Abstract
Introduction:
Pulmonary function is lower after a severe burn injury, which could influence ventilatory responses during exercise. It is unclear whether exercise training improves pulmonary function or ventilatory responses during exercise in adults with well-healed burn injuries. Therefore, we tested the hypothesis that exercise training improves pulmonary function and ventilatory responses during exercise in adults with well-healed burn injuries.
Methods:
Thirty-nine adults (28 with well-healed burn injuries & 11 non-burn-injured controls) completed six months of unsupervised, progressive exercise training including endurance, resistance, and high-intensity interval components. Before and after exercise training, we performed comprehensive pulmonary function testing and measured ventilatory responses during cycling exercise. We compared variables using two-way ANOVA (group x time (i.e., pre/post-exercise training) [repeated factor]).
Results:
Exercise training did not increase percent predicted spirometry, lung diffusing capacity, or airway resistance measures (time: p≥0.14 for all variables). However, exercise training reduced minute ventilation (; time: p≤0.05 for 50 & 75 watts) and the ventilatory equivalent for oxygen (; time: p<0.001 for 75 watts) during fixed-load exercise for both groups. The ventilatory equivalent for carbon dioxide () during exercise at 75 watts was reduced after exercise training (time: p=0.04). The percentage of age-predicted maximum heart rate at the ventilatory threshold was lower in adults with well-healed burn injuries before (p=0.002), but not after (p=0.22), exercise training. Lastly, exercise training increased and reduced during maximal exercise (time: p=0.005 for both variables).
Conclusions:
These novel findings demonstrate that exercise training can improve ventilatory responses during exercise in adults with well-healed burn injuries.
Keywords: SPIROMETRY, LUNG VOLUMES, MAXIMAL VOLUNTARY VENTILATION, LUNG DIFFUSING CAPACITY, INHALATION INJURY
INTRODUCTION
Advances in the sub-acute and acute care of burn injuries have improved survival outcomes (1). However, adults with well-healed burn injuries are at an increased risk of long-term complications (2), inclusive of respiratory morbidities, and all-cause mortality (3). Reduced pulmonary function following discharge from intensive care may be a causative factor for epidemiologic findings of low physical activity participation in adults with well-healed burn injuries (4). Indeed, full-thickness (i.e., severe) burn injuries that cover a significant portion of the body (e.g., >15% of total body surface area) and require prolonged hospitalization can contribute to lower pulmonary function for the remainder of their lives (5). Specifically, individuals who have sustained severe burn injuries have reduced forced expiratory volume in one second (FEV1), forced vital capacity (FVC), maximal voluntary ventilation (MVV), forced expiratory flow at 25-75% (FEF25-75%), and lung diffusing capacity (4, 6-14). Additionally, our laboratory recently demonstrated that adults with well-healed burn injuries have reduced percent predicted FEV1, FEV1/FVC, FEF25-75% as well as increased airway resistance decades after their burn injury (15). Furthermore, nearly half of the adults with well-healed burn-injuries evaluated in this prior study had percent predicted MVV values below the lower limit of normal. Given that decreased pulmonary function is associated with exercise intolerance, reduced quality of life, etc., there is a critical need to identify whether interventions can improve pulmonary function and ventilatory responses during exercise in adults with well-healed burn-injuries. We (16-18) and others (1, 19) have demonstrated the myriad of benefits from exercise training to ameliorate the long-term complications of burn-related trauma. However, the effects of exercise training on pulmonary function during exercise in adults who have recovered from severe burn injuries are not fully known.
In healthy adults, only swimming exercise improves pulmonary function (20). However, there is some evidence that exercise training may improve pulmonary function or lower ventilatory demand, though the data from certain clinical populations to support this notion are mixed (21-24). Exercise training, when initiated immediately after hospital discharge, improves FEV1, FVC, and MVV in children recovering from severe burn injuries (11). Additionally, both FEV1/FVC and MVV have a moderate positive relation to the peak rate of oxygen uptake () in adults with well-healed burn injuries (4), suggesting that greater FEV1/FVC and MVV values might be observed following exercise training-induced increases in . Moreover, both FEV1 and FVC have a moderate positive relation to skeletal muscle mass index in adults with well-healed burn injuries (25), suggesting that greater FEV1 and FVC values might be observed following exercise training-induced increases in skeletal muscle mass. However, 12 weeks of exercise training did not affect FEV1, FVC, or estimated MVV in a cohort of nine adults with well-healed burn injuries (10). Yet, it is plausible that the duration of exercise training necessary to elicit pulmonary function benefits is greater than 12 weeks (19), as employed in prior exercise training studies in adults with well-healed burn injuries (10, 11). Further, a larger sample size, relative to the prior work (10), may be necessary to detect improvements in pulmonary function.
We recently demonstrated that adults with well-healed burn injuries — a population with remarkably low cardiorespiratory fitness (26) — can improve their fitness similar to non-burned adults through exercise training (18). However, it is unknown whether six months of exercise training could improve pulmonary function in adults with well-healed burn injuries. Therefore, we sought to test the hypothesis that six months of exercise training would improve pulmonary function in adults with well-healed burn injuries. A key component of the scientific premise for this hypothesis was that exercise training might favorably influence certain pulmonary function measures (e.g., MVV, peak expiratory flow, FEV1), largely because they are reduced in this population and may have the potential to be improved upon. For example, exercise training challenges respiratory muscles to some extent (modest pressure generation during inspiration and expiration) (27). Thus, one can reason that such improvements in respiratory muscle function might improve the certain pulmonary function tests, such as the assessment of MVV (28, 29), particularly among a clinical population with MVV values below percent predicted as noted above.
Further, we examined how exercise training affected ventilatory responses during steady-state exercise in adults with well-healed burn injuries given the paucity of data in this unique clinical population. Such information on the ventilatory responses during exercise could allow for a better understanding of the factors that may impact exercise tolerance in adults with well-healed burn injuries (26). The sole study in this area (10) reported that 12 weeks of exercise training increased minute ventilation () during maximal treadmill exercise in adults with well-healed burn-injuries. We hypothesized that exercise training would improve ventilatory responses during cycling exercise in adults with well-healed burn injuries based on data from another clinical population (male adults with obesity) demonstrating that exercise training reduces minute ventilation () during fixed-workload submaximal exercise and increases during maximal exercise (30). Addressing these hypotheses will provide important information regarding the effectiveness of exercise as a rehabilitation strategy and could uncover previously unknown health benefits of exercise training for adults with well-healed burn injuries.
METHODS
The data presented herein are unique, yet complementary, to prior published data from this cohort of adults with well-healed burn injuries that addressed hypotheses related to microvascular function, physical function, and aerobic capacity (16-18). The exception is that we reported pulmonary function data in 53 individuals previously (15), while the present manuscript includes data from 39 of these 53 individuals who completed six months of exercise training. Additionally, the exercise training-induced changes in aerobic capacity and peak workload were reported previously from this cohort of 39 adults (under review, Watso JC et al. JAP). However, the present manuscript reports, for the first time, the effect of exercise training on ventilatory responses during fixed-load exercise (50 & 75 watts) and maximal exercise in a large cohort of adults with well-healed burn injuries.
Ethical Approval
This study protocol and informed consent were approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas (Approval identifier: STU-042014-060). All procedures conformed to standards outlined in the Declaration of Helsinki. Participants were fully informed, both verbally and in writing, of all the experimental procedures and the potential risks of participation before providing informed written consent.
Participants
We tested 28 individuals with well-healed burn injuries and 11 non-burn-injured control participants from 2014 to 2019 before and after six months of unsupervised exercise training. All adults with well-healed burn injuries were enrolled at least two years post-injury, and none had musculoskeletal impairments that limited physical activity. Based on responses to a questionnaire, both adults with well-healed burn injuries and non-burn-injured controls were sedentary and had not participated in a consistent/structured exercise training regimen at any time during the prior 12 months. Information related to burn injuries was self-reported in a medical history questionnaire. All participants were between 18 and 60 years old at screening. Exclusion criteria for all participants included current smoker, pregnancy, breastfeeding, or any overt immune, pulmonary, renal, hepatic, cardiovascular, or gastrointestinal disease. Individuals with controlled hypertension or controlled hypercholesterolemia (medication usage detailed in the RESULTS section) were permitted to enroll.
