Abstract
Ouabain preconditioning (OPC) initiated by low concentrations of the cardiac glycoside (CG) ouabain binding to Na/K-ATPase is relayed by a unique intracellular signaling and protects cardiac myocytes against ischemia/reperfusion (IR) injury. To explore more clinically applicable protocols based on CG properties, we tested whether the FDA-approved CG digoxin could trigger cardioprotective effects comparable to those of ouabain using PC and PostC protocols in the Langendorff-perfused mouse heart subjected to global ischemia and reperfusion. Ouabain or digoxin at 10 µmol/L inhibited Na/K-ATPase activity by about 30% and activated PKCε translocation by about 50%. Digoxin-induced preconditioning (DigPC), initiated by a transient exposure prior to 40 min of ischemia, was as effective as OPC as suggested by the recovery of left ventricle developed pressure (LVDP), end-diastolic pressure (EDP) and cardiac Na/K-ATPase activity after 30 min of reperfusion. DigPC also significantly decreased LDH release and reduced infarct size, comparable to OPC. PostC protocols consisting of a single bolus injection of 100 nmoles of ouabain or digoxin in the coronary tree at the beginning of reperfusion both improved significantly the recovery of LVDP and decreased LDH release, demonstrating a functional and structural protection comparable to the one provided by OPC. Given the unique signaling triggered by OPC, these results suggest that DigPostC could be considered for patients with risk factors and/or concurrent treatments that may limit effectiveness of Ischemic PostC.
Keywords: Langendorff, cardiac, Ischemia/Reperfusion, Digoxin, ouabain, Na/K-ATPase
Introduction
The Na/K-ATPase is the membrane-spanning enzyme complex that establishes and maintains cellular ion homeostasis through the active transport of Na+ and K+ ions against their electrochemical gradients [1, 2]. Cardiac glycosides (CG) are specific inhibitors of Na/K-ATPase ion-pumping function, which results in subsequent increase in intracellular Na+, increased Na+/Ca2+ exchange, and cardiac positive inotropic action [3, 4]. CG also initiate Na/K-ATPase signaling, and the activation of specific intracellular pathways leads to additional cardiac actions such as hypertrophic growth [5, 6] or resistance to ischemia-reperfusion injury. The latter can be triggered by exposure to either inotropic [7] or sub-inotropic concentrations of the CG ouabain prior to global ischemia in the isolated perfused mouse-, rat-, and rabbit hearts, or in primary culture of rat neonatal cardiac myocytes [7–11]. By analogy to ischemic preconditioning (IPC), which was the first form of preconditioning (PC) described in 1986 [12], cardioprotection by exposure to a low concentration of ouabain prior to a prolonged ischemic insult is referred to as ouabain preconditioning (OPC). In various systems, we have shown that OPC is relayed through a pathway initiated by ouabain binding to the Na/K-ATPase protein complex and transmitted from the sarcolemma to the mitochondria via key mediators that include Src, PKCε, PI3K-IA, mitochondrial KATP channel, and reactive oxygen species [7, 11, 13, 14].
In contrast to IPC, ischemic postconditioning (IPostC) can be triggered by a few very brief (< 1 min) bouts of ischemia and reperfusion at the onset of reperfusion, as discovered by Zhao, et al. in 2003 [15]. Because the onset of ischemia is unpredictable, postconditioning is more applicable in the context of myocardial infarction to reduce reperfusion injury during percutaneous interventions [16–19]. In the past few years, phase II trials have shown cardioprotective effects of IPostC in patients with acute myocardial infarction. However, there is also evidence that not all patients may benefit from IPostC given the potential influence of comorbidities and medication on IPostC cardioprotective signaling, as recently re-emphasized by Hausenloy and co-authors [20]. Our previous study in mouse heart has suggested that OPC, unlike IPC and many other forms of PC, does not require PI3K–IB or Akt activation [11]. Since disease states and/or medication differentially affect the integrity of those pathways in the heart, a CG-based approach could be a suitable alternative to IPostC in those cases, if clinically applicable. Accordingly, the present study was undertaken to assess whether the only FDA-approved CG, digoxin, could trigger cardioprotective effects comparable to those of ouabain using PC and PostC protocols in the Langendorff-perfused mouse heart subjected to global ischemia and reperfusion.
Material and Methods
Langendorff-perfused isolated mouse heart model
All animal care and experiments were approved by the Marshall University Institutional Animal Care and Use Committee (IACUC) in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. C57BL/6J male mice obtained from Jackson Laboratories (Bar Harbor, ME) were 10–14 week old at the time of experimentation. Mice were injected intraperitoneally with pentobarbital (70mg/kg) and heparin (1000 IU/kg). Hearts were rapidly removed, placed into ice cold (4 °C) Krebs–Henseleit solution and mounted on a non-recirculating Langendorff apparatus. Retrograde perfusion was performed using an oxygenated Krebs–Henseleit buffer containing (in mmol/L) NaCl (118.0), KCl (4.0), CaCl2 (1.65), KH2PO4 (1.3), MgSO4 (1.2), Ethylene glycol bis (2-aminoethylether)-N, N, N′, N′-tetraacetic acid (0.3), NaHCO3 (25), D-glucose (11.0), at a pH of 7.4. The perfusion flow rate was kept constant at about 2.5 ml/min, initially set to obtain a coronary perfusion pressure of ~ 70 mm Hg as described [21] [22]. Isovolumic left ventricular developed pressure (LVDP) was measured by inserting a water-filled polyethylene balloon into the left ventricle connected to a P23XL Becton Dickinson pressure transducer, a CP122 AC/DC strain gage amplifier and PowerLab 2/26 recording system (ADInstruments, Sydney, Australia). End diastolic pressure (EDP) was adjusted initially at 5–10 mm Hg. Hearts were paced at 9.7 Hz (4 V) with bipolar electrodes attached to the left ventricle using a Grass SD9 stimulator, and pacing was maintained throughout the experiment. After 10 to 15 min of equilibration, one of the protocols shown in Figure 1 was initiated, and LVDP and EDP were monitored throughout the experiment.
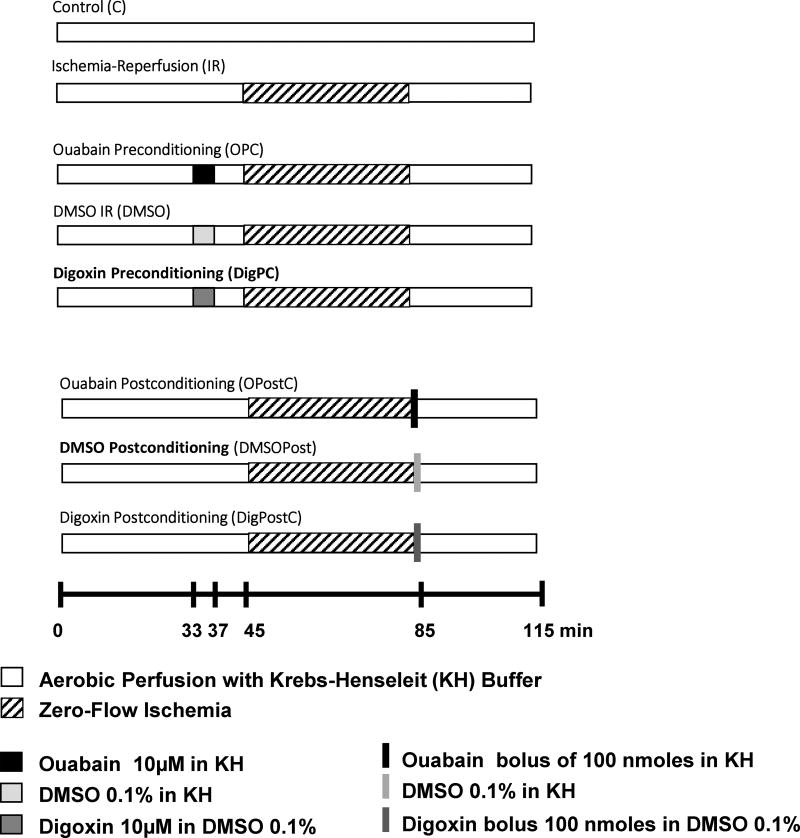
Figure 1. Experimental protocols.
