Key Points
Question
In adults at high risk for recurrence of Clostridioides difficile infection (CDI), does VE303 (a novel oral microbiome-directed therapy composed of nonpathogenic, nontoxigenic, commensal strains of Clostridia) reduce the likelihood of CDI recurrence?
Findings
Through week 8, the CDI recurrence rate (using the most inclusive, combined clinical and laboratory definition of outcome) was 13.8% for the high-dose VE303 group, 37.0% for the low-dose VE303 group, and 45.5% for the placebo group.
Meaning
High-dose VE303 prevented recurrent CDI compared with placebo.
Abstract
Importance
The effect of rationally defined nonpathogenic, nontoxigenic, commensal strains of Clostridia on prevention of Clostridioides difficile infection (CDI) is unknown.
Objective
To determine the efficacy of VE303, a defined bacterial consortium of 8 strains of commensal Clostridia, in adults at high risk for CDI recurrence. The primary objective was to determine the recommended VE303 dosing for a phase 3 trial.
Design, Setting, and Participants
Phase 2, randomized, double-blind, placebo-controlled, dose-ranging study conducted from February 2019 to September 2021 at 27 sites in the US and Canada. The study included 79 participants aged 18 years or older who were diagnosed with laboratory-confirmed CDI with 1 or more prior CDI episodes in the last 6 months and those with primary CDI at high risk for recurrence (defined as aged ≥75 years or ≥65 years with ≥1 risk factors: creatinine clearance <60 mL/min/1.73 m2, proton pump inhibitor use, remote [>6 months earlier] CDI history).
Interventions
Participants were randomly assigned to high-dose VE303 (8.0 × 109 colony-forming units [CFUs]) (n = 30), low-dose VE303 (1.6 × 109 CFUs) (n = 27), or placebo capsules (n = 22) orally once daily for 14 days.
Main Outcomes and Measures
The primary efficacy end point was the proportion of participants with CDI recurrence at 8 weeks using a combined clinical and laboratory definition. The primary efficacy end point was analyzed in 3 prespecified analyses, using successively broader definitions for an on-study CDI recurrence: (1) diarrhea consistent with CDI plus a toxin-positive stool sample; (2) diarrhea consistent with CDI plus a toxin-positive, polymerase chain reaction–positive, or toxigenic culture–positive stool sample; and (3) diarrhea consistent with CDI plus laboratory confirmation or (in the absence of a stool sample) treatment with a CDI-targeted antibiotic.
Results
Baseline characteristics were similar across the high-dose VE303 (n = 29; 1 additional participant excluded from efficacy analysis), low-dose VE303 (n = 27), and placebo (n = 22) groups. The participants’ median age was 63.5 years (range, 24-96); 70.5% were female; and 1.3% were Asian, 1.3% Black, 2.6% Hispanic, and 96.2% White. CDI recurrence rates through week 8 (using the efficacy analysis 3 definition) were 13.8% (4/29) for high-dose VE303, 37.0% (10/27) for low-dose VE303, and 45.5% (10/22) for placebo (P = .006, high-dose VE303 vs placebo).
Conclusions and Relevance
Among adults with laboratory-confirmed CDI with 1 or more prior CDI episodes in the last 6 months and those with primary CDI at high risk for recurrence, high-dose VE303 prevented recurrent CDI compared with placebo. A larger, phase 3 study is needed to confirm these findings.
Trial Registration
ClinicalTrials.gov Identifier: NCT03788434
This clinical trial compares the efficacy of 2 different doses of VE303, a defined bacterial consortium of 8 strains of commensal Clostridia, and placebo in preventing recurrence of Clostridioides difficile infection among adults at high risk.
Introduction
Clostridioides difficile infection (CDI) is the most common health care–associated infection in the US, with approximately 450 000 infections annually,1 associated with direct mortality of 5% and all-cause mortality of 15% to 20%.2,3 Antibiotics used to treat CDI also target resident commensal gut bacteria (or microbiota). This disruption of the microbiota constitutes a major risk factor for CDI recurrences by creating a favorable environment for C difficile growth and toxin production and altering the gut metabolite pool.4 Although most initial CDI episodes will respond to antibiotic treatment, up to 25% of patients will experience a first recurrence, and the risk for a subsequent recurrence can exceed 60%.5
Clinical treatment guidelines support the use of fecal microbiota transplants for patients with multiple CDI recurrences.6,7,8 However, there are logistical and safety concerns associated with fecal microbiota transplants and other donor-derived products, such as donor variability and quality and the risk of transmission of pathogenic and/or antibiotic-resistant microbes.9,10 Alternatives to donor-derived products are needed, such as defined biotherapeutic interventions of standardized composition, to restore the microbiome and safely prevent future CDI recurrences.11
Multiple agents that administer processed feces from human donors for recurrent CDI are currently under investigation12,13; the first such product was recently approved by the US Food and Drug Administration.14 An alternative live biotherapeutic product, VE303, in a phase 1 study colonized the human intestine without the use of a laxative bowel preparation prior to administration.15 VE303 is a defined bacterial consortium composed of 8 well-characterized, nonpathogenic, nontoxigenic, commensal strains of Clostridia derived from healthy human stool samples and manufactured from clonal cell banks. The primary objective of this study was to evaluate VE303 at 2 different doses in adults at high risk for recurrent CDI to determine recommendations for a phase 3 regimen.
Methods
Study Design
VE303-002 (A Double-Blind Placebo-Controlled Phase 2 Study of VE303 for Prevention of Recurrent Clostridium [Clostridioides] difficile Infection) was a randomized, double-blind, placebo-controlled, dose-finding, phase 2 study in adults with recurrent CDI and in those with primary CDI at high risk for recurrence. The trial protocol was approved by the institutional review board or ethics committee at each participating institution. This trial was conducted in accordance with Good Clinical Practice guidelines at 27 clinical sites in the US and Canada. Participants were enrolled between February 2019 and April 2021, with final follow-up in September 2021. All participants provided written informed consent. The trial protocol is in Supplement 1 and the statistical analysis plan in Supplement 2.
Intervention
VE303 is a defined live biotherapeutic product, including 5 strains from Clostridia cluster XIVa, 2 strains from cluster IV, and 1 strain from cluster XVII (eTable 1 in Supplement 3).15 These strains were selected from a microbiome strain library derived from healthy human stool samples based on individual strain- and consortium-specific colonizing properties and manufactured from clonal cell banks.15 Doses of VE303 were selected based on the results of a phase 1 study in healthy volunteers.15
Participants
The study included adults aged 18 years or older who had 1 or more prior episodes of CDI within the 6 months before the day of randomization, as well as individuals with primary CDI at high risk for recurrence, defined as age 75 years or older or age 65 years or older with at least 1 additional prespecified risk factor for recurrence (kidney dysfunction [creatinine clearance <60 mL/min/1.73 m2] at the time of the current CDI episode; regular use of a proton pump inhibitor within the past 2 months and anticipated ongoing use; or a history of CDI >6 months previously) (Figure 1). Participants must have experienced, within 30 days before randomization, onset of 3 or more loose/unformed bowel movements per 24 hours for more than 2 consecutive days or 8 or more loose/unformed bowel movements within 24 hours that was considered unlikely to have another etiology, with laboratory confirmation of CDI from a stool sample collected within 72 hours of initiation of standard-of-care antibiotic therapy. Prior to randomization, a participant had to show a successful clinical response (<3 loose/unformed bowel movements within 24 hours for >2 consecutive days) to an investigator’s choice of standard-of-care antibiotic regimen. Protocol amendments are in eTable 2 in Supplement 3. Race and ethnicity were self-reported, collected from case report forms with fixed categories, and included to better characterize the study population.
