Abstract
Aims
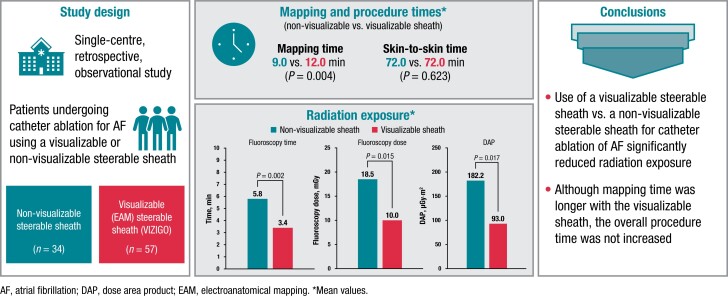
Incorporating a steerable sheath that can be visualized using an electroanatomical mapping (EAM) system may allow for more efficient mapping and catheter placement, while reducing radiation exposure, during ablation procedures for atrial fibrillation (AF). This study evaluated fluoroscopy usage and procedure times when a visualizable steerable sheath was used compared with a non-visualizable steerable sheath for catheter ablation for AF.
Methods and results
In this retrospective, observational, single-centre study, patients underwent catheter ablation for AF using a steerable sheath that is visualizable using the CARTO EAM (VIZIGO; n = 57) or a non-visualizable steerable sheath (n = 34). The acute procedural success rate was 100%, with no acute complications in either group. Use of the visualizable sheath vs. the non-visualizable sheath was associated with a significantly shorter fluoroscopy time [median (first quartile, third quartile), 3.4 (2.1, 5.4) vs. 5.8 (3.8, 8.6) min; P = 0.003], significantly lower fluoroscopy dose [10.0 (5.0, 20.0) vs. 18.5 (12.3, 34.0) mGy; P = 0.015], and significantly lower dose area product [93.0 (48.0, 197.9) vs. 182.2 (124.5, 355.0) μGy·m2; P = 0.017] but with a significantly longer mapping time [12.0 (9.0, 15.0) vs. 9.0 (7.0, 11.0) min; P = 0.004]. There was no significant difference between the visualizable and non-visualizable sheaths in skin-to-skin time [72.0 (60.0, 82.0) vs. 72.0 (55.5, 80.8) min; P = 0.623].
Conclusion
In this retrospective study, use of a visualizable steerable sheath for catheter ablation of AF significantly reduced radiation exposure vs. a non-visualizable steerable sheath. Although mapping time was longer with the visualizable sheath, the overall procedure time was not increased.
Keywords: Atrial fibrillation, Catheter ablation, Fluoroscopy time, Visualizable sheath, Radiation protection
Graphical Abstract
Graphical Abstract.
What’s new?
This retrospective study was conducted to evaluate whether a steerable catheter sheath that can be visualized using an electroanatomical mapping system affects fluoroscopy usage or procedure times in patients undergoing catheter ablation for atrial fibrillation.
Use of a visualizable steerable sheath was associated with significantly reduced radiation exposure compared with use of a non-visualizable steerable sheath, with comparable overall procedure times for both sheaths.
The overall procedural success rate was 100% with both the visualizable and non-visualizable steerable sheaths, with no acute complications observed in either group.
