Abstract
Objectives
Late presentation and delays in diagnosis and treatment consistently translate into poor outcomes in sub-Saharan Africa (SSA). The aim of this study was to collate and appraise the factors influencing diagnostic and treatment delays of adult solid tumours in SSA.
Design
Systematic review with assessment of bias using Risk of Bias in Non-randomised Studies of Exposures (ROBINS-E) tool.
Data sources
PubMed and Embase, for publications from January 1995 to March 2021.
Eligibility criteria
Inclusion criteria: quantitative or mixed-method research, publications in English, on solid cancers in SSA countries. Exclusion criteria: paediatric populations, haematologic malignancies, and assessments of public perceptions and awareness of cancer (since the focus was on patients with a cancer diagnosis and treatment pathways).
Data extraction and synthesis
Two reviewers extracted and validated the studies. Data included year of publication; country; demographic characteristics; country-level setting; disease subsite; study design; type of delay, reasons for delay and primary outcomes.
Results
57 out of 193 full-text reviews were included. 40% were from Nigeria or Ethiopia. 70% focused on breast or cervical cancer. 43 studies had a high risk of bias at preliminary stages of quality assessment. 14 studies met the criteria for full assessment and all totaled to either high or very high risk of bias across seven domains. Reasons for delays included high costs of diagnostic and treatment services; lack of coordination between primary, secondary and tertiary healthcare sectors; inadequate staffing; and continued reliance on traditional healers and complimentary medicines.
Conclusions
Robust research to inform policy on the barriers to quality cancer care in SSA is absent. The focus of most research is on breast and cervical cancers. Research outputs are from few countries. It is imperative that we investigate the complex interaction of these factors to build resilient and effective cancer control programmes.
Keywords: adult oncology, urological tumours, public health, health policy
Strengths and limitations of this study.
The study interrogated two layers of factors (context and delays) by considering the ‘Three Delays’ framework.
We used the Risk of Bias in Non-randomised Studies of Exposures (ROBINS-E) tool to evaluate the quality of studies.
We reduced heterogeneity by focusing on solid tumours, excluding awareness studies and restricting the timeframe to allow for applicability of findings to the evolving healthcare systems with time.
The quality of the studies included was largely poor; however, rigorous assessment of risk of bias across seven domains allowed deduction of key study findings that are a useful steppingstone for further investigation.
Introduction
The cancer control agenda has globally received a high level of political recognition.1 2 In sub-Saharan Africa (SSA), with an age standardised incidence and mortality rate of 128.2 and 87.2 per 100 000 people respectively, cancer is becoming a leading public health problem.3 There is growing emphasis that the successful translation of commitments to support cancer control policy into substantial reductions in cancer morbidity and mortality must occur on a locally adapted evidence-based platform but robust local research is lacking in contrast with developed nations.
Countries in SSA operate in an environment of low resources, which has resulted in cancer management largely focusing on those presenting with overt symptomatic disease.4 5 The system-level challenges are heterogenous across SSA but factors germane to all countries include limited healthcare financing, inadequate financial protection (universal health coverage), inadequate infrastructure development as well as the need for health systems to manage a dual burden of infectious disease and growing non-communicable diseases.5–8
The lack of coordination and fragmented pathways in cancer care at all stages including prevention, symptom awareness, diagnosis, treatment and post-treatment care makes cancer hard to manage in developing nations and ultimately result in high levels of premature mortality.9 Interventions occur in silos within three distinct groups: (1) across specific cancer types which are prioritised;10 (2) across prevention, treatment and palliation;11 (3) across primary, secondary and tertiary healthcare sectors.12 Additionally, building strong system linkages to coordinate cancer care across primary, secondary and tertiary sectors within country are generally overlooked and this results in critical delays.9
Fragmented pathways of care and research priorities are also reflective of the dependence on external international financial donors which tend to support their own specific agendas perpetuating silos of development.13 14 This approach can be considered reductionist as it fails to consider the system and structural drivers of inequalities in access to diagnosis and treatment.
Evaluation of the unique social, economic, geographic and cultural determinants for late diagnosis and poor treatment outcomes are imperative to provide locally generated evidence. This will ensure the effective implementation of national cancer control programmes.15 16 These factors are not just context specific (eg, country, region) but also tumour specific. An array of factors including accessibility to care (distance and cost), quality of care, coordination of care across healthcare sectors, education and training, as well as intricate personal and community relationships (values, beliefs, socioeconomic parameters, gender) need to be interpreted in each situation and considered explicitly.
Empirical work has sought to identify the factors influencing cancer diagnosis and treatment delay.17 However, to our knowledge there have been no attempts to synthesise the available evidence from primary quantitative research undertaken in the SSA context to inform cancer control policies and identify gaps in the current research literature. Gaps would include country settings, tumour types, or at-risk populations which remain under-researched. In addition, robust study designs need to be employed to help compare results between studies and provide further insights as part of the system evaluation.
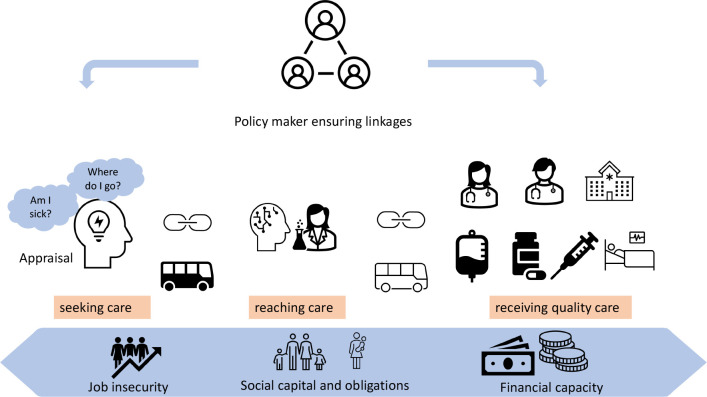
In this review we used the ‘Three Delays’ framework to support the synthesis and classification of research studies focusing on barriers to diagnosis and treatment. The Three Delays framework has been used in other health conditions, for example, child and maternal health, emergency medicine however, to date it has not been applied to cancer care delivery.18 19 The framework considers three contexts and Three Delays. The three contexts are the: patient context (perceptions of disease, barriers to care, cost of illness); provider context (care process quality and outcome evaluation, healthcare workers perceived system barriers); community context (proximity and physical accessibility of services in the community). The Three Delays are seeking care, reaching care and receiving quality care.20 Delay 1 seeking care: this is the delay in recognising illness and deciding to seek appropriate medical help outside the home. Delay 2 reaching care: this is the delay in reaching an appropriate health facility. Delay 3 receiving quality care: this is the delay in receiving quality care after reaching the health facility. The interconnection in the delays can be seen in figure 1.
Figure 1.
Three Delays framework.
The aim of this investigation was to identify common factors influencing diagnostic delays of adult solid tumours and highlight areas that require further study whether that be specific countries, tumour types or settings, in order to help target resources and inform interventions that reduce cancer survivorship disparities globally.
