Abstract
Introduction
Atopic dermatitis (AD) is a chronic, inflammatory skin condition significantly affecting quality of life. A small randomised trial showed an approximately one-third lower incidence of AD in goat milk formula-fed compared with cow milk formula-fed infants. However, due to limited statistical power, AD incidence difference was not found to be significant. This study aims to explore a potential risk reduction of AD by feeding a formula based on whole goat milk (as a source of protein and fat) compared with a formula based on cow milk proteins and vegetable oils.
Methods and analysis
This two-arm (1:1 allocation), parallel, randomised, double-blind, controlled nutritional trial shall enrol up to 2296 healthy term-born infants until 3 months of age, if parents choose to start formula feeding. Ten study centres in Spain and Poland are participating. Randomised infants receive investigational infant and follow-on formulas either based on whole goat milk or on cow milk until the age of 12 months. The goat milk formula has a whey:casein ratio of 20:80 and about 50% of the lipids are milk fat from whole goat milk, whereas the cow milk formula, used as control, has a whey:casein ratio of 60:40 and 100% of the lipids are from vegetable oils. The energy and nutrient levels in both goat and cow milk formulas are the same. The primary endpoint is the cumulative incidence of AD until the age of 12 months diagnosed by study personnel based on the UK Working Party Diagnostic Criteria. The secondary endpoints include reported AD diagnosis, measures of AD, blood and stool markers, child growth, sleep, nutrition and quality of life. Participating children are followed until the age of 5 years.
Ethics and dissemination
Ethical approval was obtained from the ethical committees of all participating institutions.
Trial registration number
Keywords: Eczema, NUTRITION & DIETETICS, Paediatric dermatology, IMMUNOLOGY
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Potential confounding is minimised due to the randomised study design.
A multicentre study design with sites in different countries increases external validity of study results.
The follow-up until 5 years of age allows to examine long-term effects of infant feeding.
Effect sizes may be limited due to the short-time period of consuming study formula as the only food.
Introduction
Atopic dermatitis (AD), also known as eczema or atopic eczema, is a chronic, inflammatory, pruritic skin condition that frequently occurs in children1 and adults. It is characterised by intense itch, recurrent eczematous lesions and a fluctuating course. AD affects 15%–30% of children in industrialised countries.2 The highest frequency of AD onset is reported for the first year of life, but it can start in later phases of childhood and even in adult age.3 4 It is reported to often be the prelude to an atopic march including food allergies, asthma and allergic rhinoconjunctivitis.5
The strongest risk factor for AD is a positive family history of AD and atopic diseases in general, with a 4.7-fold risk increase if both father and mother were affected by AD.6 This is in agreement with the identification of 34 specific genomic regions that seem associated with AD susceptibility, including the strongest genetic risk factor for AD, the semidominant null mutations in the filaggrin gene.7 This gene encodes the epidermal protein filaggrin and the mutation causes a reduction in filaggrin expression.7 Further factors influencing AD risk include climate, place of residence, household pets, diet, prolonged breast feeding, obesity, physical activity, pollution, day care attendance, basic hygiene, family size, infections in childhood, applications of antibiotics and use of emollients.8
The clinical phenotype observed in individuals with AD is variable. To support diagnosis, several sets of criteria considering the intermittent nature of AD and possible fluctuations in AD activity have been developed including the UK Working Party Criteria.9–11 Validated scoring systems such as the Scoring Atopic Dermatitis (SCORAD) or the Patient-Oriented Eczema Measure (POEM) have been introduced.12–15
Taking the considerable loss of quality of life16 and associated disease risks in children affected by AD into account, infant feeding schemes for the general population associated with a decreased risk of AD manifestation would be highly desirable. So far, no generally accepted strategies for primary prevention of AD are available. For infants at high risk of developing AD, a 4-month period of breast feeding might be advisable, but results are controversial.17 Formulas based on hydrolysed proteins, as well as prebiotics and probiotics, were reported to provide protective effects but results are inconsistent.18–20
Because of cross-reactivity, goat milk proteins can induce reactions in infants allergic to cow milk proteins, which precludes the recommendation of goat milk protein-based formulas for infants allergic to cow milk protein.21 Nevertheless, there are indications from animal studies that goat milk is less allergenic than cow milk,22 23 although such differences are not confirmed in all studies.24 The allergenic protein αs1-casein is the dominant casein in cow milk, with 12–15 g/L. In contrast, goat milk has variable levels of this protein dependent on the genotype of the goats, ranging from 0.9 to 7 g/L. In addition, caseins from goat milk are broken down to a greater extent than those from cow milk during digestion, corresponding to a potentially lower allergenic burden from goat milk.25 Although there is an 88% sequence homology between cow and goat αs1-casein, a recent study in mice found the goat milk protein less sensitising than the cow milk protein.26
A multicentre, double-blind, controlled feeding trial in Australia found that an infant formula based on cow milk proteins (n=101) and vegetable oils and a formula based on whole goat milk (n=99) were both well tolerated and supported physiological growth comparable with breastfed infants (n=101).27 This is in agreement with two other studies performed in New Zealand28 and China,29 which tested formulas based on whole goat milk and goat milk protein, respectively. The Australian study included assessment of dermatitis using SCORAD and found an incidence of 23% in the cow milk formula group compared with only 14% in the goat milk formula group.27 Although this corresponds to an approximately one-third lower incidence of AD, the difference was not statistically significant (Fisher′s exact test) given that the study was powered to evaluate potential growth differences, but not differences in AD incidence between groups.27 The addition of cow milk fat globule membranes to infant formula had shown positive effects on the neurological development of the infants and a decreased use of antipyretics, which could indicate less inflammation.30 31 In a murine model of AD, inclusion of goat milk lipids into the diet had reduced inflammation.32 The complexity of goat milk lipids, including sterols, sphingolipids and glycerophospholipids, seems similar to cow milk lipids.33 34 The different proteins and polar lipids in the formula let us expect effects on the plasma metabolome and the gut microbiome as suggested by previous human and animal studies, respectively.35–37 These biomarkers might enable mechanistic insights into associations between infant diet and the risk of AD development.
