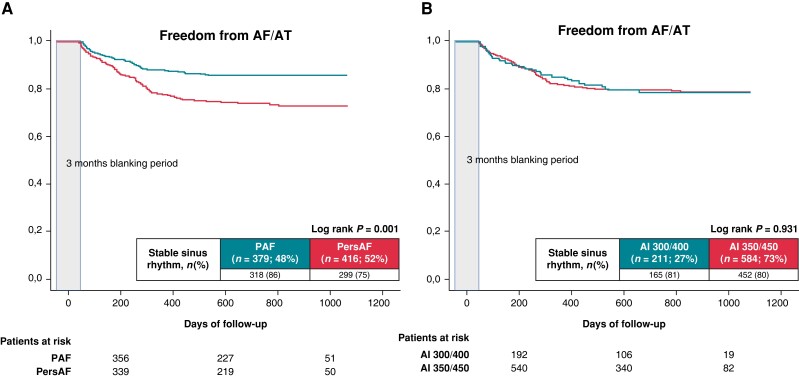
Furthermore, intrahospital AF recurrence was also comparable between paroxysmal (23 patients) and persistent AF (40 patients) (6% paroxysmal AF vs. 10% persistent AF; P = 0.064). At 12 months, 296 patients with paroxysmal AF (80%) and 295 patients with persistent AF (72%) (P = 0.010) remained free from AF recurrences. At the end of follow-up, 282 patients (76%) with paroxysmal AF and 262 patients (65%) with persistent AF (log-rank P = 0.001) remained free from AF recurrence after one AF ablation procedure, respectively (Figure 2).
Figure 2.
Proportion of patients in stable SR after the initial ablation procedure during a mean FU period of 25 ± 7 months. (A) Paroxysmal AF vs. persistent AF. (B) AI 300/400 vs. AI 350/450.
Multivariate regression analysis showed older age (HR 1.032, CI 1.014–1.050, P = 0.001), larger LA size (HR 1.026, CI 1.004–1.048, P = 0.021), persistent AF (HR 1.440, CI 1.010–2.052, P = 0.044), and extra-PV ablation targets (HR 1.552, CI 1.132–2.127, P = 0.006) as independent predictors of AF recurrence after the initial ablation procedure, but not AI 400/300 ablations (HR 0.972, CI 0.698–1.354, P = 0.867) (Table 5).
Table 5.
Univariable and multivariable hazard ratios to predict AF recurrence (n = 795)
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Predictors after the index procedure | ||||||
| ȃAge | 1.030 | 1.012–1.049 | 0.001 | 1.032 | 1.014–1.050 | 0.001 |
| ȃLarger LA size | 1.049 | 1.024–1.075 | 0.001 | 1.026 | 1.004–1.048 | 0.021 |
| ȃFemale gender | 0.820 | 0.596–1.128 | 0.222 | — | — | — |
| ȃPAF vs. PerAF | 1.490 | 1.080–2.054 | 0.015 | 1.440 | 1.010–2.052 | 0.044 |
| ȃBMI | 1.014 | 0.988–1.042 | 0.289 | — | — | — |
| ȃHypertension | 0.792 | 0.481–1.305 | 0.360 | — | — | — |
| ȃDiabetes mellitus | 1.622 | 1.090–2.412 | 0.017 | — | — | — |
| ȃCAD | 0.770 | 0.542–1.093 | 0.143 | — | — | — |
| ȃLVEF | 1.007 | 0.994–1.020 | 0.303 | — | — | — |
| ȃExtra-PV ablations | 1.722 | 1.315–2.253 | 0.001 | 1.552 | 1.132–2.127 | 0.006 |
| ȃAI 400/300 vs. 450/350 | 0.972 | 0.698–1.354 | 0.867 | — | — | — |
| Predictors after the final procedure | ||||||
| ȃAge | 1.050 | 1.025–1.075 | 0.001 | 1.043 | 1.020–1.066 | 0.001 |
| ȃLarger LA size | 1.037 | 1.008–1.067 | 0.011 | 1.037 | 1.010–1.065 | 0.007 |
| ȃFemale gender | 0.937 | 0.631–1.390 | 0.745 | — | — | — |
| ȃPAF vs. PerAF | 1.761 | 1.169–2.652 | 0.007 | 1.665 | 1.110–2.498 | 0.014 |
| ȃBMI | 1.010 | 0.980–1.041 | 0.519 | — | — | — |
| ȃHypertension | 1.553 | 0.708–3.405 | 0.272 | — | — | — |
| ȃDiabetes mellitus | 1.309 | 0.831–2.061 | 0.245 | — | — | — |
| ȃCAD | 0.936 | 0.627–1.396 | 0.745 | — | — | — |
| ȃLVEF | 1.000 | 0.984–1.016 | 0.995 | — | — | — |
| ȃExtra-PV ablations | 2.343 | 1.701–3.227 | 0.001 | 1.771 | 1.213–2.585 | 0.003 |
| ȃAI 400/300 vs. 450/350 | 0.937 | 0.624–1.408 | 0.755 | — | — | — |
Bold type indicates statistical significance P < 0.05.
BMI, body mass index; CI; confidence interval; LA, left atrium; LVEF, left ventricular ejection faction; OR, odds ratio; TIA, transient ischaemic attack.