Methods
Study design
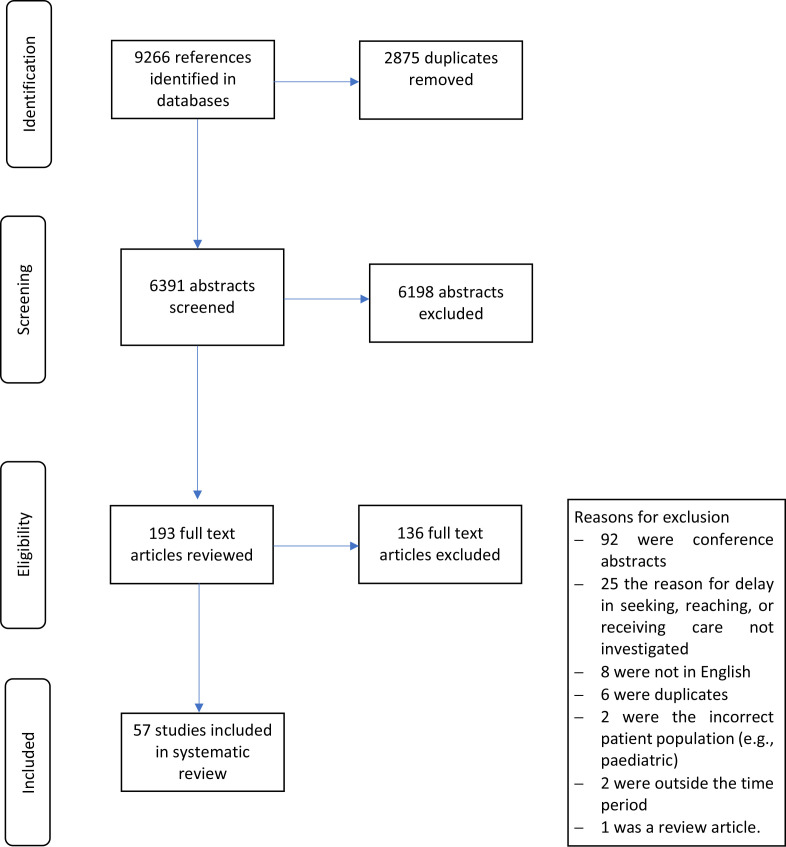
We undertook a systematic review and the findings are reported according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. The study selection flowchart diagram is presented in figure 2.
Figure 2.
Flowchart of study selection.
Search strategy
The literature search was conducted on eighth March 2021 in PubMed and Embase for articles published between January 1995 and March 2021. We restricted the timeframe to allow for relevance and applicability of findings to the evolving healthcare systems with time. The full search strategy is in the online supplemental appendix 1
bmjopen-2022-067715supp001.pdf (27.8KB, pdf)
Eligibility criteria
The study included published articles in the English language that focused on solid cancers. The primary research was focused on SSA countries. Types of studies included quantitative (surveys, observational studies) or studies using mixed-methods research methodologies. The quantitative studies had to include patients who had received a diagnosis of cancer. We excluded studies that included paediatric populations, haematologic malignancies, as well assessments of public perceptions and awareness of cancer since the focus was on patients with a cancer diagnosis and treatment pathways. Haematological malignancies have been excluded because the pathways of referral, detection, management and prognosis are very different compared with solid organ malignancies and would require a separate evaluation.
Study selection
Two reviewers (DCL and MM) screened the abstracts and full-text articles with a third reviewer (AA) to resolve any conflicts. We utilised the systematic review tool Covidence to screen, extract and validate data.21
Data abstraction and synthesis
The two primary reviewers extracted and validated the entries together before merging the outputs. Data extracted included year of article publication; country of study; demographic characteristics (age, gender, HIV status, education, marital status, employment, income level); country-level setting; disease subsite; study design; type of delay investigated, reasons for delay and primary outcomes.
Quality assessment was interrogated with Risk of Bias in Non-randomised Studies of Exposures (ROBINS-E) tool by DCL and AA.22
Patient and public involvement
None.
Results
Study characteristics
An initial search identified 6391 articles of which 193 underwent full-text review (figure 2). Fifty-seven studies were included in our final sample and data extracted.23–78 The full data extraction output is included in the online supplemental appendix 2.
Country and setting profile
The majority of studies were conducted in Nigeria, 15 (26%), Ethiopia, 8 (14%) and South Africa, 7 (12%). Five (9%) were undertaken in Uganda, four (7%) in Kenya, and three (5%) in Rwanda. Four (7%) studies were carried out in more than one country. Only 9% (n=5) of the studies were carried out at national level. Of the remaining studies, two-thirds were conducted at the hospital level (n=38) and a quarter (n=14) being conducted at regional level.
Research design
Two-thirds of included studies used a cross-sectional survey design. The rest of the studies included analysis of patient-level data collected retrospectively (23%) or prospectively (11%). Case–control and Delphi studies represented 4% of studies.
Tumour types
Breast cancer was the most studied tumour type for our research question (53%, n=29) followed by cervix (18%, n=10). About 21% of studies (n=12) evaluated multiple tumour types while there were smaller studies on colorectal cancer (n=2) and Kaposi’s sarcoma (n=1). There were no eligible studies on other high burden diseases in SSA such as prostate cancer and oesophageal cancer identified in the literature.
Participant population
Patients identified in a hospital setting were the target population in 48 out of 56 studies. In the other studies, the target populations were patients and clinicians (n=3), clinicians only (n=1), a combination of clinicians, public health opinion leaders and NGOs (n=1), patients in a community setting (n=2) and patients and health facility administrators (n=1).
Assessment of study quality
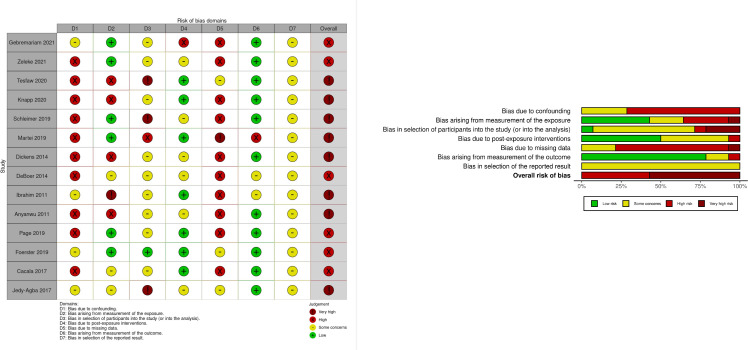
Fourteen cohort studies met the eligibility for a full assessment. The scores across the domains are illustrated in figure 3. The exposure and outcome characteristics are included in the online supplemental appendix 3. Two cohort studies did not require full interrogation as preliminary assessment of bias by asking the following three questions placed them in the very high-risk category: (1) Did the authors make any attempt to control for confounding? (2) Was the method of measuring exposure inappropriate? and (3) Was the method of measuring the outcome inappropriate? The remaining 40 were surveys. However, all the studies provided valuable insights that we used in the narrative synthesis. A similar finding on data quality from this region has been highlighted before in a contemporary systematic on the routes to diagnosis of symptomatic cancer in SSA.79 Figure 3 illustrates the different domains and proportions of bias across the studies. For the studies that were assessed comprehensively all of them had an overall judgement of high or very high risk of bias. In most studies the patient-related confounders (age, marital status and socioeconomic status such as income and education level) were collected as variables but not controlled for appropriately. Health systems factors were poorly accounted for in statistical analysis plans.
