Abstract
Objective
To evaluate the clinical efficacy and safety of leflunomide (L) added to the standard-of-care (SOC) treatment in COVID-19 patients hospitalised with moderate/critical clinical symptoms.
Design
Prospective, open-label, multicentre, stratified, randomised clinical trial.
Setting
Five hospitals in UK and India, from September 2020 to May 2021.
Participants
Adults with PCR confirmed COVID-19 infection with moderate/critical symptoms within 15 days of onset.
Intervention
Leflunomide 100 mg/day (3 days) followed by 10–20 mg/day (7 days) added to standard care.
Primary outcomes
The time to clinical improvement (TTCI) defined as two-point reduction on a clinical status scale or live discharge prior to 28 days; safety profile measured by the incidence of adverse events (AEs) within 28 days.
Results
Eligible patients (n=214; age 56.3±14.9 years; 33% female) were randomised to SOC+L (n=104) and SOC group (n=110), stratified according to their clinical risk profile. TTCI was 7 vs 8 days in SOC+L vs SOC group (HR 1.317; 95% CI 0.980 to 1.768; p=0.070). Incidence of serious AEs was similar between the groups and none was attributed to leflunomide. In sensitivity analyses, excluding 10 patients not fulfilling the inclusion criteria and 3 who withdrew consent before leflunomide treatment, TTCI was 7 vs 8 days (HR 1.416, 95% CI 1.041 to 1.935; p=0.028), indicating a trend in favour of the intervention group. All-cause mortality rate was similar between groups, 9/104 vs 10/110. Duration of oxygen dependence was shorter in the SOC+L group being a median 6 days (IQR 4–8) compared with 7 days (IQR 5–10) in SOC group (p=0.047).
Conclusion
Leflunomide, added to the SOC treatment for COVID-19, was safe and well tolerated but had no major impact on clinical outcomes. It may shorten the time of oxygen dependence by 1 day and thereby improve TTCI/hospital discharge in moderately affected COVID-19 patients.
Trial registration numbers
EudraCT Number: 2020-002952-18, NCT05007678.
Keywords: COVID-19, INFECTIOUS DISEASES, Respiratory infections
STRENGTHS AND LIMITATIONS OF THIS STUDY.
International, prospective, randomised controlled study.
Repurposing a marketed drug with established safety profile and promising dual antiviral and immunomodulating medication based on strong drug discovery data.
Study participants had milder COVID-19 disease than originally intended, thus eroding the power of the study.
Evolving standard-of-care therapy possibly diminished measurable benefit of leflunomide.
Introduction
COVID-19 pandemic caused unprecedented strain on healthcare services around the world. It has affected almost 16 million people globally and caused over 6 million deaths so far.1 Associated clinical syndromes include pneumonia, systemic inflammatory response and cardiovascular complications with high morbidity and mortality. Progressive deterioration is thought to be related to the kinetics of viral replication culminating in a surge of inflammatory mediator release, ‘cytokine storm’.2 Around 5%–10% of infected patients experience severe or life-threatening symptoms with high mortality.3
Direct-acting and host-targeting antiviral treatments are the two approaches in treating viral infections. Host targeting antiviral treatments may have an advantage over direct antivirals as they enable the body to fight against a broad spectrum of viruses by simultaneously blocking viral replication and overcoming the potential of viral mutagenesis.4 Anti-inflammatory medications have been shown to improve survival through dampening of the inappropriate immune response in susceptible patients.5 This has led to the search for a drug with such therapeutic properties.
Leflunomide is a drug licensed to treat rheumatoid arthritis (RA).6 It is widely available, cost-effective and can be easily administered both in the hospital and domestic settings. In preclinical models of cell and animal infection by SARS-CoV-2, leflunomide was shown to be a potent inhibitor of human dihydroorotate dehydrogenase (DHODH), an enzyme vital to viral replication in the host cell.7–9 It has the potential advantage of not only targeting the virus infection but also suppressing the ensuing inflammatory response which may play a role in more progressive stages of infection leading to serious complications.
The Targeting de Novo Pyrimidine Biosynthesis by Leflunomide for the Treatment of COVID-19 (DEFEAT COVID) study tested whether leflunomide added to standard care was clinically effective and safe for COVID-19 moderate/severe symptoms.
Methods
Study design
This was a multicentre, international, open-label, prospective, randomised controlled clinical trial set up at five hospitals (two in UK and three in India). The recruitment took place between September 2020 and May 2021, and was approved by all relevant ethics committees.
Participants
Patients aged 18 years and above presenting with moderate to critical symptoms of PCR-confirmed COVID-19 disease within 15 days of symptoms onset were recruited. Patients with respiratory compromise and blood oxygen saturation (SpO2) <93% on room air detected on pulse oximeter were considered to fulfil the moderate infection criteria. Patients with respiratory failure, septic shock and/or multiple organ dysfunction/failure needing assisted ventilation were considered to be critically ill. Pregnant or breastfeeding women, individuals already receiving specific monoclonal antibody therapy or those with severe immunodeficiency syndrome and hypoalbuminaemia and patients with hypersensitivity to leflunomide or liver enzymes aspartate transaminase (AST)/alanine transaminase (ALT) ≥2× upper limits of normal (ULN) were excluded from the study. All participants gave written informed consent to a member of their clinical care team.
Randomisation
Consented participants were randomised by a member of the clinical care team to either the control arm (receiving standard-of-care treatment (SOC) alone) or the intervention arm (SOC treatment+leflunomide (SOC+L)) using a stratified block randomisation web-based algorithm. Patient admission data (age </≥70; comorbidities; clinical status based on National Early Warning Score 2, NEWS2)10 were used to stratify patients into four risk categories. Group 1: high/moderate comorbidity risk with NEWS2 score ≥5; group 2: high/moderate comorbidity risk with NEWS2 score <5; group 3: low comorbidity risk with NEWS2 score ≥5 and group 4: low comorbidity risk with NEWS2 score <5.
Interventions
The definition of the SOC treatment for COVID-19 evolved nationally and internationally through the course of our study, with progressive evolution in the understanding of disease pathology and emerging treatment evidence. The SOC during the time of the study across all sites involved four main treatment domains: steroids, anticoagulation, antibiotics and antiviral medications. The intervention group (SOC+L) received oral leflunomide at a loading dose of 100 mg/day for 3 days and then 20 mg/day for 7 days as a maintenance dose. The maintenance dose was reduced to 10 mg/day if liver enzymes AST/ALT exceeded 2× ULN. Leflunomide treatment was stopped early if AST/ATL exceeded 3× ULN during the intervention. Study participants received additional COVID-19 therapies, including monoclonal antibodies, at the discretion of the direct care clinical team, even if leflunomide was initiated.