Therefore, the Goat Infant Formula Feeding and Eczema (GIraFFE) Study tests whether infant feeding with a formula based on whole goat milk (protein and fat) reduces the risk of developing AD when compared with a formula based on cow milk proteins and vegetable oils. Second, the study aims to contribute to the identification of risk factors for AD and elucidation of the mechanistic understanding of the immune system development, and to provide a resource for studying other questions related to infant nutrition and development.
Primary objective
The primary objective of this trial is to determine the relative risk of developing AD in the first 12 months in infants fed a formula based on whole goat milk compared with infants fed a formula based on cow milk.
Secondary objectives
The secondary objectives are related to AD and other atopic diseases but also to the child′s growth and well-being, including infant metabolism and gut health, in the first 5 years of life. All outcomes will be compared for an effect of the study formula treatment (goat formula vs cow formula). The study will also explore associations of AD and other atopic diseases and overall development, and aims to identify risk indicators.
Methods and analysis
Study design and population
The GIraFFE Study is a randomised, double-blind, parallel-group, superiority clinical trial to study the effect of feeding infants a whole goat milk or a whey-adjusted cow milk formula during the first year of life on the risk of allergy and other health outcomes, including growth and quality of life, in the first 5 years of life. The study is led by the key principal investigator Professor Dr Berthold Koletzko and conducted as a multicentre trial in currently four study centres in Poland and six study centres in Spain, which all have local principal investigators.
The study population consists of healthy term infants of parents who decided to start formula feeding, without a preselection for children with an increased risk of AD. The study teams proactively promote, support and protect breast feeding. Only infants of parents who decided to start formula feeding are enrolled into the study but are encouraged to continue partial breast feeding after enrolment. The infants participating need to fulfil the criteria depicted in table 1.
Table 1.
Inclusion and exclusion criteria of the GIraFFE Study
| Inclusion criteria | Exclusion criteria |
|
|
AD, atopic dermatitis; GIraFFE, Goat Infant Formula Feeding and Eczema.
Study formulas
Participants are randomly assigned to receive one of the two formulas manufactured by Dairy Goat Co-operative (NZ) (Hamilton, New Zealand). The goat milk formula is already marketed as Capricare™; it is based on whole goat milk as a source of protein (20:80 whey:casein ratio) and goat milk fat contributes 50% of total fat. The control formula is based on cow skim milk and whey protein powders (60:40 whey:casein ratio) and vegetable oils as the almost only source of fat. The study formulas are isocaloric, have the same macronutrient composition and are provided as infant and follow-on formulas (online supplemental table 1). The composition of all formulas complies with European Commission Delegated Regulation 2016/127.
bmjopen-2022-070533supp001.pdf (105.2KB, pdf)
The key differences are (1) the source of milk from cows or goats, (2) the whey:casein ratio, and (3) the fat source.
Study product intake and compliance
Feeding of study formulas can begin immediately after enrolment, but must start no later than the age of 4 months and continues until the age of 12 months. The study formula is fed ad libitum and shall be the only formula given to the participating infant. If infants do not consume at least some study formula before the infant is 4 months old, the infants are excluded from the study.
Preparation and feeding guidelines are identical for both study formulas and are in agreement with common practice. The study teams advise not to use follow-on formula prior to the infant age of 6 months, but it is the parent’s decision whether and when to introduce follow-on formula. Compliance is defined as a continuous study formula consumption over the whole intervention period without any breaks longer than 3 consecutive days and no introduction of solid foods before the age of 4 months. Compliance will be checked at all scheduled study contacts and plausibility of continuous consumption will be checked by the number of consumed cans.
Outcome measurements
The primary endpoint of the GIraFFE Study is the cumulative incidence of AD up to the age of 12 months diagnosed by study personnel, defined as meeting the UK Working Party Diagnostic Criteria for AD. The secondary endpoints are listed in table 2.
