Highlights
-
•
Healthcare workers experienced high rates of morbidity and mortality from coronavirus disease 2019
-
•
Vaccine effectiveness is moderate in those with or without prior infection with severe acute respiratory syndrome coronavirus-2.
-
•
Hybrid immunity – the protective effect of infection followed by vaccination – was strong.
-
•
Vaccination is beneficial in populations regardless of prior infection status.
Keywords: COVID-19 vaccine, Vaccine effectiveness, Hybrid immunity, Healthcare workers, Albania, Delta variant
Abstract
Background
Healthcare workers have experienced high rates of morbidity and mortality from coronavirus disease 2019 (COVID-19).
Methods
A prospective cohort study was conducted in three Albanian hospitals between 19 February and 14 December 2021. All participants underwent polymerase chain reaction (PCR) and serological testing at enrolment, regular serology throughout, and PCR testing when symptomatic.
Vaccine effectiveness (VE) against COVID-19 and against all severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infections (symptomatic or asymptomatic) was estimated. VE was estimated using a Cox regression model, with vaccination status as a time-varying variable.
Findings
In total, 1504 HCWs were enrolled in this study; 70% had evidence of prior SARS-CoV-2 infection. VE was 65.1% [95% confidence interval (CI) 37.7–80.5] against COVID-19, 58.2% (95% CI 15.7–79.3) among participants without prior SARS-CoV-2 infection, and 73.6% (95% CI 24.3–90.8) among participants with prior SARS-CoV-2 infection. For BNT162b2 alone, VE was 69.5% (95% CI 44.5–83.2). During the period when the Delta variant was predominant, VE was 67.1% (95% CI 38.3–82.5). VE against SARS-CoV-2 infection for the full study period was 36.9% (95% CI 15.8–52.7).
Interpretation
This study found moderate primary series VE against COVID-19 among healthcare workers in Albania. These results support the continued promotion of COVID-19 vaccination in Albania, and highlight the benefits of vaccination in populations with high levels of prior infection.
Introduction
Healthcare workers (HCWs) are at high risk for occupational exposures to infectious diseases, and have experienced high rates of morbidity during the coronavirus disease 2019 (COVID-19) pandemic [1], [2], [3]. They also pose a risk of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) transmission to hospitalized patients who are often at high risk of serious COVID-19 outcomes [4].
Robust mass immunization programmes, among other public health measures employed to mitigate the global impact of COVID-19, reduce both morbidity and mortality [5,6]. Since late 2020 when global vaccine distribution began, more than 13.3 billion COVID-19 vaccine doses have been administered worldwide [7]. Vaccine coverage in high-income countries (HICs) has far outpaced that in low- and middle-income countries (LMICs): in the World Health Organization (WHO) European Region, as of 21 March 2023, primary series vaccine coverage was 73.7% in HICs compared with 55.6% in upper middle-income countries and 42.0% in lower middle-income countries [8].
Similar disparities have emerged in evaluations of vaccine effectiveness (VE). To date, numerous observational VE studies have been conducted in HICs [9,10], but few have been published on VE in LMICs, and VE has not been evaluated in middle-income countries in the WHO European Region. Vaccine uptake and VE may be different in LMICs given differences in population age structures, comorbidities, and logistic capacity (such as cold chain integrity, vaccine transport, and delivery) [11].
In Albania, an upper middle-income country with a population of 2.9 million people, COVID-19 vaccination began on 11 January 2021 using the BNT162b2 (Comirnaty, Pfizer–BioNTech) vaccine. Initially, older people, nursing home residents, and HCWs were prioritized for vaccination [12]. This prospective cohort study was conducted in order to evaluate VE of the COVID-19 vaccine in a frequently exposed population prioritized for early vaccination (HCWs) in Albania.
Methods
Study design
This prospective cohort study was conducted to evaluate primary series COVID-19 VE against SARS-CoV-2 infection (both asymptomatic and symptomatic) among HCWs in three hospitals in Albania. The design and analysis were guided by the WHO/Europe VE guidance document [11], and the study was conducted within the framework of WHO's Unity platform [13]. Study implementation was conducted by the Albania Institute of Public Health.
Data collection and management
In February 2021, all HCWs at Tirana University Hospital, Durrës Regional Hospital and Fier Regional Hospital were invited to enrol in the study, regardless of their hospital role, prior infection status or their intention to receive the COVID-19 vaccine. These three large public hospitals represent three of the major metropolitan areas in the country. Enrolment was voluntary and all HCWs in the study who received COVID-19 vaccines did so through the national vaccine campaign led by the Ministry of Health.
At enrolment, participants completed a questionnaire that included demographics and health status; hospital role, including engagement in direct patient care; and COVID-19 and influenza vaccination history, as described previously [14]. In addition, at enrolment, each participant provided a blood sample for serological testing, and a respiratory sample for SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) testing, regardless of whether or not they had symptoms.
Following enrolment, participants completed a weekly symptom questionnaire, administered by study personnel; participants who reported one or more symptoms included in the Albanian Ministry of Health COVID-19 case definition [14] were tested for SARS-CoV-2 by PCR. PCR-positive samples were sent to the Institute of Virology – Charité (Berlin, Germany) where they underwent genomic sequencing.
In order to identify tests performed outside of the study, study staff monitored the Albania National SARS-CoV-2 testing database. Participants who tested positive for SARS-CoV-2 at any time during the study period were administered a follow-up questionnaire 30 days after their positive test, which included questions about symptoms, need for hospitalization, and time to resolution of symptoms [14]. All study data were entered securely and stored in REDCap (Research Electronic Data Capture, Vanderbilt University, Nashville, TN, USA) [15].