Study procedures
Patient-related clinical/investigation data, treatment compliance, outcomes and adverse events (AEs) were collected by the site investigators and recorded on the prespecified daily electronic case report form (see online supplemental appendix 1). AEs were graded according to the Common Terminology Criteria for Adverse Events.11 Blood samples were collected and processed for quantifying viral load (on days 1, 7, 11, 15, 28 or day of discharge) and for future inflammatory profiling (on days 1, 3 and 11). Liver enzymes were measured at baseline, on day 3 after the leflunomide loading and on discharge. Patient questionnaire was administered at 28 days and 90 days after randomisation to monitor the persistence of symptoms possibly associated with long COVID syndrome.12 SpO2/oxygen concentration (FiO2) data were monitored daily. The frequency of SpO2 monitoring varied with FiO2 administration. It is standard clinical practice that SpO2 is monitored every 4 hours in a clinically stable patient. The frequency increases to continuous SpO2 monitoring in a patient with oxygen requirement or ventilation support. Where multiple daily values were recorded, we selected the SpO2/FiO2 ratio reflecting increased oxygen demand.
bmjopen-2022-068179supp001.pdf (113.1KB, pdf)
Blinding
Site investigator teams and direct clinical care teams were not blinded to the randomisation outcomes, but neither were provided information about the aggregate patient outcomes.
Outcomes
The primary outcome is the time (days) from randomisation to clinical improvement (TTCI) of two points on a seven-category clinical status scale or live discharge from hospital prior to 28 days.13 The clinical status ordinal scale consisted of the following: (1) not hospitalised, resumption of normal activities; (2) not hospitalised, but unable to resume normal activities; (3) hospitalised, not requiring supplemental oxygen; (4) hospitalised, requiring supplemental oxygen; (5) hospitalised, requiring nasal high-flow oxygen therapy, non-invasive ventilation (NIV), or both; (6) hospitalised, requiring extracorporeal membrane oxygenation, invasive mechanical ventilation, or both; and (7) death.
Safety profile of leflunomide in this group of patients was assessed from incidence rates of AE deemed to be serious and/or severe (≥grade 3). Grading guidelines suggest five categories: (1) mild, asymptomatic or mild symptoms, clinical or diagnostic observations only, intervention not indicated; (2) moderate, minimal, local or non-invasive intervention indicated, limiting age-appropriate instrumental activities of daily livings (ADL); (3) severe, medically significant but not immediately life-threatening, hospitalisation or prolongation of hospitalisation indicated, disabling; limiting self-care ADL; (4) life-threatening consequences, urgent intervention indicate and (5) death related to AE. In addition, the incidences and levels of liver transaminitis (ALT, AST) were assessed.
The main secondary outcomes were focused on overall (all-cause) mortality, and oxygen dependence (duration in days) assessed by S/F ratio (ie, SPO2/supplemental FiO2) and impact on viral replication (viral load). Additional secondary outcomes included inflammatory targets such as C reactive protein (CRP), lymphocyte counts and selected cytokines (initially focusing on IL2, IL6, TNF-α). The concept of long COVID emerged during the study, so we used the data from our questionnaires at 28 days and 90 days to comment on long COVID symptoms.
Statistical analyses
Sample size calculation
The primary outcome measure was a time-to-event analysis based on an assessment of TTCI. Since our study protocol was conceived and developed during the initial peak of the global pandemic, the precise HR for major clinical outcomes related to this infection was largely unknown, and therefore, sample size calculation was based on the proportion of patients expected to meet the outcome criteria by 28 days.14 Assuming α=0.05, β=0.20 and allocation ratio=1:1, the number of patients per treatment arm was estimated to be 74. We expected a 20% attrition rate, so the total number of patients required in the study was calculated to be 178, 89 patients in each arm.
Analysis population
The full analysis set was defined according to the intention-to-treat principle (ITT). All randomised subjects were included in the ITT analysis set for the primary outcome, regardless of whether they received any dose of their allocated treatment. This analysis set was used to summarise baseline patient characteristics and to carry out all efficacy and safety assessments. Subjects were analysed according to their randomised treatment allocation. We also present a modified ITT analysis for the primary and secondary outcomes, as a sensitivity analysis, to account for study participants who were randomised in error and those who withdrew consent prior to the intervention.
Primary outcomes
The TTCI data were estimated using Kaplan-Meier survival curves. HR and 95% CIs were estimated using Cox proportional hazards regression models. The primary analysis was stratified by the randomisation strata: baseline risk indicators (age </≥70 years, comorbidities) and NEWS2 score. Log rank test was used for comparing the Kaplan-Meir curves, HRs and their CIs for the significance of the treatment effect.
Secondary outcomes
Continuous secondary outcomes were evaluated for within-groups differences using the Mann-Whitney U or Wilcoxon rank tests, respectively, depending on the data distribution identified: parametric or non-parametric. Statistical normality was assessed using the Shapiro-Wilk method. Categorical outcomes were assessed for between-group differences using the χ2 method and expressed as %. For all outcomes, statistical significance was accepted at a two-sided α of 0.05.
Adverse events
AEs were coded using MedDRA and assigned grades based on National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.03.11
Public and patient involvement
Patient volunteers were consulted regarding the study design and materials to be provided to the potential participants (patient information sheet, consent forms, questionnaires). Two lay members were appointed to the Trial Steering Committee and provided input on the conduct of the study.
