Abstract
The advent of modern synthetic-biology tools has enabled the development of cellular treatments with engineered specificity, leading to a new paradigm in anti-cancer immunotherapy. T cells have been at the forefront of such development, with six chimeric antigen receptor (CAR)-modified T cell products approved by the United States Food and Drug Administration for the treatment of hematological malignancies in the last five years. Natural killer (NK) cells are innate lymphocytes with potent cytotoxic activities, and they have become an increasingly attractive alternative to T cell therapies due to their potential for allogeneic, “off-the-shelf” applications. However, both T cells and NK cells face numerous challenges, including antigen escape, the immunosuppressive tumor microenvironment, and potential for severe toxicity. Many synthetic-biology strategies have been developed to address these obstacles, most commonly in the T-cell context. In this review, we discuss the array of strategies developed to date, their application in the NK-cell context, as well as opportunities and challenges for clinical translation.
Introduction
Surgery, radiation therapy, and chemotherapy have long served as the foundation of cancer therapy, but a number of malignancies have remained resistant to these three pillars of cancer treatment. Over the past decades, immunotherapy has emerged as a fourth pillar in the arsenal against cancer. By harnessing the patient’s own immune system, immunotherapies such as immune checkpoint blockade (1, 2), cancer vaccines (3), and adoptive transfer of immune cells engineered to target tumor antigens, have emerged as promising treatments for malignancies that are refractory to traditional therapies. Among the different immunotherapy modalities, cell-based immunotherapy has shown particular promise against hematological malignancies, but its application to the treatment of solid tumors remains work in progress (4). Multiple immune cell types, including T cells, natural killer (NK) cells, invariant natural killer T (iNKT) cells, macrophages, and neutrophils have been explored as potential chassis for cell-based immunotherapy, with T cells and NK cells as the most extensively evaluated effector cell types to date.
In contrast to small-molecule drugs, protein biologics, or radiation, cell-based immunotherapies are living drugs with the ability to persist, amplify, and traverse within the patient. These unique characteristics allow cell-based therapies to mount dynamic and complex immune responses against the tumor unattainable by other therapeutic modalities. The ability to specifically program the biological properties of therapeutic cells further expands the capabilities of engineered immune cells as cancer therapeutics. Synthetic biology is a growing discipline that generates biological systems with novel behaviors and functions, by assembling circuitries comprising synthetic biological components and/or naturally occurring biological parts repurposed for new applications. By focusing on the design, construction, and assembly of modular biological components, synthetic biology enables researchers to build biological circuits with programmable input processing and output parameters. In the context of cell therapies, integration of these circuits into immune cells enables development of products equipped with novel therapeutic functions to combat previously ‘undruggable’ or untreatable diseases. Additionally, synthetic biology can accelerate the acquisition of well-controlled biological datasets through the use of clear design rules, thus enabling better understanding of complex biological phenomena and facilitating rational biological engineering principles for translational applications.
Advancements in synthetic biology have been supported by new DNA synthesis and sequencing technologies that enable accurate and high-throughput design, assembly, and testing of biological circuitry (5, 6). Concurrently, gene-editing technologies such as with zinc finger nucleases, transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 have expanded the synthetic-biology toolkit, enabling high-throughput gene screening, targeted gene ablation and transgene insertion, and development of more complicated biological models (7). While the first generation of U.S. Food and Drug Administration (FDA)-approved cell-based cancer immunotherapies largely relies on the expression of a single transgene encoding a tumor-targeting receptor, the incorporation of biological circuits may further expand the safety and efficacy profile of cell therapies to address perpetually intractable diseases.
In this review, we assess the current state of synthetic biology applications in the field of cell-based immunotherapies, focusing specifically on advancements aimed to direct and repurpose T and NK cells against cancer.
Early Development of NK- and T-Cell Immunotherapies
NK and T cells are both lymphocytes with cytotoxic capabilities, including perforin and granzyme-mediated cytotoxicity and pro-inflammatory cytokine release, making them attractive candidates for cell-based anti-tumor therapeutics (8, 9). However, the two effector cell types also possess differential characteristics that endow each with distinct advantages (Table 1). T cells, the prototypical effectors of adaptive immunity, derive their antigen specificity from genetic recombination of the T-cell receptor, enabling highly specific recognition of foreign peptides presented on host major histocompatibility complex (MHC) molecules to induce clonal T-cell expansion and potent cytotoxicity. Importantly, T cells can establish long-term memory and persistence, thus providing the possibility of long-term surveillance against disease reemergence (10). On the other hand, NK cells are members of the innate immune system that have a relatively short lifespan and more limited proliferative potential, and they do not naturally express receptors with a broad repertoire of antigen specificity (11, 12). Instead, NK cells are regulated by a collection of germline-encoded receptors that can either activate or suppress cytotoxicity upon ligand binding, thus enabling the elimination of virally infected, transformed, or antibody-labeled cells. Importantly, NK cells express many of the signaling molecules downstream of T-cell receptor signaling (13, 14), thus enabling the adaptation of receptors designed for T-cell therapy to the NK cell context.
Table 1.
List of advantages and disadvantages for T and NK cell therapies.
| Cell Type | Advantages | Disadvantages |
|---|---|---|
| T Cells |
|
|
| NK Cells |
|
|
To date, T-cell therapy development has led the way in oncology applications, culminating in the FDA approval of six autologous chimeric antigen receptor (CAR)-T cell products for B-cell hematological malignancies and hundreds of active clinical trials against various malignancies. However, challenges accompanying autologous cell therapy, such as the risk of manufacturing failure and disease progression by patients awaiting cell manufacturing (15), have motivated research into allogeneic alternatives such as NK cells (Table 2). Unlike T cells, NK cells do not recognize MHC-presented antigens, thus avoiding the potential for graft-versus-host disease that can be triggered by allogeneic adoptive T-cell therapy. This unique property positions NK cell therapies for “off-the-self” use without the need to knock out endogenous receptors. Furthermore, unlike T cells, allogeneic NK cells can be generated from scalable sources such as cord blood, immortalized cell lines, and induced pluripotent stem cells (iPSCs), which can dramatically decrease the cost of adoptive cell therapy (16-18). Lastly, clinical evidence to date suggests NK-cell therapies can achieve anti-tumor efficacy without triggering severe cytokine release syndrome, a serious and commonly observed side effect of T-cell based therapies (17).
Table 2.
List of CAR-NK cells in clinical trials.
