Abstract
Background:
The study aimed to describe the aortic valve morphology in Chinese patients underwent transcatheter aortic valve replacement (TAVR) for symptomatic severe aortic stenosis (AS), and the impact of sizing strategies and related procedural outcomes.
Methods:
Patients with severe AS who underwent TAVR were consecutively enrolled from 2012 to 2019. The anatomy and morphology of the aortic root were assessed. “Downsize” strategy was preformed when patients had complex morphology. The clinical outcomes of patients who performed downsize strategy were compared with those received annular sizing strategy. The primary outcome was device success rate, and secondary outcomes included Valve Academic Research Consortium-3 clinical outcomes variables based on 1-year follow-up.
Results:
A total of 293 patients were enrolled. Among them, 95 patients (32.4%) had bicuspid aortic valve. The calcium volume (Hounsfield Unit-850) of aortic root was 449.90 (243.15–782.15) mm3. Calcium is distributed mostly on the leaflet level. Downsize strategy was performed in 204 patients (69.6%). Compared with the patients who performed annular sizing strategy, those received downsize strategy achieved a similar device success rate (82.0% [73] vs. 83.3% [170], P = 0.79). Aortic valve gradients (downsize strategy group vs. annular sizing group, 11.28 mmHg vs. 11.88 mmHg, P = 0.64) and percentages of patients with moderate or severe paravalvular regurgitation 2.0% (4/204) vs. 4.5% (4/89), P = 0.21) were similar in the two groups at 30 days after TAVR. These echocardiographic results were sustainable for one year.
Conclusions:
Chinese TAVR patients have more prevalent bicuspid morphology and large calcium volume of aortic root. Calcium is distributed mostly on the leaflet level. Compare with annular sizing strategy, downsize strategy provided a non-inferior device success rate and transcatheter heart valve hemodynamic performance in self-expanding TAVR procedure.
Keywords: Aortic stenosis, China, Sizing strategy, Transcatheter aortic valve replacement, Aortic valve, Morphology
Introduction
Transcatheter aortic valve replacement (TAVR) has become the main option for patients with symptomatic severe aortic stenosis (AS) at high risk for surgical aortic valve replacement (SAVR),[1–3] and an alternative option for patients with symptomatic severe AS at intermediate and low risk for SAVR.[4–8] In China, the first TAVR clinical trial started in 2012, and the morphology of the aortic valve showed many differences from Western countries. Compared with the Western registries, the proportion of bicuspid aortic valve (BiAV) among Chinese TAVR patients was higher (45.4% vs. 3.2–6.7%).[9–12] Chinese patients also had more significant heavy calcium burden. These observations motivated TAVR operators to consider different and particular TAVR strategies and device choices in clinical practice in China.[13,14] However, data on Chinese AS morphology are rare. The procedure complexity in Chinese TAVR population, particularly in BiAV subgroup, highlights the need to propose and validate a new sizing strategy to ensure a better outcome.
This study aims to describe the aortic valve morphology in Chinese patients underwent TAVR for symptomatic severe AS, and the impact of “downsize” strategy on related procedural outcomes based on a 1-year follow-up.
Methods
Ethical approval
The study was approved by the Ethics Committee of Fuwai Hospital (approval No. 2020–1290). The informed consent was exempted because of the retrospective purpose.
Patients
From October 2012 to October 2019, patients who performed TAVR with self-expanding transcatheter heart valves (THVs) for native aortic valve stenosis in Fuwai Hospital were consecutively enrolled. Patients with missing Electrocardiogram (ECG)-gated aortic computed tomography (CT) scan in the systolic phase were excluded.
CT image acquisition and analyses
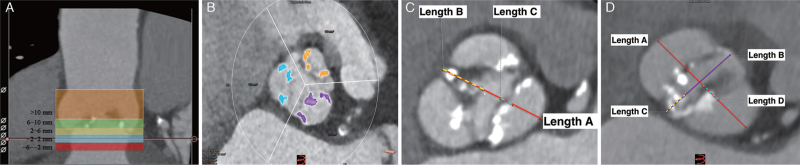
The image acquisition protocol is detailed in the supplementary materials. All examinations were analyzed on the 3mensio (3mensio Structural Heart, version 10.0, 3mensio Medical Imaging BV, the Netherlands) workstation. Analyses were performed by Fuwai Hospital core laboratory and Eagles Corelab, YingKe Medical (Beijing) Co., Ltd following the same protocol and validated by an expert physician (Dr. HJ). The BiAV morphology was classified according to Sievers Classification.[15] The annulus, sinotubular junction, ascending aorta dimensions and coronary artery heights were assessed according to the current guideline.[16] The calcium of aortic root was quantified using contrast-enhanced scans and a high Hounsfield Unit (HU-850) threshold for volumetric detection. The aortic root was divided into five specific regions: left ventricular outflow tract (LVOT) region (from 6 mm below the annular plane to 2 mm below the annular plane), annulus region (from 2 mm below the annular plane to 2 mm superior to the annular plane), lower leaflet region (from 2 mm superior to the annular plane to 6 mm superior to the annular plane), mid leaflet region (from 6 mm superior the annular plane to 10 mm superior to the annular plane), upper leaflet region (from 10 mm superior to the annular plane to the sinotubular junction level) [Figure 1A]. The aortic root was also divided into three sectors to correspond to each sinus distribution: left, right, and non-coronary for tricuspid aortic valve (TriAV), and type-1 BiAV [Figure 1B]. Length of fusion between leaflets was measured as commissure to the point the leaflet separated, and the measurement was obtained at the cross-section of 6, 8, and 10 mm above the basal plane. The presence of commissural calcium was noted. The thickness of the valve and the distance between commissure to opposite sinus were also measured at these planes [Figures 1C and 1D].