Serology
Phlebotomists collected serological specimens from participants at enrolment, and subsequently at 3-month intervals [14]. Enrolment serological samples were tested with WANTAI SARS-CoV-2 Ab enzyme-linked immunosorbent assay (WANTAI BioPharm, Beijing, China), which targets SARS-CoV-2 anti-spike protein. All serological samples were also tested with Platelia SARS-CoV-2 total antibody assay (Bio-Rad Laboratories, Hercules, CA, USA) [16,17], which measures total anti-nucleocapsid antibody and therefore can be used to identify SARS-CoV-2 infections in individuals who have received mRNA and viral vector vaccines. For both serological assays, cut-off values were determined according to the package insert. Seroconversion was defined as a positive serological result (a value above the manufacturer-determined cut-off) in a participant whose most recent serology was negative. Of the 46 participants who had a PCR-confirmed infection during the analysis period, had a negative anti-nucleocapsid antibody prior to the infection and had serology collected after their infection, 42 seroconverted (91%). The median number of days between the date of PCR-confirmed infection and serological collection was 73 [interquartile range (IQR) 39–91] days.
Vaccine effectiveness analysis
For the primary outcome, primary series VE was measured against COVID-19. Cases of COVID-19 were defined as a positive PCR result in a symptomatic participant with symptom onset between 14 days before and 4 days after PCR. As a secondary analysis, primary series VE was measured against symptomatic or asymptomatic SARS-CoV-2 infection, measured by a combined outcome of PCR-confirmed SARS-CoV-2 and/or seroconversion, defined as a positive anti-nucleocapsid antibody test in a participant who was previously seronegative. For participants who seroconverted during the study but did not report symptoms prior to their seroconversion, the time of SARS-CoV-2 infection was estimated as halfway between the last negative serological test and the subsequent positive serological test, taking into account a 3-week lag for seroconversion among asymptomatic persons [18]. For participants who had symptoms prior to their seroconversion but did not have a positive PCR, it was assumed that the infection occurred on the date of symptom onset.
In addition, the combined effect of prior SARS-CoV-2 infection and COVID-19 vaccination on VE was evaluated, using unvaccinated participants without prior infection as the reference category. For the primary analysis, participants were considered to have had prior SARS-CoV-2 infection if they reported previous PCR-confirmed infection and/or were seropositive for anti-nucleocapsid antibody at enrolment. For the secondary analysis, which included seroconversion as an outcome, participants who received CoronaVac vaccine were excluded, as anti-nucleocapsid antibodies could not be used to distinguish between natural infection and vaccine-induced immunity in these individuals.
Statistical model
VE was estimated as (1 – hazard ratio)*100. Hazard ratios comparing vaccinated and unvaccinated subjects were estimated using Cox proportional hazards models with vaccination as a time-varying exposure (vaccination status of some individuals changed over time from unvaccinated to vaccinated, and from one to two doses); as such, the same participant could contribute person-time to more than one exposure category. Study time was used as the underlying time in the Cox regression.
Unadjusted and adjusted VE estimates were calculated. Both estimates include hospital as a fixed effect. The multi-variable regression model was adjusted using a-priori fixed covariates (hospital, age, sex, prior SARS-CoV-2 infection), and potential confounders (hospital role, hands-on care, smoking, household size, chronic health condition, body mass index category) that changed the VE estimate by >5% were considered, according to the change-in-estimate approach.
Participants with prior SARS-CoV-2 infection were included in the analysis at the time point they were considered ‘at risk’ of re-infection, which was defined as 90 days after their most recent positive PCR test or, for participants without a history of a PCR-positive test, 4 weeks after their positive enrolment serology. Participants were considered to be vaccinated with their primary series ≥14 days after their second dose of COVID-19 vaccine.
Person-time contribution was from enrolment, or from the start of time at risk for those with prior SARS-CoV-2 infection, to the earliest of outcome or study exit. Person-time ended on whichever of the following came first: (1) the day of first SARS-CoV-2 infection (symptomatic infection for the primary analysis); (2) the day of receipt of third vaccine dose; or (3) the day of the last weekly questionnaire before loss to follow-up, withdrawal from the study, or censor date for the analysis period (14 December 2021). For the secondary analysis, person-time also ended on the day of the last serological result, or the day of receipt of a dose of CoronaVac. Person-time of persons vaccinated with a single dose was excluded from all analyses from the day they received their first dose, and participants vaccinated with a single dose who had any of the above outcomes were censored from further analysis.
Further analyses and sensitivity analyses
VE analyses were conducted for all vaccines combined, and specifically for the BNT162b2 vaccine. In addition, VE was evaluated during the overall study period and separately for the period in which SARS-CoV-2 B.1.617.2 (Delta variant) was predominant (1 August 2021–14 December 2021), which was defined using sequencing data from study samples along with publicly available data from GISAID [19]. Waning immunity was examined by comparing VE in the period from 14 to 180 days since the second vaccine dose with VE >180 days since the second vaccine dose.
Four sensitivity analyses were also performed: (1) the definition of ‘time at risk’ was changed from 90 days after infection to 60 days after infection, assuming a shorter duration of protection from infection; (2) ‘fully vaccinated’ was defined as 7 days after the second dose rather than 14 days after the second dose; (3) participants who were seropositive at enrolment by the WANTAI anti-spike protein assay were included in the ‘prior infection’ group for the primary analysis; and (4) the start date of the Delta-predominant period was adjusted from 1 August to 1 July 2021.
