Abstract
Background:
Data on the immunogenicity and safety of heterologous immunization schedules are inconsistent. This study aimed to evaluate the immunogenicity and safety of homologous and heterologous immunization schedules.
Methods:
Multiple databases with relevant studies were searched with an end date of October 31, 2021, and a website including a series of Coronavirus disease 2019 studies was examined for studies before March 31, 2022. Randomized controlled trials (RCTs) that compared different heterologous and homologous regimens among adults that reported immunogenicity and safety outcomes were reviewed. Primary outcomes included neutralizing antibodies against the original strain and serious adverse events (SAEs). A network meta-analysis (NMA) was conducted using a random-effects model.
Results:
In all, 11 RCTs were included in the systematic review, and nine were ultimately included in the NMA. Among participants who received two doses of CoronaVac, another dose of mRNA or a non-replicating viral vector vaccine resulted in a significantly higher level of neutralizing antibody than a third CoronaVac 600 sino unit (SU); a dose of BNT162b2 induced the highest geometric mean ratio (GMR) of 15.24, 95% confidence interval [CI]: 9.53–24.39. Following one dose of BNT162b2 vaccination, a dose of mRNA-1273 generated a significantly higher level of neutralizing antibody than BNT162b2 alone (GMR = 1.32; 95% CI: 1.06–1.64), NVX-CoV2373 (GMR = 1.60; 95% CI: 1.16–2.21), or ChAdOx1 (GMR = 1.80; 95% CI: 1.25–2.59). Following one dose of ChAdOx1, a dose of mRNA-1273 was also more effective for improving antibody levels than ChAdOx1 (GMR = 11.09; 95% CI: 8.36–14.71) or NVX-CoV2373 (GMR = 2.87; 95% CI: 1.08–3.91). No significant difference in the risk for SAEs was found in any comparisons.
Conclusions:
Relative to vaccination with two doses of CoronaVac, a dose of BNT162b2 as a booster substantially enhances immunogenicity reactions and has a relatively acceptable risk for SAEs relative to other vaccines. For primary vaccination, schedules including mRNA vaccines induce a greater immune response. However, the comparatively higher risk for local and systemic adverse events introduced by mRNA vaccines should be noted.
Registration:
PROSPERO; https://www.crd.york.ac.uk/PROSPERO/; No. CRD42021278149.
Keywords: COVID-19; 2019-nCoV vaccine mRNA-1273; BNT162 vaccine; Vaccination; Immunization schedule; Antibodies, Neutralizing; Heterologous; Immunogenicity; Network meta-analysis
Introduction
As of April 18, 2022, more than 504 million people worldwide have been infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for Coronavirus disease 2019 (COVID-19), leading to more than 6.1 million deaths.[1] Cases continue to surge as the reach of the Delta and Omicron variants becomes increasingly global.[2,3] Safe and effective vaccines are critical to preventing infection and to slowing the transmission of COVID-19. The most widely used vaccines are of the mRNA, viral vector, and inactivated types.[4] As a result of waning immunity after priming doses of vaccines and some evidence of reduced effectiveness, policymakers are considering offering booster doses.[5–7]
Manufacturing issues, shortages of raw materials, and new waves of infection have led to supply chain disruptions and inequity in vaccine distribution across regions.[8–10] In addition, age restrictions for the ChAdOx1 n-CoV-19 vaccine (ChAd; AstraZeneca, NY, USA) following the emergence of vaccine-induced thrombocytopenia and recent pauses in immunization of young people with mRNA vaccines due to concerns over myocarditis have caused changes in vaccine deployment policy and have disrupted roll-out plans.[11,12]
Heterologous prime-boost schedules have been proposed to enhance deployment flexibility and improve access to vaccines globally.[13] According to the interim guidance of the World Health Organization (WHO), heterologous COVID-19 vaccine schedules, combining multiple vaccine platforms (eg, a vectored vaccine followed by an mRNA vaccine) and combining different products from the same vaccine platform (eg, BNT162b2 followed by mRNA-1273), apply to all COVID-19 vaccines that have received a WHO emergency use listing (EUL).[14–18] However, data on the immunogenicity of heterologous schedules are inconsistent.[19,20] Although several reports have suggested more short-term reactogenicity for heterologous prime-boost schedules,[15,21] not all studies have supported this distinction.[22] Meta-analyses have largely been pairwise meta-analyses that compare two schedules that are unable to provide evidence on comparative immunogenicity and safety between multiple heterologous schedules.[23,24] Network meta-analysis (NMA) can synthesize data using indirect comparisons to provide potentially more comprehensive evidence.
Thus, we conducted a systematic review and NMA of randomized controlled trials (RCTs) to evaluate the immunogenicity and safety of homologous and heterologous immunization schedules.
Methods
We performed a systematic review and NMA according to Preferred Reporting Items of Systematic Review and Meta-analysis.[25] The protocol of this study was registered with PROSPERO (registration No: CRD42021278149).
Search strategy and study selection
We systematically searched six databases, namely, PubMed, Embase, Scopus, Web of Science, Cochrane Library, and the China National Knowledge Infrastructure. We used “COVID-19”, “SARS-2”, “vaccine”, and other relevant words and phrases as search terms [Supplementary Table 1]. The systematic search was carried out on October 31, 2021. After this, we monitored covid-nma.com[26] for the publication of new studies until March 31, 2022.
Records identified from electronic databases were independently screened by two groups of investigators (XYT and YMT; WWW and YJM). Divergences were resolved by discussion or consultation with a senior investigator (FS). The studies were divided according to the following criteria: (1) RCTs, (2) studies focusing on adults (≥18 years), (3) studies comparing heterologous vaccination regimens to each other or comparing heterologous regimens to homologous regimens, (4) those reporting immunogenicity outcomes and safety outcomes, and (5) both peer-reviewed articles and preprints. All of these were considered for inclusion. Reviews, letters, post hoc analyses, and conference abstracts were excluded.
Data extraction and quality assessment
The extracted data included basic information on the studies (publication year, first author, study region, and study phase), participant characteristics (percentage of males and mean age), immunization schedules, and outcome measurements. We were interested in immunogenicity and safety. The primary outcomes included the level of neutralizing antibodies against the original strain and serious adverse events (SAEs) following the injection of the last dose. Secondary immunogenicity outcomes included the levels of receptor-binding domain (RBD) antibody, trimetric spike protein (S-protein) antibody, and interferon-γ. We also extracted measurements of neutralizing antibodies against Delta variant strains where available, as effectiveness against variant strains is crucial for a rapidly mutating virus.[27] All of the immunogenicity outcomes were measured between 14 and 28 days after the last dose, and the data from the latest time point in the observation window was used to conduct quantitative analysis. To identify secondary safety outcomes, we extracted both systemic and local adverse events (AEs). The numbers of AEs within the first 7 days after the last dose and the sample size of observations were extracted. We only conducted NMA on the most commonly reported AEs. Data from graphics in papers were extracted using GetData Graph Digitizer version 2.25.0.32 (GetData, Sydney, NSW, Australia). The data extraction for each article was performed by two investigators (PL and WWW) independently and cross-checked by a third (YJL); inconsistencies were resolved by the third investigator by going through the text again.
