Abstract
Background:
Liver biopsy for the diagnosis of non-alcoholic steatohepatitis (NASH) is limited by its inherent invasiveness and possible sampling errors. Some studies have shown that cytokeratin-18 (CK-18) concentrations may be useful in diagnosing NASH, but results across studies have been inconsistent. We aimed to identify the utility of CK-18 M30 concentrations as an alternative to liver biopsy for non-invasive identification of NASH.
Methods:
Individual data were collected from 14 registry centers on patients with biopsy-proven non-alcoholic fatty liver disease (NAFLD), and in all patients, circulating CK-18 M30 levels were measured. Individuals with a NAFLD activity score (NAS) ≥5 with a score of ≥1 for each of steatosis, ballooning, and lobular inflammation were diagnosed as having definite NASH; individuals with a NAS ≤2 and no fibrosis were diagnosed as having non-alcoholic fatty liver (NAFL).
Results:
A total of 2571 participants were screened, and 1008 (153 with NAFL and 855 with NASH) were finally enrolled. Median CK-18 M30 levels were higher in patients with NASH than in those with NAFL (mean difference 177 U/L; standardized mean difference [SMD]: 0.87 [0.69–1.04]). There was an interaction between CK-18 M30 levels and serum alanine aminotransferase, body mass index (BMI), and hypertension (P < 0.001, P = 0.026 and P = 0.049, respectively). CK-18 M30 levels were positively associated with histological NAS in most centers. The area under the receiver operating characteristics (AUROC) for NASH was 0.750 (95% confidence intervals: 0.714–0.787), and CK-18 M30 at Youden's index maximum was 275.7 U/L. Both sensitivity (55% [52%–59%]) and positive predictive value (59%) were not ideal.
Conclusion:
This large multicenter registry study shows that CK-18 M30 measurement in isolation is of limited value for non-invasively diagnosing NASH.
Keywords: Apoptosis, Diagnosis, Cytokeratin-18, Liver histology, Non-alcoholic steatohepatitis, Non-alcoholic fatty liver disease
Introduction
Non-alcoholic fatty liver disease (NAFLD) is estimated to affect up to approximately 25% to 40% of the global adult population, particularly driven by the increasing epidemics of both obesity and type 2 diabetes mellitus.[1–3] The histological phenotypes of NAFLD extend from non-alcoholic fatty liver (NAFL) to non-alcoholic steatohepatitis (NASH),[4,5] which is histologically characterized by steatosis plus hepatocyte ballooning with or without fibrosis.[6] Over time, NASH can progress to cirrhosis, liver failure, and even hepatocellular carcinoma [7] that heralds a burdensome liver disease, which greatly increases overall mortality.[8] In a population-based study, the prevalence of advanced fibrosis related to NAFLD was around 1.1%.[9] NASH has rapidly become the second leading indication for liver transplantation in the United States and Europe and is expected to become the leading indication in the next decade.[2,10,11] In Europe, the disease burden and financial strains of NASH for the healthcare systems are high.[12]
NAFLD is histologically characterized by the accumulation of fat in >5% of hepatocytes in the absence of excessive alcohol consumption or other competing causes of hepatic steatosis.[13,14] NASH and cirrhosis can be identified and stratified based on the semi-quantitative histological features. Hence, liver biopsy is considered the “gold standard” for diagnosing NASH. However, this procedure is not a routine test due to its invasiveness and considerable costs.[15] Consequently, a non-invasive alternative to liver biopsy for diagnosing NASH is needed. Hepatocyte apoptosis is typically increased in patients with NASH, but not in those with NAFL.[16] Cytokeratin 18 (CK-18) has been proposed as a promising alternative to liver biopsy to diagnose NASH. CK-18 is the major intermediate filament protein comprising the cytoskeletal structure of hepatocytes. During hepatocyte apoptosis, effector caspase cleaved CK-18 fragments (including M30) into the bloodstream.[6,17]
Current studies on the CK-18 level and its diagnostic performance in NASH have been inconclusive. On the other hand, the most relevant studies are either meta-analyses[18,19] with missing raw data, or single-center small studies.[20] Thus, the aim of this study was to identify the utility of the CK-18 M30 level as a non-invasive alternative to liver biopsy for non-invasively diagnosing NASH and to determine its optimal clinical performance in a multi-national registry study of the largest sample to date.
Methods
Study design and ethical approval
We conducted a multicenter study to collect available individual registry data using an open online reporting form for patients with biopsy-confirmed NAFLD. The registry study and online form were widely publicized through multiple accredited gastroenterology and hepatology societies, and emails were sent directly to hepatology providers, and social media. A copy of the content of the data collection tool is available in Supplementary Table 1. It is needed to state that some of the data have been reported in previous papers,[21–31] and the details are presented in Supplementary Table 2.
The data collected contained no personal health identifiers (all subjects were digitally anonymized), and this registry was deemed not to constitute human research by the Institutional Ethics Committee.
Participants
All liver biopsies confirmed NAFLD cases from all participating centers. Subjects were excluded if any of the following conditions were met: if NAFLD was not biopsy-confirmed; if the patient had a confirmed diagnosis of other types of hepatitis (such as hepatitis A, hepatitis B, or alcohol related liver disease); or if there were missing data for any patient information, especially for CK-18 M30 measurement.
