Abstract
Objectives
Bebtelovimab is an anti-SARS-CoV-2 monoclonal antibody active against Omicron lineage variants authorized to treat high-risk outpatients with COVID-19. We sought to determine the real-world effectiveness of bebtelovimab during the Omicron phases BA.2/BA2.12.1/BA4/BA5.
Methods
We conducted a retrospective cohort study of adults with SARS-CoV-2 infection between April 6 and October 11, 2022, using health records linked to vaccine and mortality data. We used propensity scores to match of bebtelovimab-treated with untreated outpatients. The primary outcome was 28-day all-cause hospitalization. The secondary outcomes were 28-day COVID-19-related hospitalization, 28-day all-cause mortality, 28-day emergency department visits, maximum respiratory support level, intensive care unit admission, and in-hospital mortality among hospitalized patients. We used logistic regression to determine bebtelovimab treatment effectiveness.
Results
Among 22,720 patients with SARS-COV-2 infection, 3739 bebtelovimab-treated patients were matched to 5423 untreated patients. Compared with no treatment, bebtelovimab was associated with lower odds of 28-day all-cause hospitalization (1.3% vs 2.1%, adjusted odds ratio: 0.53; 95% confidence interval: 0.37-0.74, P <0.001), as well as COVID-19-related hospitalization (1.0% vs 2.0%, adjusted odds ratio: 0.44 [95% confidence interval: 0.30-0.64], P <0.001). Bebtelovimab appeared to be more beneficial in lowering the odds of hospitalization among patients with two or more comorbidities (interaction P = 0.03).
Conclusion
During the Omicron BA.2/BA.2.12.1/BA.4/BA.5 variant phase, bebtelovimab was associated with lower hospitalization.
Keywords: Anti-SARS-COV-2 monoclonal antibody, COVID-19, Outpatient, Nonhospitalized, LY-CoV1404
Background
SARS-CoV-2 neutralizing monoclonal antibodies (mAbs) have played an essential role in decreasing the risk of hospitalization and progression to severe disease among nonhospitalized patients with COVID-19 [1]. However, the emergence of new SARS-CoV-2 variants of concern continually threatens the available mAb products. For instance, the emergence of the BA.2 subvariant, which constituted more than 50% of the circulating COVID-19 cases in the United States by April 2022, rendered sotrovimab ineffective, leading to the revocation of its authorization [2,3].
Bebtelovimab is an anti-SARS-CoV-2 mAb with activity against Omicron subvariants, including BA.2 and BA.4/5 [4]. In the BLAZE-4 trial, bebtelovimab treatment enhanced viral clearance and reduced the time to sustained symptom resolution compared with placebo [5]. In February 2022, bebtelovimab was granted emergency use authorization (EUA) for the treatment of mild-moderate COVID-19 in patients at a high risk for progression to severe COVID-19 [6]. The real-world studies conducted in the early Omicron period (BA.1/2) have suggested that bebtelovimab is associated with lower odds of hospitalization and death [7,8]. Although in vitro data indicate preserved neutralization among Omicron subvariants BA.4/5, there is a lack of clinical data corroborating the effectiveness of bebtelovimab against these subvariants.
To provide additional data regarding bebtelovimab effectiveness against later Omicron subvariants (BA.4/5), we used our real-world data platform to evaluate the effectiveness of treatment with bebtelovimab on 28-day hospitalization and mortality among outpatients with early symptomatic COVID-19 during an Omicron (BA.2/BA2.12.1/BA.4/BA.5) predominant period in Colorado.
Methods
Patient population
We conducted a propensity-matched, retrospective, observational cohort study as a collaboration between the University of Colorado researchers, University of Colorado Health leaders, and the Colorado Department of Public Health and Environment. We used our existing data platform comprising electronic health data, statewide vaccination data, and mortality data, which has been described previously [3,9]. The Colorado Multiple Institutional Review Board approved this study with a waiver of written informed consent.