The risk of bias within individual studies was assessed using the Cochrane Risk of Bias tool (RoB 2.0),[28] which evaluates RCTs from six aspects, including the randomization process, deviation from intended interventions, missing outcome data, measurement of the outcome, selection of reported results, and overall bias. The quality assessment was carried out by two reviewers (PL and WWW) independently, and discrepancies were solved by discussion or consultation with a senior investigator (FS). The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process was used to rate the quality of evidence for any significant result regarding primary outcomes in the NMA.[29]
Statistical analysis
Immunogenicity outcomes were measured on a geometric scale and were transformed into a base-10 logarithmic scale. Missing means and standard deviations were estimated from the sample size, median, and interquartile range.[30] NMA was performed using the frequentist method. We calculated the mean difference and 95% confidence interval (CI) of log-scaled geometric mean titers (GMTs) and then exponentiated it to obtain the geometric mean ratio (GMR) and its 95% CI. Relative risks and 95% CIs were calculated to compare the AEs of different vaccine regimens. Heterogeneity among studies and global inconsistencies were assessed using a generalized Q test. A random-effects model was used to estimate the effect size. The probability of being at each possible ranking was estimated for all vaccines. The hierarchy of treatments was reported as the surface under the cumulative ranking curve and was summarized using a rank heat plot.[31]
Significance was set to P < 0.05, two-tailed. The statistical analyses were conducted using R 4.1.1, and NMA was performed with the netmeta package.
Results
Characteristics of included studies
We identified 17,308 records from databases and retrieved 63 more by screening the reference lists of reviews. Four studies were obtained from covid-nma.com. After screening the title, abstract, and full text, 11 studies were included in our systematic review, nine of which were used for NMA [Supplementary Figure 1]. Among the included studies, only two showed a low risk of bias, and the remaining nine were of some concern. Detailed information on the quality assessment of each domain is listed in [Supplementary Table 2]. Most of these studies were multi-centered (n = 8). They were conducted among adults in Europe (n = 6), Asia (n = 4), and South America (n = 1). The vaccination regimens involved combinations of CoronaVac (Sinovac, Beijing, China), ChAdOx1, BNT162b2 (Pfizer, NY, USA), Ad26.COV2.S (Janssen, New Brunswick, USA), mRNA-1273 (Moderna, Cambridge, USA), NVX-CoV2373 (Novavax, Gaithersburg, USA), Convidecia (CanSinoBio, Tianjin, China), and VLA2001 (Valneva, Saint Herblain, France). Detailed information on the included studies is given in [Table 1]. Comparison of the studies produced two separate evidence networks, one of which was constructed with schedules initiated with one or two doses of CoronaVac and the other of which was constructed with schedules initiated with one dose of BNT162b2, ChAdOx1, or mRNA-1273.
Table 1.
Characteristics of included studies.
| Studies | Registration number | Region | Phase | Multi/single-center | Sample size | Mean age (years) | Percentage of males | Arms | Days apart of the last dose (weeks) |
| Borobia et al[16] | NCT04860739 | Europe | Phase 2 | Multi-center | 676 | 44.0 | 43.0 | 1 dose of ChAdOx1 + BNT162b2 30 μg/1 dose of ChAdOx1 | 8–10 |
| Li et al[32] | NCT04892459 | Asia | Phase 4 | Unclear | 299 | 44.5 | 56.6 | 1 dose of CoronaVac + Convidecia 5 × 1010 viral particles/1 dose of CoronaVac + CoronaVac 600 SU/2 doses of CoronaVac + Convidecia 5 × 1010 viral particles/2 doses of CoronaVac + CoronaVac 600 SU | 4–24 |
| Liu et al[14] | ISRCTN69254139 | Europe | Phase 2 | Multi-center | 426 | 58.0 | 53.8 | 1 dose of ChAdOx1 + BNT162b2 30 μg/1 dose of ChAdOx1 + ChAdOx1 5 × 1010 viral particles/1 dose of BNT162b2 + BNT162b2 30 μg/1 dose of BNT162b2 + ChAdOx1 5 × 1010 viral particles | 4 |
| Sablerolles et al[33] | NCT04927936 | Europe | Phase 3 | Multi-center | 461 | 40.5 | 35.0 | 1 dose of Ad26.COV2.S + Ad26.COV2.S/1 dose of Ad26.COV2.S + mRNA-1273/1 dose of Ad26.COV2.S + BNT162b2/1 dose of Ad26.COV2.S | 12 |
| Munro et al[34] | ISRCTN73765130 | Europe | Phase 2 | Single-center | 2557 | 63.3 | 49.4 | 2 doses of ChAdOx1/2 doses of ChAdOx1 + ChAdOx1 5 × 1010 viral particles/2 doses of ChAdOx1 + NVX-CoV2373 5 μg/2 doses of ChAdOx1 + NVX-CoV2373 2.5 μg/2 doses of ChAdOx1 + BNT162b2 30 μg/2 doses of ChAdOx1 + VLA2001 33 AU/2 doses of ChAdOx1 + VLA2001 16.5 AU/2 doses of ChAdOx1 + BNT162b2 15 μg/2 doses of ChAdOx1 + mRNA-1273 100 μg/2 doses of ChAdOx1 + CVnCoV 12 μg/2 doses of BNT162b2/2 doses of BNT162b2 + BNT162b2 15 μg/2 doses of BNT162b2 + mRNA-1273 100 μg/2 doses of BNT162b2 + CVnCoV 12 μg/2 doses of BNT162b2 + ChAdOx1 5 × 1010 viral particles/2 doses of BNT162b2 + NVX-CoV2373 5 μg/2 doses of BNT162b2 + NVX-CoV2373 2.5 μg/2 doses of BNT162b2 + BNT162b2 30 μg/2 doses of BNT162b2 + VLA2001 33 AU/2 doses of BNT162b2 + VLA2001 16.5 AU | 12 |