Results
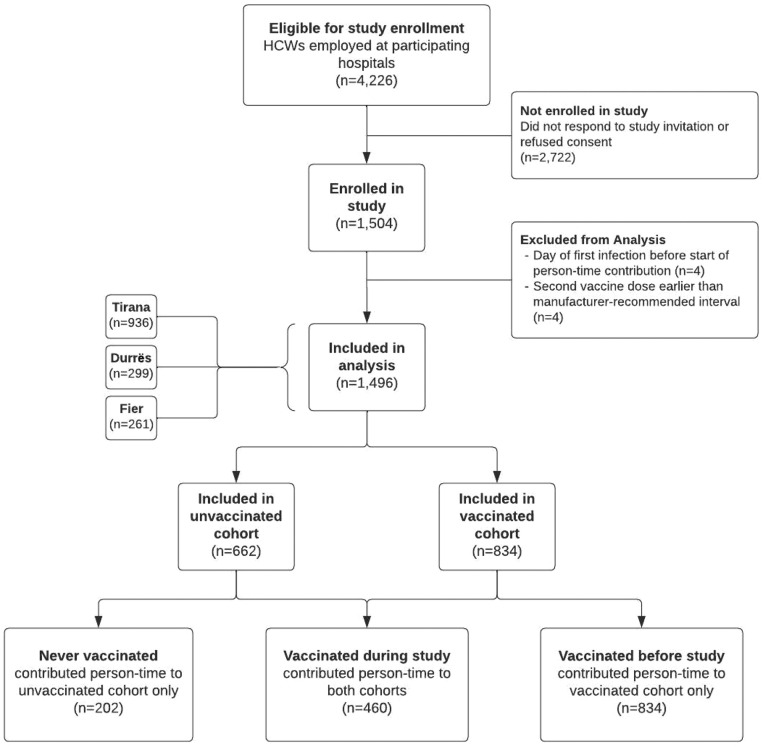
In total, 1504 participants were enrolled between 19 February and 7 May 2021. Eight participants were excluded (Figure 1). Of the 1496 HCWs included in the analysis, 936 (63%) were from Tirana, 299 (20%) were from Durrës, and 261 (17%) were from Fier. The median age was 44 (IQR 33–53) years, and 1177 (79%) participants were female (Table 1). Only 291 participants (19%) reported any underlying chronic conditions. Most HCWs were nurses or midwives [709 (47%)] or physicians [303 (20%)]. Nearly all participants [1426 (95%)] served in patient-facing roles within the hospital. Age and sex distribution, comorbid conditions and occupation were similar across sites.
Figure 1.
Flowchart illustrating the enrolment of healthcare workers in a coronavirus disease 2019 vaccine effectiveness study, Albania, 2021. HCW, healthcare worker.
Table 1.
Participant demographics and clinical characteristics by coronavirus disease 2019 (COVID-19) vaccination status on the last day of follow-upa to 14 December 2021
| Total (n=1496) | Unvaccinated (n=202) | One dose (n=74) | Two doses (n=1220) | ||
|---|---|---|---|---|---|
| Age (years), median (IQR) | 45 (35–53) | ||||
| Age group (years), n (%) | 18–29 | 224 (15) | 51 (25) | 12 (16) | 161 (13) |
| 30–39 | 388 (26) | 71 (35) | 24 (32) | 293 (24) | |
| 40–49 | 368 (25) | 31 (15) | 18 (24) | 319 (26) | |
| ≥50 | 516 (34) | 49 (24) | 20 (27) | 447 (37) | |
| Sex, n (%) | Female | 1177 (79) | 179 (89) | 60 (81) | 938 (77) |
| Pregnant | 32 (3) | 28 (16) | 1 (2) | 3 (<1) | |
| Breastfeeding | 18 (2) | 7 (4) | 1 (2) | 10 (1) | |
| Male | 319 (21) | 23 (11) | 14 (19) | 282 (23) | |
| Hospital, n (%) | Tirana | 936 (63) | 144 (71) | 50 (68) | 742 (61) |
| Durres | 299 (20) | 22 (11) | 5 (7) | 272 (22) | |
| Fier | 261 (17) | 36 (18) | 19 (26) | 206 (17) | |
| Chronic conditions, n (%)b | No | 1205 (81) | 176 (87) | 63 (85) | 966 (79) |
| Yes | 291 (19) | 26 (13) | 11 (15) | 254 (21) | |
| 1 | 221 (15) | 19 (9) | 7 (9) | 195 (16) | |
| ≥2 | 70 (5) | 7 (3) | 4 (5) | 59 (5) | |
| Smoker, n (%) | Current or previous | 273 (18) | 17 (8) | 19 (26) | 237 (19) |
| Never | 1223 (82) | 185 (92) | 55 (74) | 983 (81) | |
| BMI group, n (%) | Underweight or normal | 630 (42) | 98 (49) | 43 (58) | 489 (40) |
| Overweight | 594 (40) | 68 (34) | 19 (26) | 507 (42) | |
| Obese | 272 (18) | 36 (18) | 12 (16) | 224 (18) | |
| Self-assessed health status, n (%) | Excellent or very good | 1170 (78) | 144 (71) | 57 (77) | 969 (79) |
| Good | 192 (13) | 37 (18) | 11 (15) | 144 (12) | |
| Fair or poor | 134 (9) | 21 (10) | 6 (8) | 107 (9) | |
| Occupation/role in hospital, n (%) | Nurse or midwife | 709 (47) | 103 (51) | 23 (31) | 583 (48) |
| Physician | 303 (20) | 21 (10) | 21 (28) | 261 (21) | |
| Janitorial staff or food worker | 195 (13) | 23 (11) | 4 (5) | 168 (14) | |
| Otherc | 289 (19) | 55 (27) | 26 (35) | 208 (17) | |
| Patient facing, n (%) | Yes | 1426 (95) | 185 (92) | 68 (92) | 1173 (96) |
| No | 70 (5) | 17 (8) | 6 (8) | 47 (4) | |
| Hands-on care, n (%) | Yes | 901 (60) | 115 (57) | 38 (51) | 748 (61) |
| No | 595 (40) | 87 (43) | 36 (49) | 472 (39) | |
| Household size, n (%) | 1–3 | 575 (38) | 85 (42) | 25 (34) | 465 (38) |
| ≥4 | 921 (62) | 117 (58) | 49 (66) | 755 (62) | |
| Brand of vaccine received on the last day of follow-up, n (%) | BNT162b2 (Pfizer Comirnaty) alone | 1119 (86) | . | 43 (58) | 1076 (88) |
| ChAdOx1 nCoV-19 (Astrazeneca Vaxzevria) alone | 162 (13) | . | 28 (38) | 134 (11) | |
| Coronavac alone | 10 (1) | . | 3 (4) | 7 (1) | |
| Heterologous vaccination | 3 (<1) | . | 0 (0) | 3 (0) | |
| Interval between doses for participants who received two doses (days), median (IQR) | BNT162b2 (Pfizer Comirnaty) only | 21 (21–21) | . | . | 21 (21–21) |
| ChAdOx1 nCoV-19 (Astrazeneca Vaxzevria) only | 91 (84–98) | . | . | 91 (84–98) | |
| Coronavac only | 30 (30–30) | 30 (30–30) | |||
IQR, interquartile range; BMI, body mass index.