Exercise training
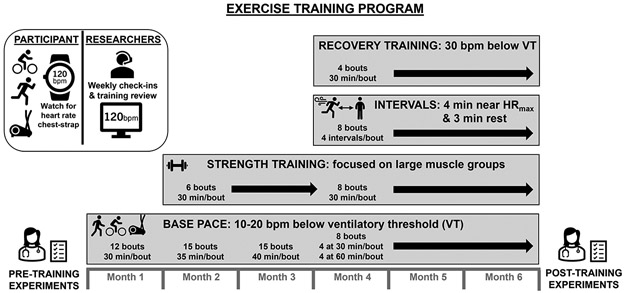
We have previously detailed this exercise training regimen (18). A schematic of this exercise training regimen is included in Figure 1. Briefly, participants completed six months of a progressive exercise training program based upon the Training Impulse approach (6) in which frequency, intensity, and duration gradually increase throughout the training period. We provided participants access to local certified personal trainer support for the first six weeks of training, which could be continued by request for the remainder of the training. Each exercise bout was preceded by a warm-up period and ended with a cool-down period. Participants self-selected exercise (e.g., cycling, walking, jogging, and/or elliptical training) as long as they achieved the heart rate prescribed that was individualized based on their heart rate responses at the ventilatory threshold and maximal heart rate, both identified during a graded exercise test (see MAXIMAL EXERCISE below). We encouraged participants to incorporate at least two of the aforementioned modes of exercise to facilitate a “cross-training” approach. We monitored training remotely using heart rate monitors (Polar Vantage XL model 145900; Polar Electro, Woodbury, NY) that provide date and time stamps. Participants performed “Base Pace” bouts of exercise (10-20 beats per minute lower than heart rate at the ventilatory threshold for 30-40 minutes each in months one through three and 60 minutes each in months four through six) three to four times per week. Additionally, “Interval Training” (four sets of four minutes “on” [within 10 beats per minute of measured maximum heart rate] and three minutes rest) and “Recovery Training” (~30 beats per minute less than heart rate at the ventilatory threshold; a comfortable exercise pace held for 30 minutes) were included in months four through six of training. Self-directed progressive resistance-based strength training, focusing on large muscle groups (including both upper and lower limbs), began in month two of the program using machines and/or free weights (as preferred by the individual). During months four through six the participants were consistently exercising five days per week for 30-60 min per day. We confirmed that participants completed the prescribed exercise training sessions through weekly consultations in combination with data from the heart rate monitor and training logs.
Figure 1. Exercise Training Program.
Participants completed six months of unsupervised, progressive exercise training including endurance, resistance, and high-intensity interval components as detailed in the figure. On a weekly basis, we checked in with participants to ensure they were compliant with the prescribed exercise via heart rate monitoring data review (base pace, intervals, and recovery training) and strength training log review. We also ensured that participants understood the exercise training scheduled for the upcoming week. VT, ventilatory threshold.
Experimental protocol
Before and after the six-month-long exercise training program, we tested participants using the following generalized schedule: day 1, steady-state exercise at two absolute workloads and maximal cardiorespiratory capacity () testing; day 2, pulmonary function testing; day 3: maximal cardiorespiratory capacity testing. During the week of testing, participants also underwent body fat assessment via Dual x-ray absorptiometry. These scans were performed by Radiology Cores at Texas Health Presbyterian Hospital Dallas or University of Texas Southwestern Medical Center. The same scanner was used pre- and post-testing for a given participant. Participants reported to the laboratory around 8:00 AM for each day of testing after abstaining from caffeine, nutritional supplements, alcohol, and physical activity for 24 hours. Participants also abstained from over-the-counter medications but were allowed to take prescription medications as needed. All testing was performed in our temperature-controlled laboratory (~22°C) at the Institute of Exercise and Environmental Medicine in Dallas, TX.
Pulmonary function assessments
All participants completed standard spirometry, MVV, lung volume, lung diffusing capacity, and airway resistance assessments (model 6200 body plethysmograph: SensorMedics, Yorba Linda, CA) according to American Thoracic Society guidelines (31, 32). Percent predicted values and z-scores, when available, were determined from the following published prediction equations from normative values: (33) for spirometry data (other than peak expiratory flow (34)); (35) for MVV in male adults; (36) for MVV in female adults; (37) for lung volumes; (38) for diffusing capacity of the lung for carbon monoxide (DLCO); and (39) for airway resistance. Additionally, we used the National Health and Nutrition Examination Survey (NHANES) III age-, sex- and ethnicity-specific reference values (40) for hemoglobin (Hb) concentrations to calculate hemoglobin-adjusted DLCO using: DLCO[predicted for Hb] = DLCO[predicted] x (1.7Hbmeasured / (0.7Hbreference + Hbmeasured)) (32).
Exercise testing
All exercise testing was performed on an electronically braked lower-body cycle ergometer (Lode Excalibur Sport, Groningen, The Netherlands) with seat and handlebar settings identical between trials. We continuously measured oxygen uptake and related ventilation measures using open-circuit indirect calorimetry (PARVO Medics True-One Metabolic Measurement System; Parvo Medics, Salt Lake City, UT).
Submaximal exercise
Participants completed two consecutive five-minute bouts of cycling at 50 and 75 watts. Ratings of perceived exertion were obtained during the fourth minute of each steady-state. Participants also completed two consecutive five-minute bouts of cycling at relative-intensity workloads (see Supplemental Digital Content).
Maximal exercise
Participants performed two graded maximal cycle exercise tests to ensure that a true was obtained during both pre-testing and post-testing. We used the higher value from the two tests during each time point (pre- and post-training) for analysis. Participants began the test by cycling at 40 watts. Thereafter, the workload was increased by 40 watts every two minutes until subjects reached volitional fatigue. We measured blood lactate concentration three minutes after test completion. Confirmation of was objectively identified based on a heart rate within 10 beats per minute of age-predicted HRmax (220 - age), a plateau in despite an increase in workload, a rating of perceived exertion of 19 or 20, and/or a respiratory exchange ratio of ≥1.0 (18, 26).
Data and statistical analysis
We determined the ventilatory equivalent for carbon dioxide for each participant by calculating the slope of the relation between and the rate of expired carbon dioxide () (41). Previous studies have estimated MVV with the product of FEV1 and ‘X’, where the constant ‘X’ ranged from 35 to 42 (42-46), but it is unknown which factor is appropriate for MVV estimation via FEV1 in adults with well-healed burn injuries. Therefore, we report the result of measured MVV divided by FEV1. We did not conduct an a priori power analysis for the assessed variables presented in this manuscript. We compared participant characteristics between groups and changes in pulmonary function values (post-training minus pre-training) using unpaired, two-tailed t-tests or Mann-Whitney tests when data failed (p<0.05) the Shapiro-Wilk normality test. For all such non-parametric analyses, we report median [interquartile range] instead of mean ± SD. We also compared the proportion of female and male adults between groups using Fisher’s exact test. We compared variables using two-way ANOVAs (group x pre/post-exercise training [repeated factor]). For post hoc analyses, we employed Šídák's multiple comparisons test when appropriate. We analyzed these data using GraphPad Prism 9.3 (GraphPad Software Inc., La Jolla, CA, USA). Finally, using Fisher’s exact test we compared the incidence of increases or decreases in the following variables from pre- to post-training that exceeded the threshold for reproducibility or the smallest measurable change between groups: FVC, FEV1, peak expiratory flow, FEF25-75%, MVV, DLCO, peak Watts, VO2peak, and HRmax (47, 48). We did not create a dichotomous line of significance/non-significance (49, 50). However, when p values were below 0.10, we considered that value along with the calculated effect size (see Figure Legends) as well as the physiological relevance for each variable to draw conclusions about the result of a given comparison.
RESULTS
Participants
We present participant demographics in Table 1 and medication usage in Table 2. Two of nine [22% of those evaluated] non-burn-injured control participants and four of 18 adults with well-healed burn injuries [22% of those evaluated] had a positive bronchodilator response during pre-testing. Exercise training did not affect body mass (Control - Pre: 87.5±19.7, Post: 87.4±20.3 vs. Burn - Pre: 82.1±19.7, Post: 82.5±19.4 kg; group p=0.46, time p=0.92, interaction p=0.76), but did reduce body fat percentage (Control - Pre: 34.0±11.7, Post: 31.9±11.8 vs. Burn - Pre: 32.4±8.5, Post: 30.5±8.8 %; group p=0.66, time p<0.001, interaction p=0.81). Compliance to exercise training did not differ between groups (Control: 92±8 vs. Burn: 93±11 %, p=0.72).
Table 1.