| Stuart et al[35] | ISRCTN27841311 | Europe | Phase 2 | Multi-center | 1072 | 62.6 | 57.9 | 1 dose of ChAdOx1 + ChAdOx1 5 × 1010 viral particles/1 dose of ChAdOx1 + mRNA-1273 100 μg/1 dose of ChAdOx1 + NVX-CoV2373 5 μg/1 dose of BNT162b2 + BNT162b2 30 μg/1 dose of BNT162b2 + mRNA-1273 100 μg/1 dose of BNT162b2 + NVX-CoV2373 5 μg | 8–12 |
| Mok et al[36] | NCT04611243 | Asia | Phase 4 | Multi-center | 80 | 51.4 | 26 | 2 doses of CoronaVac + CoronaVac 600 SU/2 doses of CoronaVac + BNT162b2 30 μg | 4 |
| Costa Clemens et al[37] | RBR–9nn3scw | South America | Phase 4 | Multi-center | 1240 | 59.6 | 39.5 | 2 doses of CoronaVac + Ad26.COV2.S 5 × 1010 viral particles/2 doses of CoronaVac + ChAdOx1 5 × 1010 viral particles/2 doses of CoronaVac + BNT162b2 30 μg/2 doses of CoronaVac + CoronaVac 600 SU | 22–24 |
| Intapiboon et al[38] | TCTR20211004001 | Asia | Phase 1 | Single-center | 91 | 39.9 | 44.0 | 2 doses of CoronaVac + BNT162b2 30 μg/2 doses of CoronaVac + BNT162b2 15 μg/2 doses of CoronaVac + BNT162b2 1/5 dose intradermally | >8 |
| Janssen et al[39] | NCT04900467 | Europe | Unclear | Multi-center | 399 | 40.3 | 58.2 | 1 dose of BNT162b2 + BNT162b2 30 μg/1 dose of BNT162b2 + mRNA-1273 100 μg/1 dose of mRNA-1273 + BNT162b2 30 μg/1 dose of mRNA-1273 + mRNA-1273 100 μg | 4–7 |
| Nanthapisal et al[40] | TCTR20210722003 | Asia | Phase 2 | Multi-center | 422 | 44.0 | 47.9 | 2 doses of CoronaVac + ChAdOx1 5 × 1010 viral particles/2 doses of CoronaVac + ChAdOx1 2.5 × 1010 viral particles | >8 |
AU: Antigen units; SU: Sino units.
Schedules initiated with CoronaVac
Five studies were included in this network, comparing eight booster vaccination regimens and two primary vaccination regimens. In all, 2132 participants were recruited in these studies, of which 43.7% were males, with a mean age of 53.5 years.
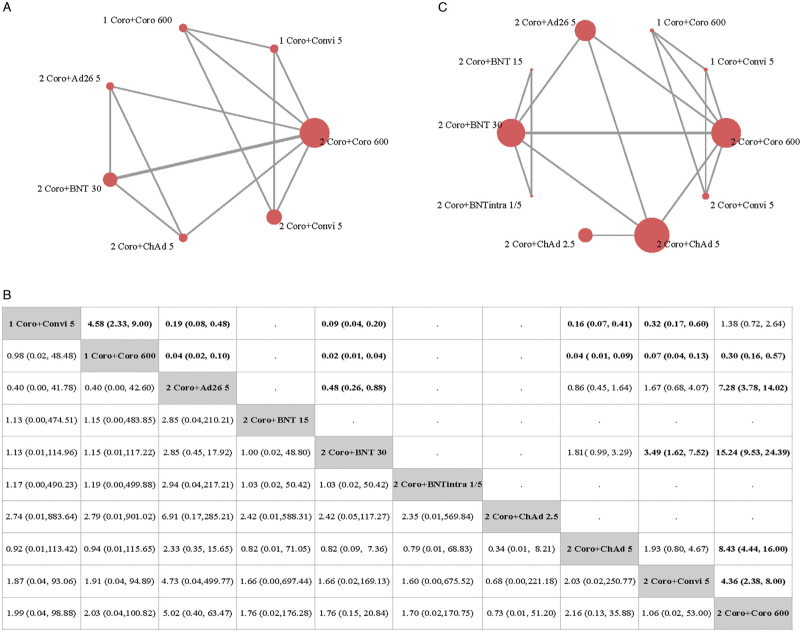
Only three studies reported the GMT of the neutralizing antibody against the original strain, incorporating seven regimens [Figure 1A]. Among participants who had received two doses of CoronaVac, another dose of mRNA or non-replicating viral vector vaccines resulted in a significantly higher level of neutralizing antibody. A following dose of BNT162b2 induced the highest GMR of 15.24 (95% CI: 9.53–24.39). Three-dose heterologous schedules induced a significantly higher level of neutralizing antibody against the original strain than two-dose schedules [Figure 1B].
Figure 1.
Results from NMA of primary outcomes of immunization schedules initiated with CoronaVac. (A) Evidence network of neutralizing antibody against an original strain. (B) Evidence network of SAEs. The figure in each cell in the upper area refers to the OR of the schedule in the row against the schedule in the column, and the figure in each cell in the lower area refers to the OR of the schedule in the column against the schedule in the row. If no relevant information on SAEs or neutralizing antibody is reported in a comparison, then a dot will be used to fill the cell in the corresponding position. (C) GMR and 95% CI of comparisons (the results for SAEs are presented in the lower area, and the results for neutralizing antibodies are presented in the upper area). 1 Coro + Convi 5: one dose of CoronaVac followed by Convidecia 5 × 1010 viral particles; 1 Coro + Coro 600: one dose of CoronaVac followed by CoronaVac 600 SU; 2 Coro + Ad26 5: two doses of CoronaVac followed by Ad26.COV2.S 5 × 1010 viral particles; 2 Coro + BNT 15: two doses of CoronaVac followed by BNT162b2 15 μg; 2 Coro + BNT 30: two doses of CoronaVac followed by BNT162b2 30 μg; 2 Coro + BNTintra 1/5: two doses of CoronaVac followed by BNT162b2 injected intradermally with 1/5 dose (6 μg); 2 Coro + ChAd 2.5: two doses of CoronaVac followed by ChAdOx1 2.5 × 1010 viral particles; 2 Coro + ChAd 5: two doses of CoronaVac followed by ChAdOx1 5 × 1010 viral particles; 2 Coro + Convi 5: two doses of CoronaVac followed by Convidecia 5 μg; 2 Coro + Coro 600: two doses of CoronaVac followed by CoronaVac 600 SU. CI: Confidence interval; GMR: Geometric mean ratio; NMA: Network meta-analysis; SAEs: Serious adverse events; SU: Sino unit.
Four studies reported the GMT of the RBD antibody. Heterologous schedules significantly enhanced the RBD antibody response, compared to homologous schedules [Supplementary Figure 2]. The measurement of S-protein antibody was only reported by one study, indicating that after two doses of CoronaVac, an additional dose of BNT162b2 caused the highest S-protein antibody level, compared to ChAdOx1, Ad26.COV2.S, and CoronaVac.