Hearts from 10–14 week old C57BL/6J male mice were isolated and perfused as shown. With the exception of the control group, all hearts were subjected to 40 min of zero-flow ischemia followed by 30 min (enzyme release and biochemical analysis) or 120 min of reperfusion (TTC staining) with Krebs-Henseleit (KH) buffer. Hearts in the preconditioning group (OPC, DigPC) were exposed to 4 min ouabain or digoxin at the indicated concentrations, followed by 8 min washout before ischemia. Hearts in the postconditioning group (OPostC, DigPostC) received 100 nmoles of the indicated drug as a bolus of 100 µL given through the aortic cannula at the onset of reperfusion. To control for possible effects of the small amount of dimethyl sulfoxide (DMSO 0.1%) present in the digoxin perfusate, a DMSO group was perfused as shown.
Experimental protocols
Hearts were subjected to control or ischemia/reperfusion protocols with or without preconditioning or postconditioning as shown in Figure 1. The control group (C) was perfused continuously for 115 min. The ischemia–reperfusion group (IR) was perfused for 45 min, subjected to 40-min zero-flow ischemia, and reperfused. Preconditioning treatments (OPC, DigPC) were induced by 4 min of 10µM ouabain or digoxin (Sigma, Saint-Louis, MO, USA) that ended 8 min before ischemia. Postconditioning treatments were induced by a bolus of 100nmoles of ouabain or digoxin at the onset of reperfusion. Digoxin perfusates contained 0.1% dimethyl sulfoxide (DMSO). Hence, corresponding DMSO groups were added and used as controls for DigPC and DigPostC. After ischemia, all hearts were reperfused with oxygenated Krebs-Henseleit solution for 30 min or 120 min (for infarct size measurements).
Lactate dehydrogenase (LDH) activity measurement
Coronary effluent was collected for 30 s at 0, 1, 2, 3, 4, 5, 10, 15, 20, and 30 min of reperfusion. LDH activity was determined colorimetrically using Cytotoxicity Detection Kit (Roche Applied Science, Indianapolis, IN, USA) as described [7].
Infarct Size Measurement
After 120 min of reperfusion, hearts were frozen (−20 °C) for 20 min, sliced into approximately 2 mm thick transverse sections and incubated in triphenyltetrazolium chloride solution (TTC, 1% in phosphate buffer, pH 7.4) at 37 °C for 20 min, then further incubated for 20 min in 10% buffered formalin phosphate (SF100-4, Fisher Scientific Co., Fair Lawn, NJ, USA). TTC stains the viable tissue in a bright-red color, and leaves nonviable as pale yellow. Quantitation was obtained the protocol that we have previously reported and that is based on Ythehus et al recommendations [23]. Briefly, the stained slices were scanned and quantified by image J (version 1.42q) software (National Institutes of Health, USA http://rsb.info.nih.gov/ij/). The area of infarct was calculated as a sum of infarct areas from all sections from the same heart using a macro as we have previously reported [24] and expressed as percentage of the area at risk (equivalent to total cardiac muscle).
Tissue Preparation, Protein Electrophoresis and Immunodetection of Na/K-ATPase isoforms
Hearts were quick-frozen in liquid nitrogen at 115 min of their respective protocols (Figure 1). Left ventricles were powdered and 100 mg of tissue were added to 1 ml of ice-cold radioimmunoprecipitation assay buffer (RIPA- 50mM Tris-base, 150mM NaCl, 1% IGEPAL, 0.25% sodium deoxycholate, 1 mM EDTA and pH 7.4) containing 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 1 mM NaF, 10 nM okadaic acid, 10 µg/ml aprotinin, 10 µg/ml leupeptin, and homogenized in a 10 ml homogenizer by 15 up-and-down strokes. The resulting lysates were centrifuged at 14,000 ×g for 15 min, and supernatants (60 mg) were dissolved in Laemmli sample buffer, incubated at 37 °C for 20min, separated on 10% SDS-PAGE, transferred to a nitrocellulose membrane and probe with anti- Na/K-ATPase α1 (α6f, Developmental Studies Hybridoma Bank at the University of Iowa), anti- Na/K-ATPase α2 (AB9094. Millipore, Billerica, MA), anti-Na/K-ATPase α3 (anti-TED antibody, a gift from Dr. T. A. Pressley, Texas Tech University Health Science Campus, Lubbock, TX), anti- Na/K-ATPase β1 (05–382. Millipore, Billerica, MA) or anti-GAPDH (sc20357, Santa Cruz, CA, USA) as described previously [7, 8].
PKCε translocation from cytosolic to particulate fraction
At time 37min of the preconditioning protocols indicated in Figure 1, hearts from different groups were snap frozen in liquid nitrogen and homogenized in buffer A containing (in mmol/L) EGTA(10), EDTA(1), DTT(0.5), PMSF(1), proteinase inhibitor cocktail, Tris-HCl(20), pH 7.5. Homogenates were centrifuged at 100,000 ×g for 1 h at 4 °C. The supernatant designated as the cytosolic fraction was removed and saved. The pellet was sonicated and centrifuged at 25,000 ×g in buffer A containing 1% Triton. The supernatant was collected as the particulate fraction as we previously described [7]. Cytosolic and particulate fractions were used in SDS-PAGE and immunoblotting using the anti-PKCε antibody C-15 (Santa Cruz, CA, USA).
Na/K-ATPase Activity
Hearts were frozen in liquid nitrogen. One hundred mg of powdered heart tissue were placed into 10ml ice-cold 1M KCl and homogenized in a 30 ml homogenizer by 15 times up-and-down strokes and again with a polytron PT-10/ST for 30s. The homogenates were centrifuged at 1000g for 10 min. The sediment was washed once with a solution containing 50 mM KCl and 50 mM Tris-HCl (pH 7.4), and twice with 50 mM Tris-HCl (pH 7.4). The final pellet was suspended in 1mM Tris-EDTA (pH 7.4). The resulting crude homogenates were incubated with the ionophore alamethicin (0.5mg/mg protein) for 10 minutes at 25°C prior to ouabain sensitive ATPase activity measurement as previously described [25, 26]. This alamethicin treatment is necessary to insure the access of substrates and inhibitors to both the ATP- and ouabain- binding sites of the enzyme in closed membrane vesicles that may form in crude homogenates. Na/K-ATPase activity was measured in 50µg of protein of alamethicin-pretreated samples by colorimetric determination of inorganic phosphate released after incubation of 10 minutes at 37°C in a reaction buffer containing (in mmol/L) Tris-HCl (20), MgCl2 (1), NaCl (100), KCl (20), EGTA-Tris (1), and NaN3(5). After addition of 2 mmol/L Mg2+/ATP, the enzymatic reaction was allowed to run for 10 min before the addition of 1 mL ice-cold 8% tricholoroacetic acid to terminate the reaction. The amount of phosphate released was determined by using an inorganic phosphate detection kit (AK-111, Biomol Research Laboratories, Inc., Plymouth Meeting, PA, USA), according to the manufacturer’s recommendation. Ouabain insensitive activity was measured in a separate reaction in the presence of 1 mmol/L ouabain in the same buffer. Ouabain sensitive Na/K-ATPase activity was then determined by substracting ouabain insensitive from total ATPase activity. To establish the ouabain dose-response curve, 50mg control hearts tissue was prepared as described above, and 13 concentrations of ouabain (ranging from 10−9 to 10−3 mol/L) were added in the reaction buffer. The resulting data were analyzed by nonlinear regression and best fitted with biphasic curve using GraphPad Prism 5.0 (GraphPad Software, Inc, La Jolla, CA).
Statistical Analysis
Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparison post hoc test. P<0.05 was considered statistically significant.