Variables and definitions
Supplementary Table 2 provides information on each center, including whether the data were used for publication, eligibility criteria, liver biopsy results reading, and the time interval between CK-18 M30 testing and liver biopsy. Liver histological specimens were scored separately at each central laboratory strictly by experienced liver histopathologists according to the NASH-Clinical Research Network scoring system.[32] The NAFLD activity score (NAS) was calculated as the sum of three histological components, namely, steatosis (grades 0–3), ballooning (grades 0–2), and lobular inflammation (grades 0–3). The stage of fibrosis was graded from 0 to 4. In order to exclude possible interference of fibrosis in the NAFL population (i.e., burned-out NASH – after progression to NASH and fibrosis, but instead NAS decreases) and to be consistent with the definition of NASH in the earliest article[20] on the use of CK-18 for NASH diagnosis, we defined the presence of NAFL and NASH as follows: individuals with a NAS ≥5 including a score of ≥1 for each of steatosis, ballooning, and lobular inflammation were diagnosed as having definite NASH; individuals with a NAS ≤2 and no fibrosis were diagnosed as having NAFL (simple steatosis). Individuals with borderline NASH, defined as a NAS of 3 to 4, were initially excluded from this primary analysis as they cannot be assigned to a clear-cut category.
Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters (kg/m2). Diabetes was defined by a fasting plasma glucose level of ≥7.0 mmol/L (≥126 mg/dL) or a hemoglobin A1c of ≥48 mmol/mol (≥6.5%) and/or current use of any anti-hyperglycemic agents. Hypertension was defined as a blood pressure of ≥130/85 mmHg and/or current use of any anti-hypertensive drugs.[33] CK-18 levels were measured using the M30-apoptosense enzyme-link immunosorbent assay kit.
Statistical analysis
All statistical analyses were conducted using IBM SPSS Statistics (version 26.0, SPSS Inc., Chicago, IL, USA) and Stata (version 11.0 SE, Stata Corporation, College Station, TX, USA). We undertook pre-specified subgroup analyses for CK-18 M30 measurements according to sex, age strata (≤44, 45–59, and ≥60 years),[34,35] ethnicity (White and non-White), BMI (<30 kg/m2 and ≥30 kg/m2), hypertension, diabetes, and serum alanine aminotransferase (ALT) (≤40 U/L and >40 U/L). Since CK-18 exhibited a right-skewed distribution, a logarithmic transformation of values was undertaken before statistical analysis. We used a two-step process for pooled effect size analysis: an odds ratio was estimated for each cohort and then the odds ratios were pooled. We also undertook tests for interaction between patient subgroups, partitioning the total heterogeneity of all cohorts into within- and between-group heterogeneity (interaction test). Because the overall sample composition varied across cohorts (i.e., in terms of ethnicity, and geographic location), a random effect model was conducted to calculate the pooled effect size. Random effect models typically result in wider confidence intervals (CIs) than the fixed effect models; however, when there is no high heterogeneity, the results of random and fixed effect models are equivalent.[36]
The receiver operating characteristic curves were used to test the diagnostic performance of CK-18 M30 for identifying NASH. The cut-off value for the CK-18 M30 level giving the optimum balance of sensitivity and specificity (i.e., Youden's index) was identified and applied to each cohort. When appraising performance at a given cut-off, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were computed and graphically presented. All reported probability values were 2-tailed, and a P value of <0.05 was considered statistically significant.
Results
We obtained data from a total of 14 cohorts in eight different countries with CK-18 M30 results and baseline characteristics in patients with biopsy-proven NAFLD. After excluding 1563 patients, a total of 1008 subjects were included in the present study [Supplementary Figure 1]. The included cohorts were located in Asia, Europe, and Oceania. The Chinese region contributed the most cohorts and most cases with 419 cases (41.6%) in 6 cohorts, followed by the French region, which contributed the second most cases with 247 (24.5%) in two cohorts; and one each from Greece, Australia, Germany, Malaysia, Switzerland, and Turkey, respectively. Among the 1008 eligible participants with biopsy-proven NAFLD, the mean age was 46.8 (range 12–79) years, 477 (47.3%) were male, and 855 (84.8%) had definite NASH. The patient characteristics of cohorts enrolled are summarized in Table 1 and Supplementary Figure 2. Table 2 lists the histological characteristics of participants: 153 (15.2%) had a NAS compatible with NAFL (simple steatosis), and none of participants in the NAFL group had liver fibrosis. Of 1008 subjects, 756 (75.0%) had varying levels of liver fibrosis, all of whom also had definite NASH. Detailed cohort information is presented in Supplementary Table 2.
Table 1.
Biopsy-proven NAFLD patient characteristics of the included cohorts.
| Centers | Age range (years) | Included patients | Male patients | NASH patients | Ln CK-18 M30 (U/L) |
| Angers (France) | 21–79 (56.4) | 199 (19.7) | 104 (52.3) | 175 (87.9) | 5.75 ± 0.75 |
| Athens (Greece) | 19–72 (46.1) | 42 (4.2) | 22 (52.4) | 17 (40.5) | 5.48 ± 0.61 |
| Sydney (Australia) | 17–79 (47.8) | 62 (6.2) | 34 (54.8) | 32 (51.6) | 5.26 ± 1.14 |
| Mainz (Germany) | 25–61 (42.7) | 7 (0.7) | 5 (71.4) | 7 (100.0) | 6.64 ± 1.09 |
| Hangzhou (China) | 24–70 (41.2) | 30 (3.0) | 23 (76.7) | 30 (100.0) | 5.33 ± 0.76 |
| Hong Kong (China) | 26–70 (46.9) | 58 (5.8) | 36 (62.1) | 33 (56.9) | 5.97 ± 0.86 |
| Kuala Lumpur (Malaysia) | 26–68 (50.6) | 90 (8.9) | 41 (45.6) | 82 (91.1) | 6.26 ± 0.76 |
| Nice (France) | 21–62 (42.6) | 48 (4.8) | 10 (20.8) | 43 (89.6) | 5.88 ± 0.59 |
| Shanghai Ruijin (China) | 21–70 (49.7) | 34 (3.4) | 20 (58.8) | 34 (100.0) | 5.35 ± 0.77 |
| Shanghai Xinhua (China) | 18–66 (39.1) | 31 (3.1) | 22 (71.0) | 24 (77.4) | 5.90 ± 0.76 |
| Bern (Switzerland) | 28–75 (55.3) | 31 (3.1) | 12 (38.7) | 28 (90.3) | 5.63 ± 0.56 |
| Tianjin (China) | 18–62 (38.4) | 17 (1.7) | 9 (52.9) | 17 (100.0) | 5.25 ± 0.98 |
| Istanbul (Turkey) | 29–70 (48.8) | 110 (10.9) | 58 (52.7) | 101 (91.8) | 4.80 ± 1.02 |
| Wenzhou (China) | 12–71 (38.4) | 249 (24.7) | 81 (32.5) | 232 (93.2) | 5.49 ± 1.12 |
| Total | 12–79 (46.8) | 1008 (100) | 477 (47.3) | 855 (84.8) | 5.58 ± 0.99 |
Values were shown as mean (range), n (%), or mean ± standard deviation. CK-18: Cytokeratin-18; NAFLD: Non-alcoholic fatty liver disease.