Three studies reported the GMT of neutralizing antibodies against the Delta strain. Heterologous schedules were more sufficient for increasing the level of neutralizing antibody against the Delta strain [Supplementary Figure 2]. Only one study reported a T-cell response after boosting, and it suggested that for participants who received two doses of CoronaVac, another dose of ChAdOx1 effectively stimulated cellular immune response. Detailed data on immunogenicity outcomes of individual studies are given in Supplementary Table 2.
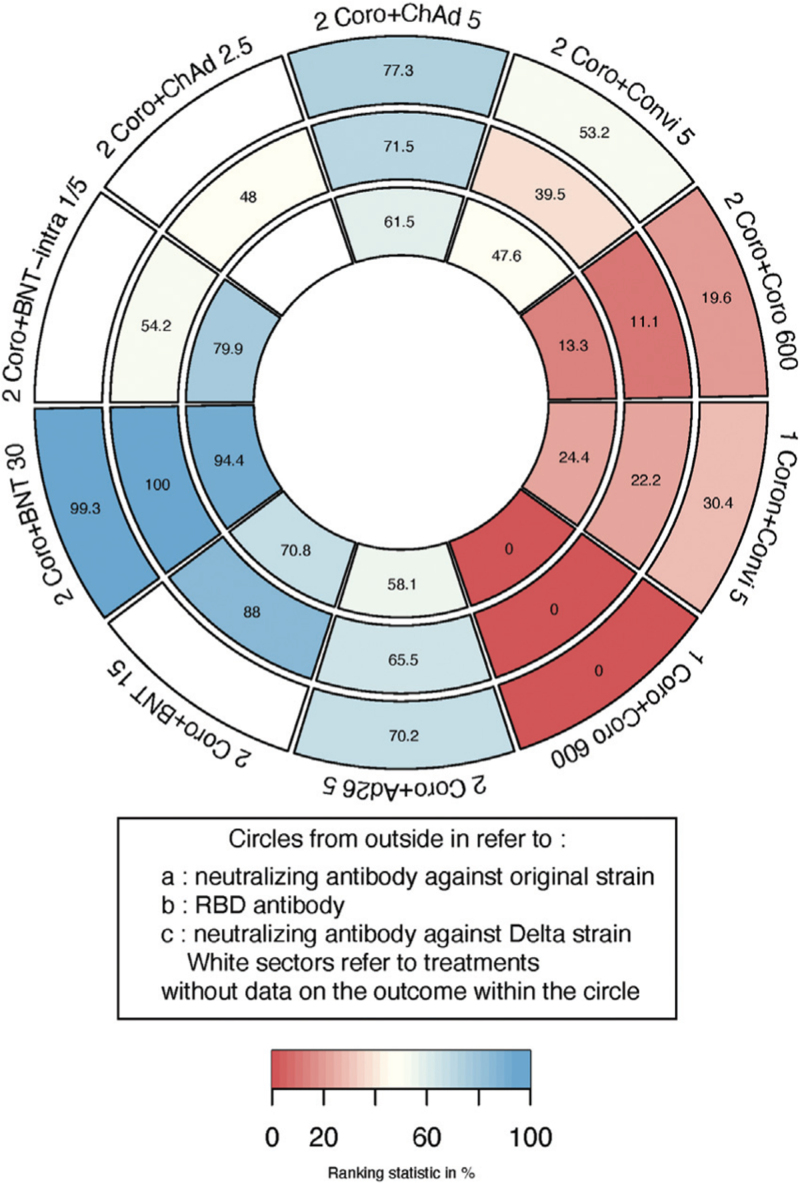
Another dose of BNT162b2 30 μg following two doses of CoronaVac took first place in the ranking of the three outcomes of interest, while two-dose schedules and the homologous three-dose schedules did not rank highly [Figure 2].
Figure 2.
Ranking heat plot of schedules initiated with CoronaVac for immunogenicity outcomes: a larger figure in a given sector means a higher probability of greater immune response in that sector. Each sector is colored according to the SUCRA value of the corresponding treatment from red (0%) to blue (100%). 1 Coro + Convi 5: one dose of CoronaVac followed by Convidecia 5 × 1010 viral particles; 1 Coro + Coro 600: one dose of CoronaVac followed by CoronaVac 600 SU; 2 Coro + Ad26 5: two doses of CoronaVac followed by Ad26.COV2.S 5 × 1010 viral particles; 2 Coro + BNT 15: two doses of CoronaVac followed by BNT162b2 15 μg; 2 Coro + BNT 30: two doses of CoronaVac followed by BNT162b2 30 μg; 2 Coro + BNTintra 1/5: two doses of CoronaVac followed by BNT162b2 injected intradermally with 1/5 dose (6 μg); 2 Coro + ChAd 2.5: two doses of CoronaVac followed by ChAdOx1 2.5 × 1010 viral particles; 2 Coro + ChAd 5: two doses of CoronaVac followed by ChAdOx1 5 × 1010 viral particles; 2 Coro + Convi 5: two doses of CoronaVac followed by Convidecia 5 μg; 2 Coro + Coro 600: two doses of CoronaVac followed by CoronaVac 600 SU. SUCRA: Surface under the cumulative ranking curve; SU: Sino unit.
Data from SAEs were extracted from all five studies and for all ten schedules [Figure 1C]. In all, three SAEs were reported for two doses of CoronaVac + Ad26.COV2.S 5 × 1010 viral particles, three for two doses of CoronaVac + BNT162b2 30 μg, and two for two doses of CoronaVac + ChAdOx1 5 × 1010 viral particles. No SAEs were reported for the other regimens. The result of NMA showed that no statistically significant differences were found in any comparison [Figure 1B].
Three local AEs and five systemic AEs that were reported frequently were assessed, namely, pain at the injection site, erythema, swelling, headache, fever, fatigue, myalgia, and arthralgia. The number of people with each AE is presented in Supplementary 3. Heterologous schedules were generally related to a higher risk for local AEs. However, a third dose of ChAdOx1 2.5 × 1010 viral particles after two doses of CoronaVac was not significantly related to a higher risk for any of the local AEs of interest [Supplementary Figure 3]. For systemic AEs, nearly all of the heterologous schedules were associated with a higher risk for fatigue. Three-dose schedules did not significantly increase the risk for systemic AEs relative to two-dose schedules, whether homologous or heterologous [Supplementary Figure 4]. Three doses of CoronaVac took first place in the ranking for most safety outcomes; meanwhile, administration of the third dose of BNT162b2 30 μg and the third dose of ChAdOx1 2.5 × 1010 viral particles following two doses of CoronaVac also showed a fair performance [Supplementary Figure 5].