We included all patients diagnosed with SARS-CoV-2 infection identified using Electronic Health Record (EHR)-based SARS-CoV-2-positive test date (either polymerase chain reaction or antigen) or date of bebtelovimab administration if a SARS-CoV-2 test result was unavailable. Patients were included if their test date was between April 6 and October 11, 2022, a period when bebtelovimab was readily available, and the dominant variant was Omicron. The study end date allowed at least 28 days of follow-up for all patients. University of Colorado Health COVID-19 treatment guidance recommended bebtelovimab as an alternative to nirmatrelvir-ritonavir if treatment eligibility was not met; however, the ultimate decision to initiate treatment was made by patients and clinicians.
The main exclusion criteria were: (i) order or administration of molnupiravir or nirmatrelvir-ritonavir, or administration of another SARS-CoV-2 monoclonal antibody (sotrovimab or tixagevimab/cilgavimab [within 10 days of SARS-CoV-2 positive date]), or outpatient remdesivir; (ii) hospitalization at the time of SARS-CoV-2 positive test; or (iii) a positive SARS-CoV-2 test more than 10 days before the bebtelovimab administration date. We retained patients who died on the same day as their observed SARS-CoV-2-positive test, given the ubiquitous use of self-testing during the period. We did not exclude patients who did not meet EUA criteria based on the available EHR data because not all eligibility criteria were consistently available.
Due to the increased use of home antigen tests, most (80.3%) patients treated with bebtelovimab did not have a SARS-CoV-2-positive test date in the EHR. Because the treatment with EUA bebtelovimab requires a positive SARS-CoV-2 test, we assumed that testing occurred outside the health system for these patients. In the primary analysis, the dates were imputed from a random sample of the existing length of days between positive SARS-CoV-2 testing and mAb treatment, and we conducted a sensitivity analysis to this assumption.
We balanced for potential confounders using the nearest neighbor propensity matching, with a maximum 1:2 treated-to-untreated ratio using a caliper of 0.2 and logistic regression, with treatment status as the outcome using the R package MatchIt v4.5.2 [10,11]. The propensity model included categorical age, sex, race/ethnicity, insurance status, immunocompromised status, obesity status, number of comorbid conditions other than immunocompromised and obesity, number of vaccinations at the time of infection, and categorical week. Variables with a standardized mean difference above 0.1 after propensity matching were adjusted for in all outcome models to account for the residual imbalance [12].
Outcomes
The primary outcome was 28-day all-cause hospitalization measured from a positive SARS-CoV-2 test based on the actual or imputed test date. As a secondary outcome, we defined 28-day COVID-19-related hospitalization as the presence of one or more of the following: COVID-19 International Classification of Diseases, Tenth Revision codes (U07.1, J12.82, M35.81, Z20.822, M35.89), inpatient administration of remdesivir, or use of any supplemental oxygen. Other secondary outcomes were 28-day all-cause mortality and 28-day all-cause emergency department (ED) visits. In the hospitalized subset, the exploratory outcomes included disease severity based on the maximum respiratory support level, intensive care unit admission rates, and in-hospital mortality.
Variable definitions
Hospitalization was defined as any inpatient or observation encounter documented in the EHR. We selected the first hospitalization that occurred the same day or any day after a SARS-CoV-2 positive test for untreated patients or after the order date for bebtelovimab-treated patients. ED visits were defined as any visit to the ED, with or without an associated inpatient or observation encounter. We defined COVID-19 disease severity as the maximum level of respiratory support received in the following order from lowest to highest severity: no supplemental oxygen, standard (nasal cannula/face mask) oxygen, high-flow nasal cannula or noninvasive ventilation, and invasive mechanical ventilation [13]. In-hospital mortality was the highest level of disease severity.
