To the editor,
Liver transplantation (LT) is the only therapeutic option for both HCC and cirrhosis. Despite careful patient selection, HCC-R_LT occurs in as much as 20%, and is often characterized by an aggressive clinical course and high mortality. We therefore designed a proof-of-concept study aimed to analyze the safety of combination therapy with nivolumab and bevacizumab in patients with HCC-R_LT, whose tumor progressed in most cases while on sorafenib therapy. In particular, we focused on the risk of rejection and overall survival (OS).
METHODS
We evaluated the feasibility and safety of nivolumab (240 mg every 14 d) and bevacizumab (5 mg/kg every 14 d) in HCC-R_LT. Candidate patients underwent hepatic, renal, and cardiac function tests (at baseline ad after each infusion), echocardiography, and total-body CT (at baseline and every 3 months unless otherwise indicated). Bevacizumab was added if nivolumab was well tolerated and no rejection occurred after the first 2 nivolumab infusions. Patients unsuitable for nivolumab/bevacizumab were treated with regorafenib (160 mg orally once daily for 3 weeks out of each 4-week cycle). Adverse events (AE) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v.5.0.
The biological aggressiveness of the tumor at transplantation and the plausibility of antiangiogenic therapy were evaluated as indicated in Supplementary Methods, http://links.lww.com/LVT/A334.
Endpoints and statistics
Efficacy assessments were made using Response Evaluation Criteria in Solid Tumors 1.1. Outcome endpoints were defined as stable disease ≥6 months, partial response, complete response or progressive disease (ie, ≥20% increase in tumor size against a known baseline lesion or new intrahepatic or extrahepatic lesions in the liver, lymph nodes, lungs, and bones). Overall survival (OS) was evaluated by the Kaplan–Meier method after the initiation of second-line therapy. Statistical analysis was performed with IBM SPSS Statistics for Macintosh, Version 28.0.
Declaration of ethical approval
Combination therapy received nominal authorization after a formal review of the health records of each patient by the county off-label drug Committee [NOP]. Informed written consent was obtained from each subject. The study complied with good clinical practice guidelines, the Declaration of Helsinki and Instanbul, and the applicable local laws.
RESULTS
Between February 2018 and September 2021, 22 patients with HCC-R_LT were consecutively referred to the Gastroenterology Unit of Azienda Ospedaliero-Universitaria di Modena, Italy, after a median period of 14.5 months (range, 4–106 mo) from LT. Demographic, biochemical, clinical data, pretransplant downstaging interventions, HCC characteristics at transplant, and histochemical staining for Angiopoietin-2 and VEGF_A are reported in Supplementary Table 1, http://links.lww.com/LVT/A335 and Supplementary Figure 1, http://links.lww.com/LVT/A333.
Of the 22 patients, 4 (18.1%) underwent best supportive care (BSC) alone (all for Eastern Cooperative Oncology Group Performance Status Scale=3). The other 18 (81.8%) started treatment with sorafenib.
Seventeen/18 (94.4%) progressed after a median of 6 months. Twelve/17 (70.6%) were switched to regorafenib, not being eligible for Nivolumab or Bevacizumab for the following: Nivolumab, hyperthyroidism n=2; cardiac ejection fraction <45% n=2; Bevacizumab: low neutrophils count n=3; arterial hypertension on treatment n=2, and chronic heart failure n=1. The other 5 patients (29.4%) received nivolumab as initial treatment. One patient received only 1 infusion. Three weeks later, bilirubin levels increased, liver and lung tumoral invasion rapidly progressed, and massive neoplastic ascites developed together with acute kidney insufficiency. The patient died 2 weeks later.
All the other 4 patients tolerated nivolumab well. One patient experienced after the first infusion G2 bilirubin and G3 aspartate aminotransferase increase. A moderate-to-severe rejection was present in the liver biopsy (Figure 1, A). First, Methylprednisolone (1 g/day) for 3 days, then prednisone 50 mg/day for 1 week tapered and discontinued after 3 weeks resolved the clinical event. Nivolumab was continued without recurrence of rejection. Once stable on nivolumab and with no evident AE, the 4 patients started bevacizumab.
FIGURE 1.