We did not detect significant between-study heterogeneity or between-design inconsistency (P > 0.05) for neutralizing antibodies against the original strain and safety outcomes. We addressed moderate confidence in three comparisons (two doses of CoronaVac followed by Ad26.COV2.S 5 ×1010 viral particles vs. two doses of CoronaVac followed by CoronaVac 600 SU; two doses of CoronaVac followed by BNT162b2 30 mg vs. two doses of CoronaVac followed by CoronaVac 600 SU; two doses of CoronaVac followed by ChAdOx1 5×1010 viral particles vs. two doses of CoronaVac followed by CoronaVac 600 SU) according to the GRADE. All other comparisons were considered to have low or very low confidence [Supplementary Table 3].
Schedules initiated with mRNA or non-replicating viral vector vaccines
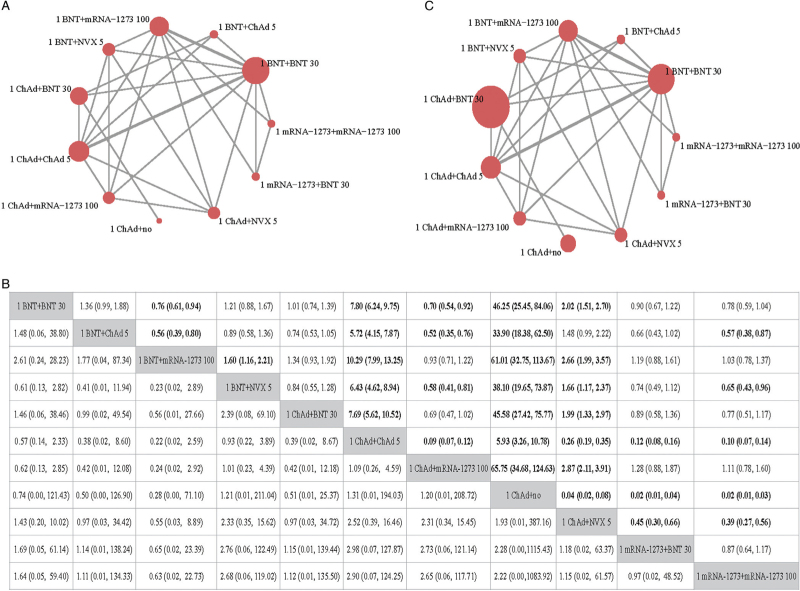
Four studies were included in this network, comparing 11 schedules [Figure 3A]. All of the schedules included in this network were primary vaccination regimens consisting of two doses of vaccines. The participants in this network received one dose of BNT162b2, ChAdOx1, or mRNA1273. In total, 2573 participants were enrolled in the studies, and 53.6% of them were males. The mean age was 53.5 years.
Figure 3.
Results from NMA of primary outcomes of schedules initiated with mRNA or non-replicating viral vector. (A) Evidence network of the neutralizing antibody against an original strain. (B) Evidence network of SAEs. The figure in each cell in the upper area refers to the OR of the schedule in the row against the schedule in the column, and the figure in each cell in the lower area refers to the OR of the schedule in the column against the schedule in the row. If no relevant information on SAEs or neutralizing antibody is reported in a comparison, the a dot will be used to fill the cell in the corresponding position. (C) GMR and 95% CI of comparisons (the results for SAEs are presented in the lower area, and the results for neutralizing antibodies are presented in the upper area). 1 ChAd + NVX 5: one dose of ChAdOx1 followed by NVX-CoV2373 5 μg; 1 mRNA-1273 + BNT 30: one dose of mRNA-1273 followed by BNT162b2 30 μg; 1 mRNA-1273 + mRNA-1273 100: one dose of mRNA-1273 followed by mRNA-1273 100 μg; 1 BNT + BNT 30: one dose of BNT162b2 followed by BNT162b1 30 μg; 1 BNT + ChAd 5: one dose of BNT162b2 followed by ChAdOx1 5 × 1010 viral particles; 1 BNT + mRNA-1273 100: one dose of BNT162b2 followed by mRNA-1273 100 μg; 1 BNT + NVX 5: one dose of BNT162b2 followed by NVX-CoV2373 5 μg; 1 ChAd + BNT 30: one dose of ChAdOx1 followed by BNT162b2 30 μg; 1 ChAd + ChAd 5: one dose of ChAdOx1 followed by ChAdOx1 5 × 1010 viral particles; 1 ChAd + mRNA-1273 100: one dose of ChAdOx1 followed by mRNA-1273 100 μg; 1 ChAd + no: one dose of ChAdOx1 only. CI: Confidence interval; GMR: Geometric mean ratio; NMA: Network meta-analysis; SAEs: Serious adverse events.
All four studies reported neutralizing antibodies against the original strain. After one dose of BNT162b2 vaccination, another dose of mRNA-1273 induced a significantly higher level of neutralizing antibody than BNT162b2 (GMR = 1.32; 95% CI: 1.06–1.64), NVX-CoV2373 (GMR = 1.60; 95% CI: 1.16–2.21), and ChAdOx1 (GMR = 1.80; 95% CI: 1.25–2.59). For participants who received one dose of ChAdOx1, one dose of mRNA-1273 was more effective for improving antibody levels, than the second dose of ChAdOx1 (GMR = 11.09; 95% CI: 8.36–14.71) or NVX-CoV2373 (GMR = 2.87; 95% CI: 1.08–3.91) [Figure 3B].
Four studies reported S-protein antibodies, and their results were similar to those in regard to neutralizing antibodies against the original strain [Supplementary Figure 5]. Three studies reported information on T-cell response. Heterologous schedules were suggested to be associated with a stronger T-cell response than homologous schedules [Supplementary Figure 5]. A neutralizing antibody against the Delta strain was reported in two studies, indicating that another dose of mRNA-1273 after one dose of ChAdOx or BNT162b2 results in a higher level of antibodies. Detailed data on the immunogenicity outcomes of the individual study are given in [Supplementary 2].