Figure 1. Screening, Randomization, and Follow-up Through Week 24.
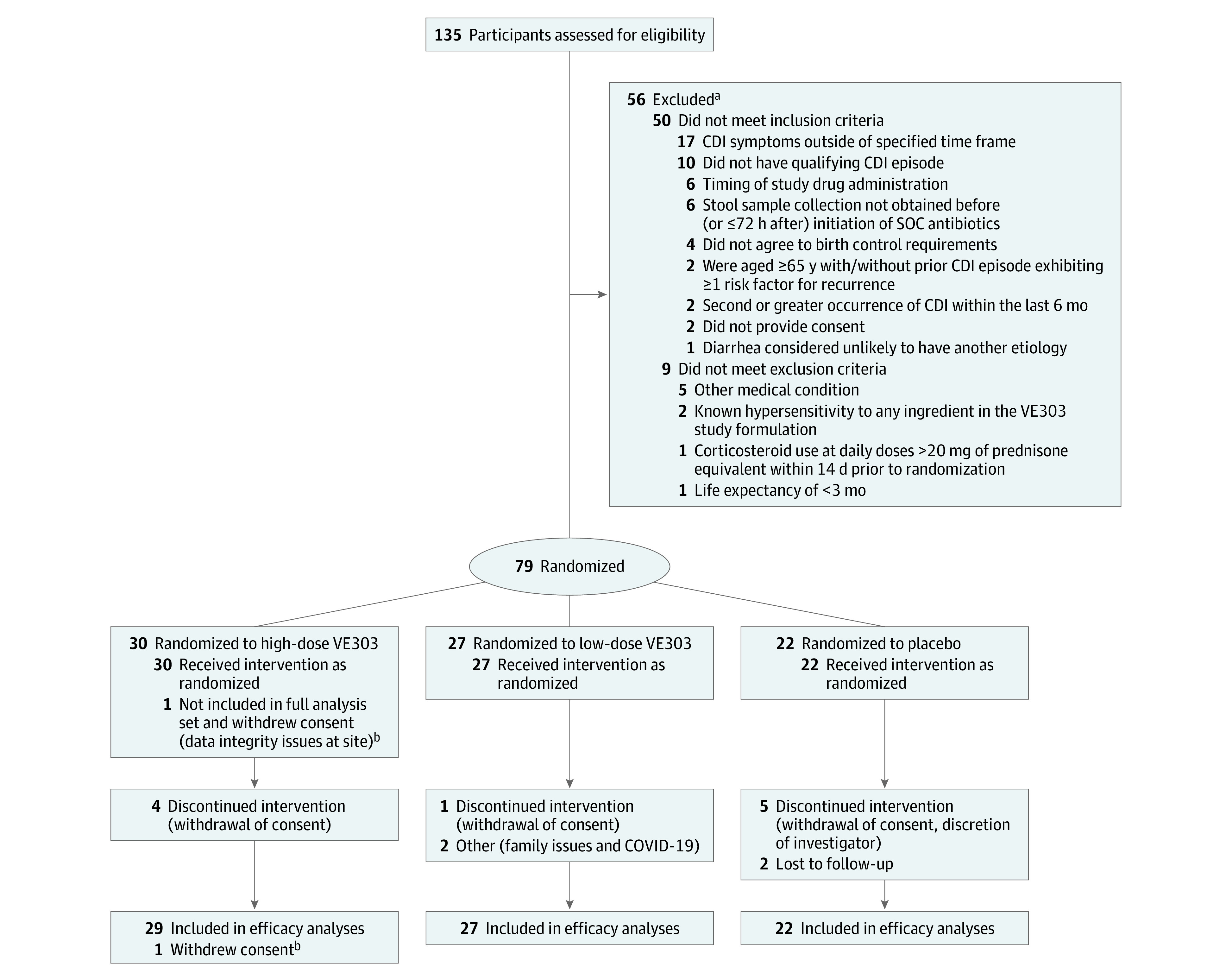
CDI indicates Clostridioides difficile infection; SOC, standard of care.
aThe number of reasons for exclusion exceed the number excluded because individuals could have had multiple reasons for not enrolling in the study.
bData integrity issues at 1 study site led to 1 participant being excluded from the full analysis set and from all analyses of efficacy and strain colonization; this participant was included in the safety analysis set.
Trial Procedures
The trial consisted of a screening period of up to 3 weeks before randomization, an 8-week primary efficacy period starting when the study drug was initiated, followed by a 16-week follow-up period to assess safety, collect stool samples for colonization dynamics analysis, and identify late CDI recurrences. The first dose of study drug was administered at the study site within 24 hours after completing antibiotic treatment for the qualifying CDI episode; remaining doses were taken at home. During the dosing period, participants returned for follow-up visits on days 7 and 14; between visits, study personnel performed daily check-ins by telephone to assess compliance and monitor potential adverse events (AEs) and CDI recurrences. After dosing, participants had in-person visits at weeks 4, 8, and 24 and telephone visits at weeks 12, 16, and 20. Fecal samples were collected at screening, after treatment for the qualifying CDI episode, and longitudinally through week 24. Metagenomic sequencing was performed to determine VE303 strain colonization and changes in microbiome diversity, as previously described.15
Randomization and Blinding
Eligible participants were randomized in a 2:1:2:1 (high dose to placebo to low dose to placebo) ratio to receive once-daily high-dose VE303 (10 capsules [8.0 × 109 colony-forming units {CFUs}] daily; cumulative dose, 1.1 × 1011 CFUs), high-dose placebo (10 capsules), low-dose VE303 (2 capsules [1.6 × 109 CFUs] daily; cumulative dose, 2.2 × 1010 CFUs), and low-dose placebo (2 capsules) for 14 days. Pooling of the 2 placebo groups for analysis resulted in functional 1:1:1 randomization. Placebo capsules were physically indistinguishable from VE303 capsules. Participants were stratified based on CDI history (primary CDI at high risk for recurrence vs recurrent CDI), antibiotic used for treatment of the qualifying CDI episode (vancomycin 4 times daily vs other antibiotic, including alternative vancomycin dosing regimens), and CDI testing used to confirm the qualifying episode (free toxin [enzyme immunoassay {EIA} for toxin A/B or cell cytotoxicity neutralization assay {CCNA}] vs other [polymerase chain reaction {PCR} or toxigenic culture]). Randomization was performed using a centralized interactive web response system. Study participants and site staff were unblinded to the number of daily capsules but remained blinded to treatment group; sponsor personnel who interacted with sites remained blinded to capsules per day and treatment assignments until the database was locked.
Outcomes
The primary objective was to determine a recommended dose of VE303 for a planned phase 3 study. The primary efficacy end point was the CDI recurrence rate through week 8 in 3 prespecified analyses with successively broader, inclusive definitions for CDI recurrence (eFigure 1 in Supplement 3).