Results
Recruitment, randomisation, assignment of therapy and follow-up
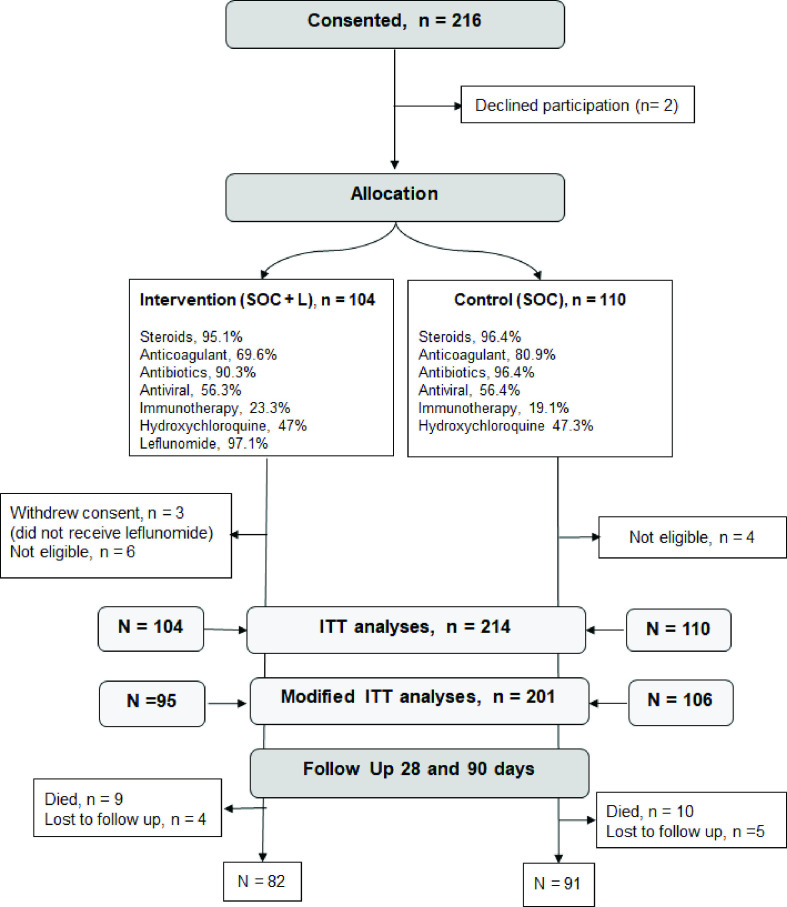
Between September 2020 and May 2021, 214 patients were recruited to the study from 2 UK Hospitals (n=66, 31%; Ashford and St Peters’ National Health Service (NHS) Trust, Surrey; Kingston Hospital NHS Trust, London) and 3 Hospitals in India; (n=148, 69%; Max Hospital, Delhi; Meditrina Institute, Nagpur; Noble Hospital, Pune). Due to the wavering new COVID-19 infections, the UK recruitment came to a halt in February 2021 and patients at the three Indian sites were recruited in the remaining period. Of the 214 participating patients, 104 were randomised to the intervention (SOC+L) group and 110 to the control (SOC) group. In the SOC+L group, three patients withdrew study consent after randomisation, and did not receive leflunomide therapy. During the data cleaning process, 10 patients were flagged as not meeting the inclusion criteria (6 in SOC+L; 4 in SOC), as they did not have moderate COVID-19 symptoms at the time of randomisation. Daily clinical data were collected for all patients during hospitalisation and the patients were asked to complete follow-up questionnaires at 28 days and 90 days after randomisation, as shown in figure 1.
Figure 1.
Randomisation, treatment assignment and follow-up of study participants. Immunotherapy included tocilizumab, bevacizumab and interferon alpha and beta. ITT, intention to treat; SOC, standard of care.
Baseline patient characteristics were similar between the SOC+L and SOC groups, summarised in table 1.
Table 1.
Baseline patient characteristics at the time of randomisation
| Characteristics | SOC+L n=104 | SOC n=110 |
| Age, years, mean±SD | 55.2±14.7 | 56.4±15.2 |
| BMI, kg/m2, mean±SD | 27.3±5.1 | 27.7±5.6 |
| Female gender at birth, % | 28.8 | 37.3 |
| Ethnicity, % | ||
| South Asian | 75 | 69 |
| White | 24 | 30 |
| Arab | – | 0.91 |
| Comorbidities, % | ||
| BMI≥40 kg/m2 | 2.9 | 4.6 |
| Age ≥70 years | 18.3 | 20 |
| Chronic respiratory disease | 8.7 | 15.5 |
| Chronic cardiovascular disease (including hypertension) | 38.5 | 39.1 |
| Chronic renal disease | 2.9 | 2.7 |
| Diabetes | 23.1 | 20.9 |
| Immunosuppressive diseases | 6.7 | 6.4 |
| Others | ||
| Malignant neoplasm | 3.9 | 2.7 |
| Chronic haematological disease | 1 | 0.9 |
| Chronic neurological disorder | 10.6 | 3.7 |
| Malnutrition | 1 | 0.9 |
| Smoking (present or past) | 21.1 | 20 |
| Symptom duration, day, median (IQR) | 6 (4–8) | 6 (5–8) |
| Time from admission, day, median (IQR) | 2 (1–4) | 2 (1–3) |
| Non-invasive ventilation, % | 4.8 | 7.3 |
| Invasive ventilation, % | 1 | 1.8 |
| NEWS 2 score median (IQR) | 6 (4–8) | 5 (4–8) |
| CRP, mg/L, median (IQR) | 28 (9–77) | 32 (13–64) |
| Transaminase, >ULN, % | ||
| ALT | 44.7 | 31.7 |
| AST | 35.4 | 28.4 |
| Stratification, % | ||
| Group 1 | 12.5 | 14.5 |
| Group 2 | 14.4 | 16.4 |
| Group 3 | 48.1 | 46.4 |
| Group 4 | 25 | 22.7 |
Group 1: high/moderate comorbidity risk with NEWS2 score ≥5; group 2: high/moderate comorbidity risk with NEWS2 score <5; group 3: low comorbidity risk with NEWS2 score ≥5 and group 4: low comorbidity risk with NEWS2 score <5. ULN values: ALT 49 U/L; AST 48 U/L; Immunosuppressive diseases: asplenia, rheumatological disorder.
ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; CRP, C reactive protein; NEWS2, National Early Warning Score 2; SOC, standard of care; ULN, upper limits of normal.
Baseline characteristics were similar in both arms but there were significantly more patients with chronic neurological disorders in the SOC+L group. None of the patients with this condition had contraindication to NIV.
Treatment assignment and compliance
Full course of leflunomide therapy was completed by 81/104 patients (78%). Of the 19 patients (16 in UK, 3 in India) who did not complete treatment, 3 patients did not receive a single dose of leflunomide as they withdrew consent soon after randomisation, 5 patients died prior to completion of the full course, 8 patients stopped leflunomide early when ALT/AST exceeded 3× ULN laboratory reference range, 1 patient had tocilizumab introduced to replace leflunomide, 1 patient self-discharged early and 1 refused final two doses. Leflunomide treatment compliance appeared to be better in participants from Indian centres as 92% of them received the full dose of leflunomide compared with 52% of patients in the UK centres which was largely due to a higher incidence of liver enzyme transaminitis and mortality observed in the UK cohort.