Figure 1.
Calcium assessment and leaflets measurement of aortic stenosis patients. (A) The aortic root was divided into five specific regions. LVOT region: from 6 mm below the annular plane to 2 mm below the annular plane; Annulus region: from 2 mm below the annular plane to 2 mm superior to the annular plane; Lower leaflet region: from 2 mm superior to the annular plane to 6 mm superior to the annular plane; Mid leaflet region: from 6 mm superior to the annular plane to 10 mm superior to the annular plane; Upper leaflet region: from 10 mm superior to the annular plane to the sinotubular junction level; (B) Calcium quantification by leaflet sector: Purple, yellow and blue represent the left, right and none coronary leaflet sectors respectively; (C) Leaflets thickness and fusion between measurements for TAV and Type-1 BiAV: Length A (red line): Commissure to opposite sinus measurement; Length B (yellow imaginary line): Fusion length between leaflets (measured as commissure to the very point the leaflet separated); Length C (blue imaginary line): Thickness of leaflet (measured as the high-density or low-density tissue between the sinus of Valsalva and aortic valve orifice); (D) Leaflets thickness and fusion between measurements for Type-0 BiAV: Length A (red line): Mid sinus-sinus measurement; Length B (purple line): Inter-commissural distance (the distance between commissures of the respective opposing leaflets); Length C (yellow imaginary line): Fusion between leaflets (measured as commissure to the very point the leaflet separated); Length D (blue imaginary line): Thickness of leaflet (measured as the high-density or low-density tissue between the sinus of Valsalva and aortic valve orifice). LC: Left coronary; LVOT: Left ventricular outflow tract; NC: Non coronary; RC: Right coronary; TAV: Transcatheter aortic valve; Type-1 BiAV: Sievers type-1 bicuspid aortic valve; Type-0 BiAV: Sievers type-0 bicuspid aortic valve.
TAVR procedure
TAVR procedures were performed with three self-expanding THVs, Venus A (Venus Medtech Inc., Hangzhou, China), VitaFlow valve (Shanghai MicroPort CardioFlow Medtech Co., Ltd., Shanghai, China), and Taurus One valve (PeiJia Med Co. Ltd. Suzhou, China). THV size was selected by the heart team based on patients’ annular perimeter, morphology of the aortic valve, calcium volume of the aortic root, and thickness of leaflets. Downsize strategy is defined as using a THV whose size is smaller than annulus sizing. Patients with BiAV, severe calcification at leaflets’ level, dramatic leaflet thickening, and fusions that were difficult to separate preferred to use the downsize strategy. Clinical outcomes were recorded according to Valve Academic Research Consortium-3 criteria,[17] up to 1-year follow-up.
Statistical analysis
Continuous variables were reported as mean ± standard deviation or median (interquartile range [Q1, Q3]) depending on the distribution of data. Kolmogorov–Smirnov test was used to examine whether continuous variables were normally distributed. For those with normal distribution compared across independent groups, an independent sample t-test was employed. For non-normally distributed continuous variables compared across independent groups, a Kruskal–Wallis test was used. Categorical variables were reported as number (percentage) and compared using the chi-squared test. A two-sided P < 0.05 was considered statistically significant and all statistical analyses were performed using SPSS software (version 24; SPSS, Inc., Chicago, IL, USA).[7]
Results
A total of 293 patients were enrolled. There were 198 (67.6%) TriAV patients and the remaining 95 patients (32.4%) had a bicuspid morphology. Among the 95 BiAV patients, 49 (51.6%) were categorized as Sievers Type 1 and 46 (48.4%) were Sievers Type 0. Baseline characteristics are summarized in Table 1.
Table 1.