Progression and regression of atrial fibrillation duration during follow-up
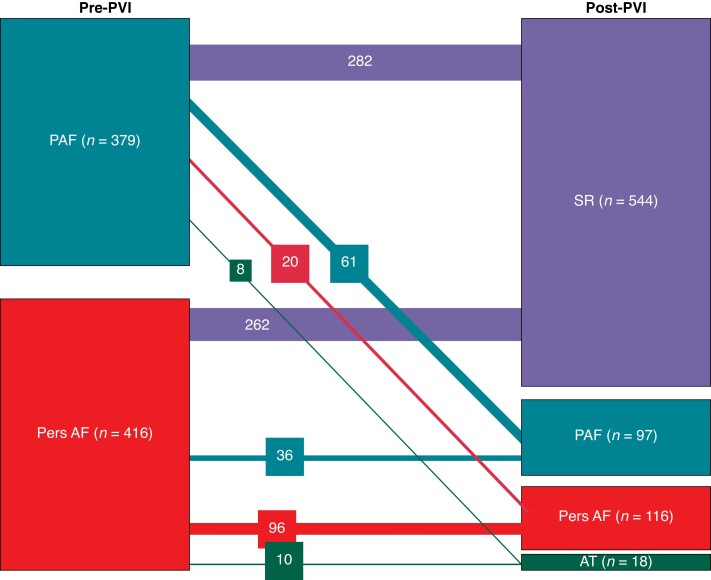
Eighty-nine of the 231 patients with recurrences of atrial tachyarrhythmias initially had paroxysmal AF (39%). Of those 89 patients, 61 had again documented recurrent paroxysmal AF (=16% of all patients with initial paroxysmal AF), 20 persistent AF (=5.3%), and 8 macro-reentrant atrial tachycardias (=2.1%). One hundred forty-two of the 231 patients with recurrences initially had persistent AF (61%). Thirty-six out of 416 patients showed regression to paroxysmal AF (9% of all patients with initial persistent AF), 96/416 patients showed again recurrent persistent AF (23%), and 10/416 patients showed recurrent macro-reentrant tachycardias (2.4%) (Figure 3).
Figure 3.
Sankey diagram showing progression and regression of AF types after initial AF ablation procedures. AT, macro-reentrant atrial tachycardia; PAF, paroxysmal atrial fibrillation; Pers AF, persistent atrial fibrillation; PVI, pulmonary vein isolation; SR, sinus rhythm.
Reconnection patterns and second ablation procedure
In total, 103 of all patients received a redo procedure (13% of all patients; 45% of all patients with AF recurrences; 46% initial paroxysmal AF vs. 54% initial persistent AF) after a median of 321 ± 182 days after the index procedure (see Table 4). In 81 patients (79%), reconnection of at least one PV was documented (10% of the overall cohort) without a significant difference between AI groups (82% vs. 77%; P = 0.589) or paroxysmal vs. persistent AF (81% vs. 77%, P = 0.616). Mean number of reconnected PVs was 1.8 ± 1.3 without any significant differences among target AI values (1.8 ± 1.2 vs. 1.8 ± 1.4; P = 0.945) or AF types (1.8 ± 1.3 vs. 1.8 ± 1.4; P = 0.985). The reconnection rate of the right-sided PVs was significantly higher than the left sided PVs, however comparable between target AI groups and AF types. Reconnections at the inferior LPs were higher among AI 400/300 ablations (26% vs. 6%; P = 0.019); all other LPV sites were comparable (roof P = 0.619; ridge P = 0.924; anterior P = 0.570; posterior P = 0.570). At the RPVs, AI 400/300 procedures had higher reconnection rates at the anterior site (33% vs. 7%; P = 0.001) and lower reconnection sites posterior (21% vs. 51%; P = 0.004). Roof and inferior reconnections were comparable among groups (roof P = 0.731; inferior 0.276) (Figure 4A and B).
Table 4.