End of the person-time included in the analysis for each participant. The person-time ended on: (1) the day of the first infection, (2) the day of receipt of a third vaccine dose, (3) the day of receipt of a second vaccine dose if the interval between the first and second doses was shorter than the manufacturer's recommendation, or (4) the day of the last weekly questionnaire before complete loss to follow-up, withdrawal or censor date (14 December 2021).
Include cancer, chronic heart disease, high blood pressure/hypertension, chronic kidney disease, chronic liver disease (such as cirrhosis, hepatitis, fatty liver disease), chronic lung disease (such as asthma, chronic obstructive pulmonary disease), diabetes, immunocompromised (including solid organ transplant and human immunodeficiency virus), neurologic disease (including cerebrovascular disease, epilepsy, multiple sclerosis), obesity, autoimmune disorder.
Includes accountant, administration staff, chemist, courier, driver, economist, general director, lawyer, psychologist, public health specialist, specialist, storekeeper.
At enrolment, 1054 (70%) HCWs had evidence of prior or current SARS-CoV-2 infection through PCR and/or serological assays; 417 (28%) participants reported a PCR-confirmed infection before enrolment, and 18 (1%) had a PCR-positive respiratory sample at enrolment. At enrolment, 981 (66%) participants were positive for SARS-CoV-2 anti-nucleocapsid antibodies. Of the 1097 participants who were seropositive at enrolment by any assay, most [964 (88%)] were positive for both anti-spike antibody and anti-nucleocapsid antibody, indicating prior SARS-CoV-2 infection regardless of vaccination status.
At enrolment, 858 (57%) participants had already received one dose of COVID-19 vaccine (99% ≤5 days prior to enrolment), eight (1%) participants had received two doses, and 630 (42%) participants were unvaccinated. At the end of the analysis period, 1220 (82%) participants had received two doses, 74 (5%) participants had received one dose, and 202 (14%) participants remained unvaccinated. Seventy-one (5%) particpants had received a third dose. Of the 1220 participants who had received two doses by the end of the analysis period, most [1119 (86%)] had received BNT162b2 (Comirnaty, Pfizer), while 162 (13%) had received ChAdOx1 nCoV-19 (Vaxzevria, AstraZeneca), 10 (1%) had received CoronaVac (Sinovac Life Sciences), and three (<1%) reported heterologous vaccination (Table 1). At the end of the analysis period, compared with participants who had received two doses of vaccine, unvaccinated participants were younger [35 years (IQR 29–49) vs 45 years (IQR 35–53)] and more likely to be female [179/202 (89%) vs 938/1220 (77%)], but otherwise demographic, health and occupation characteristics were similar between vaccinated and unvaccinated participants (Table 1).
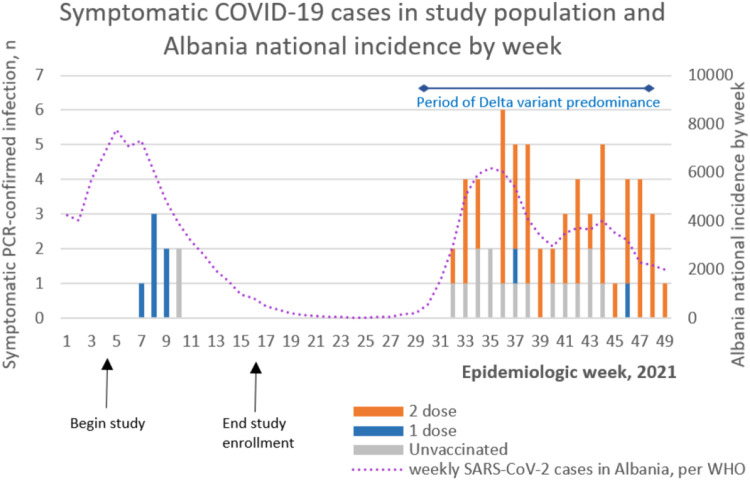
Seventeen unvaccinated participants developed COVID-19 during the study period (incidence: 24.2 symptomatic infections per 100,000 person-days), and 42 vaccinated participants developed COVID-19 (incidence: 15.8 per 100,000 person-days) (Table 2). Of the 71 cases that occurred during the study period among all participants (including eight cases that occurred in partially vaccinated participants, one case that occurred during the washout period, and three cases that occurred among participants who had received a booster dose), 63 (90%) occurred between 1 August and 14 December 2021, a period during which the Delta variant was predominant (Figure 2). During the study period, 19/28 sequenced SARS-CoV-2 PCR-positive study samples and 24/28 general surveillance SARS-CoV-2 samples from Albania were the Delta variant. The remaining nine samples were the Alpha variant (n=4), Omicron variant (n=2) or Lineage B (n=3).