There was no significant difference in the assignment of SOC treatment between the SOC+L and SOC groups as shown in figure 1. It included corticosteroids, anticoagulants, antibiotics and antiviral therapies. Overall, steroid uptake was >95% in both treatment arms with different protocols used at participating study centres: dexamethasone 4 mg/day for 3 days; dexamethasone 6 mg/day for 7–10 days; methylprednisolone 80 mg/day for 7 days and methylprednisolone 120 mg/day for 5 days. However, there was no difference in the steroid treatment assigned between the control and the treatment groups. There were some differences in the proportions of patients receiving additional adjunct therapies such as hydroxychloroquine and immunotherapy (online supplemental table 1). Overall, hydroxychloroquine was prescribed to similar proportion of patients in the intervention and the control group (47%) but the proportions of patients receiving it in the UK was much smaller, 3% compared with 67% in India. A small number of patients received immunomodulating drugs such as interferon alpha and beta (n=20 in India), tocilizumab and bevacizumab (n=5 in the UK, n=2 in India).
bmjopen-2022-068179supp002.pdf (30.5KB, pdf)
Primary outcomes
TTCI of two points on a clinical status scale/discharge before 28 days
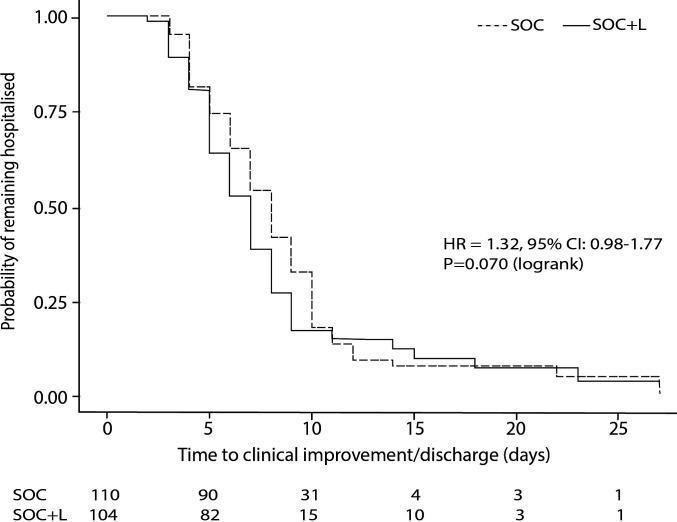
In the ITT analyses (n=214), SOC+L group did not have a significantly shorter TTCI than the SOC group within 28 days of randomisation; the median was 7.0 (IQR 7.0–8.0) days vs 8.0 (IQR 7.0–9.0) days, respectively; with an HR of 1.32 (95% CI 0.98 to 1.77), p=0.070 (figure 2).
Figure 2.
Time to clinical improvement of two points on a clinical status scale or discharge prior 28 days in a stratified ITT analysis (primary outcome). Patients who died were censored at the time their death occurred, while all surviving patients who did not reach TTCI criteria by day 28 were right censored at that point. Most of the patients were discharged within the first 10 days of admission. ITT, intention to treat; SOC, standard of care; TTCI, time to clinical improvement.
In modified ITT population (n=201) where 3 patients who withdrew consent after being randomised to the SOC+L group but never received leflunomide treatment and 10 patients who did not fulfil moderate COVID-19 symptoms at randomisation were excluded from analysis, the median TTCI was significantly shorter in the SOC+L group than SOC group by 1.0 day, median 7.0 days (IQR 7.0 −8.0) vs 8.0 (IQR 7.0–9.0), respectively, with an HR of 1.42 (95% CI 1.04 to 1.94); p=0.028.
Safety
Incidences of AE of all grades are summarised in table 2.
Table 2.
Incidence of reported adverse events in both treatment arms
| Adverse events | SOC+L (n=104) | SOC (n=110) |
| Adverse events (n)/patients (n) | 121/56 | 91/42 |
| Grade 1 (mild) | 58/39 | 48/32 |
| Grade 2 (moderate) | 23/13 | 17/9 |
| Grade 3 (severe)/grade 4 (life-threatening events) | 31/15 | 16/9 |
| Grade 5 (deaths) | 9/9 | 10/10 |
SOC, standard of care.
The table shows the number of AEs recorded in the study and the number of patients affected by at least one AE.
At least one AE was reported in 98/214 participants, and most of them were mild in severity. AEs of moderate grade were reported in 13/104 patients in SOC+L group and 9/110 patients in SOC group. Serious AEs (n=47) were reported in 15/104 patients in SOC+L groups and 9/110 in SOC group and 19 patients died (9 in SOC+L group, 10 in the SOC group). There was no significant difference in the incidence of AE reported between the two groups. No Serious AEs were attributed to leflunomide. A online supplemental table 2 lists all AEs recorded in the study according to MedDRA terms.
bmjopen-2022-068179supp003.pdf (117.7KB, pdf)
Liver function
At baseline, more patients with greater than ULN levels of ALT and AST were randomised in the SOC+L group than the SOC group (ALT: 46 vs 33, p=0.049; AST: 31 vs 24, p=0.340). By day 3/4, following the initial loading of leflunomide therapy in the SOC+L group, there was a significantly higher number of patients with greater than ULN level of ALT and AST in the SOC+L than the SOC group (64 vs 38, p<0.001; and 51 vs 24, p<0.001). By discharge, the difference in the number of patients with ALT and AST transaminitis between the SOC+L and SOC groups was no longer significant (28 vs 27, p=0.633; and 20 vs 17, p=0.318) (online supplemental table 3). Leflunomide therapy was terminated early if transaminase levels exceeded 3× ULN. However, there were five patients in India who continued with leflunomide therapy at the discretion of the researcher and direct care team with close monitoring of their liver function. Interestingly, in this subset of patients, the transaminase levels improved despite continuation of therapy. There were no AEs related to clinically significant liver injury due to leflunomide. AEs related to liver dysfunction were reported in 16/104 (15.4%) patients in SOC+L group, 7 were mild, 8 were moderate and 1 was severe. Of these, 10 were deemed possibly treatment related and leflunomide treatment was discontinued in 9 patients. Comparatively, in the control group, 6/110 (5.5%) patients had liver dysfunction related AE. Five of them were mild and one case was severe.
bmjopen-2022-068179supp004.pdf (68.8KB, pdf)
Secondary outcomes
A modified ITT approach was used for data from 201 patients for all secondary outcomes. This included 95 patients in the SOC+L group and 106 patients in SOC group. For these analyses, we excluded 3 patients in the SOC+L group who withdrew consent and never received leflunomide and 10 patients (6 SOC+L; 4 SOC) who did not fulfil moderate COVID symptoms inclusion criterion (did not show respiratory compromise and blood SpO2<93% on room air).