Results
Characterization of the cardiac glycoside sensitivity and the isoenzyme composition of hearts of C57BL/6J male mice
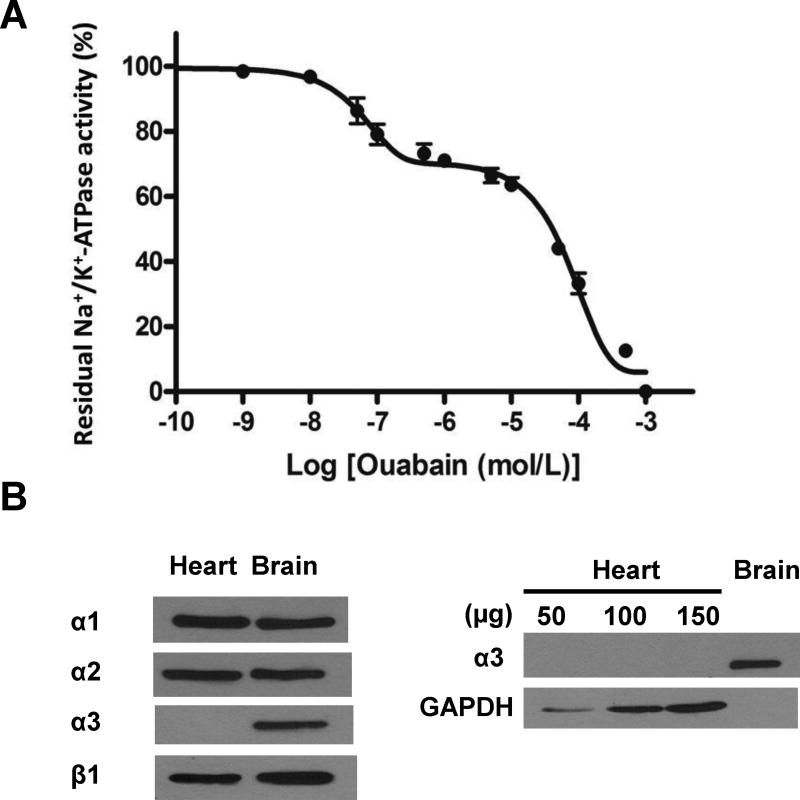
The catalytic activity of the entire pool of cellular Na/K-ATPase was measured in crude homogenates of hearts prepared using a high salt solution as detailed in Methods. The ouabain-sensitive ATPase activity corresponding to Na/K-ATPase activity was 7.8±0.6 µmol Phosphate (Pi)/h/mg protein. The dose-response curve to ouabain measured as described in Methods revealed a biphasic inhibitory curve after best-fit analysis, indicating at least two isoforms with differed ouabain sensitivity. As shown in Figure 2A,a site of high affinity with an IC50 of 6.3 × 10−8 mol/L accounted for about 29% of the total Na/K-ATPase activity, with the remaining 71% corresponding to the activity of the site of low affinity (1.0 × 10−4 mol/L) typically observed in rodent tissues. These findings are in accordance with what has been reported in the literature [27]. Generally speaking, 71% corresponding to the activity of the site of low affinity is viewed as the ouabain-resistant rodent α1 predominant isoform (about 80%) of the α-catalytic subunit in the adult rodent heart, the remainder being ouabain-sensitive α2 [27–30]. However, expression of ouabain-sensitive α3 has also been reported in adult mice heart [31]. Thus, possible expression of α3 was also carefully considered in this study. The molecular identity of Na/K-ATPase isoenzymes composed of α and β subunits in the C57BL/6J mouse heart was assessed by western blotting using isoform-specific antibodies in whole heart homogenates. As shown in Figure 2B, the three isoforms of the catalytic subunit were detected in the mouse brain, which was used as a positive control. In the C57BL/6J heart, Na/K-ATPase α1, α2 and β1 were readily detected, but not α3. Alpha 3 remained undetectable when the amount of heart lysate proteins was increased from 50 to 150 µg. These argued against a significant expression of the α3 isoform in the C57BL/6J heart.
Figure 2. Cardiac glycosides sensitivity and isoenzyme composition of cardiac Na/K-ATPase in C57BL/6J male mice.
A. Dose-response curve of Na/K-ATPase activity vs. ouabain concentrations. Heart crude homogenates were prepared as described in Methods. Na/K-ATPase activity was measured in the presence of the ionophore alamethicin and the indicated concentrations of ouabain. Values are mean ± SEM (n=4). B. Na/K-ATPase isoform detection in heart and brain crude homogenates. Representative blots from 3 independent experiments are shown.
Comparative effects of Ouabain and Digoxin on Na/K-ATPase enzyme and signaling functions
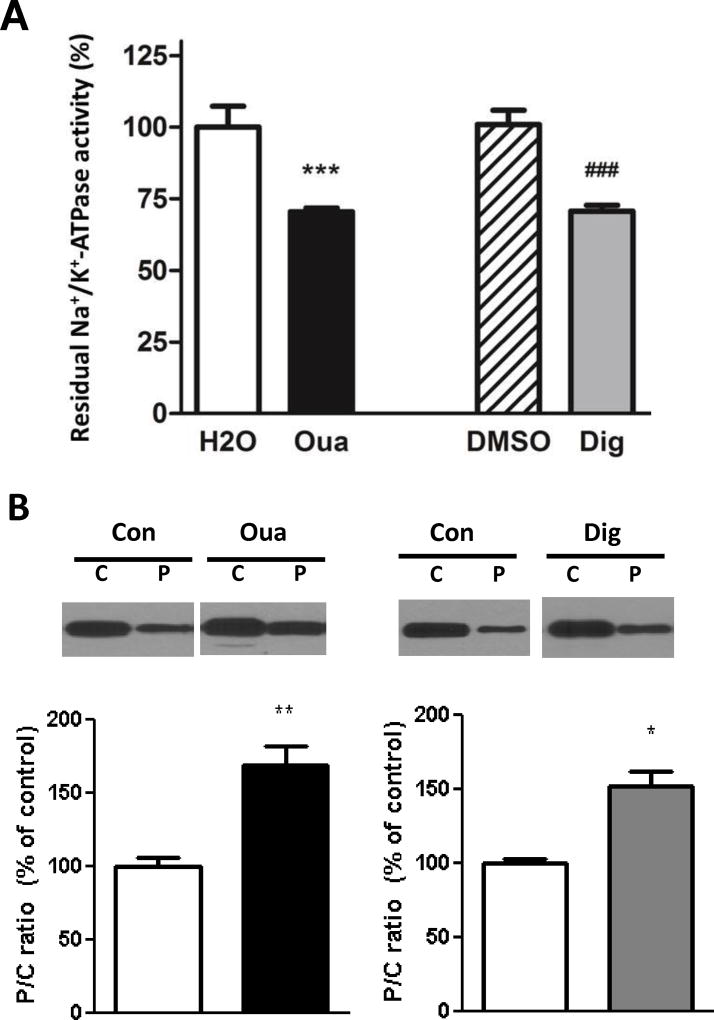
As shown in Figure 3A, addition of 10µmol/L ouabain and digoxin to a crude cardiac homogenate resulted in a comparable inhibition of the Na/K-ATPase of about 30% (ouabain 29.5±1.2 % and digoxin 28.2±1.6 %) compared to their respective controls. Although DMSO 0.1% was used as a control for the digoxin group, it is important to note that no difference was observed between Na/K-ATPase activities measured in the presence of absence of 0.1% DMSO (not shown). We further tested whether exposure to 4 min of digoxin 10µmol/L could trigger PKCε translocation from the cytosolic to the particulate fraction, a required step of the ouabain preconditioning pathway [24]. The data presented in Figure 3B suggest that indeed, this treatment of digoxin led to PKCε response comparable to that observed when ouabain was given in the same conditions.
Figure 3. Comparative effects of Ouabain and Digoxin on Na/K-ATPase enzyme and signaling functions.