Table 2.
Secondary endpoints of the GIraFFE Study
| Secondary endpoints | Time frame (age) |
| Cumulative incidence of study personnel-diagnosed AD, defined as meeting the UK Working Party Diagnostic Criteria for AD | up to 24 and 60 months |
| Cumulative incidence of parental-reported diagnosis of AD defined as meeting the UK Working Party Diagnostic Criteria for AD, in a telephone interview or parental report of a non-study doctor diagnosis in addition to the study diagnosis of AD | up to 12, 24 and 60 months |
| Point incidence of study diagnosed and parental-reported AD, defined as meeting the UK Working Party Diagnostic Criteria for AD | at 4, 6, 12, 24 and 60 months |
| Age at first study diagnosis, parental report-based study diagnosis or parental report of a diagnosis of AD by a non-study doctor | up to 12, 24 and 60 months |
| AD severity in children with diagnosed (study diagnosis or reported diagnosis) AD, using SCORAD questionnaire completed by study personnel at all face-to-face visits | 4, 6, 12, 24 and 60 months |
| AD severity in children with diagnosed (study diagnosis or reported diagnosis) AD, using POEM questionnaire completed by parents at all scheduled contacts | 4, 6, 8, 10, 12, 18, 24, 36, 48 and 60 months |
| Cumulative use of eczema-related medication or skin care | up to 12, 24 and 60 months |
| Parental report of a clinical diagnosis of food allergy | 12, 24 and 60 months |
| Parental reported hay fever and asthma-related diseases | up to 12, 24 and 60 months |
| Anthropometric measures (weight-for-age, length-for-age and BMI-for-age z-scores) at baseline | at 4, 6, 12, 24 and 60 months |
| Parental report of gastrointestinal symptoms (Infant Gastrointestinal Symptom Questionnaire) and sleep (Brief Infant Sleep Questionnaire) | at 4, 6 and 12 months |
| Quality of life in children using the Infant Toddler Quality Of Life Questionnaire filled by parents | at 4, 12, 24 and 60 months |
| Nutrition questionnaire | at 4, 6, 8, 10, 12 and 60 months |
| Allergic sensitisation (total and specific IgEs including cow milk protein and goat milk) | at 12 and 60 months |
| Blood lipids, metabolome, lipidome and further exploratory markers | at 4, 12 and 60 months |
| Gut microbiome | at 4, 12 and 60 months |
AD, atopic dermatitis; BMI, body mass index; GIraFFE, Goat Infant Formula Feeding and Eczema; POEM, Patient-Oriented Eczema Measure; SCORAD, Scoring Atopic Dermatitis.
Sample size
The number of subjects to be studied was based on the incidence of AD in the population and the effect size to be detected. Reported AD incidence estimates for young children in Spain and Poland are 13% and 17%, respectively.38 39 The previous study comparing goat and cow milk formulas had indicated a risk reduction for AD incidence of 30%.27 Thus, we assume a cumulative incidence of AD at 15% in the first 12 months of life, based on the cited data, and a 30% clinically relevant risk reduction by whole goat milk formula. A sample size of 861 infants per group is required to set the significance level to 0.05 and statistical power to 80%. We estimate the dropout rate until the age of 12 months to be 25%. Thus, 1148 infants per group (in total 2296) need to be studied. If the dropout rate turns out to differ markedly from the assumption, the number of infants to be recruited may be adjusted during the study.
Recruitment
Precautions are taken to ensure that recruitment does not undermine breastfeeding intentions and practice. Due to differences in healthcare systems and local infrastructure, the way to approach and recruit subjects is different for each study centre. In most cases, those families who expressed their decision to partially or fully formula feed are made aware of the study in paediatric practices or primary healthcare centres. In any case, parents are not informed about the study until they had decided to feed the baby with formula or both formula and breastmilk, in order not to interfere with breast feeding. The recruitment of study participants has started in January 2021 and is currently ongoing in all 10 study centres.
Blinding and randomised allocation of study formulas
The study is double blinded using four different three-character codes, two for each study product. Study personnel, biostatistician, data manager, trial monitor, laboratory analysts and all persons involved in the organisation and conduct of the study and study participants are blinded. Study products are shipped to the participating families and the sites by logistic partners.