Table 2.
Histological characteristics of biopsy-proven NAFLD patients.
| Liver histology features | All patients (n = 1008) | NAFL patients (n = 153) | NASH patients (n = 855) |
| Steatosis grades, n (%) | |||
| 1 (5–33%) | 200 (19.8) | 137 (89.5) | 63 (7.4) |
| 2 (34–66%) | 310 (30.8) | 16 (10.5) | 294 (34.4) |
| 3 (>66%) | 498 (49.4) | 0 | 498 (58.2) |
| Lobular inflammation grades, n (%) | |||
| 0 | 124 (12.3) | 124 (81.0) | 0 |
| 1 | 462 (45.8) | 29 (19.0) | 433 (50.6) |
| 2 | 377 (37.4) | 0 | 377 (44.1) |
| 3 | 45 (4.5) | 0 | 45 (5.3) |
| Ballooning grades, n (%) | |||
| 0 | 130 (12.9) | 130 (85.0) | 0 |
| 1 | 417 (41.4) | 23 (15.0) | 394 (46.1) |
| 2 | 461 (45.7) | 0 | 461 (53.9) |
| Fibrosis stages, n (%) | |||
| 0 | 252 (25.0) | 153 (100.0) | 99 (11.6) |
| 1 | 339 (33.6) | 0 | 339 (39.6) |
| 2 | 209 (20.7) | 0 | 209 (24.4) |
| 3 | 155 (15.4) | 0 | 155 (18.1) |
| 4 | 53 (5.3) | 0 | 53 (6.2) |
NAFL: Non-alcoholic fatty liver; NASH: Non-alcoholic steatohepatitis; NAFLD: Non-alcoholic fatty liver disease.
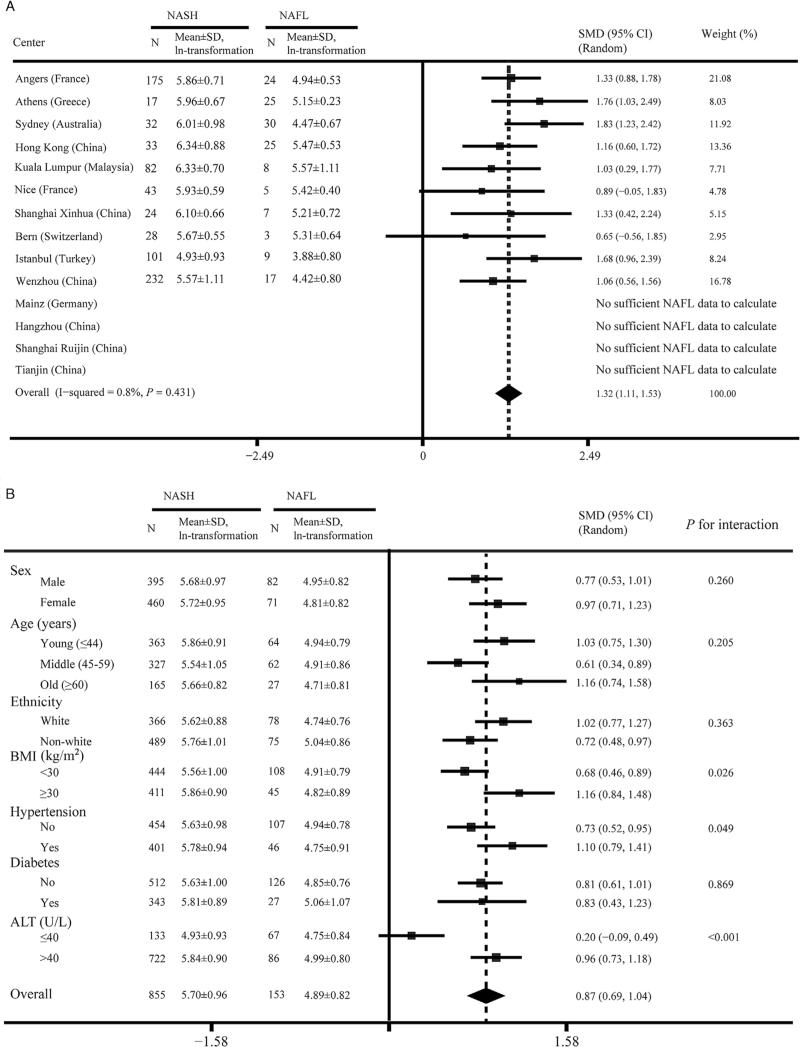
CK-18 M30 levels in NAFL and NASH
Figure 1A shows the circulating levels of CK-18 M30 in the NASH and NAFL groups from each cohort (four cohorts did not present data due to an insufficient NAFL population). Except for cohorts from Nice (France) and Bern (Switzerland), all cohorts showed significantly higher levels of CK-18 M30 in patients with NASH than in those with NAFL. Overall, in our multicenter registry study, CK-18 M30 levels were on average 177 units higher in the NASH group than in NAFL (standardized mean difference: 0.87, 95% CI: 0.69–1.04; median of 315 and 138 U/L in the two patient groups, respectively). Additionally, as shown in Supplementary Table 3, we compared the serum CK-18 M30 levels in NAFL, borderline NASH, and NASH populations. Both in the pooled cohort and in most individual cohorts, serum CK-18 M30 concentrations were highest in NASH, intermediate in borderline NASH, and lowest in NAFL.