Baseline characteristics
| Non-burn-injured controls | Adults with well-healed burn injuries |
p | Cohen’s d | |
|---|---|---|---|---|
| Number of participants | 11 (5 F / 6 M) | 28 (12 F / 16 M) | >0.99 | - |
| Age, yrs | 35 [28-39] (20-52) | 42 [26-54] (21-60) | 0.13 | 0.60 |
| Body height, cm | 173 [166-182] (165-189) | 170 [163-174] (151-190) | 0.25 | 0.49 |
| Body mass, kg | 84 ± 25 (39-122) | 82 ± 20 (47-126) | 0.86 | 0.06 |
| Body mass index, kg/m2 | 29 ± 6 (20-38) | 28 ± 5 (19-43) | 0.78 | 0.09 |
| Body surface area, m2 | 2.0 ± 0.3 (1.4-2.4) | 1.9 ± 0.3 (1.4-2.5) | 0.68 | 0.33 |
| Body surface area grafted, % | - | 46 ± 21 (14-84) | - | - |
| Time since burn injury, yrs | - | 17 ± 12 (2-50) | - | - |
We present data as median [IQR] or mean ± SD with ranges in parentheses. We compared the proportion of female and male adults between groups using Fisher’s exact test. We compared group values using Mann-Whitney tests for age and body height and using unpaired, two-tailed t-tests for body mass, body mass index, and body surface area.
Table 2.
Medication usage at screening
| Non-burn-injured Controls |
Adults with well-healed burn injuries |
|
|---|---|---|
| Vitamins | 4 (36%) | 6 (21%) |
| Depressants | 0 | 5 (18%) |
| Contraceptives | 0 | 4 (14%) |
| Nutraceuticals | 2 (18%) | 3 (11%) |
| Mineral supplements | 0 | 3 (11%) |
| Thyroid drugs | 2 (18%) | 2 (7%) |
| Statins | 1 (9%) | 2 (7%) |
| Selective serotonin reuptake inhibitors | 0 | 2 (7%) |
| Proton pump inhibitors | 0 | 2 (7%) |
| Antihistamines | 0 | 2 (7%) |
| Angiotensin converting enzyme inhibitors | 1 (9%) | 1 (4%) |
| Central nervous system stimulants | 2 (18%) | 0 |
| *See list in the table legend | 0 | 1 (4%) |
We report the number (and proportion) of participants in each group using medication in each medication class. One adult with well-healed burn injuries began taking a thiazide diuretic after exercise training started.
The incidence of zero (control) vs one (adults with well-healed burn injuries) applies for each of the following medication classes: Laxatives, Aminopenicillins, 5HT3 receptor antagonists, Anticholinergic antiemetics, Thiazide diuretics, NSAIDs, Benzodiazepines, Narcotic analgesic combinations, Protein powder supplement, GABA analogs, Topical steroids, Estrogens, Adrenergic bronchodilators, Miscellaneous analgesics, Calcium channel blocking agents, Miscellaneous antidepressants, Pyrrolidine anticonvulsants, Narcotic analgesics, Androgens and anabolic steroids.
As previously, reported (Watso JC et al. JAP), adults with well-healed burn injuries had a lower (Control - Pre: 33.3±10.9, Post: 38.3±11.9 vs. Burn - Pre: 27.4±4.9, Post: 30.4±6.3 mL O2/kg/min; main effect of group p=0.01) and peak workload (Control - Pre: 256±56, Post: 295±54 vs. Burn - Pre: 205±45, Post: 234±50 watts; main effect of group p=0.002) than non-burn-injured controls regardless of time point. Exercise training increased and peak workload (main effect of time p<0.001 for both). The magnitude of the increase in (interaction effect p=0.19) and peak workload (interaction effect p=0.40) from exercise training was not different between groups.
Pulmonary function testing
Adults with well-healed burn injuries had lower absolute FEV1, absolute FEF25-75%, and absolute peak expiratory flow (Table 3). Exercise training decreased absolute FVC, percent predicted FVC, absolute vital capacity, as well as increased absolute functional residual capacity and percent predicted functional residual capacity (Table 3). It is notable that the changes in FVC and functional residual capacity are inconsequentially small. Exercise training increased MVV/FEV1 (Control - Pre: 37.7±6.6, Post: 41.3±9.2 vs. Burn - Pre: 36.5±8.2, Post: 37.2±9.0; group p=0.37, time p=0.04, interaction p=0.15). Adults with well-healed burn-injuries had a lower percent predicted peak expiratory flow and percent predicted MVV (Table 3).
Table 3.
Absolute and percent predicted pulmonary function values
| Non-burn-injured controls |
Adults with well-healed burn injuries |
p values | |||||
|---|---|---|---|---|---|---|---|
| Pre-training | Post-training | Pre-training | Post-training | Group | Time | Interaction | |
| Forced vital capacity, L | 4.96 ± 1.14 | 4.84 ± 1.11 | 4.30 ± 0.97 | 4.21 ± 0.95 | 0.09 | 0.02 | 0.67 |
| Change (post-training - pre-training) | −0.12 ± 0.24 | −0.08 ± 0.21 | 0.67 | ||||
| Percent predicted | 105 ± 14 | 103 ± 15 | 101 ± 14 | 99 ± 13 | 0.39 | 0.01 | 0.76 |
| z-score | 0.609 ± 1.173 | 0.372 ± 1.247 | 0.104 ± 1.078 | −0.039 ± 0.999 | 0.25 | 0.02 | 0.53 |
| Forced expiratory volume in one second, L | 4.02 ± 0.88 | 3.93 ± 0.93 | 3.32 ± 0.78 | 3.29 ± 0.77 | 0.03 | 0.07 | 0.41 |
| Change (post-training - pre-training) | −0.09 ± 0.17 | −0.03 ± 0.19 | 0.41 | ||||
| Percent predicted | 104 ± 10 | 101 ± 13 | 96 ± 17 | 95 ± 16 | 0.22 | 0.06 | 0.50 |
| z-score | 0.451 ± 0.909 | 0.247 ± 1.166 | −0.267 ± 1.304 | −0.336 ± 1.222 | 0.14 | 0.08 | 0.38 |
| Forced expiratory volume in one second/forced vital capacity, (%) | 82 ± 6 | 81 ± 6 | 79 ± 11 | 78 ± 8 | 0.34 | 0.46 | 0.66 |
| Change (post-training - pre-training) | 0 [−2 - 2] | 0 [−3 - 1] | 0.62 | ||||
| Percent predicted | 98 ± 7 | 98 ± 7 | 96 ± 12 | 95 ± 9 | 0.44 | 0.47 | 0.66 |
| z-score | −0.239 ± 0.851 | −0.232 ± 0.891 | −0.651 ± 1.083 | −0.549 ± 1.061 | 0.32 | 0.54 | 0.59 |
| Forced expiratory flow 25-75%, L/s | 4.16 ± 1.05 | 4.07 ± 1.21 | 3.19 ± 1.24 | 3.18 ± 1.24 | 0.04 | 0.53 | 0.61 |
| Change (post-training - pre-training) | −0.04 [−0.41 - 0.15] | −0.06 [−0.30 - 0.19] | 0.78 | ||||