Mortality
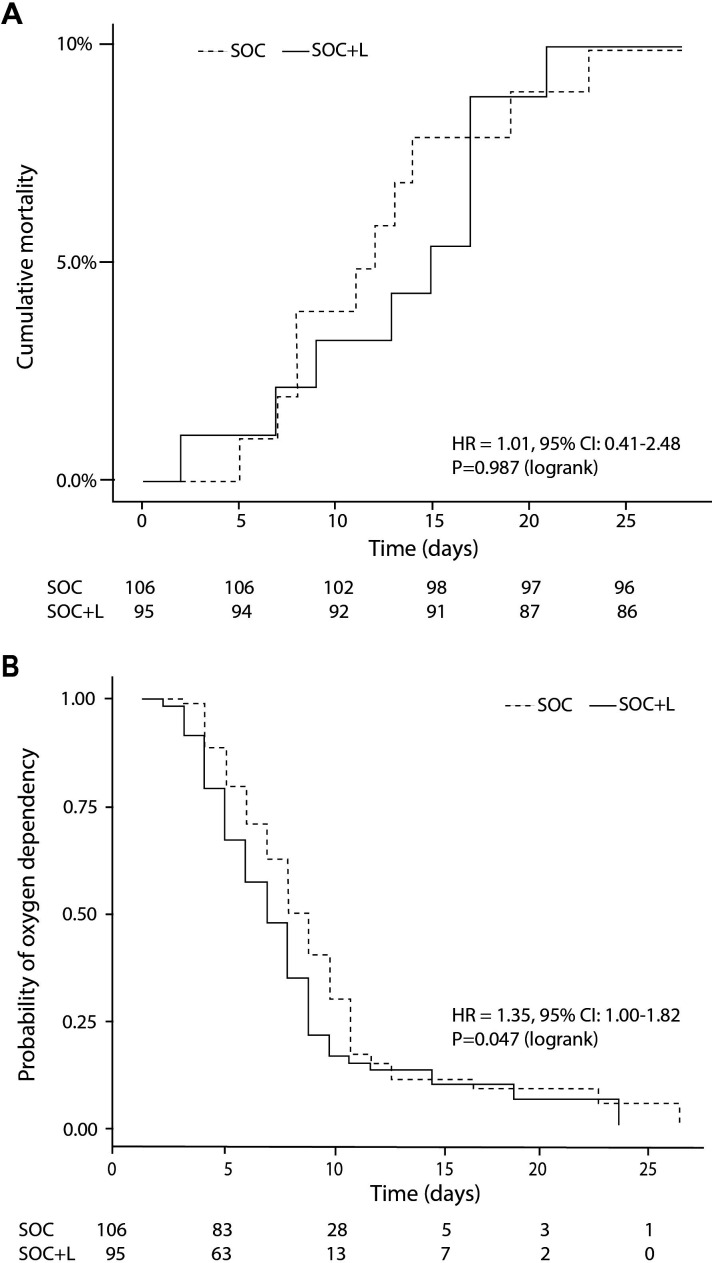
There was no difference in all-cause mortality within 28 days of randomisation between the treatment arms as 9/95 (9.47%) of patients died in SOC+L group compared with 10/106 (9.43%) in SOC groups. The survival curves diverge in favour of the SOC+L group after 10 days of hospital treatment, but the curves converged again after 3 weeks (when majority of the patients have been discharged). All deaths were attributed to complications related to COVID-19 (figure 3, panel A).
Figure 3.
Cumulative all-cause mortality (A) oxygen dependence (B) by 28 days. SOC, standard of care.
Oxygenation and assisted ventilation
Oxygen independence is defined by maintenance of SpO2/FiO2 Air ratio >4.43. There was a difference in the median time the participants required to be completely weaned off oxygen therapy between groups; 6.0 (IQR 4.0–8.0) days in the SOC+L group vs 7.0 (5.0–10.0) days in the SOC group, p=0.047 (figure 3, panel B).
NIV was required for 14.4% of patients in SOC+L group vs 16.4% in the SOC group. The duration of NIV was 6.0 (IQR 2.0–9.0) days in the SOC+L group compared with 4.5 days (IQR 2.3–6.8) in the SOC group. Similar proportion of patients required NIV at the time of study enrolment (4.8% in SOC+L group vs 7.3% in SOC group, p=0.45).
The proportion of patients admitted to level-2 intensive care unit (ICU) was 8.7% in the SOC+L group and 8.2% in the SOC group. The median time spent at ICU was 8.0 (IQR 5.0–10.0) days vs 9.0 (IQR 5.0–13.0) days, respectively. Invasive ventilation was required for 3.9% of patients in the SOC+L group and 5.5% in the SOC group with median duration of 6 (IQR 4.8–6.0) days vs 7.0 (IQR 5.3–11.8) days, respectively. None of the between group comparisons were statistically significant. Patients recruited in India were significantly less likely to require invasive or NIV or be admitted to ICU compared with patients recruited in the UK (p<0.001).
Viral load
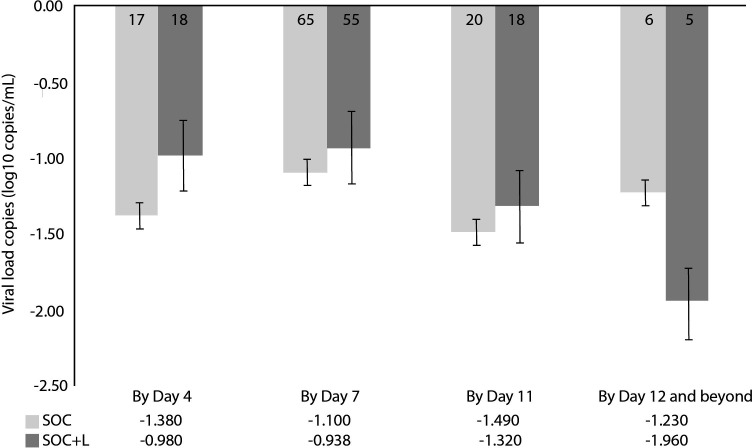
Quantitative SARS-COV-2 PCR measurements from nasopharyngeal swabs at baseline showed no difference in median log10 viral loads (copies/mL) between the two groups, SOC+L 4.68 (IQR 4.45–4.85) vs SOC 4.76 (IQR 4.48–4.92), p=027. We clustered the serial samples to reflect the crucial time intervals during the hospital stay: time coinciding with finishing leflunomide loading dose (by day 4), time to 75% patients being discharged from hospital (by day 7), time to finishing leflunomide maintenance dose (by day 11) and beyond (figure 4). Viral loads were significantly reduced in both treatment arms. There was no significant difference in the overall rate of the viral load clearance between the two groups by day 11 and beyond. Viral loads were significantly reduced in both treatment arms by day 7, p<0.001; and by day 11, p<0.030. The rate of viral load reduction between groups by day 11 appeared to be similar.
Figure 4.
Mean changes in log10 viral load (copies/mL) from baseline. Error bars represent SE. Numbers in the bars represent the number of samples available for measurements. SOC, standard of care.
Cytokines, CRP and lymphocytes
Cytokine levels were assessed separately for UK and Indian sites as two laboratories using different assays processed the samples. The median baseline levels of IL 2, IL 6 and TNF-α levels (UK: IL2 0.43 (IQR 0.30–0.62) pg/mL; IL6 6.2 (IQR 2.9–9.7) pg/mL; TNF-α 10.1 (IQR 7.8–13.5) pg/mL; India: IL2 4.3 (IQR 2.8–5.8) pg/mL; IL6 12.6 (IQR 6.5–43.1) pg/mL; TNF-α 6.1 (IQR 4.9–7.1) pg/mL) were not significantly raised from normal reference ranges and were not different between treatment groups in both countries. The cytokine levels were reduced during hospitalisation, though the clinical significance of these changes within the normal range is uncertain. There was no significant difference in the trends observed between treatment arms.