A. Na/K-ATPase Activity. Residual Na/K-ATPase activity was compared in crude mouse heart homogenates subjected to 10 µmol/L ouabain or digoxin. Values are mean ± SEM of residual Na/K-ATPase-specific activity expressed as percentage of total Na/K-ATPase-specific activity (n=4–6). B. Na/K-ATPase signaling. Mouse hearts were perfused for 4 min with or without ouabain or digoxin 10 µmol/L and frozen in liquid nitrogen. Cytosolic (C) and particulate (P) fractions were prepared from heart lysates as described in Methods and P/C ratios of PKCε contents were compared. Values are mean ± SEM of 4–7 separate experiments. *P < 0.05 and **P< 0.01 vs. control. Con: control, Oua: ouabain 10 µmol/L; Dig: digoxin 10 µmol/L.
Comparative effects of Ouabain and Digoxin Preconditioning on Cardiac Contractile Function
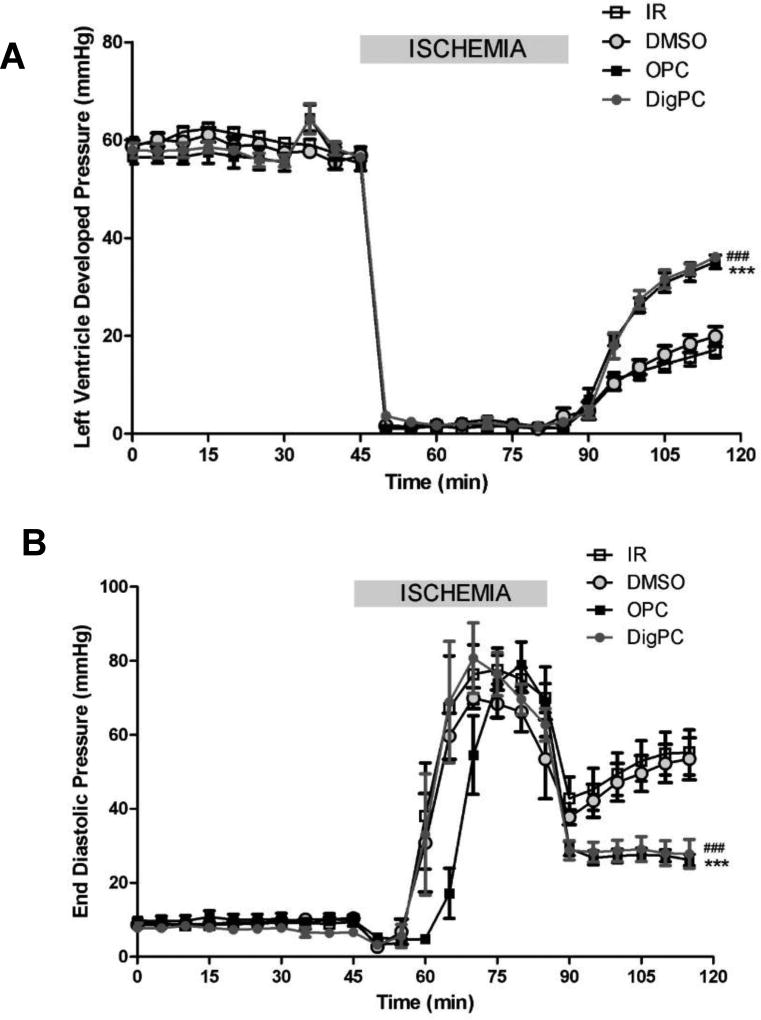
Following a 10–30 min equilibration period, cardiac performance of Langendorff-perfused C57BL/6J mouse hearts was stable and randomly assigned to different treatment groups. In control hearts, LVDP was 58±1.4 mmHg, EDP was 9.7±0.7, and dP/dt was 5238 ±112 mmHg/s, with less than 10% variation over the 205 min perfusion period (not shown). There was no significant difference in baseline contractile performance between groups (not shown). Four min of exposure to 10µmol/L ouabain or digoxin caused a similar transient increase in LVDP (21.2±2.1 % and 23.4±6.1 %, respectively) with return to basal level after 8 min of washout (time-point 45 min, Figure 1). Ischemia resulted in cardiac arrest in all groups. At reperfusion, cardiac function was significantly compromised in all groups, and the rigor-type ischemic contracture was observed as shown by the significant raise in EDP (time = 75 min, Figure 3B). Cardiac contracture resumed within seconds of reperfusion in all groups. OPC and DigPC improved cardiac contractile performance within minutes of reperfusion. At the end of 30 min of reperfusion, the LVDP recovery was 29.6±3.7% in the non-treated group and 33.8±3.4% in the DMSO group, again suggesting that the trace amount of DMSO used to increase digoxin solubility did not affect IR injury in these conditions. OPC and DigPC treatments yielded to significantly higher recoveries of LVDP at 30 min (62.1±1.4 % and 62.5±1.9 %, respectively, P<0.05 vs. respective controls). This was correlated with a similar protection against IR-induced increased in EDP of about 25% by OPC and DigPC (P< 0.05 vs. respective controls), as shown in Figure 4B at t=120 min.
Figure 4. Comparative effects of OPC and DigPC on Left ventricular developed pressure (LVDP, A) and end diastolic pressure (EDP, B) recovery.
Mice hearts were retroperfused in Langendorff mode and subjected to 40 min of global ischemia and 30 min of reperfusion without preconditioning treatment (IR or DMSO), or following exposure to ouabain preconditioning (OPC), or digoxin preconditioning (DigPC) according to the protocols described in Figure 1. Values are means ± SEM (n=5–6). *** P<0.001 vs. IR, ### P<0.001 vs. DMSO.
Reduced Myocardial Cell Death in Ouabain and Digoxin Preconditioned Hearts
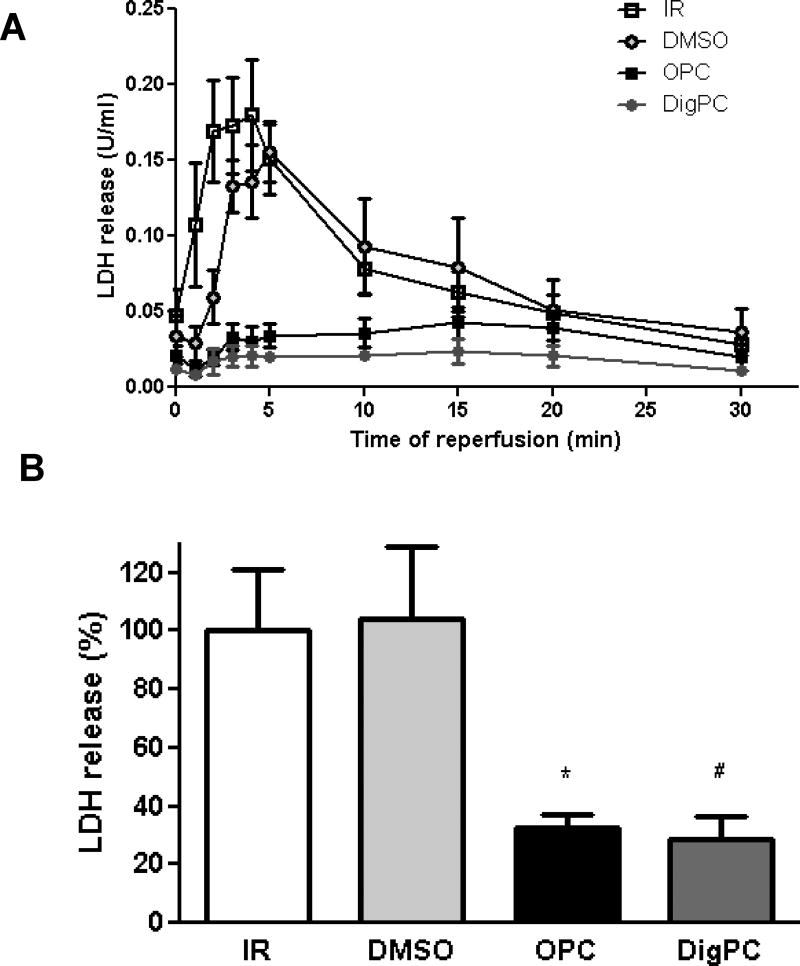
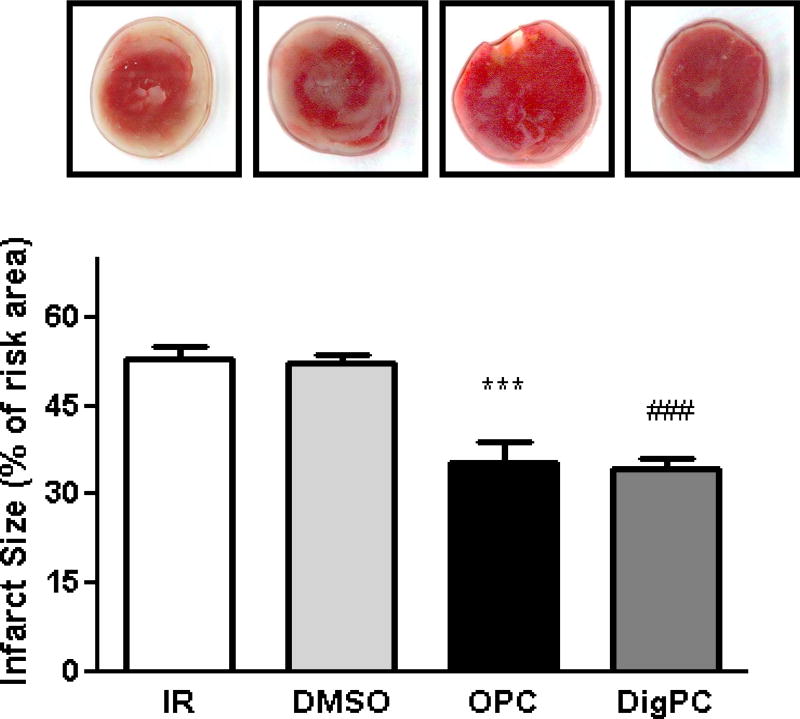