Figure 3.
Quality assessment of studies (n=14). McGuinness and Higgins.86
Three Delays framework
We synthesised the empirical studies into the Three Delay areas: seeking, reaching and receiving quality cancer care. About 37% (n=21) of the studies investigated all Three Delays while 42% (n=24) focused on 2 delays and 21% (n=12) on 1 delay. Table 1 outlines how the various studies addressed the components of the Three Delays framework.
Table 1.
Three Delays framework distribution of studies
| First author name | Year | Cancer type | Country | N | Setting | Design | Three Delays |
| Gebremariam et al34 | 2021 | Breast | Ethiopia | 223 | Regional | Retrospec | C |
| Zeleke et al46 | 2021 | Cervical | Ethiopia | 410 | Hospital | Retrospec | A |
| Mapanga et al31 | 2021 | Lung | S.Africa | 27 | Regional | Delphi | A, B, C |
| Nakaganda et al29 | 2020 | Multisite* | Uganda | 359 | Hospital | Survey | A, B, C |
| Tesfaw et al63 | 2020 | Breast | Ethiopia | 426 | Regional | Retrospec | A, C |
| Tesfaw et al65 | 2020 | Breast | Ethiopia | 371 | Regional | Survey | A, C |
| Reibold et al25 | 2020 | Breast | Ethiopia | 51 | Hospital | Survey | C |
| Knapp et al54 | 2020 | Breast | Nigeria | 609 | Hospital | Retrospec | A, B |
| Leng et al62 | 2020 | Multisite† | Nigeria | 186 | Hospital | Survey | A, B, C |
| Togawa et al55 | 2020 | Breast | Namibia Nigeria Uganda Zambia |
1518 | Hospital | Survey | A, C |
| Swanson et al76 | 2020 | cervical | Uganda | 268 | Hospital | Survey | C |
| Foerster et al40 | 2020 | Breast | Uganda, Zambia, Namibia, Nigeria | 1429 | Hospital | Survey | A, B, C |
| Dereje et al45 | 2020 | Cervical | Ethiopia | 212 | Regional | Survey | A, C |
| Dereje et al44 | 2020 | Cervical | Ethiopia | 231 | Regional | Survey | A, B |
| Agodirin et al78 | 2020 | Breast | Nigeria | 420 | Regional | Survey | A, B, C |
| Martin et al28 | 2019 | cancer type not specified | Rwanda | 73 | National | Survey | C |
| Page et al66 | 2019 | cervical | Kenya | 505 | Regional | Prospect | A, B |
| Low et al43 | 2019 | Multisite‡ | Uganda | 100 | Hospital | Survey | A, B |
| Wambalaba et al69 | 2019 | Multisite§ | Kenya | 1048 | National | Retrospec | A, C |
| Grosse Frie et al57 | 2019 | Breast | Mali | 124 | Regional | Survey | A, B, C |
| Yang et al41 | 2019 | Breast | Tanzania | 196 | Hospital | Survey | B |
| Schleimer et al27 | 2019 | Breast | Rwanda | 151 | Regional | Retrospec | A, B, C |
| Foerster et al61 | 2019 | Breast | Uganda Nigeria Namibia |
1335 | Hospital | Prospect | A, B, C |
| Tapera et al56 | 2019 | cervical | Zimbabwe | 78 | Regional | Survey | A, B, C |
| Agodirin et al60 | 2019 | Breast | Nigeria | 237 | Regional | Survey | A, B, C |
| Rayne et al33 | 2019 | Breast | S.Africa | 252 | Hospital | Survey | A, B |
| Subramanian et al52 | 2019 | Breast | Kenya | 800 | Regional | Survey | A, B, C |
| Olarewaju et al42 | 2019 | breast | Nigeria | 275 | Hospital | Survey | A, B, C |
| Ajah et al30 | 2019 | Multisite¶ | Nigeria | 95 | Hospital | Survey | A |
| Martei et al59 | 2019 | Multisite** | Botswana | 286 | Hospital | Retrospec | A |
| Herbst et al24 | 2018 | Colorectal | S.Africa | 162 | Hospital | Retrospec | C |
| Anakwenze et al50 | 2018 | Multisite†† | Botswana | 214 | Hospital | Survey | A, B |
| Moodley et al53 | 2018 | Breast | S.Africa | 201 | Hospital | Survey | A, B |
| Joffe et al26 | 2018 | Breast | S.Africa | 499 | Hospital | Survey | A, B, C |
| Awofeso et al70 | 2018 | Breast, Cervical | Nigeria | 105 | Hospital | Survey | A, B, C |
| Bhatia et al67 | 2018 | Multisite‡‡ | Botswana | 214 | Hospital | Survey | A, B |
| Oladeji et al32 | 2017 | Multisite§§ | Nigeria | 218 | Hospital | Survey | A, B, C |
| Jedy-Agba et al39 | 2017 | Breast | Nigeria | 316 | National | Case-control | A, B |
| Alatise et al58 | 2017 | colorectal | Nigeria | 127 | Hospital | Survey | A, B, C |
| Cacala et al51 | 2017 | Breast | S.Africa | 172 | Hospital | Prospect | A, B |
| Brinton et al48 | 2016 | Breast | Ghana | 1184 | Regional | Survey | A, B |
| Mlange et al64 | 2016 | Cervical | Tanzania | 202 | Hospital | Survey | A, B |
| Mwaka et al73 | 2015 | Cervical | Uganda | 149 | Hospital | Survey | A, B |
| Long et al47 | 2015 | Multisite¶¶ | Cameroon | 220 | Hospital | Survey | A, B, C |
| Pace et al37 | 2015 | Breast | Rwanda | 144 | National | Survey | A, B, C |
| Tadesse74 | 2015 | cervical | Ethiopia | 198 | Hospital | Survey | B, C |
| Dickens et al75 | 2014 | Breast | S.Africa | 1071 | Hospital | Retrospec | B |
| De Boer et al72 | 2014 | K.Sarcoma | Uganda | 161 | Hospital | Retrospec | A, B |
| Ntirenganya et al71 | 2014 | Breast | Rwanda Sierra Leone |
6820 | National | Survey | A, B |
| Fasunla et al49 | 2013 | Sinonasal | Nigeria | 61 | Hospital | Survey | A, B, C |
| Ibrahim et al68 | 2011 | cervical | Sudan | 197 | Hospital | Retrospec | B |
| Anyanwu et al23 | 2011 | breast | Nigeria | 275 | Hospital | Retrospec | B, C |
| Otieno et al35 | 2010 | Breast | Kenya | 166 | Hospital | Survey | A, B, C |
| Ezeome et al38 | 2009 | Breast | Nigeria | 164 | Hospital | Survey | A, B |
| Clegg-Lamptey et al77 | 2009 | breast | Ghana | 101 | Hospital | Survey | A, B, C |
| Ukwenya et al36 | 2008 | Breast | Nigeria | 111 | Hospital | Survey | A, B, C |
*Cervix, Kaposi’s sarcoma, breast, prostate, oesophagus.