Pulmonary vein reconnection patterns and LA substrate modification during first redo procedures (n = 103)
| Characteristic | PAF (n = 379; 48%) | PersAF (n = 416; 52%) | All patients (n = 795) | P value | AI 400/300 (n = 211; 27%) | AI 450/350 (n = 584; 73%) | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Repeat procedure | 47 | (12) | 56 | (13) | 103 | (13) | 0.657 | 33 | (16) | 70 | (12) | 0.176 |
| Numbers of PV accessed | 186 | — | 230 | — | 416 | — | — | 130 | — | 286 | — | — |
| Reconnection PVs | ||||||||||||
| ȃLSPV | 20 | (44) | 20 | (36) | 40 | (39) | 0.424 | 11 | (33) | 29 | (42) | 0.400 |
| ȃLIPV | 14 | (30) | 14 | (25) | 28 | (27) | 0.541 | 7 | (21) | 21 | (30) | 0.329 |
| ȃRSPV | 24 | (52) | 33 | (59) | 57 | (55) | 0.494 | 20 | (61) | 37 | (54) | 0.506 |
| ȃRIPV | 27 | (59) | 34 | (61) | 61 | (59) | 0.435 | 22 | (67) | 39 | (57) | 0.328 |
| ȃNo PV | 9 | (19) | 13 | (23) | 22 | (21) | 0.616 | 6 | (18) | 16 | (23) | 0.589 |
| ȃTotal | 85 | (46) | 101 | (44) | 186 | (45) | 0.716 | 60 | (46) | 126 | (44) | 0.690 |
| Number of PV reconnections per patient | ||||||||||||
| ȃ0 | 9 | (19) | 13 | (23) | 22 | (21) | 0.957 | 6 | (18) | 16 | (23) | 0.175 |
| ȃ1 | 9 | (19) | 9 | (16) | 18 | (17) | 4 | (12) | 14 | (20) | ||
| ȃ2 | 17 | (36) | 19 | (34) | 36 | (35) | 17 | (52) | 19 | (27) | ||
| ȃ3 | 6 | (13) | 6 | (11) | 12 | (12) | 2 | (6) | 10 | (14) | ||
| ȃ4 | 6 | (13) | 9 | (16) | 15 | (15) | 4 | (12) | 11 | (16) | ||
| ȃMean PVs | 1.8 ± 1.3 | 1.8 ± 1.4 | 1.8 ± 1.3 | 0.985 | 1.8 ± 1.2 | 1.8 ± 1.4 | 0.945 | |||||
| LA substrate | 39 | (83) | 42 | (75) | 81 | (79) | 0.325 | 31 | (94) | 50 | (71) | 0.009 |
| ȃRoof line | 7 | (15) | 15 | (27) | 22 | (21) | 0.142 | 4 | (12) | 18 | (26) | 0.116 |
| ȃAnterior line | 15 | (32) | 20 | (36) | 35 | (34) | 0.685 | 12 | (36) | 23 | (33) | 0.726 |
| ȃPosterior wall | 20 | (43) | 18 | (32) | 38 | (37) | 0.275 | 18 | (55) | 20 | (29) | 0.011 |
| ȃSVC | 15 | (32) | 22 | (39) | 37 | (36) | 0.437 | 11 | (33) | 26 | (37) | 0.707 |
| ȃCTI | 2 | (4) | 11 | (20) | 13 | (13) | 0.019 | 5 | (15) | 8 | (11) | 0.595 |
Bold values indicate statistical significance.
AF, atrial fibrillation; AI, ablation index; CTI, cavotricuspidal isthmus; LA, left atrium; PAF, paroxysmal AF; PersAF, persistent AF; PV, pulmonary vein; SVC, superior vena cava.
Figure 4.
(A) Diagram showing the number of segments with reconnection identified within each region during redo procedures after target AI 400/300 procedures (n = 33). (B) Diagram showing the number of segments with reconnection identified within each region during redo procedures after target AI 450/350 procedures (n = 70).
During redo, initial Group 1 procedures received significantly more often LA substrate modification (94% vs. 71%; P = 0.009), mainly due to higher rates of additional substrate modification at the posterior wall (55% vs. 29%; P = 0.011). Left atrium substrate modification was comparable between AF types during redo procedures; however, patients with persistent AF received more often additional CTI ablation due to documented typical RA flutter (20% vs. 4%; P = 0.019).
During the first redo procedures, six complications (5.8%) occurred: two patients suffered from post-interventional stroke (both with persistent AF; one patient with hemiplegia right after PVI with ischaemia proven via MRI and one patient with seizures after PVI and proven ischaemia via MRI; both had pre-interventional transoesophageal echocardiography for thrombus exclusion and had ACT >300 during AF ablation), one patient with persistent AF had post-interventional symptomatic sinus node dysfunction and received a dual chamber pacemaker implantation, one patient had peri-interventional air embolism without any sequelae and two patients had post-interventional Category 1 EDEL.
Clinical outcome after second ablation procedure
After the second procedure, 18/103 (17%) patients developed AF recurrence. Of them, 7/48 (15%) patients initially diagnosed with PAF and 11/55 (20%) patients initially diagnosed with persistent AF developed atrial arrhythmia recurrences. Correspondingly, 9/33 (27%) patients initially ablated with AI 400/300 and 9/70 (13%) patients initially ablated with AI 450/350 developed recurrent atrial tachyarrhythmias. Following, AF recurrence rates between AI group procedures (P = 0.072) as well as between paroxysmal and persistent AF (P = 0.470) were comparable after the second procedure. Thirty-nine percent of all patients showed paroxysmal, 50% persistent AF, and 11% atrial tachycardias as type of recurrence.
Three and more ablation procedures
In five patients (all with initially persistent AF), a third procedure was undertaken during follow-up period after a median of 175 ± 52 days after the second procedure. Only one patient showed reconnection of PVs (RSPV and RIPV) undergoing reisolation (this patient was in the AI 400/300 group). In the other four patients, all PVs were still isolated. In three patients, a posterior wall isolation via box lesion was performed. One patient received a roof and anterior line, and one patient only a roof line. No major complications occurred.
After the third procedure, two patients (40%) remained in stable SR, and three patients (60%) developed recurrent atrial arrhythmias.
Overall clinical outcome and predictors of success after multiple procedures
The freedom from AF after a mean of 1.1 ± 0.4 procedures at the end of follow-up was 86% for paroxysmal AF and 75% for persistent AF, respectively (log-rank P = 0.001). Comparable rates of SR were present for initial target AI 400/300 and 450/350 procedures at the end of follow-up (81% vs. 80%; log-rank P = 0.931) (Figure 5).
Figure 5.