Tweet: use of a visualizable steerable sheath during catheter ablation for Afib reduces radiation exposure
Introduction
Catheter ablation for the treatment of atrial fibrillation (AF) is a well-established procedure that is typically performed with radiofrequency (RF) or cryothermic energy sources.1,2 Pulmonary vein isolation (PVI) is considered the cornerstone of catheter ablation procedures to treat AF.1,2 Pulmonary vein isolation procedures may be limited by AF recurrence, which occurs in approximately one-third of patients with paroxysmal AF within 1 year and necessitates redoing the procedure.3 Despite advances in catheter ablation technologies, ablation procedures may still be associated with rare, but potentially serious, complications such as pulmonary vein stenosis, phrenic nerve palsy, and atrial oesophageal fistulas,1,2,4,5 which may be more likely to emerge when high-fidelity lesion set continuity is interrupted by a lack of appropriate catheter visualization. Medical staff performing catheter ablations may experience chronic musculoskeletal pain, with spine problems reported in 42% and hip, knee, and ankle problems reported in 27% due to the need to wear heavy lead-lined protective garments during extended procedures.6 In addition, patients are exposed to high levels of radiation, corresponding to the equivalent of approximately 830 chest X-rays during an RF ablation.7
Use of steerable sheaths for catheter positioning has been shown to improve the safety and efficacy of PVI procedures compared with conventional fixed sheaths.8–10 Additionally, incorporation of three-dimensional electroanatomical mapping (EAM) systems can be used to reduce radiation exposure during ablation procedures.11,12 The CARTO VIZIGO Bi-directional Guiding Sheath (Biosense Webster, Inc., Irvine, CA, USA) is a steerable sheath that can be directly visualized using an EAM system (CARTO 3 System; Biosense Webster, Inc.) to facilitate navigation and ablation catheter placement without depending wholly on fluoroscopy. Previous reports have shown that this visualizable steerable sheath increases PVI success rates, improves procedural efficiency, and reduces fluoroscopy time compared with non-steerable sheaths in patients undergoing catheter ablation for paroxysmal AF or complex left atrial arrhythmias.10,13–15 Additionally, a previous analysis of the US Food and Drug Administration’s Manufacturer And User Facility Device Experience database showed a comparable safety profile for this visualizable steerable sheath and a steerable sheath that is not visualizable on the EAM system (Agilis NxT Steerable Introducer; Abbott Laboratories, Chicago, IL, USA).16
This study was performed to assess differences in fluoroscopy usage and procedure times when a visualizable steerable sheath was used compared with a non-visualizable steerable sheath in patients undergoing catheter ablation for AF.
Methods
Study design
This was a retrospective, observational, single-centre study comparing use of a visualizable steerable sheath (VIZIGO) vs. a non-visualizable steerable sheath (Agilis) in patients undergoing catheter ablation. This study complies with the Declaration of Helsinki, and ethical approval for study conduct was obtained from the Mater Misericordiae University Hospital/Mater Private Hospital (MPH) Institutional Review Board (Ethics Approval Reference 1/378/2283 TMR).
Data sources and patients
Patient data were obtained from the MPH AF registry (https://www.cvridublin.ie/research/outcomes-research/mph-atrial-fibrillation-(af)-registry/). Only cases requiring PVI alone were included; patients with PVI plus additional ablations and patients undergoing redo ablation procedures were excluded. Patients in the visualizable sheath group were recruited in a continuous manner after introduction of the visualizable steerable sheath to our practice; the initial 10 cases where that sheath was used were excluded to allow for differences due to any initial learning curve in the use of the sheath. The control group was a contemporaneous operator-matched group of controls who underwent catheter ablation for AF. The only difference in the treatment received between the two groups pertained to the use of a visualizable vs. a non-visualizable steerable sheath.
Procedures
All patients underwent preprocedural computed tomography of the left atrium (LA) to delineate anatomy. All cases were performed under general anaesthesia. Transoesophageal echocardiography was utilized to guide transseptal puncture and to exclude left atrial thrombus. Vascular ultrasound (where required) was used to introduce a 7Fr sheath, a 63 cm SL0 sheath (Swartz SL0 Transseptal Guiding Introducer; Abbott Laboratories) and a steerable sheath into the right femoral vein.
Patients who were previously receiving warfarin continued without interruption. Patients receiving direct oral anticoagulants (DOACs) did not receive their dose on the day of the procedure; DOAC administration was recommenced after a post-procedure echocardiogram to exclude effusion 3 h following the procedure. For periprocedural anticoagulation, 125 units of unfractionated heparin per kilogram were administered after femoral puncture, with a target-activated clotting time of 300–350 s. Heparin was infused at 1000 units per hour via two long sheaths. The heparin bolus was given following venous access and prior to access to the LA.
A deflectable decapolar catheter (Dynamic Deca; Boston Scientific, Natick, MA, USA) was positioned in the coronary sinus (CS) under fluoroscopy guidance. Before placement of the CS catheter in patients in the visualizable sheath group, a short fast anatomical map was created within the right atrium. This was to ensure that the EAM system had adequate geometry and anatomy on the septal aspect of the LA to accurately locate the sheath. Transseptal puncture was performed using a 71 cm BRK-1 XS Transseptal Needle (Abbott Laboratories) via the SL0 sheath or via the steerable sheath, per operator preference, in which case a 98 cm needle was utilized. Whenever possible, the first puncture was double wired. If the patient was experiencing AF, they were cardioverted (200 J synchronized) after transseptal puncture. Prior to ablation, a three-dimensional map of the LA was created with CS pacing at 600 ms with both voltage and activation data using a Lasso Circular Mapping Catheter (Biosense Webster, Inc.) and the CARTO EAM system.