Figure 1.
Differences in the CK-18 M30 level between NASH and NAFL patient groups. (A) In each registry center. (B) Subgroup analysis by main characteristics. ALT: Alanine aminotransferase; BMI: Body mass index; CI: Confidence interval; CK-18: Cytokeratin-18; NAFL: Non-alcoholic fatty liver; NASH: Non-alcoholic steatohepatitis; SD: Standard deviation; SMD: Standardized mean difference.
In pre-specified subgroups of patients [Figure 1B], CK-18 M30 concentrations remained significantly higher in NASH patients than in those with NAFL, irrespective of sex, age strata, ethnicity, and presence of obesity, hypertension, or diabetes. Patients with elevated serum ALT levels (ALT >40 U/L) also had significantly increased CK-18 M30 levels in the NASH group (P < 0.001). Furthermore, for each of these pre-specified patient subgroups, interaction tests were conducted. We detected significant interactions with serum ALT level, BMI, and hypertension (F = 16.35, P < 0.001; F = 5.00, P = 0.026; F = 3.88, P = 0.049; respectively).
Univariable correlation analyses were undertaken between CK-18 M30 level and histological NAS in each cohort [Supplementary Figure 3]. These results were consistent with those in Figure 1A to some extent, indicating no significant association between CK-18 M30 and NAS in Bern (Switzerland) (r = 0.19, P = 0.307).
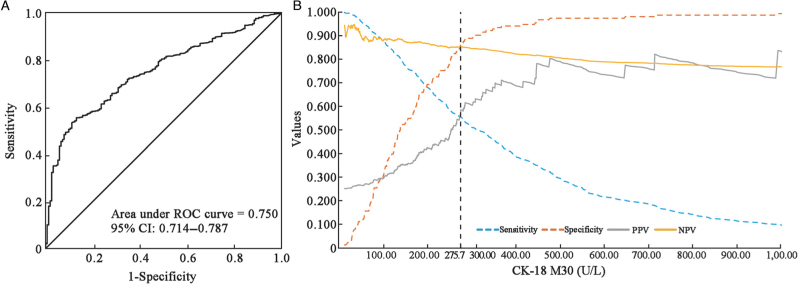
Diagnosis of NASH using the best cut-off of CK-18 M30 level
Multiple results showed significant differences in CK-18 M30 levels between the NASH and NAFL patient groups; hence, its reliability for the diagnosis of NASH in NAFLD patients was further investigated. The optimum cut-off values of CK-18 M30 to diagnose NASH were determined using the area under receiver operator characteristic (AUROC) curve analysis and Youden's index. Different cohort data gave different optimal values, and finally, a CK-18 M30 level of 275.7 U/L was determined as the best cut-off in the whole sample [Table 3 and Figure 2A].
Table 3.
AUROC curve analysis of plasma CK-18 M30 levels for NASH diagnosis.
| Centers | AUROC (95% CI) | Best cut-off (U/L) | Sensitivity (%) | Specificity (%) |
| Angers (France) | 0.849 (0.776–0.921) | 275.8 | 62.9 | 91.7 |
| Athens (Greece) | 0.879 (0.766–0.992) | 215.8 | 82.4 | 84.0 |
| Sydney (Australia) | 0.886 (0.795–0.977) | 203.0 | 84.4 | 86.7 |
| Mainz (Germany)∗ | – | – | – | – |
| Hangzhou (China)∗ | – | – | – | – |
| Hong Kong (China) | 0.813 (0.703–0.924) | 285.3 | 81.8 | 76.0 |
| Kuala Lumpur (Malaysia) | 0.759 (0.508–1.000) | 253.0 | 90.2 | 75.0 |
| Nice (France) | 0.758 (0.584–0.932) | 327.4 | 60.5 | 100.0 |
| Shanghai Ruijin (China)∗ | – | – | – | – |
| Shanghai Xinhua (China) | 0.815 (0.648–0.983) | 457.8 | 54.2 | 100.0 |
| Bern (Switzerland) | 0.655 (0.347–0.962) | 386.0 | 35.7 | 100.0 |
| Tianjin (China)∗ | – | – | – | – |
| Istanbul (Turkey) | 0.901 (0.814–0.988) | 79.4 | 72.3 | 100.0 |
| Wenzhou (China) | 0.810 (0.721–0.899) | 169.5 | 67.2 | 88.2 |
| Total | 0.750 (0.714–0.787) | 275.7 | 55.4 | 86.9 |
The cohort does not have enough NAFL data to calculate the best cut-off for plasma CK-18 M30 levels.
AUROC: Area under receiver operating characteristic; CK-18: Cytokeratin-18; CI: Confidence interval; NASH: Non-alcoholic steatohepatitis; –: Not applicable.