The covariates of interest included treatment status, categorical age in years, sex, race/ethnicity, insurance status, obesity status, immunocompromised status, number of additional comorbid conditions, number of vaccinations, and Omicron subvariant (BA.4/BA.5). EHR evidence of comorbid conditions (obesity, hypertension, cardiovascular disease, diabetes mellitus, pulmonary disease, and liver disease) was based on the Charlson and Elixhauser comorbidity indexes. Immunocompromised status was coded as reported previously [3]. The number of comorbid conditions was the sum of individual comorbid conditions. Obesity and immunocompromised status were kept as separate comorbid conditions. Vaccination status was categorized based on the number of vaccinations (0, 1, 2, or ≥3) administered before the SARS-CoV-2 index test date. Based on the Colorado surveillance data, we considered patients with a SARS-CoV-2-positive test after June 18, 2022 to be in the BA.4/BA.5 period, given that the statewide proportion of BA.4/5 was above 50% by that date [14].
Statistical analysis
We used Firth logistic regression to assess the association between treatment and 28-day hospitalization, 28-day mortality, and 28-day ED visits. The Firth logistic regression (R package logistf V 1.24) addresses the estimation issues related to complete separation from low event rates [15], [16], [17]. All models were adjusted for age, sex, race/ethnicity, insurance status, obesity status, immunocompromised status, number of additional comorbid conditions, number of vaccinations, and Omicron subvariant. We fit an adjusted logistic regression for the 28-day hospitalization secondary outcomes to assess the association between treatment and the odds of being transferred to the intensive care unit. Due to the small number of hospitalized participants, we present only descriptive statistics for the respiratory disease severity.
We estimated the adjusted treatment effects for six subgroups by fitting the interaction models that were adjusted for all variables of interest. The subgroups were binary age (<65 vs ≥65 years), binary and three-level immunocompromised status, binary number of comorbidities (0-1 vs ≥2), binary vaccination status (0-2 vs ≥3), and Omicron subvariant period (before BA.4/5 and BA.4/5).
We performed two sensitivity analyses (Appendix). First, we repeated the primary analysis but restricted the cohort to patients with EHR-derived data that confirmed an EUA-eligible condition. Second, we performed a sensitivity analysis of our imputation method by subtracting 7 days from the bebtelovimab administration date (the maximum time difference allowed by EUA). Statistical analyses were performed using R (v3.6.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Between April 6 and October 11, 2022, 17,386 patients met the study inclusion and were available for analysis (Appendix Figure 1, Appendix Table 1). Upon propensity matching, 9162 patients were included in the primary matched cohort (3739 bebtelovimab, 5423 untreated). The propensity score matching reduced the imbalance in all key covariates, including age, race/ethnicity, obesity status, immunocompromised status, number of comorbid conditions, number of vaccinations, insurance status, and week of infection. The standardized mean differences before and after propensity matching are available in Appendix Table 2.
Characteristics of the bebtelovimab patients in the primary cohort
In the propensity-matched cohort, bebtelovimab-treated patients reflected characteristics consistent with patients at a high risk for progression to severe COVID-19 (Table 1 ). Among patients who received bebtelovimab (n = 3739), 1702 (45.5%) were aged ≥65 years, 390 (10.4%) were Non-Hispanic Black or Hispanic, 1266 (32.8%) were immunocompromised, 1051 (28.1%) were obese, and 1652 (44.2%) had two or more comorbid conditions. Among immunocompromised patients, 42.0% were categorized as mild, whereas 58.0% were moderate-severe. A small proportion of bebtelovimab-treated patients received treatment presumably due to chronic immunosuppressants whose interactions precluded the use of nirmatrelvir-ritonavir, such as mammalian target of rapamycin inhibitors (everolimus or sirolimus) or calcineurin inhibitors (cyclosporine or tacrolimus) (n = 41, 1.1%) and mycophenolate (n = 19, 0.5%). Few patients received B-cell-depleting therapy with rituximab (n = 18, 0.5%).
Table 1.