†Breast, cervical, head and neck, prostate.
‡KS, cervical cancer, breast cancer, esophageal cancer, head and neck cancer, non-Hodgkin lymphoma, vulvovaginal, prostate, conjunctival squam cell ca, penile, melanoma.
§Cervix, breast, esophagus, prostate, ovary, colon, thyroid, pancreatic, lung, liver.
¶Cervical, ovarian, endometrial, vulva, choriocarcinoma, leiomyosarcoma.
**Cervical, breast, prostate, esophageal, lung, uterine, ovarian, colorectal, head and neck cancers, Kaposi sarcoma.
††Cervical, breast, head and neck, vulvar, aposi sarcoma, endometrial, penile, anal, oesophageal, lymphoma, prostate.
‡‡Cervical, breast, head and neck, vulvar, Kaposi’s sarcoma, endometrial, penile, anal, oesophageal, lymphoma, prostate.
§§Uterine cervix, breast, head and neck, prostate, GIT.
¶¶Skin, breast, colorectal, gynecologic, anal; Three Delays codes.
A, seeking care; B, reaching care; C, receiving quality care; K. Sarcom, Kaposi sarcoma; N, sample size; Prospect, prospective; Retrospec, retrospective; S. Africa, South Africa.
The reasons of the delays amalgamated from the studies and identified as contributing to each type of systems delay are outlined in table 2. They are further synthesised into economic, psychological, sociocultural, health services and geography subthemes and referenced appropriately in the text. The comprehensive output with outcomes of the data extraction is included as online supplemental appendix 2.
Table 2.
Reasons for Three Delays
| Reasons for seeking care delay | Reasons for reaching care delay | Reasons for receiving quality care delay |
| Psychological | ||
| Belief in witchcraft | Preference for alternative treatment | Defaulting because of side effects of drugs |
| Denial | Declining treatment | |
| Embarrassment | Fear of wasting doctor’s time | |
| Fear of being asked to stop habits for example, smoking | Fear of treatment (eg, mastectomy) | |
| Stigma | Lack of consent | |
| Secrecy | Preference to observe | |
| Putting others needs first | Preference for alternative therapies (herbal, Chinese, acupuncture, food supplements) | |
| Prior bad experience at health centre of hospital | ||
| Preference for care abroad | ||
| Lack of trust in health system | ||
| Fear of doctors, diagnosis, dying, job loss, losing part of body, missing family commitments because of treatment, telling people of illness, treatment | ||
| Sociocultural | ||
| Family and friends’ disapproval | Family responsibilities | Communication barriers |
| Busy schedule | Lack of a caregiver to accompany to facilities | Family commitments |
| Anticipated long waiting time at clinic | Obligations at home | Language barrier |
| Preference for prayers and spiritual intervention | No relative to care for them during treatment | |
| Preference for food supplements/organic foods | Patients changing mobile numbers so cannot be contacted for further management | |
| Preference for alternative therapies (herbal, homeopathy, Chinese, acupuncture) | Ignorance on available treatment | |
| No one to look after children | ||
| Low education | ||
| Lack of personal initiative | ||
| Ignorance on how to seek healthcare | ||
| Lack of awareness of symptoms | ||
| Economic | ||
| Impact of taking time off work | Dependence on others for transport | Cancer not priority |
| Anticipated expense of treatment | Difficulty making appointment or reaching doctor | Failure to come back for follow-up diagnostic or treatment appointments |
| Transport challenges (eg, cost) | High cost of prediagnostic costs | Failure to find accommodation as outpatients close to treatment centre |
| Prioritising day to day survival over seeking help | High cost of transport | Financial incapability |
| Obligations at home | Inability to afford clinic visits | High cost of medicines |
| No health insurance | Lack of money (for transport) | Paying out of pocket expenses |
| Financial incapability | Work commitments | Poor nutrition |
| Geography | ||
| Distance | Distance | |
| Travelled away from home (out of comfort zone) | Lack of knowledge of estimated distance to nearest service | |
| Health service | ||
| Lack of cancer awareness programmes and screening | Lack of navigation in primary care | Absence of multidisciplinary team care |
| Long investigation time at first contact | Burnout and disinterest of healthcare workers | |
| Misdiagnosis at lower levels | Diagnostic delay | |
| Was told by healthcare worker there was no treatment for disease | Chemotherapy stock outs | |
| Turned away from clinics for arriving late | Few specialists | |
| High patient volume compared with resources | ||
| Lack of continuity of care by same healthcare workers | ||
| Lack of palliative care and counselling services | ||
| Lack of pathology and screening services | ||
| Lack of smoking cessation clinics | ||
| Lack of specific appointments with specialists | ||
| Unwelcoming, demotivated and uncommitted staff turn patients away | ||
| Long appointments, waiting periods | ||
| Misdiagnosis | ||
| No bed space | ||
| Not healthy enough to continue treatment | ||
| Patients changing mobile numbers so cannot be contacted for further management | ||
| Poorly trained staff | ||
| Power outages | ||
| Unavailability of treatment modality | ||
| Surgeon/operating room unavailability | ||
| Pre-referral diagnosis not communicated | ||
| Poor collaboration among healthcare workers | ||
Seeking care
Reasons for delays in seeking care included a lack of awareness about cancer and low health literacy which manifested itself as fears, false perceptions and beliefs and embarrassment about cancer.26 28 31 32 35 37 38 40 42 42 44 51 55 58 60 64 65 67 70 71 73 77 78 There was also a preference for seeking treatment from traditional or faith-based healers.27 30 32 35–38 42 44 46 48 49 51 55 57 58 65 70 71 77 78 Participants in the various studies recounted the belief they had not been sick enough or did not have adequate money to justify abandoning their obligations (both financial and social);26 27 29 31 37 38 42 45 51 52 55 56 58 72 77 78 they rather reassured themselves about the seriousness of symptoms (eg, lumps) as the symptoms did not cause disability or pain in the early stages of disease and that it was self-limiting.26 31 36–38 42 44 47 51 53 67 73 78 Additionally, not knowing where or how to enter the health system for symptoms before they cause life-threatening conditions contributed to delays in seeking treatment.31 37 44 46 The unknown costs of managing cancer was also noted to intimidate patients and delay presentation as a result.26 44
Reaching care
The physical distance to appropriate care was cited as a major barrier for patients who have to take into consideration transport costs to specialist facilities, accommodation and subsistence costs.23 27–29 32 33 37 40–42 47 50–52 55 56 60 62 71–73 77 78 Even when transport is made available, they carry the cost of being away from their jobs and families. Other than geographical distance, low levels of cancer care knowledge among primary-level healthcare staff was also a barrier for referral of patients.31 37 45 70 74 78 This was identified as a source of misdiagnosis and underlay the lack of recognition for the urgency of transferring care to tertiary institutions. In one study, participants had reported that they had been misinformed at the primary level that their condition was incurable.38
Receiving quality care
The paucity of infrastructure, equipment, medication and human resources needed for cancer care underpinned the barriers to receiving quality cancer care.28 62 69 We noted a lack of availability or poor quality diagnostic equipment and treatment facilities were also challenges identified.58 62 70 Other factors included demotivated and burnt-out staff and the lack of specialist training of staff in cancer.25 28 31 32 38 56 62 Tensions and mistrust of the system as a whole between the patients and healthcare providers operating in constrained environments were reported as contributing to factors that drove patients to alternate medicine or even simply abandon treatment.31 47 52 In addition, the lack of availability of essential resources lead to high prices and catastrophic out of pocket expenses for the patients.23 29 31 32 36 42 47 49 52 55 56 61 62 76 77