Ablation was performed with a SmartTouch Surround Flow DF Catheter (Biosense Webster, Inc.) and guided by Ablation Index (AI),17 with a targeted AI of 350 and power of 35 W on the posterior and inferior regions, a targeted AI of 450 and power of 45 W on the anterior and superior regions, and a targeted inter-lesion distance (ILD) of 4 mm and maximum ILD of 6 mm. Regarding ablation lesions, all patients included in the study received PVI only, performed using bilateral wide antral circumferential ablation lines.18,19
Validation was performed by remapping the LA after a 20-min waiting period, ensuring both entry and exit blocks into all pulmonary veins. Where linear ablation lesions were created, a bidirectional block was confirmed across these lines using appropriate differential pacing.
Assessments
The following procedural and fluoroscopy data were collected: (i) setup time, defined as the time from the patient entering the room until the operator began vascular/left atrial access (including anaesthetic time and time to position electrocardiogram and EAM system patches on the patient); (ii) access time, defined as the time from the operator beginning vascular access to successful and safe transseptal puncture and securing access to the patient’s LA; (iii) mapping time, defined as the time spent from the end of access time to completion of the initial anatomical map (including mapping of the septal aspect of the right atrium for patients in whom the visualizable sheath was used, which was necessary to allow for collection of an EAM system positional matrix to ensure reliable visualization); (iv) ablation time, defined as the time from completion of the anatomical map and start of ablation to completion of first pass ablation; and (v) validation time, defined as the time to completion of the initial ablation [including validation remap of the LA, checking for exit/entry block, and (if necessary in the cases where first pass isolation was not obtained) additional ablation]. Additional parameters collected included skin-to-skin procedure time (sum of the access, mapping, ablation, and validation times), fluoroscopy time, fluoroscopy dose, dose area product (DAP), RF time, and ablation application count.
Statistical analysis
Continuous variables were tested for normality using the Shapiro–Wilk test. Variables with a normal distribution were expressed as mean (standard deviation), while those without a normal distribution were expressed as median (first quartile, third quartile). Statistical comparisons were conducted using the Wilcoxon–Mann–Whitney test or Student’s t-test as appropriate. R 4.1.2 (R Core Team, 2021) was used for all statistical analyses.
Results
Study population
Baseline characteristics for the 34 patients treated using the non-visualizable sheath and the 57 treated using the visualizable sheath are summarized in Table 1. Mean age was 66.4 years for patients in the non-visualizable sheath group and 64.5 years for those in the visualizable sheath group; most patients in both groups were male (76.5 and 70.2%, respectively). No statistical differences across the two groups were observed for any of the baseline parameters, including sex; age; or congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischaemic attack, vascular disease, age 65–74 years, and sex categories (CHA2DS2-VASc) score.
Table 1.
Patient baseline demographic characteristics
| Parameter | Non-visualizable sheath (n = 34) | Visualizable sheath (n = 57) | P value |
|---|---|---|---|
| Age, years, mean (SD) | 66.4 (8.3) | 64.5 (9.1) | 0.311 |
| Female, n (%) | 8 (23.5) | 17 (29.8) | 0.683 |
| CHA2DS2-VASc score, mean (SD) | 2.0 (1.3) | 1.8 (1.2) | 0.580 |
| Congestive heart failure, n (%) | 4 (11.8) | 11 (19.3) | 0.519 |
| Hypertension, n (%) | 20 (58.8) | 29 (50.9) | 0.604 |
| Diabetes mellitus, n (%) | 4 (11.8) | 2 (3.5) | 0.272 |
| Vascular disease, n (%) | 4 (11.8) | 9 (15.8) | 0.825 |
| Stroke or TIA (%) | 1 (2.9) | 0 | 0.793 |
CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke or TIA, vascular disease, age 65–74 years, sex category; SD, standard deviation; TIA, transient ischaemic attack.