| Clinical Trial Identifier |
Trial Name | Antigen Target |
Disease Indication |
NK Cell Source |
Trial Status |
Year Trial Started |
Armor |
|---|---|---|---|---|---|---|---|
| NCT03056339 | Umbilical & Cord Blood (CB) Derived CAR-Engineered NK Cells for B Lymphoid Malignancies | CD19 | Stem Cell Transplant Patients with Relapsed and/or Refractory B-Cell Lymphoma or Leukemia | Cord Blood | Active, not recruiting | 2017 | iCasp9 and IL-15 |
| NCT03692663 | Study of Anti-PSMA CAR NK Cell (TABP EIC) in Metastatic Castration-Resistant Prostate Cancer | PSMA | Metastatic Castration-Resistant Prostate Cancer | Unknown | Recruiting | 2018 | N/A |
| NCT04623944 | NKX101, Intravenous Allogeneic CAR NK Cells, in Adults With AML or MDS | NKG2D Ligands | Relapsed or Refractory Acute Myeloid Leukemia or Intermediate, High and Very High Risk Relapsed or Refractory MDS | Either Haplo-Matched Related Donor Derived or Unrelated Off-the-Shelf Donor Derived | Recruiting | 2020 | Membrane-Bound IL-15 |
| NCT04887012 | Clinical Study of HLA Haploidentical CAR-NK Cells Targeting CD19 in the Treatment of Refractory/Relapsed B-cell NHL | CD19 | B-cell Non-Hodgkin's lymphoma | HLA haploidentical | Recruiting | 2021 | N/A |
| NCT05008575 | Anti-CD33 CAR NK Cells in the Treatment of Relapsed/Refractory Acute Myeloid Leukemia | CD33 | Acute Myeloid Leukemia | Unknown | Recruiting | 2021 | N/A |
| NCT05008536 | Anti-BCMA CAR-NK Cell Therapy for the Relapsed or Refractory Multiple Myeloma | BCMA | Multiple Myeloma, Refractory | Umbilical and Cord Blood | Recruiting | 2021 | N/A |
| NCT04847466 | Immunotherapy Combination: Irradiated PD-L1 CAR-NK Cells Plus Pembrolizumab Plus N-803 for Subjects With Recurrent/Metastatic Gastric or Head and Neck Cancer | PD-L1 | Gastroesophageal Junction Cancers Advanced HNSCC | NK-92 | Recruiting | 2021 | N/A |
| NCT04796675 | Cord Blood Derived Anti-CD19 CAR-Engineered NK Cells for B Lymphoid Malignancies | CD19 | Relapsed or Refractory Hematological Malignancies | Cord Blood | Recruiting | 2021 | N/A |
| NCT05020678 | NKX019, Intravenous Allogeneic Chimeric Antigen Receptor Natural Killer Cells (CAR NK), in Adults With B-cell Cancers | CD19 | Relapsed or Refractory Non-Hodgkin Lymphoma, Chronic Lymphocytic Leukemia or B cell Acute Lymphoblastic Leukemia | Either Haplo-Matched Related Donor Derived or Unrelated Off-the-Shelf Donor Derived | Recruiting | 2021 | Membrane-Bound IL-15 |
| NCT05247957 | NKG2D CAR-NK Cell Therapy in Patients With Relapsed or Refractory Acute Myeloid Leukemia | NKG2D | Recurrent Refractory Acute Myeloid Leukemia | Umbilical Cord | Recruiting | 2022 | N/A |
| NCT05410717 | CLDN6-CAR-NK Cell Therapy for Advanced Solid Tumors | Claudin6 | Advanced Solid Tumors | Autologous PBMC | Recruiting | 2022 | IL7 + CCL19 or scFvs Against PD1/CTLA4/Lag3 |
| NCT05213195 | NKG2D CAR-NK Cell Therapy in Patients With Refractory Metastatic Colorectal Cancer | NKG2D | Refractory Metastatic Colorectal Cancer | Unknown | Recruiting | 2022 | N/A |
| NCT05215015 | Study of Anti-CD33/CLL1 CAR-NK in Acute Myeloid Leukemia | CD33/CLL1 | Acute Myeloid Leukemia | Unknown | Recruiting | 2022 | N/A |
| NCT05194709 | Study of Anti-5T4 CAR-NK Cell Therapy in Advanced Solid Tumors | 5T4 | Advanced Solid Tumors | Unknown | Recruiting | 2022 | N/A |
| NCT05410041 | Anti-CD19 CAR-Engineered NK Cells in the Treatment of Relapsed/Refractory B-cell Malignancies | CD19 | Recurrent or Refractory CD19-Positive B-Cell Malignant Tumors | Unknown | Recruiting | 2022 | N/A |
| NCT05379647 | Natural Killer (NK) Cell Therapy for B-Cell Malignancies | CD19 | Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia and Relapsed or Refractory B-cell Lymphoma | Unknown | Recruiting | 2022 | N/A |
| NCT05182073 | FT576 in Subjects With Multiple Myeloma | BCMA | Multiple Myeloma | iPSC | Recruiting | 2022 | Modified CD16, IL-15 Receptor Fusion, Elimination of CD38 |
Early clinical trials evaluating the adoptive transfer of NK cells relied on their innate ability to identify transformed cells for anti-tumor efficacy, leading to modest outcomes (19, 20). However, advances in synthetic biology and the remarkable efficacy of CAR-T cells have provided a template for engineering more targeted and potent NK cell therapies. Early development of synthetic activating receptors in NK cells utilized prototypical CARs developed for T cells and validated the ability to redirect NK-cell cytotoxicity in an antigen-specific manner (21). Similarly, the earliest CAR-NK cell clinical trials utilized prototypical, CD19-targeting CARs (NCT00995137, NCT01974479). While the recent successes with CAR-T cells have reinvigorated the field of cellular therapy, more sophisticated strategies are needed to broaden its therapeutic outlook and synthetic biology is expected to be key in facilitating the engineering of these next-generation therapies.
Synthetic Biology in CAR Engineering
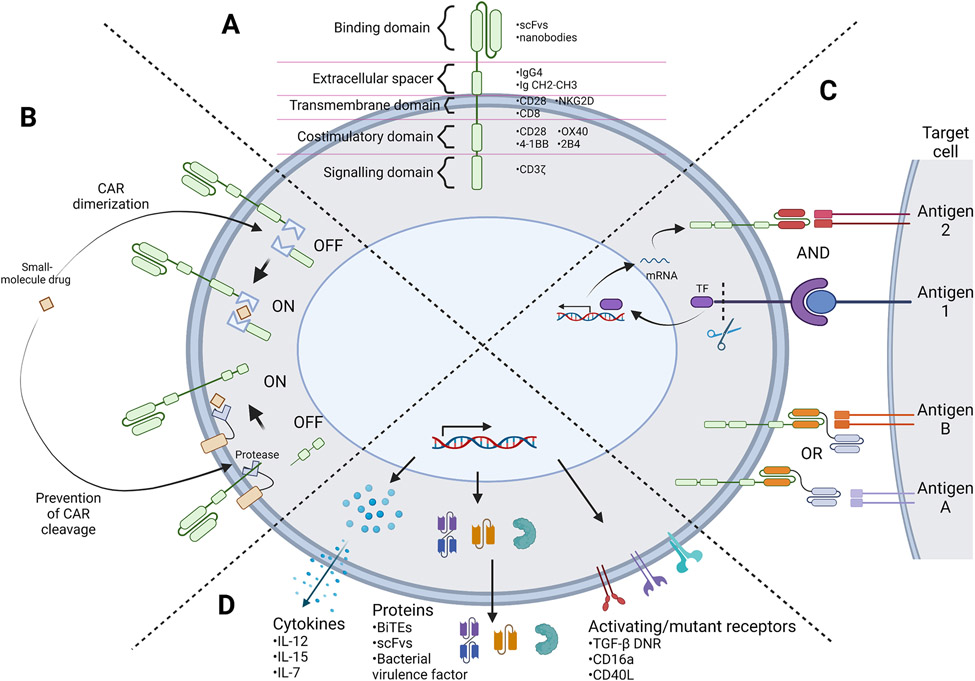
CARs are synthetic receptors that enable immune cells to recognize and initiate antigen-specific cytotoxic responses (22). The adoptive transfer of CAR-T cells has demonstrated remarkable clinical efficacy in treating various B-cell hematological malignancies, becoming the first genetically modified cell therapy to receive FDA approval in 2017 (23). CARs are transmembrane proteins comprising of four major components: an extracellular antigen-binding domain, extracellular spacer domain, transmembrane domain, and intracellular signaling domain (Fig. 1A). This architecture allows for transduction of an extracellular antigen-binding event into an intracellular signaling cascade, resulting in downstream cell activation and subsequent target-cell killing. First-generation CARs, which only utilize CD3ζ as an intracellular signaling domain, failed to elicit potent anti-tumor activity in clinical trials (24, 25). In response, one or two intracellular co-stimulatory domains were incorporated in second- and third-generation CARs, respectively, to enhance cytokine production, proliferation, and in vivo persistence (26-28). Although the early development of CAR-NK cells directly adopted CARs developed for T cells, the modular design of CARs has also allowed development of novel CARs containing alternative transmembrane and signaling domains that may be better suited for NK-cell function. For example, “NK-CARs” containing an NKG2D transmembrane domain and a 2B4 co-stimulatory domain, both NK-cell–related proteins, have shown promising anti-tumor function in pre-clinical settings. In comparison to T cells expressing prototypical “T-CARs” comprising CD28 transmembrane and CD28 plus 4-1BB costimulatory domains, iPSC-derived NK cells expressing NK-CARs achieved stronger efficacy and reduced off-tumor in a mouse xenograft model of ovarian cancer (29). These results suggest that tailoring of CAR components for optimal signaling in NK cells may further increase the efficacy of CAR-NK cell therapies for solid tumors.
Figure 1.
Representation of synthetic biology strategies for engineering next-generation T and NK cell therapies. (A) Schematic depicting the CAR protein architecture along with commonly employed domains in each modular component. (B) Example of CAR regulation strategies. A small-molecule drug can act as a dimerizing agent to assemble a full CAR protein, or stabilize CAR protein expression by inhibiting a protease to prevent CAR protein cleavage. (C) AND and OR Boolean logic-gate strategies for multi-antigen sensing. AND-gated strategies require the sensing of multiple tumor antigens to trigger target cell killing. For example, recognition of antigen 1 can prompt the release of a transcription factor (TF) that triggers expression of a CAR specific to antigen 2, thus both antigens 1 and 2 must be present in order to trigger CAR-T cell activation. OR-gated strategies require the sensing of any one antigen among multiple antigens to trigger target cell killing. For example, tandem bispecific OR-gate CARs have two binding domains that recognize two different antigens, such that recognition of either antigen A or B will induce activation of the CAR-expressing cell. (D) Armoring strategies in which transgenic payloads are expressed together with the CAR transgene. These payloads can be cytokines, chemokines, receptors, or other proteins that aim to improve the function of the T or NK cell and/or neighboring immune cells, frequently with the goal of reprogramming the surrounding tumor microenvironment. (Figure created with BioRender.)
