To the Editor: Given the high rates of pre-diseases and various initiating causes, critical illnesses have become the most heterogeneous group in clinical practice, which multiplies treatment challenges. Despite different types of injurious “hit,” this response to various acute critical conditions is relatively homogenous and may eventually progress to a critical illness.
By studying the pathophysiology of critical illnesses, we have identified a basic similarity during their development: the underlying co-pathophysiology is the host/organ unregulated response (HOUR). Infection, trauma, heart attacks, etc. are usually described as the “initiating cause” or “first hit” that directly induces the critical illness or triggers a host/organ response, leading to the activation or suppression of multiple endothelial, hormonal, bioenergetic, metabolic, and immune pathways. This subsequent response is the “second hit.” Although the response is initially adaptive/protective, if the injury persists and attains some magnitude with or without the inappropriate interventions, the response becomes dysregulated, ultimately resulting in critical illness. This article attempts to summarize the current knowledge base for HOUR as a common cause of critical illness and discuss therapeutic strategies to regulate HOUR.
Several elements act together to promote the progression of a disease to a severe illness. Based on the characteristics and regularity, critical illness can be divided into four stages [Supplementary Figure 1]:
Pre-disease: This stage refers to the underlying pathophysiologic disturbances/chronic comorbidities (eg, hypertension, diabetes, congenital heart disease, and asthma), age, sex, and specific immunosuppressive status (eg, acute leukemia, autoimmune disease during hormone or immunosuppressive therapy, tumors during chemotherapy or targeted drug therapy, post-transplantation during anti-rejection therapy, and acquired immune deficiency syndrome). These premorbid factors related to the individual can have a substantial impact on the outcome of critical illness, modifying both the disease process and the approach to treatment.
Initiating causes: This stage refers to some directed injury or acute illness such as trauma, infections, cardiac surgery, stroke, hemorrhage, and acute coronary syndrome. Depending on the nature and extent of the insults, these initiating causes can directly induce critical illness (eg, severe traumatic brain injury, massive pulmonary embolism, and acute myocardial infarction) or act as the “first hit” (eg, infection and pancreatitis), triggering the dysregulated host response.
Common cause-HOUR: This stage refers to the nature and magnitude of the host response. The host response acts as a sensor of danger signal. When the initiating factors persist and attain some magnitude—potentially exacerbated by inappropriate or delayed interventions—the host response may become dysregulated, leading to the “second hit” to the body and eventually resulting in critical illness. Studies have shown that patients do not usually die of their initial disease but of the physiological consequences of the disease, such as sepsis.[1]
Critical illness: This stage refers to the degree of concomitant organ dysfunction. Life-threatening organ dysfunction represents a critical status. The sequential organ failure assessment score can be used to quantitatively describe the degree of organ dysfunction that develops over the course of a critical illness.
The host response refers to the host's defense mechanism against exogenous stimulation. When the body suffers stimulation, the host response system reacts quickly to help the body return to a new equilibrium point. However, when the adaptive/protective host response suffers a severe, overwhelming insult that is beyond its capability, the response becomes dysregulated. These initiating causes can be divided into three types: infective causes, non-infective causes, and truamatic brain injury (TBI).
Microorganisms or their constituents represent pathogen-associated molecular patterns that activate pattern recognition receptors, thereby triggering a downstream inflammatory response. Sepsis is the most typical syndrome in this category, which is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection,[1] emphasizing the crucial role of the innate and adaptive immune response in the development of this clinical syndrome.
Trauma can result in the uncontrolled local and systemic release of endogenous mediators that act as damage-associated molecular patterns (DAMPs). The recognition of DAMPs by the innate immune system triggers both an intense pro-inflammatory immune response and a concomitant anti-inflammatory response. While excessive inflammation promotes the development and propagation of secondary tissue injuries beyond the initial traumatic foci, the anti-inflammatory response leads to host defense impairment, thereby contributing to multiple organ dysfunctions and, ultimately, to death.[2] Pancreatitis is another example of inflammation characterized by the absence of pathogens during the initial stage.
TBI is also a result of direct and indirect mechanisms. The indirect or secondary injury of TBI involves the initiation of an acute inflammatory response. However, the specificity of TBI compared with trauma is that it directly insults the central nervous system (CNS) and activates CNS response. It is well known that the stress response involves central, peripheral, and cellular systems that work together to keep the body informed and ready to react to changes based on external or internal cellular environments. TBI directly causes neurologic deficits and simultaneously induces neural, hormonal, and immune responses from the very start. Accordingly, TBI is a “destined” critical illness.