Proportion of patients in stable SR after multiple ablation procedures (mean 1.1 ± 0.4) during a mean FU period of 25 ± 7 months. (A) Paroxysmal AF vs. persistent AF. (B) AI 300/400 vs. AI 350/45.
Multivariable Cox regression analysis revealed older age (HR 1.043, CI 1.020–1.066, P = 0.001), larger LA size (HR 1.037, CI 1.010–1.065, P = 0.007), persistent AF (HR 1.665, CI 1.110–2.498, P = 0.014), and extra-PV ablation targets (HR 1.771, CI 1.213–2.585, P = 0.003) as independent predictors of AF recurrence after multiple ablation procedures, but not AI 400/300 (HR 0.937, CI 0.624–1.408, P = 0.755) (Table 5).
Discussion
Main findings
The present study investigates safety and long-term follow-up of a real-life cohort after AF ablation using a HPSD protocol with 50 W RF ablations in a large single-centre retrospective study design. These results suggest that AF ablation with HPSD using a target AI of 400/450 for non-posterior and 300/350 for posterior LA wall ablations results in short procedure, fluoroscopy, and ablation times. High-power short-duration AF ablation in a highly experienced ablation centre is safe with low overall complication rates. Using a lower target AI for posterior wall ablation (300) yielded comparable efficacy but superior oesophageal safety in our cohort of patients. Long-term success rates are promising but 80% of patients with recurrent atrial arrhythmias show PV reconnections irrespective of target AI of 400/300 or 450/350. Significant predictors of AF recurrence after first as well after multiple procedures were increasing age, larger LA size, and persistent AF as well as extra-PV ablation targets in multivariable regression analysis.
Efficacy of high-power short-duration AF ablation
Despite recent developments in ablation technologies such as automated lesion assessment, contact force catheters, and new ablation methods, PV reconnection following initial successful PVI remains a significant clinical problem of AF recurrences.12 Several experimental in vitro and in vivo models on HPSD AF ablation have been performed.6,7,13 Transmural atrial lesions could be obtained using 50 W for 5 s at a contact force of 10 g and lesions’ depth between conventional energy delivery and HPSD ablation did not differ. This is not unexpected, because HPSD ablation shifts tissue heating from conductive towards resistive heating compared to conventional RF ablation settings. Thereby, a better predictable and more shallow but superficially wider lesion geometry can be generated. This might also result in improved linear lesion continuity.14
The high efficacy and safety rates of HPSD AF ablation compared to conventional ablations with 20–30 W were investigated in two randomized controlled trials (RCT). Leo et al. compared lesion size index (LSI)–guided AF ablation with 40–20 W ablations with different LSIs (4 or 5). Forty watt ablations had shorter procedural and RF times and had significantly more first-pass PVI and less acute PV reconnections. Importantly, HPSD ablations had significantly less AF recurrences after 3 years.15 Another RCT was conducted by Shin et al. who could show that 50 W ablations resulted in shorter procedure and ablation times for PV isolation with a comparable safety profile compared to 30 W and 40 W ablations.16 The 12-month follow-up was comparable with AF recurrences in 16% of all patients and no difference between paroxysmal and persistent AF.16 Several other publications with conventional AF ablations as comparators confirm high efficacy and safety rates of AF ablation using HPSD with different ablation settings and parameters.13,17–19 All of them report significantly reduced RF and procedure times with high first-pass isolation rates and reduced acute PV reconnections.20 Recently, also very high-power short-duration (vHPSD) ablations with 60–70 W were found to be comparably safe and even more efficient than ablations with conventional power.21
Our results encourage the positive trend for the high efficacy of a HPSD approach with 76% freedom from AF after 12 months after one procedure and 81% after multiple procedures at the end of follow-up. However, results are not directly comparable to other studies with different ablation settings and target AIs. It is crucial to envision HPSD ablation not as a single strategy but rather multiple different energy-duration-contact-force settings which may have different effects on safety and efficacy. An individual test of a dedicated set of parameters should be performed.
Ablation index–guided high-power short-duration AF ablation
Recently, a quantitative ablation lesion marker (AI) has been introduced and several reports have shown that AI-guided HPSD AF ablation might be an efficient and safe ablation strategy8,9 in an attempt to standardize local ablation lesion. A recent meta-analysis could show significant reduction of total procedure time (mean −23 min) and ablation time (mean −11 min) as well as fluoroscopy time (mean −8 min). Furthermore, freedom from AF after 6 months was higher compared to conventional RF AF ablation settings.22 Sufficient AI values with cut-off value of 370 for posterior wall and 480 for anterior wall were associated with durable lesions in the first clinical study using AI-guided PVI.23 Recent studies targeting AI values of 550 for anterior wall and 400 for posterior wall were associated with high procedure success rates and low PV reconnection rates.8,24 In the previously mentioned RCT of Shin et al., different AI values were identified as independent predictors of arrhythmia recurrence. Therefore, the use of AI with an optimal regional target value might further improve clinical outcomes. The present study used target AI of 300 or 350 for posterior and 400 or 450 for non-posterior wall ablation. This could possibly be the reason for lower first-pass isolation rates within the present study compared to AI 550/400 ablations. Furthermore, intraprocedural PV reconnection was high in nearly 20% of all patients. We used a CLOSE-guided approach with intertag distance of 6 mm. However, this approach was evaluated with target AI values of 550/400 resulting in durable PV isolation in 41–62% during redo procedures compared to only 18–23% within our cohort.25 Considering this, a reduction of the intertag distance could be discussed with possible heat stacking increasing the risk for EDEL on the other hand. However, lower AI values appear not necessarily associated with higher posterior PV reconnections and clinical AF recurrences (AI 550/400 with 73–80% 12-month success rate;8,24 AI 450/350 with 76% 12-month success rate; and 400/300 with also 76% 12-month success rate). Nevertheless, the primary target of every AF ablation procedure should be durable PV isolation and with this regard, our ablation approach might be too conservative. Whereas posterior AI targets should aim to produce more shallow lesions without thermal energy ‘overshoot’ to the oesophagus, a less stringent safety margin for non-posterior wall AI may be tolerated. Ablation index cut-offs have not been implemented into the generator software for automated shut-off of energy application but this may be concerned for future strategies as a safety tool. Novel insights might reveal a personalized ablation protocol with adaption of the AI to the respective left atrial wall thickness. First results of this strategy seem promising with high first-pass isolation rates, beneficial long-term result short procedure, and RF times.26 A multicentre trial is ongoing delivering more robust results.