To further test whether tissue viability was preserved by the preconditioning treatments, lactate dehydrogenase release was measured at reperfusion as detailed in Methods. The amount of LDH released during the first 30 min of reperfusion (calculated as the area under the curve) was comparable in the IR and DMSO groups (Figure 5), suggesting that the small amount of DMSO used as vehicle did not interfere with the assay or significantly affect cell viability. DigPC-induced decrease in LDH released was significantly lower than IR, undistinguishable from that measured in the OPC group (Figure 5). These findings were corroborated by infarct size measurements after triphenyltetrazolium chloride (TTC) staining in hearts reperfused for two hours (Figure 6). Indeed, infarcts resulting from 40 min of global ischemia and 2 hours of reperfusion were comparable in untreated hearts and in hearts treated with DMSO only (52.3±2.2% and 52.1±1.4% of the area at risk, respectively (Figure 6), but significantly decreased in OPC (35.3±3.7 % P<0.001 vs. IR) and DigPC hearts (34.4±1.6 %, P<0.001 vs. DMSO).
Figure 5. Comparative effects of OPC and DigPC on Ischemia/Reperfusion-induced lactate dehydrogenase (LDH) release in coronary effluents.
A. Time-dependent release of LDH in coronary effluents throughout the 30min of reperfusion. B. Total LDH release calculated from the 30 min area-under-the curves and expressed as percentage of the IR group. Values are means ± SEM (n=5–6). * P<0.05 vs. IR, # P<0.05 vs. DMSO. IR: ischemia/reperfusion, OPC: ouabain preconditioning, DMSO: DMSO + ischemia/reperfusion, DigPC: digoxin preconditioning.
Figure 6. Comparative effects of OPC and DigPC on Ischemia/Reperfusion-induced infarction.
Infarct sizes are expressed as percentage of the area risk (equivalent to whole myocardium) and representative center cross-sections are shown. Bars represent means ± SEM of 6 independent experiments for each group. *** P<0.01 vs. IR, ### P<0.01 vs. DMSO. IR: ischemia/reperfusion, OPC: ouabain preconditioning, DMSO: DMSO + ischemia/reperfusion, DigPC: digoxin preconditioning.
Preserved Post-ischemic Cardiac Na/K-ATPase Enzymatic Activity with Ouabain or Digoxin Preconditioning
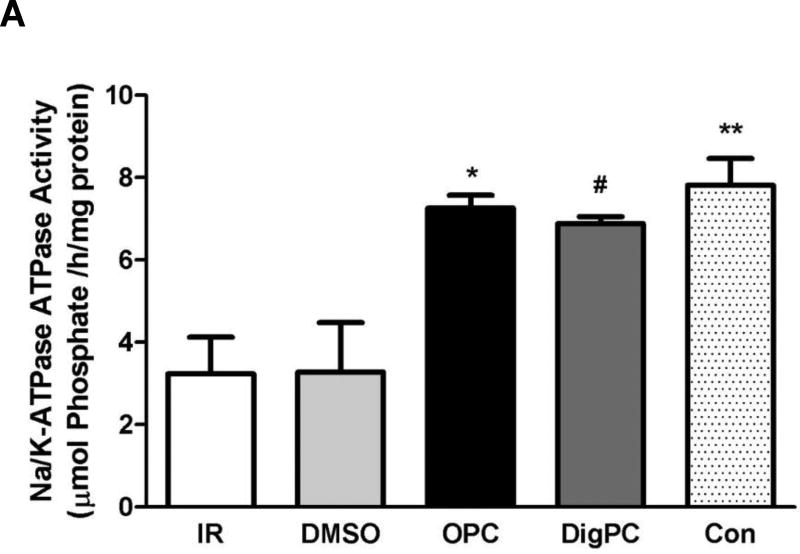
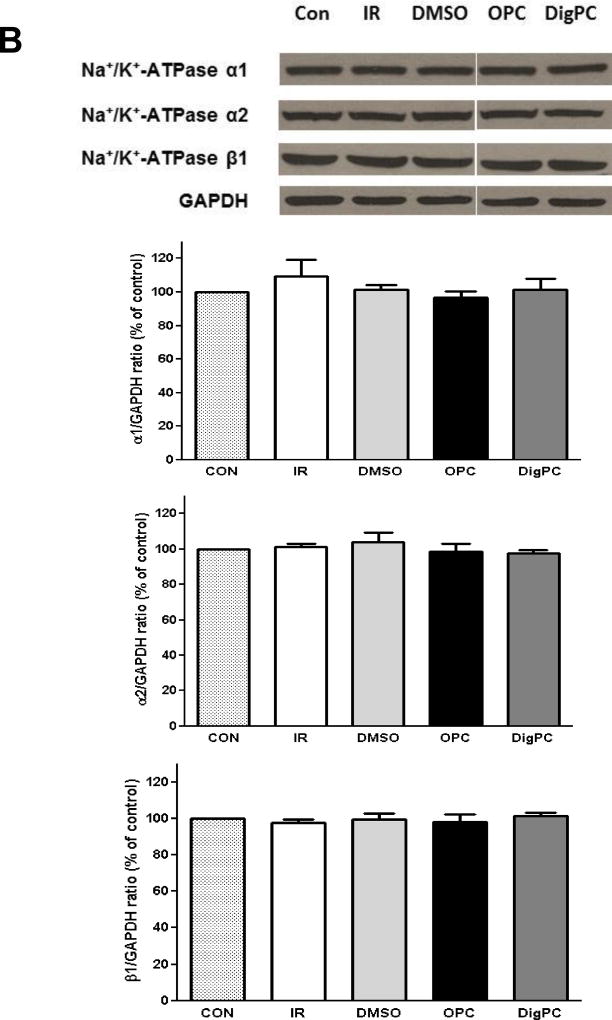
To test whether treatment with the selected CG could protect mouse myocardial Na/K-ATPase against IR-induced alteration, the maximal ouabain-sensitive Na/K-ATPase activity was measured in crude homogenates prepared from hearts exposed to 40 min of ischemia and 30 min of reperfusion. As expected, Na/K-ATPase activity was significantly decreased by about 60% in IR and DMSO-IR groups (3.2±0.9 µmol Pi/h/mg and 3.3±1.2 µmol Pi/h/mg, P<0.01 compared to 7.8±0.6 µmol Pi/h/mg in control hearts). As shown in Figure 7, OPC and DigPC had a significantly higher Na/K-ATPase activity (7.3±0.3 µmol Pi/h/mg and 6.9±0.2 µmol Pi/h/mg, P<0.05 vs. IR or DMSO+IR). This activity was undistinguishable from that of control hearts (P>0.05). As depicted in Figure 7, these changes in activity following ischemia/reperfusion or preconditioning were not associated to any detectable change in Na/K-ATPase α1, α2 or β1/GAPDH ratios in whole heart homogenates, suggesting that changes in total protein levels of Na/K-ATPase subunits did not occur.