Discussion
The impact of delays in the cancer care pathway on persistent high mortality rates are well recognised. Countries in SSA are called on to accelerate the establishment and implementation of their cancer control plans and it is pertinent to recognise that while respecting the unique aspects of each nation, utilisation of a common knowledge base avoids duplication and allows for prudent efficient use of scarce resources.2 16 In this regard, results from research using a robust methodological approach provides a foundation for common knowledge that is applicable broadly.17
However, our systematic review of studies in SSA investigating the barriers to access to cancer care demonstrates a very limited number of studies despite the importance of this subject area, with heterogeneity in study design which limits their translational impact. The publications we found were clustered to the Northern and West African regions and given the heterogenous factors influencing the SSA region data cannot reliably be extrapolated across the continent. In addition, 70% of the studies focused on breast and cervical cancer with major causes of cancer-related mortality and morbidity such as prostate and oesophageal cancer not addressed which is of major concern. The results highlight the need for a coordinated approach to manage these evidence gaps with no studies addressing the barriers to diagnosis and treatment of cancer identified in 35 of 48 countries in SSA.
The capacity to conduct robust research is increasingly possible across countries in SSA but it requires considerable efforts to coordinate these resources to support a common agenda based on country and regional-level priorities.80 81 Presently, a discordance between research needs and research funding priorities across the continent has been accelerated by the synthetic external agendas in individual countries rather than supporting endogenous solutions driven by those experiencing the problems.82 83 This is exemplified by our findings which show research is concentrated on a pool of four or five better resourced countries and two main tumour types likely related to the availability of external funding.
Most published data have been obtained through cross-sectional surveys, which detail the prevalence of reasons for delays but fail to account for important cofounding factors and system-level processes to enable the effective problem solving. Nonetheless they still provide a valuable baseline insight that we integrated into a ‘Three Delays’ model.
The common roots of the reasons for delays at each level of seeking, reaching and receiving quality care as listed in table 2 are first fear (apprehension or mistrust) and second, a lack of resources (financial, human or infrastructure). Across all delays, cost is a major factor that influences the interval between the stages in the cancer pathway. Out of pocket expenses are high with patients requiring cover for transport, accommodation, diagnostic tests and medicines. A significant number of patients live under the poverty line and it may seem unrealistic for the families to spend on what is perceived to be an incurable disease in the first instance.84 A recent study demonstrated the threat of catastrophic health expenditure that accompanies a cancer diagnosis even with the basic drugs in low and middle income countries.85
In seeking care, fear is compounded by the lack of awareness (knowledge) on the disease, availability of services or how to navigate the pathways to quality healthcare. It can drive patients to rely on familiar systems of alternative medicines (traditional healers, ‘Chinese’ medicine, faith-based healers). In addition to these challenges taking time off from work or domestic obligations to attend healthcare appointments is often relegated in terms of priorities due to financial and social implications. Societal expectations also create fear of stigmatism and promote secrecy that hinder free information flow between those seeking it and its custodians.
For reaching care the lack of adequate coordination of services was the dominant theme. Poorly trained staff or lack of support for primary healthcare practitioners delayed referrals to more specialised services and the health system in such a scenario could possibly discourage patients on the curability of the condition. Links and relationships are essential between primary and secondary/tertiary healthcare as most patients will present first to local clinics or health posts. This is particularly important where systems are not electronically linked for results to be easily attainable between practitioners.
To receive quality care, patients need access to a healthcare system with appropriate human resource and infrastructure (diagnostic and treatment). A lack of human resource encompasses both the competence of the workforce for tertiary services as well as the actual low numerical value of specialised knowledgeable staff leading to burnout. Equally a skilled and competent workforce without appropriate infrastructure or sufficient medication and surgical supplies cannot be expected to deliver quality care. Another aspect to consider for receiving quality care includes patient factors like good nutritional status, financial capacity and social capital to undergo treatment. Acceptance and adherence to treatment are also integral to a successful intervention as investigated by Anyanwu et al.23
The findings from our study suggest that reasons for delays are interlinked both at an individual level and population level (figure 1). An individual with vulnerabilities at the seeking-level phase would most likely experience repetitive barriers in reaching care as well as receiving quality care. An underdeveloped health system with poor linkages between primary healthcare and tertiary-level care will inevitably have a large proportion of patients falling through the cracks between phases of care. This could be due to untimely referrals and inability to support diagnostic costs thereby relying on the patient to raise funds.
Limitations
A major limitation in the interpretation and application of the findings of this research output is the quality of the included studies. Recognition of this limitation and application of additional triangulation has assisted us to utilise what is available in this space. Future directions based on our findings would be to conduct more research studies that will provide quality data for policy formation and effective implementation.
Conclusion
To see a reduction in cancer mortality in SSA health systems need to address delays within the cancer pathway from initial presentation and appraisal to completion of treatment and the survivorship pathway. Holistic support for the patient as well as the workforce across the continuum and longitudinally in each phase is important to achieve good outcomes. Cognizance of the multiple barriers presents for individual patients from developing a cancer to its treatment is important for policymakers and experts to build resilient and effective cancer control programmes. With an individual in mind an effective population approach can be achieved. Due to the paucity of organised data in SSA, the starting point of research is often extrapolated from other regions who have different realities. In carrying out this systematic review we intend to provide an organised pool of information that will provide a robust resource for other researchers seeking to conduct studies in SSA.
bmjopen-2022-067715supp002.pdf (194.2KB, pdf)
bmjopen-2022-067715supp003.pdf (33.6KB, pdf)
Supplementary Material
Footnotes
Contributors: DCL, MM and AA were involved in all aspects. SM, VB, MS, ASS, RM, JS and ADM participated in study design, data interpretation, preparation and revision of manuscript. DCL is responsible for the overall content as guarantor.