Procedural data
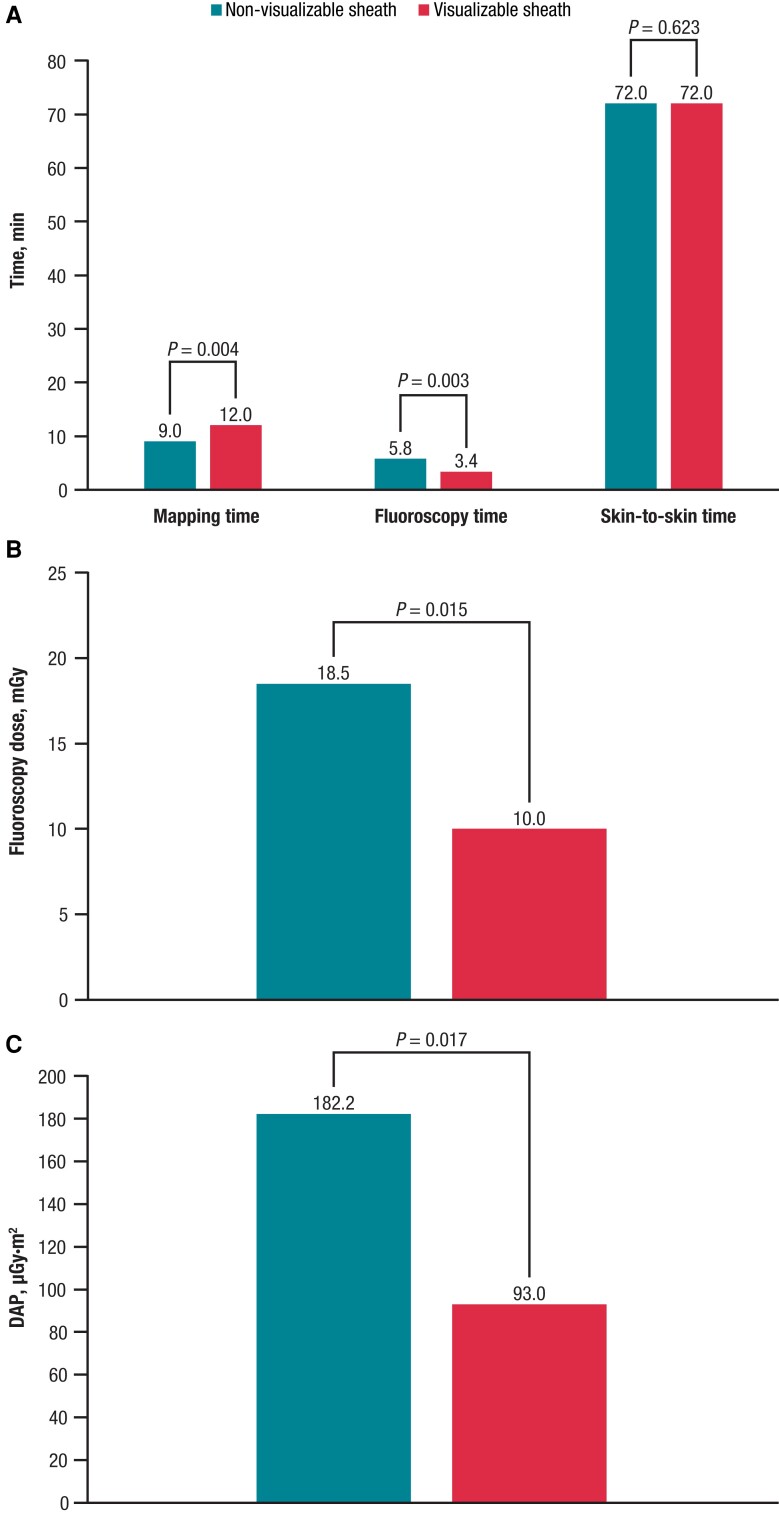
The acute procedural success rate was 100%. Procedural characteristics are summarized in Table 2. Compared with the non-visualizable sheath, use of the visualizable sheath was associated with a significantly shorter fluoroscopy time (P = 0.003; Figure 1A), lower fluoroscopy dose (P = 0.015; Figure 1B), and lower DAP (P = 0.017; Figure 1C); however, the mapping time was significantly longer with the visualizable vs. the non-visualizable sheath (P = 0.004; Figure 1A). There were no other significant differences between the two treatment techniques for any measured parameters, including overall skin-to-skin time (P = 0.623; Figure 1A). The only parameter that passed the test for normality (per the Shapiro–Wilk test) was skin-to-skin time.