For the allocation of the subjects to the four study codes minimisation, randomisation (1:1:1:1 ratio) is applied with centres as the only strata.40 41 The dynamic randomisation method minimises imbalances in age at randomisation and sex. A random element makes assignment unpredictable with a maximal group difference of ±4 children allowed. The randomised allocation sequence is provided as part of the study management tool by CSAM MedSciNet UK (Reading, UK) based on a published procedure.42
Data collection, management and analysis
Data collection and management
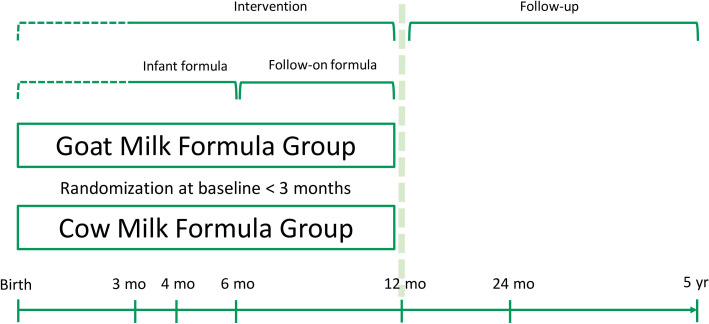
During the intervention, study centre visits are planned at enrolment (=baseline and randomisation), at 4, 6 and 12 months of age, and during the follow-up visits at 24 and 60 months (figure 1). Telephone contacts after enrolment and at 2 and 3 months of age are done, depending on the age at enrolment. After the face-to-face visits at age 4 and 6 months, which aim to collect data during the phase of dominating formula feeding (4 months) and the age of high incidence of AD in the Australian study at age 6 months,27 phone calls are scheduled at age 8 and 10 months for further data collection and to support protocol compliance and study logistics during the intervention period. During the follow-up, telephone calls are performed at 18, 36 and 48 months of age to collect data and enhance contacts with the participating families.
Figure 1.
Schematic representation of the study design.
An initial screening for eligibility is performed at the first contact with potentially participating families, and at the enrolment visit prior to randomisation, the subject’s suitability according to inclusion and exclusion criteria is confirmed. Families willing to participate sign the informed consent form. A template of informed consent form is enclosed in the online supplemental material. At the baseline visit, information about atopic diseases of parents and siblings, pregnancy information, birth data, socioeconomic background, the home environment, the child’s medical history and details of feeding practices since birth are collected. At all study visits, anthropometric measurements are performed.
bmjopen-2022-070533supp002.pdf (1.4MB, pdf)
The UK Working Party Diagnostic Criteria are used for AD diagnosis at enrolment and at all subsequent visits and telephone calls until the age of 60 months. Criteria are adapted for children under the age of 12 months in respect to time frame and body areas considered and at the telephone calls, when no direct visual inspection is possible and parental report at the visit day is documented.
Two questionnaires are used for the assessment of the severity of diagnosed AD (study diagnosis or reported diagnosis): (1) SCORAD questionnaire at all face-to-face visits, and (2) POEM questionnaire at all examination time points up to 60 months of age. For all children who were ever study diagnosed with AD, this reflects the objective view of trained medical personnel (SCORAD)13 14 and the more subjective view of the parents (POEM).12 15
The introduction of complementary feeding, use of cow milk and cow milk products, allergenic foods, use of beverages and food preferences is assessed with questionnaires at 4, 6, 8, 10 and 12 months. At the 12-month and 60-month visits, Food Frequency Questionnaires (FFQs) are used for a more detailed assessment of dietary habits. The FFQ was modified according to the age of children, based on an FFQ applied in the Identification and prevention of Dietary- and lifestyle-induced health EFfects In Children and infantS Project.43
During all telephone calls and visits in the intervention period, intake and acceptance of the formula is assessed as compliance indicator. For adverse event (AE) recording, participating families are asked in all scheduled visits and telephone calls for hospitalisation, illness and any medication of the child.
Parents are explicitly asked for a doctor’s diagnosis of food allergies at 12, 24 and 60 months with a specific focus on cow milk, egg, peanuts, soy and fish. Furthermore, asthma, bronchitis/bronchiolitis, wheezing and allergic rhinitis at the 60-month visit with distinction between self-observation and doctor diagnosis are assessed.
During the intervention period, questionnaires about sleep (Brief Infant Sleep Questionnaire)44 and gastrointestinal problems (Infant Gastrointestinal Symptom Questionnaire)45 are applied at the face-to-face visits. Furthermore, the Infant Toddler Quality Of Life Questionnaire46 is completed at 4, 12, 24 and 60 months by parents. Parents are asked at enrolment, and at all contacts from 4 months on about general skin care and if there has been a prescription of topical treatment like corticosteroid or other immunosuppressive therapies by a physician since last visit.
Data are collected primarily with a web-based online database developed by CSAM MedSciNet UK (Reading, UK) with direct data entry by study personnel and participating families as default option. The use of paper forms is limited to situations where the direct input into the database is technically not possible or not wished by parents. Furthermore, copies of signed consent forms are stored electronically. All procedures are checked for general data protection regulation conformity by a Ludwig Maximilian University (LMU) data protection officer.
Biosamples
Blood collection is planned at the 4-month, 12-month and 60-month visits. Standard operating procedures (SOPs) are in place. Highest priority is given to the analysis of atopy-related parameters such as total IgE, specific IgEs for cow and goat milk protein, as well as further frequent allergens and inflammation markers. Serum lipids (total cholesterol, high-density lipoprotein-cholesterol, triglycerides) and lipidomic and metabolomic analyses aim at describing the metabolism of the infants in respect to formula consumed and for the identification of biochemical risk markers or eventual metabolic consequences of AD. As a safety indicator, full blood count is taken from all blood samples. If corresponding consent has been obtained, filaggrin genotype will be determined and further genetic analysis performed if additional funding is granted.