Baseline characteristics by bebtelovimab treatment status for primary matched cohort.
| Bebtelovimab (n = 3739) |
Untreated (n = 5423) |
|
|---|---|---|
| Age groupa | ||
| 18-44 years | 877 (23.5%) | 1643 (30.3%) |
| 45-64 years | 1160 (31.0%) | 1795 (33.1%) |
| ≥65 years | 1702 (45.5%) | 1985 (36.6%) |
| Female sex | 2196 (58.7%) | 3221 (59.4%) |
| Race/Ethnicity | ||
| Non-Hispanic White | 3200 (85.6%) | 4566 (84.2%) |
| Hispanic | 289 (7.7%) | 486 (9.0%) |
| Non-Hispanic Black | 101 (2.7%) | 138 (2.5%) |
| Other | 149 (4.0%) | 233 (4.3%) |
| Insurance statusa | ||
| Private/Commercial | 1834 (49.1%) | 3193 (58.9%) |
| Medicare | 1728 (46.2%) | 1920 (35.4%) |
| Medicaid | 113 (3.0%) | 196 (3.6%) |
| Other (None/Uninsured/Unknown) | 64 (1.7%) | 114 (2.1%) |
| Immunocompromiseda | ||
| Mild | 515 (13.8%) | 610 (11.2%) |
| Moderate/severe | 711 (19.0%) | 748 (13.8%) |
| Obesea | 1052 (28.1%) | 1301 (24.0%) |
| Number of other comorbid conditions | ||
| One | 1005 (26.9%) | 1644 (30.3%) |
| Two or more | 1652 (44.2%) | 1840 (33.9%) |
| Diabetes mellitus | 737 (19.7%) | 788 (14.5%) |
| Cardiovascular disease | 987 (26.4%) | 1088 (20.1%) |
| Pulmonary disease | 1233 (33.0%) | 1514 (27.9%) |
| Renal disease | 581 (15.5%) | 482 (8.9%) |
| Hypertension | 1785 (47.7%) | 2,234 (41.2%) |
| Liver disease | ||
| Mild | 455 (12.2%) | 488 (9.0%) |
| Severe | 66 (1.8%) | 33 (0.6%) |
| Number of vaccinations prior to SARS-CoV-2+ datea | ||
| 0 | 578 (15.5%) | 955 (17.6%) |
| 1 | 128 (3.4%) | 204 (3.8%) |
| 2 | 479 (12.8%) | 708 (13.1%) |
| 3+ | 2554 (68.3%) | 3556 (65.6%) |
Variables used in the propensity matching, along with cohort week (not listed).
Primary outcome
The crude rates of hospitalization are displayed in Table 2 . During the study period, the incidence of 28-day hospitalization in our primary matched cohort was 1.6% (n = 60/3842) before the BA.4/5 period, rising to 2.0% (n = 104/5320) in the BA.4/5 predominant period. In the adjusted analysis, treatment with bebtelovimab was associated with reduced odds of 28-day all-cause hospitalization (adjusted odds ratio [aOR] 0.53, [95% confidence interval (CI) 0.37-0.74], P <0.001, Table 2). Of the total hospitalizations, 88.4% met our definition of COVID-related. Treatment with bebtelovimab remained associated with reduced odds of 28-day COVID-related hospitalization in the adjusted analysis (aOR 0.44 [95% CI 0.30-0.64], P <0.001). The results of the sensitivity analysis restricted to patients with an EUA-qualifying condition were consistent with the primary outcome (aOR 0.53 [95% CI 0.37-0.74], P <0.001) (Appendix Table 3). In addition, these findings remained consistent in the sensitivity analysis using the secondary 7-day imputation method (aOR 0.57 [95% CI 0.40-0.80], P <0.001) (Appendix Table 4).
Table 2.
Primary and secondary outcomes for bebtelovimab for primary matched cohort.
| Outcome |
Bebtelovimab | Untreated | Adjusted odds ratio (95% confidence interval) | P-value |
|---|---|---|---|---|
| Overall sample | n = 3739 | n = 5423 | – | – |
| All-cause hospitalization within 28-days | 48 (1.3%) | 116 (2.1%) | 0.53 (0.37-0.74) | <0.001 |
| COVID-related hospitalization within 28 days | 38 (1.0%) | 107 (2.0%) | 0.44 (0.30-0.64) | <0.001 |
| All-cause emergency department visit within 28 days | 260 (7.0%) | 276 (5.1%) | 1.35 (1.13-1.62) | 0.001 |
| All-cause mortality within 28 days | 3 (0.1%) | 11 (0.2%) | 0.43 (0.11-1.30) | 0.139 |
| Hospitalized sample | n = 48 | n = 116 | – | – |
| Intensive care unit visit during hospitalization | 6 (12.5%) | 21 (18.1%) | – | – |
All regression models adjusted for age, sex, race/ethnicity, obesity, immunocompromised status, number of comorbidities, insurance status, vaccination status, and subvariant.