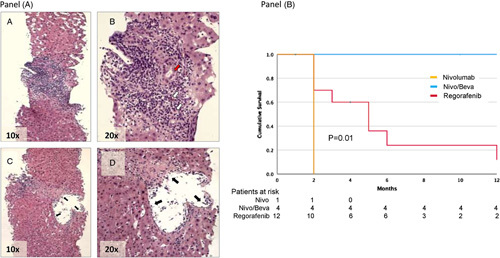
(A) Liver biopsy performed 10 days after the first nivolumab infusion. HE staining shows prominent portal mixed inflammation with interface activity ([A], ×10, [B] ×20) and eosinophil infiltrate (B, white arrows). There is a presence of cytoplasmatic vacuolization of the duct epithelium consistent with bile duct damage ([B], red arrow). In [C (×10)] and [D (×20)], a representative example of subendothelial lymphocytic inflammation with lifting up of the endothelium compatible with endothelitis is shown (black arrows). (B), Kaplan–Meier curve for the survival of patients with recurrence of HCC after liver transplant after starting second-line therapy with Nivolumab/Bevacizumab (blue line), or Regorafenib (red line). Differences in survival were compared by the log-rank test. The orange line indicates the patient, who received only 1 Nivolumab infusion but died of rapid tumoral progression.
Stable disease at the hepatic, lung, and lymph node levels was achieved in all 4 patients. Two patients with bone metastasis experienced progression after 8 and 10 months, respectively, from starting nivolumab/bevacizumab. Both patients underwent 2 courses of radiotherapy 5 months apart, obtaining satisfactory pain control.
All 4 patients are still on combination therapy: the mean treatment period has been so far 13.4±5.1 months (median 16 mo). OS from the initiation of sorafenib has been 26.5±10.4 for patients on nivolumab/bevacizumab versus 9.5±5.5 for those on regorafenib (p=0.02). The survival of patients in BSC has been 5.5±5.2 months. OS from the initiation of second-line therapy has been 16.0±4.5 months for patients on nivolumab/bevacizumab versus 5.8±6.1 months for those on regorafenib (p=0.01)(Figure 1, B; Table 1).
TABLE 1.
Clinical characteristics of patients at the time of HCC recurrence after LT divided by different types of second-line treatment (nivolumab, nivolumab/bevacizumab, or regorafenib)
| Features | Nivolumab (n=1) | Nivo/Beva (n=4) | Regorafenib (n=12) | p a |
|---|---|---|---|---|
| Age at LT (mean, SD) | 51 | 56.7±8.5 | 60.1±9.1 | 0.52 |
| Age at recurrence (mean, SD) | 53 | 58.5±9.2 | 62.3±8.9 | 0.48 |
| Sex (male/female) | 0/1 | 3/1 | 11/2 | 0.66 |
| Blood type | ||||
| A | 1 | 1 (20.0) | 5 (38.4) | 0.49 |
| B | – | – | 2 (15.3) | – |
| AB | – | – | 2 (15.3) | – |
| 0 | – | 3 (60.0) | 4 (30.7) | – |
| Etiology of Liver disease, n (%) | ||||
| HCV | 1 | 1 (25.0) | 6 (46.2) | 0.60 |
| HBV | – | 1 (25.0) | 3 (25.0) | – |
| Other causes | – | 2 (50.0) | 3 (25.0) | – |
| Bilirubin (mg/dL) (mean, SD) | 0.9 | 0.5±0.2 | 0.5±0.1 | 0.94 |
| Albumin (g/dL)(mean, SD) | 4.1 | 3.9±0.6 | 3.7±0.4 | 0.68 |
| Creatinine (mg/dL) (mean, SD) | 0.7 | 0.9±0.2 | 1.0±0.2 | 0.31 |
| INR | 1.0 | 1.0±0.2 | 1.0±.01 | 0.86 |
| AFP (ng/mL) (mean, SD) | 53 | 25±35 | 149±403 | 0.56 |
| Edmondson-Steiner grading, n (%) | ||||
| 1 | – | – | 1 (8.3) | 0.83 |
| 2 | 1 | 2 (50.0) | 5 (41.7) | – |
| 3 | – | 2 (50.0) | 6 (50.0) | – |
| Presence of viable tumor at explant, n (%) | 0 | 3 (75.0) | 10 (76.9) | 0.32 |
| Post-transplant complications, n (%) | ||||
| Biliary stenosis | 0 | 2 (50.0) | 3 (23.0) | 0.81 |
| Re-transplant | 0 | 1 (25.0) | 1 (7.6) | – |
| Immunosuppression at recurrence, n (%) | ||||
| Everolimus | 0 | 1 (25.0) | 6 (50.0) | 0.40 |
| Sirolimus | 1 | 2 (50.0) | 2 (16.7) | – |
| Tacrolimus | 0 | 1 (25.0) | 4 (33.3) | – |
| Tumor burden at recurrence (volume, mm3) | ||||
| Liver | 1,677 | 827±319 | 1053±1152 | 0.83 |
| Lung | 3,350 | 5481±4271 | 3116±4869 | 0.41 |
| Lymph node | 29,300 | 7195±6481 | 10,221±11,685 | 0.74 |
| Bone | 18,392 | 5598±7280 | 8350±10,677 | 0.79 |