For microbiome analyses, stool samples are collected at 4, 12 and 60 months in a subgroup of 600 infants. At enrolment, interested families receive the stool collection material as well as written instructions. A questionnaire is used to record the classification of the stool sample on the Brussels Infant and Toddler Stool Scale and the administration of probiotics. According to the standardised procedures, samples should be frozen at −20°C within less than 15 min after collection; samples have to be transferred to a −80°C freezer within a week. The details of microbiome analyses have not been fully defined yet, but will apply established DNA extraction and amplification methods and corresponding bioinformatics tools.
Adverse events
AEs are recorded according to a standardised protocol including an opinion on the assumed relation to the intervention and a categorisation of the AEs. During the intervention and until 30 days after study product intake, all safety events fulfilling the following criteria are reported as AEs.
-
Child was treated with:
Medication >14 days.
Oral antibiotics.
Inhalation therapy.
Steroids, salbutamol, antihistamines, montelukast.
Child was hospitalised.
Child was treated with a special diet >7 days.
Child interrupted the intake of the study product >1 day or completely discontinued consumption.
From 31 days after the last product intake, only safety events, fulfilling the applicable criteria, and considered as potentially related to the intervention or that may influence study outcomes, are reported as AEs.
Any AE that results in death, is life-threatening, requires hospitalisation or results in persistent or significant disabilities is classified as serious AE (SAE). The principal investigators of the individual study centres review all SAEs at their centre and provide an opinion, including a comment on the relation to the intervention.
A clinical trial insurance has been set up.
Monitoring
An external monitor performed the study monitoring during the first 10 months of the study recruitment. After this period, monitoring activities were taken over by LMU researchers. Monitoring should improve the quality of the collected data but mainly focuses on the compliance of all local study procedures with the protocol, established SOPs and good clinical practice. Besides on-site monitoring, additional remote monitoring is also performed.
A Data and Safety Monitoring Board (DSMB) has been established, with the primary responsibility of reviewing and evaluating data for participant safety and study progress including a critical review of the findings after the first 128 participants have completed the intervention period. The DSMB review focuses on interim/cumulative data of study-related AEs, individual centre performance, protocol deviations and external factors such as scientific or therapeutic developments that may have an impact on participant safety or raise ethical concerns. Based on the accumulated study data, the board makes recommendations concerning continuation, modification or eventual termination of the GIraFFE Study.
The DSMB consists of three members who have no direct involvement in the conduct of the study, financial, professional or other interests that may affect independent decision-making.
If the recruitment rate is less than 50% of the expected rate after 12 months, or if the primary objective yields no effect of the intervention, the study may be terminated prematurely. After consulting with the trial steering committee, the sponsor and key principal investigator will decide about the premature study termination.
Statistical analysis
Statistical analyses are scheduled when all recruited infants have passed 12-month, 24-month and 60-month visits, respectively. All primary and secondary analyses including methods to deal with missing data and subgroup analyses are to be specified in a Statistical Analysis Plan, which is finalised prior to database lock and unblinding.
As primary statistical analysis, a comparison of the cumulative incidence of children with AD until 12 months of age between the goat milk formula group and cow milk formula group is planned. For this analysis, a generalised estimating equation Poisson model with a log link and robust SEs by sandwich estimators of variance will be used.47
The findings are compared with further adjusted models that include major influencing factors of AD frequency, including country, sex, filaggrin genotype, parental atopic diseases, parental AD, antibiotic usage, family size and socioeconomic status. Furthermore, interactions of filaggrin mutations, the number of immediate family members with AD or other atopic disease with AD frequency shall be investigated. If effect modification by one of the mentioned predictive covariates is significant at the 5% level, subgroup analyses for each category will be presented.
Secondary analyses will look at the secondary objectives with similar statistical approaches.
Ethics and dissemination
Ethical approval was obtained from the ethical committee of the LMU Hospital Munich, Germany (number 20-188; ethikkommission@med.uni-muenchen.de) and the ethical committees of all 10 study centres: Hospital Universitario La Paz, Madrid (ref. 47/322688.9/20; ref: 47/748801.9/21); CEIC Aragón, Zaragoza (CP-CI PI20/098); Hospital Clínico Universitario de Valencia (ref. CEIm 2020/219); Institut d’Investigació Sanitària Pere Virgili, Reus/Tarragona (ref. CEIM: 057/2020); CEIM/CEI Andalucía, Delegación Provincial de Granada (ref. CEIM/CEI: 1134-M1-20); Hospital Universitario Torrecárdenas, Almeria (ref. CEIM: 109/2019), Warmińsko–Mazurskiej Izbie Lekarskiej w Olsztynie (number 1/2020/VII); Poznań University of Medical Sciences (number 436/20); University of Rzeszów (number 05/07/2020); Instytucie ‘Pomnik-Centrum Zdrowia Dziecka’ (12/KBE/2020).