Figure 7. Comparative effects of ouabain and digoxin post conditioning on cardiac Na/K-ATPase activity and subunit expression.
A. Na/K-ATPase activity. Mouse hearts were Langendorff-perfused according to the protocols described in Figure 1. After 30 min of reperfusion, crude homogenates were prepared and Na/K-ATPase activity was measured in the presence of the ionophore alamethicin as described in Methods. Values are means ± SEM (n=3). * P<0.05 vs. IR, # P<0.05 vs. DMSO. B. Na/K-ATPase isoform expression. Mouse hearts were Langendorff-perfused according to the protocols described in Figure 1. After 30 min of reperfusion, heart lysates were prepared and subjected to western blot analysis with Na/K-ATPase isoform-specific antibodies. Representative blots and compiled Na/K-ATPase alpha1/GAPDH, alpha2/GAPDH, and beta1/GAPDH ratios expressed as percentage of control are shown. Values are means ± SEM (n=4). No significant difference was detected.
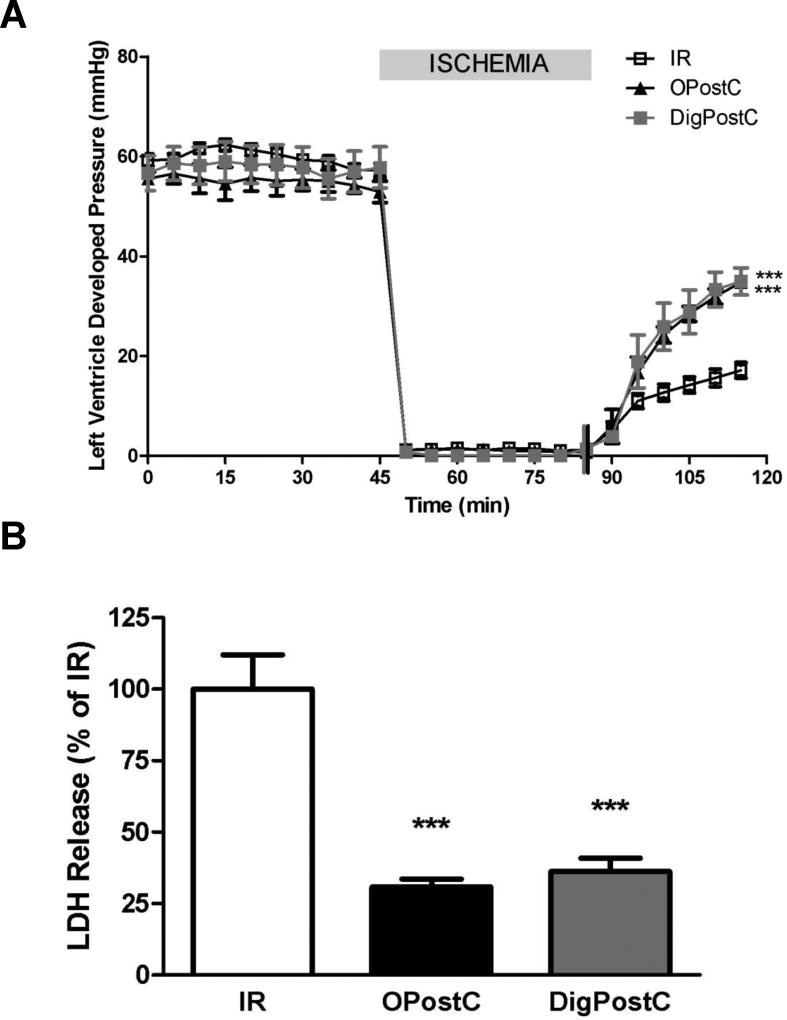
Cardioprotective Effects of Post-conditioning by Ouabain and Digoxin
Many substances that can trigger preconditioning (PC) are also able to trigger postconditioning (PostC) through similar signaling pathways [16–19]. To test whether selected CG could also trigger postconditioning, a bolus infusion of 100 nmoles ouabain was given just before the beginning of reperfusion (Figure 1). As shown in Figure 8, at the end of 30min reperfusion, the LVDP in ouabain postconditioning (OPostC) group was recovered significantly compared to IR group (62.5 ±1.4 % vs. 29.6±3.7%, P< 0.001) and the extent of recovery was comparable to that of OPC (62.1±1.4 %). Accordingly, the total amount of LDH released during 30min reperfusion was also decreased significantly in OPostC group to 30.8±2.8% of the amount in IR group (P<0.001) . The decrease of LDH in OPostC was also comparable to OPC group (32.3±5.0 % of IR). These results indicated that OPostC was as potent as OPC in terms of cardioprotection in face of ischemia-reperfusion injury. Due to the similarity between ouabain and digoxin in biochemical and signaling properties as shown in Fig. 3, the same PostC protocol was used for digoxin (bolus injection of 100nmoles digoxin). At the end of 30 min reperfusion, DigPostC also significantly improved LVDP recovery (62.2±5.5%, 29.6±3.7% in IR, P<0.001) and significantly lowered LDH release (36.2±4.6% of IR, P<0.001 vs. IR). Compared to OPostC, the LVDP and LDH release in DigPostC were very similar. These results suggest that OPostC and DigPostC afforded comparable levels of protection against ischemia-reperfusion injury.
Figure 8. Comparative effects of OPostC and DigPostC on Left ventricular developed pressure (LVDP, A) recovery and LDH release (B).
Mice hearts were retroperfused in Langendorff mode and subjected to 40 min of global ischemia and 30 min of reperfusion without postconditioning treatment (IR), or following exposure to ouabain postconditioning (OPostC) or digoxin preconditioning (DigPostC) according to the protocols described in Figure 1. Values are means ± SEM (n=5–6). *** P<0.001 vs. IR.
Discussion
Basic and clinical ischemic-reperfusion research continues to support effectiveness and feasibility of cardioprotection beyond timely reperfusion, but there is still no cardioprotective drug available [32]. Translation of experimental results into the clinical setting has been challenging for a number of reasons, the least of which is the impact of cardiovascular co-morbidities such as hypertension or diabetes and their treatments on most known cardioprotective mechanisms [33]. Based on our 2015 study [11], the specific signaling pathway triggered by OPC would be uniquely suited for patients with diseases and/or treatments that impair PI3-KIA or Akt-dependent signaling. Before any further consideration could be given to this concept, two key questions had to be addressed. First, could cardioprotective signaling and favorable tissue response be triggered with digoxin, the only FDA-approved CG, which has been used clinically in the management of heart failure and atrial fibrillation for decades [34]. The second question, also translational in nature, was whether or not a CG-based post-conditioning (DigPostC) protocol could be established and afford a significant cardioprotection comparable to that of an ischemic post-conditioning protocol.