| Adrenal gland | – | 2567 | – | |
| Tumor burden at progression, before starting second-line therapy (volume, mm3) | ||||
| Liver | 1,593 | 1139±585 | 1203±1394 | 0.94 |
| Lung | 6,688 | 11,917±14,868 | 10,497±20,423 | 0.91 |
| Lymph node | 13,346 | 16,279±21,455 | 16,033±34,392 | 0.99 |
| Bone | 50,240 | 31,081±21,395 | 14,527±12,372 | 0.31 |
| Adrenal gland | – | 5558±1423 | 10,321±5606 | 0.43 |
| Treatments performed after HCC recurrence before systemic therapy, n (%) | ||||
| Surgery | – | – | – | 0.65 |
| Lung resection | 1 | – | 4 (30.7) | – |
| Adrenal gland removal | – | 1 (25.0) | 2 (15.3) | – |
| Hepatic hilar lymph node resection | – | 1 (25.0) | – | – |
| Cutaneous metastasis removal | – | – | 1 (7.6) | – |
| Locoregional Treatments | – | – | 1 (7.6) | – |
| None | – | 2 (25.0) | 5 (38.4) | – |
| Timing of events (months, mean±SD) | ||||
| Recurrence-free survival | 10 | 21.5±25.8 | 25.7±24.1 | 0.77 |
| Interval from recurrence to sorafenib | 12 | 3.0±1.4 | 3.8±8.0 | 0.89 |
| Time between sorafenib initiation and discontinuation for progression | 2 | 7.5±1.9 | 6.7±3.1 | 0.68 |
| Time from progression to nivo/beva or regorafenib initiation | 2 | 2.5±0.7 | 2.0±1.0 | 0.59 |
| Time from nivo/beva or regorafenib to death or last visit | 2 | 16.0±4.5 | 5.8±6.1 | 0.01 |
Note: The patient who received a single infusion of Nivolumab is reported individually.
The significance is referred to the comparison between nivolumab/bevacizumab and regorafenib.
Abbreviations: AFP indicates alpha-fetoprotein; Beva, bevacizumab; BSC, Best supportive care; Nivo, nivolumab.
Two/18 patients on sorafenib experienced G1 diarrhea and 2 patients experienced G1 palmar-plantar erythrodysesthesia. After the switch to regorafenib, 3/12 had G2 diarrhea and 1 had G1 fatigue. With nivolumab/bevacizumab, apart from the patient who developed moderate–severe liver rejection after the first nivolumab infusion, the only AE were G1 alanine-aminotransferase increase in 1 patient with nivolumab and G1 proteinuria in another after bevacizumab, not requiring active intervention apart from temporary dose reduction.
DISCUSSION
HCC recurrence after LT represents a dramatic occurrence, only moderately influenced by tyrosine kinase inhibitors.1,2 Indeed, the survival figures reported in the literature indicate an 18-month median survival in sorafenib responders2 and 13 months in regorafenib-treated sorafenib failures,3 with an OS less than 3 months for those on BSC.1 Despite increased tumor stabilization and improved survival obtained in non-LT patients with immune checkpoint inhibitors and bevacizumab,3 LT patients are excluded from experimental protocols for concerns over the potential interaction between anticancer and immunosuppressive drugs. Although preliminary, our data show that the combination of nivolumab/bevacizumab can achieve a stabilization of the disease in the liver and in the lungs, with a gain in survival for patients who have failed sorafenib and would otherwise have only been amenable to BSC or regorafenib. The switch to regorafenib in 12 patients not eligible to nivolumab/bevacizumab was associated with significantly lower survival rates. This is even more relevant because of the notable biological aggressiveness of HCC-R_LT, demonstrated by the extremely high angiopoietin-2 expression, especially in hepatic sinusoidal endothelia.4 VEGF was expressed at a much lower level in tumor tissue, with elevated expression in the surrounding nontumoral tissue.5 This suggests that the relevant clinical effect of bevacizumab could be mediated through an indirect effect on the surrounding nontumoral tissues, preventing further VEGF-mediated signaling.