Funding: This work was supported by the London School of Hygiene and Tropical Medicine’s Wellcome Institutional Strategic Support Fund (grant reference 204928/Z/16/Z).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study. The data extraction output is available as supplementary material.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable. This study was a systematic review so did not directly involve animal or human participants. Patient consent for publication was not required.
References
- 1.58th world health assembly. WHA58.22 cancer prevention and control; 2005.
- 2.70th world health assembly. WHA70.12 cancer prevention and control in the context of an integrated approach; 2017.
- 3.GLOBOCAN . Sub-Saharan Africa hub source: globocan 2020 cancer statistics. 2020. Available: https://gco.iarc.fr/today/data/factsheets/populations/971-sub-saharan-africa-hub-fact-sheets.pdf [Accessed 10 Apr 2022].
- 4.Azevedo MJ. The state of health system(s) in Africa: challenges and opportunities. In: Historical Perspectives on the State of Health and Health Systems in Africa, Volume II. Cham: Springer International Publishing, 2017: 1–73. Available: http://link.springer.com/10.1007/978-3-319-32564-4_1 [Google Scholar]
- 5.Ifeagwu SC, Yang JC, Parkes-Ratanshi R, et al. Health financing for universal health coverage in sub-Saharan Africa: a systematic review. Glob Health Res Policy 2021;6:8. 10.1186/s41256-021-00190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asante A, Wasike WSK, Ataguba JE. Health financing in sub-Saharan Africa: from analytical frameworks to empirical evaluation. Appl Health Econ Health Policy 2020;18:743–6. 10.1007/s40258-020-00618-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oleribe OO, Momoh J, Uzochukwu BS, et al. Identifying key challenges facing healthcare systems in Africa and potential solutions. Int J Gen Med 2019;12:395–403. 10.2147/IJGM.S223882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeltner T, Riahi F, Huber J. Acute and chronic health challenges in sub-Saharan Africa: an unfinished agenda. In: Groth H, May JF, eds. Africa’s Population: In Search of a Demographic Dividend. Cham: Springer International Publishing, 2017: 283–97. Available: http://link.springer.com/10.1007/978-3-319-46889-1_18 [Google Scholar]
- 9.World Health Organisation . WHO report on cancer: setting priorities, investing wisely and providing care for all. Geneva, 2020. [Google Scholar]
- 10.McKenzie F, Zietsman A, Galukande M, et al. African breast cancer-disparities in outcomes (ABC-DO): protocol of a multicountry mobile health prospective study of breast cancer survival in sub-saharan africa. BMJ Open 2016;6:e011390. 10.1136/bmjopen-2016-011390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel-Wahab M, Bourque J-M, Pynda Y, et al. Status of radiotherapy resources in Africa: an international atomic energy agency analysis. Lancet Oncol 2013;14:e168–75. 10.1016/S1470-2045(12)70532-6 [DOI] [PubMed] [Google Scholar]
- 12.Orem J. Building modern cancer care services in sub-Saharan Africa based on a clinical-research care model. Am Soc Clin Oncol Educ Book 2022;42:1–6. 10.1200/EDBK_349953 [DOI] [PubMed] [Google Scholar]
- 13.Khan MS, Meghani A, Liverani M, et al. How do external donors influence national health policy processes? Experiences of domestic policy actors in cambodia and pakistan. Health Policy Plan 2018;33:215–23. 10.1093/heapol/czx145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ollila E. Global health priorities - priorities of the wealthy? Global Health 2005;1:6. 10.1186/1744-8603-1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero Y, Trapani D, Johnson S, et al. National cancer control plans: a global analysis. Lancet Oncol 2018;19:e546–55. 10.1016/S1470-2045(18)30681-8 [DOI] [PubMed] [Google Scholar]
- 16.World Health Organisation . National cancer control programmes. policies and managerial guidelines. 2nd edition. 2002. [Google Scholar]
- 17.Walter F, Webster A, Scott S, et al. The andersen model of total patient delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Policy 2012;17:110–8. 10.1258/jhsrp.2011.010113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah B, Krishnan N, Kodish SR, et al. Applying the three delays model to understand emergency care seeking and delivery in rural bangladesh: a qualitative study. BMJ Open 2020;10:e042690. 10.1136/bmjopen-2020-042690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serbanescu F, Goodwin MM, Binzen S, et al. Addressing the first delay in saving mothers, giving life districts in Uganda and Zambia: approaches and results for increasing demand for facility delivery services. Glob Health Sci Pract 2019;7:S48–67. 10.9745/GHSP-D-18-00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thaddeus S, Maine D. Too far to walk: maternal mortality in context. Soc Sci Med 1994;38:1091–110. 10.1016/0277-9536(94)90226-7 [DOI] [PubMed] [Google Scholar]
- 21.Covidence. n.d. Available: https://app.covidence.org
- 22.ROBINS-E Development Group . Risk of bias in non-randomized studies - of exposure (ROBINS-E). Launch version; 2022. Available: https://www.riskofbias.info/welcome/robins-e-tool#h.trqnh6qozyhl
- 23.Anyanwu SNC, Egwuonwu OA, Ihekwoaba EC. Acceptance and adherence to treatment among breast cancer patients in eastern nigeria. Breast 2011;20 Suppl 2:S51–3. 10.1016/j.breast.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 24.Herbst C, Miot JK, Moch SL, et al. Access to colorectal cancer (CRC) chemotherapy and the associated costs in a South African public healthcare patient cohort. Journal of Cancer Policy 2018;15:18–24. 10.1016/j.jcpo.2017.11.005 [DOI] [Google Scholar]
- 25.Reibold CF, Tariku W, Eber-Schulz P, et al. Adherence to newly implemented tamoxifen therapy for breast cancer patients in rural Western Ethiopia. Breast Care (Basel) 2021;16:484–90. 10.1159/000512840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joffe M, Ayeni O, Norris SA, et al. Barriers to early presentation of breast cancer among women in Soweto, South Africa. PLoS ONE 2018;13:e0192071. 10.1371/journal.pone.0192071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schleimer LE, Vianney Dusengimana J-M, Butonzi J, et al. Barriers to timely surgery for breast cancer in Rwanda. Surgery 2019;166:1188–95. 10.1016/j.surg.2019.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin AN, Kaneza K-M, Kulkarni A, et al. Cancer control at the district hospital level in sub-saharan africa: an educational and resource needs assessment of general practitioners. J Glob Oncol 2019;5:1–8. 10.1200/JGO.18.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakaganda A, Solt K, Kwagonza L, et al. Challenges faced by cancer patients in uganda: implications for health systems strengthening in resource limited settings. J Cancer Policy 2021;27:100263. 10.1016/j.jcpo.2020.100263 [DOI] [PubMed] [Google Scholar]