Regulatable CAR platforms for improved safety
Unlike antibodies and small-molecule drugs, cell-based therapies use living cells that can proliferate, persist, and circulate within the body, thereby mounting dynamic and complex immune responses against target cells. However, the dynamism and potency of cell-based therapies also pose unique safety challenges in the clinic, with the potential for severe toxicities such as cytokine release syndrome and immune-effector cell associated neurotoxicity syndrome (30). A potential strategy to reduce unintended toxicity is to implement regulatory devices that can control either the expression or the function of CAR proteins, and consequently regulate the activity of CAR-expressing cells.
The regulation of gene expression or protein activity using small-molecule drugs has a long history in mammalian synthetic biology (31, 32), and drug-regulatable platforms have been used to enable on-demand cessation of CAR signaling activity without permanently ablating the engineered cell population (Fig. 1B). For example, ON switches can be engineered by splitting the CAR protein into two nonfunctional domains such that CARs are in the inactive OFF state by default, and expression can be turned on through the administration of a dimerizing drug (33-35). Alternatively, OFF switches can be engineered such that CARs fused to degradation domains are in a default ON state, but administration of small-molecule drugs can induce CAR proteasomal degradation to turn expression off (36, 37). Although drug-regulatable expression systems provide increased flexibility and control, common challenges for these switch designs include: ‘leaky’ (i.e., high baseline) activity in the OFF state, reduced CAR expression and potency compared to constitutive systems, and poor dynamic range between the ON and OFF states. Various engineering strategies are under active evaluation to address these obstacles. A recently reported drug-regulatable platform termed signal neutralization by an inhibitable protease, or SNIP, has been shown to have a tight OFF state, improved dynamic range, and improved potency compared to constitutively active CARs in multiple hematological and solid tumor models (38). Carefully designed clinical trials will be required to evaluate the tunability of drug-regulated CAR designs in human patients, where additional variables such as cell quality, tumor burden, and drug pharmacokinetics could individually and jointly impact the behavior of engineered cells.
Multi-antigen sensing for improved safety and specificity
In addition to toxicities associated with overly active immune responses, off-tumor toxicity presents an additional challenge to cell-based cancer immunotherapy. The lack of targetable tumor-restricted antigens necessitates the targeting of tumor-associated antigens (TAAs) that are not exclusively expressed on tumor cells, thus exposing healthy tissues that express the same antigen to ‘on-target off-tumor’ toxicity. For example, CD19 CAR-T cell and CD19 CAR-NK cell therapies both result in B-cell aplasia in responding patients, as the CD19-targted immune cells simultaneously eliminate CD19-expressing healthy and malignant B cells (17, 39). B-cell aplasia is a clinically manageable condition (40), but analogous on-target, off-tumor toxicities against other antigens such as HER2, mesothelin, and carcinoembryonic antigen have led to early trial terminations and patient fatalities (41, 42).
Tumor-targeting specificity can be improved by biological circuitry that computes AND- or AND-NOT–gated Boolean logic, which requires the engagement of multiple TAAs in a specific combination before triggering target-cell killing (Fig. 1C). An AND-gate CAR requires that two or more antigens are present to trigger CAR signaling. One way to achieve this is to distribute the CD3ζ activation domain and co-stimulatory domain (e.g., CD28 or 4-1BB) of a typical second-generation CAR into two different receptors, one first-generation CAR containing only the CD3ζ chain, and a chimeric costimulatory receptor (CCR) containing only the co-stimulatory domain (43). Both receptors must be bound to their respective ligands in order to trigger full-intensity T-cell responses against the target cell. Another approach to achieving AND-gate logic requires sequential detection of multiple antigens, such as with the synthetic Notch (synNotch) or synthetic intramembrane proteolysis receptor (SNIPR) systems (44-46). These designs require two gene-expression cassettes. The first cassette constitutively expresses a synthetic receptor containing a transcription factor that is cleaved and released when the receptor binds its ligand (antigen A). The released transcription factor translocates to the nucleus and drives the inducible expression of a conventional CAR, which subsequently targets antigen B. Such AND-gated designs have been shown to prevent killing of off-tumor targets that express only one antigen but not the other. However, AND-gate designs remain capable of off-tumor killing if healthy tissue expressing the CAR-targeted antigen is colocalized with the tumor cells, as shown in the case of a synNotch-controlled ROR1 CAR-T cell therapy that triggered severe toxicity in the Raji lymphoma model due to simultaneous destruction of ROR1-expressing lymphoma and healthy tissue in the bone marrow (47).
As an alternative strategy, AND-NOT–gate CARs have also been shown to increase target specificity. AND-NOT gates require both the presence of antigen A and the absence of antigen B in order to trigger cell activation (48, 49). This is accomplished by co-expressing a conventional, activating CAR (aCAR) targeting antigen A with an inhibitory CAR (iCAR) targeting antigen B. iCAR signaling triggered by antigen B overrides aCAR signaling, thus inhibiting cell activation when antigen B is present. By using an aCAR that targets a TAA and an iCAR that targets HLA-A2, this AND-NOT–gate strategy has been applied to target tumor cells that have downregulated MHC expression, thus simultaneously increasing tumor specificity while addressing a potential immune escape mechanism (49, 50).
Despite significant potential advantages, AND- and AND-NOT–gated CAR designs also face important caveats. First, each of the circuits described above require the expression of multiple transgenes, and the increased genetic payload size can significantly reduce the efficiency of transgene integration (51). Second, early synthetic-biology attempts at making computation circuits, exemplified by the synNotch system, often utilized non-human components such as viral transcription factors, and the potential immunogenicity of such designs presents a significant barrier to clinical translation. Finally, by increasing the number of antigens that must be present or absent in a specific combination, the system increases the probability for antigen escape, as the tumor now only needs to alter one of multiple antigens’ expression pattern to avoid detection.
Multi-antigen sensing to prevent tumor escape
Although tumor specificity—i.e., ability to specifically recognize tumor cells and not healthy tissue—is a critical consideration in therapy design due to its safety implications, the flip side of the coin—i.e., inability to recognize all tumor cells by targeting a single antigen—also presents a key challenge in cell-based therapy. Antigen escape can arise from either natural tumor heterogeneity or antigen downregulation in response to selective pressure imposed by therapy. For example, a substantial fraction of patients with B-cell malignancies relapse after CD19 CAR-T cell therapy, and up to 94% of relapsing patients have CD19-negative tumors (52-54). Antigen escape poses an even greater challenge in solid tumors such as glioblastoma multiforme (GBM) due to intrinsic heterogeneity in antigen expression (55). Consequently, antigen loss has been observed as a major mechanism of CAR-T cell treatment resistance in patients with GBM (56, 57). Several methodologies have been proposed to address antigen escape, including incorporating tandem bispecific CARs or multiple CARs targeting different antigens into a single cell (OR-gate Boolean logic), or simultaneously or sequentially administering multiple cell products that target different antigens. In most head-to-head comparisons, tandem bispecific CARs have exhibited greater anti-tumor efficacies compared to co-expressing two CARs in a cell or administering a pooled T-cell population (58-60). In OR-gate Boolean logic, therapeutic cells are engineered to recognize two or more antigens, and the presence of any recognizable antigen would trigger cell-mediated toxicity, thus requiring the tumor to lose all recognizable antigens in order to successfully evade detection. For example, tandem scFv bispecific CARs have demonstrated reduced tumor relapse and superior anti-tumor activity against B-cell malignancies susceptible to CD19 antigen loss (61, 62). Early success with CD19/CD20- and CD19/CD22-targeting bispecific CARs have led to the initiation of numerous ongoing clinical trials (NCT04007029, NCT04700319, NCT03241940). Multi-antigen targeting has also been extended to pre-clinical and clinical studies in other malignancies, including multiple myeloma (e.g., NCT04162353) (60) and GBM (58). It should be noted that, depending on the choice of target antigens, OR-gated CAR designs have the potential to exacerbate off-tumor toxicity, as a wider range of healthy tissue may become recognizable to engineered cells that target multiple antigens.
