Abstract
Introduction
Epidemiological evidence supports an association between higher levels of physical activity and improved cancer survival. Trial evidence is now needed to demonstrate the effect of exercise in a clinical setting. The Exercise during CHemotherapy for Ovarian cancer (ECHO) trial is a phase III, randomised controlled trial, designed to determine the effect of exercise on progression-free survival and physical well-being for patients receiving first-line chemotherapy for ovarian cancer.
Methods and analysis
Participants (target sample size: n=500) include women with newly diagnosed primary ovarian cancer, scheduled to receive first-line chemotherapy. Consenting participants are randomly allocated (1:1) to either the exercise intervention (plus usual care) or usual care alone, with stratification for recruitment site, age, stage of disease and chemotherapy delivery (neoadjuvant vs adjuvant). The exercise intervention involves individualised exercise prescription with a weekly target of 150 minutes of moderate-intensity, mixed-mode exercise (equivalent to 450 metabolic equivalent minutes per week), delivered for the duration of first-line chemotherapy through weekly telephone sessions with a trial-trained exercise professional. The primary outcomes are progression-free survival and physical well-being. Secondary outcomes include overall survival, physical function, body composition, quality of life, fatigue, sleep, lymphoedema, anxiety, depression, chemotherapy completion rate, chemotherapy-related adverse events, physical activity levels and healthcare usage.
Ethics and dissemination
Ethics approval for the ECHO trial (2019/ETH08923) was granted by the Sydney Local Health District Ethics Review Committee (Royal Prince Alfred Zone) on 21 November 2014. Subsequent approvals were granted for an additional 11 sites across Queensland, New South Wales, Victoria and the Australian Capital Territory. Findings from the ECHO trial are planned to be disseminated via peer-reviewed publications and international exercise and oncology conferences.
Trial registration number
Australian New Zealand Clinical Trial Registry (ANZCTRN12614001311640; https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367123&isReview=true).
Keywords: GYNAECOLOGY, ONCOLOGY, PUBLIC HEALTH
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Randomised controlled study design.
Evaluation of exercise therapy delivered using an equitable mode of delivery, that is, telehealth delivery that is accessible to all.
Focus on a cancer type (specifically, ovarian cancer) that is understudied and under-represented in exercise oncology trials to date.
Adequately powered evaluation of practice-changing outcomes including progression-free survival, cost-effectiveness and chemotherapy adherence.
At the time of study design, it was unclear whether women receiving chemotherapy for newly diagnosed ovarian cancer could safely meet exercise prescription targets and the extent to which exercise dose completed would influence exercise effect.
Introduction
The benefits of exercise for people with cancer, both during and following treatment, have been extensively investigated.1 2 Consistently, high-quality evidence supports the beneficial impact of exercise on a wide range of physical, psychosocial and emotional health outcomes, but particularly for physical function, health-related quality of life, fatigue, anxiety and depression.1 Epidemiological evidence also supports a positive association between physical activity and survival outcomes. Specifically, findings from a meta-analysis including mostly data from observational studies found that risk of cancer-specific and all-cause mortality was 58% and 63% lower, respectively, for those in the highest category of physical activity post-cancer compared with those in the lowest physical activity category.3 Further, exploratory findings from two randomised controlled trials involving patients with breast cancer have also shown a positive effect of exercise on survival outcomes.4 5
The compelling evidence base supporting the role of exercise in improving the quality, and potentially quantity, of cancer survivorship has been used to develop internationally endorsed guidelines.1 2 6 These guidelines recommend that cancer survivors participate in 150 min per week of moderate-intensity, mixed-mode (aerobic and resistance) exercise. Yet, neither the scientific evidence nor the availability and promotion of these guidelines have enacted widespread integration of exercise therapy into cancer care globally or within any given country. Lack of workforce capacity, access to exercise oncology services and referral pathways, as well as associated costs (and lack of public reimbursement), have all been proposed as reasons preventing advancements in cancer care through exercise.7 However, there also remain significant evidence gaps that cannot be ignored.
The current exercise oncology evidence base is largely derived from trials involving cancer types associated with good prognosis. Specifically, 89% of trials conducted to date involve cancers with 5-year survival rates that are higher than the 5-year survival rate for all cancers combined.8 9 There is also a participant bias likely present in the majority of exercise trials, whereby participants tend to be younger, and have fewer comorbidities and a history of exercise participation, than the population from which they are drawn.10 11 Consequently, the generalisability of findings to the wider population with cancer is unclear.10 Additionally, approximately two-thirds of the interventions evaluated in exercise oncology trials are highly supervised, conducted in a gymnasium or clinic setting, and/or involve weekly exercise targets that do not align with current guidelines (eg, involve aerobic exercise only).8 The dearth of evaluation of pragmatic, translational interventions limits the external validity and transferability of findings into standard practice. Further, adequately powered clinical trials evaluating the effect of exercise on survival, cost-effectiveness and adherence to standard oncology treatment are also lacking, yet it is precisely this evidence that is needed to promote equitable and widespread uptake of exercise into standard cancer care.
Unlike those diagnosed with common solid cancers, patients with ovarian cancer are more likely diagnosed with advanced stage disease and can expect poorer prognosis.12–15 Ovarian cancer treatment typically involves extensive abdominal surgery and combination chemotherapy,13 16 which is associated with acute and persistent side effects that lead to morbidity and declines in quality of life, driven by declines in physical well-being.17–20 A population-based ovarian cancer study demonstrated that only 32% of patients who were prescribed chemotherapy completed their regimen without dose reductions or delays.13 Completion of the full chemotherapy dose is associated with longer survival, while delays, dose reductions and early cessation are associated with poorer outcomes.16 21 The current 5-year ovarian cancer survival rate is 48%, which remains lower than the 5-year survival rate achieved in 1975 for all cancers combined.22
Data collected from prospective, longitudinal cohort studies show low and decreasing levels of physical activity following diagnosis of ovarian cancer for the majority (up to 80%23 24), and that lower physical activity levels were associated with reduced quality of life and chemotherapy completion.25 As such, patients with ovarian cancer arguably represent a group with the most to gain through exercise, yet they also represent one of the least studied cancer cohorts in exercise oncology trials. Findings from a 2020 systematic review which included data from five studies (one randomised controlled trial and four pre/post-trials) evaluating the effect of an exercise intervention in women with ovarian cancer provide preliminary evidence supporting exercise as being safe and feasible, and may lead to benefits in health-related quality of life, fatigue, physical and mental function, strength, sleep and chemotherapy completion rates.26 There is a clear need for further research that seeks to reduce morbidity and improve quality of life and survival following ovarian cancer, and exercise intervention has potential for benefit.