Table 2.
Procedural characteristicsa
| Variable | Non-visualizable sheath (n = 34) | Visualizable sheath (n = 57) | P valueb |
|---|---|---|---|
| Setup time, min | 32.0 (29.3, 37.0) | 31.0 (27.0, 39.0) | 0.828 |
| Skin-to-skin time, min | 72.0 (55.5, 80.8) | 72.0 (60.0, 82.0) | 0.623c |
| Access time, min | 22.0 (17.0, 26.5) | 21.0 (19.0, 28.0) | 0.522 |
| Mapping time, min | 9.0 (7.0, 11.0) | 12.0 (9.0, 15.0) | 0.004 |
| Ablation time, mind | 24.5 (19.3, 27.0) | 23.0 (18.9, 28.0) | 0.679 |
| Validation time, min | 12.5 (6.3, 18.8) | 11.0 (7.0, 17.0) | 0.765 |
| Fluoroscopy time, min | 5.8 (3.0, 8.6) | 3.4 (2.1, 5.4) | 0.003 |
| Fluoroscopy dose, mGy | 18.5 (12.3, 34.0) | 10.0 (5.0, 20.0) | 0.015 |
| DAP, μGy·m2 | 182.2 (124.5, 355.0) | 93.0 (48.0, 197.9) | 0.017 |
| RF time, min | 16.5 (14.7, 19.6) | 16.0 (13.9, 19.4) | 0.501 |
| Ablation application count | 77.0 (70.3, 92.0) | 78.0 (69.0, 92.0) | 0.879 |
| Major complications | 0 | 0 | NA |
| Minor complications | 1 | 3 | >0.9999e |
DAP, dose area product; NA, not applicable; RF, radiofrequency.
Data are presented as median (first quartile, third quartile).
Statistical comparisons were conducted using the Wilcoxon–Mann–Whitney test unless otherwise stated.
Ablation time refers to the time from completion of initial map to completion of all ablations. RF time refers to total cumulative duration of energy delivery.
Student’s t-test was also used to compare skin-to-skin time; differences were not significant (P = 0.73).
Fisher’s exact test.
Figure 1.
Procedural data for patients treated using non-visualizable and visualizable sheaths. (A) Median mapping time, fluoroscopy time, and skin-to-skin time. (B) Fluoroscopy dose. (C) DAP. DAP, dose area product.
Safety
No major acute complications were reported for either group. Four patients described minor vascular complications (bruising and haematoma at the vascular access site) that resolved spontaneously (Tables 2 and 3). There was no significant difference found in safety outcomes between the two groups (Table 3), although the study was insufficiently powered to determine a difference in safety outcomes.
Table 3.
Complications
| Complications | Non-visualizable sheath (n = 34) | Visualizable sheath (n = 57) | P valuea |
|---|---|---|---|
| Major | 0 | 0 | NA |
| ȃDeath | 0 | 0 | NA |
| ȃMI | 0 | 0 | NA |
| ȃStroke/TIA | 0 | 0 | NA |
| ȃSevere bleeding | 0 | 0 | NA |
| ȃAE fistula | 0 | 0 | NA |
| ȃTamponade | 0 | 0 | NA |
| ȃPhrenic nerve palsy | 0 | 0 | NA |
| ȃAV fistula/pseudoaneurysm | 0 | 0 | NA |
| Minor | 1 | 3 | >0.9999 |
| ȃMinor haematoma | 1 | 3 | >0.9999 |
AE, adverse event; AV, arteriovenous; MI, myocardial infarction; NA, not applicable; TIA, transient ischaemic attack.
Fisher’s exact test.