To date, designs that enable regulated or logic-gated CAR activities have largely been demonstrated in the T-cell setting. However, such designs may become increasingly important in CAR-NK cell engineering as this treatment modality expands beyond B-cell malignancies and into indications with greater toxicity potential or more heterogeneous antigen-expression profiles (63). Despite the largely safe clinical profile of NK-cell therapies reported to date (17, 64), biological designs that enable regulated CAR activity and logic-gated signal computation remain useful resources as CAR-NK cells advance to a broader array of disease indications.
Armoring NK and T cells to improve efficacy
To date, much of the synthetic biology efforts in cell-based therapies have focused on the engineering of CAR proteins or circuitries that revolve around CAR proteins. However, numerous biological pathways work in concert to impact the anti-tumor activities of T cells and NK cells, thus providing a wide variety of engineering targets that can potentially enhance efficacy. To date, solid tumors remain intractable challenges for cell-based therapies due to various immunosuppressive and immune-evading mechanisms unique to the tumor microenvironment (TME), such as overexpression of inhibitory ligands and cytokines, hypoxia, a poor nutrient profile, and dysfunctional immune or stromal cells (65). To address this, CAR-expressing immune cells have been “armored” with cytokines, chemokines, receptors, or other transgenic molecules to enhance T-cell cytotoxicity, persistence, or tumor infiltration, or to remodel the TME to a more proinflammatory state that favors anti-tumor activity (66) (Fig. 1D). CAR-T cells have been engineered to co-express cytokines such as interleukin (IL)-12 (67), IL-7 (68), and IL-15 (69), as well as payloads such as bispecific T-cell engagers (70, 71) and bacterial virulence factors (72) to improve targeting of solid tumors with heterogeneous antigen expression. Many armored CAR-T cells are currently being evaluated in the clinic (Table 3), and promising preliminary results have begun to emerge. In a phase-I clinical trial (NCT03198546), six patients with liver, pancreatic, or ovarian cancer were treated with mesothelin- or GPC3-targeting CAR-T cells armored with IL-7 and CCL19. Preliminary report notes one complete response and one partial response among the treated patients, with fever and fatigue as common side effects but “no grade 2–4 adverse events or major complications” reported (73). Although the number of patients treated with armored CAR-T cells to date is relatively small, the potential to achieve response without severe toxicity in patients with solid tumors is highly encouraging.
Table 3.
List of armored CAR-T cells in clinical trials.
| Clinical Trial Identifier |
Trial Name | Antigen Target | Disease Indication |
T Cell Source |
Trial Status |
Year Trial Started |
Armor |
|---|---|---|---|---|---|---|---|
| NCT01822652 | 3rd Generation GD-2 Chimeric Antigen Receptor and iCaspase Suicide Safety Switch, Neuroblastoma, GRAIN (GRAIN) | GD2 | Relapsed or Refractory Neuroblastoma | Autologous | Active, not recruiting | 2013 | iCaspase9 |
| NCT01953900 | iC9-GD2-CAR-VZV-CTLs/Refractory or Metastatic GD2-positive Sarcoma and Neuroblastoma (VEGAS) | GD2 | Advanced Osteosarcoma and Neuroblastoma | Autologous Varicella Zoster Virus (VZV) Specific T Cells | Active, not recruiting | 2013 | iCaspase9 |
| NCT02498912 | Cyclophosphamide Followed by Intravenous and Intraperitoneal Infusion of Autologous T Cells Genetically Engineered to Secrete IL-12 and to Target the MUC16ecto Antigen in Patients With Recurrent MUC16ecto+ Solid Tumors | MUC16 | MUC16-Positive Solid Tumors | Autologous | Active, not recruiting | 2015 | fIL-12 |
| NCT03356782 | Safety and Efficacy Evaluation of 4th Generation Safety-engineered CAR T Cells Targeting Sarcomas | CD133, GD2, Muc1, CD117 or Others | Relapsed or Late-Staged Sarcoma | Autologous | Recruiting | 2017 | anti-PD-1 Ig, anti-PD-L1 Ig, iCaspase9 |
| NCT03198546 | GPC3-CAR-T Cells for Immunotherapy of Cancer With GPC3 Expression | GPC3 and/or Soluble TGFβ | GPC3-Positive Hepatocellular Carcinoma | Autologous | Recruiting | 2017 | IL7 + CCL19 or scFvs Against PD1/CTLA4/TIGIT |
| NCT03016377 | Administration of Autologous CAR-T CD19 Antigen With Inducible Safety Switch in Patients With Relapsed/Refractory ALL | CD19 | Relapsed or Refractory Acute Lymphoblastic Leukemia | Autologous | Recruiting | 2017 | iCaspase9 |
| NCT03373071 | Anti-CD19 CAR T Cells in Pediatric Patients Affected by Relapsed/Refractory CD19+ ALL and NHL | CD19 | Pediatric Relapsed or Refractory B- Acute Lymphoblastic Leukemia or Non-Hodgkin’s Lymphoma with Measurable Bone Marrow Involvement | Autologous | Active, not recruiting | 2017 | Suicide switch |
| NCT03089203 | CART-PSMA-TGFβRDN Cells for Castrate-Resistant Prostate Cancer | PSMA | Metastatic Castrate Resistant Prostate Cancer | Autologous | Recruiting | 2017 | TGFβ DNR |
| NCT03721068 | Study of CAR T-Cells Targeting the GD2 With IL-15+iCaspase9 for Relapsed/Refractory Neuroblastoma or Relapsed/Refractory Osteosarcoma | GD2 | Relapsed or Refractory Neuroblastoma or Osteosarcoma | Autologous | Recruiting | 2018 | IL-15 + iCaspase9 |
| NCT03602157 | Study of CAR-T Cells Expressing CD30 and CCR4 for r/r CD30+ HL and CTCL | CD30 | Relapsed or Refractory CD30+ Hodgkin’s Lymphoma and cutaneous T-cell lymphoma | Autologous | Recruiting | 2018 | CCR4 |
| NCT03778346 | Integrin β7, BCMA, CS1, CD38 and CD138 as the Single or Compound Targets for the Fourth Generation of CAR-T Cells | Multiple | Relapsed or Refractory Multiple Myeloma | Autologous | Recruiting | 2018 | IL-7, CCL19 |
| NCT03635632 | C7R-GD2.CART Cells for Patients With Relapsed or Refractory Neuroblastoma and Other GD2 Positive Cancers (GAIL-N) | GD2 | Relapsed or Refractory Neuroblastoma and other GD2-positive Solid Cancers | Autologous | Recruiting | 2018 | IL-7 Receptor |
| NCT03696784 | Anti-CD19 CAR-T Cells With Inducible Caspase 9 Safety Switch for B-cell Lymphoma | CD19 | Relapsed or Refractory B-Cell Lymphoma | Autologous | Recruiting | 2018 | iCaspase9 |
| NCT03741127 | Long-Term Follow-Up Study for Subjects Treated With P-BCMA-101 | BCMA | Multiple Myeloma | Autologous | Active, not recruiting | 2018 | iCaspase9 |
| NCT03932565 | Interventional Therapy Sequential With the Fourth-generation CAR-T Targeting Nectin4/FAP for Malignant Solid Tumors | Nectin4/FAP | Nectin4-Positive Advanced Malignant Solid Tumors | Autologous | Recruiting | 2019 | IL-7, CCL19, or / and IL-12 |
| NCT04186520 | CAR-20/19-T Cells in Patients With Relapsed Refractory B Cell Malignancies | CD19/CD20 | Relapsed, refractory B-Cell Non-Hodgkin Lymphoma or Chronic Lymphocytic Leukemia | Autologous | Recruiting | 2019 | IL-7 + IL-15 |
| NCT03814447 | The Fourth Generation CART-cell Therapy for Refractory-Relapsed Ovarian Cancer | MSLN | Refractory or Relapsed Ovarian Cancer | Autologous | Recruiting | 2019 | Unknown |
| NCT04016129 | CAR-T Immunotherapy Targeting CD19- ALL | CD22/CD123/CD38/CD10/CD20/TSLPR | Patients Who Have Relapsed after CD19 CAR-T Therapy or Have CD19-Negative B cell Malignancies | Autologous | Recruiting | 2019 | Unknown |
| NCT04099797 | C7R-GD2.CAR T Cells for Patients With GD2-expressing Brain Tumors (GAIL-B) | GD2 | GD2-Positive or H3K27M-Mutant Diffuse Intrinsic Pontine Glioma, High Grade Glioma, Embryonal Tumors, or Ependymal Tumors | Autologous | Recruiting | 2019 | IL-7 Receptor |