Baseline and anatomic characteristics of AS patients receiving TAVR for both annular sizing and downsize groups.
| Items | Total (n = 293) | Annular sizing (n = 89) | Downsize (n = 204) | P-value |
| Baseline characteristics | ||||
| Age (years) | 75.86 ± 7.36 | 76.93 ± 6.41 | 75.40 ± 7.71 | 0.29 |
| Male | 176 (60.1) | 50 (56.2) | 126 (61.8) | 0.36 |
| BMI (kg/m2) | 23.54 ± 3.79 | 23.89 ± 3.64 | 23.39 ± 3.85 | 0.56 |
| STS score | 5.2 ± 2.2 | 4.8 ± 2.0 | 5.7 ± 2.4 | 0.32 |
| NYHA ≥ III | 255 (87.0) | 76 (85.4) | 179 (87.8) | 0.59 |
| Coronary heart disease | 119 (40.6) | 40 (45.0) | 79 (38.7) | 0.32 |
| Previous stroke or TIA | 41 (14.0) | 10 (11.2) | 31 (15.2) | 0.36 |
| Diabetes | 79 (27.0) | 23 (25.8) | 56 (27.5) | 0.78 |
| Hypertension | 182 (62.1) | 55 (61.8) | 127 (62.3) | 0.94 |
| Hyperlipidemia | 173 (59.0) | 58 (65.2) | 115 (56.4) | 0.15 |
| Peripheral vascular disease | 31 (10.6) | 12 (13.5) | 19 (9.3) | 0.29 |
| COPD | 39 (13.3) | 10 (11.2) | 29 (14.2) | 0.49 |
| AF/AFL | 50 (17.1) | 18 (20.2) | 32 (15.7) | 0.34 |
| Permanent pacemaker | 12 (4.1) | 6 (6.7) | 6 (2.9) | 0.13 |
| Aortic-valve area (cm2) | 0.68 ± 0.33 | 0.67 ± 0.28 | 0.68 ± 0.36 | 0.35 |
| Aortic-valve gradient (mmHg) | 55.83 ± 17.18 | 55.77 ± 16.98 | 55.85 ± 17.32 | 0.99 |
| Left ventricular ejection fraction (%) | 55.96 ± 13.94 | 57.83 ± 14.88 | 52.05 ± 7.96 | <0.01 |
| Moderate or severe regurgitation | ||||
| Aortic | 100 (34.1) | 28 (31.5) | 72 (35.3) | 0.52 |
| Mitral | 67 (22.9) | 17 (19.1) | 50 (24.5) | 0.31 |
| Anatomic characteristics | ||||
| Bicuspid aortic valve | 95 (32.4) | 23 (25.8) | 72 (35.3) | 0.06 |
| Sievers Type 1 | 49 (16.7) | 13 (14.6) | 36 (17.7) | 0.06 |
| Sievers Type 0 | 46 (15.7) | 10 (11.2) | 36 (17.7) | 0.05 |
| Systolic annular measurement | ||||
| Long-axial diameter (mm) | 27.00 (25.20–29.20) | 26.50 (25.00–28.30) | 27.35 (25.25–29.35) | 0.33 |
| Short-axial diameter (mm) | 20.80 (19.10–22.60) | 20.80 (18.90–22.10) | 20.90 (19.10–22.70) | 0.08 |
| Perimeter (mm) | 75.60 (70.63–81.75) | 74.60 (69.40–80.50) | 76.25 (70.95–82.45) | 0.18 |
| Area (mm2) | 436.50 (381.45–513.50) | 429.20 (372.90–492.90) | 443.90 (387.55–518.45) | 0.18 |
| Eccentricity of annulus | 0.24 (0.19–0.27) | 0.23 (0.19–0.26) | 0.24 (0.19–0.28) | 0.27 |
| Sinotubular junction diameter (mm) | 29.50 (26.80–32.20) | 28.40 (26.20–30.90) | 29.98 (27.28–32.50) | <0.01 |
| Ascending aorta (mm) | 38.40 (35.00–42.80) | 37.60 (34.50–40.60) | 39.15 (35.25–43.65) | 0.03 |
| LVOT perimeter (mm) | 78.00 (70.85–86.80) | 77.20 (69.70–85.00) | 78.15 (71.65–87.25) | 0.34 |
| LVOT area (mm2) | 443.70 (367.70–557.00) | 432.40 (353.30–524.60) | 450.85 (373.40–568.60) | 0.22 |
| LCA ostium (mm) | 13.90 (11.50–15.95) | 13.30 (11.10–15.80) | 14.00 (11.65–16.05) | 0.39 |
| RCA ostium (mm) | 16.60 (14.70–18.80) | 16.70 (14.70–18.80) | 16.55 (14.80–18.80) | 0.85 |
| Calcium volume (mm3) | 449.00 (243.15–782.15) | 379.00 (210.70–828.50) | 456.05 (267.85–729.60) | 0.64 |
Data are expressed as n (%), mean ± standard deviation, or median (Q1, Q3). AF: Atrial fibrillation; AFL: Atrial flutter; AS: Aortic stenosis; BMI: Body mass index; COPD: Chronic obstructive pulmonary disease; LCA: Left coronary artery; LVOT: Left ventricular outflow tract; NYHA: New York heart association; RCA: Right coronary artery; STS score: The Society of Thoracic Surgeons risk score; TAVR: Transcatheter aortic valve replacement; TIA: Transient ischemic attack.