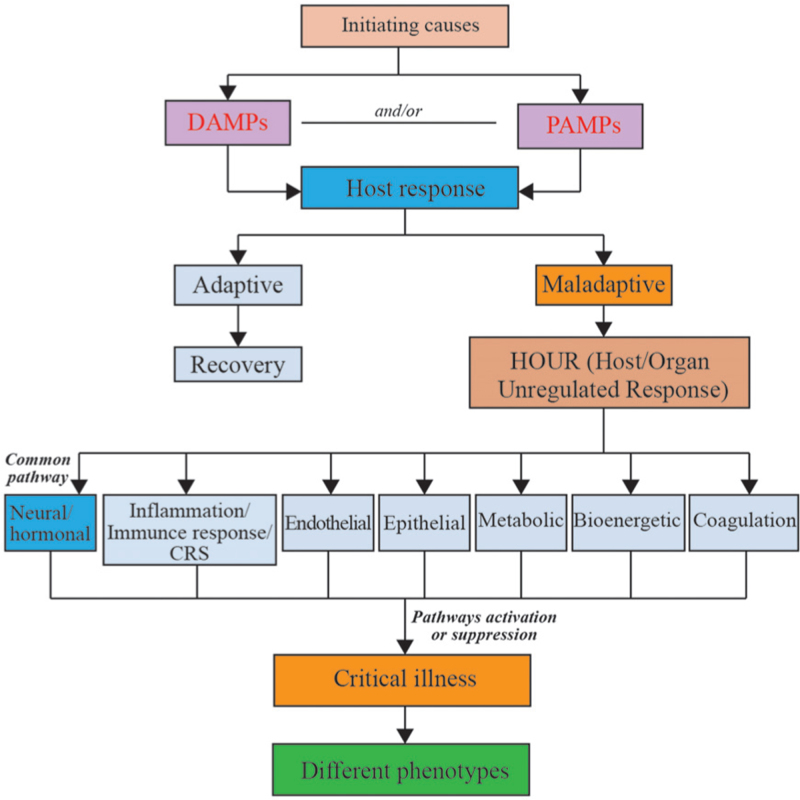
The effects of HOUR are not identical across individuals, which is due to the diverse downstream pathways and affected organs [Figure 1]. However, they have at least shared one response that is associated with the nervous system. The initiating causes may be seen as strong stimulation to the body, and neuroendocrine hormones play major roles in the regulation of both basal homeostasis and responses to threats. The stress response is mediated by the stress system, which is partly located in the CNS and partly in peripheral organs. Hormone production is markedly affected by critical illness and influences multiple systems, including the cardiovascular, immune, bioenergetic, and metabolic systems. Some specialists hold the view that critical illness may represent a stress-related decompensation syndrome mediated by the neural, endocrine, bioenergetic, and immune systems.[3]
Figure 1.
The effect of HOUR on critical illness. CRS: cytokine release syndrome; DAMPs: Damage associated molecular patterns; HOUR: Host/organ unregulated response; PAMPs: Pathogen-associated molecular patterns.
The immune and inflammatory responses may share the same downstream pathways. For instance, cytokine release syndrome (CRS) is the common pathway of immune and inflammatory responses and can induce a wide range of clinical and laboratory abnormalities. All cases of CRS represent secondary organ dysfunction, often involving the renal, hepatic, or pulmonary systems.
Mitochondria generate most of cell's supply of adenosine triphosphate. The stress response in critical illness acts at the cellular level through mitochondria as a common pathway.[4] Thus, mitochondria could be considered as the final effector in the host/organ response. Mitochondria perform other supplementary functions such as cell signaling and the activation of cell death pathways, which have influenced the evolution of cellular and organ function in critical illnesses.[5]
Different types of injuries induce the release of catecholamine and cytokine to damage the endothelium, which is responsible for endothelial breakdown, resulting in glycocalyx shedding, the breakdown of tight junctions with capillary leakage, and a procoagulant microvasculature. There are also downstream irregularities in the microvasculature with patchy areas of constriction and dilatation that activate the clotting pathways.[6]
Individuals with different initiating causes can represent different or similar host responses. The predominant response may determine the main phenotypic feature, for example, neural, immune, inflammatory, cytokine, coagulation, metabolic, or a mix of these. As a result, the clinical manifestations are diverse due to the final target organs. The main phenotypic feature may be cardiovascular, with a hyperkinetic heart and vasoplegia, but could also be acute respiratory distress syndrome (ARDS).
Although our understanding of the pathophysiology of critical illness has progressed during recent decades, the options for successful therapeutic interventions remain restricted. There are few initiating causes that can be addressed, and the majority of the initiating causes require time for recovery. Therefore, the management of HOUR warrants attention.
During the acute phase of critical illness, the aim of treatment is to prevent adverse changes in normal physiological status. Once the critical phase is established, the therapy target should directly support the intrinsic cellular mechanisms. Pathophysiological responses should not be considered simply as disturbances of physiology, but rather as factors that accommodate the body to stimulation. In other words, management of the host response could help the body return to homeostasis or establish new homeostasis. However, it should be noted that interventions have both a therapeutic effect as well as a re-injury effect. Moreover, a remaining challenge is to identify an optimal timepoint for intervention that will not interrupt the adaptive host response process. Therefore, we recommend therapeutic principles based on recognition of HOUR [Supplementary Table 1].
Critically ill patients suffer from pain and various stimuli. Interventional pharmacological and mechanical supports could also be stressor agents for such patients. The goals of sedation and analgesia are to relieve pain and anxiety, attenuate stress response, and improve patient compliance. Analgesic drugs, such as opioids and nonsteroidal anti-inflammatory drugs, act on the production of stimulation, directly blocking afferent impulses. Sedatives, such as midazolam, propofol, dexmedetomidine, and ketamine, regulate the balance between excitation and inhibition in the brain. Antisympathetic drugs influence effectors, with beta-blockers and dexmedetomidine commonly used in clinical practice.