| NCT04162119 | Safety and Efficiency Study of BCMA-PD1-CART Cells in Relapsed/Refractory Multiple Myeloma | BCMA | Relapsed or Refractory Multiple Myeloma | Autologous | Recruiting | 2019 | Mutant PD-1Fc Fusion Protein |
| NCT04489862 | αPD1-MSLN-CAR T Cells for the Treatment of MSLN-positive Advanced Solid Tumors | MSLN | MSLN-Positive Solid Tumors | Autologous | Recruiting | 2020 | Secreted anti-PD-1 Nanobody |
| NCT04684563 | huCART19-IL18 in NHL/CLL Patients | CD19 | Non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia | Autologous | Recruiting | 2020 | IL-18 |
| NCT04429438 | Multi-CAR-T Cells Targeting B Cell Lymphomas | CD19 + Others | Primary Mediastinal B Cell Lymphoma and B Cell Lymphoma Involving the Central Nervous System | Autologous | Recruiting | 2020 | iCaspase9 |
| NCT04430530 | 4SCAR-T Therapy Post CD19-targeted Immunotherapy | CD22/CD123/CD38/CD10/CD20 | CD19 Negative B-cell Malignancies | Autologous | Recruiting | 2020 | iCaspase9 |
| NCT04381741 | CD19 CAR-T Expressing IL7 and CCL19 Combined With PD1 mAb for Relapsed or Refractory Diffuse Large B Cell Lymphoma (CICPD) | CD19 | Diffuse Large B-cell Lymphoma | Autologous | Recruiting | 2020 | IL-7, CCL19 |
| NCT04430595 | Multi-4SCAR-T Therapy Targeting Breast Cancer | Her2, GD2, or CD44v6 | Breast Cancer | Autologous | Recruiting | 2020 | iCaspase9 |
| NCT04577326 | Mesothelin-targeted CAR T-cell Therapy in Patients With Mesothelioma | MSLN | MSLN-Positive Malignant Pleural Mesothelioma | Autologous | Active, not recruiting | 2020 | PD-1 DNR |
| NCT04650451 | Safety and Activity Study of HER2-Targeted Dual Switch CAR-T Cells (BPX-603) in Subjects With HER2-Positive Solid Tumors | HER2 | Previously Treated, Locally Advanced or Metastatic HER2-Amplified/Overexpressed Solid Tumors | Autologous | Recruiting | 2020 | iCaspase9 |
| NCT04249947 | P-PSMA-101 CAR-T Cells in the Treatment of Subjects With Metastatic Castration-Resistant Prostate Cancer (mCRPC) and Advanced Salivary Gland Cancers (SGC) | PSMA | Metastatic Castration-Resistant Prostate Cancer and Advanced Salivary Gland Cancers | Autologous | Recruiting | 2020 | iCaspase9 |
| NCT04227275 | A Study of CART-PSMA-TGFβRDN in Patients With Metastatic Castration Resistant Prostate Cancer | PSMA | Metastatic Castrate Resistant Prostate Cancer | Autologous | Active, not recruiting | 2020 | TGFβ DNR |
| NCT04377932 | Interleukin-15 Armored Glypican 3-specific Chimeric Antigen Receptor Expressed in T Cells for Pediatric Solid Tumors | GPC3 | Relapsed or Refractory GPC3-Positive Solid Tumors | Autologous | Recruiting | 2021 | IL-15 |
| NCT04706936 | Novel BCMA-targeted CAR-T Cell Therapy for Multiple Myeloma | BCMA | Relapsed or Refractory Multiple Myeloma | Autologous | Recruiting | 2021 | Unknown (4th gen) |
| NCT04842812 | Engineered TILs/CAR-TILs to Treat Advanced Solid Tumors | HER2, MSLN, PSCA, MUC1, Lewis-Y, GPC3, AXL, EGFR, Claudin18.2/6, ROR1, GD1, or B7-H3 | Advanced Solid Tumors | Tumor Infiltrating Lymphocytes | Recruiting | 2021 | scFvs that Target PD1 and CTLA4 |
| NCT04850560 | Sequential Low-dose Decitabine With PD-1/CD28 CD19 CAR-T in Relapsed or Refractory B-cell Lymphoma | CD19 | CD19-Positive Relapsed or Refractory B-Cell Lymphoma | Autologous | Recruiting | 2021 | PD-1/CD28 Switch Receptor |
| NCT04960579 | P-BCMA-ALLO1 Allogeneic CAR-T Cells in the Treatment of Subjects With Multiple Myeloma (MM) | BCMA | Relapsed or Refractory Multiple Myeloma | Allogeneic T cells | Recruiting | 2021 | iCaspase9 |
| NCT05166070 | An Exploratory Clinical Study of RD133 in Subjects With Relapsed or Refractory MSLN-Positive Solid Tumors | MSLN | Relapsed or Refractory MSLN-Positive Solid Tumors | Autologous | Recruiting | 2021 | TGFβ DNR |
| NCT05141253 | Safety and Efficacy of RD133 in Subjects With Relapsed or Refractory MSLN-Positive Solid Tumors | MSLN | Relapsed or Refractory MSLN-Positive Solid Tumors | Autologous | Recruiting | 2021 | TGFβ DNR |
| NCT05373147 | αPD1-MSLN-CAR T Cells for the Treatment of MSLN-positive Advanced Solid Tumors | MSLN | Advanced Solid Tumors with >10% MSLN | Autologous | Recruiting | 2022 | Secreted anti-PD-1 Nanobody |
| NCT05239143 | P-MUC1C-ALLO1 Allogeneic CAR-T Cells in the Treatment of Subjects With Advanced or Metastatic Solid Tumors | MUC1-C | Advanced or Metastatic Solid Tumors | Allogeneic T cells | Recruiting | 2022 | iCaspase9 |
| NCT05487495 | Donor-Derived CD5 CAR T (CT125B) Cells for Relapsed or Refractory T- Cell Acute Lymphoblastic Leukemia/Lymphoma | CD5 | Relapsed or Refractory T-Cell Acute Lymphoblastic Leukemia/Lymphoma | Autologous | Recruiting | 2022 | Thymidine Kinase (HSV-TK) Suicide Switch |
Likewise, NK cells have been engineered to express armors that exploit their biological characteristics to further improve therapeutic outcomes. In a landmark clinical trial, cord-blood NK cells were engineered to express a second-generation CD19 CAR plus IL-15 to enhance survival, as well as inducible caspase 9 as a safety switch (17). The therapy was efficacious and well tolerated in a large cohort of lymphodepleted patients with relapsed or refractory CD19-postive malignancies, with a 64% complete response rate and CAR-NK cells remaining detectable in peripheral blood for >12 months. This persistence level contrasts with earlier adoptive NK-cell transfer regimens, where NK cell persistence was limited to days or weeks without cytokine supplementation (19, 74). Armoring strategies designed to promote the proliferation and persistence of therapeutic cells may be particularly critical in the allogeneic setting, where the durability of response has been a major concern. Whereas autologous CAR-T cells have been shown to persist in treated patients for a decade (75), the durability of response in patients treated with allogeneic CAR-T and CAR-NK cell is too early to fully assess as clinical data points remain relatively low in number. The fact that NK cells do not establish long-term memory like T cells, and that allogeneic CAR-T cells remain vulnerable to immune rejection despite genetic knockout of endogenous T-cell receptor and/or MHC molecules (76-79), render engineering strategies that can artificially booster cell persistence of particular interest.
In addition to armoring T cells and NK cells with cytokines to drive proliferation, one could also combine therapeutic cells with other forms of immunotherapy to further enhance the immune response. Preclinical studies have suggested that combination of CAR-T cell therapy with anti–PD-1 immune checkpoint blockade could promote the functional persistence of CAR-T cells (80, 81). However, early clinical results reported to date have not yet demonstrated definitive advantage of combining CAR-T cell therapy with checkpoint blockade (82, 83). Pursuing a different modality of combination therapy, an ongoing phase-I clinical trial by BioNTech (NCT04503278) is evaluating the effect of an mRNA vaccine designed to target claudin-6 (CLDN-6) expression to dendritic cells, which can subsequently boost the activation and proliferation of anti–CLDN-6 CAR-T cells. Early results from the trial suggest promising efficacy and safety (84), and the clinical experience generated from such trials will prove highly valuable as the field explores different combination strategies to tackle solid tumors.