Figure 2.
Diagnostic performance of the CK-18 M30 level. (A) AUROC curve of CK-18 M30 levels for the diagnosis of NASH. (B) Sensitivity, specificity, PPV, and NPV versus all possible CK-18 M30 levels. AUROC: Area under receiver operating characteristic; CI: Confidence interval; CK-18: Cytokeratin-18; NASH: Non-alcoholic steatohepatitis; NPV: Negative predictive value; PPV: Positive predictive value; ROC: receiver operating characteristic.
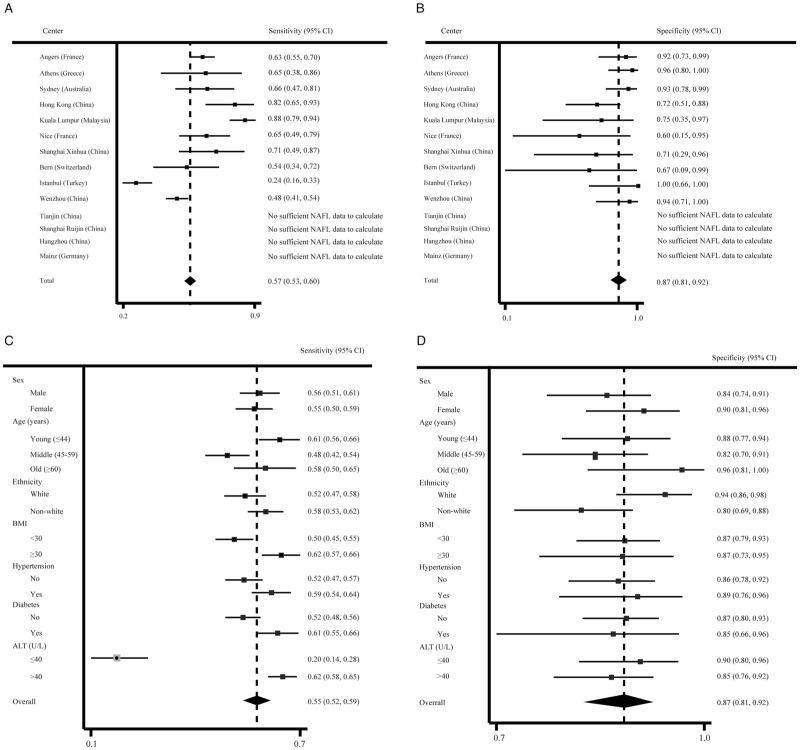
We recalculated sensitivity and specificity using this optimal threshold for each cohort and subgroup [Figure 3]. As shown in Table 3 and Figure 2A, the AUROC for CK-18 M30 to diagnose the presence of NASH was 0.750 (95% CI: 0.714–0.787). Even at the best cut-off value, the sensitivity (55% [95% CI: 52–59%]) of CK-18 M30 levels for diagnosing NASH was low, although the specificity was 87% (95% CI: 81–92%).
Figure 3.
Diagnostic performance of the CK-18 M30 level at the optimal cut-off value of 275.7 U/L. (A) Sensitivity in each registry center. (B) Specificity in each registry center. (C) Sensitivity in subgroups by main characteristics. (D) Specificity in subgroups by main characteristics. ALT: Alanine aminotransferase; BMI: Body mass index; CI: Confidence interval; CK-18: Cytokeratin-18; NAFL: Non-alcoholic fatty liver.
Further subgroup analyses were performed to estimate the sensitivity and specificity, as given in Table 3 and Figures 3A, 3B. In pre-specified patient subgroups, the specificity was high in all patient subgroups, but low sensitivity was observed even in the population with a BMI of ≥30 kg/m2 (sensitivity: 62% [57–66%], specificity: 87% [73–95%]) or in old (sensitivity: 58% [50–65%], specificity: 96% [81–100%]). In addition, as shown in Figure 2B, at different cut-off values, the changes in sensitivity, specificity, PPV, and NPV of CK-18 M30 levels for the diagnosis of NASH are presented. With increases in the cut-off value, the corresponding specificity and PPV increased, the sensitivity gradually decreased, while the overall NPV changed little. At the optimal cut-off point of the CK-18 M30 level, the PPV was low (59%), although the NPV was better (85%).
Discussion
In this large, multinational cohort of patients with biopsy-proven NAFLD, our aim was to assess the utility of the CK-18 M30 level as a non-invasive alternative to distinguish between NASH and NAFL and to further determine the optimal cut-off value for NASH diagnosis. Data from different cohorts around the world enriched the present study, which were both large and multi-ethnic with a wide age range. Unfortunately, although we used strict entry criteria and our results showed a significant difference in CK-18 M30 levels between the NASH and NAFL groups, the present data do not support a role of the CK-18 M30 level alone to accurately diagnose NASH.
The global prevalence of NASH has been steadily increasing, and although histological changes are currently the gold standard, liver biopsy is infrequently performed due to its important limitations.[37] Since liver fat accumulation and inflammation in NASH are more patchy than in chronic hepatitis C, liver biopsy is prone to sampling errors, and its utility is further limited by significant intra- and inter-observer variabilities.[38,39] Moreover, liver biopsy also represents a significant physical and financial burden on patients and healthcare providers, with associated morbidity and even mortality. These risks, as stated by Davison et al,[40] especially in the context of clinical studies, lead to inherent limitations in conducting larger NASH research.