Oesophageal safety profile of ablation index–guided high-power short-duration ablations
Careful titration of ablation energy especially at the posterior wall is crucial to balance between durable transmural contiguous ablation lesions on the one hand and collateral oesophageal thermal injury on the other hand. However, optimal ablation power and time are still unclear. For conventional RF ablation strategies with 25–35 W, rates of EDEL of about 18% were reported with slight improvements for ablation techniques with increased power and shorter duration.9,11,27,28 The novel vHPSD ablation technique using 90 W seems to be associated with dramatic decline of EDEL rates in the first in-human trial and our own experience.29,30 This is not unexpected, because HPSD ablation results in increased resistive heating with potentially reducing collateral tissue damage from conductive heat transfer.7 Conflicting evidence was recently reported from Nakagawa et al. who could show significantly smaller lesion size and lower tissue temperatures in thigh muscle preparations as well as in beating animal hearts. Furthermore, the ratio of diameter and depth is greater with 90 W ablations resulting in shallower lesions.31 Actual tissue temperatures with LSI-guided 50 W ablations appear to be higher than those with standard 30 W ablation measured in a porcine model with thermocouples.32 With better predictable tissue ablation effects, collateral oesophageal injuries might be prevented without sacrificing ablation success due to insufficient ablation lesions at posterior wall with subsequent PV reconnection. Reduced ablation time per lesion possibly alleviates effects of catheter instability and instable contact force resulting in more homogeneous, transmural ablation sites. Transmural lesions are key points of short-term ablation success resulting in lower oedema, acute PV reconnections, and decreased need for re-ablation points at the same sites. Therefore, HPSD ablation results in a lower risk of oesophageal injury with comparable clinical efficacy profile as it has been shown by previous results with only 3–6% thermal oesophageal injuries.33 Data from our study indicate that AI-guided ablation may also be safe in regard to creating low incidences of EDELs by potentially reducing local energy and ablation time to a minimum needed for adequate lesion formation but not more. Competing results were published recently by Francke et al. showing significantly higher EDEL rates of nearly 12% with a CLOSE-guided 50 W protocol with target AI values of 550/400.34 Notably, again posterior box lesion did not increase EDEL risk. Reduction of target AI for posterior lesions from 380 to 350/320 was associated with reduced occurrence of EDEL as well as gastroparesis.35 This emphasizes the importance of critical evaluation of ablation protocols and its respective AI target values for posterior lesions. However, further RCTs for AI-guided ablations documenting improved efficacy on the one hand and safety on the other hand are lacking. Our data consistently show that an AI cut-off of 300 at posterior-wall sites effectively creates PVI with decreased incidences of EDEL. Higher AI cut-off values only increased EDEL risk without increasing efficacy.
Predictors of AF recurrence after high-power short-duration AF ablation
Several factors for AF recurrence after initial successful AF ablation like non-paroxysmal AF, diabetes mellitus, and increased LA size have been published.36 Our study results are consistent with previous analyses attributing patients with increasing age, larger LA size, persistent AF, and additional extra-PV ablation targets worse outcome not only after a single, but also after multiple procedures. This finding is consistent throughout all major ablation techniques including standard RF protocols, single-shot devices, or upcoming newer technologies.
Redo procedures
AF represents a chronic disease, and a significant part of patients will require a redo procedure after initial successful AF ablation. Currently, to the authors’ best knowledge, there is no evidence of higher risk for complications during redo AF ablations; however, the overall complication rate during redo procedures in this study was nearly 6% including thermal oesophageal injury (3% complications if excluding EDEL). There were two patients with post-interventional stroke despite continued OAK treatment and pre-interventional imaging-based LA/LAA thrombus exclusion deserves attention.37 Of note, we included EDEL in our overall complication rates hampering direct comparability with other studies.
There was a statistical trend towards decreased efficacy of AI 400/300 ablations after the second procedures resulting in double AF recurrences as in the AI 450/350 group. Although numbers were small, it can be discussed that in patients with ‘difficult’ PV isolation or rather high PV reconnections due to demanding catheter stability or thicker atrial myocardium, application of AI 400/300 may result in less durable long-term isolation.