Discussion
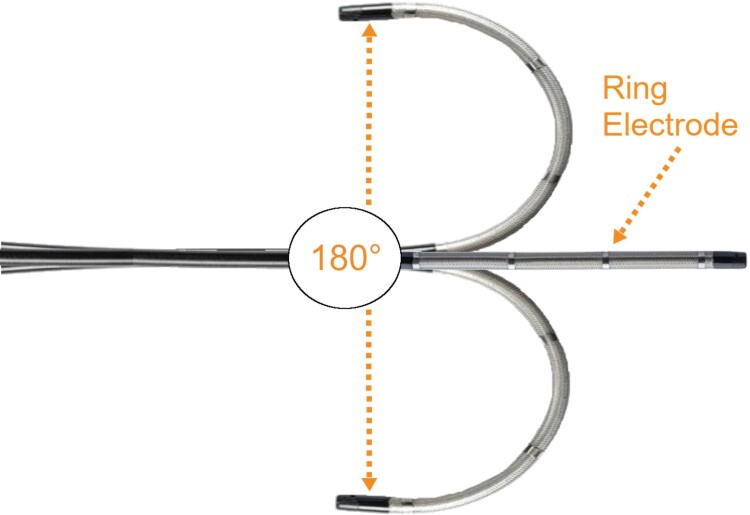
In this retrospective, observational study, use of a visualizable steerable sheath (Figure 2) for catheter ablation procedures for AF was associated with significantly reduced fluoroscopy time, fluoroscopy dose, and DAP and significantly increased mapping time compared with use of a non-visualizable sheath. Despite the increase in mapping time, no increase in overall procedure time was observed with the visualizable compared with the non-visualizable sheath. Additionally, a 100% acute procedural success rate was observed with both the visualizable and non-visualizable steerable sheaths, and no major complications occurred in either treatment group.
Figure 2.
The distal portion of the VIZIGO visualizable sheath in its neutral position and in maximal bidirectional flexion. Note the four-ring electrodes, which allow for visualization of the sheath regardless of orientation or degree of flexion. Image courtesy of @Biosense Webster, Inc. All rights reserved. CARTO VlZlGO is a trademark of Biosense Webster, Inc.
Although catheter ablation procedures are routinely performed to treat AF, these procedures may be associated with serious complications, including pulmonary vein stenosis, phrenic nerve palsy, and atrial oesophageal fistualas.1,2,4,5 Additionally, due to the use of fluoroscopic imaging to visualize the catheter during placement, radiation exposure levels are high during the procedures, leading to acute radiation exposures in patients7 and the need for medical staff to wear bulky lead-lined garments for protection.6 No acute complications were observed in this study, with only minor complications reported with either steerable sheath. Moreover, as indicated by the significant reductions in the fluoroscopy time and dose, use of the visualizable sheath was associated with significant reductions in radiation exposure compared with use of the non-visualizable sheath in this study. These results suggest that visualizable sheaths can be used safely and effectively, while offering reduced radiation exposure, in patients undergoing catheter ablation procedures for AF.
Results from the current study are consistent with previous reports showing that the VIZIGO visualizable steerable sheath had similar safety to the Agilis non-visualizable sheath,16 with shorter fluoroscopy times and lower fluoroscopy doses in patients undergoing PVI for the treatment of AF.15,20 As was reported here, overall procedure time was similar between the visualizable and non-visualizable sheath groups in previous studies.15,20
The lack of difference in the overall procedure time between the visualizable and non-visualizable sheath groups is reassuring. Operators considering the use of a visualizable sheath may have concerns that the additional mapping required for setup would translate into an increased risk of complications due to a longer procedure time. This study has shown that not to be the case; while the visualizable sheath requires longer mapping times, the overall procedure times are no longer than with a non-visualizable sheath due to the accumulation of efficiencies in other components of the procedure. However, these individual time savings do not meet statistical significance.
In relation to radiation exposure, benefits are seen with use of the visualizable sheath, while the individual differences in the times and doses are small, and the accumulation over an operator’s career could be significant, particularly for high-volume operators. The authors would also suggest that operators pursuing a zero or near-zero fluoroscopy approach would find use of the visualizable sheath to be beneficial. The quantification of such benefits has yet to be fully explored.
Limitations
This study may have been subject to certain limitations. First, 1-year outcome data were not collected; however, given that both groups of patients benefited from AI-guided procedures, durability and long-term procedural success are expected to be high and similar in both groups. Additionally, patients were not matched in the visualizable and non-visualizable sheath groups; however, baseline characteristics (e.g. sex, CHA2DS2-VASc scores, and age) did not differ significantly between groups. Finally, these results should be interpreted with caution due to the relatively small number of patients included and retrospective single-centre design of this study. The outcomes observed here should be confirmed in larger, prospective, randomized, multicentre studies.