The Exercise during CHemotherapy for Ovarian cancer (ECHO) trial is a randomised controlled clinical trial evaluating the value of physical exercise during chemotherapy for patients with newly diagnosed ovarian cancer. We hypothesise that compared with usual care, exercise therapy during chemotherapy will result in improved progression-free survival and higher scores for physical well-being at 6 and 12 months post-randomisation, and lower morbidity and more efficient use of healthcare resources compared with usual care.
Methods and analysis
This manuscript was written in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines27 (SPIRIT checklist provided in online supplemental file 1). Ethics approval was granted by the Sydney Local Health District Ethics Review Committee (2019/ETH08923), approved on 21 November 2014 (online supplemental file 2). This trial was registered with the Australian New Zealand Clinical Trial Registry (ANZCTRN12614001311640). The approved study protocol, and the participant information and consent form can be found in online supplemental files 3 and 4, respectively.
bmjopen-2022-067925supp001.pdf (119.6KB, pdf)
bmjopen-2022-067925supp002.pdf (249.5KB, pdf)
bmjopen-2022-067925supp003.pdf (1.2MB, pdf)
bmjopen-2022-067925supp004.pdf (301.8KB, pdf)
The ECHO trial is a national, multicentre, phase III randomised controlled trial being conducted in collaboration with the National Health and Medical Research Council Clinical Trials Centre (NHMRC CTC) and the Australia New Zealand Gynaecological Oncology Group. The ECHO trial aims to evaluate the enduring effects of an individualised, telehealth-delivered exercise intervention (plus usual care) versus usual care alone, during first-line chemotherapy for patients with ovarian cancer.
Patient and public involvement
Consumer representatives (authors HO, MW) are embedded within the ECHO trial. They have been involved in all stages of trial development, funding submissions and study implementation. Specifically, our consumers provided perspectives on study design, chosen outcome of interests, timing and duration of the intervention, delivery mode of intervention, and have reviewed and edited all participant-facing study documentation to minimise participant burden. Further, involvement of our consumers throughout the implementation phase continues via participation in teleconference meetings, and our consumers will lead dissemination of trial findings to consumer groups.
Eligibility criteria/participants
To be eligible, participants are required to meet all the inclusion criteria and none of the exclusion criteria presented in table 1.
Table 1.
Eligibility criteria for participation in the ECHO trial
| A patient is eligible if they meet: | |
| All the following: | None of the following: |
|
|
ECHO, Exercise during CHemotherapy for Ovarian cancer; ECOG, Eastern Cooperative Oncology Group.
Recruitment, registration and randomisation
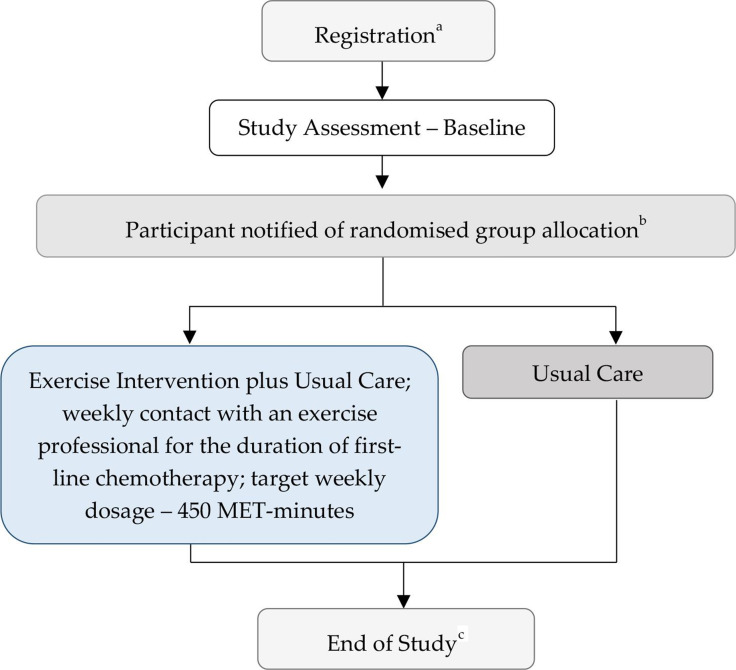
Participating sites along with their relevant ethical approvals granted are shown in online supplemental table 1 and participant flow is shown in figure 1. Potentially eligible patients are identified by clinical trial nurses, or directly referred to Griffith University by their treating gynaecological/medical oncologist. The trial is introduced and discussed with potentially eligible patients, thereafter providing patients with the participant information and consent forms, confirming eligibility status, and collecting written informed consent for all interested and eligible patients. Consenting patients are registered into a centrally administered database and randomised 1:1 to the exercise intervention (exercise intervention plus usual care) or usual care group using the Flexetrials Enrollment System (computerised, password-protected randomisation system with the NHMRC CTC). Randomisation is stratified by recruitment site, age (<70 vs ≥70 years), International Federation of Gynecology and Obstetrics stage of disease (I–II vs III–IV) and chemotherapy delivery (adjuvant vs neoadjuvant plus adjuvant). Demographic, disease and treatment-related characteristics are extracted from medical records and entered into a centralised database (OpenClinica) via an electronic case report form. Newly recruited participants are contacted via telephone, to collect a full medical history (including comorbidities and medications) and schedule a baseline assessment.
Figure 1.
Participant flow through the Exercise during CHemotherapy for Ovarian cancer trial. aRegistration includes randomisation with stratification for recruitment site, age (<70 vs ≥70 years), stage of disease (I–II vs III–IV) and chemotherapy delivery (adjuvant vs neoadjuvant plus adjuvant). Staff involved with baseline assessments and participants remain blinded to randomisation outcome at this stage. bStaff involved with data collection remain blinded to randomisation outcome. cProgression status and survival status will be updated periodically throughout trial duration and at approximately 6 months prior to end of trial. MET, metabolic equivalent.
bmjopen-2022-067925supp005.pdf (69.7KB, pdf)
Blinding and bias
Group allocation is concealed from consenting patients until post-baseline assessment. The nature of exercise prevents the participant and exercise professionals delivering the intervention from being blinded to group allocation; however, all clinicians and research staff involved with recruitment, data extraction from medical records and data entry are blinded to group allocation. Research staff collecting objectively assessed outcomes are trained to follow a validated protocol and are blinded to treatment group and previous scores.