Limitations
The major limitation of this study is the observational design. Lost to follow-up rate was low with 2%. The results of this study may not be transferrable to other centres using different ablation protocols with different catheters or settings. Both groups contained different operators with different ablation approaches and protocols, influencing the direct comparability of both groups. Patients with private health insurance were mostly allocated to Group 1. These patients tend to reveal higher socio-economic status with lower prevalence of cardiovascular diseases such as arterial hypertension, diabetes mellitus, or lower BMI.38
Mean ablation values such as contact force, temperature, and ablation time were not available for the cohort. No cerebral imaging was performed in this study, so the risk of ‘silent’ cerebral events is not available if different between AI settings. Additionally, position and distance between atrium and oesophagus were not investigated and therefore not included in our analysis. A major concern is that ablations were manually titrated towards the proposed AI cut-off but no automated stop mechanism is available even though this may be considered a safety tool. Additional substrate modification might increase waiting period in those patients and more PV reconnections could be detected.
Follow-up was mostly performed with 24-h Holter ECGs possibly overestimating success rates after AF ablation affecting both target AI groups.
Conclusions
High-power short-duration AF ablation with target AI of 400 for non-posterior wall and 300 for posterior wall lesions resulted in comparable PV reconnection rates and long-term results to AI 450/350 ablations with a significantly lower risk for thermal oesophageal injury. The lower AI cut-off to protect the oesophagus was not associated with higher AF recurrences with freedom from any atrial arrhythmia in 81% of all patients after a mean of 1.1 procedures. Older age, larger LA size, persistent AF, and extra-PV ablation targets were identified as multivariate risk factors for AF recurrences and higher AI cut-off, total ablation time, and larger LA size were associated with a higher risk for EDEL.
Contributor Information
Julian Müller, Clinic for Interventional Electrophysiology, Heart Centre Bad Neustadt, Von-Guttenberg-Straße 11, 97616 Bad Neustadt a. d. Saale, Germany; Department of Cardiology and Angiology, Philipps-University Marburg, Baldingerstraße, 35034 Marburg, Germany.
Karin Nentwich, Clinic for Interventional Electrophysiology, Heart Centre Bad Neustadt, Von-Guttenberg-Straße 11, 97616 Bad Neustadt a. d. Saale, Germany; Department of Cardiology and Angiology, Philipps-University Marburg, Baldingerstraße, 35034 Marburg, Germany.
Artur Berkovitz, Clinic for Interventional Electrophysiology, Heart Centre Bad Neustadt, Von-Guttenberg-Straße 11, 97616 Bad Neustadt a. d. Saale, Germany.
Elena Ene, Clinic for Interventional Electrophysiology, Heart Centre Bad Neustadt, Von-Guttenberg-Straße 11, 97616 Bad Neustadt a. d. Saale, Germany.
Kai Sonne, Clinic for Interventional Electrophysiology, Heart Centre Bad Neustadt, Von-Guttenberg-Straße 11, 97616 Bad Neustadt a. d. Saale, Germany.
Vitaly Zhuravlev, Clinic for Interventional Electrophysiology, Heart Centre Bad Neustadt, Von-Guttenberg-Straße 11, 97616 Bad Neustadt a. d. Saale, Germany.
Ivaylo Chakarov, Clinic for Interventional Electrophysiology, Heart Centre Bad Neustadt, Von-Guttenberg-Straße 11, 97616 Bad Neustadt a. d. Saale, Germany.
Sebastian Barth, Clinic for Interventional Electrophysiology, Heart Centre Bad Neustadt, Von-Guttenberg-Straße 11, 97616 Bad Neustadt a. d. Saale, Germany; Department of Cardiology and Angiology, Philipps-University Marburg, Baldingerstraße, 35034 Marburg, Germany.
Christian Waechter, Department of Cardiology and Angiology, Philipps-University Marburg, Baldingerstraße, 35034 Marburg, Germany.
Michael Behnes, First Department of Medicine, University Medical Centre Mannheim (UMM), Theodor-Kutzer-Ufer 1-3, 68167 Mannheim, Germany.
Philipp Halbfass, Department of Cardiology and Angiology, Philipps-University Marburg, Baldingerstraße, 35034 Marburg, Germany; Department of Cardiology, Oldenburg, Rahel-Straus-Straße 10, 26133 Oldenburg, Germany.
Thomas Deneke, Clinic for Interventional Electrophysiology, Heart Centre Bad Neustadt, Von-Guttenberg-Straße 11, 97616 Bad Neustadt a. d. Saale, Germany.
Data availability
The data are available upon reasonable request from the authors.