| Percent predicted | 104 ± 17 | 102 ± 23 | 92 ± 33 | 92 ± 33 | 0.29 | 0.61 | 0.70 |
| z-score | 0.198 ± 0.688 | 0.099 ± 0.936 | −0.360 ± 1.217 | −0.362 ± 1.205 | 0.20 | 0.56 | 0.58 |
| Peak expiratory flow, L/s | 9.4 ± 2.0 | 9.4 ± 2.1 | 7.3 ± 2.1 | 7.2 ± 1.6 | 0.003 | 0.49 | 0.93 |
| Change (post-training - pre-training) | −0.1 ± 0.7 | −0.1 ± 0.8 | 0.93 | ||||
| Percent predicted | 106 ± 8 | 104 ± 7 | 87 ± 23 | 86 ± 18 | 0.005 | 0.55 | 0.99 |
| Maximal voluntary ventilation, L/min | 148 ± 27 | 157 ± 34 | 120 ± 36 | 121 ± 35 | 0.01 | 0.16 | 0.22 |
| Change (post-training - pre-training) | 8 [−7 - 23] | 2 [−13 - 10] | 0.15 | ||||
| Percent predicted | 105 ± 20 | 112 ± 24 | 88 ± 28 | 89 ± 26 | 0.03 | 0.14 | 0.30 |
| Total lung capacity, L | 6.6 ± 1.3 | 6.5 ± 1.3 | 6.0 ± 1.2 | 5.9 ± 1.3 | 0.17 | 0.16 | 0.56 |
| Change (post-training - pre-training) | −0.1 ± 0.2 | 0.0 ± 0.3 | 0.56 | ||||
| Percent predicted | 102 ± 9 | 101 ± 11 | 97 ± 11 | 96 ± 11 | 0.16 | 0.15 | 0.62 |
| z-score | 0.194 ± 0.793 | 0.057 ± 0.933 | −0.299 ± 0.942 | −0.369 ± 0.952 | 0.17 | 0.14 | 0.62 |
| Residual volume/total lung capacity, (%) | 24 ± 4 | 25 ± 5 | 28 ± 6 | 28 ± 7 | 0.08 | 0.42 | 0.90 |
| Change (post-training - pre-training) | 0 [−1 - 2] | 0 [−1 - 4] | 0.54 | ||||
| Percent predicted | 104 ± 17 | 107 ± 19 | 109 ± 19 | 111 ± 19 | 0.48 | 0.47 | 0.95 |
| z-score | 0.079 ± 0.646 | 0.081 ± 0.829 | 0.358 ± 0.796 | 0.410 ± 0.754 | 0.23 | 0.83 | 0.84 |
| Vital capacity, L | 5.1 ± 1.2 | 5.0 ± 1.2 | 4.4 ± 1.0 | 4.3 ± 1.0 | 0.07 | 0.05 | 0.81 |
| Change (post-training - pre-training) | −0.1 ± 0.2 | −0.1 ± 0.3 | 0.81 | ||||
| Percent predicted | 102 ± 11 | 100 ± 13 | 94 ± 14 | 92 ± 13 | 0.09 | 0.06 | 0.91 |
| z-score | 0.170 ± 0.909 | −0.011 ± 1.049 | −0.488 ± 1.107 | −0.660 ± 1.051 | 0.09 | 0.07 | 0.96 |
| Functional residual capacity, L | 2.9 ± 0.8 | 3.1 ± 0.9 | 2.7 ± 0.6 | 2.9 ± 0.8 | 0.49 | 0.009 | 0.89 |
| Change (post-training - pre-training) | 0.2 ± 0.4 | 0.2 ± 0.5 | 0.89 | ||||
| Percent predicted | 91 ± 18 | 98 ± 23 | 88 ± 18 | 95 ± 20 | 0.70 | 0.01 | 0.86 |
| z-score | −0.559 ± 1.076 | −0.210 ± 1.219 | −0.637 ± 0.963 | −0.320 ± 1.010 | 0.80 | 0.01 | 0.90 |
| DLCO, mL/min/mmHg | 28.8 ± 5.0 | 29.7 ± 7.2 | 26.4 ± 6.9 | 26.1 ± 5.9 | 0.21 | 0.64 | 0.42 |
| Change (post-training - pre-training) | −0.7 [−2.4 - 1.6] | −0.2 [−2.1 - 1.2] | 0.69 | ||||
| Percent predicted | 101 ± 18 | 99 ± 18 | 99 ± 22 | 99 ± 21 | 0.87 | 0.73 | 0.56 |
| Percent predicted with Hb-adjustment | 100 ± 3 | 101 ± 3 | 100 ± 3 | 101 ± 2 | 0.79 | 0.15 | 0.48 |
| z-score | 0.419 ± 1.136 | 0.578 ± 1.594 | −0.069 ± 1.233 | −0.077 ± 1.045 | 0.20 | 0.63 | 0.60 |
| Valv, L | 5.7 ± 1.1 | 5.6 ± 1.2 | 5.2 ± 1.2 | 5.2 ± 1.2 | 0.35 | 0.71 | 0.53 |
| Change (post-training - pre-training) | −0.1 [−0.4 - 0.2] | 0.0 [−0.2 - 0.2] | 0.78 | ||||
| Percent predicted | 97 ± 12 | 96 ± 15 | 91 ± 13 | 91 ± 12 | 0.23 | 0.63 | 0.55 |
| z-score | −0.268 ± 1.070 | −0.419 ± 1.371 | −0.865 ± 1.218 | −0.856 ± 1.123 | 0.24 | 0.57 | 0.52 |
| Airway resistance, cmH2O/L/s | 2.2 ± 0.7 | 2.2 ± 0.7 | 2.6 ± 1.0 | 2.6 ± 1.0 | 0.19 | 0.83 | 0.90 |
| Change (post-training - pre-training) | −0.1 [−0.3 - 0.3] | 0.1 [−0.3 - 0.5] | 0.52 | ||||
| Percent predicted | 165 ± 39 | 164 ± 43 | 185 ± 62 | 183 ± 62 | 0.30 | 0.83 | 0.93 |
| Specific airway resistance, cmH2O/L/s | 7.4 ± 1.6 | 7.8 ± 1.9 | 9.0 ± 3.7 | 9.4 ± 3.9 | 0.17 | 0.36 | 0.95 |
| Change (post-training - pre-training) | 0.4 ± 1.3 | 0.4 ± 2.6 | 0.95 | ||||
| Percent predicted | 177 ± 42 | 185 ± 46 | 218 ± 82 | 218 ± 87 | 0.16 | 0.69 | 0.69 |
We present data as mean ± SD or Median [IQR]. We compared values using two-way ANOVAs (group x time [repeated factor]). Hb, hemoglobin; DLCO, diffusing capacity of the lung for carbon monoxide. For non-burn-injured controls vs. adults with well-healed burn injuries, respectively: n=11 vs. n=25 for spirometry, n=11 vs. n=24 for maximal voluntary ventilation, n=11 vs. n=24 for lung volumes, n=10 vs. 23 for lung diffusing capacity, and n=11 vs. for 26 for airway resistance.
Ventilatory measures during fixed-workload lower-body cycling exercise
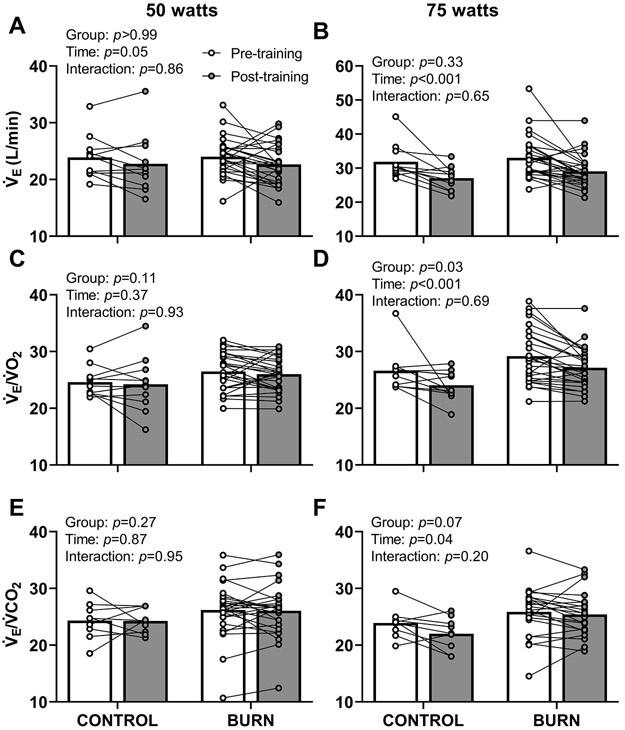
As previously reported (18), exercise training reduces during cycling exercise at 50 (Control - Pre: 0.94±0.10, Post: 0.93±0.10 vs. Burn - Pre: 0.92±0.13, Post: 0.87±0.11 L O2/kg/min; group p=0.31, time p=0.05, interaction p=0.37) and 75 watts (Control - Pre: 1.16±0.10, Post: 1.11±0.11 vs. Burn - Pre: 1.12±0.13, Post: 1.06±0.11 L O2/kg/min; group p=0.22, time p<0.001, interaction p=0.66) as well as the associated ratings of perceived exertion (data included in Table 4). New analyses in the present manuscript detail that exercise training decreased , via decreased tidal volume (Table 4), during exercise at 50 (Figure 2A) and 75 watts (Figure 2B). Adults with well-healed burn injuries had a higher respiratory rate during cycling exercise at 75 watts, which was unchanged after exercise training (Table 4). Exercise training did not affect the ventilatory equivalent for oxygen () during exercise at 50 watts in either group (Figure 2C). Adults with well-healed burn injuries had higher during exercise at 75 watts, which was reduced after exercise training (Figure 2D). The ventilatory equivalent for carbon dioxide () during cycling at 50 watts was not different between groups or time points (Figure 2E). Exercise training reduced the ventilatory equivalent for carbon dioxide during cycling at 75 watts (Figure 2F).
Table 4.