Secondary outcomes
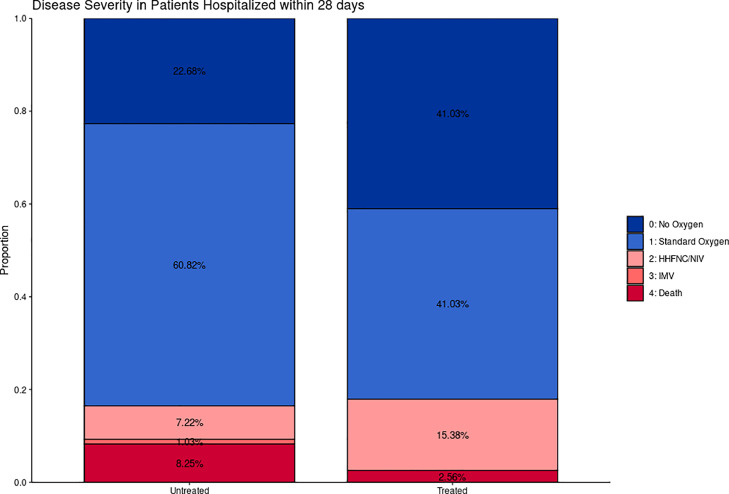
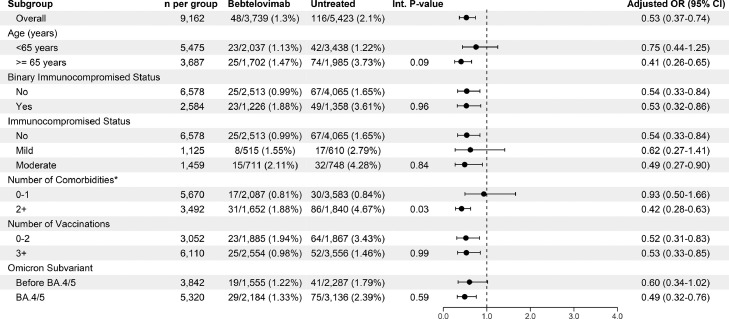
The observed rates of need for standard oxygen, high-flow nasal oxygen, invasive mechanical ventilation, or death were nominally lower in bebtelovimab-treated patients than untreated subjects (63.6% vs 73.9%) (Figure 1 ). In the test for interaction by subgroups, heterogeneity was observed; patients with two or more comorbidities were associated with treatment effect (Figure 2 ), whereas the treatment effect was no longer significant in those with one or no comorbidities. Marginal heterogeneity was observed with age (P = 0.09), with age ≥65 years associated with statistically significant treatment benefit, whereas age <65 years was not. No heterogeneity was observed within immunocompromised status (binary and three-level), number of vaccinations, or Omicron subvariant phase.
Figure 1.
Maximum respiratory support by monoclonal antibody treatment status among patients hospitalized within 28 days within the propensity-matched cohort. Comparing severity of hospitalizations for bebtelovimab-treated (n = 48) and untreated patients (n = 116), the maximum level of respiratory support appeared lower for bebtelovimab-treated patients, but inferential statistics were not able to be performed. Abbreviations: HFNC, high-flow nasal cannula oxygen; IMV, invasive mechanical ventilation; NIV, noninvasive ventilation.
Figure 2.
Forest plot for Omicron infected outpatients subgroup analysis of the propensity-matched cohort. Int, interaction, OR, odds ratio. The Int terms for age and number of vaccinations were significantly different.