References
- 1. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei Bet al. . 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- 2. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga Let al. . 2017 HRS/EHRA/ECAS/APHRS/SOLAECE Expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace 2018;20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kautzner J, Neuzil P, Lambert H, Peichl P, Petru J, Cihak Ret al. . EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace 2015;17:1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuck KH, Albenque JP, Chun KJ, Fürnkranz A, Busch M, Elvan Aet al. . Repeat ablation for atrial fibrillation recurrence post cryoballoon or radiofrequency ablation in the FIRE AND ICE Trial. Circ Arrhythm Electrophysiol 2019;12:e007247. [DOI] [PubMed] [Google Scholar]
- 5. Kuck KH, Hoffmann BA, Ernst S, Wegscheider K, Treszl A, Metzner Aet al. . Impact of complete versus incomplete circumferential lines around the pulmonary veins during catheter ablation of paroxysmal atrial fibrillation: results from the Gap-Atrial Fibrillation-German Atrial Fibrillation Competence Network 1 Trial. Circ Arrhythm Electrophysiol 2016;9:e003337. [DOI] [PubMed] [Google Scholar]
- 6. Leshem E, Zilberman I, Tschabrunn CM, Barkagan M, Contreras-Valdes FM, Govari Aet al. . High-power and short-duration ablation for pulmonary vein isolation: biophysical characterization. JACC Clin Electrophysiol 2018;4:467–79. [DOI] [PubMed] [Google Scholar]
- 7. Barkagan M, Contreras-Valdes FM, Leshem E, Buxton AE, Nakagawa H, Anter E. High-power and short-duration ablation for pulmonary vein isolation: safety, efficacy, and long-term durability. J Cardiovasc Electrophysiol 2018;29:1287–96. [DOI] [PubMed] [Google Scholar]
- 8. Hussein A, Das M, Riva S, Morgan M, Ronayne C, Sahni Aet al. . Use of ablation index-guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients: the PRAISE study results. Circ Arrhythm Electrophysiol 2018;11:e006576. [DOI] [PubMed] [Google Scholar]
- 9. Chen S, Chun KRJ, Tohoku S, Bordignon S, Urbanek L, Willems Fet al. . Esophageal endoscopy after catheter ablation of atrial fibrillation using ablation-index guided high-power: Frankfurt AI-HP ESO-I. JACC Clin Electrophysiol 2020;6:1253–61. [DOI] [PubMed] [Google Scholar]
- 10. Phlips T, Taghji P, El Haddad M, Wolf M, Knecht S, Vandekerckhove Yet al. . Improving procedural and one-year outcome after contact force-guided pulmonary vein isolation: the role of interlesion distance, ablation index, and contact force variability in the ‘CLOSE'-protocol. Europace 2018;20:f419–f27. [DOI] [PubMed] [Google Scholar]
- 11. Halbfass P, Pavlov B, Müller P, Nentwich K, Sonne K, Barth Set al. . Progression from esophageal thermal asymptomatic lesion to perforation complicating atrial fibrillation ablation: a single-center registry. Circ Arrhythm Electrophysiol 2017;10(8):e005233. [DOI] [PubMed] [Google Scholar]
- 12. Macle L, Khairy P, Weerasooriya R, Novak P, Verma A, Willems Set al. . Adenosine-guided pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: an international, multicentre, randomised superiority trial. Lancet 2015;386:672–9. [DOI] [PubMed] [Google Scholar]
- 13. Winkle RA, Mohanty S, Patrawala RA, Mead RH, Kong MH, Engel Get al. . Low complication rates using high power (45–50 W) for short duration for atrial fibrillation ablations. Heart Rhythm 2019;16:165–9. [DOI] [PubMed] [Google Scholar]
- 14. Bourier F, Duchateau J, Vlachos K, Lam A, Martin CA, Takigawa Met al. . High-power short-duration versus standard radiofrequency ablation: insights on lesion metrics. J Cardiovasc Electrophysiol 2018;29:1570–5. [DOI] [PubMed] [Google Scholar]
- 15. Leo M, Pedersen M, Rajappan K, Ginks MR, Hunter RJ, Bowers Ret al. . Power, lesion size index and oesophageal temperature alerts during atrial fibrillation ablation: a randomized study. Circ Arrhythm Electrophysiol 2020;13:e008316. [DOI] [PubMed] [Google Scholar]
- 16. Shin DG, Ahn J, Han SJ, Lim HE. Efficacy of high-power and short-duration ablation in patients with atrial fibrillation: a prospective randomized controlled trial. Europace 2020;22:1495–501. [DOI] [PubMed] [Google Scholar]
- 17. Kottmaier M, Popa M, Bourier F, Reents T, Cifuentes J, Semmler Vet al. . Safety and outcome of very high-power short-duration ablation using 70 W for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Europace 2020;22:388–93. [DOI] [PubMed] [Google Scholar]
- 18. Winkle RA, Mead RH, Engel G, Kong MH, Salcedo J, Brodt CRet al. . High-power, short-duration atrial fibrillation ablations using contact force sensing catheters: outcomes and predictors of success including posterior wall isolation. Heart Rhythm 2020;17:1223–31. [DOI] [PubMed] [Google Scholar]
- 19. Kaneshiro T, Kamioka M, Hijioka N, Yamada S, Yokokawa T, Misaka Tet al. . Characteristics of esophageal injury in ablation of atrial fibrillation using a high-power short-duration setting. Circ Arrhythm Electrophysiol 2020;13:e008602. [DOI] [PubMed] [Google Scholar]
- 20. Winkle RA. HPSD ablation for AF high-power short-duration RF ablation for atrial fibrillation: a review. J cardiovasc Electrophysiol 2021;32:2813–23. [DOI] [PubMed] [Google Scholar]