Anatomic analysis
As shown in Supplementary Table 1, BiAV patients had a longer aortic valve annular perimeter (77.50 [72.90–85.00] mm vs. 74.65 [70.00–80.50] mm, P < 0.01) and larger area (462.60 [404.80–551.00] mm2vs. 428.65 [374.10–492.60] mm2, P < 0.01) with less elliptical annuli according to the eccentricity ratio compared with TriAV ones (0.21 [0.16–0.27] vs. 0.24 [0.21–0.27], P < 0.01). Annulus calcifications were more common in BiAV patients (54/95 [56.8%] vs. 71/198 [35.9%], P < 0.01). The TriAV patients had smaller LVOT, sinotubular junction, and ascending aorta dimension when compared with BiAV patients. Sievers Type 1 BiAV patients had smaller ascending aorta dimension than Type 0 BiAV morphologies (41.80 [38.00–46.60] mm vs. 44.25 [40.30–48.20] mm, P = 0.03). LVOT and sinotubular junction dimensions did not show statistically significant differences among the two BiAV subtypes. Distance between coronary ostium and aortic annulus plane were longer in BiAV patients compared with TriAV patients, left (15.20 [12.00–17.95] mm vs. 13.40 [11.30–15.30] mm, P < 0.01), right (17.70 [15.15–19.35] mm vs. 16.10 [14.70–18.20] mm, P = 0.01) [Supplementary Table 1].
Calcium distribution
The median total calcium volume (HU-850) was 449.90 (243.15–782.15) mm3. The distribution of calcium by sector was as follows: left 93.70 (32.80–197.90) mm3, right 104.50 (37.90–221.73) mm3, and none coronary distributions (179.10 [81.30–377.70] mm3) [Supplementary Figure 1]. Patients with bicuspid aortic valve had more severe calcium burden compared with TriAV (581.30 [417.50–934.95] mm3vs. 358.45 [206.40–668.40] mm3, P < 0.01) [Supplementary Table 2 and Supplementary Figure 2].
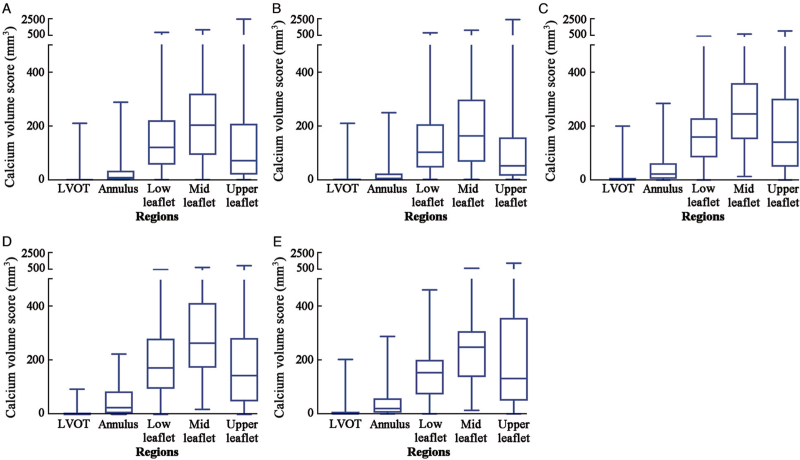
Calcifications were present rarely at LVOT region and the annulus region, but more at lower leaflet region and mid leaflet region. The median calcium volumes in these latter regions were 119.20 (55.60–219.95) mm3 and 201.80 (91.40–318.20) mm3, respectively, which were larger than that in the annulus region (7.50 [0.70–33.30] mm3) and LVOT region (0 [0–1.30] mm3). The vertical distribution of calcification was similar in different morphologies, despite the fact that BiAV morphologies had more burden of calcium [Supplementary Table 2 and Figure 2].
Figure 2.
Calcium volume scores at different levels of the aortic root in aortic stenosis patients. (A) Volume scores in all patients. (B) Volume scores in patients with TriAV. (C) Volume scores in patients with BiAV. (D) Volume scores in patients with Sievers type-1 bicuspid aortic valve. (E) Volume scores in patients with Sievers type-0 bicuspid aortic valve. BiAV: Bicuspid aortic valve; LVOT: Left ventricular outflow tract; TriAV: Tricuspid aortic valve.