Assessments and intervention timing
Assessments of participant-reported and objectively measured outcomes are conducted at baseline (prior to second cycle of chemotherapy), and at 6 and 12 months post-randomisation (figure 1 and online supplemental table 2). Assessments for objectively measured outcomes are conducted at a university-based or hospital-based clinic or the participant’s home. Data related to chemotherapy completion rate and chemotherapy-related adverse events are extracted from medical records post-baseline assessment and updated at 12 months post-randomisation. Participants who withdraw or stop the exercise intervention early continue completing follow-up assessments, if they agree to do so.
bmjopen-2022-067925supp006.pdf (68.8KB, pdf)
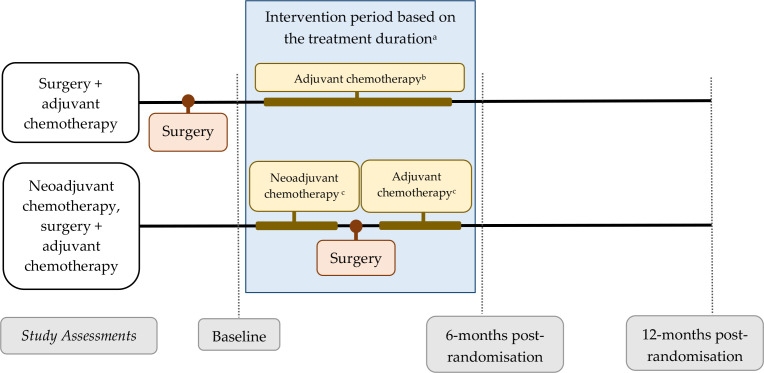
Following baseline assessment, each participant receives a group allocation letter and an Ovarian Cancer Australia ‘Resilience Pack’. The Resilience Kit contains information relating to the diagnosis and treatment of ovarian cancer, support services and staying well (including through physical activity).28 Those in the exercise group are also assigned an exercise oncology professional (ExP) and are mailed intervention materials, including an exercise tracker and a Borg Rating of Perceived Exertion Scale. Participants in the exercise group are then contacted by their allocated ExP and begin the intervention within 7 days of baseline assessment. The exercise intervention is designed to span the duration of first-line chemotherapy, which is typically scheduled over an 18-week period, but may be longer to accommodate interruptions (eg, surgery for those who have had neoadjuvant chemotherapy) or dose delays (figure 2). Should chemotherapy cease earlier due to disease progression, shorter planned chemotherapy or any other reason, continued access to the intervention as appropriate and desired by the participant, up to a maximum of 18 weeks (from commencement of intervention), is provided.
Figure 2.
Trial assessment schedule and timing of intervention for women who receive surgery plus adjuvant chemotherapy versus women who receive neoadjuvant chemotherapy, surgery plus adjuvant chemotherapy. aParticipant may commence chemotherapy prior to intervention commencement (intervention commencement is anchored to baseline assessment). Intervention may continue beyond 6 months post-randomisation assessment if treatment delays occur. bTypically six cycles of chemotherapy scheduled over 18 weeks. cTypically three cycles of neoadjuvant chemotherapy scheduled over 9 weeks, followed by three cycles of adjuvant chemotherapy scheduled over 9 weeks.
Outcomes and timing of assessment
Outlined in table 2 are the outcomes of interest, methods and timing of assessment, and a priori defined clinically relevant thresholds. Progression-free survival and physical well-being are the primary outcomes. Progression-free survival has been recommended as an appropriate survival endpoint for first-line trials in ovarian cancer, provided that the intervention is not overly toxic and that important secondary endpoints are also assessed (including overall survival, patient-reported outcomes, severity of adverse effects and economic evaluation), as is the case for the ECHO trial.29 The advantages of progression-free survival as the primary endpoint (compared with overall survival) include an earlier and more sensitive signal of anti-tumour efficacy, a lower likelihood of influence by competing risks (particularly relevant for older participants) and reduced chance of confounding from treatments received after progression.30–34 Improving progression-free survival is also a direct, tangible and important benefit to patients, as it delays cancer-related symptoms and side effects of second-line treatment.35 Physical well-being was also chosen as a primary outcome as chemotherapy-related side effects contribute to declines in physical well-being, which in turn drive declines in overall quality of life.17–20 Further, there is strong evidence from trials outside of the ovarian cancer setting, as well as from pilot work undertaken in preparation for the ECHO trial, that demonstrate exercise during chemotherapy can improve physical well-being.1 36 Secondary outcomes (listed in table 2) include outcomes that matter to the patient and their clinicians, are lacking in level 1 evidence beyond common cancer types and are significant determinants of subsequent uptake of exercise in routine clinical practice. A more detailed description of the methods of assessment for each outcome can be found in online supplemental table 3. Participants are also invited to provide blood samples for future exploratory analyses of biomarkers that are potentially prognostic and/or predictive of a response to exercise. Questionnaires and trial assessment forms (used to record objectively assessed outcome data) are scanned and imported into the trial database at the NHMRC CTC.
Table 2.