Ventilatory responses and rating of perceived exertion during exercise.
| Non-burn-injured controls (n=11) |
Adults with well-healed burn injuries (n=27) |
p values | |||||
|---|---|---|---|---|---|---|---|
| Pre-training | Post-training | Pre-training | Post-training | Group | Time | Interaction | |
| 50 watts | |||||||
| Respiratory rate, breaths/min | 16 ± 4 | 18 ± 5 | 20 ± 5 | 20 ± 5 | 0.07 | 0.16 | 0.22 |
| Tidal volume, L | 1.6 ± 0.7 | 1.4 ± 0.5 | 1.3 ± 0.4 | 1.2 ± 0.3 | 0.08 | 0.002 | 0.09 |
| Heart rate, beats/min | 104 ± 16 | 98 ± 18 | 101 ± 20 | 95 ± 18 | 0.60 | 0.001 | 0.89 |
| Oxygen pulse, mL/beat | 9.5 ± 1.7 | 9.9 ± 2.2 | 9.5 ± 2.5 | 9.6 ± 2.1 | 0.84 | 0.21 | 0.48 |
| Respiratory exchange ratio | 0.87 ± 0.09 | 0.81 ± 0.09 | 0.88 ± 0.09 | 0.85 ± 0.08 | 0.36 | <0.001 | 0.21 |
| Rating of perceived exertion | 10 ± 2 | 9 ± 1 | 11 ± 2 | 9 ± 2 | 0.08 | 0.004 | 0.80 |
| 75 watts | |||||||
| Respiratory rate, breaths/min | 19 ± 4 | 19 ± 5 | 23 ± 7 | 22 ± 5 | 0.03 | 0.57 | 0.54 |
| Tidal volume, L | 1.8 ± 0.4 | 1.6 ± 0.5 | 1.5 ± 0.4 | 1.4 ± 0.3 | 0.09 | 0.002 | 0.47 |
| Respiratory exchange ratio | 0.94 ± 0.08 | 0.87 ± 0.04 | 0.95 ± 0.08 | 0.91 ± 0.07 | 0.23 | <0.001 | 0.18 |
| Oxygen pulse, mL/beat | 10.5 ± 1.6 | 10.9 ± 2.7 | 10.5 ± 3.3 | 10.6 ± 2.2 | 0.86 | 0.39 | 0.51 |
| Heart rate, beats/min | 116 ± 18 | 107 ± 21 | 115 ± 27 | 106 ± 20 | 0.89 | <0.001 | 0.85 |
| Rating of perceived exertion | 11 ± 2 | 10 ± 1 | 12 ± 2 | 11 ± 2 | 0.09 | 0.003 | 0.52 |
| Maximal exercise | |||||||
| Respiratory rate, breaths/min | 49 ± 8 | 50 ± 8 | 47 ± 9 | 48 ± 10 | 0.40 | 0.49 | 0.92 |
| Tidal volume, L | 2.6 ± 0.7 | 2.7 ± 0.6 | 2.1 ± 0.5 | 2.2 ± 0.5 | 0.01 | 0.12 | 0.98 |
| Heart rate, beats/min | 189 ± 9 | 184 ± 12 | 175 ± 14 | 172 ± 14 | 0.006 | 0.02 | 0.55 |
| Oxygen pulse, mL/beat | 14.9 ± 3.9 | 17.3 ± 4.0 | 12.9 ± 3.8 | 14.3 ± 3.5 | 0.06 | <0.001 | 0.14 |
| Respiratory exchange ratio | 1.24 ± 0.14 | 1.19 ± 0.07 | 1.22 ± 0.11 | 1.17 ± 0.09 | 0.58 | 0.004 | 0.73 |
| Rating of perceived exertion | 20 ± 1 | 19 ± 1 | 19 ± 1 | 19 ± 2 | 0.21 | 0.29 | 0.76 |
We present data as mean ± SD. We compared values using two-way ANOVAs (group x time [repeated factor]).
Figure 2. Ventilatory Responses During Submaximal Fixed-Workload Lower-body Cycling Exercise.
Exercise training decreased minute ventilation () during cycling exercise at 50 (panel A, group: F1,36=0.000043, time: F1,36=4.2, interaction: F1,36=0.032) and 75 watts (panel B, group: F1,36=0.97, time: F1,36=22, interaction: F1,36=0.20). Exercise training did not affect /rate of oxygen uptake () during cycling exercise at 50 watts (panel C, group: F1,36=2.7, time: F1,36=0.83, interaction: F1,36=0.0080). Adults with well-healed burn injuries had higher during cycling exercise at 75 watts, which was reduced after exercise training (panel D, group: F1,36=4.9, time: F1,36=13, interaction: F1,36=0.16). The ventilatory equivalent for carbon dioxide (minute ventilation ()/expired carbon dioxide ()) during cycling at 50 watts was not different between groups or time points (panel E; group: F1,33=1.3, time: F1,33=0.029, interaction: F1,33=0.0037). Exercise training reduced the ventilatory equivalent for carbon dioxide during cycling at 75 watts (panel F; group: F1,29=3.6, time: F1,29=4.4, interaction: F1,29=1.7). We present data as means with individual responses. Sample sizes for panels A-D: n=11 non-burn-injured controls & n=27 adults with well-healed burn injuries. Sample sizes for panels E & F: n=9 non-burn-injured controls & n=26 adults with well-healed burn injuries for 50 watts; n=9 non-burn-injured controls & n=22 adults with well-healed burn injuries for 75 watts. We compared data using two-way ANOVAs (group x time [repeated factor]) with Šídák's multiple comparisons test when appropriate. CONTROL, adults without a prior burn injury; BURN, adults with well-healed burn injuries.
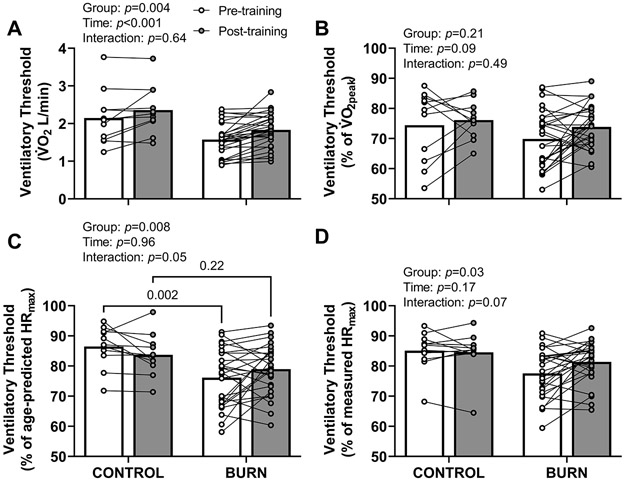
Oxygen uptake and heart rate at the ventilatory threshold
The (Figure 3A), but not the percentage of (Figure 3B), at the ventilatory threshold was lower in adults with well-healed burn injuries and increased with exercise training. The percentage of age-predicted HRmax at the ventilatory threshold was lower in adults with well-healed burn injuries before, but not after, exercise training (Figure 3C). The percentage of measured HRmax at the ventilatory threshold was lower in adults with well-healed burn injuries (Figure 3D).
Figure 3. Ventilatory Threshold.
The rate of oxygen uptake () (panel A, group: F1,36=9.6, time: F1,36=21, interaction: F1,36=0.23), but not the percentage of the peak rate of oxygen uptake () (panel B, group: F1,36=1.7, time: F1,36=3.0, interaction: F1,36=0.49), at ventilatory threshold was lower in adults with well-healed burn injuries and increased with exercise training. The percentage of age-predicted maximum heart rate (HRmax) at ventilatory threshold was lower in adults with well-healed burn injuries before, but not after, exercise training (panel C, group: F1,36=8.0, time: F1,36=0.0024, interaction: F1,36=4.1). The percentage of measured HRmax at the ventilatory threshold was lower in adults with well-healed burn injuries before, but not after, exercise training (panel D, group: F1,36=5.1, time: F1,36=1.9, interaction: F1,36=3.5). We present data as means with individual responses. Sample sizes: n=11 non-burn-injured controls & n=27 adults with well-healed burn injuries. We compared data using two-way ANOVAs (group x time [repeated factor]) with Šídák's multiple comparisons test when appropriate. CONTROL, adults without a prior burn injury; BURN, adults with well-healed burn injuries.