- 30.Ajah L, Ezeome IV, Umeh UA, et al. Complementary and alternative medicine. use and challenges among gynaecological cancer patients in nigeria: experiences in a tertiary health institution - preliminary results. Eur J Gynaecol Oncol 2019;40:101–5. 10.12892/ejgo4429.2019 [DOI] [Google Scholar]
- 31.Mapanga W, Norris SA, Chen WC, et al. Consensus study on the health system and patient-related barriers for lung cancer management in South Africa. PLoS One 2021;16:e0246716. 10.1371/journal.pone.0246716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oladeji A, Atalabi O, Jimoh M, et al. Delay in presentation of cancer patients for diagnosis and management: an institutional report. Internet J Oncol 2017;13. 10.5580/IJO.44745 [DOI] [Google Scholar]
- 33.Rayne S, Schnippel K, Kruger D, et al. Delay to diagnosis and breast cancer stage in an Urban South African breast clinic. S Afr Med J 2019;109:159–63. 10.7196/SAMJ.2019.v109i3.13283 [DOI] [PubMed] [Google Scholar]
- 34.Gebremariam A, Assefa M, Addissie A, et al. Delayed initiation of adjuvant chemotherapy among women with breast cancer in addis Ababa, Ethiopia. Breast Cancer Res Treat 2021;187:877–82. 10.1007/s10549-021-06131-9 [DOI] [PubMed] [Google Scholar]
- 35.Otieno ES, Micheni JN, Kimende SK, et al. Delayed presentation of breast cancer patients. East Afr Med J 2010;87:147–50. 10.4314/eamj.v87i4.62410 [DOI] [PubMed] [Google Scholar]
- 36.Ukwenya AY, Yusufu LMD, Nmadu PT, et al. Delayed treatment of symptomatic breast cancer: the experience from Kaduna, Nigeria. S Afr J Surg 2008;46:106–10. [PubMed] [Google Scholar]
- 37.Pace LE, Mpunga T, Hategekimana V, et al. Delays in breast cancer presentation and diagnosis at two rural cancer referral centers in Rwanda. Oncologist 2015;20:780–8. 10.1634/theoncologist.2014-0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ezeome ER. Delays in presentation and treatment of breast cancer in Enugu, Nigeria. Niger J Clin Pract 2010;13:311–6. [PubMed] [Google Scholar]
- 39.Jedy-Agba E, McCormack V, Olaomi O, et al. Determinants of stage at diagnosis of breast cancer in nigerian women: sociodemographic, breast cancer awareness, health care access and clinical factors. Cancer Causes Control 2017;28:685–97. 10.1007/s10552-017-0894-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foerster M, McKenzie F, Zietsman A, et al. Dissecting the journey to breast cancer diagnosis in sub-saharan africa: findings from the multicountry ABC-DO cohort study. Int J Cancer 2021;148:340–51. 10.1002/ijc.33209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang K, Msami K, Calixte R, et al. Educational opportunities for down-staging breast cancer in low-income countries: an example from Tanzania. J Cancer Educ 2019;34:1225–30. 10.1007/s13187-019-01587-2 [DOI] [PubMed] [Google Scholar]
- 42.Olarewaju SO, Oyekunle EO, Bamiro AO. Effect of sociodemographic variables on patient and diagnostic delay of breast cancer at the foremost health care institution in Nigeria. J Glob Oncol 2019;5:1–8. 10.1200/JGO.19.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Low DH, Phipps W, Orem J, et al. Engagement in HIV care and access to cancer treatment among patients with HIV-associated malignancies in Uganda. J Glob Oncol 2019;5:1–8. 10.1200/JGO.18.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dereje N, Addissie A, Worku A, et al. Extent and predictors of delays in diagnosis of cervical cancer in addis Ababa, Ethiopia: a population-based prospective study. JCO Glob Oncol 2020;6:277–84. 10.1200/JGO.19.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dereje N, Gebremariam A, Addissie A, et al. Factors associated with advanced stage at diagnosis of cervical cancer in addis Ababa, Ethiopia: a population-based study. BMJ Open 2020;10:e040645. 10.1136/bmjopen-2020-040645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeleke S, Anley M, Kefale D, et al. Factors associated with delayed diagnosis of cervical cancer in tikur anbesa specialized hospital, ethiopia, 2019: cross-sectional study. Cancer Manag Res 2021;13:579–85. 10.2147/CMAR.S285621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long C, Titus Ngwa Tagang E, Popat RA, et al. Factors associated with delays to surgical presentation in north-west cameroon. Surgery 2015;158:756–63. 10.1016/j.surg.2015.04.016 [DOI] [PubMed] [Google Scholar]
- 48.Brinton L, Figueroa J, Adjei E, et al. Factors contributing to delays in diagnosis of breast cancers in Ghana, West Africa. Breast Cancer Res Treat 2017;162:105–14. 10.1007/s10549-016-4088-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fasunla AJ, Ogunkeyede SA. Factors contributing to poor management outcome of sinonasal malignancies in south-west nigeria. Ghana Med J 2013;47:10–5. [PMC free article] [PubMed] [Google Scholar]
- 50.Anakwenze C, Bhatia R, Rate W, et al. Factors related to advanced stage of cancer presentation in botswana. J Glob Oncol 2018;4:1–9. 10.1200/JGO.18.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Čačala SR, Gilart J. Factors relating to late presentation of patients with breast cancer in area 2 Kwazulu-Natal, South Africa. J Glob Oncol 2017;3:497–501. 10.1200/JGO.2016.008060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Subramanian S, Gakunga R, Jones M, et al. Financial barriers related to breast cancer screening and treatment: a cross-sectional survey of women in Kenya. Journal of Cancer Policy 2019;22:100206. 10.1016/j.jcpo.2019.100206 [DOI] [Google Scholar]
- 53.Moodley J, Cairncross L, Naiker T, et al. From symptom discovery to treatment - women’s pathways to breast cancer care: a cross-sectional study. BMC Cancer 2018;18:312. 10.1186/s12885-018-4219-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knapp GC, Tansley G, Olasehinde O, et al. Geospatial access predicts cancer stage at presentation and outcomes for patients with breast cancer in southwest nigeria: a population-based study. Cancer 2020;127:1432–8. 10.1002/cncr.33394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Togawa K, Anderson BO, Foerster M, et al. Geospatial barriers to healthcare access for breast cancer diagnosis in sub-saharan african settings: the african breast cancer-disparities in outcomes cohort study. Int J Cancer 2020;148:2212–26. 10.1002/ijc.33400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tapera O, Dreyer G, Kadzatsa W, et al. Health system constraints affecting treatment and care among women with cervical cancer in Harare, Zimbabwe. BMC Health Serv Res 2019;19:829. 10.1186/s12913-019-4697-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grosse Frie K, Kamaté B, Traoré CB, et al. Health system organisation and patient pathways: breast care patients’ trajectories and medical doctors’ practice in Mali. BMC Public Health 2019;19:204. 10.1186/s12889-019-6532-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alatise OI, Fischer SE, Ayandipo OO, et al. Health-seeking behavior and barriers to care in patients with rectal bleeding in Nigeria. J Glob Oncol 2017;3:749–56. 