Trial outcomes, methods of assessment, clinically relevant effect size* and timing of assessment
| Outcomes of interest, assessments and clinically relevant effect (CRE) size | Time point | |||
| B | 6m | 12m | Y† | |
| Primary outcomes | ||||
| Progression-free survival | ||||
| As determined by the responsible clinician, according to pragmatic clinical criteria including imaging, serum Ca125 levels and/or commencement of further anti-cancer treatments.42 43 CRE: 25% reduction in hazard rate.40 |
X | X | ||
| Physical well-being | ||||
| Physical well-being subscale of the FACT–O (range=0–28); CRE: 2 units.44 45 | X | X | X | |
| Secondary outcomes | ||||
| Overall survival | ||||
| Date of death abstracted from medical records or death registry; CRE: 25% reduction in the hazard rate.40 | X | X | ||
| Physical function | ||||
|
Objectively measured outcomes Fitness—Six-Minute Walk Test; CRE: ≥50-metre increase.46 47 Upper-body muscular endurance—YMCA Bench Press; CRE: 0.5 SD.48 49 Lower-body muscular endurance—30-second Sit to Stand; CRE: 2 repetitions.50 Balance—Single Leg Stance (eyes closed); CRE: 0.5 SD.48 49 Participant-reported outcome Short Form Health Survey; CRE: 0.5 SD.51 |
X | X | X | |
| Body composition | ||||
| Fat mass and fat-free mass as measured by bioimpedance spectroscopy; CRE: relative improvement of 5%.52 53 Lymphoedema status as measured by bioimpedance spectroscopy; CRE: 5% absolute difference in % with evidence of lymphoedema between groups.54 55 |
X | X | X | |
| Quality of life | ||||
| FACT–O (range=0–156); CRE: 8 units.45 Measure of Ovarian Symptoms and Treatment Concerns41 56; CRE: 0.5 SD.48 49 PROMIS–Global Health57; CRE: 0.5 SD.48 49 EQ-5D (range=0–1)58; CRE: 0.06.59 |
X | X | X | |
| Fatigue | ||||
| Functional Assessment of Chronic Illness Therapy–Fatigue; CRE: 3 units.60 | X | X | X | |
| Sleep | ||||
| Insomnia Severity Index; CRE: 6 units.61 Pittsburgh Sleep Quality Index; CRE: 3 units.62 |
X | X | X | |
| Anxiety and depression | ||||
| Hospital and Anxiety Depression Scale; CRE: 1.5 units.63 | X | X | X | |
| Chemotherapy completion rate | ||||
| Relative dose intensity (RDI) of each agent (total delivered dose/week) as a % of the initial target dose (standard dose intensity calculated for each regimen by summing RDI for each agent given when calculating average). CRE: 5% absolute difference between groups.64 | X | |||
| Chemotherapy-related adverse events (AEs) | ||||
| Proportion of grade 3/4 AEs related to treatment of ovarian cancer as measured by the standard CTC-AE V.4 and recorded in medical records65; CRE: 0.5 SD.48 49 FACT–O Trial Outcome Index (range=0–72); CRE: 4 units.44 |
X | X‡ | X | |
| Healthcare use | ||||
| Healthcare usage data will be obtained from study clinical records and Services Australia (Medicare) claims. These will include treatments, appointments/tests, AEs and hospitalisation. Costs will be assigned to hospitalisation using national cost schedules (eg, AR-DRGs). Resources for implementing the intervention will be recorded by the trial managers. Costs to patients (lost employment and out-of-pocket costs including exercise-related costs) will be assessed using validated questions developed for populations with cancer.66 67 |
X | |||
| Tertiary outcomes | ||||
| Biomarkers | ||||
| Serum (plain blood collection tube), plasma (EDTA blood collection tube) and whole blood (Paxgene). | X | X | X | |
*In the absence of established cut-offs, clinically important changes were defined as 0.5 SD difference between follow-up and baseline measures.48 49
†At approximately the 4.5-year mark of the trial timeline.
‡Data related to chemotherapy completion rate and chemotherapy-related AEs are extracted from medical records monthly across the chemotherapy period per participant.
AR-DRGs, Australian Refined Diagnosis Related Groups; B, baseline; CTC-AE, Common Terminology Criteria for Adverse Events; EQ-5D, EuroQol 5-Dimension; FACT–O, Functional Assessment of Cancer Therapy–Ovarian; 6m, 6 months post-randomisation; 12m, 12 months post-randomisation; PROMIS, Patient-Reported Outcomes Measurement Information System; Y, yearly thereafter.
bmjopen-2022-067925supp007.pdf (129.3KB, pdf)
Exercise intervention
The exercise intervention (online supplemental table 4) is delivered by tertiary (minimum 3-year undergraduate training in exercise science or physiotherapy) and trial-trained ExP for the duration of first-line chemotherapy. The weekly intervention target is to accumulate at least 150 min per week of moderate-intensity, mixed-mode (aerobic and resistance-based) exercise, with participants encouraged to complete at least two resistance-based exercise sessions per week.1 2 6 This is equivalent to an exercise dosage of 450 metabolic equivalent minutes per week. The starting exercise dosage (and parameters comprising dosage: frequency, intensity, duration and type) and pace of progression to this weekly target are individualised according to each participant’s circumstance. Progression may occur through modification of duration and number of sessions per day, mode and/or intensity.
bmjopen-2022-067925supp008.pdf (135.3KB, pdf)
The intervention is delivered via weekly telephone sessions with an ExP. Patients experiencing difficulty adhering to the prescribed weekly exercise dosage are offered more frequent telehealth sessions. Less frequent contact may occur for those with high exercise self-efficacy, typically meeting the weekly exercise target and who are equipped to accommodate exercise barriers appropriately, that is, for whom weekly contact is therefore unnecessary or burdensome. When possible and when participants approve, the telehealth intervention is supported by five face-to face visits with an ExP (either trial trained or an accredited exercise physiologist or physiotherapist) scheduled throughout the intervention. This intervention feature aligns with Australia’s Chronic Disease Management Plan, which allows for up to five reimbursable visits per year with an allied health professional.
The ExP uses a patient-centred approach in the delivery of the intervention by following the Chronic Disease Self-management Intervention Model (CDSM), adapted from our previous work in breast cancer.37 The CDSM emphasises collaborative interactions with the ExP providing education, support and exercise prescription guidance according to each patient’s individual circumstances (considering health history, previous exercise behaviours, diagnosis and treatment characteristics, and treatment-related side effects), while concurrently acknowledging the participant’s expertise in knowing what works best in the context of their life and their effort versus reward willingness and expectations. Each session involves (1) an assessment of the previous week’s completed exercise, barriers to exercise, changes to medications or chemotherapy, presence and change to treatment-related side effects (and review of potential relationship with exercise) and details related to the occurrence of any exercise-related adverse event; (2) provision of advice including exercise prescription for the coming week and recommendations for exercise modifications in response to treatment-related side effects or exercise-related adverse events if relevant, as well as provision of education on exercise topics (table 3); and (3) assistance relating to overcoming barriers (including treatment-related side effects) or dealing with exercise-related adverse events. Over the course of the intervention, the goal for the ExP is to develop independent, regular exercisers, capable of achieving and adhering to the weekly exercise target beyond the duration of the intervention without regular ExP support. Further, ExPs are instructed to remain within their scope of practice during sessions with participants, and to refer patients back to their treating team if unmanaged disease-related or treatment-related side effects present.