Table 2.
Two-dose vaccine effectiveness (VE) against symptomatic coronavirus disease 2019 (COVID-19), confirmed by polymerase chain reaction, for the full cohort, and stratified by previous severe acute respiratory syndrome coronavirus-2 infection status and for BNT162b2 alone
| Two doses (all vaccines) | n | Total person-time (days) | Symptomatic COVID-19 infection | Incidence per 100,000 person-days | Unadjusted VE | (95% CI) | Adjusted VE | (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Total cohort | 1474 | . | . | . | . | . | . | . |
| Unvaccinated | 70,367 | 17 | 24.2 | . | . | . | . | |
| ≥14 days from second dose | 266,464 | 42 | 15.8 | 62.2 | (34.3–78.3) | 65.1 | (37.7–80.5) | |
| Participants without prior infection | 440 | . | . | . | . | . | . | . |
| Unvaccinated | 18,829 | 12 | 63.7 | . | . | . | . | |
| ≥14 days from second dose | 81,169 | 34 | 41.9 | 60.6 | (21.5–80.3) | 58.2 | (15.7–79.3) | |
| Participants with prior infection | 1034 | . | . | . | . | . | . | . |
| Unvaccinated | 51,538 | 5 | 9.7 | . | . | . | . | |
| ≥14 days from second dose | 185,295 | 8 | 4.3 | 72.9 | (23.6–90.4) | 73.6 | (24.3–90.8) | |
| Two doses (BNT162b2) | n | Total person-time (days) | Symptomatic COVID-19 infection | Incidence per 100,000 person-days | Unadjusted VE | (95% CI) | Adjusted VE | (95% CI) |
| Total cohort | 1430 | . | . | . | . | . | . | . |
| Unvaccinated | . | 70,367 | 17 | 24.2 | . | . | . | . |
| ≥14 days from second dose | . | 248,388 | 35 | 14.1 | 65.2 | (38.3–80.4) | 69.5 | (44.5–83.2) |
| Participants without prior infection | 440 | . | . | . | . | . | . | . |
| Unvaccinated | . | 18,829 | 12 | 63.7 | . | . | . | . |
| ≥14 days from second dose | . | 74,545 | 28 | 37.6 | 63.7 | (25.5–82.3) | 65.5 | (29.2–83.2) |
| Participants with prior infection | 990 | . | . | . | . | . | . | . |
| Unvaccinated | . | 51,538 | 5 | 9.7 | . | . | . | . |
| ≥14 days from second dose | . | 173,843 | 7 | 4 | 73.5 | (23.4–90.8) | 71.3 | (16–90.2) |
CI, confidence interval.
Figure 2.
Epicurve showing coronavirus disease 2019 (COVID-19) cases by vaccination status in the study population, and national COVID-19 incidence in Albania, by epidemiologic week, 2021. PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; WHO, World Health Organization.
All 71 participants with COVID-19 completed the 30-day follow-up questionnaire after their positive test. Eighteen participants sought medical care, including four who sought care at the emergency department. None were hospitalized and all reported full recovery at the time of the follow-up survey. The median number of days sick was 4 (IQR 3–7).
Vaccine effectiveness: prevention of symptomatic COVID-19
Adjusted primary series VE against PCR-confirmed symptomatic COVID-19 in the total cohort was 65.1% [95% confidence interval (CI) 37.7–80.5]. Among participants without prior infection, VE was 58.2% (95% CI 15.7–79.3), and among those with prior SARS-CoV-2 infection, VE was 73.6% (95% CI 24.3–90.8) (Table 2). For participants who received the BNT162b2 vaccine, overall VE was 69.5% (95% CI 44.5–83.2). In those without prior infection, BNT162b2 VE was 65.5% (95% CI 29.2–83.2), and in those with prior infection, BNT162b2 VE was 71.3% (95% CI 16.0–90.2).
During the Delta-predominant period, VE of all vaccines combined against COVID-19 in the full cohort was 67.1% (95% CI 38.3–82.5); among those without prior infection, VE in this period was 67.0% (95% CI 27.7–84.9), and among those with prior infection, VE was 68.1% (95% CI 0.9–89.7). Finally, during the Delta-predominant period, BNT162b2-only VE in the full cohort was 68.0% (95% CI 39.7–83.0); among those without prior infection, BNT162b2 VE was 73.0% (95% CI 39.7–87.9), and among those with prior infection, BNT162b2 VE was 65.9% (95% CI -6.0–89.0).
Compared with unvaccinated participants with no evidence of prior SARS-CoV-2 infection, VE against COVID-19 was 62.1% (95% CI 25.3–80.8) for vaccinated participants with no prior infection, and 95.7% (95% CI 89.5–98.3) for vaccinated participants who also had prior infection (hybrid immunity). Protection against COVID-19 re-infection was 84.4% (95% CI 55.5–94.5) for unvaccinated participants who had been infected previously. Findings were similar when the analysis was limited to BNT162b2 alone, the Delta-predominant period alone, and the Delta-predominant period and BNT162b2.
Vaccine effectiveness: prevention of any (asymptomatic or symptomatic) SARS-CoV-2 infection
In the secondary analysis of VE against all SARS-CoV-2 infections, there were 71 SARS-CoV-2 infections (23 PCR-confirmed infections and 48 seroconversions) among unvaccinated participants (122 per 100,000 person-days), and 190 infections (43 PCR-confirmed infections and 147 seroconversions) in vaccinated participants (88 per 100,000 person-days). VE against any infection was 36.9% (95% CI 15.8–52.7) in the full cohort, 53.9% (95% CI 30.4–69.5) among those without prior infection, and 13.7% (95% CI -37.8–46.0) in those with prior infection. For participants who had received BNT162b2 vaccine, VE against infection was 33.3% (95% CI 10.2–50.4) in the full cohort, 53.9% (95% CI 38.0–68.9) in those without prior infection, and 7.9% (95% CI -49.3–43.3) in those with prior infection (Table 3).