If recurrent CDI was suspected, a stool sample was collected for laboratory confirmation. CDI recurrence was defined as 3 or more unformed bowel movements per day for more than 2 consecutive days or 8 or more unformed bowel movements within 24 hours, unlikely to have another etiology, and a decision by the blinded investigator to initiate C difficile–directed antimicrobial therapy. Safety of VE303 was evaluated for 24 weeks total.
Secondary efficacy end points included CDI recurrence at week 24 and stool microbiome end points, including VE303 strain colonization and fecal microbiome diversity.
Microbiome Analyses
Prespecified analyses of the total abundance and detection of VE303 strains were performed to determine the effects of dose on strain colonization and the association of these results with on-study CDI recurrences. VE303 strain presence in stool samples was evaluated using a specialized bioinformatics assay based on the detection and frequency of highly curated genomic marker regions, as previously described.15 A prespecified analysis of the changes in microbiota diversity among the treatment groups was also performed (eMethods and eTables 3-6 in Supplement 3).15 Diversity was calculated using the Shannon Diversity Index, an ecological measure of the number of species in a community and their relative abundance (eMethods in Supplement 3).
A post hoc analysis was performed to evaluate the association between VE303 exposure and response, with each VE303-dosed participant defined as having low or high colonization based on the number of VE303 strains detected. A cutpoint analysis was conducted to determine meaningful colonization categories, calculating time-to-event curves and log-rank P values for all possible colonization cutpoints (eTable 4 in Supplement 3). To preserve balanced analysis subgroups, we reported the model with the number of detected strains in high or low colonized participants being above or below the median for all VE303-dosed participants (eMethods in Supplement 3).
Statistical Analysis
The original sample size planned for the study was 146 participants, with plans to increase enrollment based on the results of an interim analysis and sample size reestimation. Enrollment was slower than planned and further exacerbated by the COVID-19 pandemic. Thus, it was decided to forgo the original interim analysis and sample size reestimation and instead implement 3 unblinded administrative interim analyses. The interim analyses did not change the design, objectives, end points, or conduct of the study. Unblinding was limited to a small group in senior leadership at Vedanta Biosciences who were not directly involved with the study to guide future development strategy and inform the potential design of subsequent trials. No statistical power calculations were performed to define the final number of participants enrolled in the study. The sample size target was decreased to 60 to 80 participants. This decision was made in accordance with considerations of the Food and Drug Administration’s guidance on conduct of clinical trials during the COVID-19 pandemic.16
The placebo recipients were pooled for all efficacy, safety, and microbiome analyses. Continuous variables were summarized with descriptive statistics (means with SDs; medians with IQRs) by treatment group. Categorical data were summarized by frequency counts and percentages by treatment group. The hypothesis test for CDI recurrence (no treatment effect vs ≥1 effective dose; α = .05, 1-sided) was conducted using a Cochran-Mantel-Haenszel test (stratified for the factors used in randomization). SAS version 9.4 (SAS Institute Inc) was used for statistical analyses.
For the efficacy analyses, the full analysis set was defined as all study participants who were randomized and received at least 1 dose of study drug, according to their randomized study drug assignment. The primary efficacy end point was analyzed in 3 prespecified analyses (efficacy analyses 2 and 3 were prespecified sensitivity analyses) that used successively broader case definitions for an on-study CDI recurrence (eFigure 1 in Supplement 3): (1) an episode of diarrhea consistent with CDI and a toxin-positive stool sample (EIA for toxin A/B or CCNA); (2) cases included in analysis 1 or an episode of diarrhea consistent with CDI that included a positive PCR or toxigenic culture test result (ie, all laboratory-confirmed recurrences), followed by treatment with a CDI-targeted antibiotic; and (3) cases included in analysis 2 or an episode of diarrhea consistent with CDI in the absence of laboratory confirmation, followed by treatment with a CDI-targeted antibiotic as prescribed by the investigator. Both VE303 groups (high and low doses) were compared separately with the pooled placebo group. Missing values for the primary efficacy end point were not imputed, but additional supportive analyses were performed in which participants who did not experience a recurrence prior to week 8 and who discontinued the trial for any reason prior to the week 8 visit were treated as having had a CDI recurrence.
The safety analysis set was defined as all participants who were randomized and received 1 or more doses of study drug, according to the study drug actually received.
Results
Participant Characteristics
Of the 135 participants screened, 79 were randomized and analyzed for safety; 1 participant was excluded from the full analysis set and from the efficacy and microbiome analyses because of early closure of the study site (Figure 1). Of the 78 remaining participants, 74 (94.9%) completed 8 weeks of follow-up. The median age was 63.5 years (range, 24-96); most participants were female (70.5%); and 1.3% were Asian, 1.3% Black, 2.6% Hispanic, and 96.2% White; baseline demographics and clinical characteristics were comparable across treatment groups (Table 1) and recurrent CDI and primary CDI at high risk for recurrence subgroups.
Table 1. Demographics and Baseline Characteristics (Full Analysis Set).
| Parameter | Patients with CDI qualifying episode, No. (%) | |||||
|---|---|---|---|---|---|---|
| High-dose VE303 (n = 29) | Low-dose VE303 (n = 27) | Placebo (n = 22) | ||||
| Primary (n = 4) | Recurrent (n = 25) | Primary (n = 6) | Recurrent (n = 21) | Primary (n = 3) | Recurrent (n = 19) | |
| Age at consent, y | ||||||
| Median (range) | 79 (77-87) | 58 (32-85) | 75 (66-82) | 63 (24-96) | 79 (73-79) | 62 (36-85) |
| <65 | 0 | 16 (64.0) | 0 | 12 (57.1) | 0 | 12 (63.2) |
| ≥65 | 4 (100) | 9 (36.0) | 6 (100) | 9 (42.9) | 3 (100) | 7 (36.8) |
| Sex | ||||||
| Female | 2 (50.0) | 16 (64.0) | 5 (83.3) | 15 (71.4) | 3 (100) | 14 (73.7) |
| Male | 2 (50.0) | 9 (36.0) | 1 (16.7) | 6 (28.6) | 0 | 5 (26.3) |
| Race | ||||||
| Asian | 0 | 1 (4.0) | 0 | 0 | 0 | 0 |
| Black or African American | 0 | 0 | 0 | 1 (4.8) | 0 | 0 |
| White | 4 (100) | 24 (96) | 6 (100) | 19 (90.4) | 3 (100) | 19 (100) |
| Othera | 0 | 0 | 0 | 1 (4.8) | 0 | 0 |
| Hispanic/Latinx ethnicity | 0 | 2 (8.0) | 0 | 0 | 0 | 0 |
| Total No. of prior CDI episodes at baseline (including current CDI qualifying episode) | ||||||
| Median (IQR) | 0 | 3.0 (2-4) | 0 | 3.0 (2-3) | 0 | 3.0 (2-3) |
| Standard-of-care antibiotic treatment for qualifying episode | ||||||
| Vancomycin 4 times daily | 3 (75.0) | 19 (76.0) | 4 (66.7) | 15 (71.4) | 2 (66.7) | 13 (68.4) |
| Other regimens | ||||||
| Fidaxomicin | 1 (25.0) | 4 (66.7) | 2 (100) | 5 (83.3) | 0 | 4 (66.7) |
| Metronidazole | 0 | 2 (33.3) | 0 | 0 | 0 | 1 (16.7) |
| Vancomycin, not 4 times daily | 0 | 0 | 0 | 1 (16.7) | 1 (100) | 1 (16.7) |
| CDI laboratory diagnostic method for qualifying episode | ||||||
| Free toxin (EIA, CCNA) | 3 (75.0) | 14 (56.0) | 3 (50.0) | 14 (66.7) | 0 | 15 (78.9) |
| Other (PCR, toxigenic culture)b | 1 (25.0) | 11 (44.0) | 3 (50.0) | 7 (33.3) | 3 (100) | 4 (21.1) |
Abbreviations: CCNA, cell cytotoxicity neutralization assay; CDI, Clostridioides difficile infection; EIA, enzyme immunoassay; PCR, polymerase chain reaction.