Fusion and leaflets thickening
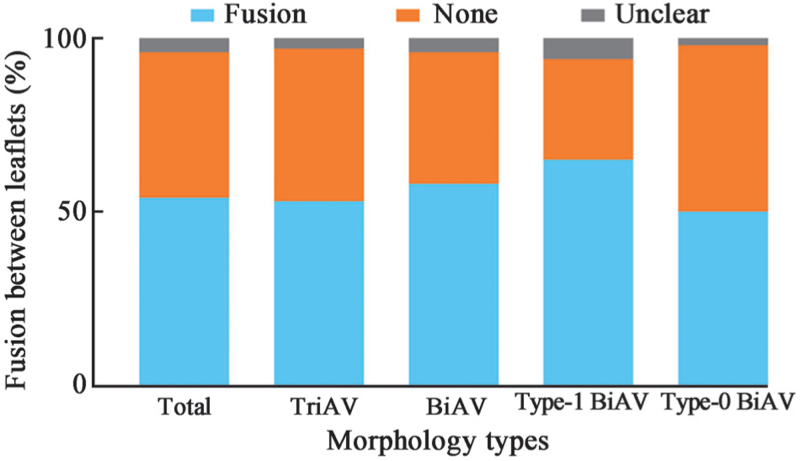
Fusion between leaflets were found in 160 (54.6%) patients. Among the 198 TriAV patients, 105 (53.0%) had fusion between leaflets. Moreover, 32 patients (65.3%) with type 1 BiAV morphology and 23 patients (50.0%) with type 0 BiAV had fusion between leaflets [Figure 3]. The TriAV patients had thinner leaflets when compared with BiAV morphologies (5.03 ± 2.83 mm vs. 6.78 ± 3.05 mm, P < 0.01) [Supplementary Figure 3].
Figure 3.
Fusion between leaflets of aortic stenosis patients. BiAV: Bicuspid aortic valve; TriAV: Tricuspid aortic valve; Type-1 BiAV: Sievers type-1 bicuspid aortic valve; Type-0 BiAV: Sievers type-0 bicuspid aortic valve.
Procedure
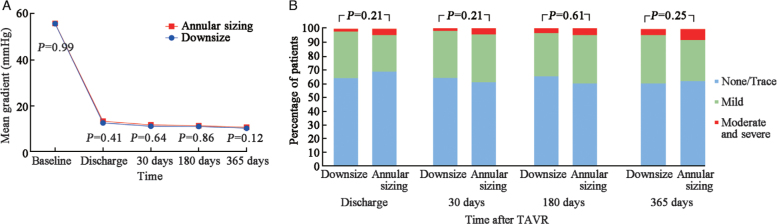
TAVR with self-expanding THV was performed in 293 patients. The overall device success rate was 82.9% (n = 243). Downsize strategy was used in 204 patients (69.6%) with a similar percentage of patients in TriAV and BiAV groups (132/198 [66.7%] vs. 72/95 [75.8], P = 0.11). Patients in the downsize group had poorer left ventricular ejection fraction (52.05 ± 7.96% vs. 57.83 ± 14.88%, P < 0.01), larger sinotubular junction (29.98 [27.28–32.50] mm vs. 28.40 [26.20–30.90] mm, P < 0.01) and larger ascending aorta dimension (39.15 [35.25–43.65] mm vs. 37.60 [34.50–40.60] mm, P = 0.03). The calcium volume was tended to be larger in the downsize group (456.05 [267.85–729.60] mm3vs. 379.00 [210.70–828.50] mm3, P = 0.64) [Table 1]. There was no statistical difference between the two sizing approaches neither in terms of technical success (annular sizing group vs. downsize group, 82.0% [73] vs. 83.3% [170], P = 0.79), nor in terms of death, perivalvular leakage (PVL), second valve implantation, or prosthetic aortic valve stenosis 30 days after the procedure [Table 2]. At 30 days after TAVR, the mean aortic-valve gradient was 11.28 mmHg in the downsize strategy group as compared to 11.88 mmHg in the annular sizing group (P = 0.64). The percentage of patients with moderate or severe paravalvular regurgitation did not differ significantly between the downsize strategy group and the annular sizing group (2.0% [4/204] vs. 4.5% [4/89], P = 0.21) at 30 days. These echocardiographic results were sustainable for one year [Figure 4]. The two sizing approaches achieved not statistically significant results in clinical and echographic results in TriAV and BiAV patients [Supplementary Table 3] as well as in all BiAV morphology types [Supplementary Table 4].
Table 2.
VARC endpoints of AS patients 30 days after receiving TAVR: annular sizing vs. downsize approaches.
| Items | Annular sizing (n = 89) | Downsize (n = 204) | P-value |
| Technical success | 73 (82.0) | 170 (83.3) | 0.79 |
| Device success | 64 (71.9) | 153 (75.0) | 0.58 |
| Death | 2 (2.3) | 4 (2.0) | 0.88 |
| Second valve implantation | 10 (11.2) | 23 (11.3) | 0.99 |
| PVL | 4 (4.5) | 4 (2.0) | 0.21 |
| New permanent pacemaker implantation | 9 (10.1) | 13 (6.4) | 0.27 |
| Repeat procedure | 7 (7.9) | 16 (7.8) | 0.99 |
| Major vascular complication | 2 (2.3) | 6 (2.9) | 0.73 |
| Prosthetic aortic valve stenosis | 4 (4.5) | 13 (6.4) | 0.52 |
Data are expressed as n (%). AS: Aortic Stenosis; PVL: Perivalvular leakage; TAVR: Transcatheter aortic valve replacement; VARC: Valve Academic Research Consortium.
Figure 4.
Echocardiographic findings of aortic stenosis patients. (A) Mean pressure gradients from baseline to one year for patients in annulus sizing and downsize groups. (B) Paravalvular aortic regurgitation incidence. The incidence of paravalvular aortic regurgitation in the downsize versus annulus sizing groups is shown at various follow-up time points. TAVR: Transcatheter aortic valve replacement.