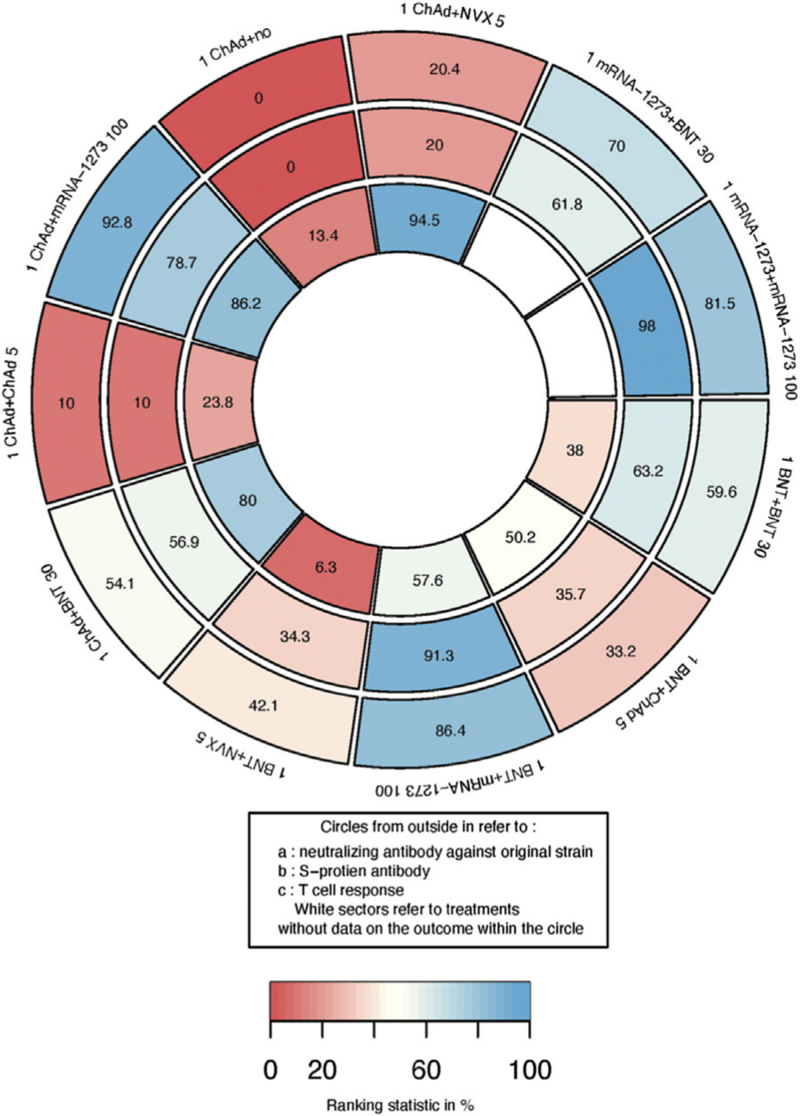
For participants who received one dose of BNT162b2, ChAdOx1, or mRNA-1273, another dose of mRNA-1273 performed well in the ranking of immunogenicity outcomes [Figure 4].
Figure 4.
Ranking heat plot of schedules initiated with mRNA or non-replicating viral vector for immunogenicity outcomes: a larger figure in a given sector means a higher probability of a greater immune response in that sector. Each sector is colored according to the SUCRA value of the corresponding treatment and outcome from red (0%) to blue (100%). 1 ChAd + NVX 5: one dose of ChAdOx1 followed by NVX-CoV2373 5 μg; 1 mRNA-1273 + BNT 30: one dose of mRNA-1273 followed by BNT162b2 30 μg; 1 mRNA-1273 + mRNA-1273 100: one dose of mRNA-1273 followed by mRNA-1273 100 μg; 1 BNT + BNT 30: one dose of BNT162b2 followed by BNT162b1 30 μg; 1 BNT + ChAd 5: one dose of BNT162b2 followed by ChAdOx1 5 × 1010 viral particles; 1 BNT + mRNA-1273 100: one dose of BNT162b2 followed by mRNA-1273 100 μg; 1 BNT + NVX 5: one dose of BNT162b2 followed by NVX-Cov2373 5 μg; 1 ChAd + BNT 30: one dose of ChAdOx1 followed by BNT162b2 30 μg; 1 ChAd + ChAd 5: one dose of ChAdOx1 followed by ChAdOx1 5 × 1010 viral particles; 1 ChAd + mRNA-1273 100: one dose of ChAdOx1 followed by mRNA-1273 100 μg; 1 ChAd + no: one dose of ChAdOx1 only. SUCRA: Surface under the cumulative ranking curve.
We extracted SAEs from all four included studies, as shown in [Figure 3C]. Four were reported for one dose of ChAdOx1 + ChAdOx1 5 × 1010 viral particles, three for one dose of ChAdOx1 + mRNA-1273 100 μg, one for one dose of ChAdOx1 + NVX-CoV2373 5 μg, two for one dose of BNT162b2 + BNT162b2 30 μg, and three for one dose of BNT162b2 + NVX-CoV2373 5 μg. The results of NMA did not show any significant difference between regimens [Figure 3B].
The same eight AEs were also assessed. The number of people presenting with each AE is presented in [Supplementary 3]. Another dose of mRNA-1273 following one dose of ChAdOx1 was associated with a higher risk for local AEs than other vaccines [Supplementary Figure 6]. This was also related to a higher risk for fatigue and myalgia [Supplementary Figure 7]. Schedules that included mRNA-1273 as the second dose had a lower rank in terms of safety outcomes, while the second dose of NVX-CoV2373 and the homologous regimen of ChAdOx1 performed well [Supplementary Figures 8, 9].
No significant heterogeneity or inconsistency (P > 0.05) was detected in networks for any immunogenicity or safety outcomes, except for arthralgia (P = 0.037). According to the GRADE, the results for all of the comparisons had very low confidence [Supplementary Table 4].
Discussion
Considering the significant international interest in heterologous COVID-19 vaccine schedules, it is important to assess emerging evidence on their immunogenicity and reactogenicity. Our NMA with 11 trials and 7723 participants suggests that a heterologous vaccine schedule with mRNA or non-replicating viral vector vaccines is more highly immunogenic in individuals who have previously received two doses of CoronaVac with increased transient local and systemic reactogenicity. Notably, the heterologous regimens also induce protection against the Delta variant. For participants primed with mRNA or non-replicating viral vector vaccines, the subsequent receipt of mRNA-1273 provides a limited, higher immunogenic effect than a homogeneous booster in both humoral and cellular immune responses. However, regimens that include mRNA-1273 might increase the risk for AEs.