Given the many features shared by all CG, the answer to the first question may seem predictable at first. Indeed, all CG share a unique core structure. More specifically, digoxin and ouabain share the same five-membered lactone ring, but have different hydroxyl group substitutes and different glycosylation connected to the steroid core [35]. All CG are also known to bind to and inhibit Na/K-ATPase and increase the cardiac force of contraction. On the other hand, they are numerous reports that CG do trigger diverse-, if not antagonistic, biological responses [36]. The underlying mechanisms, but also the crucial physiological and pharmacological consequences of this diversity are currently the focus of intense investigation; particularly in cardiovascular and cancer research [35, 37, 38]. In terms of cardioprotective signaling against ischemia-reperfusion injury, the focus has been on OPC and the underlying pathway involving Src kinase, PKCε, mitoK-ATP opening, and PI3K–IA [11]. Although encouraging initial results have been reported by d’Urso et al. [9], it has remained unknown whether a transient exposure to a subinotropic concentration of digoxin would trigger PKCε translocation and subsequent preconditioning. As shown in figure 3, ouabain and digoxin at a concentration of 10 µmol/L produced a similar level of inhibition of the mouse cardiac Na/K-ATPase when applied to a crude extract. At this concentration, they also triggered a comparable stimulation of about 50% of the cardioprotective PKCε translocation from the cytosolic to the particulate fraction after 4 min. DigPC with 10 µmol/L digoxin afforded protection against I/R induced cardiac dysfunction and cell death, as well as a protection of Na/K-ATPase activity comparable to OPC.
Accordingly, we set out to address the second objective of this study, which was to test whether a CG-based post conditioning protocol could be developed with digoxin. Perhaps not surprisingly, initial attempts using 10 µmol/L of ouabain for 4 minutes at the onset of reperfusion yielded negative results (data not shown). Based on our recent study in the rat [14], this concentration of ouabain may have triggered an unsustainable level of inhibition of Na/K-ATPase α2- and α1-containing isoenzymes in the ischemic heart and hinder the recovery of ion homeostasis at reperfusion. This initial finding, along with hints from IPostC algorithms and pharmacological PostC reporting brief intermittent periods of exposure to treatment as most effective at reperfusion, especially in small species [39], prompted us to opt for a brief, one-time (bolus) injection of 100 nmoles of ouabain at reperfusion. As shown in figure 8, this postconditioning protocol was as effective as OPC in protecting cardiac structure and function, and could be triggered by both ouabain and digoxin.
Based on in vitro studies in epithelial cells [40], Na/K-ATPase α1, rather than α2, would be the critical receptor for CG-based postconditioning. Although this remains to be firmly established, our findings suggest that CG-induced cardioprotective signaling is still achievable despite the isoform-specific endocytosis of Na/K-ATPase α1 that we and others have observed early during the ischemia reperfusion process [10, 41].
In sum, we report a novel DigPostC protocol that is highly protective against IR injury in the Langendorff-perfused mouse heart. Further pre-clinical investigation in vivo is warranted, and we propose that it should focus on digoxin rather than ouabain as a trigger. Indeed, decades of use of FDA-approved digoxin, still prescribed as the only oral inotropic agent available and the only inotropic agent associated with favorable effects on outcomes in patients with heart failure [42–45], shall greatly facilitate further translation of this cardioprotective effects into the clinical arena. Additionally, with DigPostC effects observed with a very small bolus injection in the coronary tree (Figure 8), concerns of toxicity associated with digoxin’s relatively low therapeutic index would be significantly curtailed.
Acknowledgments
This study was supported by NIH P01HL036573 and MIIR Funds.
Footnotes
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Mobasheri A, Avila J, Cózar-Castellano I, Brownleader MD, Trevan M, Francis MJ, Lamb JF, Martín-Vasallo P. Na+, K+-ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Bioscience reports. 2000;20(2):51–91. doi: 10.1023/a:1005580332144. [DOI] [PubMed] [Google Scholar]
- 2.Skou JC. The Na, K-pump. Methods in enzymology. 1988;156:1. doi: 10.1016/0076-6879(88)56004-4. [DOI] [PubMed] [Google Scholar]
- 3.Reuter H, Henderson SA, Han T, Ross RS, Goldhaber JI, Philipson KD. The Na+-Ca2+ exchanger is essential for the action of cardiac glycosides. Circ Res. 2002;90(3):305–8. doi: 10.1161/hh0302.104562. [DOI] [PubMed] [Google Scholar]
- 4.Shattock MJ, Ottolia M, Bers DM, Blaustein MP, Boguslavskyi A, Bossuyt J, Bridge JH, Chen-Izu Y, Clancy CE, Edwards A, Goldhaber J, Kaplan J, Lingrel JB, Pavlovic D, Philipson K, Sipido KR, Xie ZJ. Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. J Physiol. 2015;593(6):1361–82. doi: 10.1113/jphysiol.2014.282319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L, Li J, Liu J, Yuan Z, Pierre SV, Qu W, Zhao X, Xie Z. Involvement of Na+/K+-ATPase in hydrogen peroxide-induced hypertrophy in cardiac myocytes. Free Radic Biol Med. 2006;41(10):1548–56. doi: 10.1016/j.freeradbiomed.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Zhao X, Pierre SV, Askari A. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol. 2007;293(5):C1489–97. doi: 10.1152/ajpcell.00158.2007. [DOI] [PubMed] [Google Scholar]
- 7.Pasdois P, Quinlan CL, Rissa A, Tariosse L, Vinassa B, Costa AD, Pierre SV, Dos Santos P, Garlid KD. Ouabain protects rat hearts against ischemia-reperfusion injury via pathway involving src kinase, mitoKATP, and ROS. Am J Physiol Heart Circ Physiol. 2007;292(3):H1470–8. doi: 10.1152/ajpheart.00877.2006. [DOI] [PubMed] [Google Scholar]
- 8.Morgan EE, Li Z, Stebal C, Belliard A, Tennyson G, Salari B, Garlid KD, Pierre SV. Preconditioning by subinotropic doses of ouabain in the Langendorff perfused rabbit heart. J Cardiovasc Pharmacol. 2010;55(3):234–9. doi: 10.1097/FJC.0b013e3181ce5e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Urso G, Frascarelli S, Zucchi R, Biver T, Montali U. Cardioprotection by ouabain and digoxin in perfused rat hearts. J Cardiovasc Pharmacol. 2008;52(4):333–7. doi: 10.1097/FJC.0b013e3181884448. [DOI] [PubMed] [Google Scholar]
- 10.Belliard A, Sottejeau Y, Duan Q, Karabin JL, Pierre SV. Modulation of cardiac Na+,K+-ATPase cell surface abundance by simulated ischemia-reperfusion and ouabain preconditioning. Am J Physiol Heart Circ Physiol. 2013;304(1):H94–103. doi: 10.1152/ajpheart.00374.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan Q, Madan ND, Wu J, Kalisz J, Doshi KY, Haldar SM, Liu L, Pierre SV. Role of phosphoinositide 3-kinase IA (PI3K-IA) activation in cardioprotection induced by ouabain preconditioning. J Mol Cell Cardiol. 2015;80:114–25. doi: 10.1016/j.yjmcc.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):112436. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 13.Garlid KD, Costa AD, Quinlan CL, Pierre SV, Dos Santos P. Cardioprotective signaling to mitochondria. J Mol Cell Cardiol. 2009;46(6):858–66. doi: 10.1016/j.yjmcc.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belliard A, Gulati GK, Duan Q, Alves R, Brewer S, Madan N, Sottejeau Y, Wang X, Kalisz J, Pierre SV. Ischemia/reperfusion-induced alterations of enzymatic and signaling functions of the rat cardiac Na+/K+-ATPase: protection by ouabain preconditioning. Physiol Rep. 2016;4(19) doi: 10.14814/phy2.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579–88. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 16.Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3beta. Anesthesiology. 2005;103(5):987–95. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Jang Y, Xi J, Wang H, Mueller RA, Norfleet EA, Xu Z. Postconditioning prevents reperfusion injury by activating delta-opioid receptors. Anesthesiology. 2008;108(2):243–50. doi: 10.1097/01.anes.0000299437.93898.4a. [DOI] [PubMed] [Google Scholar]