Critical illnesses are complex, temporally dynamic, and dependent on both stimulating factors (eg, pathogens) and host factors (eg, genetic susceptibility). Due to such challenges, therapies involving immune or inflammation modulation remain controversial. Studies in children with coronavirus disease 2019 suggest that anti-immune and anti-inflammatory therapies are beneficial for the targeted patients.[7,8] Blood purification with adsorption was also thought to be a potential adjunctive therapy for sepsis and systematic inflammation to regulate the excessive cytokine storm or reduce endotoxin activity.[9]
Hypothermia reduces the release of excitatory amino acids and the production of free radicals. According to international guidelines,[10] targeted temperature management is the only neuroprotective intervention currently recommended after out-of-hospital cardiac arrest.
Nutrition also plays a key role in modulating the inflammatory response. The benefits of nutrition support may vary depending on severity of organ dysfunction. For ARDS patients, the combination of high organ failure rates and high-calorie delivery is associated with an increase in mortality.[11] Timely and adequate nutrition support may optimize the host response and thereby minimize nutrion-related complications while improving overall outcome.[12]
Several antioxidants that target mitochondria have been shown to protect against critical illness. Novel therapies with the potential to prevent and reverse mitochondrial dysfunction during critical illness are currently being studied, including various mitochondiral targeted drugs and agents that induce mitochondrial biogenesis or mitophagy.[5] The translation of these emerging therapies to the bedside may lead to major advances in critical care medicine.
To summarize, critical illness is a momentary pause before death that gives clinicians an opportunity to save patients’ lives. Critical illness can be defined as any state in an individual (pre-disease) with HOUR induced by initiating causes (injury or acute disease). HOUR activates or suppresses diverse downstream pathways, leading to multiple organ dysfunction. Adequate host response aids tissue repair and general recovery; however, dysregulated host response induces decompensated damage. Although management of HOUR can benefit critically ill patients, this has not yet been established in routine clinical practice.
Funding
This work was supported by a grant from the Key Project of Central Health Care Scientific Research (No. 2020ZD08).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Huang W, Liu D, Zhang H, Ding X, Wang X. Focus on host/organ unregulated response: a common cause of critical illness. Chin Med J 2023;136:108–110. doi: 10.1097/CM9.0000000000002374
Supplemental digital content is available for this article.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sauaia A, Moore FA, Moore EE. Postinjury inflammation and organ dysfunction. Crit Care Clin 2017; 33:167–191. doi: 10.1016/j.ccc.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuesta JM, Singer M. The stress response and critical illness: a review. Crit Care Med 2012; 40:3283–3289. doi: 10.1097/CCM.0b013e31826567eb. [DOI] [PubMed] [Google Scholar]
- 4.Manoli I, Alesci S, Blackman MR, Su YA, Rennert OM, Chrousos GP. Mitochondria as key components of the stress response. Trends Endocrinol Metab 2007; 18:190–198. doi: 10.1016/j.tem.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Supinski GS, Schroder EA, Callahan LA. Mitochondria and critical illness. Chest 2020; 157:310–322. doi: 10.1016/j.chest.2019.08.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iba T, Levy JH. Sepsis-induced coagulopathy and disseminated intravascular coagulation. Anesthesiology 2020; 132:1238–1245. doi: 10.1097/ALN.0000000000003122. [DOI] [PubMed] [Google Scholar]
- 7.Ouldali N, Toubiana J, Antona D, Javouhey E, Madhi F, Lorrot M, et al. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA 2021; 325:855–864. doi: 10.1001/jama.2021.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Son MBF, Murray N, Friedman K, Young CC, Newhams MM, Feldstein LR, et al. Multisystem inflammatory syndrome in children – initial therapy and outcomes. N Engl J Med 2021; 385:23–34. doi: 10.1056/NEJMoa2102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Y, Peng JY, Peng Z. Blood purification in sepsis and systemic inflammation. Curr Opin Crit Care 2021; 27:582–586. doi: 10.1097/MCC.0000000000000890. [DOI] [PubMed] [Google Scholar]
- 10.Nolan JP, Sandroni C, Böttiger BW, Cariou A, Cronberg T, Friberg H, et al. European resuscitation council and european society of intensive care medicine guidelines 2021: post-resuscitation care. Intensive Care Med 2021; 47:369–421. doi: 10.1007/s00134-021-06368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson SJ, McKeever L, Lateef OB, Freels S, Fantuzzi G, Braunschweig CA. Combination of high-calorie delivery and organ failure increases mortality among patients with acute respiratory distress syndrome. Crit Care Med 2019; 47:69–75. doi: 10.1097/CCM.0000000000003476. [DOI] [PubMed] [Google Scholar]
- 12.Sharma K, Mogensen KM, Robinson MK. Pathophysiology of critical illness and role of nutrition. Nutr Clin Pract 2019; 34:12–22. doi: 10.1002/ncp.10232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.