The median baseline levels of CRP were similar in both groups, 28 (IQR 8–71) in SOC+L vs 34 (14–71) mg/L in SOC. By 1 week of treatment, there were similar levels of reduction between groups.
The median baseline lymphocytes levels were lower than normal reference range in both groups (0.99 (IQR 0.6–1.6)×109/L in SOC+L vs 0.95 (IQR 0.6–1.6)×109/L in SOC. By 1 week of treatment, levels rose to normal range in both groups. There was no significant difference in the trends observed between groups.
Twenty-eight days and 90 days follow-up
At 28 days, 59/81 patients (71.2%) in the SOC+L group and 60/91 patients (65.9%) in the SOC group experienced at least 1 of 9 common long-COVID symptoms (fatigue, cough, anxiety, chest pain, brain fog, breathlessness, disturbed sleep, palpitations, joint pain); with sleep quality (48.2% vs 38.5%), breathlessness (40.7% vs 42.9%), joint pain (32.1% vs 33%), fatigue (29.6% vs 31.9%) and anxiety (24.7% vs 19.8%) being the most common symptoms experienced (online supplemental table 4). At 90 days, there was a reduction in overall prevalence of symptoms as 42/81 patients (51.2%) in the SOC+L group and 37/91 (40.7%) patients in the SOC group and any of the residual symptoms were of reduced severity. There was no significant difference in these outcomes between the treatment arms.
bmjopen-2022-068179supp005.pdf (40.2KB, pdf)
Myalgia symptoms were comparably reduced between the two groups at 90 days. Anosmia and loss of taste were still reported by two and seven patients, respectively, in the SOC+L group, but none in the SOC group.
At 28 days, 41.5% patients in the SOC+L group and 52.8% in SOC group, reported being moderately to severely dyspnoeic (grade 4: stops for breath after walking 100 m; grade 5: too breathless to leave the house or breathless when dressing). These proportions were further reduced at 90 days, to 22% in the SOC+L group compared with 19.8% in SOC group. These differences were not significant in between group comparisons.
Mental health issues were highlighted by reports of feeling depressed and losing interest in doing things. Comparable proportions of patients in the SOC+L group and SOC group reported those problems at 28 days (17.9% vs 16.0%; 11.6% vs 14.2%, respectively) which were further reduced in both groups at 90 days (11.6% vs 9.4%; 9.5% vs 6.6%, respectively).
At 28 days participants in the SOC+L group scored their current health as being 80%±25% of the usual which increased to 89%±17% at 90 days. In the SOC group, the scores were similar, 82%±23% and 90%±17% at 28 and 90 days, respectively.
Discussion
This study is the first prospective, multicentre, randomised, controlled clinical trial investigating the clinical efficacy and safety of leflunomide in treating acute COVID-19 infection. The study showed that a course of leflunomide (3 days of 100 mg/day loading dose followed by 7 days of 20 mg/day maintenance dose) added to the standard care treatment (steroids, anticoagulants, antibiotics and antiviral therapy), did not influence the primary outcome of the trial and the acute clinical outcomes at 28 days, or the prevalence of long-COVID symptoms at 28 and 90 days. However, participants who received leflunomide as an adjunct therapy were weaned off oxygen earlier, which translated to reduced hospital stay by 1 day. The medication appeared to be safe and well tolerated with no severe AEs attributable to it. A small proportion of patients in our study were still burdened by COVID-19-related symptoms 90 days after randomisation.
This multicentre trial advances the evidence base on the impact of leflunomide, a repurposed RA medication, on COVID-19 infection. Leflunomide was a potentially attractive therapeutic choice from early preclinical and clinical experience reported from hospitals in Wuhan, China. DHODH, located in the inner mitochondrial membrane is a rate-limiting enzyme in de novo pyrimidine biosynthesis. In virus-infected cells, a large intracellular nucleotide pool is consumed by rapid viral replication. RNA viruses need unique UMP but not TMP in their genomes. As UMP is the particular nucleoside produced by DHODH, RNA viruses are sensitive to reduced DHODH activity. Preclinical models of cell and animal infection by SARS-CoV-2 demonstrated that leflunomide attenuates viral genome replication, suppresses inflammatory response and the release of proinflammatory cytokines and chemokines.7–9 Early reports from China advocated major clinical benefits in patients treated with leflunomide both in terms of less severe outcomes and duration of infection.15–17 While the current study did not reproduce these overall benefits in the ITT analysis regarding the primary outcome, it confirmed some positive effects in those patients who received the trial intervention (in modified ITT analysis).
Our results are likely explained by the changing landscape and evolution of the routine COVID-19 treatment protocols in the standard arm of the study and the resultant severity of the COVID-19 outcomes in general. The initial phase of the COVID-19 pandemic was characterised by severe respiratory and systemic infections and poor outcomes due to the development of acute respiratory distress syndrome, multiorgan failure and eventual death.18 19 Contrary to this early experience with COVID-19 management, the in-hospital mortality in this study was much lower, less than 10% in both groups. The majority of patients in both treatment arms improved during hospitalisation and were discharged within a week of admission. Inclusion of prognostically significant COVID-19 therapies in both pharmacological and non-pharmacological SOC treatments undoubtedly contributed to a reduction in severe complications and better overall outcomes. During patient recruitment of the current trial, various therapies have been introduced, including steroids, which were received by more than 95% of the study population as SOC.
Theoretical considerations suggest that leflunomide may effectively inhibit viral replication. The initial pilot study during the early outbreak of COVID-19 in Wuhan, China reported reduced viral shedding time following leflunomide treatment during acute infection compared with the SOC therapy.15 Similarly, viral shedding duration was reduced in leflunomide treated patients who remained qPCR positive 1 month after the initial infection.16 Our study addressed the viral load reduction at prespecified time points. Values of viral load were reduced over time but there was no difference between the treatment arms. Both methodological considerations and the inclusion of comprehensive pharmacological treatment regimens in the SOC could explain these differences. For instance, corticosteroid therapy was absent in the early study from Wuhan, but the later study refers to the use of hydroxychloroquine, interferon-alpha and antiviral medications as part of acute SOC therapy.15 16 However, our results are in line with other reports from China which showed that duration of viral shedding was not affected by leflunomide added to nebulised interferon alpha therapy for treating long-term positive COVID-19 after 4 weeks of in-hospital treatment.17 Interestingly, one-third of these patients received corticosteroid therapy during the initial acute treatment.16 17
Beyond the issue of therapeutic efficacy and viral load, our study confirms overall safety of leflunomide in COVID-19 infection. The safety profile of leflunomide is well established in the treatment of RA.6 When repurposed for the COVID-19 treatment, it was well tolerated since no serious AEs were attributed to it. Similar findings were reported in other studies.15 16 20 Mild transaminitis following long-term leflunomide use in the RA population is recognised, and usually resolves after medication is terminated. The mechanism is likely to be modulation of interleukins which may hinder the protection of hepatocytes from injury rather than direct toxicity.21 There were comparable incidences of transaminitis in both treatment arms in our study. However, more patients in the UK cohort had raised liver function tests leading to modification or termination of leflunomide treatment. This may be accounted for by the difference in the severity of COVID-19 disease and spectrum of comorbidities between UK and Indian participants rather than genetic polymorphism in drug metabolism. Overall, the proposed leflunomide regimen was well tolerated.