Based on these concerns, a non-invasive alternative to liver biopsy is urgently needed. Since there is such a mechanism in hepatocytes that apoptotic cells are associated with caspase-cleaved CK-18 fragments being released into the blood. Increased apoptotic activity is frequently observed in NASH, but not in NAFL.[17,41,42] Hence, the conjecture that CK-18 fragments in blood might be useful to distinguish between NASH and NAFL has attracted increasing attention. However, from the available published studies, research on the value of CK-18 M30 in NASH has produced mixed results. It showed promising results in some,[41–43] but not all, studies.[1,44,45] In the present multicenter registry study, circulating levels of CK-18 M30 were markedly different between NASH and NAFL, which in turn confirms the plausibility of the mechanism triggering CK-18 release as described before. However, further analysis from the perspective of a screening test, its efficacy may be more limited than previously thought, that is, at least the measurement of the CK-18 M30 level alone appears to be not accurate enough to diagnose NASH, although its diagnostic performance is much better than that of serum aspartate aminotransferase (AST) levels for the non-invasive identification of NASH [Supplementary Figure 4].
Of note, we do not rule out the possibility that the CK-18 M30 level, when used in combination with other non-invasive tests, might play a role in the non-invasive identification of NASH without the need to resort to liver biopsy. For example, some studies put forward a composite score index MACK-3, comprising hemoglobin A1c and serum AST levels,[22,24,40] or combination with metabolic syndrome and ALT,[21] or the combined use with controlled attenuation parameter.[4] In addition, there are new non-invasive markers proposed, which do not involve CK-18, such as circulating oxidized fatty acids[46,47] based on the theory of cell death and oxidative stress, novel liquid biopsy marker[48] based on the mass spectrometry, and flow cytometry as well as the acNASH index[49] based on statistical models. Of course, from a practical point of view, we would prefer to find a single, high-efficacy, non-invasive biomarker for accurately diagnosing NASH.
Additionally, in the present study, we used the definition of NASH as a NAS >5 and excluded the “borderline NASH” population in order to exclude possible interference of fibrosis in the NAFL population (i.e., burned-out NASH) and to be consistent with the definition of NASH used in the original paper[20]. However, we acknowledge that there are other definitions of NASH currently available, and so we undertook additionally analysis: individuals with a NAS ≥4 with a score of ≥1 for each steatosis, ballooning, and lobular inflammation being diagnosed as having definite NASH; individuals with a NAS ≤3 diagnosed as having definite NAFL, regardless of the presence of fibrosis. Notably, the results were similar, thereby suggesting that although CK-18 M30 was significantly different in the two populations, it was not ideal for the non-invasive diagnosis of NASH (data not shown).
We acknowledge that there are some important limitations to this study. First, as a multicenter registry study, both liver biopsies and laboratory tests for measuring CK-18 M30 levels were performed and measured independently by individual laboratories and analyzed by experienced liver pathologists at each center (note well, this is similar practice to other multicenter registry studies, where blinded evaluation of all liver biopsy specimens by independent pathologists at a central laboratory is not undertaken). Second, even though our study included 14 different cohorts, the data may not be representative of all global ethnic groups. Third, since most of the subjects who agreed to undergo liver biopsy were patients with certain liver diseases, and all patients included in this study were patients with NAFLD, there was no comparison with a non-NAFLD healthy population. However, as previously stated,[43] it is not possible or ethical to obtain liver tissue in healthy individuals.
In conclusion, this large multinational registry study shows that the CK-18 M30 level is significantly different between patients with NASH and those with NAFL. However, CK-18 M30 measurement in isolation is of limited value for diagnosing NASH. We do not rule out the possibility that the CK-18 M30 level could be a promising diagnostic biomarker of NASH when used in combination with other non-invasive tests, but we prefer to find a single, cost-effective, efficient, non-invasive indicator in the future studies.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82070588), High-Level Creative Talents from the Department of Public Health in Zhejiang Province (No. S2032102600032), and Project of New Century 551 Talent Nurturing in Wenzhou. G. Targher is supported in part by grants from the University School of Medicine of Verona, Verona, Italy. C.D. Byrne is supported in part by the Southampton NIHR Biomedical Research Centre (No. IS-BRC-20004), UK. ME and JG are supported by the Robert W. Storr Bequest to the Sydney Medical Foundation, University of Sydney; a National Health and Medical Research Council of Australia (NHMRC) Program Grant (No. APP1053206) and Project and ideas grants (Nos. APP2001692, APP1107178, and APP1108422).
Data sharing statement
The data underlying the results of this study are available upon request because they contain potentially sensitive information. Interested researchers can contact the corresponding author for data access requests via email (zhengmh@wmu.edu.cn), or by contacting the author in each registry.
Supplementary Material
Footnotes
How to cite this article: Zhang H, Rios RS, Boursier J, Anty R, Chan WK, George J, Yilmaz Y, Wong VW, Fan J, Dufour JF, Papatheodoridis G, Chen L, Schattenberg JM, Shi J, Xu L, Wong GL, Lange NF, Papatheodoridi M, Mi Y, Zhou Y, Byrne CD, Targher G, Feng G, Zheng M. Hepatocyte apoptosis fragment product cytokeratin-18 M30 level and non-alcoholic steatohepatitis risk diagnosis: an international registry study. Chin Med J 2023;136:341–350. doi: 10.1097/CM9.0000000000002603
Supplemental digital content is available for this article.