Table 3.
Two-dose vaccine effectiveness (VE) against severe acute respiratory syndrome coronavirus disease-2 (SARS-CoV-2) infection, documented by polymerase chain reaction (PCR) or seroconversion, for the full cohort, and stratified by previous SARS-CoV-2 infection status and for BNT162b2 alone
| Two doses (all vaccines) | n | Total person-time (days) | PCR-confirmed infection | Seroconversion with Platelia | PCR+ or seroconversion with Platelia | Incidence per 100,000 person-days | Unadjusted VE | (95% CI) | Adjusted VE | (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Total cohort | 1412 | |||||||||
| Unvaccinated | 58,260 | 23 | 48 | 71 | 122 | |||||
| ≥14 days from second dose | 214,763 | 43 | 147 | 190 | 88 | 39.7 | (20.4–54.3) | 36.9 | (15.8–52.7) | |
| Participants without prior infection | 400 | |||||||||
| Unvaccinated | 13,945 | 16 | 30 | 46 | 330 | |||||
| ≥14 days from second dose | 55,507 | 34 | 77 | 111 | 200 | 54.4 | (34.9–68) | 53.9 | (30.4–69.5) | |
| Participants with prior infection | 1012 | |||||||||
| Unvaccinated | 44,315 | 7 | 18 | 25 | 56 | |||||
| ≥14 days from second dose | 159,256 | 9 | 70 | 79 | 50 | 16.5 | (-31.5–47) | 13.7 | (-37.8–46) | |
| Two doses (BNT162b2) | n | Total person-time (days) | PCR-confirmed infection | Seroconversion with Platelia | PCR+ or seroconversion with Platelia | Incidence per 100,000 person-days | Unadjusted VE | (95% CI) | Adjusted VE | (95% CI) |
| Total cohort | 1373 | |||||||||
| Unvaccinated | 58,260 | 23 | 42 | 65 | 112 | |||||
| ≥14 days from second dose | 202,724 | 35 | 135 | 170 | 84 | 35.2 | (13.3–51.5) | 33.3 | (10.2–50.4) | |
| Participants without prior infection | 400 | |||||||||
| Unvaccinated | 13,945 | 16 | 26 | 42 | 301 | |||||
| ≥14 days from second dose | 51,907 | 26 | 70 | 96 | 185 | 51.8 | (30–66.8) | 53.9 | (38–68.9) | |
| Participants with prior infection | 973 | |||||||||
| Unvaccinated | 44,315 | 7 | 16 | 23 | 52 | |||||
| ≥14 days from second dose | 150,817 | 9 | 65 | 74 | 49 | 6.8 | (-49.3–41.8) | 7.9 | (-49.3–43.3) | |
CI, confidence interval.
Vaccine effectiveness: change in VE by time since vaccination
Sixteen participants who were 14–180 days post-vaccination had COVID-19, of which 11 (69%) cases occurred during the Delta-predominant period; 26 participants who were >180 days post-vaccination had COVID-19, of which 24 (92%) cases occurred during the Delta-predominant period. VE against COVID-19 was 65.7% (95% CI 26.5–84.0) for participants who were 14–180 days post-vaccination, and 64.6% (95% CI 31.7–81.7) for those who were >180 days post-vaccination. During the Delta-predominant period, VE was 70.2% (95% CI 29.0–87.5) and 66.0% (95% CI 33.0–82.7) for participants who were 14–180 and >180 days post-vaccination, respectively.
Vaccine effectiveness: sensitivity analyses
When the period after infection during which participants were considered not to be at-risk for re-infection was decreased from 90 to 60 days, results were similar to the 90-day analysis. When a participant was considered to be fully vaccinated at 7 days instead of 14 days, VE was also similar. Finally, when the definition of prior infection at enrolment was expanded to include a positive WANTAI anti-spike antibody test for unvaccinated participants and participants who received their first vaccine ≤5 days prior to enrolment, and when the start date of the Delta-predominant period was redefined as 1 July 2021 (instead of 1 August 2021), VE against COVID-19 was largely unchanged.
Discussion
This study of COVID-19 VE among HCWs in Albania, which – to the authors’ knowledge – is the first COVID-19 VE study in an upper middle-income country in Europe, found moderate VE against PCR-confirmed COVID-19 among HCWs, most of whom had received two doses of BNT162b2 vaccine, during the Delta-predominant period. In contrast to previous COVID-19 VE studies conducted among HCWs in HICs that enrolled cohorts with relatively low rates of prior SARS-CoV-2 infection [20], [21], [22], HCWs in this study had a high rate of prior infection only 1 year after the start of the pandemic, consistent with trends among HCWs in the Eastern European region, as well as high seroprevalence among the general population in Albania at this time [23]. In this population of previously infected HCWs, VE against COVID-19 was also moderate. This point estimate was higher than the estimated VE among HCWs with no prior infection, which contrasts with other published findings during this timeframe [20,24]. However, this study found very high VE (95.7%) against COVID-19 among HCWs who had been infected previously and later received two doses of COVID-19 vaccine when compared with unvaccinated HCWs without previous infection. These findings of strong protection conferred by hybrid immunity are similar to results of recently published studies in Sweden [25], the UK [9] and Brazil [26]. It is notable that prior SARS-CoV-2 infection reduced the risk of re-infection even in the absence of vaccination, and the present findings of the protective effect of infection alone in the pre-Omicron period have also been demonstrated in other studies [27]. However, infection is not recommended as a strategy to prevent future infections; COVID-19 disease has a number of risks, including severe disease, death and post-infection complications [28].