Table 3.
Educational topics covered during the ECHO trial exercise intervention
| Topics | |
| What to expect during the exercise intervention | Exercise frequency |
| Exercise and treatment side effects | Exercise intensity |
| Why should I be active? | Exercise session components |
| When not to exercise | Goal setting |
| Being active safely | Motivation and support |
| What and how much should I do? | Identify and problem-solve barriers |
| Starting and progressing | Getting back on track after an interruption to exercise |
| Types of exercise | Exercise after the intervention |
| Frequency, Intensity, Time and Type | |
ECHO, Exercise during CHemotherapy for Ovarian cancer.
A trial-specific case management folder (CMF) is maintained by the ExPs for each participant to facilitate comprehensive recording of intervention delivery and adherence, exercise compliance and the occurrence of exercise-related adverse events. The CMF provides a record of: contacts (dates, mode (telephone, face-to-face, texts, emails or video conferencing) and duration of contact); education topics covered (see table 3) and when; voluntary purchases made by the participant related to participating in the trial; detailed notes related to the ‘assess, advise and assist’ components of the CDSM; exercise dosage prescribed and dose completed each week through recording daily exercise mode (including number and type of exercises for resistance exercise and specific mode of aerobic exercise), duration and intensity (according to 0–10 Rating of Perceived Exertion Scale)38 39; and exercise-related adverse events including type, grade, causality (relationship to the intervention) and impact (eg, hospitalisation required and/or impact to intervention). Completed CMFs are reviewed for completeness and assessment of intervention fidelity by the senior trial ExP, are used to facilitate case discussions at monthly trial ExP meetings and will be used to quantify intervention safety and feasibility. Each exercise group participant is provided with an exercise logbook, which they are advised to use to record prescribed and completed exercise throughout the intervention.
Usual care
Participants in the usual care group may be provided with physical activity advice during and after treatment from members of their treating team or may access physical activity advice from other sources (eg, Ovarian Cancer Australia’s Resilience Kit, cancer organisations, private accredited exercise physiologists, etc). Therefore, participation in this trial does not preclude patients in the usual care group from exercising regularly; however, exercise participation is neither assessed nor monitored throughout their chemotherapy.
Sample size justification
A total accrual of approximately 500 participants recruited over 6 years, with an additional follow-up of 2 years, provides over 80% power to detect a difference of 6.7 months in median progression-free survival, assuming a median progression-free survival in the usual care group of 20 months (HR: 0.75, 400 observed progression-free survival events), with a two-sided type 1 error rate of 0.045. This includes overaccrual of approximately 26 participants to account for ineligibility, withdrawal prior to baseline assessment and missing data. This degree of progression-free survival improvement is similar or greater than those seen in pivotal trials of expensive and targeted therapies in ovarian cancer, which have been translated into routine clinical practice and reimbursement by regulatory authorities.40 41
Approximately 210 participants per group also provide 80% power to detect an absolute minimum clinically important difference of 2 points between the mean physical well-being scores of the groups at 6 and 12 months post-randomisation, assuming an SD of 5.6, a two-sided type 1 error rate of 0.005, with an allowance of approximately 15% for missing data.
Statistical methods and analysis
Efficacy analyses will be by intention-to-treat including all randomised participants. The presence of baseline group imbalances in outcomes and potential confounders, although not expected, will be explored for potential adjustment in sensitivity analyses. Attrition and contamination in both groups will be tracked with validated physical activity instruments completed at each assessment. Kaplan-Meier curves will be used to describe progression-free and overall survival and the log-rank test will be used to test for differences between treatment and usual care groups. Provided relevant assumptions are met, physical well-being and the secondary outcomes will be analysed using Generalised Estimating Equations, accounting for correlations between repeated measurements in the same subject, and allowing examination of partially complete records, to test the effects of treatment group and time. Effects of treatment (eg, HRs, differences in mean patient-reported outcomes, ORs), with appropriate 95% CIs, will be used to describe the effects of exercise. We will undertake a within-trial cost-utility analysis and compare the costs and quality-adjusted life years (QALYs) for the treatment and usual care groups to produce an incremental cost per QALY ratio. Cost-utility analysis will employ non-parametric bootstrapping statistics using the bias-corrected approach. One-way and probabilistic sensitivity analyses will be used to address data uncertainty and identify scenarios which produce value for money.
Trial management
The Trial Management Committee (TMC) oversees trial planning, monitoring, progress, review of information from related research and implementation of recommendations from other trial committees and external bodies (eg, ethics committees). The TMC meets two to four times per year and considers whether to continue the trial as planned, modify or stop it, based on available information.
Ethics and dissemination
Written informed consent was provided by all participants after eligibility was established and prior to baseline assessment. The trial was conducted according to the guidelines of the Declaration of Helsinki and approved by the Sydney Local Health District Ethics Review Committee (2019/ETH08923), 21 November 2014).
The ECHO trial aims to recruit a representative sample of patients newly diagnosed with ovarian cancer, who are on average, insufficiently active at diagnosis. The first ECHO participant was recruited on 30 June 2015 and we anticipate achieving our recruitment target in the first quarter of 2023. The evidence-based exercise intervention is delivered via telehealth, facilitating equitable access for all patients, including patients who live in more rural, regional areas of Australia. Further, the trial is adequately powered to evaluate survival, alongside a range of outcomes that matter to patients with ovarian cancer and their multidisciplinary team. As such, positive trial findings have significant potential to accelerate improvements in survival and well-being not just for patients with ovarian cancer, but for the broader population with cancer. It is possible that on trial completion, the null hypothesis will be accepted. An insufficient or unfeasible weekly exercise dosage, too short intervention or simply that exercise is unable to shift survival outcomes for patients with ovarian cancer all represent plausible reasons for null findings. However, the ECHO sample size, alongside the comprehensive data collection protocol for exercise safety and feasibility, will allow for in-depth exploration of the relationship between exercise dose and outcomes and whether this relationship is mediated by patient, diagnosis, treatment and behavioural characteristics. The collection of blood samples will also provide rich samples for the subsequent analysis of potential biological mechanisms through which exercise may benefit. In addition, the feasibility data will be used to assess and describe what exercise patients are willing, able and interested in doing in endeavours to improve survival and/or quality of life outcomes. However, should the null hypothesis be rejected, the ECHO trial findings are poised to force policy change, and to fundamentally shift patient and clinician perceptions about the need for exercise therapy in cancer care.