10.1200/JGO.2016.006601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martei YM, Grover S, Bilker WB, et al. Impact of essential medicine stock outs on cancer therapy delivery in a resource-limited setting. J Glob Oncol 2019;5:1–11. 10.1200/JGO.18.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agodirin O, Olatoke S, Rahman G, et al. Impact of primary care delay on progression of breast cancer in a black african population: a multicentered survey. J Cancer Epidemiol 2019;2019:2407138. 10.1155/2019/2407138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foerster M, Anderson BO, McKenzie F, et al. Inequities in breast cancer treatment in sub-Saharan Africa: findings from a prospective multi-country observational study. Breast Cancer Res 2019;21:93. 10.1186/s13058-019-1174-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leng J, Ntekim AI, Ibraheem A, et al. Infrastructural challenges lead to delay of curative radiotherapy in Nigeria. JCO Glob Oncol 2020;6:269–76. 10.1200/JGO.19.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tesfaw A, Getachew S, Addissie A, et al. Late-stage diagnosis and associated factors among breast cancer patients in south and southwest ethiopia: a multicenter study. Clin Breast Cancer 2021;21:e112–9. 10.1016/j.clbc.2020.08.011 [DOI] [PubMed] [Google Scholar]
- 64.Mlange R, Matovelo D, Rambau P, et al. Patient and disease characteristics associated with late tumour stage at presentation of cervical cancer in northwestern Tanzania. BMC Womens Health 2016;16:5. 10.1186/s12905-016-0285-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tesfaw A, Demis S, Munye T, et al. Patient delay and contributing factors among breast cancer patients at two cancer referral centres in Ethiopia: a cross-sectional study. J Multidiscip Healthc 2020;13:1391–401. 10.2147/JMDH.S275157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Page CM, Ibrahim S, Park LP, et al. Patient factors affecting successful linkage to treatment in a cervical cancer prevention program in Kenya: a prospective cohort study. PLoS ONE 2019;14:e0222750. 10.1371/journal.pone.0222750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhatia RK, Rayne S, Rate W, et al. Patient factors associated with delays in obtaining cancer care in botswana. J Glob Oncol 2018;4:1–13. 10.1200/JGO.18.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ibrahim A, Rasch V, Pukkala E, et al. Predictors of cervical cancer being at an advanced stage at diagnosis in sudan. Int J Womens Health 2011;3:385–9. 10.2147/IJWH.S21063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wambalaba FW, Son B, Wambalaba AE, et al. Prevalence and capacity of cancer diagnostics and treatment: a demand and supply survey of health-care facilities in Kenya. Cancer Control 2019;26:1073274819886930. 10.1177/1073274819886930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Awofeso O, Roberts AA, Salako O, et al. Prevalence and pattern of late-stage presentation in women with breast and cervical cancers in Lagos university teaching Hospital, Nigeria. Niger Med J 2018;59:74–9. 10.4103/nmj.NMJ_112_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ntirenganya F, Petroze RT, Kamara TB, et al. Prevalence of breast masses and barriers to care: results from a population-based survey in rwanda and sierra leone. J Surg Oncol 2014;110:903–6. 10.1002/jso.23726 [DOI] [PubMed] [Google Scholar]
- 72.De Boer C, Niyonzima N, Orem J, et al. Prognosis and delay of diagnosis among kaposi’s sarcoma patients in uganda: a cross-sectional study. Infect Agent Cancer 2014;9:17. 10.1186/1750-9378-9-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mwaka AD, Garimoi CO, Were EM, et al. Social, demographic and healthcare factors associated with stage at diagnosis of cervical cancer: cross-sectional study in a tertiary hospital in northern Uganda. BMJ Open 2016;6:e007690. 10.1136/bmjopen-2015-007690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tadesse SK. Socio-economic and cultural vulnerabilities to cervical cancer and challenges faced by patients attending care at tikur anbessa hospital: a cross sectional and qualitative study. BMC Womens Health 2015;15:75. 10.1186/s12905-015-0231-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dickens C, Joffe M, Jacobson J, et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: a south african public hospital case series of over 1,000 women. Int J Cancer 2014;135:2173–82. 10.1002/ijc.28861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swanson M, Nakalembe M, Chen L-M, et al. Surgical candidacy and treatment initiation among women with cervical cancer at public referral hospitals in Kampala, Uganda: a descriptive cohort study. BMJ Open 2020;10:e039946. 10.1136/bmjopen-2020-039946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clegg-Lamptey J, Dakubo J, Attobra YN. Why do breast cancer patients report late or abscond during treatment in Ghana? A pilot study. Ghana Med J 2009;43:127–31. [PMC free article] [PubMed] [Google Scholar]
- 78.Agodirin O, Olatoke S, Rahman G, et al. Presentation intervals and the impact of delay on breast cancer progression in a black african population. BMC Public Health 2020;20:962. 10.1186/s12889-020-09074-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martins T, Merriel SWD, Hamilton W. Routes to diagnosis of symptomatic cancer in sub-saharan africa: systematic review. BMJ Open 2020;10:e038605. 10.1136/bmjopen-2020-038605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nyirenda T, Bockarie M, Machingaidze S, et al. Strengthening capacity for clinical research in sub-Saharan Africa: partnerships and networks. Int J Infect Dis 2021;110:54–61. 10.1016/j.ijid.2021.06.061 [DOI] [PubMed] [Google Scholar]
- 81.Graef KM, Okoye I, Ohene Oti NO, et al. Operational strategies for clinical trials in africa. JCO Glob Oncol 2020;6:973–82. 10.1200/JGO.19.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.The-nature-of-and-motive-for-academic-research-in-higher-edu.ris. [Google Scholar]
- 83.Mutapi F. Africa should set its own health-research agenda. Nature 2019;575:567. 10.1038/d41586-019-03627-9 [DOI] [PubMed] [Google Scholar]
- 84.McCormack V, Newton R. Research priorities for social inequalities in cancer in sub-saharan africa. International Agency for Research on Cancer, 2019. Available: https://www.ncbi.nlm.nih.gov/books/NBK566204 [PubMed] [Google Scholar]
- 85.Fundytus A, Sengar M, Lombe D, et al. Access to cancer medicines deemed essential by oncologists in 82 countries: an international, cross-sectional survey. Lancet Oncol 2021;22:1367–77. 10.1016/S1470-2045(21)00463-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McGuinness LA, Higgins JPT. Risk-of-bias visualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 2020;12:1–7. 10.1002/jrsm.1411 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-067715supp001.pdf (27.8KB, pdf)
bmjopen-2022-067715supp002.pdf (194.2KB, pdf)
bmjopen-2022-067715supp003.pdf (33.6KB, pdf)
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study. The data extraction output is available as supplementary material.