Ventilatory measures during maximal lower-body cycling exercise
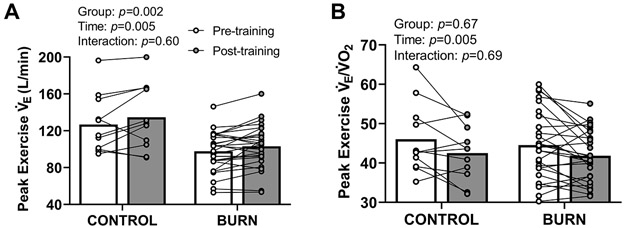
Adults with well-healed burn injuries had a lower during maximal exercise, which was increased, via increased tidal volume (Table 4), after exercise training (Figure 4A). Exercise training reduced during maximal exercise (Figure 4B). Exercise training did not affect /maximal voluntary ventilation in either group (Control - Pre: 71±16, Post: 71±21 vs. Burn - Pre: 69±14, Post: 71±16 %; group p=0.83, time p=0.42, interaction p=0.72). Exercise training did not affect during maximal exercise (Control - Pre: 35±5, Post: 36±5 vs. Burn - Pre: 36±5, Post: 36±5; group p=0.91, time p=0.83, interaction p=0.48).
Figure 4. Minute Ventilation () and /Oxygen Uptake () During Maximal Lower-body Cycling Exercise.
Adults with well-healed burn injuries had a lower during maximal cycling exercise, which was increased after exercise training (panel A, group: F1,36=11, time: F1,36=8.9, interaction: F1,36=0.28). Exercise training reduced during maximal cycling exercise (panel B, group: F1,36=0.18, time: F1,36=9.1, interaction: F1,36=0.16). We present data as means with individual responses. Sample sizes: n=11 non-burn-injured controls & n=27 adults with well-healed burn injuries. We compared data using two-way ANOVAs (group x time [repeated factor]). CONTROL, adults without a prior burn injury; BURN, adults with well-healed burn injuries.
The incidence of increases or decreases, from pre- to post-training that exceeded the threshold for reproducibility or the smallest measurable change (see Supplemental Table S2, Supplemental Digital Content, Smallest measurable changes for select variables) was not different between groups for FVC, FEV1, peak expiratory flow, FEF25-75%, MVV, DLCO, peak Watts, VO2peak, and HRmax (p≥0.16 for all statistical results).
DISCUSSION
The purpose of this investigation was two-fold. First, we examined whether six months of unsupervised exercise training improves pulmonary function in adults with well-healed burn injuries. We found that exercise training does not improve percent predicted spirometry, lung diffusing capacity, or airway resistance measures. Second, we examined whether six months of unsupervised exercise training improves ventilatory responses during exercise in adults with well-healed burn injuries. We found that 1) exercise training reduced (50 & 75 watts) and the ventilatory equivalent for oxygen () (75 watts) during fixed-workload exercise, 2) at the ventilatory threshold was lower in adults with well-healed burn-injuries and increased after exercise training, 3) the ventilatory equivalent for carbon dioxide during exercise at 75 watts was reduced after exercising training, 4) the percentage of age-predicted HRmax at the ventilatory threshold was lower in adults with well-healed burn-injuries before, but not after, exercise training, and 5) exercise training reduced during maximal exercise. Taken together, the present data suggest that six months of unsupervised exercise training in adults with well-healed burn injuries do not improve pulmonary function, but do improve ventilatory responses during submaximal and maximal exercise. These novel findings highlight that exercise training can improve ventilatory responses during exercise in the burn-injured population.
Pulmonary function testing
Severe burn injuries result in lower FEV1, FVC, MVV, FEF25-75%, and lung diffusing capacity relative to both non-burn-injured populations and published population values (4, 6-14). Additionally, our laboratory recently demonstrated that adults with well-healed burn injuries have reduced percent predicted FEV1, FEV1/FVC, FEF25-75%, and MVV as well as increased airway resistance decades after their burn injury (15). Therefore, it is critical to identify interventions that improve pulmonary function in adults with well-healed burn injuries. Exercise training for 12 weeks following a severe burn injury improved FVC, FEV1, and MVV in children aged 7-17 years old (11). However, exercise training for 12 weeks did not improve pulmonary function in nine adults with well-healed burn injuries (10). We reasoned that a longer exercise training intervention (i.e., 26 weeks) would improve pulmonary function in a larger cohort of adults with well-healed burn injuries. This rationale was further supported by published evidence of a positive relation between MVV and (4), as well as between FEV1 and skeletal muscle mass index (25), in adults with well-healed burn injuries because and skeletal muscle mass increase with exercise training (51). In contrast to our hypothesis, six months of exercise training did not improve percent predicted spirometry, lung diffusing capacity, or airway resistance measures.
Previous studies have estimated MVV with the product of FEV1 and a constant ‘X’, where ‘X’ ranged from 35 to 42 (42-46). Our data suggest that 37 is an appropriate constant when calculating MVV from FEV1. Additionally, we found modest reductions in FVC and modest increases in functional residual capacity with exercise training. However, we posit that these modest changes are not meaningful because they are within the variability of measurement. Thus, we would summarize the findings to indicate that our exercise training program does not meaningfully improve pulmonary function in adults with well-healed burn injuries. Such a lack of improvements could be due to the mean values for adults with well-healed burn injuries being below 90% of predicted for only three variables (peak expiratory flow, MVV, and functional residual capacity). That is, with percent predicted values being greater than 90% for the bulk of the variables, there could be little room for improvement from exercise training - an intervention with only some evidence for pulmonary function benefits (21-24).
Ventilatory responses during fixed-workload lower-body cycling exercise
There is a paucity of data related to ventilatory responses during submaximal exercise in adults with well-healed burn injuries. Examining these variables is clinically important because of the potential for reduced spirometry measures (15, 20-24) to modify ventilatory responses during submaximal exercise. For example, if there is an augmented for a given workload, this can increase the rating of perceived breathlessness and the rating of perceived exertion (52). Given the influence of subjective responses during exercise on exercise enjoyment (30, 52), altered ventilatory responses during exercise bouts could deter an adult with well-healed burn injuries from engaging in and maintaining a physically active lifestyle.
In the present study, we found that adults with well-healed burn injuries had a higher , driven by a higher respiratory rate (as opposed to tidal volume), during cycling exercise at 75 watts. While the exaggerated respiratory rate during exercise at this fixed workload was unchanged by exercise training, adults with well-healed burn injuries gained several benefits from exercise training during fixed-workload exercise. Namely, exercise training decreased and , driven by decreased tidal volume, during cycling exercise at 75 watts. Given the context in the prior paragraph about how ventilatory responses during exercise have the potential to affect subjective variables that can influence exercise participation, we contend that such physiological changes are favorable.
We also found that the ventilatory equivalent for carbon dioxide during exercise at 75 watts was reduced after exercising training. Prior work in adults with obesity suggested that exercise training, with associated weight loss, reduces ventilatory demand during exercise (30). In the present work, we found that exercise training reduced ventilatory demand among adults with well-healed burn injuries, as suggested by the lower ventilatory equivalent for carbon dioxide during exercise at 75 watts. Because ventilatory demand was reduced without concomitant weight loss, such improvements are likely due to exercise training-related factors unrelated to weight loss in the present cohort. One possible contributor to reduced ventilatory demand during fixed-workload lower-body exercise is increased skeletal muscle oxidative enzyme (citrate synthase & cytochrome c oxidase) activity in the vastus lateralis, as previously reported in a subset of this cohort (18). A second possible contributor to reduced ventilatory demand during fixed-workload lower-body exercise is improved lower extremity strength, as previously reported in this cohort (17). While the present results are insightful, our findings should be confirmed in future work because, as mentioned earlier, this study represents only the first investigation of ventilatory responses during submaximal exercise in adults with well-healed burn injuries.
Oxygen uptake and heart rate at the ventilatory threshold
We found that adults with well-healed burn injuries were at a lower , but not percent of , at the ventilatory threshold. Similar to the non-burn-injured control group, adults with well-healed burn injuries had a higher , but not percent of , at the ventilatory threshold after exercise training. Importantly, the percentage of age-predicted HRmax at the ventilatory threshold was lower in adults with well-healed burn injuries before, but not after, exercise training. Hence, exercise training ‘normalized’ the percentage of age-predicted HRmax at which adults with well-healed burn injuries attain their ventilatory threshold. This finding suggests that adults with well-healed burn injuries can comfortably (i.e., without excessive ventilation) exercise at a higher percentage of age-predicted HRmax, which may have positive implications for the cardiovascular health benefits they can achieve with exercise training. In short, adults with well-healed burn injuries who are exercise-trained can exercise at a higher percentage of their age-predicted HRmax before approaching the ventilatory threshold, similar to their non-burn-injured counterparts.