Conclusions
In this retrospective study, use of a visualizable steerable sheath for catheter ablation in the treatment of AF led to significantly reduced radiation exposure as compared with a non-visualizable steerable sheath. Although mapping time was increased with the visualizable sheath, the overall procedure time was not increased. Additionally, 100% acute procedural success was observed, and there was no difference in terms of acute safety outcomes between groups, with complications limited to minor groin haematomas. Taken together, these results suggest that use of a steerable sheath that is visualizable on an EAM system allows for successful catheter ablation, with the potential for increased safety for clinicians and patients based on the reduction in radiation exposure compared with a non-visualizable steerable sheath.
Acknowledgements
Medical writing and editorial assistance was provided in accordance with Good Publication Practice (GPP3) guidelines by Michelle Hughes, PhD, of Lumanity Communications Inc. (Yardley, PA, USA) and was funded by Biosense Webster, Inc. (Irvine, CA, USA).
Contributor Information
Noel Fitzpatrick, Atrial Fibrillation Institute, Mater Private Network, 72 Eccles Street, Dublin 7, D07 RD8P, Ireland.
Ashish Mittal, Atrial Fibrillation Institute, Mater Private Network, 72 Eccles Street, Dublin 7, D07 RD8P, Ireland.
Joseph Galvin, Atrial Fibrillation Institute, Mater Private Network, 72 Eccles Street, Dublin 7, D07 RD8P, Ireland; Health Sciences Centre, UCD School of Medicine, University of College Dublin, Belfield, Dublin 4, D04 V1W8, Ireland.
Gael Jauvert, Atrial Fibrillation Institute, Mater Private Network, 72 Eccles Street, Dublin 7, D07 RD8P, Ireland.
John Keaney, Health Sciences Centre, UCD School of Medicine, University of College Dublin, Belfield, Dublin 4, D04 V1W8, Ireland; Acute Cardiology Unit, Mater Misericordiae University Hospital, Eccles St, Dublin 7, D07 R2WY, Ireland.
Edward Keelan, Atrial Fibrillation Institute, Mater Private Network, 72 Eccles Street, Dublin 7, D07 RD8P, Ireland.
Jim O’Brien, Atrial Fibrillation Institute, Mater Private Network, 72 Eccles Street, Dublin 7, D07 RD8P, Ireland.
Gábor Széplaki, Atrial Fibrillation Institute, Mater Private Network, 72 Eccles Street, Dublin 7, D07 RD8P, Ireland; Royal College of Surgeons in Ireland, 123 St Stephen's Green, Dublin 2, D02 YN77, Ireland.
Funding
Medical writing and editorial assistance for this manuscript was funded by Biosense Webster (Irvine, CA, USA).
Data availability
Data in this article will be shared on reasonable request to the corresponding author.