Supplementary Material
Acknowledgments
This project was made possible as a consequence of funding support from Cancer Australia, Cancer Council Australia, World Cancer Research Fund, Cancer Council Queensland and Griffith University, and by the women who agree to participate in this trial during a particularly difficult period in their life. Further, we would like to acknowledge the invaluable support given to this trial by gynaecological oncologists and medical oncologists across Australia (including Niara Oliveira, Kathryn Middleton, Anna Kuchel, Alison Brand, Paul Harnett, Phillip Beale, Bo Gao, Di Adams, Archana Rao, Amy Tang, Russell Land, Naven Chetty, Nisha Jagasia, Nimithri Cabraal, Marcelo Nascimento and Helen Green) and the dedicated trial coordinators and research nurses (including Sara Baniahmadi, Tim Roque, Vanessa Taylor and Vanessa Behan), the trial exercise professionals and site research staff, all of whom have made this trial possible while never forgetting the patients.
Footnotes
Twitter: @_SandiHayes, @louisagord
Collaborators: The ECHO trial collaborative includes: The Queensland Centre for Gynaecological Cancer Research authors: Trudi Cattley, James Nicklin, Andrea Garrett, Piksi Singh, Jeff Goh, Catherine Shannon, Lewis Perrin (Queensland Centre for Gynaecological Cancer Research, University of Queensland, Brisbane, Australia). Amasy Alkhateeb (University of Sydney, NHMRC Clinical Trials Centre, Camperdown, New South Wales, Australia). Dirkje Sommeijer (University of Sydney, NHMRC Clinical Trials Centre, Camperdown, New South Wales, Australia; Department of Medical Oncology, Amsterdam University Medical Center; Department of Internal Medicine, Flevoziekenhuis, Almere, the Netherlands). Melissa Creed, Sheree Rye, Nicole McDonald (School of Health Sciences and Social Work, Menzies Health Institute Queensland, Griffith University, Brisbane, Australia).
Contributors: All authors contributed to one or more of the following: study design, protocol development, study implementation, and TMC membership and participation; all authors have reviewed and approved this manuscript. The following authors were involved in: study design—SH, AO, LM, AD, LGG, EE, MJ, VLB, EHB, DV, PW, JA, AB, MF, HO, MW, DS and MS; trial recruitment, registration and randomisation—SH, AO, LM, AD, RRS, CS, TJ, JA, AB, YCL, HO, MW, AG, AA, CSh, JN, JG, LP, PS, NM, SR and TC; data collection—SH, AO, LGG, MJ, VLB, RRS, CS, TJ, KP, HO, MW, AG, AA, CSh, JN, JG, LP, PS, NM, SR and TC; and intervention development and delivery—SH, EE, RRS, CS, TJ, KP, MC and SR.
Funding: The ECHO trial has been supported by grant funding from Cancer Australia (reference #: 1063509), the World Cancer Research Fund (reference #: 2016/1617), Cancer Australia and Cancer Council Australia (reference #: 1158566), Cancer Council Queensland and Griffith University (reference #: ACCR-0000000077), as well as charitable funding from Gynaecologic Cancer RED (Research Education and Development Society). SH has been supported by fellowship funding from Cancer Council Queensland (2014-18).
Disclaimer: Funding sources did not participate in or influence study design or implementation and will not influence subsequent analysis and interpretation of data.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: The ECHO trial, Trudi Cattley, James Nicklin, Andrea Garrett, Piksi Singh, Jeff Goh, Catherine Shannon, Lewis Perrin, Amasy Alkhateeb, Dirkje Sommeijer, Melissa Creed, Sheree Rye, and Nicole McDonald
Ethics statements
Patient consent for publication
Not required.
References
- 1.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 2019;51:2375–90. 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayes SC, Newton RU, Spence RR, et al. The exercise and sports science australia position statement: exercise medicine in cancer management. J Sci Med Sport 2019;22:1175–99. 10.1016/j.jsams.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 3.Friedenreich CM, Stone CR, Cheung WY, et al. Physical activity and mortality in cancer survivors: a systematic review and meta-analysis. JNCI Cancer Spectr 2020;4:pkz080. 10.1093/jncics/pkz080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courneya KS, Segal RJ, McKenzie DC, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc 2014;46:1744–51. 10.1249/MSS.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 5.Hayes SC, Steele ML, Spence RR, et al. Exercise following breast cancer: exploratory survival analyses of two randomised, controlled trials. Breast Cancer Res Treat 2018;167:505–14. 10.1007/s10549-017-4541-9 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO guidelines on physical activity and sedentary behaviour. Geneva, 2020. [PubMed] [Google Scholar]
- 7.Kennedy MA, Bayes S, Newton RU, et al. Implementation barriers to integrating exercise as medicine in oncology: an ecological scoping review. J Cancer Surviv 2022;16:865–81. 10.1007/s11764-021-01080-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buffart LM, Kalter J, Sweegers MG, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 rcts. Cancer Treat Rev 2017;52:91–104. 10.1016/j.ctrv.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 9.Australian Institute of Health and Welfare . Cancer in australia 2019. Canberra: AIHW, 2019. [Google Scholar]
- 10.Spence RR, Sandler CX, Newton RU, et al. Physical activity and exercise guidelines for people with cancer: why are they needed, who should use them, and when? Semin Oncol Nurs 2020;36:151075. 10.1016/j.soncn.2020.151075 [DOI] [PubMed] [Google Scholar]
- 11.Spence RR, Disipio T, Schmitz KH. Is unsupervised exercise following breast cancer safe for all women? Int J Phys Med Rehabil 2014;2. 10.4172/2329-9096.1000197 [DOI] [Google Scholar]
- 12.Australian Institute of Health and Welfare . Ovarian cancer in australia: an overview, 2010. Canberra: AIHW, 2010. [Google Scholar]
- 13.Jordan S, Steer C, DeFazio A, et al. Patterns of chemotherapy treatment for women with invasive epithelial ovarian cancer--a population-based study. Gynecol Oncol 2013;129:310–7. 10.1016/j.ygyno.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 14.Keim-Malpass J, Mihalko SL, Russell G, et al. Problems experienced by ovarian cancer survivors during treatment. J Obstet Gynecol Neonatal Nurs 2017;46:544–54. 10.1016/j.jogn.2017.04.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ovarian Cancer Research Foundation . State of the nation in ovarian cancer: research audit; 2020.
