To the Editor: Mineral supplements are usually regarded as compounds crucial to promoting overall health status and longevity. Systemic mineral status has been demonstrated to be associated with life expectancy in many observational studies, while the causal effects of mineral status on life expectancy have not yet been systematically elucidated. In this study, we investigated the causal effects of six minerals (calcium, magnesium, iron, copper, zinc, and selenium) on life expectancy with large-scale genome-wide association study (GWAS) summary data using two-sample Mendelian randomization (MR) analysis.
We derived the data on calcium-related genetic variation from 17 population-based GWAS results (n = 39,400) and covered additionally 21,679 individuals to identify relevant genetic loci.[1] In addition, we obtained the data on iron-related genetic variation from the GWAS results of 23,986 individuals from 11 population-based cohorts of European descent in nine participating centers.[2] The magnesium-related genetic variation data were derived from the European ancestry GWAS (n = 15,366) from the International Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium, and these single-nucleotide polymorphisms (SNPs) were confirmed using an additional 8463 individuals of European ancestry.[3] The genetic variations related to blood copper, zinc, and selenium concentrations were derived from Australian (the Queensland Institute of Medical Research, QIMR) and UK (the Avon Longitudinal Study of Parents and Children, ALSPAC) data.[4]
We used the published SNPs with a strong relationship (P < 5.0×10–8) and independent inheritance (r2 < 0.01) for the blood mineral concentrations serving as an instrumental variable in the MR analysis (https://www.ebi.ac.uk/gwas). Two respective SNPs that were significantly related to blood copper ions (rs1175550 and rs2769264), blood selenium ions (rs921943 and rs7700970), and blood zinc ions (rs1532423 and rs2120019) were identified and selected. The summary statistics of the association of mineral-related SNPs with parental lifespan were derived from a meta-analysis of the UK biobank (https://www.ukbiobank.ac.uk/) and LifeGen consortium (n = 1,012,240).[5] All the original studies received due ethical approval, and all the participants provided informed consent.
Using the genetic data of offspring, effect sizes observed under a Cox proportional hazards model were doubled to reflect the expected parental genotype effects.[6] We multiplied the effect estimates in this study by ten to estimate the absolute changes in life expectancy. We presented the exposure–outcome associations by estimating the variations of the parental life expectancy with their 95% confidence interval (CI) per 1-standard deviation (SD) increase of genetically evaluated mineral status. A two-sided P value <0.05 indicated statistically significant evidence for a causal association. We conducted all analyses using R software (V.3.5.1, R Development Core Team, Vienna, Austria).
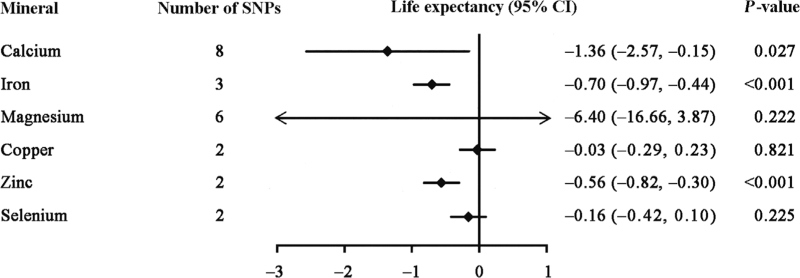
We used the inverse-variance weighting (IVW) MR method to estimate the causal associations of genetically predicted mineral status with the parental life expectancy. As shown in Figure 1, genetically predicted higher serum calcium, serum iron, blood zinc status appeared to have decreased the life expectancy. The association of a 1-SD increase in genetically predicted mineral status with lifespan years was –1.36 for serum calcium (95% CI: –2.57, –0.15; P = 0.027), –0.70 for serum iron (95% CI: –0.97, –0.44; P <0.001), and –0.56 for blood zinc (95% CI: –0.82, –0.30; P <0.001). However, a 1-SD increase in genetically elevated blood copper (beta coefficient = −0.03; 95% CI: −0.29, 0.23; P = 0.821), serum magnesium (beta coefficient = −6.40; 95% CI: −16.66, 3.87; P = 0.222), and blood selenium (beta coefficient = −0.16; 95% CI: −0.42, 0.10; P = 0.225) were not causally associated with parental lifespan. We performed sensitivity analyses using MR-Egger regression, the weight median method, mode-based estimation (MBE), and MR pleiotropy residual sum and outlier (MR-PRESSO). In sensitivity analyses, we observed substantial inverse associations of serum iron with parental lifespan using the penalized IVW (P < 0.001), weighted median (P < 0.001), and weighted MBE (P < 0.001), but no statistically significant association using the MR-Egger method (P = 0.072). We observed substantial inverse associations of serum magnesium with parental lifespan using the penalized IVW (P = 0.004), weighted median (P = 0.014), and weighted MBE (P = 0.018), whereas neither reached statistical significance using the MR-Egger (P = 0.106) and MR-PRESSO (P = 0.089) methods. We observed substantial inverse associations for serum calcium with parental lifespan using the penalized IVW (P = 0.037) and MR-PRESSO methods (P = 0.047), whereas no statistically significant associations were found using the MR-Egger (P = 0.757), weighted median (P = 0.259), and weighted MBE methods (P = 0.169). Sensitivity analyses were not performed for blood copper, zinc, and selenium because these methods require more than two variants.