Currently, protocol version 1.1 has been valid since 25 June 2020. The ethical committees will approve all protocol amendments prior to implementation.
Patient and public involvement
The protocol for the study including all procedures related to subject safety and protection of personal data was predominantly developed at a public hospital, but without specific patient consultations.
Public dissemination and data availability
Researchers and sponsor are committed to publish the study findings in peer-reviewed international scientific journals. Dissemination of study results may also include posting of a synopsis online, abstracts submitted to and presentations at scientific conferences, and other dissemination activities including social media.
After a delay period for full scientific evaluation, the remaining biosamples and associated data of participants, for whom respective consent is available, will be transferred into a registered biobank (Hauner biobank, LMU Munich). Data and samples will be accessible for other researchers according to the biobank regulations.
Supplementary Material
Acknowledgments
The members of the GIraFFE Study group contributed to the realisation of the study. The GIraFFE Study group consists of the following members: Carme Rubio-Torrents, Ester Parada-Ricart, Natalia Ferré, Veronica Luque (Tarragona/Reus); Encarnación López-Ruzafa, Melinda Moriczi (Almeria); Elena Crehuá-Gaudiza, Cecilia Martínez-Costa (Valencia); Gerardo Rodriguez, María Luisa Álvarez, Cristina Guillén, María Perán, Laura García, Sheila García (Zaragoza); Bibiana Chinea, Ariadna Witte, Esperanza Escribano (Madrid); Jose Antonio García-Santos, Mireia Escudero-Marín, Rocío Bonillo-León (Granada); Janusz Książyk, Alicja Syc, Aleksandra Żyła-Pawlak (Warsaw); Artur Mazur (Rzeszów); Małgorzata Jamka, Aleksandra Lisowska (Poznań). Colin Prosser (DGC), Philipp Schwarzfischer and Sandra Unterschemmann (LMU) contributed to the development or the implementation of the protocol.
Footnotes
Contributors: The conception and design of the study were developed by BK, VG, HD, UH and SG. JMF produced the first draft of the manuscript and all coauthors RG-M, CC, MSdP, EJ-C, JW, BR, JE, MG, PG, DG, II, VG, HD, UH, SG and BK critically reviewed the manuscript and approved the final version. RG-M, CC, MSdP, EJ-C, JW, BR, JE, MG, PG, DG and II participated in the set-up of the study.
Funding: The study products are manufactured and provided to participants by the study sponsor (Dairy Goat Co-operative (NZ), Hamilton, New Zealand). The sponsor has allocated a fixed budget to each of the institutions hosting the study centre and the key principal investigator with his team to conduct the study. This work was supported by Dairy Goat Co-operative (NZ) Limited and the New Zealand Ministry for Primary Industries as part of the Caprine Innovations NZ Sustainable Food & Fibre Futures Partnership Programme (grant number PGP06-16001). BK is the Else Kröner Seniorprofessor of Paediatrics at LMU – University of Munich, financially supported by the charitable Else Kröner-Fresenius-Foundation, LMU Medical Faculty and LMU University Hospitals.
Disclaimer: The sponsor, the site principal investigators and the key principal investigator have agreed and fixed in the study protocol that the final decision-making power on the study rests with the trial steering committee, which includes the key principal investigator, all site principal investigators and the sponsor. The trial steering committee also takes decisions on further grant applications to fund additional analyses of data and biosamples generated in GIraFFE.
Competing interests: SG is an employee of DGC (Dairy Goat Co-operative (NZ), Hamilton, New Zealand).