Other indicates that the participant did not report a specific race.
Other CDI laboratory diagnostic methods included PCR (n = 28) and toxigenic culture (n = 1).
Efficacy
For efficacy analysis 1, which included participants with a toxin-positive stool sample, the high-dose VE303 group recorded 4 CDI recurrences among 29 participants (13.8%) compared with 5 recurrences among 22 participants (22.7%) in the placebo group (adjusted absolute risk reduction [ARR], 11.0% [90% CI, −9% to 27%]; P = .29), evident at week 8 (Figure 2A; eFigure 2A in Supplement 3); low-dose VE303 participants (9/27 [33.3%]) had more CDI recurrences than those in the placebo group (ARR, −6.8% [90% CI, −3.2% to 10%]; P = .55).
Figure 2. Recurrence Rates of Clostridioides difficile Infection (CDI) Through Week 8.
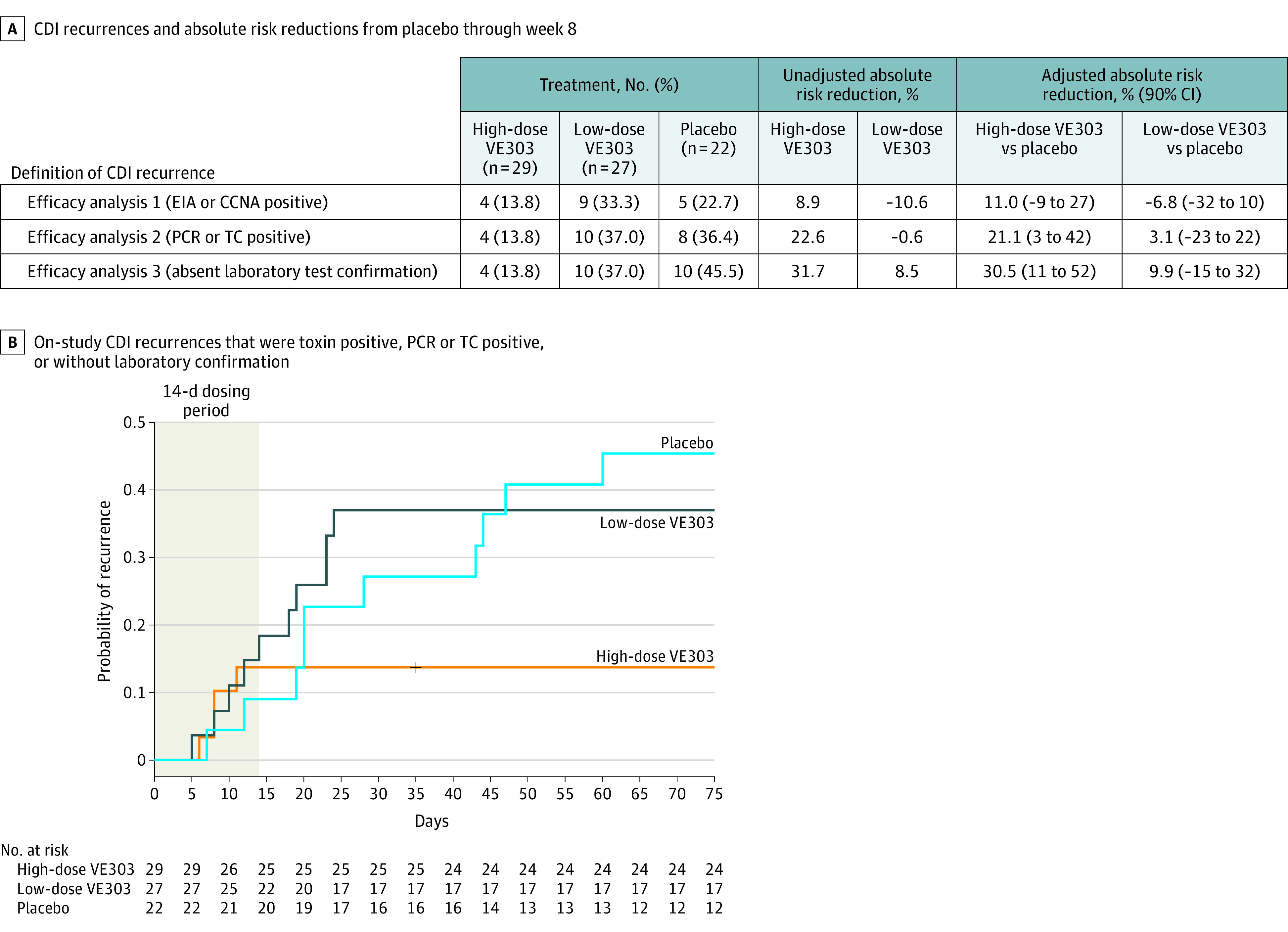
A, Two participants with on-study recurrences were included only in efficacy analysis 3: both participants were assigned to the placebo group and had stool samples taken on day 12 and day 14 following their suspected on-study CDI recurrence. Laboratory tests for these 2 samples were negative, but both participants were treated with antibiotics based on clinical symptoms; thus, both participants were included in the efficacy analysis. Adjustment was for the stratification factors.
B, Participants in the high-dose VE303 group had fewer recurrences (4/29 [13.8%]) than participants in the placebo group (10/22 [45.5%]) (adjusted absolute risk reduction, 30.5% [90% CI, 11% to 52%]; P = .006). Recurrence rates in the low-dose VE303 group (10/27 [37.0%]) were comparable with those in the placebo group (adjusted absolute risk reduction, 9.9% [90% CI, −15% to 32%]; P = .30). Per the prespecified statistical analysis plan, study week 8 included recurrences with onset up to day 63.
CCNA indicates cell cytotoxicity neutralization assay; EIA, enzyme immunoassay; PCR, polymerase chain reaction; and TC, toxigenic culture.
For efficacy analysis 2, the sensitivity analysis that included all recurrences in efficacy analysis 1 plus participants with a positive PCR or toxigenic culture stool test result, the high-dose VE303 group had fewer recurrences (4/29 [13.8%]) compared with the placebo group (8/22 [36.4%]) (ARR, 21.1% [90% CI, 3% to 42%]; P = .04) (Figure 2A; eFigure 2B in Supplement 3); CDI recurrences among the low-dose VE303 group (10/27 [37.0%]) were similar to the placebo group (ARR, 3.1% [90% CI, −23% to 22%]; P = .72).