References
- 1.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011; 140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 3.Zheng KI, Fan JG, Shi JP, Wong VW, Eslam M, George J, et al. From NAFLD to MAFLD: a “redefining” moment for fatty liver disease. Chin Med J 2020; 133:2271–2273. doi: 10.1097/CM9.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alhinai A, Qayyum-Khan A, Zhang X, Samaha P, Metrakos P, Deschenes M, et al. Non-alcoholic steatohepatitis in liver transplant recipients diagnosed by serum cytokeratin 18 and transient elastography: a prospective study. World J Hepatol 2021; 13:2179–2191. doi: 10.4254/wjh.v13.i12.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999; 94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 6.Balakrishnan M, Loomba R. The role of noninvasive tests for differentiating NASH from NAFL and diagnosing advanced fibrosis among patients with NAFLD. J Clin Gastroenterol 2020; 54:107–113. doi: 10.1097/MCG.0000000000001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rios RS, Zheng KI, Zheng MH. Non-alcoholic steatohepatitis and risk of hepatocellular carcinoma. Chin Med J 2021; 134:2911–2921. doi: 10.1097/CM9.0000000000001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology 2020; 158:1611–1625. e12. doi: 10.1053/j.gastro.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 9.Huber Y, Schulz A, Schmidtmann I, Beutel M, Pfeiffer N, Munzel T, et al. Prevalence and risk factors of advanced liver fibrosis in a population-based study in Germany. Hepatol Commun 2022; 6:1457–1466. doi: 10.1002/hep4.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikolasevic I, Filipec-Kanizaj T, Mijic M, Jakopcic I, Milic S, Hrstic I, et al. Nonalcoholic fatty liver disease and liver transplantation – where do we stand? World J Gastroenterol 2018; 24:1491–1506. doi: 10.3748/wjg.v24.i14.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 12.Schattenberg JM, Lazarus JV, Newsome PN, Serfaty L, Aghemo A, Augustin S, et al. Disease burden and economic impact of diagnosed non-alcoholic steatohepatitis in five European countries in 2018: a cost-of-illness analysis. Liver Int 2021; 41:1227–1242. doi: 10.1111/liv.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012; 142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Wang TY, Wang RF, Bu ZY, Targher G, Byrne CD, Sun DQ, et al. Association of metabolic dysfunction-associated fatty liver disease with kidney disease. Nat Rev Nephrol 2022; 18:259–268. doi: 10.1038/s41581-021-00519-y. [DOI] [PubMed] [Google Scholar]
- 15.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology 2009; 49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 16.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 2003; 125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 17.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology 2009; 50:1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwok R, Tse YK, Wong GL, Ha Y, Lee AU, Ngu MC, et al. Systematic review with meta-analysis: Non-invasive assessment of non-alcoholic fatty liver disease – the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther 2014; 39:254–269. doi: 10.1111/apt.12569. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Vali Y, Boursier J, Duffin K, Verheij J, Brosnan MJ, et al. Accuracy of cytokeratin 18 (M30 and M65) in detecting non-alcoholic steatohepatitis and fibrosis: a systematic review and meta-analysis. PLoS One 2020; 15:e0238717.doi: 10.1371/journal.pone.0238717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology 2006; 44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 21.Anty R, Iannelli A, Patouraux S, Bonnafous S, Lavallard VJ, Senni-Buratti M, et al. A new composite model including metabolic syndrome, alanine aminotransferase and cytokeratin-18 for the diagnosis of non-alcoholic steatohepatitis in morbidly obese patients. Aliment Pharmacol Ther 2010; 32:1315–1322. doi: 10.1111/j.1365-2036.2010.04480.x. [DOI] [PubMed] [Google Scholar]
- 22.Boursier J, Anty R, Vonghia L, Moal V, Vanwolleghem T, Canivet CM, et al. Screening for therapeutic trials and treatment indication in clinical practice: MACK-3, a new blood test for the diagnosis of fibrotic NASH. Aliment Pharmacol Ther 2018; 47:1387–1396. doi: 10.1111/apt.14621. [DOI] [PubMed] [Google Scholar]
- 23.Chan WK, Sthaneshwar P, Nik Mustapha NR, Mahadeva S. Limited utility of plasma M30 in discriminating non-alcoholic steatohepatitis from steatosis - A comparison with routine biochemical markers. PLoS One 2014; 9:e105903.doi: 10.1371/journal.pone.0105903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuah KH, Wan Yusoff WNI, Sthaneshwar P, Nik Mustapha NR, Mahadeva S, Chan WK. MACK-3 (combination of hoMa, Ast and CK18): a promising novel biomarker for fibrotic non-alcoholic steatohepatitis. Liver Int 2019; 39:1315–1324. doi: 10.1111/liv.14084. [DOI] [PubMed] [Google Scholar]
- 25.Huber Y, Pfirrmann D, Gebhardt I, Labenz C, Gehrke N, Straub BK, et al. Improvement of non-invasive markers of NAFLD from an individualised, web-based exercise program. Aliment Pharmacol Ther 2019; 50:930–939. doi: 10.1111/apt.15427. [DOI] [PubMed] [Google Scholar]
- 26.Papatheodoridis GV, Hadziyannis E, Tsochatzis E, Georgiou A, Kafiri G, Tiniakos DG, et al. Serum apoptotic caspase activity in chronic hepatitis C and nonalcoholic Fatty liver disease. J Clin Gastroenterol 2010; 44:e87–e95. doi: 10.1097/MCG.0b013e3181c0945a. [DOI] [PubMed] [Google Scholar]