- 18.Ke JJ, Yu FX, Rao Y, Wang YL. Adenosine postconditioning protects against myocardial ischemia-reperfusion injury though modulate production of TNF-alpha and prevents activation of transcription factor NF-kappaB. Mol Biol Rep. 2011;38(1):531–8. doi: 10.1007/s11033-010-0137-8. [DOI] [PubMed] [Google Scholar]
- 19.Ebner B, Ebner A, Reetz A, Bohme S, Schauer A, Strasser RH, Weinbrenner C. Pharmacological postconditioning by bolus injection of phosphodiesterase-5 inhibitors vardenafil and sildenafil. Mol Cell Biochem. 2013;379(1–2):43–9. doi: 10.1007/s11010-013-1625-7. [DOI] [PubMed] [Google Scholar]
- 20.Hausenloy DJ, Botker HE, Engstrom T, Erlinge D, Heusch G, Ibanez B, Kloner RA, Ovize M, Yellon DM, Garcia-Dorado D. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J. 2017;38(13):935–941. doi: 10.1093/eurheartj/ehw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutherland FJ, Shattock MJ, Baker KE, Hearse DJ. Mouse isolated perfused heart: characteristics and cautions. Clin Exp Pharmacol Physiol. 2003;30(11):867–78. doi: 10.1046/j.1440-1681.2003.03925.x. [DOI] [PubMed] [Google Scholar]
- 22.Bai Y, Morgan EE, Giovannucci DR, Pierre SV, Philipson KD, Askari A, Liu L. Different roles of the cardiac Na+/Ca2+-exchanger in ouabain-induced inotropy, cell signaling, and hypertrophy. Am J Physiol Heart Circ Physiol. 2013;304(3):H427–35. doi: 10.1152/ajpheart.00462.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ytrehus K, Liu Y, Tsuchida A, Miura T, Liu GS, Yang X, Herbert D, Cohen MV, Downey JM. Rat, and rabbit heart infarction: effects of anesthesia, perfusate, risk zone, and method of infarct sizing. American Journal of Physiology-Heart and Circulatory Physiology. 1994;267(6):H2383–H2390. doi: 10.1152/ajpheart.1994.267.6.H2383. [DOI] [PubMed] [Google Scholar]
- 24.Pierre SV, Yang C, Yuan Z, Seminerio J, Mouas C, Garlid KD, Dos-Santos P, Xie Z. Ouabain triggers preconditioning through activation of the Na+,K+-ATPase signaling cascade in rat hearts. Cardiovasc Res. 2007;73(3):488–96. doi: 10.1016/j.cardiores.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang W, Rhee HM, Chiu TH, Askari A. Re-evaluation of the relationship between the positive inotropic effect of ouabain and its inhibitory effect on (Na+ + K+)-dependent adenosine triphosphatase in rabbit and dog hearts. J Pharmacol Exp Ther. 1979;211(3):571–82. [PubMed] [Google Scholar]
- 26.Xie ZJ, Wang YH, Ganjeizadeh M, McGee R, Jr, Askari A. Determination of total (Na+ + K+)-ATPase activity of isolated or cultured cells. Anal Biochem. 1989;183(2):215–9. doi: 10.1016/0003-2697(89)90470-3. [DOI] [PubMed] [Google Scholar]
- 27.Dostanic I, Schultz Jel J, Lorenz JN, Lingrel JB. The alpha 1 isoform of Na,K-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart. J Biol Chem. 2004;279(52):54053–61. doi: 10.1074/jbc.M410737200. [DOI] [PubMed] [Google Scholar]
- 28.Lucchesi PA, Sweadner KJ. Postnatal changes in Na,K-ATPase isoform expression in rat cardiac ventricle. Conservation of biphasic ouabain affinity. J Biol Chem. 1991;266(14):9327–31. [PubMed] [Google Scholar]
- 29.He S, Shelly DA, Moseley AE, James PF, James JH, Paul RJ, Lingrel JB. The alpha(1)- and alpha(2)-isoforms of Na-K-ATPase play different roles in skeletal muscle contractility. Am J Physiol Regul Integr Comp Physiol. 2001;281(3):R917–25. doi: 10.1152/ajpregu.2001.281.3.R917. [DOI] [PubMed] [Google Scholar]
- 30.Berry RG, Despa S, Fuller W, Bers DM, Shattock MJ. Differential distribution and regulation of mouse cardiac Na+/K+-ATPase alpha1 and alpha2 subunits in T-tubule and surface sarcolemmal membranes. Cardiovasc Res. 2007;73(1):92–100. doi: 10.1016/j.cardiores.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Hilgenberg LG, Pham B, Ortega M, Walid S, Kemmerly T, O’Dowd DK, Smith MA. Agrin regulation of alpha3 sodium-potassium ATPase activity modulates cardiac myocyte contraction. J Biol Chem. 2009;284(25):16956–65. doi: 10.1074/jbc.M806855200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidavalur R, Swarnakar S, Thirunavukkarasu M, Samuel SM, Maulik N. Ex vivo and in vivo approaches to study mechanisms of cardioprotection targeting ischemia/reperfusion (i/r) injury: useful techniques for cardiovascular drug discovery. Current drug discovery technologies. 2008;5(4):269–278. doi: 10.2174/157016308786733555. [DOI] [PubMed] [Google Scholar]
- 33.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacological reviews. 2007;59(4):418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 34.Gheorghiade M, van Veldhuisen DJ, Colucci WS. Contemporary use of digoxin in the management of cardiovascular disorders. Circulation. 2006;113(21):2556–64. doi: 10.1161/CIRCULATIONAHA.105.560110. [DOI] [PubMed] [Google Scholar]
- 35.Dvela M, Rosen H, Feldmann T, Nesher M, Lichtstein D. Diverse biological responses to different cardiotonic steroids. Pathophysiology. 2007;14(3):159–166. doi: 10.1016/j.pathophys.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Feldmann T, Glukmann V, Medvenev E, Shpolansky U, Galili D, Lichtstein D, Rosen H. Role of endosomal Na+-K+-ATPase and cardiac steroids in the regulation of endocytosis. American Journal of Physiology-Cell Physiology. 2007;293(3):C885–C896. doi: 10.1152/ajpcell.00602.2006. [DOI] [PubMed] [Google Scholar]
- 37.Nesher M, Shpolansky U, Viola N, Dvela M, Buzaglo N, Ben-Ami HC, Rosen H, Lichtstein D. Ouabain attenuates cardiotoxicity induced by other cardiac steroids. British journal of pharmacology. 2010;160(2):346–354. doi: 10.1111/j.1476-5381.2010.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blaustein MP. Endogenous ouabain: role in the pathogenesis of hypertension. Kidney international. 1996;49(6):1748–1753. doi: 10.1038/ki.1996.260. [DOI] [PubMed] [Google Scholar]
- 39.Penna C, Mancardi D, Raimondo S, Geuna S, Pagliaro P. The paradigm of postconditioning to protect the heart. Journal of cellular and molecular medicine. 2008;12(2):435–458. doi: 10.1111/j.1582-4934.2007.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie J, Ye Q, Cui X, Madan N, Yi Q, Pierre SV, Xie Z. Expression of rat Na-K-ATPase α2 enables ion pumping but not ouabain-induced signaling in α1-deficient porcine renal epithelial cells. American Journal of Physiology-Cell Physiology. 2015;309(6):C373–C382. doi: 10.1152/ajpcell.00103.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hilgemann DW, Fine M, Linder ME, Jennings BC, Lin M-J. Massive endocytosis triggered by surface membrane palmitoylation under mitochondrial control in BHK fibroblasts. Elife. 2013;2:e01293. doi: 10.7554/eLife.01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289(7):871–8. doi: 10.1001/jama.289.7.871. [DOI] [PubMed] [Google Scholar]
- 43.D.I.G. The effect of digoxin on mortality and morbidity in patients with heart failure. The Digitalis Investigation Group. N Engl J Med. 1997;336(8):525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 44.Gheorghiade M, Braunwald E. Reconsidering the role for digoxin in the management of acute heart failure syndromes. JAMA. 2009;302(19):2146–7. doi: 10.1001/jama.2009.1657. [DOI] [PubMed] [Google Scholar]
- 45.Hood WB, Jr, Dans AL, Guyatt GH, Jaeschke R, McMurray JJ. Digitalis for treatment of congestive heart failure in patients in sinus rhythm: a systematic review and meta-analysis. J Card Fail. 2004;10(2):155–64. doi: 10.1016/j.cardfail.2003.12.005. [DOI] [PubMed] [Google Scholar]