For efficacy analysis 3, the sensitivity analysis that included all recurrences in efficacy analysis 2 plus those that were treated with an antibiotic for CDI but without a confirmatory diagnostic laboratory test, participants in the high-dose VE303 group had fewer recurrences (4/29 [13.8%]) than those in the placebo group (10/22 [45.5%]) (ARR, 30.5% [90% CI, 11% to 52%]; α = .05, 1-sided; P = .006); the CDI recurrence rate in the low-dose VE303 group (10/27 [37.0%]) was similar to that in the placebo group (10/22 [45.5%]) (ARR, 9.9% [90% CI, −15% to 32%]; P = .30) (Figure 2A). Two participants assigned to placebo, with clinical evidence of CDI recurrences on day 28 and day 44, were included in efficacy analysis 3 only. In both instances, the on-study recurrences could not be confirmed through laboratory tests due to delayed collection and logistical issues with the stool samples (eFigure 3C in Supplement 3).
In the high-dose VE303 group, no CDI recurrences occurred from day 12 through week 8, whereas recurrences in the placebo group occurred through week 8. The odds ratio of CDI recurrence up to week 8 for the high-dose VE303 group vs placebo was 0.19 (90% CI, 0.05 to 0.71; 1-sided P = .008).
In follow-up through week 24 (eFigure 3 in Supplement 3), 2 additional CDI recurrences were reported: in the high-dose VE303 group on day 110 and in the low-dose VE303 group on day 154 (eFigure 3B in Supplement 3).
Safety and Adverse Effects
Most participants (76/79 [96.2%]) experienced 1 or more treatment-emergent AEs (TEAEs), which were generally of mild or moderate intensity and were mainly gastrointestinal (Table 2). There were no reported grade 4 TEAEs and no deaths. Treatment-related TEAEs were reported by more participants in the high-dose VE303 group (16/30 [53.3%]) than in the low-dose VE303 group (8/27 [29.6%]) or the placebo group (7/22 [31.8%]). Serious TEAEs were reported in 7 participants (8.9%) across treatment groups (total of 11 serious TEAEs): 1 participant in the high-dose VE303 group reported 1 event (transfusion reaction), 4 participants in the low-dose VE303 group reported 5 events (CDI, breast cancer, exacerbation of chronic obstructive pulmonary disease, and dizziness and cerebrovascular accident), and 2 participants in the placebo group reported 5 events (abdominal pain, nausea, and vomiting; Escherichia coli bacteremia and pyelonephritis). Based on review by both the investigator and the Vedanta Biosciences medical monitor, none of these serious TEAEs was considered treatment related (Table 2). Seven participants (4 in the high-dose VE303 group, 2 in the low-dose VE303 group, and 1 in the placebo group) experienced TEAEs leading to study drug discontinuation; all were events of diarrhea (Table 2). All AEs of special interest observed in the study were gastrointestinal (diarrhea, abdominal pain, flatulence, and vomiting); no bacterial infection AEs of special interest were observed.
Table 2. Overall Summary of Adverse Events and Related Treatment-Emergent Adverse Events (TEAEs) by System Organ Class and Preferred Term (Safety Analysis Set).
| Adverse eventa | No. (%) | ||
|---|---|---|---|
| High-dose VE303 (n = 30) | Low-dose VE303 (n = 27) | Placebo (n = 22) | |
| Serious TEAEsb | 1 (3.3) | 4 (14.8) | 2 (9.1) |
| Treatment-related | 0 | 0 | 0 |
| ≥1 TEAEc | 28 (93.3) | 27 (100) | 21 (95.5) |
| Treatment-related | 16 (53.3) | 8 (29.6) | 7 (31.8) |
| Adverse events of special interestd | 4 (13.3) | 0 | 1 (4.5) |
| Treatment-related | 4 (13.3) | 0 | 1 (4.5) |
| Grade 1 TEAEs (mild) | 28 (93.3) | 27 (100) | 20 (90.9) |
| Treatment-related | 14 (46.7) | 8 (29.6) | 7 (31.8) |
| Grade 2 TEAEs (moderate) | 12 (40.0) | 16 (59.3) | 11 (50.0) |
| Treatment-related | 4 (13.3) | 0 | 1 (4.5) |
| Grade 3 TEAEs (severe) | 2 (6.7) | 4 (14.8) | 3 (13.6) |
| Treatment-related | 1 (3.3) | 0 | 0 |
| Grade 4 TEAEs (life-threatening) | 0 | 0 | 0 |
| Treatment-related | 0 | 0 | 0 |
| COVID-19–associated TEAEs | 0 | 0 | 0 |
| Deaths | 0 | 0 | 0 |
| TEAEs leading to study treatment discontinuation | 4 (13.3) | 2 (7.4) | 1 (4.5) |
| Adverse events reported in ≥5% of participantse | |||
| Gastrointestinal disorders | 26 (86.7) | 25 (92.6) | 21 (95.5) |
| Diarrhea | 21 (70.0) | 23 (85.2) | 19 (86.4) |
| Nausea | 6 (20.0) | 6 (22.2) | 2 (9.1) |
| Nervous system disorders | 5 (16.7) | 7 (25.9) | 3 (13.6) |
| Investigations | 5 (16.7) | 3 (11.1) | 1 (4.5) |
| Abdominal pain | 5 (16.7) | 2 (7.4) | 2 (9.1) |
| General disorders and administration site conditions | 4 (13.3) | 6 (22.2) | 2 (9.1) |
| Skin and subcutaneous tissue disorders | 4 (13.3) | 1 (3.7) | 1 (4.5) |
| Infections and infestations | 3 (10.0) | 8 (29.6) | 8 (36.4) |
| Constipation | 3 (10.0) | 1 (3.7) | 0 |
| Metabolism and nutrition disorders | 2 (6.7) | 4 (14.8) | 1 (4.5) |
| Musculoskeletal and connective tissue disorders | 2 (6.7) | 3 (11.1) | 1 (4.5) |
| Headache | 2 (6.7) | 3 (11.1) | 0 |
| Chills | 2 (6.7) | 2 (7.4) | 0 |
| Abdominal distension | 2 (6.7) | 1 (3.7) | 2 (9.1) |
| Fatigue | 2 (6.7) | 1 (3.7) | 0 |
| Flatulence | 2 (6.7) | 1 (3.7) | 2 (9.1) |
| Irritable bowel syndrome | 2 (6.7) | 1 (3.7) | 0 |
| Dysgeusia | 2 (6.7) | 0 | 2 (9.1) |
| Upper abdominal pain | 2 (6.7) | 0 | 0 |
| Blood potassium increased | 2 (6.7) | 0 | 0 |
| Vomiting | 1 (3.3) | 4 (14.8) | 3 (13.6) |
| Injury, poisoning, and procedural complications | 1 (3.3) | 4 (14.8) | 3 (13.6) |
| Respiratory, thoracic, and mediastinal disorders | 1 (3.3) | 4 (14.8) | 2 (9.1) |
| Surgical and medical procedures | 1 (3.3) | 3 (11.1) | 1 (4.5) |
| Urinary tract infection | 1 (3.3) | 2 (7.4) | 4 (18.2) |
| Dyspepsia | 1 (3.3) | 2 (7.4) | 1 (4.5) |
| Cough | 1 (3.3) | 2 (7.4) | 0 |
| Dehydration | 1 (3.3) | 2 (7.4) | 0 |
| Pyrexia | 0 | 4 (14.8) | 0 |
| Dizziness | 0 | 3 (11.1) | 1 (4.5) |
| Abdominal discomfort | 0 | 2 (7.4) | 0 |
| Eructation | 0 | 2 (7.4) | 0 |
| Malaise | 0 | 2 (7.4) | 0 |
| Fall | 0 | 2 (7.4) | 0 |
| Benign, malignant, and unspecified neoplasms (including cysts and polyps) | 0 | 2 (7.4) | 0 |
| Edema peripheral | 0 | 1 (3.7) | 2 (9.1) |
| Lower abdominal pain | 0 | 0 | 3 (13.6) |
| Vascular disorders | 0 | 0 | 3 (13.6) |
| Oropharyngeal pain | 0 | 0 | 2 (9.1) |
Adverse events were coded using the Medical Dictionary for Regulatory Activities (version 21.1).