Discussion
The main findings of this study can be summarized as follows: (1) Chinese TAVR patients have an extremely high frequency of BiAV morphology; (2) Chinese TAVR patients suffer from a heavy calcium burden, especially in BiAV morphology; (3) The calcium burden distributes mainly on the leaflets level instead of annular or LVOT level in Chinese patients; (4) The leaflets thickening and fusion are frequent in China; and (5) Downsize strategy was applied in most patients undergoing self-expanding TAVR procedure, providing a non-inferior device success rate and hemodynamic outcomes compared to annular sizing strategy in the overall cohort BiAV patients as well as in all patients with BiAV morphology types, up to 1-year follow-up.
In this cohort, among the 293 TAVR patients, bicuspid morphology was identified in 95 (32.4%) cases, which is higher than that reported in Western country registries.[9–11] Furthermore, the calcium volume of the aortic root in all the population in our study is 449.90 (243.15–782.15) mm3 based on a contrast scan with an 850 HU threshold for detection, which is much larger than the calcium volume reported in Western countries (ranged between 146 and 381 mm3).[18–20] Difference also exists in the distribution of calcification, and annular calcification was present in 78.8% and 81.2% of bicuspid and tricuspid patients in the Transcatheter Valve Therapy Registry.[11] In the present study, the calcium volume was more severe in BiAV patients and predominant on the leaflets region; annulus calcification was only present in 125/293 (42.7%) of the total population, 54/95 (56.8%) and 71/198 (35.9%) in bicuspid and tricuspid patients, respectively. The fusion between leaflets exists in nearly half of the population, and the leaflet thickening occurs in more than half of the patients which pushed the surgeon and interventional cardiologist to adopt the downsize strategy more frequently. A possible explanation for the difference in morphology type and thickening of the leaflets might be due to the etiology difference of native valve heart disease between China and Western countries. The AS was the most frequent valve disease (2152/5219 patients, 41.2%) in the EURObservational Research Programme Valvular Heart Disease II Survey, nearly 90% of those patients were caused by degenerative pathogenesis.[21] Nevertheless, AS was less frequent in the Chinese population (305/5983, 5.1%), and degeneration of the aortic valve causes only 69.2% of the above AS cases; while congenital and rheumatic heart disease cause 15.7% and 9.2% of moderate or greater AS, respectively.[22] Congenital heart disease, including bicuspid morphologies, was more common in the Chinese population, which could partially explain the high percentage of BiAV among Chinese TAVR patients. Nevertheless, patients included in the present study were relatively younger compared with Western country registries.
Chinese TAVR patients had an extremely high frequency of BiAV morphology combined with severe calcium burden, leaflets thickening, and fusion between leaflets. Although TAVR is globally performed in many BiAV patients, even in low surgical risk severe AS patients,[23,24] BiAV patients still encounter a relatively low device success rate when compared with TriAV patients, especially with regards to the need for second valve implantation and moderate or greater PVL.[11,25,26] Particularly in these registries, BiAV with calcified raphe and excess leaflet calcification were associated with increased risk of procedural complications and mid-term mortality.[23] Furthermore, the calcium volume of both aortic leaflets and device landing zones are highly associated with PVL.[18,27] Therefore, the distinctive aortic morphology and anatomy characteristics made it difficult to perform TAVR with annulus sizing among Chinese patients, which highlights the need to propose and validate a new sizing strategy.
Downsize strategy was based on supra-annular morphology and anatomy characteristics. Major interference between the implanted prosthesis and the aortic valve anatomy occurred at a level above the annulus, especially in patients with severe calcification at the leaflets level.[28] As prosthesis under expansion is constantly observed in BiAV, selecting a smaller THV size based on intercommissural distance and annular size could avoid excessive oversizing, and lead to prosthesis under expansion.[29] Therefore, up to 70% of the TAVR procedure in our institution was performed using downsize THV strategy, the device success rate of which did not differ between the two groups even the calcium burden tended to be heavier in the downsize group. Echocardiographic findings show that the aortic-valve mean gradient and the rate of patients with moderate or severe paravalvular regurgitation did not differ significantly between the two groups of patients until one year after the TAVR procedure. It is generally thought that using smaller size THVs might lead to a higher incidence of PVL or risk of embolization, but such an issue did not occur in the present study. We believe that although in the downsize strategy, it uses the super-annular plane as a stable landing zone, and the annular plane as the sealing area, which means the THVs reserve a smaller oversize percentage at the annular plane, which is why the PVL rate or risk of embolization didn’t increase in the downsize group. Also, with a smaller percentage of oversizing, the THVs might lead to a higher expansion rate of the frame, which guarantees the effective orifice area (EOA) of THVs.