Yet another strategy of armoring immune cells is to equip the cells with receptors that can either abrogate immunosuppression or convert suppressive signals in the TME into stimulatory ones for the engineered cells. For example, transforming growth factor-β (TGF-β) is a potent inhibitory cytokine in the TME. To overcome this obstacle, researchers have evaluated strategies to ablate TGF-β signaling, such as CRISPR/Cas9-mediated knock-out of the endogenous TGF-β receptor chain II (NCT04976218). Alternatively, expression of the dominant-negative TGF-β receptor (DNR), which is a truncated TGF-β receptor chain II that lacks the intracellular domain, is also under investigation. The DNR has been co-expressed with tumor-targeting receptors in both T cells (85) and NK cells (86), and preclinical data indicate the DNR can robustly abrogate endogenous TGF-β signaling by both serving as a sink for TGF-β binding and by poisoning the heterodimeric TGF-β receptor complex to abolish signaling. A phase-I clinical trial (NCT03089203) treated patients with prostate cancer using T cells that co-express the DNR with a PSMA-targeting CAR (87). In this trial, the best response was stable disease, achieved by five of 13 patients. Of note, the only patient to receive the highest dose in the trial (1–3 x 108 m−2 CAR-T cells) experienced clonal CAR-T cell expansion and a 98% reduction in serum PSA levels, but rapidly developed grade-4 CRS and died due to complications from sepsis and multimodal immunosuppression. The underlying mechanisms that drove the dramatic clonal T-cell expansion and severe toxicity in this individual patient remain unresolved at this time, but this study highlights the need to further explore both the potential and the risks associated with armoring CAR-T cells to overcome immunosuppression.
Taking it one step further, researchers have engineered synthetic receptors that convert TGF-β binding into immunostimulatory signaling. For example, Burga et al. fused the DNR to DNAX-activation protein 12 (DAP12), and demonstrated that NK cells bearing the fusion protein demonstrated improved efficacy and persistence compared to the DNR in mice bearing TGF-β–secreting neuroblastoma xenografts (88). As another example, Chang et al. developed CARs that can respond to soluble antigens and demonstrated the ability to activate T cells in response to TGF-β through the expression of a TGF-β–binding CAR (89). CARs responsive to additional soluble antigens can potentially broaden this signal-conversion strategy to overcome a variety of immunosuppressive cytokines in the TME (90).
Finally, endogenous receptors can also be modified to enhance effector cell function. For example, Zhu et al. demonstrated that the NK-cell activating receptor FcγRIIIa (CD16a), which is responsible for NK-cell–mediated antibody-dependent cellular cytotoxicity (ADCC), can be mutated to prevent proteolytic cleavage by ADAM17 (91). iPSC-derived NK cells expressing the mutated form of CD16a significantly outperformed unmodified peripheral-blood NK cells in controlling Raji xenografts when co-administered with an anti-CD20 antibody (rituximab) in a repeated-dosing study. An NK-cell product (FT596) containing both the “NK-CAR” and optimized CD16a, along with membrane-tethered IL-15, is currently under clinical evaluation. Preliminary results from this trial are encouraging: 20 patients with relapsed and/or refractory B-cell lymphoma were treated with escalating doses of 20–300 million engineered NK cells, with or without co-treatment with rituximab, resulting in >50% objective response rate among 17 efficacy-evaluable patients, including seven complete responses (16). Two patients who achieved a complete response had previously relapsed after CAR-T cell therapy, highlighting the potential for this therapy to serve as an alternative to CAR-T cells. This remarkable outcome underscores the utility of systematically investigating and incorporating synthetic payloads rooted in biological relevance, a methodology that may lead to next-generation cellular therapies equipped to overcome currently intractable malignancies.
Conclusion
As the synthetic-biology toolkit continues to expand, the generation of increasingly sophisticated biological circuits has become possible in the engineering of cell-based therapies for cancer. Exciting pre-clinical and clinical data for NK and T cell-based therapies are now emerging, demonstrating higher specificity, safety, and durability. Increasing complexity in biological design is frequently coupled with increasing complexity in manufacturing as well as vulnerability to unintended consequences due to system crosstalk or component failures that compromise overall system function. Therefore, the implementation of multi-layered biological circuits in the cell-based therapy context must be done in a judicious manner to maximize robustness and minimize unnecessary complexity. Nevertheless, the wide array of novel biological functions made accessible by synthetic biology approaches offer potential solutions to many roadblocks currently limiting the broad application cell-based immunotherapies, and provides avenues to extend these therapies to more patients with advanced cancers.
Acknowledgments
JDC is supported by the UCLA Tumor Immunology Training Grant (USHHS Ruth L. Kirschstein Institutional National Research Service Award # T32CA009120). TAG is supported by the National Science Foundation Graduate Research Fellowship Program (fellowship to TAG) and the Mark Foundation for Cancer Research (18-029-ELA, grant to YYC). YYC is supported by the Parker Institute for Cancer Immunotherapy, Jean and Stephan Kaplan, and Cancer Research Institute (CRI2701).
Abbreviations List:
- aCAR
activating CAR
- ADCC
antibody-dependent cellular cytotoxicity
- CAR
chimeric antigen receptor
- CLDN6
claudin-6
- CRISPR
clustered regularly interspaced short palindromic repeats
- DAP12
DNAX-activation protein 12
- DNR
dominant-negative TGF-β receptor
- FDA
Food and Drug Administration
- GBM
glioblastoma multiforme
- iCAR
inhibitory CAR
- IL
interleukin
- iNKT cells
invariant natural killer T cells
- iPSCs
induced pluripotent stem cells
- NK cells
natural killer cells
- SNIPR
synthetic intramembrane proteolysis receptor
- synNotch
synthetic Notch
- TAAs
tumor-associated antigens
- TALENs
transcription activator-like effector nucleases
- TGF-β
transforming growth factor-β
Footnotes
Conflict of Interest Statement
Y.Y.C. is an inventor on a patent application for CART19/20 and holds several patent applications in the area of CAR-T cell therapy. Y.Y.C. is a founder of, holds equity in, and receives consulting fees from ImmPACT Bio. She is a member of the scientific advisory board of and holds equity in Catamaran Bio, Notch Therapeutics, Pluto Immunotherapeutics, Prime Medicine, Sonoma Biotherapeutics, and Waypoint Bio. The other authors declare no conflicts of interest.