None of these events were considered treatment related.
TEAEs were defined as adverse events that started or increased in severity at the time of or after administration of the first dose of study treatment, up to week 24. TEAEs were classified in severity by Common Terminology Criteria for Adverse Events grade. TEAEs were defined as those reported as possibly related, probably related, definitely related, or missing. Missing severity was imputed as severe (grade 3).
Adverse events of special interest were defined as any clinically significant (grade ≥2) event, either gastrointestinal adverse event or bacterial infection, that were considered related to study treatment. All AESIs were gastrointestinal (diarrhea, abdominal pain, flatulence, and vomiting); no bacterial infections were observed.
Presented in descending order by high-dose group.
Changes in the Microbiome
At the end of study treatment on day 14, VE303 fecal colonization, as measured by the number of consortium strains detected (Figure 3A) and the sum of the strain relative abundances (Figure 3B), was significantly increased (P < .001, Wilcoxon rank-sum test) in the VE303-dosed groups compared with the placebo group (eTable 3 in Supplement 3). VE303 detection was significantly increased in the high-dose VE303 vs low-dose VE303 group (P < .05, Wilcoxon test; Figure 3A; eTable 3 in Supplement 3). Likewise, total VE303 relative abundance in the high-dose VE303 group (5.2%) was greater than in the low-dose VE303 group (0.8%) (P = .09, Wilcoxon rank-sum test, eTable 3 in Supplement 3), indicating a dose-exposure effect (Figure 3B). An ad hoc analysis combined data from the active treatment groups, comparing the probability of CDI recurrence in participants with high colonization vs those with low colonization based on strain detection. Higher early colonization of VE303 strains among dosed participants was associated with greater clinical efficacy: participants with high VE303 colonization at day 14 (5 to 8 strains detected) had a lower probability of CDI recurrence compared with participants who had low VE303 strain colonization (0 to 4 strains detected; log rank test, P = .08) (Figure 3C; eTables 4 and 5 in Supplement 3). Species alpha diversity (using the Shannon Diversity Index; see the Methods section and eMethods in Supplement 3) was increased in nonrecurrent vs recurrent participants by the end of dosing (P = .02; linear mixed effects [LME] model; Figure 3D; eTable 6 in Supplement 3). Diversity recovery was most notable in the high-dose VE303 group; the relative diversity rise (delta) in nonrecurrent vs recurrent participants increased with dose and was highest for the high-dose VE303 group (high-dose VE303: delta = 0.7, P = .08; low-dose VE303: delta = 0.4, P = .39; placebo: delta = 0.1, P = .29; LME, eTable 6 in Supplement 3). Microbial diversity recovered to greater than screening levels by day 14 in the high-dose VE303 group (P = .004, LME, eTable 6 in Supplement 3), whereas the difference was not significant in the low-dose VE303 and placebo groups (P > .20, LME, eTable 6 in Supplement 3). Day 14 diversity was increased in the high-dose VE303 group vs placebo (P = .02, LME, eTable 6 in Supplement 3), whereas that in the low-dose VE303 group was not significantly different from placebo.
Figure 3. VE303 Strain Colonization and Fecal Microbiome Diversity.
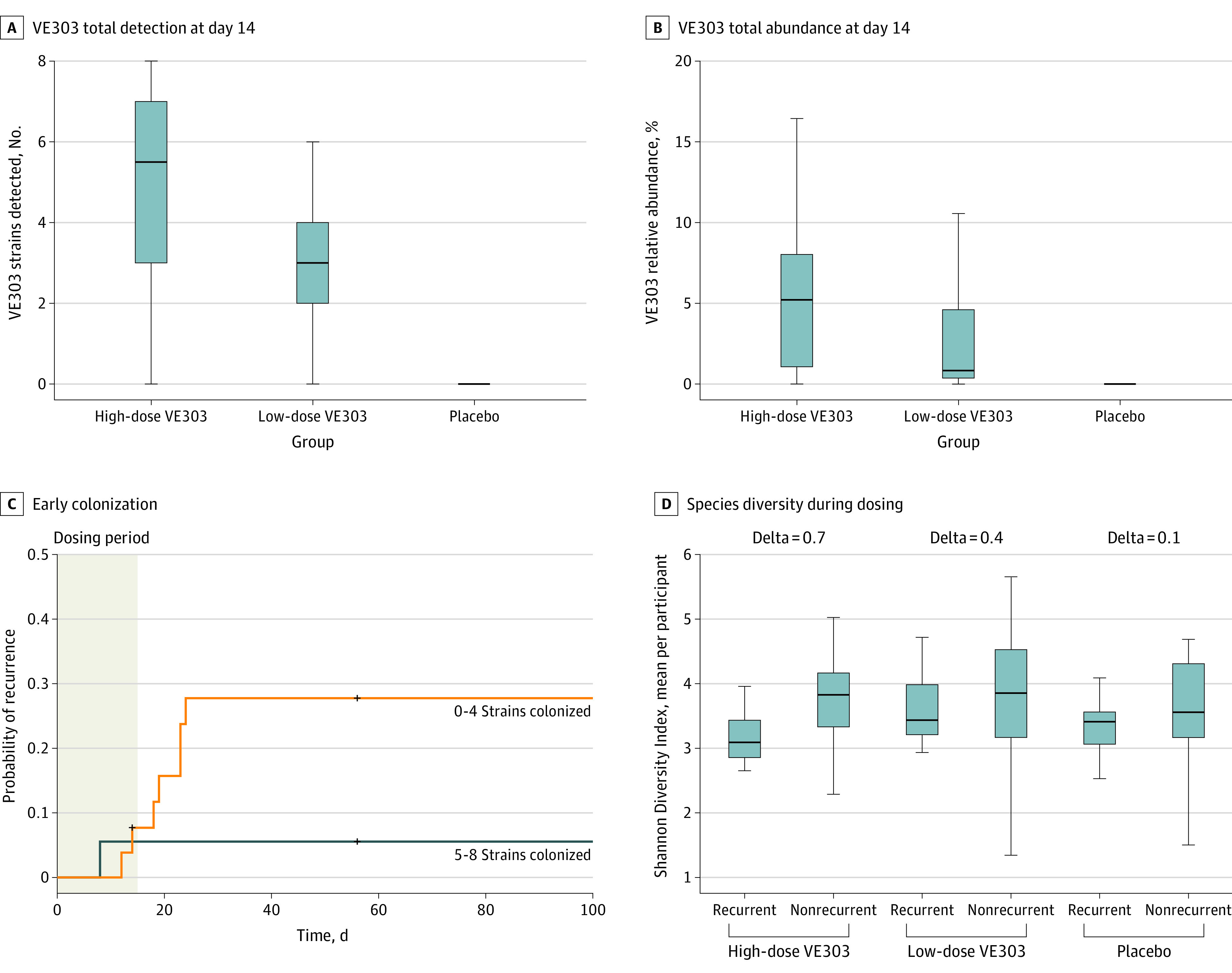
Total VE303 strain detection (A) and total VE303 strain relative abundance (B) at the end of dosing were significantly increased in VE303-dosed groups compared with the placebo group (P < .001, Wilcoxon test). C, In VE303 recipients, higher colonization (5 to 8 strains colonized) at the end of dosing was associated with a lower Clostridioides difficile infection recurrence rate and lower probability of recurrence (log-rank test, P = .08, hazard ratio for low colonization/high colonization = 5.32). D, Species alpha diversity during the 2 weeks after antibiotic treatment, shown as the time-averaged Shannon Diversity Index per participant, was associated with clinical efficacy (P = .02, linear mixed-effects model for all treatment groups). All individual participants are plotted. The box-and-whisker plots depict the median (lines within the boxes), IQR (top and bottom of the boxes), and reasonable extreme values at 1.5 × IQR in the data set (where the vertical lines end).