Due to the heavy calcium burden and fusion between leaflets, downsize strategy was performed in the majority of TAVR patients in the present study, even in TriAV patients. The success rates of the procedure and clinical outcomes of downsize group were similar in TriAV and BiAV. Although bicuspid morphology was thought to be the main reason for THVs downsize,[29] but heavy calcium burden, leaflets fusion, and thickening will occupy the space of the sinus, especially those “functional” bicuspid aortic valves, which lead to an under expansion of the THV frame like BiAV morphology. Therefore, TriAV patients with heavy calcium burden and fusion between leaflets will also benefit from downsize strategy.
TAVR procedures in this cohort were performed using the first generation THVs with no retrievable function, this explains in part the relatively low procedural success rate.
There are some limitations of this study. It is a single-center study, however, as the national cardiovascular center in China, the patients came from all around the country; therefore, our results could partially represent the real landscape of Chinese AS patients. The Hounsfield unit cutoffs for calcium assessment on contrast multi-detector computed tomography (MDCT) have not been well established and attenuation can vary significantly across scans. Nonetheless, 850-HU was one of the acceptable thresholds for detection of the calcium volume in contrast to CT scans. All patients included in the present study had followed the standard MDCT scan protocol, with a strict image quality evaluation. This should mitigate the differences among patients. All the THVs used in the present study were first-generation devices.
In conclusion, the CT assessment of Chinese TAVR patients shows a high frequency of BiAV morphology. Chinese AS patients had a severe calcium burden at the aortic root, especially in BiAV morphology. The calcium is mostly distributed on the leaflets instead of the annular level. The severe leaflet thickening and fusion between leaflets were frequent. In this setting, downsize strategy was more applied by Chinese physicians, providing a non-inferior device success rate and prosthesis hemodynamic performance when compared with the annular sizing strategy in self-expanding TAVR procedures.
Funding
This study was funded by the National Key Research & Development Program of China (No. 2020YFC2008100) and the Project of Capital Clinical Treatment Technology Research and Translation Application (No. Z201100005520068).
Conflicts of interest
Dr. Walid Ben Ali has received a research grant from Medtronic and Edward LifeSciences. Dr. Thomas Modine serves as a consultant to Abbott, Boston Scientific, Edwards Lifesciences, GE, Medtronic, and MicroPort.
Supplementary Material
Footnotes
How to cite this article: Niu G, Ali WB, Wang M, Jilaihawi H, Zhang H, Zhang Q, Ye Y, Liu X, Yao J, Zhao Q, Wang Y, Zhou Z, Zhang L, Ren X, An Y, Lu B, Modine T, Wu Y, Song G. Anatomical morphology of the aortic valve in Chinese aortic stenosis patients and clinical results after downsize strategy of transcatheter aortic valve replacement. Chin Med J 2022;135:2968–2975. doi: 10.1097/CM9.0000000000002517
Supplemental digital content is available for this article.
References
- 1.Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 2.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 3.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 4.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017; 376:1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 5.Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016; 387:2218–2225. doi: 10.1016/s0140-6736(16)30073-3. [DOI] [PubMed] [Google Scholar]
- 6.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016; 374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 7.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019; 380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 8.Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019; 380:1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 9.Zahn R, Werner N, Gerckens U, Linke A, Sievert H, Kahlert P, et al. Five-year follow-up after transcatheter aortic valve implantation for symptomatic aortic stenosis. Heart 2017;103:1970–1976. doi: 10.1136/heartjnl-2016-311004. [DOI] [PubMed] [Google Scholar]
- 10.Kochman J, Huczek Z, Scislo P, Dabrowski M, Chmielak Z, Szymanski P, et al. Comparison of one- and 12-month outcomes of transcatheter aortic valve replacement in patients with severely stenotic bicuspid versus tricuspid aortic valves (results from a multicenter registry). Am J Cardiol 2014; 114:757–762. doi: 10.1016/j.amjcard.2014.05.063. [DOI] [PubMed] [Google Scholar]
- 11.Halim SA, Edwards FH, Dai D, Li Z, Mack MJ, Holmes DR, et al. Outcomes of transcatheter aortic valve replacement in patients with bicuspid aortic valve disease: a report from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation 2020; 141:1071–1079. doi: 10.1161/CIRCULATIONAHA.119.040333. [DOI] [PubMed] [Google Scholar]
- 12.Song G, Jilaihawi H, Wang M, Chen M, Wang J, Wang W, et al. Severe symptomatic bicuspid and tricuspid aortic stenosis in China: characteristics and outcomes of transcatheter aortic valve replacement with the venus-A valve. Struct Heart 2018; 2:60–68. doi: 10.1080/24748706.2017.1398437. [Google Scholar]