A recent systematic review that evaluated the effectiveness of heterologous and homologous COVID-19 vaccine regimens found that a two-dose CoronaVac with one dose of a BNT162b2 regimen was highly effective[41]; our results are consistent with those results. One plausible explanation is that the heterologous vaccine may induce a significantly higher level of cross-binding and cross-neutralizing antibody titers, as well as RBD-specific memory B cells or S1-specific T cells than three doses of inactivated vaccines.[42] If so, this would imply that a heterologous vaccination regimen has advantages for diversifying immune responses that could be considered a promising vaccination roll-out strategy.[43] Although the WHO recommended EUL vectored or mRNA vaccines for subsequent doses as a heterologous booster following the CoronaVac primary series, our study provides evidence for prioritizing the use of mRNA vaccines.[44]
Heterologous schedules had greater immunogenic effects than ChAd/ChAd, which is similar to the findings of three previous meta-analyses.[19,23,24] A prospective cohort study reported that SARS-CoV-2 pseudovirus neutralization capacity against the alpha and beta variants shows significantly increased median infectious dose (ID50) titers after heterologous ChAd/BNT vaccination compared to homologous regimens.[22] Evidence also supports the use of a heterologous ChAd/BNT or BNT/ChAd schedule to induce a better T cell response than homologous BNT/BNT vaccination.[22,23] Vector-based SARS-CoV-2 vaccines tend to induce higher CD8+ T cell responses than BNT162b2.[34,45–47] However, in contrast to a previous meta-analysis,[23] we did not observe a higher level of neutralizing antibody for the heterologous ChAd/BNT schedule than the BNT/BNT schedule. One possible explanation is the difference in the detection methods of neutralizing antibodies and the intervals of the second dose between the RCTs we included, as well as the cohorts that the previous meta-analysis focused on.[48–50] Regimens including mRNA-1273 lead to a slightly higher risk for local AEs and systemic AEs, as reported in a previous observational study.[51] The dose of mRNA-1273 that has been recommended to be half might account for the higher reaction in the current analysis.[52] Notably, although heterologous schedules, including those employing mRNA-1273, also significantly enhance the immunogenicity response compared to homologous regimens of mRNA vaccine, the range of enhancement is not as great as those of inactivated primer and non-replicating viral vector primer.
Given that both humoral and cellular immune responses were markedly improved by heterologous schedules, we speculate that the heterologous mRNA vaccine could provide superior protection, regardless of initial vaccination.
Compared to previous relevant meta-analyses, a major strength of our study is that it is substantially more comprehensive having included multiple heterologous COVID-19 vaccine schedules in networks and a large number of RCTs. Furthermore, we provided rankings for immunogenicity and the reactogenicity of different heterologous schedules to support decision-making. In addition, we assessed the quality of evidence and incorporated it into an explanation of the results according to the GRADE framework.
However, there are several limitations to mention. First, most comparisons were assessed as being of very low quality in the GRADE framework, showing wide CIs owing to sparse data, which could restrict the interpretation of the results. However, these data are still valuable for making optimal use of a diverse vaccine portfolio. When more data on ongoing trials are available, we will update this analysis. Second, the methodology of some included trials was poor. More than 80% of trials were of some concern due to their deviation from the intended interventions. Thus, this may have introduced bias, and our results should be interpreted with caution. Third, to include and make comparisons between as many studies as possible, we used titers of pseudotype virus-neutralizing antibody against the original strain. These are normally lower than titers of live virus-neutralizing antibody; however, we believe that by calculating the relative treatment effect, GMR, we should have counteracted this difference. Finally, due to the sparsity of data and lack of access to original trial data, we could not perform detailed NMA subgroup analyses, meta-regressions, or individual patient data meta-analyses to properly address the potentially relevant effect modifiers, such as age, race, and prime-boost interval.
The findings from this NMA are the most comprehensive evidence currently available to guide choices about primary series and booster vaccination schedules for combating the COVID-19 pandemic.
Conclusions
After vaccination with two doses of CoronaVac, a dose of BNT162b2 as a booster substantially enhances immunogenicity reactions and has a relatively acceptable risk for SAEs compared to other vaccines. For primary vaccination, schedules initiated with one dose of an mRNA vaccine induce better immunogenicity reactions than one dose of ChAdOx1, and the following dose of mRNA vaccine can improve immunogenicity reactions compared to ChAdOx1. However, the comparatively higher risk for local and systemic AEs introduced by mRNA vaccines should be noted.
Funding
This work was supported by a grant from the National Key R&D Program of China (No. 2021YFC2301601).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Li P, Wang W, Tao Y, Tan X, Li Y, Mao Y, Gao L, Feng L, Zhan S, Sun F. Immunogenicity and reactogenicity of heterologous immunization schedules with COVID-19 vaccines: a systematic review and network meta-analysis. Chin Med J 2023;136:24–33. doi: 10.1097/CM9.0000000000002567
Pei Li and Weiwei Wang contributed equally to this work.
Supplemental digital content is available for this article.
References
- 1.Johns Hopkins University. Coronavirus Resource Center 2022. Available from: https://coronavirus.jhu.edu/map.html. [Accessed 2022]. [Google Scholar]
- 2.Mlcochova P, Kemp SA, Dhar MS, Papa G, Meng B, Ferreira IATM, et al. SARS-CoV-2 B.1.617. 2 Delta variant replication and immune evasion. Nature 2021; 599:114–119. doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viana R, Moyo S, Amoako DG, Tegally H, Scheepers C, Althaus CL, et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022; 603:679–686. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan YJ, Chan KH, Hung IF. Safety and efficacy of COVID-19 vaccines: a systematic review and meta-analysis of different vaccines at phase 3. Vaccines 2021; 9:989.doi: 10.3390/vaccines9090989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chemaitelly H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med 2021; 385:e83.doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta KD, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med 2021; 27:2127–2135. doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 2021; 398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padma TV. COVID vaccines to reach poorest countries in 2023 despite recent pledges. Nature 2021; 595:342–343. doi: 10.1038/d41586-021-01762-w. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Ghadami A, Drake JM, Rohani P, Epureanu BI. Mathematical model of the feedback between global supply chain disruption and COVID-19 dynamics. Sci Rep 2021; 11:15450.doi: 10.1038/s41598-021-94619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padma TV. India's COVID-vaccine woes by the numbers. Nature 2021; 592:500–501. doi: 10.1038/d41586-021-00996-y. [DOI] [PubMed] [Google Scholar]
- 11.Paterlini M. COVID-19: Sweden, Norway, and Finland suspend use of Moderna vaccine in young people “as a precaution”. BMJ 2021; 375:n2477.doi: 10.1136/bmj.n2477. [DOI] [PubMed] [Google Scholar]