Discussion
This study reported findings from a randomized, double-blind, placebo-controlled study of VE303, a novel defined bacterial consortium composed of 8 strains of commensal Clostridia, for prevention of CDI. To our knowledge, this is the first double-blind, placebo-controlled study to demonstrate efficacy with a defined bacterial consortium in any therapeutic indication.
Compared with placebo, high-dose VE303 prevented CDI among high-risk participants. In the high-dose VE303 group, all CDI recurrences through week 8 occurred by day 11, likely due to subsequent colonization of VE303 strains and VE303-induced restoration of the gut microbiota community. Most participants experienced sustained cure through week 24, suggesting that VE303 has a durable effect. Low-dose VE303 did not prevent CDI recurrence compared with placebo during the 8-week primary efficacy period. The results of a phase 1 study demonstrated dose-dependent colonization with VE303,15 which was corroborated by the lower levels of colonization in the low-dose VE303 group and subsequent lack of clinical efficacy observed in this trial. The observed recurrence rate of 13.8% in the high-dose VE303 group compared with placebo and adjusted absolute risk reduction of 30.5% compared favorably with those of fecal microbial transplant (28% recurrence rate),17 bezlotoxumab (absolute risk reduction, 10%),18 RBX2660 (absolute risk reduction, 12.3%),14 and investigational agents that rely on human fecal donors—with absolute risk reductions relative to placebo of 27.4% and 13.0% for SER-109 and CP101, respectively.12,13
The CDI recurrence rate of 22.7% in the placebo group based on toxin positivity alone was lower than expected, given the high-risk populations enrolled. By contrast, the recurrence rate in the placebo group when based on all laboratory-confirmed cases (36.4%) or a combined laboratory and clinical approach (45.5%) was consistent with the rates and diagnostic approaches of most other studies.12,13 This latter definition of CDI recurrence reflects a pragmatic approach used clinically, with treatment often initiated based on PCR and/or symptoms, in addition to EIA results.1 Two participants had clinical recurrences that were treated but not laboratory confirmed, due to pandemic-related challenges; their stool samples were not obtained until 12 and 14 days, respectively, after onset of the suspected recurrence and antibiotic treatment. Test results were negative, likely due to treatment and late sample collection.
In this study, 72% of participants were treated with vancomycin 4 times daily and 21% with fidaxomicin for the qualifying CDI episode, which mirrors current clinical practice. Due to recent updates to treatment guidance that have expanded recommendations for fidaxomicin use,6,7,8 recurrence rates in future CDI trials could be lower than historical reference rates. Studies have shown that although fidaxomicin has similar cure rates to oral vancomycin, it is associated with significantly lower rates of recurrent CDI.19,20 Nevertheless, in patients with multiple CDI recurrences who received prior vancomycin therapy but were treated with fidaxomicin, CDI recurrence rates remain suboptimal.12,21 This highlights the need for additional approaches to prevention.
VE303 was generally well tolerated. Fewer than half of VE303 recipients reported a treatment-related TEAE, and approximately 10% reported a severe AE or a serious TEAE. Most TEAEs were gastrointestinal and mild in intensity. No treatment-related serious TEAEs or deaths were reported. The favorable tolerability profile of VE303 was expected, based on prior reports of fecal microbiota transplants and other live biotherapeutic products.12,13,22,23
The VE303 component strains colonized rapidly during the dosing period. VE303 strains showed significantly greater abundance and prevalence in both of the VE303 groups compared with placebo. Receipt of a higher dose significantly increased total VE303 strain colonization, and participants with high VE303 colonization had a lower probability of CDI recurrence than those with low colonization, suggesting a positive exposure-response relationship. Individuals with CDI display gut dysbiosis characterized by low species diversity and dominance of putative pathogens, which is further exacerbated by first-line treatment with antibiotics.24,25 By contrast, a healthy gut resilient to perturbations is characterized by high diversity and increased Clostridia cluster IV and XIVa species. The data from the current study suggest that VE303 expedited recovery of the endogenous microbiota, highlighting a structural ecologic role of Clostridia in colonization resistance.
Limitations
This study had limitations. First, the definition of CDI recurrence in efficacy analysis 3 included 2 participants whose recurrences were diagnosed clinically, without confirmation by positive laboratory testing. Some commonly used tests, such as EIA for toxin A/B, have high specificity but low sensitivity, while others, such as PCR, have high sensitivity but suboptimal specificity. The combined use of laboratory testing plus clinical assessment is endorsed by professional society guidelines but the study definitions could have misclassified some diarrheal episodes as CDI.
Second, study enrollment was hindered by the COVID-19 pandemic and other factors, including availability of open-label fecal microbial transplant in many areas, which negatively affected the sample size goal.
Third, the study’s demographic distribution was comparable with the epidemiology seen in CDI26; however, the predominantly White population may limit the generalizability of these findings. An adequately sized and powered phase 3 study is planned, with an emphasis on enrolling a more diverse study population.
Conclusions
Among adult participants with laboratory-confirmed CDI with 1 or more prior CDI episodes in the last 6 months and those with primary CDI at high risk for recurrence, high-dose VE303 prevented recurrent CDI compared with placebo. A larger, phase 3 study is needed to confirm these findings.