- 21. Popa MA, Bourier F, Lengauer S, Krafft H, Bahlke F, Förschner LVet al. . Safety profile and long-term efficacy of very high-power short-duration (60–70 W) catheter ablation for atrial fibrillation: results of a large comparative analysis. Europace 2023;16;25(2):408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu X, Gui C, Wen W, He Y, Dai W, Zhong G. Safety and efficacy of high power shorter duration ablation guided by ablation index or lesion size index in atrial fibrillation ablation: a systematic review and meta-analysis. J Interv Cardiol 2021;2021:5591590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Das M, Loveday JJ, Wynn GJ, Gomes S, Saeed Y, Bonnett LJet al. . Ablation index, a novel marker of ablation lesion quality: prediction of pulmonary vein reconnection at repeat electrophysiology study and regional differences in target values. Europace 2017;19:775–83. [DOI] [PubMed] [Google Scholar]
- 24. Taghji P, El Haddad M, Phlips T, Wolf M, Knecht S, Vandekerckhove Yet al. . Evaluation of a strategy aiming to enclose the pulmonary veins with contiguous and optimized radiofrequency lesions in paroxysmal atrial fibrillation: a pilot study. JACC Clin Electrophysiol 2018;4:99–108. [DOI] [PubMed] [Google Scholar]
- 25. De Pooter J, Strisciuglio T, El Haddad M, Wolf M, Phlips T, Vandekerckhove Yet al. . Pulmonary vein reconnection no longer occurs in the majority of patients after a single pulmonary vein isolation procedure. JACC Clin Electrophysiol 2019;5:295–305. [DOI] [PubMed] [Google Scholar]
- 26. Teres C, Soto-Iglesias D, Penela D, Jáuregui B, Ordoñez A, Chauca Aet al. . Personalized paroxysmal atrial fibrillation ablation by tailoring ablation index to the left atrial wall thickness: the ‘Ablate by-LAW’ single-centre study-a pilot study. Europace 2022;24:390–9. [DOI] [PubMed] [Google Scholar]
- 27. Halbfass P, Lehmkuhl L, Foldyna B, Berkovitz A, Sonne K, Nentwich Ket al. . Correlation of magnetic resonance imaging and post-ablation endoscopy to detect oesophageal thermal injury in patients after atrial fibrillation ablation: MRI-EDEL-study. Europace 2020;22:1009–16. [DOI] [PubMed] [Google Scholar]
- 28. Halbfass P, Nentwich K, Krug J, Roos M, Sonne K, Ene Eet al. . Impact of surround flow catheter tip irrigation in contact force ablation on the incidence of asymptomatic oesophageal lesions after atrial fibrillation ablation: a prospective comparative study. Europace 2017;19:1116–22. [DOI] [PubMed] [Google Scholar]
- 29. Reddy VY, Grimaldi M, De Potter T, Vijgen JM, Bulava A, Duytschaever MFet al. . Pulmonary vein isolation with very high power, short duration, temperature-controlled lesions: the QDOT-FAST trial. JACC Clin Electrophysiol 2019;5:778–86. [DOI] [PubMed] [Google Scholar]
- 30. Halbfass P, Wielandts JY, Knecht S, de Waroux JB LP, Tavernier R, De Wilde Vet al. . Safety of very high-power short-duration radiofrequency ablation for pulmonary vein isolation: a two-centre report with emphasis on silent oesophageal injury. Europace 2022;24(3):400–405. [DOI] [PubMed] [Google Scholar]
- 31. Nakagawa H, Ikeda A, Sharma T, Govari A, Ashton J, Maffre Jet al. . Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with high power-short duration and moderate power-moderate duration: effects of thermal latency and contact force on lesion formation. Circ Arrhythm Electrophysiol 2021;14:e009899. [DOI] [PubMed] [Google Scholar]
- 32. Otsuka N, Okumura Y, Kuorkawa S, Nagashima K, Wakamatsu Y, Hayashida Set al. . In vivo tissue temperature during lesion size index-guided 50W ablation versus 30W ablation: a porcine study. J Cardiovasc Electrophysiol 2023;34(2):369-378 [DOI] [PubMed] [Google Scholar]
- 33. Müller J, Berkovitz A, Halbfass P, Nentwich K, Ene E, Sonne Ket al. . Acute oesophageal safety of high-power short duration with 50 W for atrial fibrillation ablation. Europace 2022;24:928–37. [DOI] [PubMed] [Google Scholar]
- 34. Francke A, Naumann G, Weidauer MC, Scharfe F, Schoen S, Wunderlich Cet al. . Esophageal safety in CLOSE-guided 50 W high-power-short-duration pulmonary vein isolation: the PREHEAT-PVI-registry. J Cardiovasc Electrophysiol 2022;33:2276–84. [DOI] [PubMed] [Google Scholar]
- 35. List S, Meinhardt C, Mueller J, Deneke T, Barth S, Waechter Cet al. . Incidence of ablation-induced esophageal lesions and gastroparesis in patients undergoing ablation index guided high power short duration atrial fibrillation ablation. J Cardiovasc Electrophysiol.2023;34(1):82-89 [DOI] [PubMed] [Google Scholar]
- 36. Kim YG, Choi JI, Boo KY, Kim DY, Oh SK, Park HSet al. . Clinical and echocardiographic risk factors predict late recurrence after radiofrequency catheter ablation of atrial fibrillation. Sci Rep 2019;9:6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen MJ, Arora R, Jalife J. Atrial myopathy. JACC Basic Transl Sci 2019;4:640–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara Pet al. . Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation 2018;137:2166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available upon reasonable request from the authors.