- 12.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021; 384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu NC, Chi H, Tu YK, Huang YN, Tai YL, Weng SL, et al. To mix or not to mix? A rapid systematic review of heterologous prime-boost COVID-19 vaccination. Expert Rev Vaccines 2021; 20:1211–1220. doi: 10.1080/14760584.2021.1971522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Shaw RH, Stuart ASV, Greenland M, Aley PK, Andrews NJ, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet 2021; 398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw RH, Stuart A, Greenland M, Liu X, Nguyen Van-Tam JS, Snape MD, et al. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet 2021; 397:2043–2046. doi: 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borobia AM, Carcas AJ, Pérez-Olmeda M, Castaño L, Bertran MJ, García-Pérez J, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021; 398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benning L, Töllner M, Hidmark A, Schaier M, Nusshag C, Kälble F, et al. Heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccination induces strong humoral responses among health care workers. Vaccines 2021; 9:857.doi: 10.3390/vaccines9080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Hou L, Guo X, Jin P, Wu S, Zhu J, et al. Heterologous prime-boost immunization with CoronaVac and Convidecia. medRxiv 2021; doi: 10.1101/2021.09.03.21263062. [Google Scholar]
- 19.Parker EPK, Desai S, Marti M, O’Brien KL, Kaslow DC, Kochhar S, et al. Emerging evidence on heterologous COVID-19 vaccine schedules-To mix or not to mix? Lancet Infect Dis 2022; 22:438–440. doi: 10.1016/S1473-3099(22)00178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barros-Martins J, Hammerschmidt SI, Cossmann A, Odak I, Stankov MV, Morillas Ramos G, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med 2021; 27:1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Normark J, Vikström L, Gwon YD, Persson IL, Edin A, Björsell T, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination. N Engl J Med 2021; 385:1049–1051. doi: 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillus D, Schwarz T, Tober-Lau P, Vanshylla K, Hastor H, Thibeault C, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med 2021; 9:1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lv J, Wu H, Xu J, Liu J. Immunogenicity and safety of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine: a systematic review. Infect Dis Poverty 2022; 11:53.doi: 10.1186/s40249-022-00977-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen TT, Quach THT, Tran TM, Phuoc HN, Nguyen HT, Vo TK, et al. Reactogenicity and immunogenicity of heterologous prime-boost immunization with COVID-19 vaccine. Biomed Pharmacother 2022; 147:112650.doi: 10.1016/j.biopha.2022.112650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71.doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Available from: https://covid-nma.com/vaccines/. [Accessed 2022] [Google Scholar]
- 27.Zeng B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med 2022; 20:200.doi: 10.1186/s12916-022-02397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019). Cochrane, 2019. Available from https://www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS One 2014; 9:e99682.doi: 10.1371/journal.pone.0099682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018; 27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 31.Veroniki AA, Straus SE, Fyraridis A, Tricco AC. The rank-heat plot is a novel way to present the results from a network meta-analysis including multiple outcomes. J Clin Epidemiol 2016; 76:193–199. doi: 10.1016/j.jclinepi.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Hou L, Guo X, Jin P, Wu S, Zhu J, et al. Heterologous AD5-nCOV plus CoronaVac versus homologous CoronaVac vaccination: a randomized phase 4 trial. Nat Med 2022; 28:401–409. doi: 10.1038/s41591-021-01677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sablerolles RSG, Rietdijk WJR, Goorhuis A, Postma DF, Visser LG, Geers D, et al. Immunogenicity and reactogenicity of vaccine boosters after Ad26.COV2.S priming. N Engl J Med 2022; 386:951–963. doi: 10.1056/NEJMoa2116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munro APS, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021; 398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stuart ASV, Shaw RH, Liu X, Greenland M, Aley PK, Andrews NJ, et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet 2022; 399:36–49. doi: 10.1016/S0140-6736(21)02718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mok CKP, Chen C, Yiu K, Chan TO, Lai KC, Ling KC, et al. A randomized clinical trial using CoronaVac or BNT162b2 vaccine as a third dose in adults vaccinated with two doses of CoronaVac. Am J Respir Crit Care Med 2022; 205:844–847. doi: 10.1164/rccm.202111-2655LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa Clemens SA, Weckx L, Clemens R, Almeida Mendes AV, Ramos Souza A, Silveira MBV, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet 2022; 399:521–529. doi: 10.1016/S0140-6736(22)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Intapiboon P, Seepathomnarong P, Ongarj J, Surasombatpattana S, Uppanisakorn S, Mahasirimongkol S, et al. Immunogenicity and safety of an intradermal BNT162b2 mRNA vaccine booster after two doses of inactivated SARS-CoV-2 vaccine in healthy population. Vaccines 2021; 9:1375.doi: 10.3390/vaccines9121375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janssen C, Cachanado M, Ninove L, Lachatre M, Michon J, Epaulard O, et al. Immunogenicity and reactogenicity of heterologous and homologous mRNA-1273 and BNT162b2 vaccination: a multicenter non-inferiority randomized trial. EClinicalMedicine 2022; 48:101444.doi: 10.1016/j.eclinm.2022.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nanthapisal S, Puthanakit T, Jaru-Ampornpan P, Nantanee R, Sodsai P, Himananto O, et al. A randomized clinical trial of a booster dose with low versus standard dose of AZD1222 in adult after 2 doses of inactivated vaccines. Vaccine 2022; 40:2551–2560. doi: 10.1016/j.vaccine.2022.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Au WY, Cheung PP. Effectiveness of heterologous and homologous COVID-19 vaccine regimens: living systematic review with network meta-analysis. BMJ 2022; 377:e069989.doi: 10.1136/bmj-2022-069989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuo F, Abolhassani H, Du LK, Piralla A, Bertoglio F, de Campos-Mata L, et al. Heterologous immunization with inactivated vaccine followed by mRNA-booster elicits strong immunity against SARS-CoV-2 Omicron variant. Nat Commun 2022; 13:2670.doi: 10.1038/s41467-022-30340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mallapaty S. China's COVID vaccines have been crucial now immunity is waning. Nature 2021; 598:398–399. doi: 10.1038/d41586-021-02796-w. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. Interim Recommendations for Heterologous COVID-19 Vaccine Schedules. WHO, 2021. Available from https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-heterologous-schedules. [Google Scholar]
- 45.Chandrashekar A, Yu J, McMahan K, Jacob-Dolan C, Liu J, He X, et al. Vaccine protection against the SARS-CoV-2 Omicron variant in macaques. Cell 2022; 185:1549–1555. e11. doi: 10.1016/j.cell.2022.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, et al. Homologous and heterologous COVID-19 booster vaccinations. N Engl J Med 2022; 386:1046–1057. doi: 10.1056/NEJMoa2116414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collier AY, Yu J, McMahan K, Liu J, Chandrashekar A, Maron JS, et al. Differential kinetics of immune responses elicited by COVID-19 vaccines. N Engl J Med 2021; 385:2010–2012. doi: 10.1056/NEJMc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 2021; 397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parry H, Bruton R, Stephens C, Bentley C, Brown K, Amirthalingam G, et al. Extended interval BNT162b2 vaccination enhances peak antibody generation. NPJ Vaccines 2022; 7:14.doi: 10.1038/s41541-022-00432-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity 2010; 33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt T, Klemis V, Schub D, Mihm J, Hielscher F, Marx S, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med 2021; 27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.US Food and Drug Administration. Moderna COVID-19 Vaccine Health Care Provider Fact Sheet. FDA, 2021. Available from https://www.fda.gov/media/144637/download. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.