Trial Protocol
Statistical Analysis Plan
eMethods
eFigure 1. Prespecified Efficacy Analyses
eFigure 2. Recurrence Rates of Clostridioides difficile Infection Through Week 8
eFigure 3. Recurrence Rates of Clostridioides difficile Infection Through Week 24
eTable 1. VE303 Strain Identity
eTable 2. Summary of Study Protocol Changes
eTable 3. Model Summary: Comparison of Total VE303 Abundance and Proportion Across Dosed and Placebo Groups
eTable 4. Cut-point Models Summary: Recurrence-Free Probability in All Possible High vs Low Colonization Categories
eTable 5. Cox Model Summary: Comparison of Recurrence-Free Probability in High vs Low Colonizing Participants
eTable 6. Linear Mixed Model Summary: Comparison of Diversity Across Treatment and Response Groups over Time
eAppendix. List of Investigators
Data Sharing Statement
References
- 1.Guh AY, Mu Y, Winston LG, et al. ; Emerging Infections Program Clostridioides difficile Infection Working Group . Trends in US burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;382(14):1320-1330. doi: 10.1056/NEJMoa1910215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lofgren ET, Cole SR, Weber DJ, Anderson DJ, Moehring RW. Hospital-acquired Clostridium difficile infections: estimating all-cause mortality and length of stay. Epidemiology. 2014;25(4):570-575. doi: 10.1097/EDE.0000000000000119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feuerstadt P, Das R, Brandt LJ. The evolution of urban C difficile infection (CDI): CDI in 2009-2011 is less severe and has better outcomes than CDI in 2006-2008. Am J Gastroenterol. 2014;109(8):1265-1276. doi: 10.1038/ajg.2014.167 [DOI] [PubMed] [Google Scholar]
- 4.Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology. 2014;146(6):1547-1553. doi: 10.1053/j.gastro.2014.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21-27. doi: 10.1111/1469-0691.12046 [DOI] [PubMed] [Google Scholar]
- 6.Johnson S, Lavergne V, Skinner AM, et al. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis. 2021;73(5):e1029-e1044. doi: 10.1093/cid/ciab549 [DOI] [PubMed] [Google Scholar]
- 7.Kelly CR, Fischer M, Allegretti JR, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol. 2021;116(6):1124-1147. doi: 10.14309/ajg.0000000000001278 [DOI] [PubMed] [Google Scholar]
- 8.van Prehn J, Reigadas E, Vogelzang EH, et al. ; Guideline Committee of the European Study Group on Clostridioides difficile . European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin Microbiol Infect. 2021;27(suppl 2):S1-S21. doi: 10.1016/j.cmi.2021.09.038 [DOI] [PubMed] [Google Scholar]
- 9.DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381(21):2043-2050. doi: 10.1056/NEJMoa1910437 [DOI] [PubMed] [Google Scholar]
- 10.Zellmer C, Sater MRA, Huntley MH, Osman M, Olesen SW, Ramakrishna B. Shiga toxin-producing Escherichia coli transmission via fecal microbiota transplant. Clin Infect Dis. 2021;72(11):e876-e880. doi: 10.1093/cid/ciaa1486 [DOI] [PubMed] [Google Scholar]
- 11.Taroncher-Oldenburg G, Jones S, Blaser M, et al. Translating microbiome futures. Nat Biotechnol. 2018;36(11):1037-1042. doi: 10.1038/nbt.4287 [DOI] [PubMed] [Google Scholar]
- 12.Feuerstadt P, Louie TJ, Lashner B, et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N Engl J Med. 2022;386(3):220-229. doi: 10.1056/NEJMoa2106516 [DOI] [PubMed] [Google Scholar]
- 13.Gilbert JA. Microbiome therapy for recurrent Clostridioides difficile. Lancet Microbe. 2022;3(5):e334. doi: 10.1016/S2666-5247(22)00096-9 [DOI] [PubMed] [Google Scholar]
- 14.Khanna S, Assi M, Lee C, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs. 2022;82(15):1527-1538. doi: 10.1007/s40265-022-01797-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dsouza M, Menon R, Crossette E, et al. Colonization of the live biotherapeutic product VE303 and modulation of the microbiota and metabolites in healthy volunteers. Cell Host Microbe. 2022;30(4):583-598.e8. doi: 10.1016/j.chom.2022.03.016 [DOI] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration . FDA guidance on conduct of clinical trials of medical products during the COVID-19 public health emergency. Published August 2021. Accessed July 1, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-public-health-emergency
- 17.Baunwall SMD, Lee MM, Eriksen MK, et al. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: an updated systematic review and meta-analysis. EClinicalMedicine. 2020;29-30:100642. doi: 10.1016/j.eclinm.2020.100642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilcox MH, Gerding DN, Poxton IR, et al. ; MODIFY I and MODIFY II Investigators . Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med. 2017;376(4):305-317. doi: 10.1056/NEJMoa1602615 [DOI] [PubMed] [Google Scholar]
- 19.Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(suppl 2)(suppl 2):S154-S161. doi: 10.1093/cid/cis462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louie TJ, Miller MA, Mullane KM, et al. ; OPT-80-003 Clinical Study Group . Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431. doi: 10.1056/NEJMoa0910812 [DOI] [PubMed] [Google Scholar]
- 21.Skinner AM, Tan X, Sirbu BD, Danziger LH, Gerding DN, Johnson S. A tapered-pulsed fidaxomicin regimen following treatment in patients with multiple Clostridioides difficile infection recurrences. Clin Infect Dis. 2021;73(6):1107-1109. doi: 10.1093/cid/ciab233 [DOI] [PubMed] [Google Scholar]
- 22.Baxter M, Colville A. Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect. 2016;92(2):117-127. doi: 10.1016/j.jhin.2015.10.024 [DOI] [PubMed] [Google Scholar]
- 23.Rapoport EA, Baig M, Puli SR. Adverse events in fecal microbiota transplantation: a systematic review and meta-analysis. Ann Gastroenterol. 2022;35(2):150-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280. doi: 10.1371/journal.pbio.0060280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seekatz AM, Young VB. Clostridium difficile and the microbiota. J Clin Invest. 2014;124(10):4182-4189. doi: 10.1172/JCI72336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lessa FC, Mu Y, Winston LG, et al. Determinants of Clostridium difficile infection incidence across diverse United States geographic locations. Open Forum Infect Dis. 2014;1(2):ofu048. doi: 10.1093/ofid/ofu048 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods
eFigure 1. Prespecified Efficacy Analyses
eFigure 2. Recurrence Rates of Clostridioides difficile Infection Through Week 8
eFigure 3. Recurrence Rates of Clostridioides difficile Infection Through Week 24
eTable 1. VE303 Strain Identity
eTable 2. Summary of Study Protocol Changes
eTable 3. Model Summary: Comparison of Total VE303 Abundance and Proportion Across Dosed and Placebo Groups
eTable 4. Cut-point Models Summary: Recurrence-Free Probability in All Possible High vs Low Colonization Categories
eTable 5. Cox Model Summary: Comparison of Recurrence-Free Probability in High vs Low Colonizing Participants
eTable 6. Linear Mixed Model Summary: Comparison of Diversity Across Treatment and Response Groups over Time
eAppendix. List of Investigators
Data Sharing Statement