Discussion
In this retrospective study of outpatients diagnosed with COVID-19 during a SARS-CoV-2 Omicron predominant phase, including BA.4/BA.5, bebtelovimab was associated with a lower incidence of 28-day all-cause hospitalization and COVID-related hospitalization. These results were consistent across several clinically meaningful subgroups, including those with mild and moderate-severe immunocompromised states. Notably, significant treatment heterogeneity was observed in subgroups, where benefit was apparent in patients with two or more comorbid conditions. Finally, our findings were consistent with the primary outcome when restricted to those with confirmed EUA eligibility or using a secondary imputation method.
Our findings are similar to other studies, which have examined the effectiveness of bebtelovimab, albeit during earlier periods of the Omicron phase. In a study by McCreary et al., bebtelovimab was associated with lower odds of hospitalization or death than no treatment [7]. Notably, this study showed potential heterogeneity in the treatment effect in that older but not younger patients had lower odds of hospitalization. Our study similarly found treatment heterogeneity in those patients with more than two comorbidities who may derive the most significant benefit from treatment. Additional study is needed to optimally determine which subgroups derive benefit from outpatient COVID-19 therapeutics, particularly with extensive population immunity from vaccination and prior infection.
The National Institutes of Health guidelines for the treatment of nonhospitalized adults with COVID-19 previously recommended bebtelovimab as an alternative therapy to nirmatrelvir-ritonavir and remdesivir when either was unavailable or not feasible [13]. We observed that a small proportion of patients were on immunosuppressants with a significant drug interaction with ritonavir, which likely precluded the use of nirmatrelvir-ritonavir in these patients. Other large analyses have estimated that approximately 15% of patients with a high risk for progression to severe disease have at least one potential contraindication to nirmatrelvir-ritonavir [18,19]. As such, there is an urgent, continued need for the development of mAbs and other therapeutics, particularly with the advent of Omicron sublineages XBB and BQ.1/BQ.1.1, which resulted in recent revocation of the bebtelovimab EUA.
Limitations
Several important limitations should be considered. Using an EHR from a single health system could result in treatment and outcomes occurring elsewhere, leading to misclassification. However, this potential is minimized because the state's health system is the largest, and mortality data linked from statewide data is robust. In addition, patient symptom duration was unavailable in our dataset. Test results for SARS-CoV-2 were missing in most bebtelovimab-treated patients, possibly due to the use of rapid SARS-CoV-2 antigen tests at home. As such, for patients in the bebtelovimab-treated group, we imputed the test date based on the date of bebtelovimab order and performed sensitivity analyses to these assumptions, which were consistent with the main finding. Finally, as a single-health system retrospective study, we cannot exclude the possibility of residual and unmeasured confounding.
Conclusion
During the Omicron BA.2/BA.2.12.1/BA.4/BA.5 variant phase in Colorado, bebtelovimab was associated with lower all-cause and COVID-19-related hospitalization, most prominently in patients with two or more comorbid conditions.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This study was funded by the National Center for Advancing Translational Sciences of the US National Institutes of Health (grant numbers UL1TR002525, UL1TR002535-03S3, and UL1TR002535-04S2).
Ethical approval
The Colorado Multiple Institutional Review Board approved this study with a waiver of written informed consent.
Acknowledgments
This study was supported by the Health Data Compass Data Warehouse project (healthdatacompass.org).
Author contributions
AAG conceived and obtained funding for the study. KCM, VK LEB, NEC, NRA, and AAG designed the study. VK, LEB, and NEC analyzed the data. VK, LEB, TDB, NEC, DAM, and SR accessed and verified the data. KCM drafted the original version of the manuscript. All authors had full access to the data, reviewed the manuscript, contributed to data interpretation, approved the final version, and accept responsibility to submit for publication.
Statement of data availability
Deidentified participant data and a data dictionary defining each field in the set, as well as a statistical analysis plan, will be made available to others with publication with a signed data access agreement and approval by the project steering committee through communication with the corresponding author (kyle.molina@cuanschutz.edu) for researchers to reproduce results.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.04.396.