- 13.Zhao ZG, Feng Y, Liao YB, Li YJ, Xiong TY, Ou YW, et al. Reshaping bicuspid aortic valve stenosis with an hourglass-shaped balloon for transcatheter aortic valve replacement: a pilot study. Catheter Cardiovasc Interv 2020; 95: (Suppl 1): 616–623. doi: 10.1002/ccd.28726. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, He Y, Zhu Q, Gao F, He W, Yu L, et al. Supra-annular structure assessment for self-expanding transcatheter heart valve size selection in patients with bicuspid aortic valve. Catheter Cardiovasc Interv 2018; 91:986–994. doi: 10.1002/ccd.27467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007; 133:1226–1233. doi: 10.1016/j.jtcvs.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 16.Blanke P, Weir-McCall JR, Achenbach S, Delgado V, Hausleiter J, Jilaihawi H, et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR): an expert consensus document of the society of cardiovascular computed tomography. JACC Cardiovasc Imaging 2019; 12:1–24. doi: 10.1016/j.jcmg.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 17.VARC-3 WRITING COMMITTEE, Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, et al. Valve academic research consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J 2021; 42:1825–1857. doi: 10.1093/eurheartj/ehaa799. [DOI] [PubMed] [Google Scholar]
- 18.Jilaihawi H, Makkar RR, Kashif M, Okuyama K, Chakravarty T, Shiota T, et al. A revised methodology for aortic-valvar complex calcium quantification for transcatheter aortic valve implantation. Eur Heart J Cardiovasc Imaging 2014; 15:1324–1332. doi: 10.1093/ehjci/jeu162. [DOI] [PubMed] [Google Scholar]
- 19.Bettinger N, Khalique OK, Krepp JM, Hamid NB, Bae DJ, Pulerwitz TC, et al. Practical determination of aortic valve calcium volume score on contrast-enhanced computed tomography prior to transcatheter aortic valve replacement and impact on paravalvular regurgitation: Elucidating optimal threshold cutoffs. J Cardiovasc Comput Tomogr 2017; 11:302–308. doi: 10.1016/j.jcct.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Kim WK, Renker M, Rolf A, Liebetrau C, Van Linden A, Arsalan M, et al. Accuracy of device landing zone calcium volume measurement with contrast-enhanced multidetector computed tomography. Int J Cardiol 2018; 263:171–176. doi: 10.1016/j.ijcard.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 21.Iung B, Delgado V, Rosenhek R, Price S, Prendergast B, Wendler O, et al. Contemporary presentation and management of valvular heart disease: The EURObservational Research Programme Valvular Heart Disease II Survey. Circulation 2019; 140:1156–1169. doi: 10.1161/circulationaha.119.041080. [DOI] [PubMed] [Google Scholar]
- 22.Zhang B, Xu H, Zhang H, Liu Q, Ye Y, Hao J, et al. Prognostic value of N-terminal pro-B-type natriuretic peptide in elderly patients with valvular heart disease. J Am Coll Cardiol 2020; 75:1659–1672. doi: 10.1016/j.jacc.2020.02.031. [DOI] [PubMed] [Google Scholar]
- 23.Yoon SH, Kim WK, Dhoble A, Milhorini Pio S, Babaliaros V, Jilaihawi H, et al. Bicuspid aortic valve morphology and outcomes after transcatheter aortic valve replacement. J Am Coll Cardiol 2020; 76:1018–1030. doi: 10.1016/j.jacc.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Forrest JK, Ramlawi B, Deeb GM, Zahr F, Song HK, Kleiman NS, et al. Transcatheter aortic valve replacement in low-risk patients with bicuspid aortic valve stenosis. JAMA Cardiol 2021; 6:50–57. doi: 10.1001/jamacardio.2020.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blackman DJ, Van Gils L, Bleiziffer S, Gerckens U, Petronio AS, Abdel-Wahab M, et al. Clinical outcomes of the Lotus Valve in patients with bicuspid aortic valve stenosis: an analysis from the RESPOND study. Catheter Cardiovasc Interv 2019; 93:1116–1123. doi: 10.1002/ccd.28120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Biase C, Mastrokostopoulos A, Philippart R, Desroche LM, Blanco S, Rehal K, et al. Aortic valve anatomy and outcomes after transcatheter aortic valve implantation in bicuspid aortic valves. Int J Cardiol 2018; 266:56–60. doi: 10.1016/j.ijcard.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 27.John D, Buellesfeld L, Yuecel S, Mueller R, Latsios G, Beucher H, et al. Correlation of device landing zone calcification and acute procedural success in patients undergoing transcatheter aortic valve implantations with the self-expanding CoreValve prosthesis. JACC Cardiovasc Interv 2010; 3:233–243. doi: 10.1016/j.jcin.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Xiong TY, Li YJ, Feng Y, Liao YB, Zhao ZG, Mylotte D, et al. Understanding the interaction between transcatheter aortic valve prostheses and supra-annular structures from post-implant stent geometry. JACC Cardiovasc Interv 2019; 12:1164–1171. doi: 10.1016/j.jcin.2019.02.051. [DOI] [PubMed] [Google Scholar]
- 29.Tchetche D, de Biase C, van Gils L, Parma R, Ochala A, Lefevre T, et al. Bicuspid aortic valve anatomy and relationship with devices: the BAVARD Multicenter Registry. Circ Cardiovasc Interv 2019; 12:e007107.doi: 10.1161/circinterventions.118.007107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.