One of the motivations of the current trial employing leflunomide was to benefit from the anti-inflammatory effects of this drug. In this context, hydrocortisone has been demonstrated as an effective therapy in severe COVID-19 infections and recent trials also demonstrated the benefit of tocilizumab, a selective IL-6 inhibitor and a different disease modifying RA medication. However, such finding is not universal as the benefit of tocilizumab is mainly demonstrated in critically to moderately ill patients.22 23 A recent meta-analysis showed that the benefit of IL-6 receptor antagonist was encountered only in patients who were also treated with glucocorticoids.24 This is in keeping with observations that a broader spectrum of proinflammatory cytokines, macrophages and T cell response have all been documented in severely ill patients demonstrating the role of a more complex inflammatory response. It is exactly this broader inflammatory reaction that could be targeted by leflunomide as its effect on cytokines is not restricted to IL 6 and it may also have an impact on activated T cell response.8 9 25 Such phenomena might contribute to the benefits of reduced oxygen dependence in patients who have received leflunomide treatment. However, it is conceivable that the full benefit of such anti-inflammatory effect may be more pronounced in severely ill patients, but this population was under-represented in our trial and the (inadvertent) inclusion of patients with milder symptoms may have led to some attrition of statistical power in our study. A more detailed analysis of the cytokine and metabolic profiles of our trial population is underway to clarify these important issues.
Another important consideration when discussing the potential benefits of leflunomide is the mutation ability of the SARS-CoV-2 virus.26 So far, the mutations observed in different strains worldwide have largely been confined to the part of the spike protein affecting the virus’s ability of cell entry as opposed to a region targeted by neutralising antibodies. However, the possibility of mutations in different regions cannot be excluded. Targeting the host’s pyrimidine biosynthesis pathway by leflunomide, rather than using drugs with direct antiviral action, remains an advantage offering protection against a broader spectrum of viruses and potentially overcoming resistance. Indeed, DHODH inhibitors such as leflunomide have shown broad-spectrum antiviral effects against various RNA viruses in cell models.7 Leflunomide may, therefore, be considered a viable pharmacological treatment for COVID-19 patients given it is well tolerated, safe, economical and widely available. Its clinical effectiveness measured against recognised selective IL-6 inhibitors in the more severely/critically ill patients needs to be further explored as leflunomide may be the preferred option in countries where other immunomodulating agents, such as talizumab, may not be practical or widely available.
Limitations
This study has several limitations. In order to balance the needs of the trial with clinical care and to minimise disruption to already overstretched clinical resources during COVID-19 pandemic, we chose to adopt an open-label study design. This design may have affected the data collection and clinical management of the patients and potentially introduced a bias. However, it also allowed early detection of significant AEs and a potential outcome benefit. This was an important consideration when testing an off-label use of a medication in COVID-19, a disease with high morbidity and mortality.
The study was set out to recruit more severely and critically affected patients in a single country. However, due to recruitment restrictions because of national prioritisation of critically ill patients to only a few studies together with scarcity of NHS resources during the pandemic, the study was extended abroad, ultimately recruiting less affected patients with heterogeneous clinical profiles. Although patient characteristics and medications received as part of SOC did not differ between the randomised arms, the more heterogeneous population, milder COVID-19 disease and more effective SOC treatments most likely impacted on the hypothesised effect size and the ability of finding a difference in our recruited sample. Finally, the COVID-19 restrictions affected our protocolised laboratory investigations, such as the serial viral load and comprehensive inflammatory profiling. Nevertheless, studies focusing on the more severely affected participants are underway and will be the subject of a separate submission.
Secondary outcomes assessing organ and multiorgan endpoints set out in the original protocol were not reported because the data are incomplete for meaningful analyses.
Conclusion
Leflunomide had no major impact on the clinical outcomes when administered together with the currently established but evolving therapies in moderately affected COVID-19 patients. It may shorten duration of oxygen dependence thereby affecting the TTCI and hospital discharge. Transaminitis associated with leflunomide therapy did not lead to excess AEs compared with the control group and may have arisen in part due to the severity of clinical infection. Further studies are needed to investigate the potential benefits of leflunomide in the critically ill patients and the biological mechanisms involved.
Supplementary Material
Acknowledgments
We are grateful to all the patients who took part in the study and contributed their data to our research. We also thank their clinical care teams who helped with data collection. We are grateful for the support from medical research charity LifeArc as part of its initiatives to address the need for new therapies against COVID-19 against the backdrop of world pandemic. We would like to acknowledge the vital roles of the following: data monitoring committee: David Morgan (Chair), Lajos Szentgyorgyi, Yee Cheah. Trial Steering Committee: Brendan Madden (Chair), Patrick Yong, Sreenivasa Rao Kondapally Seshasai, Matt Mendes, Janice Rodrigues-Mendes, Rod Hughes, Sharanpal Jeetle, Subash Somalanka. Clinical Research Organisation: Innovate Research CRO in India helped with site and patient recruitment, project management, monitoring and data collation: Devesh Kumar (Director), Piyush Kumar Pandey (Head Clinical Operations), Shaitan Singh (Assistant Project Manager), Charanpreet Arora (Clinical research associate).
Footnotes
Collaborators: DEFEAT COVID Investigators: Shashank Sharma, Megan McGee, Kieran Brack, Maia Aquino, Rita Pereira, Vicky Frost, Kirsty, Gibson, Maria Croft, Fatima Omar, Kapila Ranasinghe (Ashford and St Peter’s Hospital NHS Foundation Trust, Ashford, UK). Siva Mahendran, Anna Joseph, Maggie Grout (Kingston Hospital NHS Foundation Trust, Kingston upon Thames, UK). Arun Dewan, Ritesh Aggarwal (Max Hospital, New Delhi India). Ajay Bulle (Meditrina Institute, Nagpur, India). Aparna Kodre (Noble Hospital, Pune, India).
Contributors: All designated authors meet all four ICMJE criteria for authorship. ZC, NM, HLL, SRKS, LL, JDB, SL, IJ, DF and PS made substantial contribution to the conception and design of the study. SS, AK, AB, RA, KB, MM, KR, FO, KL, AW, AL, SRKS, JDB, HLL, NM, IK-H and ZC made substantial contribution to the acquisition, analysis and interpretation of the data for the study. All authors contributed to the drafting, revision and final approval of the manuscript. ZC, NM and LL are responsible for the overall content and are the guarantors of the study.