References
- 1.Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):3801. Epub 2020/08/01. doi: 10.1038/s41467-020-17670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68. Epub 2020/05/21. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. Epub 2019/02/19. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyiadzis MM, Dhodapkar MV, Brentjens RJ, Kochenderfer JN, Neelapu SS, Maus MV, et al. Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J Immunother Cancer. 2018;6(1):137. Epub 2018/12/06. doi: 10.1186/s40425-018-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buermans HP, den Dunnen JT. Next generation sequencing technology: Advances and applications. Biochim Biophys Acta. 2014;1842(10):1932–41. Epub 2014/07/06. doi: 10.1016/j.bbadis.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Hughes RA, Ellington AD. Synthetic DNA Synthesis and Assembly: Putting the Synthetic in Synthetic Biology. Cold Spring Harb Perspect Biol. 2017;9(1). Epub 2017/01/05. doi: 10.1101/cshperspect.a023812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalil AM. The genome editing revolution: review. J Genet Eng Biotechnol. 2020;18(1):68. Epub 2020/10/31. doi: 10.1186/s43141-020-00078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen MH, Schrama D, Thor Straten P, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006;126(1):32–41. Epub 2006/01/19. doi: 10.1038/sj.jid.5700001. [DOI] [PubMed] [Google Scholar]
- 9.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–10. Epub 2008/04/22. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 10.Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nature Reviews Immunology. 2014;14(1):24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moretta A, Marcenaro E, Parolini S, Ferlazzo G, Moretta L. NK cells at the interface between innate and adaptive immunity. Cell Death & Differentiation. 2008;15(2):226–33. doi: 10.1038/sj.cdd.4402170. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Wallace DL, de Lara CM, Ghattas H, Asquith B, Worth A, et al. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121(2):258–65. Epub 2007/03/10. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moingeon P, Lucich JL, McConkey DJ, Letourneur F, Malissen B, Kochan J, et al. CD3 zeta dependence of the CD2 pathway of activation in T lymphocytes and natural killer cells. Proceedings of the National Academy of Sciences. 1992;89(4):1492–6. doi: doi: 10.1073/pnas.89.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson P, Caligiuri M, Ritz J, Schlossman SF. CD3-negative natural killer cells express ε TCR as part of a novel molecular complex. Nature. 1989;341(6238):159–62. doi: 10.1038/341159a0. [DOI] [PubMed] [Google Scholar]
- 15.Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16(6):372–85. Epub 2019/03/07. doi: 10.1038/s41571-019-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachanova V, Ghobadi A, Patel K, Park JH, Flinn IW, Shah P, et al. Safety and Efficacy of FT596, a First-in-Class, Multi-Antigen Targeted, Off-the-Shelf, iPSC-Derived CD19 CAR NK Cell Therapy in Relapsed/Refractory B-Cell Lymphoma. Blood. 2021;138(Supplement 1):823-. doi: 10.1182/blood-2021-151185. [DOI] [Google Scholar]
- 17.Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med. 2020;382(6):545–53. Epub 2020/02/06. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15(12):1563–70. Epub 2013/10/08. doi: 10.1016/j.jcyt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–7. Epub 2005/01/06. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 20.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011;17(19):6287–97. Epub 2011/08/17. doi: 10.1158/1078-0432.CCR-11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran AC, Zhang D, Byrn R, Roberts MR. Chimeric zeta-receptors direct human natural killer (NK) effector function to permit killing of NK-resistant tumor cells and HIV-infected T lymphocytes. J Immunol. 1995;155(2):1000–9. Epub 1995/07/15. [PubMed] [Google Scholar]
- 22.Gao TA, Chen YY. Engineering Next-Generation CAR-T Cells: Overcoming Tumor Hypoxia and Metabolism. Annu Rev Chem Biomol Eng. 2022;13:193–216. Epub 2022/06/15. doi: 10.1146/annurev-chembioeng-092120-092914. [DOI] [PubMed] [Google Scholar]
- 23.O'Leary MC, Lu X, Huang Y, Lin X, Mahmood I, Przepiorka D, et al. FDA Approval Summary: Tisagenlecleucel for Treatment of Patients with Relapsed or Refractory B-cell Precursor Acute Lymphoblastic Leukemia. Clin Cancer Res. 2019;25(4):1142–6. Epub 2018/10/13. doi: 10.1158/1078-0432.CCR-18-2035. [DOI] [PubMed] [Google Scholar]
- 24.Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15(4):825–33. Epub 2007/02/15. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 25.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14(11):1264–70. Epub 2008/11/04. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haynes NM, Trapani JA, Teng MlWL, Jackson JT, Cerruti L, Jane SM, et al. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood. 2002;100(9):3155–63. doi: 10.1182/blood-2002-04-1041. [DOI] [PubMed] [Google Scholar]
- 27.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–84. Epub 2004/02/13. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 28.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21(2):215–23. Epub 2009/03/31. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell. 2018;23(2):181–92 e5. Epub 2018/08/08. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. Epub 2018/12/12. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burslem GM, Crews CM. Small-Molecule Modulation of Protein Homeostasis. Chem Rev. 2017;117(17):11269–301. Epub 2017/08/05. doi: 10.1021/acs.chemrev.7b00077. [DOI] [PubMed] [Google Scholar]
- 32.Chen YY, Jensen MC, Smolke CD. Genetic control of mammalian T-cell proliferation with synthetic RNA regulatory systems. Proc Natl Acad Sci U S A. 2010;107(19):8531–6. Epub 2010/04/28. doi: 10.1073/pnas.1001721107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung WH, Gay J, Martin U, Garrett TE, Horton HM, Certo MT, et al. Sensitive and adaptable pharmacological control of CAR T cells through extracellular receptor dimerization. JCI Insight. 2019;5. Epub 2019/05/01. doi: 10.1172/jci.insight.124430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350(6258):aab4077. Epub 2015/09/26. doi: 10.1126/science.aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zajc CU, Dobersberger M, Schaffner I, Mlynek G, Puhringer D, Salzer B, et al. A conformation-specific ON-switch for controlling CAR T cells with an orally available drug. Proc Natl Acad Sci U S A. 2020;117(26):14926–35. Epub 2020/06/20. doi: 10.1073/pnas.1911154117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juillerat A, Tkach D, Busser BW, Temburni S, Valton J, Duclert A, et al. Modulation of chimeric antigen receptor surface expression by a small molecule switch. BMC Biotechnol. 2019;19(1):44. Epub 2019/07/05. doi: 10.1186/s12896-019-0537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richman SA, Wang LC, Moon EK, Khire UR, Albelda SM, Milone MC. Ligand-Induced Degradation of a CAR Permits Reversible Remote Control of CAR T Cell Activity In Vitro and In Vivo. Mol Ther. 2020;28(7):1600–13. Epub 2020/06/20. doi: 10.1016/j.ymthe.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labanieh L, Majzner RG, Klysz D, Sotillo E, Fisher CJ, Vilches-Moure JG, et al. Enhanced safety and efficacy of protease-regulated CAR-T cell receptors. Cell. 2022;185(10):1745–63 e22. Epub 2022/04/29. doi: 10.1016/j.cell.2022.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. Epub 2014/10/16. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reagan PM, Neelapu SS. How I Manage: Pathophysiology and Management of Toxicity of Chimeric Antigen Receptor T-Cell Therapies. J Clin Oncol. 2021;39(5):456–66. Epub 2021/01/13. doi: 10.1200/jco.20.01616. [DOI] [PubMed] [Google Scholar]
- 41.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–51. Epub 2010/02/25. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19(3):620–6. Epub 2010/12/16. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31(1):71–5. Epub 2012/12/18. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, et al. Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell. 2016;164(4):770–9. Epub 2016/02/03. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sukumaran S, Watanabe N, Bajgain P, Raja K, Mohammed S, Fisher WE, et al. Enhancing the Potency and Specificity of Engineered T Cells for Cancer Treatment. Cancer Discov. 2018;8(8):972–87. Epub 2018/06/09. doi: 10.1158/2159-8290.CD-17-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu I, Liu R, Garcia JM, Hyrenius-Wittsten A, Piraner DI, Alavi J, et al. Modular design of synthetic receptors for programmed gene regulation in cell therapies. Cell. 2022;185(8):1431–43 e16. Epub 2022/04/16. doi: 10.1016/j.cell.2022.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srivastava S, Salter AI, Liggitt D, Yechan-Gunja S, Sarvothama M, Cooper K, et al. Logic-Gated ROR1 Chimeric Antigen Receptor Expression Rescues T Cell-Mediated Toxicity to Normal Tissues and Enables Selective Tumor Targeting. Cancer Cell. 2019;35(3):489–503 e8. Epub 2019/03/20. doi: 10.1016/j.ccell.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5(215):215ra172. Epub 2013/12/18. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandberg ML, Wang X, Martin AD, Nampe DP, Gabrelow GB, Li CZ, et al. A carcinoembryonic antigen-specific cell therapy selectively targets tumor cells with HLA loss of heterozygosity in vitro and in vivo. Sci Transl Med. 2022;14(634):eabm0306. Epub 2022/03/03. doi: 10.1126/scitranslmed.abm0306. [DOI] [PubMed] [Google Scholar]