Ventilatory responses during maximal lower-body cycling exercise
Most (14), but not all (10), studies examining the effects of severe burn injuries on ventilatory responses during maximal exercise were conducted in children. In the single report from adults with well-healed burn injuries (10), 12 weeks of exercise training raised peak by 47%, whilst raising relative by 16%, during maximal treadmill exercise. The greater exercise training-induced increase in peak in the previous study relative to the present work (6% increase in peak in our cohort of adults with well-healed burn injuries) is likely due, in part, to participant selection. Namely, in the previous study (10) adults with well-healed burn injuries were recruited if they were still experiencing a functional deficit that persisted following standard rehabilitation, whereas participants in the present work did not need to meet this criterion. So, participants in the present work may have been in better health and may not have had as great of room for improvement. Nevertheless, the present data establish that while adults with well-healed burn injuries have a lower peak , unsupervised exercise training can improve this variable. Such a change in peak was likely due to participants attaining a higher peak workload and (53). Finally, it is noteworthy that exercise training reduced during maximal exercise. Taken together, these data suggest that exercise training improves ventilatory responses during maximal exercise adults with well-healed burn injuries.
Other rehabilitation strategies
It could be that whole-body and/or respiratory muscle exercise training increases respiratory muscle strength and the ability to expand the lungs and chest wall. Indeed, recent work shows promise for inspiratory muscle strength training to improve respiratory muscle strength among adults with well-healed burn injuries (54). Such an intervention may complement traditional exercise training among adults with well-healed burn injuries, a group with disproportionately low cardiorespiratory fitness and physical activity levels (4, 26). When considering the lack of improvement in pulmonary function in the present work, future studies should integrate exercise training along with respiratory muscle-specific training to evoke improvements in both ventilatory responses during exercise and, potentially, measures of pulmonary function.
Experimental Considerations
First, there were fewer adults in the non-burn-injured control group relative to the burn-injured group. That said, the primary focus of this investigation was whether adults with well-healed burn injuries can attain pulmonary function benefits from six months of unsupervised exercise training. It is possible that the assessed non-burn-injured control adults do not represent responses that would be observed in a much larger population because this group was recruited to be sedentary to match the adults with well-healed burn injuries, was prescribed the same exercise training for the same duration as adults with well-healed burn injuries, and was tested using identical equipment as adults with well-healed burn injuries in our laboratory. Second, we did not assess maximal inspiratory and expiratory pressures in the present study. This is relevant because maximal inspiratory and expiratory pressure can be reduced after a severe burn injury (7, 25). Additionally, exercise training can increase maximal inspiratory and expiratory pressure. While these variables would have been insightful, our study design allowed us to evaluate a wide range of pulmonary function measures and ventilatory responses during various exercise tests (present work), microvascular function (16), physical function and muscular strength (17), aerobic capacity and skeletal muscle oxidative enzyme activity (18), and cardiovascular adaptations (under review, Watso JC et al. JAP) after six months of exercise training in a unique clinical population. Nonetheless, future work is warranted to explore the effects of exercise training on maximal inspiratory and expiratory pressures in adults with well-healed burn injuries. Third, related to the fact that these data address a secondary research question of a larger project, we do not have complete smoking history data available for all participants, particularly in the non-burn-injured control group. In the individuals with well-healed burn injuries, two adults reported a smoking history (three pack-years and 12 pack-years). Future research is necessary to address the aforementioned knowledge gaps.
Perspectives and significance
The present data suggest that six months of unsupervised exercise training does not improve pulmonary function in adults with well-healed burn injuries. However, these data demonstrate that exercise training improves ventilatory responses during submaximal and maximal exercise in this unique clinical population. Taken together, rehabilitation strategies, other than exercise training, are needed to improve pulmonary function in adults with well-healed burn injuries. Additionally, the present data highlight novel benefits of exercise training related to ventilatory responses during submaximal and maximal exercise for adults with well-healed burn injuries.
CONCLUSIONS AND FUTURE DIRECTIONS
Additional research is necessary to interrogate the mechanisms (e.g., chemoreceptor feedback and mechanical sensing) by which exercise training induces favorable reductions in ventilatory drive and rating of perceived exertion during submaximal exercise without concomitant improvements in standard pulmonary function tests in adults with well-healed burn injuries. Additionally, the effects of exercise training on airway responsiveness to pharmacological bronchodilators remain unclear in adults with well-healed burn injuries. Therefore, such studies are warranted because this population has FEV1, FEF25-75%, and MVV values lower than predicted based on normative data.
Supplementary Material
Table S1. Ventilatory responses and rating of perceived exertion during exercise at 40 and 70% VO2peak.
Table S2. Smallest measurable changes for select variables
Figure S1. Minute Ventilation () and /Oxygen Uptake () During Submaximal Fixed Relative Intensity Lower-body Cycling Exercise.
Acknowledgments
We would like to thank the individuals who participated in this research. We would also like to thank Jan Karel Petric, Naomi Kennedy, Amy Adams, Sarah Bailey, Zaid Mohammed, Laasya Madana, and Elias Johnson for their assistance with the study. The current address for Matthew N. Cramer is Defence Research and Development Canada–Toronto Research Centre, Toronto, ON, Canada M3K 2C9. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Conflict of Interest and Funding Source:
This research was supported by NIH R01GM068865 (CGC), Department of Defense W81XWH-15-1-0647 (CGC), NIH F32HL154559 (JCW), NIH F32GM117693 (SAR), and NIH Administrative Supplements to Promote Diversity in Health-Related Research (SAR, GM, and DPW), and American Heart Association Postdoctoral Fellowship (BNB). The authors have no competing interests to declare. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Availability of data and material
Data are available upon reasonable request to the principal investigator after institutional data transfer approvals.
SUPPLEMENTAL DIGITAL CONTENT
SDC 1: Supplemental Digital Content.docx
Supplemental methods, results, and discussion
REFERENCES
- 1.Porter C, Hardee JP, Herndon DN, Suman OE. The role of exercise in the rehabilitation of patients with severe burns. Exerc Sport Sci Rev. 2015;43(1):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duke JM, Randall SM, Fear MW, Boyd JH, Rea S, Wood FM. Respiratory morbidity after childhood burns: a 10-year follow-up study. Pediatrics. 2016;138(4):e20161658. [DOI] [PubMed] [Google Scholar]
- 3.Mason SA, Nathens AB, Byrne JP, et al. Increased rate of long-term mortality among burn survivors: a population-based matched cohort study. Ann Surg. 2019;269(6):1192–9. [DOI] [PubMed] [Google Scholar]
- 4.Willis CE, Grisbrook TL, Elliott CM, Wood FM, Wallman KE, Reid SL. Pulmonary function, exercise capacity and physical activity participation in adults following burn. Burns. 2011;37(8):1326–33. [DOI] [PubMed] [Google Scholar]
- 5.Whitener DR, Whitener LM, Robertson KJ, Baxter CR, Pierce AK. Pulmonary function measurements in patients with thermal injury and smoke inhalation. Am Rev Respir Dis. 1980;122(5):731–9. [DOI] [PubMed] [Google Scholar]
- 6.Won YH, Cho YS, Joo SY, Seo CH. Respiratory characteristics in patients with major burn injury and smoke inhalation. J Burn Care Res. 2022;43(1):70–6. [DOI] [PubMed] [Google Scholar]
- 7.Özkal Ö, Topuz S, Karahan S, Erdem MM, Konan A, Yastı A. Clinical predictors of pulmonary functions, respiratory/peripheral muscle strength and exercise capacity at discharge in adults with burn injury. Disabil Rehabil. 2021;43(20):2875–81. [DOI] [PubMed] [Google Scholar]
- 8.Won YH, Cho YS, Joo SY, Seo CH. The effect of a pulmonary rehabilitation on lung function and exercise capacity in patients with burn: a prospective randomized single-blind study. J Clin Med. 2020;9(7):2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Björnhagen V, Schüldt Ekholm K, Larsen F, Ekholm J. Burn survivors' pulmonary and muscular impairment, exercise tolerance and return-to-work following medical-vocational rehabilitation: a long-term follow-up. J Rehabil Med. 2018;50(5):465–71. [DOI] [PubMed] [Google Scholar]