Funding: This work was supported by LifeArc, a UK registered charity number 1015243 (grant number not applicable).
Disclaimer: The funders and the sponsor of the study had no role in the analyses or the interpretation of the results.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
DEFEAT-COVID Investigators:
Shashank Sharma, Megan McGee, Kieran Brack, Maia Aquino, Rita Pereira, Vicky Frost, Kirsty Gibson, Maria Croft, Fatima Omar, Kapila Ranasinghe, Siva Mahendran, Anna Joseph, Maggie Grout, Arun Dewan, Ritesh Aggarwal, Ajay Bulle, and Aparna Kodre
Data availability statement
Data are available on reasonable request. The anonymised data may be made available on request following approval from the Trial Management Group and the Sponsor.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by South Central-Berkshire Research Ethics Committee, Bristol REC Centre, reference number 20/SC/02642), Max HealthCare Ethics Committee, reference number RS/MSSSH/GMHRCCMS/MHEC/CCM/20-233), Meditrina Institute Ethics Committee, reference number ECR/605/Inst/MH/2014/RR4), Noble Hospital Institutional Ethics Committee, reference number NHIEC/FEB/2021/238). Participants gave informed consent to participate in the study before taking part.
References
- 1. WHO . Available: https://COVID19.who.int/
- 2. Chen LYC, Quach TTT. COVID-19 cytokine storm syndrome: a threshold concept. Lancet Microbe 2021;2:e49–50. 10.1016/S2666-5247(20)30223-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, et al. Emerging treatment strategies for COVID-19 infection. Clin Exp Med 2021;21:167–79. 10.1007/s10238-020-00671-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dwek RA, Butters TD, Platt FM, et al. Targeting glycosylation as a therapeutic approach. Nat Rev Drug Discov 2002;1:65–75. 10.1038/nrd708 [DOI] [PubMed] [Google Scholar]
- 5. Angus DC, Berry S, Lewis RJ, et al. The REMAP-CAP (randomized embedded multifactorial adaptive platform for community-acquired pneumonia) study. rationale and design. Ann Am Thorac Soc 2020;17:879–91. 10.1513/AnnalsATS.202003-192SD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alfaro-Lara R, Espinosa-Ortega HF, Arce-Salinas CA, et al. Systematic review and meta-analysis of the efficacy and safety of leflunomide and methotrexate in the treatment of rheumatoid arthritis. Reumatol Clin (Engl Ed) 2019;15:133–9. 10.1016/j.reuma.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 7. Xiong R, Zhang L, Li S, et al. Novel and potent inhibitors targeting DHODH are broad-spectrum antivirals against RNA viruses including newly-emerged coronavirus SARS-CoV-2. Protein Cell 2020;11:723–39. 10.1007/s13238-020-00768-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yao HW, Li J, Chen JQ, et al. A 771726, the active metabolite of leflunomide, inhibits TNF-alpha and IL-1 from Kupffer cells. Inflammation 2004;28:97–103. 10.1023/b:ifla.0000033025.73363.d3 [DOI] [PubMed] [Google Scholar]
- 9. Li L, Liu J, Delohery T, et al. The effects of teriflunomide on lymphocyte subpopulations in human peripheral blood mononuclear cells in vitro. J Neuroimmunol 2013;265:82–90. 10.1016/j.jneuroim.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 10. Royal College of Physicians . Available: https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2
- 11. Cancer Therapy Evaluation Program . Available: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_60
- 12. Mandal S, Barnett J, Brill SE, et al. “Long-COVID”: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021;76:396–8. 10.1136/thoraxjnl-2020-215818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. WHO . Available: http://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/
- 14. Machin D, Campbell M, Fayers P, et al. Sample size tables for clinical studies, 2nd ed. In: Blackwell Science. 1997: 176–7. [Google Scholar]
- 15. Hu K, Wang M, Zhao Y, et al. A small-scale medication of leflunomide as a treatment of COVID-19 in an open-label blank-controlled clinical trial. Virol Sin 2020;35:725–33. 10.1007/s12250-020-00258-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Q, Guo H, Li Y, et al. Efficacy and safety of leflunomide for refractory COVID-19: a pilot study. Front Pharmacol 2021;12:581833. 10.3389/fphar.2021.581833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang M, Zhao Y, Hu W, et al. Treatment of coronavirus disease 2019 patients with prolonged postsymptomatic viral shedding with leflunomide: a single-center randomized controlled clinical trial. Clin Infect Dis 2021;73:e4012–9. 10.1093/cid/ciaa1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Group ICC. COVID-19 symptoms at hospital admission vary with age and sex: ISARIC multinational study. MedRxiv 2020:2020.10.26.20219519. 10.1101/2020.10.26.20219519 [DOI] [Google Scholar]
- 20. Chakravarty EF, Sanchez-Yamamoto D, Bush TM. The use of disease modifying antirheumatic drugs in women with rheumatoid arthritis of childbearing age: a survey of practice patterns and pregnancy outcomes. J Rheumatol 2003;30:241–6. [PubMed] [Google Scholar]
- 21. Ogata A, Kato Y, Higa S, et al. Il-6 inhibitor for the treatment of rheumatoid arthritis: a comprehensive review. Mod Rheumatol 2019;29:258–67. 10.1080/14397595.2018.1546357 [DOI] [PubMed] [Google Scholar]
- 22. Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med 2021;384:1491–502. 10.1056/NEJMoa2100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abani O, Abbas A, Abbas F. Tocilizumab in patients admitted to hospital with COVID-19 (recovery): a randomised, controlled, open-label, platform trial. Lancet 2021;397:1637–45. 10.1016/S0140-6736(21)00676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020;324:1330–41. 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kraan MC, Smeets TJM, van Loon MJ, et al. Differential effects of leflunomide and methotrexate on cytokine production in rheumatoid arthritis. Ann Rheum Dis 2004;63:1056–61. 10.1136/ard.2003.014738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang S, Xu X, Wei C, et al. Molecular evolutionary characteristics of SARS-CoV-2 emerging in the United States. J Med Virol 2022;94:310–7. 10.1002/jmv.27331 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-068179supp001.pdf (113.1KB, pdf)
bmjopen-2022-068179supp002.pdf (30.5KB, pdf)
bmjopen-2022-068179supp003.pdf (117.7KB, pdf)
bmjopen-2022-068179supp004.pdf (68.8KB, pdf)
bmjopen-2022-068179supp005.pdf (40.2KB, pdf)
Data Availability Statement
Data are available on reasonable request. The anonymised data may be made available on request following approval from the Trial Management Group and the Sponsor.