Figure 1.
Changes in parental lifespan for genetically predicted blood minerals concentrations. The associations were assessed using the inverse-variance weighted method. Estimates are the effect of genetically predicted a 1-SD increase of each blood mineral on life expectancy change. CI: Confidence interval; SD: Standard deviation; SNPs: Single-nucleotide polymorphisms.
According to MR-Egger regression, no obvious heterogeneity was observed in the SNPs of serum calcium (Q = 4.65, P = 0.590) and serum iron (Q = 2.79, P = 0.090). The Cochran's Q heterogeneity test was used to explore the possible biases related to the inclusion of pleiotropic variants. No evidence of directional pleiotropy was observed for the association of serum calcium (intercept=−0.03, P = 0.461), iron (intercept=0.21, P = 0.284), and magnesium (intercept=0.12, P = 0.220) with parental lifespan. We observed no measurement errors in SNP-exposure effect estimates of serum calcium ( statistic=97.1%), iron ( statistic = 91.0%), and magnesium ( statistic =97.2%). The value of (0 and 1) indicates the possible biases of MR-Egger; the closer to the value of 1, the stronger evidence that the causal association cannot be affected by possible biases.
The limitations of this study are as follows. First, the SNP biomarkers used in our study all came from European or Austrian ancestry populations, which may limit the generalization of the findings to other populations. Studies that enroll larger samples with racially diversified populations are needed to explore the genetic locus and blood minerals relevant to life expectancy. Another potential limitation is that in the sensitivity analyses, the mineral status demonstrated no statistically significant effects on life expectancy when we used some MR methods; further validation is required in future studies with large sample sizes or a well-designed randomized controlled trial to confirm our findings.
In conclusion, findings of this MR study showed that genetically higher serum calcium, serum iron, and blood zinc concentrations may be associated with a reduced life expectancy. Meanwhile, there was no association of genetically predicted blood magnesium, copper, and selenium concentrations with life expectancy. Our genetic evidence suggests that people without mineral deficiencies are unlikely to benefit from supplementation. This study will help address some controversies on the associations between minerals and life expectancy.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81971091 and 81901177), Beijing Natural Science Foundation (No. 7182049), Beijing Hospitals Authority Youth Programme (Nos. QML20190501 and QML20200501), and the Young Elite Scientist Sponsorship Program from China Association for Science and Technology (No. 2019QNRC001).
Conflicts of interest
None.
Footnotes
How to cite this article: Fang H, Chen W, Jin A, Wang M, Yan H, Xiang X, Pan Y. Effects of genetically determined mineral status on life expectancy: a Mendelian randomization study. Chin Med J 2023;136:242–244. doi: 10.1097/CM9.0000000000002436
Hongjuan Fang and Weiqi Chen contributed equally to this work.
References
- 1.O'Seaghdha CM, Wu H, Yang Q, Kapur K, Guessous I, Zuber AM, et al. Meta-analysis of genome-wide association studies identifies six new loci for serum calcium concentrations. PLoS Genet 2013; 9:e1003796.doi: 10.1371/journal.pgen.1003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benyamin B, Esko T, Ried JS, Radhakrishnan A, Vermeulen SH, Traglia M, et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat Commun 2014; 5:4926.doi: 10.1038/ncomms5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer TE, Verwoert GC, Hwang SJ, Glazer NL, Smith AV, van Rooij FJ, et al. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six loci influencing serum magnesium levels. PLoS Genet 2010; 6:e1001045.doi: 10.1371/journal.pgen.1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans DM, Zhu G, Dy V, Heath AC, Madden PA, Kemp JP, et al. Genome-wide association study identifies loci affecting blood copper, selenium, and zinc. Hum Mol Genet 2013; 22:3998–4006. doi: 10.1093/hmg/ddt239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timmers PR, Mounier N, Lall K, Fischer K, Ning Z, Feng X, et al. Genomics of 1 million parent lifespans implicates novel pathways and common diseases and distinguishes survival chances. Elife 2019; 8:e39856.doi: 10.7554/eLife.39856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joshi PK, Pirastu N, Kentistou KA, Fischer K, Hofer E, Schraut KE, et al. Genome-wide meta-analysis associates HLA-DQA1/DRB1 and LPA and lifestyle factors with human longevity. Nat Commun 2017; 8:910.doi: 10.1038/s41467-017-00934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]