A contribution of this study is the use of serology in addition to PCR to define prior SARS-CoV-2 infection and to estimate VE against all SARS-CoV-2 infections – both symptomatic and asymptomatic. This combined outcome provided an opportunity to define prior infection comprehensively in the study population. In addition, collecting serological samples quarterly enabled the authors to capture asymptomatic infections or symptomatic infections that were not detected by PCR. In this study, the VE point estimate against PCR-confirmed symptomatic COVID-19 was higher than that for VE against all SARS-CoV-2 infection, defined by the combined outcome, although the 95% CIs overlapped. This trend towards higher COVID-19 VE against symptomatic illness compared with a combined endpoint of symptomatic and asymptomatic infection is consistent with previous findings [29,30]. These results are also consistent with numerous studies showing that COVID-19 vaccines are more effective at preventing severe outcomes, such as hospitalization and death, than they are at preventing milder disease.
While there was a high prevalence (70%) of prior infection in the study population, only 28% were identified by PCR testing prior to enrolment. In addition, 274 (18%) participants seroconverted during the study period without positive PCR tests. These observations suggest that asymptomatic infection may be common, and highlights the importance of robust infection control practices in healthcare settings as part of a larger strategy to reduce nosocomial SARS-CoV-2 transmission from asymptomatic HCWs to staff and patients.
In addition to the use of serology to detect prior infection before enrolment, and to estimate asymptomatic infections during the study period, the strengths of this study include the prospective cohort design and the low number of participants who withdrew or were lost to follow-up. In addition, there was rigorous adherence to the protocol: >95% of participants completed the symptom questionnaire during most weeks; all participants who reported symptoms on their weekly questionnaire had a specimen collected for PCR testing; and serological samples were collected from nearly all participants at enrolment (100%), 3 months (98%) and 6 months (91%). As a result, there were few missing data.
However, this study has some limitations. First, this study measured VE against laboratory-confirmed infection, but was not powered to estimate VE against less common but more severe outcomes, including hospitalization and death. The low number of events also led to notable variability in the results, as reflected in the wide CIs seen for the VE estimates, in particular among participants with prior infection. Additionally, the authors were unable to adjust for timing of prior infections, or variant of prior infection, because the majority of participants had previous infection confirmed only by seropositivity at enrolment, and did not report the date of infection. Finally, the study population may be subject to selection bias – enrolment was voluntary and therefore the HCWs in this study may not fully represent HCWs in Albania or elsewhere (the authors were not able to evaluate representativeness, as demographic information for all eligible HCWs at the study sites was not available). However, two-dose COVID-19 vaccine coverage in this study at the end of the analysis (82%) was similar to two-dose coverage among HCWs in Albania as a whole during the same time period (84%) [31].
In conclusion, this prospective cohort study in an upper-middle income European country found that primary series COVID-19 vaccination was effective in preventing COVID-19 in hospital-based HCWs. The findings support current vaccine policy and highlight the need to continue to promote vaccine uptake, particularly in countries such as Albania, where, as of 19 March 2023, less than half the population has completed a primary vaccine series [8]. In addition, the study findings highlight the added benefit of vaccination in settings with high seroprevalence. Finally, this study is ongoing; future analyses will evaluate VE during the period of Omicron predominance, against future variants of concern, and for additional vaccine doses.
Conflict of interest statement
None declared.
Acknowledgments
Acknowledgements
The authors wish to thank Jörn Beheim-Schwarzbach, Victor M. Corman and Terry C. Jones (Institute of Virology, Charité–Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt – Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany; and German Centre for Infection Research, Partner Site Charité, Berlin, Germany); and Marta Valenciano and Alain Moren (Epiconcept, France).
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding
This study was funded by the Task Force for Global Health [US Centers for Disease Control and Prevention (CDC) Cooperative Agreement #NU51IP000873) and the WHO Regional Office for Europe. Staff at CDC and WHO Regional Office for Europe were involved in the design of the study, analysis and interpretation of the results.
Ethics and study registration
The study protocol was approved by the WHO Ethical Review Committee (Reference Number CERC.0097A) and the Albania Institute of Public Health Ethical Review Committee (Reference Number 156). The CDC Humans Review determined the activity to be a public health evaluation. All participants provided written informed consent. The study is also registered with clinicaltrials.gov (Identifier NCT04811391).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2023.04.009.
Appendix. Supplementary materials
References
- 1.Gouda D, Singh PM, Gouda P, Goudra B. An overview of health care worker reported deaths during the COVID-19 pandemic. J Am Board Fam Med. 2021;34(Suppl):S244–S246. doi: 10.3122/jabfm.2021.S1.200248. [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay S, Baticulon RE, Kadhum M, Alser M, Ojuka DK, Badereddin Y, et al. Infection and mortality of healthcare workers worldwide from COVID-19: a systematic review. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chutiyami M, Bello UM, Salihu D, Ndwiga D, Kolo MA, Maharaj R, et al. COVID-19 pandemic-related mortality, infection, symptoms, complications, comorbidities, and other aspects of physical health among healthcare workers globally: an umbrella review. Int J Nurs Stud. 2022;129 doi: 10.1016/j.ijnurstu.2022.104211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.SAGE Working Group on COVID-19 vaccines . World Health Organization; Geneva: 2020. WHO SAGE roadmap for prioritizing uses of COVID-19 vaccines in the context of limited supply.https://www.who.int/docs/default-source/immunization/sage/covid/sage-prioritization-roadmap-covid19-vaccines.pdf Version 1.1Available at. accessed 6 January 2022. [Google Scholar]
- 5.Meslé MM, Brown J, Mook P, Hagan J, Pastore R, Bundle N, et al. Estimated number of deaths directly averted in people 60 years and older as a result of COVID-19 vaccination in the WHO European Region, December 2020 to November 2021. Eurosurveillance. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.47.2101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilches TN, Moghadas SM, Sah P, Fitzpatrick MC, Shoukat A, Pandey A, et al. Estimating COVID-19 infections, hospitalizations, and deaths following the US vaccination campaigns during the pandemic. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2021.42725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO coronavirus (COVID-19) dashboard. Geneva: WHO. Available at: https://covid19.who.int/. https://covid19.who.int/(accessed 8 April 2023).