- 50.Tokatlian T, Asuelime GE, Mock JY, DiAndreth B, Sharma S, Toledo Warshaviak D, et al. Mesothelin-specific CAR-T cell therapy that incorporates an HLA-gated safety mechanism selectively kills tumor cells. J Immunother Cancer. 2022;10(1). Epub 2022/01/30. doi: 10.1136/jitc-2021-003826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sweeney NP, Vink CA. The impact of lentiviral vector genome size and producer cell genomic to gag-pol mRNA ratios on packaging efficiency and titre. Molecular Therapy - Methods & Clinical Development. 2021;21:574–84. doi: 10.1016/j.omtm.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Majzner RG, Mackall CL. Clinical lessons learned from the first leg of the CAR T cell journey. Nat Med. 2019;25(9):1341–55. Epub 2019/09/11. doi: 10.1038/s41591-019-0564-6. [DOI] [PubMed] [Google Scholar]
- 53.Xu X, Sun Q, Liang X, Chen Z, Zhang X, Zhou X, et al. Mechanisms of Relapse After CD19 CAR T-Cell Therapy for Acute Lymphoblastic Leukemia and Its Prevention and Treatment Strategies. Front Immunol. 2019;10:2664. Epub 2019/12/05. doi: 10.3389/fimmu.2019.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu H, Sotillo E, Harrington C, Wertheim G, Paessler M, Maude SL, et al. Repeated loss of target surface antigen after immunotherapy in primary mediastinal large B cell lymphoma. Am J Hematol. 2017;92(1):E11–E3. Epub 2016/10/26. doi: 10.1002/ajh.24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–401. Epub 2014/06/14. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown CE, Badie B, Barish ME, Weng L, Ostberg JR, Chang WC, et al. Bioactivity and Safety of IL13Ralpha2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin Cancer Res. 2015;21(18):4062–72. Epub 2015/06/11. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399). Epub 2017/07/21. doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hegde M, Corder A, Chow KK, Mukherjee M, Ashoori A, Kew Y, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther. 2013;21(11):2087–101. Epub 2013/08/14. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest. 2021;131(13). Epub 2021/07/02. doi: 10.1172/JCI152477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zah E, Nam E, Bhuvan V, Tran U, Ji BY, Gosliner SB, et al. Systematically optimized BCMA/CS1 bispecific CAR-T cells robustly control heterogeneous multiple myeloma. Nat Commun. 2020;11(1):2283. Epub 2020/05/10. doi: 10.1038/s41467-020-16160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–8. Epub 2017/11/21. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol Res. 2016;4(6):498–508. Epub 2016/04/10. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Portillo AL, Hogg R, Poznanski SM, Rojas EA, Cashell NJ, Hammill JA, et al. Expanded human NK cells armed with CAR uncouple potent anti-tumor activity from off-tumor toxicity against solid tumors. iScience. 2021;24(6):102619. Epub 2021/06/24. doi: 10.1016/j.isci.2021.102619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SC, Shimasaki N, Lim JSJ, Wong A, Yadav K, Yong WP, et al. Phase I Trial of Expanded, Activated Autologous NK-cell Infusions with Trastuzumab in Patients with HER2-positive Cancers. Clin Cancer Res. 2020;26(17):4494–502. Epub 2020/06/12. doi: 10.1158/1078-0432.CCR-20-0768. [DOI] [PubMed] [Google Scholar]
- 65.Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front Immunol. 2020;11:940. Epub 2020/06/06. doi: 10.3389/fimmu.2020.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hong M, Clubb JD, Chen YY. Engineering CAR-T Cells for Next-Generation Cancer Therapy. Cancer Cell. 2020;38(4):473–88. Epub 2020/08/01. doi: 10.1016/j.ccell.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71(17):5697–706. Epub 2011/07/12. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 68.Shum T, Omer B, Tashiro H, Kruse RL, Wagner DL, Parikh K, et al. Constitutive Signaling from an Engineered IL7 Receptor Promotes Durable Tumor Elimination by Tumor-Redirected T Cells. Cancer Discov. 2017;7(11):1238–47. Epub 2017/08/24. doi: 10.1158/2159-8290.CD-17-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33(1):74–82. Epub 2014/11/19. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi BD, Yu X, Castano AP, Bouffard AA, Schmidts A, Larson RC, et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019;37(9):1049–58. Epub 2019/07/25. doi: 10.1038/s41587-019-0192-1. [DOI] [PubMed] [Google Scholar]
- 71.Yin Y, Rodriguez JL, Li N, Thokala R, Nasrallah MP, Hu L, et al. Locally secreted BiTEs complement CAR T cells by enhancing killing of antigen heterogeneous solid tumors. Mol Ther. 2022;30(7):2537–53. Epub 2022/05/17. doi: 10.1016/j.ymthe.2022.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin C, Ma J, Ramachandran M, Yu D, Essand M. CAR T cells expressing a bacterial virulence factor trigger potent bystander antitumour responses in solid cancers. Nat Biomed Eng. 2022;6(7):830–41. Epub 2022/04/06. doi: 10.1038/s41551-022-00875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pang N, Shi J, Qin L, Chen A, Tang Y, Yang H, et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J Hematol Oncol. 2021;14(1):118. Epub 2021/07/31. doi: 10.1186/s13045-021-01128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69(9):4010–7. Epub 2009/04/23. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Melenhorst JJ, Chen GM, Wang M, Porter DL, Chen C, Collins MA, et al. Decade-long leukaemia remissions with persistence of CD4(+) CAR T cells. Nature. 2022;602(7897):503–9. Epub 2022/02/04. doi: 10.1038/s41586-021-04390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caribou Biosciences Announces Positive Initial Data for CB-010 Anti-CD19 Allogeneic CAR-T Cell Therapy [Internet]. 12 May 2022. Available from: https://investor.cariboubio.com/news-releases/news-release-details/caribou-biosciences-announces-positive-initial-data-cb-010-anti
- 77.Precision BioSciences Reports Clinical Program Updates for Its Allogeneic CAR T Pipeline [Internet]. 11 December 2021. Available from: https://investor.precisionbiosciences.com/news-releases/news-release-details/precision-biosciences-reports-clinical-program-updates-its
- 78.Allogene Therapeutics Presents Positive Phase 1 Data on ALLO-501 and ALLO-501A in Relapsed/Refractory Non-Hodgkin Lymphoma at the 2021 Annual Meeting of the American Society of Clinical Oncology [Internet]. 4 June 2021. Available from: https://ir.allogene.com/news-releases/news-release-details/allogene-therapeutics-presents-positive-phase-1-data-allo-501 [Google Scholar]
- 79.CRISPR Therapeutics Reports Positive Results from its Phase 1 CARBON Trial of CTX110™ in Relapsed or Refractory CD19+ B-cell malignancies [Internet]. 12 October 2021. Available from: https://crisprtx.gcs-web.com/news-releases/news-release-details/crispr-therapeutics-reports-positive-results-its-phase-1-carbon#
- 80.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126(8):3130–44. Epub 2016/07/28. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.John LB, Devaud C, Duong CP, Yong CS, Beavis PA, Haynes NM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19(20):5636–46. Epub 2013/07/23. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 82.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6(261):261ra151. Epub 2014/11/08. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heczey A, Louis CU, Savoldo B, Dakhova O, Durett A, Grilley B, et al. CAR T Cells Administered in Combination with Lymphodepletion and PD-1 Inhibition to Patients with Neuroblastoma. Mol Ther. 2017;25(9):2214–24. Epub 2017/06/13. doi: 10.1016/j.ymthe.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carvalho T. mRNA vaccines boost BioNTech's CAR T cell therapy. Nat Med. 2022. Epub 2022/09/02. doi: 10.1038/d41591-022-00091-3. [DOI] [PubMed] [Google Scholar]
- 85.Bollard CM, Rossig C, Calonge MJ, Huls MH, Wagner HJ, Massague J, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99(9):3179–87. Epub 2002/04/20. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- 86.Yvon ES, Burga R, Powell A, Cruz CR, Fernandes R, Barese C, et al. Cord blood natural killer cells expressing a dominant negative TGF-beta receptor: Implications for adoptive immunotherapy for glioblastoma. Cytotherapy. 2017;19(3):408–18. Epub 2017/01/23. doi: 10.1016/j.jcyt.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 87.Narayan V, Barber-Rotenberg JS, Jung IY, Lacey SF, Rech AJ, Davis MM, et al. PSMA-targeting TGFbeta-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat Med. 2022;28(4):724–34. Epub 2022/03/23. doi: 10.1038/s41591-022-01726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burga RA, Yvon E, Chorvinsky E, Fernandes R, Cruz CRY, Bollard CM. Engineering the TGFbeta Receptor to Enhance the Therapeutic Potential of Natural Killer Cells as an Immunotherapy for Neuroblastoma. Clin Cancer Res. 2019;25(14):4400–12. Epub 2019/04/24. doi: 10.1158/1078-0432.CCR-18-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang ZL, Lorenzini MH, Chen X, Tran U, Bangayan NJ, Chen YY. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat Chem Biol. 2018;14(3):317–24. Epub 2018/01/30. doi: 10.1038/nchembio.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chang ZL, Hou AJ, Chen YY. Engineering primary T cells with chimeric antigen receptors for rewired responses to soluble ligands. Nat Protoc. 2020;15(4):1507–24. Epub 2020/02/28. doi: 10.1038/s41596-020-0294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu H, Blum RH, Bjordahl R, Gaidarova S, Rogers P, Lee TT, et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood. 2020;135(6):399–410. Epub 2019/12/20. doi: 10.1182/blood.2019000621. [DOI] [PMC free article] [PubMed] [Google Scholar]