Figure 2. Recurrence Rates of Clostridioides difficile Infection (CDI) Through Week 8.
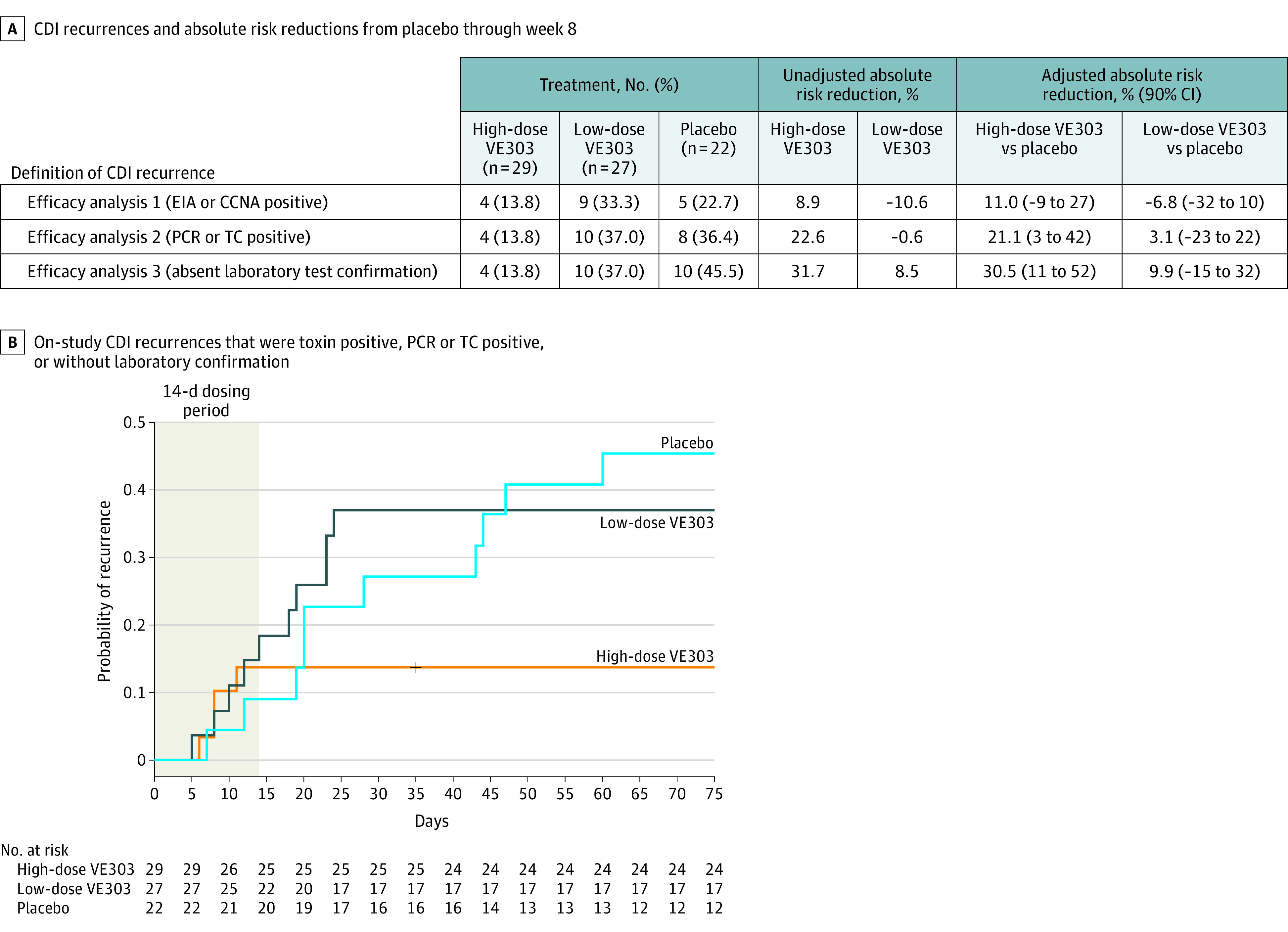
A, Two participants with on-study recurrences were included only in efficacy analysis 3: both participants were assigned to the placebo group and had stool samples taken on day 12 and day 14 following their suspected on-study CDI recurrence. Laboratory tests for these 2 samples were negative, but both participants were treated with antibiotics based on clinical symptoms; thus, both participants were included in the efficacy analysis. Adjustment was for the stratification factors.
B, Participants in the high-dose VE303 group had fewer recurrences (4/29 [13.8%]) than participants in the placebo group (10/22 [45.5%]) (adjusted absolute risk reduction, 30.5% [90% CI, 11% to 52%]; P = .006). Recurrence rates in the low-dose VE303 group (10/27 [37.0%]) were comparable with those in the placebo group (adjusted absolute risk reduction, 9.9% [90% CI, −15% to 32%]; P = .30). Per the prespecified statistical analysis plan, study week 8 included recurrences with onset up to day 63.
CCNA indicates cell cytotoxicity neutralization assay; EIA, enzyme immunoassay; PCR, polymerase chain reaction; and TC, toxigenic culture.