- 16.Högberg T, Glimelius B, Nygren P, et al. A systematic overview of chemotherapy effects in ovarian cancer. Acta Oncol 2001;40:340–60. 10.1080/02841860151116420 [DOI] [PubMed] [Google Scholar]
- 17.Arriba LN, Fader AN, Frasure HE, et al. A review of issues surrounding quality of life among women with ovarian cancer. Gynecol Oncol 2010;119:390–6. 10.1016/j.ygyno.2010.05.014 [DOI] [PubMed] [Google Scholar]
- 18.Ross TL, DeFazio A, Friedlander M, et al. Insomnia and its association with quality of life in women with ovarian cancer. Gynecol Oncol 2020;158:760–8. 10.1016/j.ygyno.2020.06.500 [DOI] [PubMed] [Google Scholar]
- 19.Beesley VL, Ross TL, King MT, et al. Evaluating patient-reported symptoms and late adverse effects following completion of first-line chemotherapy for ovarian cancer using the most (measure of ovarian symptoms and treatment concerns). Gynecol Oncol 2022;164:437–45. 10.1016/j.ygyno.2021.12.006 [DOI] [PubMed] [Google Scholar]
- 20.Beesley VL, Webber K, Nagle CM, et al. When will I feel normal again? Trajectories and predictors of persistent symptoms and poor wellbeing after primary chemotherapy for ovarian cancer. Gynecol Oncol 2020;159:179–86. 10.1016/j.ygyno.2020.07.029 [DOI] [PubMed] [Google Scholar]
- 21.Wright J, Doan T, McBride R, et al. Variability in chemotherapy delivery for elderly women with advanced stage ovarian cancer and its impact on survival. Br J Cancer 2008;98:1197–203. 10.1038/sj.bjc.6604298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Australia . Ovarian cancer statistics in Australia. Australian Government; 2021. Available: https://www.canceraustralia.gov.au/cancer-types/ovarian-cancer/statistics [Google Scholar]
- 23.Fleming S, Jones T, Janda M, et al. Physical activity trajectories following gynecological cancer: results from a prospective, longitudinal cohort study. Int J Gynecol Cancer 2020;30:1784–90. 10.1136/ijgc-2020-001543 [DOI] [PubMed] [Google Scholar]
- 24.Jones T, Sandler C, Vagenas D, et al. Physical activity levels among ovarian cancer survivors: a prospective longitudinal cohort study. Int J Gynecol Cancer 2021;31:553–61. 10.1136/ijgc-2020-002107 [DOI] [PubMed] [Google Scholar]
- 25.Beesley V, Ross T, Nina N, et al. Does physical activity improve chemotherapy completion in women receiving chemotherapy for ovarian cancer? ASIA Pac J Clin Oncol 2019;15:82–3. [Google Scholar]
- 26.Jones TL, Sandler CX, Spence RR, et al. Physical activity and exercise in women with ovarian cancer: a systematic review. Gynecol Oncol 2020;158:803–11. 10.1016/j.ygyno.2020.06.485 [DOI] [PubMed] [Google Scholar]
- 27.Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooch E, Angle A, Moore R. Resilience kit. 3 ed. Melbourne, Victoria: Ovarian Cancer Australia Limited, 2018. [Google Scholar]
- 29.Karam A, Ledermann JA, Kim J-W, et al. Fifth ovarian cancer consensus conference of the gynecologic cancer intergroup: first-line interventions. Ann Oncol 2017;28:711–7. 10.1093/annonc/mdx011 [DOI] [PubMed] [Google Scholar]
- 30.Begg CB. Justifying the choice of endpoints for clinical trials. J Natl Cancer Inst 2013;105:1594–5. 10.1093/jnci/djt289 [DOI] [PubMed] [Google Scholar]
- 31.Cheson BD. The international harmonization project for response criteria in lymphoma clinical trials. Hematol Oncol Clin North Am 2007;21:841–54. 10.1016/j.hoc.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 32.Michiels S, Saad ED, Buyse M. Progression-free survival as a surrogate for overall survival in clinical trials of targeted therapy in advanced solid tumors. Drugs 2017;77:713–9. 10.1007/s40265-017-0728-y [DOI] [PubMed] [Google Scholar]
- 33.Oza AM, Castonguay V, Tsoref D, et al. Progression-free survival in advanced ovarian cancer: a canadian review and expert panel perspective. Curr Oncol 2011;18 Suppl 2:S20–7. 10.3747/co.v18i6.998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Y, Bycott P, Akerborg O, et al. Interpreting overall survival results when progression-free survival benefits exist in today’s oncology landscape: a metastatic renal cell carcinoma case study. Cancer Manag Res 2014;6:365–71. 10.2147/CMAR.S67249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutman S, Piper M, Grant M, et al. Progression-free survival: what does it mean for psychological well-being or quality of life?, Report no: 13-EHC074-EF. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. [PubMed] [Google Scholar]
- 36.Newton MJ, Hayes SC, Janda M, et al. Safety, feasibility and effects of an individualised walking intervention for women undergoing chemotherapy for ovarian cancer: a pilot study. BMC Cancer 2011;11:389. 10.1186/1471-2407-11-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayes S, Rye S, Battistutta D, et al. Design and implementation of the exercise for health trial -- a pragmatic exercise intervention for women with breast cancer. Contemp Clin Trials 2011;32:577–85. 10.1016/j.cct.2011.03.015 [DOI] [PubMed] [Google Scholar]
- 38.Borg G. Borg’s perceived exertion and pain scales: human kinetics; 1998.