A limitation of this observational study resides in the low number of enrolled patients. The results show that the combination of nivolumab/bevacizumab is feasible for LT patients, with few and manageable AE. The favorable results observed in terms of survival suggest that a larger and controlled study could be pursued. Bone metastasis remains an active and unsolved problem, indicating the need for alternative approaches.
Supplementary Material
Acknowledgments
FUNDING INFORMATION
This study was supported by the AIRC under IG 2020 ID. 24858 Project —P.I. Erica Villa.
CONFLICT OF INTEREST
The authors have no conflicts to report.
Footnotes
Abbreviations: AE, Adverse events; AFP, alpha-fetoprotein; BSC, best supportive care; HCC-R_LT, HCC recurrence after LT; LT, Liver transplantation; OS, overall survival.
Lorenza Di Marco and Alessandra Pivetti equally contributed.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.ltxjournal.com
Contributor Information
Lorenza Di Marco, Email: lor.dimarco@gmail.com.
Alessandra Pivetti, Email: pivetti.alessandra@aou.mo.it.
Francesco Giuseppe Foschi, Email: francesco.foschi@auslromagna.it.
Roberto D’Amico, Email: roberto.damico@unimore.it.
Filippo Schepis, Email: Filippo.schepis@unimore.it.
Cristian Caporali, Email: caporali.cristian@policlinico.mo.it.
Federico Casari, Email: federico.casari@hotmail.it.
Simone Lasagni, Email: simone.lasagni@unimore.it.
Rosina Maria Critelli, Email: rosinamaria.critelli@unimore.it.
Fabiola Milosa, Email: fabiola.milosa@unimore.it.
Adriana Romanzi, Email: adriana.romanzi@unimore.it.
Gemma Marcelli, Email: gemma.marcelli@unimore.it.
Nicola De Maria, Email: demaria.nicola@policlinico.mo.it.
Dante Romagnoli, Email: romagnoli.dante@unimore.it.
Barbara Catellani, Email: catellani.barbara@aou.mo.it.
Filippo Scianò, Email: sciano.filippo@aou.mo.it.
Paolo Magistri, Email: paolo.magistri@unimore.it.
Antonio Colecchia, Email: antonio.colecchia@unimore.it.
Pamela Sighinolfi, Email: sighinolfi.pamela@aou.mo.it.
Fabrizio Di Benedetto, Email: fabrizio.dibenedetto@unimore.it.
Maria-Luz Martinez-Chantar, Email: mlmartinez@cicbiogune.es.
Erica Villa, Email: erica.villa@unimore.it.
REFERENCES
- 1. Invernizzi F, Iavarone M, Zavaglia C, Mazza S, Maggi U, Cesarini L, et al. Experience with early sorafenib treatment with mTOR inhibitors in hepatocellular carcinoma recurring after liver transplantation. Transplantation. 2020;104:568–574. [DOI] [PubMed] [Google Scholar]
- 2. Iavarone M, Invernizzi F, Ivanics T, Mazza S, Zavaglia C, Sanduzzi-Zamparelli M, et al. Regorafenib efficacy after sorafenib in patients with recurrent hepatocellular carcinoma after liver transplantation: a retrospective study. Liver Transpl. 2021;27:1767–1778. [DOI] [PubMed] [Google Scholar]
- 3. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus Bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. [DOI] [PubMed] [Google Scholar]
- 4. Villa E, Critelli R, Lei B, Marzocchi G, Cammà C, Giannelli G, et al. Neoangiogenesis-related genes are hallmarks of fast-growing hepatocellular carcinomas and worst survival. Results from a prospective study. Gut. 2016;65:861–869. [DOI] [PubMed] [Google Scholar]
- 5. Fodor D, Jung I, Turdean S, Satala C, Gurzu S. Angiogenesis of hepatocellular carcinoma: an immunohistochemistry study. World J Hepatol. 2019;11:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]