- 27.Shen F, Zheng RD, Mi YQ, Shi JP, Wang XY, Hu XQ, et al. Value of a two-step approach with cytokeratin-18 and controlled attenuation parameter in noninvasive differential diagnosis of nonalcoholic steatohepatitis (in Chinese). Chin J Hepatol 2016; 24:429–434. doi: 10.3760/cma.j.issn.1007-3418.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Shen J, Chan HL, Wong GL, Chan AW, Choi PC, Chan HY, et al. Assessment of non-alcoholic fatty liver disease using serum total cell death and apoptosis markers. Aliment Pharmacol Ther 2012; 36:1057–1066. doi: 10.1111/apt.12091. [DOI] [PubMed] [Google Scholar]
- 29.Shen J, Chan HL, Wong GL, Choi PC, Chan AW, Chan HY, et al. Non-invasive diagnosis of non-alcoholic steatohepatitis by combined serum biomarkers. J Hepatol 2012; 56:1363–1370. doi: 10.1016/j.jhep.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 30.Sun DQ, Zheng KI, Xu G, Ma HL, Zhang HY, Pan XY, et al. PNPLA3 rs738409 is associated with renal glomerular and tubular injury in NAFLD patients with persistently normal ALT levels. Liver Int 2020; 40:107–119. doi: 10.1111/liv.14251. [DOI] [PubMed] [Google Scholar]
- 31.Zhou YJ, Gao F, Liu WY, Wong GL, Mahadeva S, Raihan Nik Mustapha N, et al. Screening for compensated advanced chronic liver disease using refined Baveno VI elastography cutoffs in Asian patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther 2021; 54:470–480. doi: 10.1111/apt.16487. [DOI] [PubMed] [Google Scholar]
- 32.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 33.Miao L, Targher G, Byrne CD, Valenti L, Qi X, Zheng MH. Portal hypertension in NAFLD: challenges and perspectives. Portal Hypertens Cirrhosis 2022; 1:57–65. doi: 10.1002/poh2.8. [Google Scholar]
- 34. Truelsen T, Begg S, Mathers C. The Global Burden of Cerebrovascular Disease. World Health Organization; 2006. Available from https://www.researchgate.net/publication/228551377. [Google Scholar]
- 35.Wang D, Zhang H, Ma H, Zhang L, Yang L, Xu L. Hearing threshold levels and hearing loss among people in Zhejiang, China: a population-based cross-sectional study. BMJ Open 2019; 9:e027152.doi: 10.1136/bmjopen-2018-027152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heneghan C, Ward A, Perera R, Self-Monitoring Trialist C, Bankhead C, Fuller A, et al. Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data. Lancet 2012; 379:322–334. doi: 10.1016/S0140-6736(11)61294-4. [DOI] [PubMed] [Google Scholar]
- 37.Schattenberg JM, Straub BK. On the value and limitations of liver histology in assessing non-alcoholic steatohepatitis. J Hepatol 2020; 73:1592–1593. doi: 10.1016/j.jhep.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 38.Davison BA, Harrison SA, Cotter G, Alkhouri N, Sanyal A, Edwards C, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol 2020; 73:1322–1332. doi: 10.1016/j.jhep.2020.06.025. [DOI] [PubMed] [Google Scholar]
- 39.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005; 128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 40.Davison BA, Edwards C, Loomba R, Harrison SA, Cotter G, Alkhouri N, et al. Noninvasive measure of treatment response in non-alcoholic steatohepatitis: insights from EMMINENCE and meta-analysis. JGH Open 2021; 5:740–749. doi: 10.1002/jgh3.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diab DL, Yerian L, Schauer P, Kashyap SR, Lopez R, Hazen SL, et al. Cytokeratin 18 fragment levels as a noninvasive biomarker for nonalcoholic steatohepatitis in bariatric surgery patients. Clin Gastroenterol Hepatol 2008; 6:1249–1254. doi: 10.1016/j.cgh.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feldstein AE, Alkhouri N, De Vito R, Alisi A, Lopez R, Nobili V. Serum cytokeratin-18 fragment levels are useful biomarkers for nonalcoholic steatohepatitis in children. Am J Gastroenterol 2013; 108:1526–1531. doi: 10.1038/ajg.2013.168. [DOI] [PubMed] [Google Scholar]
- 43.Kim YS, Jung ES, Hur W, Bae SH, Choi JY, Song MJ, et al. Noninvasive predictors of nonalcoholic steatohepatitis in Korean patients with histologically proven nonalcoholic fatty liver disease. Clin Mol Hepatol 2013; 19:120–130. doi: 10.3350/cmh.2013.19.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cusi K, Chang Z, Harrison S, Lomonaco R, Bril F, Orsak B, et al. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol 2014; 60:167–174. doi: 10.1016/j.jhep.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 45.Francque SMA, Verrijken A, Mertens I, Hubens G, Van Marck E, Pelckmans P, et al. Noninvasive assessment of nonalcoholic fatty liver disease in obese or overweight patients. Clin Gastroenterol Hepatol 2012; 10:1162–1168. doi: 10.1016/j.cgh.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 46.Tamimi TIAR, Elgouhari HM, Alkhouri N, Yerian LM, Berk MP, Lopez R, et al. An apoptosis panel for nonalcoholic steatohepatitis diagnosis. J Hepatol 2011; 54:1224–1229. doi: 10.1016/j.jhep.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zein CO, Lopez R, Fu X, Kirwan JP, Yerian LM, McCullough AJ, et al. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism. Hepatology 2012; 56:1291–1299. doi: 10.1002/hep.25778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angelini G, Panunzi S, Castagneto-Gissey L, Pellicanò F, De Gaetano A, Pompili M, et al. Accurate liquid biopsy for the diagnosis of non-alcoholic steatohepatitis and liver fibrosis. Gut 2023; 72:392–403. doi: 10.1136/gutjnl-2022-327498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu XX, Zheng KI, Boursier J, Chan WK, Yilmaz Y, Romero-Gomez M, et al. acNASH index to diagnose nonalcoholic steatohepatitis: a prospective derivation and global validation study. EClinicalMedicine 2021; 41:101145.doi: 10.1016/j.eclinm.2021.101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.