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Solman L, Lloyd-Lavery A, Grindlay DJC, et al. What’s new in atopic eczema? An analysis of systematic reviews published in 2016. Part 1: treatment and prevention. Clin Exp Dermatol 2019;44:363–9. 10.1111/ced.13885 [DOI] [PubMed] [Google Scholar]
- 2.Bieber T. Mechanisms of disease: atopic dermatitis. N Engl J Med 2008;358:1483–94. 10.1056/NEJMra074081 [DOI] [PubMed] [Google Scholar]
- 3.Illi S, von Mutius E, Lau S, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol 2004;113:925–31. 10.1016/j.jaci.2004.01.778 [DOI] [PubMed] [Google Scholar]
- 4.Garmhausen D, Hagemann T, Bieber T, et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy 2013;68:498–506. 10.1111/all.12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider L, Hanifin J, Boguniewicz M, et al. Study of the atopic March: development of atopic comorbidities. Pediatr Dermatol 2016;33:388–98. 10.1111/pde.12867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apfelbacher CJ, Diepgen TL, Schmitt J. Determinants of eczema: population-based cross-sectional study in Germany. Allergy 2011;66:206–13. 10.1111/j.1398-9995.2010.02464.x [DOI] [PubMed] [Google Scholar]
- 7.Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers 2018;4:1. 10.1038/s41572-018-0001-z [DOI] [PubMed] [Google Scholar]
- 8.Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab 2015;66 Suppl 1:8–16. 10.1159/000370220 [DOI] [PubMed] [Google Scholar]
- 9.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol 1980;60:44–7. 10.2340/00015555924447 [DOI] [Google Scholar]
- 10.Williams HC, Burney PG, Hay RJ, et al. The U.K. working party’s diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol 1994;131:383–96. 10.1111/j.1365-2133.1994.tb08530.x [DOI] [PubMed] [Google Scholar]
- 11.Fleming S, Bodner C, Devereux G, et al. An application of the United Kingdom working party diagnostic criteria for atopic dermatitis in scottish infants. J Invest Dermatol 2001;117:1526–30. 10.1046/j.0022-202x.2001.01579.x [DOI] [PubMed] [Google Scholar]
- 12.Spuls PI, Gerbens LAA, Simpson E, et al. Patient-oriented eczema measure (POEM), a core instrument to measure symptoms in clinical trials: a harmonising outcome measures for eczema (home) statement. Br J Dermatol 2017;176:979–84. 10.1111/bjd.15179 [DOI] [PubMed] [Google Scholar]
- 13.Kunz B, Oranje AP, Labrèze L, et al. Clinical validation and guidelines for the SCORAD index: consensus report of the European task force on atopic dermatitis. Dermatology 1997;195:10–9. 10.1159/000245677 [DOI] [PubMed] [Google Scholar]
- 14.Oranje AP, Glazenburg EJ, Wolkerstorfer A, et al. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol 2007;157:645–8. 10.1111/j.1365-2133.2007.08112.x [DOI] [PubMed] [Google Scholar]
- 15.Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004;140:1513–9. 10.1001/archderm.140.12.1513 [DOI] [PubMed] [Google Scholar]
- 16.Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract 2006;60:984–92. 10.1111/j.1742-1241.2006.01047.x [DOI] [PubMed] [Google Scholar]
- 17.Høst A, Halken S, Muraro A, et al. Dietary prevention of allergic diseases in infants and small children. Pediatr Allergy Immunol 2008;19:1–4. 10.1111/j.1399-3038.2007.00680.x [DOI] [PubMed] [Google Scholar]
- 18.Greer FR, Sicherer SH, Burks AW, et al. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics 2019;143:e20190281. 10.1542/peds.2019-0281 [DOI] [PubMed] [Google Scholar]
- 19.Li L, Han Z, Niu X, et al. Probiotic supplementation for prevention of atopic dermatitis in infants and children: a systematic review and meta-analysis. Am J Clin Dermatol 2019;20:367–77. 10.1007/s40257-018-0404-3 [DOI] [PubMed] [Google Scholar]
- 20.Gappa M, Filipiak-Pittroff B, Libuda L, et al. Long-term effects of hydrolyzed formulae on atopic diseases in the GINI study. Allergy 2021;76:1903–7. 10.1111/all.14709 [DOI] [PubMed] [Google Scholar]
- 21.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) . Scientific opinion on the suitability of goat milk protein as a source of protein in infant formulae and in follow‐on formulae. EFS2 2012;10. 10.2903/j.efsa.2012.2603 [DOI] [Google Scholar]
- 22.Sanz Ceballos L, Sanz Sampelayo MR, Gil Extremera F, et al. Evaluation of the allergenicity of goat milk, cow milk, and their lactosera in a guinea pig model. Journal of Dairy Science 2009;92:837–46. 10.3168/jds.2008-1125 [DOI] [PubMed] [Google Scholar]
- 23.Kapila R, Kavadi PK, Kapila S. Comparative evaluation of allergic sensitization to milk proteins of cow, buffalo and goat. Small Rumin Res 2013;112:191–8. 10.1016/j.smallrumres.2012.11.028 [DOI] [Google Scholar]
- 24.Restani P. Goat milk allerginicity. J Pediatr Gastroenterol Nutr 2004;39:323–4. 10.1097/00005176-200410000-00003 [DOI] [PubMed] [Google Scholar]
- 25.Hodgkinson AJ, Wallace OAM, Boggs I, et al. Gastric digestion of cow and goat milk: impact of infant and young child in vitro digestion conditions. Food Chemistry 2018;245:275–81. 10.1016/j.foodchem.2017.10.028 [DOI] [PubMed] [Google Scholar]