- 39.Norton K, Norton L, Sadgrove D. Position statement on physical activity and exercise intensity terminology. J Sci Med Sport 2010;13:496–502. 10.1016/j.jsams.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 40.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011;365:2484–96. 10.1056/NEJMoa1103799 [DOI] [PubMed] [Google Scholar]
- 41.King MT, Stockler MR, Butow P, et al. Development of the measure of ovarian symptoms and treatment concerns: aiming for optimal measurement of patient-reported symptom benefit with chemotherapy for symptomatic ovarian cancer. Int J Gynecol Cancer 2014;24:865–73. 10.1097/IGC.0000000000000167 [DOI] [PubMed] [Google Scholar]
- 42.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 43.Rustin GJS, Vergote I, Eisenhauer E, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and Ca 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int J Gynecol Cancer 2011;21:419–23. 10.1097/IGC.0b013e3182070f17 [DOI] [PubMed] [Google Scholar]
- 44.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. J Clin Oncol 2001;19:1809–17. 10.1200/JCO.2001.19.6.1809 [DOI] [PubMed] [Google Scholar]
- 45.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570–9. 10.1200/JCO.1993.11.3.570 [DOI] [PubMed] [Google Scholar]
- 46.Rasekaba T, Lee AL, Naughton MT, et al. The six-minute walk test: a useful metric for the cardiopulmonary patient. Intern Med J 2009;39:495–501. 10.1111/j.1445-5994.2008.01880.x [DOI] [PubMed] [Google Scholar]
- 47.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006;54:743–9. 10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 48.Hayes SC, Rye S, Disipio T, et al. Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res Treat 2013;137:175–86. 10.1007/s10549-012-2331-y [DOI] [PubMed] [Google Scholar]
- 49.Norman GR, Sloan JA, Wyrwich KW. The truly remarkable universality of half a standard deviation: confirmation through another look. Expert Rev Pharmacoecon Outcomes Res 2004;4:581–5. 10.1586/14737167.4.5.581 [DOI] [PubMed] [Google Scholar]
- 50.Zanini A, Crisafulli E, D’Andria M, et al. Minimal clinically important difference in 30 second sit-to-stand test after pulmonary rehabilitation in patients with COPD. Eur Respir J 2018;52(suppl 62):OA5199. 10.1183/13993003.congress-2018.OA5199 [DOI] [PubMed] [Google Scholar]
- 51.Ware JE, Kosinski M, Gandek B. SF-36 health survey: manual and interpretation guide. Lincoln, RI: QualityMetric, 2005. [Google Scholar]
- 52.Reeves MM, Terranova CO, Winkler EAH, et al. Effect of a remotely delivered weight loss intervention in early-stage breast cancer: randomized controlled trial. Nutrients 2021;13:4091. 10.3390/nu13114091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012;62:243–74. 10.3322/caac.21142 [DOI] [PubMed] [Google Scholar]
- 54.Steele ML, Janda M, Vagenas D, et al. Normative interlimb impedance ratios: implications for early diagnosis of uni- and bilateral, upper and lower limb lymphedema. Lymphat Res Biol 2018;16:559–66. 10.1089/lrb.2017.0082 [DOI] [PubMed] [Google Scholar]
- 55.Steele ML, Janda M, Vagenas D, et al. A bioimpedance spectroscopy-based method for diagnosis of lower-limb lymphedema. Lymphat Res Biol 2020;18:101–9. 10.1089/lrb.2018.0078 [DOI] [PubMed] [Google Scholar]
- 56.King MT, Stockler MR, O’Connell RL, et al. Measuring what matters most: validation of the measure of ovarian symptoms and treatment, a patient-reported outcome measure of symptom burden and impact of chemotherapy in recurrent ovarian cancer. Qual Life Res 2018;27:59–74. 10.1007/s11136-017-1729-8 [DOI] [PubMed] [Google Scholar]
- 57.Hays RD, Bjorner JB, Revicki DA, et al. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res 2009;18:873–80. 10.1007/s11136-009-9496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and vas scores in cancer. Health Qual Life Outcomes 2007;5:70. 10.1186/1477-7525-5-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cella D, Eton DT, Lai J-S, et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the functional assessment of cancer therapy (fact) anemia and fatigue scales. J Pain Symptom Manage 2002;24:547–61. 10.1016/s0885-3924(02)00529-8 [DOI] [PubMed] [Google Scholar]
- 61.Yang M, Morin CM, Schaefer K, et al. Interpreting score differences in the insomnia severity index: using health-related outcomes to define the minimally important difference. Curr Med Res Opin 2009;25:2487–94. 10.1185/03007990903167415 [DOI] [PubMed] [Google Scholar]
- 62.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 63.Puhan MA, Frey M, Büchi S, et al. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes 2008;6:46. 10.1186/1477-7525-6-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Terada Y, Nakamae H, Aimoto R, et al. Impact of relative dose intensity (RDI) in CHOP combined with rituximab (R-CHOP) on survival in diffuse large B-cell lymphoma. J Exp Clin Cancer Res 2009;28:116. 10.1186/1756-9966-28-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.National Cancer Institute . Common terminology criteria for adverse events v4.0. NIH Publication, 2009. [Google Scholar]
- 66.Gordon L, Ferguson M, Chambers S, et al. Fuel, beds, meals and meds: out-ofpocket expenses for patients with cancer in rural queensland. Cancer Forum 2009;33:202–8. [Google Scholar]
- 67.Gordon LG, Lynch BM, Beesley VL, et al. The working after cancer study (WACS): a population-based study of middle-aged workers diagnosed with colorectal cancer and their return to work experiences. BMC Public Health 2011;11:604. 10.1186/1471-2458-11-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-067925supp001.pdf (119.6KB, pdf)
bmjopen-2022-067925supp002.pdf (249.5KB, pdf)
bmjopen-2022-067925supp003.pdf (1.2MB, pdf)
bmjopen-2022-067925supp004.pdf (301.8KB, pdf)
bmjopen-2022-067925supp005.pdf (69.7KB, pdf)
bmjopen-2022-067925supp006.pdf (68.8KB, pdf)
bmjopen-2022-067925supp007.pdf (129.3KB, pdf)
bmjopen-2022-067925supp008.pdf (135.3KB, pdf)