Appendix. Supplementary materials
References
- 1.Lin WT, Hung SH, Lai CC, Wang CY, Chen CH. The impact of neutralizing monoclonal antibodies on the outcomes of COVID-19 outpatients: a systematic review and meta-analysis of randomized controlled trials. J Med Virol. 2022;94:2222–2229. doi: 10.1002/jmv.27623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UD Food and Drug Administration. FDA updates Sotrovimab emergency use authorization, https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-use-authorization; 2022 [accessed 08 November 2022].
- 3.Aggarwal NR, Beaty LE, Bennett TD, et al. Change in effectiveness of sotrovimab for preventing hospitalization and mortality for at-risk COVID-19 outpatients during an omicron BA.1 and BA.1.1-predominant phase. Int J Infect Dis. 2022;128:310–317. doi: 10.1016/j.ijid.2022.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora P, Kempf A, Nehlmeier I, et al. Augmented neutralisation resistance of emerging omicron subvariants BA.2.12.1, BA.4, and BA.5. Lancet Infect Dis. 2022;22:1117–1118. doi: 10.1016/S1473-3099(22)00422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dougan M, Azizad M, Chen P, et al. Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19. medRxiv. 12 November 2022. https://www.medrxiv.org/content/10.1101/2022.03.10.22272100v1 [accessed 15 January 2023].
- 6.Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for Bebtelovimab, https://www.fda.gov/media/156152/download 2022 [accessed 15 January 2023].
- 7.McCreary EK, Kip KE, Collins K, et al. Evaluation of bebtelovimab for treatment of Covid-19 during the SARS-CoV-2 omicron variant era. Open Forum Infect Dis. 2022;9 doi: 10.1093/ofid/ofac517. ofac517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razonable RR, O'Horo JC, Hanson SN, et al. Comparable outcomes for bebtelovimab and ritonavir-boosted nirmatrelvir treatment in high-risk patients with coronavirus Disease-2019 during severe acute respiratory syndrome coronavirus 2 BA.2 Omicron Epoch. J Infect Dis. 2022;226:1683–1687. doi: 10.1093/infdis/jiac346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal NR, Beaty LE, Bennett TD, et al. Real-world evidence of the neutralizing monoclonal antibody sotrovimab for preventing hospitalization and mortality in COVID-19 outpatients. J Infect Dis. 2022;226:2129–2136. doi: 10.1093/infdis/jiac206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Soft. 2011;42:1–28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- 11.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TL, Collins GS, Spence J, et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol. 2017;17:78. doi: 10.1186/s12874-017-0338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institutes of Health . 2022. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines.https://www.covid19treatmentguidelines.nih.gov/ [accessed 01 November 2022] [PubMed] [Google Scholar]
- 14.CDPHE . 2021. Colorado Department of Public Health and Environment.https://covid19.colorado.gov/data Covid-19 data. [accessed 26 October 2022] [Google Scholar]
- 15.Puhr R, Heinze G, Nold M, Lusa L, Geroldinger A. Firth's logistic regression with rare events: accurate effect estimates and predictions? Stat Med. 2017;36:2302–2317. doi: 10.1002/sim.7273. [DOI] [PubMed] [Google Scholar]
- 16.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med. 2002;21:2409–2419. doi: 10.1002/sim.1047. [DOI] [PubMed] [Google Scholar]
- 17.Heinze G, Ploner M, Jiricka L. logistf: Firth's Bias-Reduced Logistic Regression. v1.24. R package; 2020. https://CRAN.R-project.org/package=logistf.
- 18.Lim S, Tignanelli CJ, Hoertel N, Boulware DR, Usher MG. Prevalence of medical contraindications to Nirmatrelvir/Ritonavir in a cohort of hospitalized and nonhospitalized patients with COVID-19. Open Forum Infect Dis. 2022;9:ofac389. doi: 10.1093/ofid/ofac389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoertel N, Boulware DR, Sánchez-Rico M, Burgun A, Limosin F. Prevalence of contraindications to Nirmatrelvir-Ritonavir among hospitalized patients with COVID-19 at risk for progression to severe disease. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.42140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.