- 26.Zhang K, Zhang LN, Zhou RY, et al. Cow’s milk alpha(S1)-casein is more sensitizing than goat’s milk alpha(S1)-casein in a mouse model. Food Funct 2022;13:6484–97. 10.1039/d2fo01136k [DOI] [PubMed] [Google Scholar]
- 27.Zhou SJ, Sullivan T, Gibson RA, et al. Nutritional adequacy of goat milk infant formulas for term infants: a double-blind randomised controlled trial. Br J Nutr 2014;111:1641–51. 10.1017/S0007114513004212 [DOI] [PubMed] [Google Scholar]
- 28.Grant C, Rotherham B, Sharpe S, et al. Randomized, double-blind comparison of growth in infants receiving goat milk formula versus cow milk infant formula. J Paediatr Child Health 2005;41:564–8. 10.1111/j.1440-1754.2005.00722.x [DOI] [PubMed] [Google Scholar]
- 29.Xu M, Wang Y, Dai Z, et al. Comparison of growth and nutritional status in infants receiving goat milk–based formula and cow milk–based formula: a randomized, double-blind study. Food & Nutrition Research 2015;59:28613. 10.3402/fnr.v59.28613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timby N, Hernell O, Vaarala O, et al. Infections in infants fed formula supplemented with bovine milk fat globule membranes. J Pediatr Gastroenterol Nutr 2015;60:384–9. 10.1097/MPG.0000000000000624 [DOI] [PubMed] [Google Scholar]
- 31.Timby N, Domellöf E, Hernell O, et al. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial. Am J Clin Nutr 2014;99:860–8. 10.3945/ajcn.113.064295 [DOI] [PubMed] [Google Scholar]
- 32.Woods K, Cait A, Gell K, et al. Goat milk-derived lipids restrain NK T cell-dependent eosinophilic inflammation in a murine model of atopic dermatitis. J Invest Dermatol 2022;142:2541–3. 10.1016/j.jid.2022.03.006 [DOI] [PubMed] [Google Scholar]
- 33.Lagutin K, MacKenzie A, Bloor S, et al. HPLC-MS, GC and NMR profiling of bioactive lipids of human milk and milk of dairy animals (cow, sheep, goat, buffalo, camel, red deer). Separations 2022;9:145. 10.3390/separations9060145 [DOI] [Google Scholar]
- 34.Gallier S, Tolenaars L, Prosser C. Whole goat milk as a source of fat and milk fat globule membrane in infant formula. Nutrients 2020;12:3486. 10.3390/nu12113486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Q, Yin Q, Xie Q, et al. Elucidating gut microbiota and metabolite patterns shaped by goat milk-based infant formula feeding in mice colonized by healthy infant feces. Food Chemistry 2023;410:135413. 10.1016/j.foodchem.2023.135413 [DOI] [PubMed] [Google Scholar]
- 36.Tannock GW, Lawley B, Munro K, et al. Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk-based formula, or breast milk. Appl Environ Microbiol 2013;79:3040–8. 10.1128/AEM.03910-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He X, Parenti M, Grip T, et al. Metabolic phenotype of breast-fed infants, and infants fed standard formula or bovine MFGM supplemented formula: a randomized controlled trial. Sci Rep 2019;9. 10.1038/s41598-018-36292-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Draaisma E, Garcia-Marcos L, Mallol J, et al. A multinational study to compare prevalence of atopic dermatitis in the first year of life. Pediatr Allergy Immunol 2015;26:359–66. 10.1111/pai.12388 [DOI] [PubMed] [Google Scholar]
- 39.Kamer B, Pasowska R, Dółka E, et al. Prevalence of atopic dermatitis in infants during the first six months of life: authors’ observations. Postepy Dermatol Alergol 2013;30:277–81. 10.5114/pdia.2013.38355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White SJ, Freedman LS. Allocation of patients to treatment groups in a controlled clinical study. Br J Cancer 1978;37:849–57. 10.1038/bjc.1978.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott NW, McPherson GC, Ramsay CR, et al. The method of minimization for allocation to clinical trials: a review. Control Clin Trials 2002;23:662–74. 10.1016/s0197-2456(02)00242-8 [DOI] [PubMed] [Google Scholar]
- 42.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103–15. [PubMed] [Google Scholar]
- 43.Lanfer A, Hebestreit A, Ahrens W, et al. Reproducibility of food consumption frequencies derived from the children’s eating habits questionnaire used in the IDEFICS study. Int J Obes (Lond) 2011;35 Suppl 1:S61–8. 10.1038/ijo.2011.36 [DOI] [PubMed] [Google Scholar]
- 44.Sadeh A. A brief screening questionnaire for infant sleep problems: validation and findings for an internet sample. Pediatrics 2004;113:e570–7. 10.1542/peds.113.6.e570 [DOI] [PubMed] [Google Scholar]
- 45.Riley AW, Trabulsi J, Yao M, et al. Validation of a parent report questionnaire: the infant gastrointestinal symptom questionnaire. Clin Pediatr 2015;54:1167–74. 10.1177/0009922815574075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Landgraf JM, Vogel I, Oostenbrink R, et al. Parent-reported health outcomes in infants/toddlers: measurement properties and clinical validity of the ITQOL-SF47. Qual Life Res 2013;22:635–46. 10.1007/s11136-012-0177-8 [DOI] [PubMed] [Google Scholar]
- 47.Pedroza C, Truong VTT. Estimating relative risks in multicenter studies with a small number of centers-which methods to use? A simulation study. Trials 2017;18:512. 10.1186/s13063-017-2248-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-070533supp001.pdf (105.2KB, pdf)
bmjopen-2022-070533supp002.pdf (1.4MB, pdf)