- 8.World Health Organization Regional Office for Europe. WHO/Europe COVID-19 vaccine programme monitor. Copenhagen: WHO Regional Office for Europe. Available at: https://worldhealthorg.shinyapps.io/EURO_COVID-19_vaccine_monitor/(accessed 8 April 2023).
- 9.Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJM, Simmons R, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benenson S, Oster Y, Cohen MJ, Nir-Paz R. BNT162b2 mRNA Covid-19 vaccine effectiveness among health care workers. N Engl J Med. 2021;384:1775–1777. doi: 10.1056/NEJMc2101951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization Regional Office for Europe . WHO Regional Office for Europe; Copenhagen: 2021. Cohort study to measure COVID-19 vaccine effectiveness among healthcare workers in the WHO European Region: guidance document. [Google Scholar]
- 12.Ministry of Health and Instituti i Shëndetit Publik . Ministry of Health and Instituti i Shëndetit Publik; Tirana: 2020. Plani Kombëtar i Vaksinimit ndaj COVID-19 [National Vaccination Plan against COVID-19]https://shendetesia.gov.al/wp-content/uploads/2021/02/4-SHQIP.pdf . Available at. accessed 8 November 2021. [Google Scholar]
- 13.World Health Organization. Coronavirus disease (COVID-19) technical guidance: the unity studies: early investigation protocols. Geneva: WHO. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/early-investigations (accessed 8 November 2021).
- 14.Sridhar S, Fico A, Preza I, Hatibi I, Sulo J, Kissling E, et al. COVID-19 vaccine effectiveness among healthcare workers in Albania (COVE-AL): protocol for a prospective cohort study and cohort baseline data. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-057741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WANTAI SARS-CoV-2 Ab ELISA package insert. Revised 2021. Available at: fda.gov/media/140929/download (accessed 6 January 2022).
- 17.Platelia SARS-CoV-2 Total Ab package insert. Available at: https://www.fda.gov/media/137493/download (accessed 6 January 2022).
- 18.Wajnberg A, Mansour M, Leven E, Bouvier NM, Patel G, Firpo-Betancourt A, et al. Humoral response and PCR positivity in patients with COVID-19 in the New York City region, USA: an observational study. Lancet Microbe. 2020;1:e283–e289. doi: 10.1016/S2666-5247(20)30120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khare S, Gurry C, Freitas L, Schultz MB, Bach G, Diallo A, et al. GISAID's role in pandemic response. China CDC Wkly. 2021;3:1049–1051. doi: 10.46234/ccdcw2021.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, et al. Protection against SARS-CoV-2 after COVID-19 vaccination and previous infection. N Engl J Med. 2022;386:1207–1220. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lumley SF, O'Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huynen P, Grégoire C, Gofflot S, Seidel L, Maes N, Vranken L, et al. Long-term longitudinal evaluation of the prevalence of SARS-CoV-2 antibodies in healthcare and university workers. Sci Rep. 2022;12:5156. doi: 10.1038/s41598-022-09215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sulcebe G, Ylli A, Cenko F, Kurti-Prifti M. Rapid increase of SARS-CoV-2 seroprevalence during the 2020 pandemic year in the population of the city of Tirana, Albania. medRxiv. doi: https://doi.org/10.1101/2021.02.18.21251776
- 24.Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman LS, Ash N, et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med. 2022;386:2201–2212. doi: 10.1056/NEJMoa2118946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordström P, Ballin M, Nordström A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: a retrospective, total population cohort study in Sweden. Lancet Infect Dis. 2022;22:781–790. doi: 10.1016/S1473-3099(22)00143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerqueira-Silva T, Andrews JR, Boaventura VS, Ranzani OT, de Araújo Oliveira V, Paixão ES, et al. Effectiveness of CoronaVac, ChAdOx1 nCoV-19, BNT162b2, and Ad26.COV2.S among individuals with previous SARS-CoV-2 infection in Brazil: a test-negative, case–control study. Lancet Infect Dis. 2022;22:791–801. doi: 10.1016/S1473-3099(22)00140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.COVID-19 Forecasting Team Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. Lancet. 2023;401:833–842. doi: 10.1016/S0140-6736(22)02465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bobrovitz N, Ware H, Ma X, Li Z, Hosseini R, Cao C, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the Omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023;23:556–567. doi: 10.1016/S1473-3099(22)00801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashmawy R, Kamal E, Sharaf S, Elsaka N. Effectiveness and safety of inactivated SARS-CoV-2 vaccine (BBIPB-CorV) among healthcare workers: a seven month follow-up study at fifteen hospitals, Research Square. doi: https://doi.org/10.21203/rs.3.rs-1431715/v1. [DOI] [PMC free article] [PubMed]
- 30.Tang L, Hijano DR, Gaur AH, Geiger TL, Neufeld EJ, Hoffman JM, et al. Asymptomatic and symptomatic SARS-CoV-2 infections after BNT162b2 vaccination in a routinely screened workforce. JAMA. 2021;325:2500–2502. doi: 10.1001/jama.2021.6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albania Institute of Public Health . Albania Institute of Public Health; December 2021. Weekly report. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.