References
- 1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga Let al. . 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist Cet al. . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 3. Van Der Zee SA, d’Avila A. Redo procedures in patients with paroxysmal atrial fibrillation. J Innov Card Rhythm Manag 2010;1:44–52. [Google Scholar]
- 4. Muthalaly RG, John RM, Schaeffer B, Tanigawa S, Nakamura T, Kapur Set al. . Temporal trends in safety and complication rates of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2018;29:854–60. [DOI] [PubMed] [Google Scholar]
- 5. Deneke T, Schade A, Diegeler A, Nentwich K. Esophago-pericardial fistula complicating atrial fibrillation ablation using a novel irrigated radiofrequency multipolar ablation catheter. J Cardiovasc Electrophysiol 2014;25:442–3. [DOI] [PubMed] [Google Scholar]
- 6. Goldstein JA, Balter S, Cowley M, Hodgson J, Klein LW; Interventional Committee of the Society of Cardiovascular Interventions . Occupational hazards of interventional cardiologists: prevalence of orthopedic health problems in contemporary practice. Catheter Cardiovasc Interv 2004;63:407–11. [DOI] [PubMed] [Google Scholar]
- 7. Picano E, Piccaluga E, Padovani R, Antonio Traino C, Grazia Andreassi M, Guagliumi G. Risks related to fluoroscopy radiation associated with electrophysiology procedures. J Atr Fibrillation 2014;7:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hutchinson MD, Garcia FC, Mandel JE, Elkassabany N, Zado ES, Riley MPet al. . Efforts to enhance catheter stability improve atrial fibrillation ablation outcome. Heart Rhythm 2013;10:347–53. [DOI] [PubMed] [Google Scholar]
- 9. Santangeli P, Lin D. Catheter ablation of paroxysmal atrial fibrillation: have we achieved cure with pulmonary vein isolation? Methodist Debakey Cardiovasc J 2015;11:71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo R, Jia R, Cen Z, Lu S, Yang C, Han Set al. . Effects of the visualized steerable sheath applied to catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol 2022;64:511–8. [DOI] [PubMed] [Google Scholar]
- 11. Huo Y, Christoph M, Forkmann M, Pohl M, Mayer J, Salmas Jet al. . Reduction of radiation exposure during atrial fibrillation ablation using a novel fluoroscopy image integrated 3-dimensional electroanatomic mapping system: a prospective, randomized, single-blind, and controlled study. Heart Rhythm 2015;12:1945–55. [DOI] [PubMed] [Google Scholar]
- 12. Reddy VY, Morales G, Ahmed H, Neuzil P, Dukkipati S, Kim Set al. . Catheter ablation of atrial fibrillation without the use of fluoroscopy. Heart Rhythm 2010;7:1644–53. [DOI] [PubMed] [Google Scholar]
- 13. Kozluk E, Lojewska K, Hiczkiewicz J. First experience with left atrial arrhthmia ablation using a bi-directional steerable transseptal sheath (Vizigo) visible in the CARTO system as a method to reduce fluoroscopy. Eur J Transl Clin Med 2020;3:18–21. [Google Scholar]
- 14. Osorio J, Zei P, Rajendra A, Morales G. Zero fluoroscopy atrial fibrillation ablation and the role of a new steerable sheath visualizable on the electroanatomic mapping system. Presented at the 12th Annual Asia Pacific Heart Rhythm Society (APHRS) Scientific Session, October 24–27, 2019, Bangkok, Thailand.
- 15. Wakamatsu Y, Nagashima K, Kurokawa S, Otsuka N, Hayashida S, Yagyu Set al. . Impact of the combined use of intracardiac ultrasound and a steerable sheath visualized by a 3D mapping system on pulmonary vein isolation. Pacing Clin Electrophysiol 2021;44:693–702. [DOI] [PubMed] [Google Scholar]
- 16. Kewcharoen J, Shah K, Bhardwaj R, Contractor T, Turagam MK, Mandapati Ret al. . Post-FDA approval “real-world” safety profile of different steerable sheaths during catheter ablation: a Food and Drug Administration MAUDE database study. Heart Rhythm 2022;19:856–7. [DOI] [PubMed] [Google Scholar]
- 17. Das M, Loveday JJ, Wynn GJ, Gomes S, Saeed Y, Bonnett LJet al. . Ablation index, a novel marker of ablation lesion quality: prediction of pulmonary vein reconnection at repeat electrophysiology study and regional differences in target values. Europace 2017;19:775–83. [DOI] [PubMed] [Google Scholar]
- 18. Marrouche NF, Dresing T, Cole C, Bash D, Saad E, Balaban Ket al. . Circular mapping and ablation of the pulmonary vein for treatment of atrial fibrillation: impact of different catheter technologies. J Am Coll Cardiol 2002;40:464–74. [DOI] [PubMed] [Google Scholar]
- 19. Ouyang F, Ernst S, Chun J, Bansch D, Li Y, Schaumann Aet al. . Electrophysiological findings during ablation of persistent atrial fibrillation with electroanatomic mapping and double Lasso catheter technique. Circulation 2005;112:3038–48. [DOI] [PubMed] [Google Scholar]
- 20. Janosi K, Debreceni D, Janosa B, Simor T, Kupo P. Visualizable vs. standard, non-visualizable steerable sheath for pulmonary vein isolation procedures: randomized, single-center trial. Europace 2022;24:i403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data in this article will be shared on reasonable request to the corresponding author.