- 10.Grisbrook TL, Wallman KE, Elliott CM, Wood FM, Edgar DW, Reid SL. The effect of exercise training on pulmonary function and aerobic capacity in adults with burn. Burns. 2012;38(4):607–13. [DOI] [PubMed] [Google Scholar]
- 11.Suman OE, Mlcak RP, Herndon DN. Effect of exercise training on pulmonary function in children with thermal injury. J Burn Care Rehabil. 2002;23(4):288–93. [DOI] [PubMed] [Google Scholar]
- 12.Mlcak R, Desai MH, Robinson E, Nichols R, Herndon DN. Lung function following thermal injury in children--an 8-year follow up. Burns. 1998;24(3):213–6. [DOI] [PubMed] [Google Scholar]
- 13.Nylen E, Jeng J, Jordan M, et al. Late pulmonary sequela following burns: persistence of hyperprocalcitonemia using a 1–57 amino acid N-terminal flanking peptide assay. Respir Med. 1995;89(1):41–6. [DOI] [PubMed] [Google Scholar]
- 14.Mlcak R, Desai M, Robinson E, McCauley R, Richardson J, Herndon D. Increased physiological dead space/tidal volume ratio during exercise in burned children. Burns. 1995;21(5):337–9. [DOI] [PubMed] [Google Scholar]
- 15.Watso JC, Romero SA, Moralez G, et al. Adults with well-healed burn injuries have lower pulmonary function values decades after injury. Physiol Rep. 2022;10(10):e15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero SA, Moralez G, Jaffery MF, et al. Exercise training improves microvascular function in burn injury survivors. Med Sci Sports Exerc. 2020;52(11):2430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang M, Moralez G, Romero SA, et al. The benefits of an unsupervised exercise program in persons with well-healed burn injuries within the International Classification of Functioning, Disability and Health (ICF). Burns. 2020;46(6):1280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero SA, Moralez G, Jaffery MF, et al. Progressive exercise training improves maximal aerobic capacity in individuals with well-healed burn injuries. Am J Physiol Regul Integr Comp Physiol. 2019;317(4):R563–R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lateur BJ, Shore WS. Exercise following burn injury. Phys Med Rehabil Clin N Am. 2011;22(2):347–50, vii. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Y, Macera C, Addy C, Sy F, Wieland D, Blair SN. Effects of physical activity on exercise tests and respiratory function. Br J Sports Med. 2003;37(6):521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belman MJ, Kendregan BA. Physical training fails to improve ventilatory muscle endurance in patients with chronic obstructive pulmonary disease. Chest. 1982;81(4):440–3. [DOI] [PubMed] [Google Scholar]
- 22.Vogiatzis I, Williamson AF, Miles J, Taylor IK. Physiological response to moderate exercise workloads in a pulmonary rehabilitation program in patients with varying degrees of airflow obstruction. Chest. 1999;116(5):1200–7. [DOI] [PubMed] [Google Scholar]
- 23.Orenstein DM, Franklin BA, Doershuk CF, et al. Exercise conditioning and cardiopulmonary fitness in cystic fibrosis. The effects of a three-month supervised running program. Chest. 1981;80(4):392–8. [DOI] [PubMed] [Google Scholar]
- 24.Alison JA, Donnelly PM, Lennon M, et al. The effect of a comprehensive, intensive inpatient treatment program on lung function and exercise capacity in patients with cystic fibrosis. Phys Ther. 1994;74(6):583–91; discussion 591-3. [DOI] [PubMed] [Google Scholar]
- 25.Won YH, Cho YS, Kim DH, Joo SY, Seo CH. Relation between low pulmonary function and skeletal muscle index in burn patients with major burn injury and smoke inhalation: a retrospective study. J Burn Care Res. 2020;41(3):695–9. [DOI] [PubMed] [Google Scholar]
- 26.Ganio MS, Pearson J, Schlader ZJ, et al. Aerobic fitness is disproportionately low in adult burn survivors years after injury. J Burn Care Res. 2015;36(4):513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harms CA, Wetter TJ, McClaran SR, et al. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol (1985). 1998;85(2):609–18. [DOI] [PubMed] [Google Scholar]
- 28.Rafferty GF, Lou Harris M, Polkey MI, Greenough A, Moxham J. Effect of hypercapnia on maximal voluntary ventilation and diaphragm fatigue in normal humans. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1567–71. [DOI] [PubMed] [Google Scholar]
- 29.Bai TR, Rabinovitch BJ, Pardy RL. Near-maximal voluntary hyperpnea and ventilatory muscle function. J Appl Physiol Respir Environ Exerc Physiol. 1984;57(6):1742–8. [DOI] [PubMed] [Google Scholar]
- 30.Chlif M, Chaouachi A, Ahmaidi S. Effect of aerobic exercise training on ventilatory efficiency and respiratory drive in obese subjects. Respir Care. 2017;62(7):936–46. [DOI] [PubMed] [Google Scholar]
- 31.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49(1):1600016. [DOI] [PubMed] [Google Scholar]
- 33.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. [DOI] [PubMed] [Google Scholar]
- 35.Kory RC, Callahan R, Boren HG, Syner JC. The Veterans Administration-Army cooperative study of pulmonary function. I. Clinical spirometry in normal men. Am J Med. 1961;30:243–58. [DOI] [PubMed] [Google Scholar]
- 36.Grimby G, Soderholm B. Spirometric studies in normal subjects: III. Static lung volumes and maximum voluntary ventilation in adults with a note on physical fitness 1. Acta Medica Scandinavica. 1963;173(2):199–206. [PubMed] [Google Scholar]
- 37.Hall GL, Filipow N, Ruppel G, et al. Official ERS technical standard: Global Lung Function Initiative reference values for static lung volumes in individuals of European ancestry. Eur Respir J. 2021;57(3):2000289. [DOI] [PubMed] [Google Scholar]
- 38.Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J. 2017;50(3):1700010. [DOI] [PubMed] [Google Scholar]
- 39.Dubois AB, Botelho SY, Comroe JH Jr., A new method for measuring airway resistance in man using a body plethysmograph: values in normal subjects and in patients with respiratory disease. J Clin Invest. 1956;35(3):327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollowell JG, van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer C. Hematological and iron-related analytes--reference data for persons aged 1 year and over: United States, 1988-94. Vital Health Stat 11. 2005(247):1–156. [PubMed] [Google Scholar]
- 41.Sun X-G, Hansen JE, Garatachea N, Storer TW, Wasserman K. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med. 2002;166(11):1443–8. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy MC. A practical measure of the maximum ventilatory capacity in health and disease. Thorax. 1953;8(1):73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cara M [Physical basis for test of ventilation mechanism with application to kinesitherapy]. Poumon. 1953;9(5-6):371–428. [PubMed] [Google Scholar]
- 44.Campbell SC. A comparison of the maximum voluntary ventilation with the forced expiratory volume in one second: an assessment of subject cooperation. J Occup Med. 1982;24(7):531–3. [PubMed] [Google Scholar]
- 45.Gandevia B, Hugh-Jones P. Terminology for measurements of ventilatory capacity; a report to the thoracic society. Thorax. 1957;12(4):290–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dillard TA, Hnatiuk OW, McCumber TR. Maximum voluntary ventilation. Spirometric determinants in chronic obstructive pulmonary disease patients and normal subjects. Am Rev Respir Dis. 1993;147(4):870–5. [DOI] [PubMed] [Google Scholar]
- 47.Lavin KM, Guenette JA, Smoliga JM, Zavorsky GS. Controlled-frequency breath swimming improves swimming performance and running economy. Scand J Med Sci Sports. 2015;25(1):16–24. [DOI] [PubMed] [Google Scholar]
- 48.Straub AM, Midgley AW, Zavorsky GS, Hillman AR. Ramp-incremented and RPE-clamped test protocols elicit similar VO2max values in trained cyclists. Eur J Appl Physiol. 2014;114(8):1581–90. [DOI] [PubMed] [Google Scholar]
- 49.Gandevia S Publications, replication and statistics in physiology plus two neglected curves. J Physiol. 2021;599(6):1719–21. [DOI] [PubMed] [Google Scholar]
- 50.Curran-Everett D Evolution in statistics: P values, statistical significance, kayaks, and walking trees. Adv Physiol Educ. 2020;44(2):221–4. [DOI] [PubMed] [Google Scholar]
- 51.Howden EJ, Perhonen M, Peshock RM, et al. Females have a blunted cardiovascular response to one year of intensive supervised endurance training. J Appl Physiol (1985). 2015;119(1):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balmain BN, Weinstein K, Bernhardt V, Marines-Price R, Tomlinson AR, Babb TG. Multidimensional aspects of dyspnea in obese patients referred for cardiopulmonary exercise testing. Respir Physiol Neurobiol. 2020;274:103365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blackie SP, Fairbarn MS, McElvaney NG, Wilcox PG, Morrison NJ, Pardy RL. Normal values and ranges for ventilation and breathing pattern at maximal exercise. Chest. 1991;100(1):136–42. [DOI] [PubMed] [Google Scholar]
- 54.Abazarnejad E, Froutan R, Ahmadabadi A, Mazlom SR. Improving respiratory muscle strength and health status in burn patients: a randomized controlled trial. Qual Life Res. 2022;31(3):769–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Ventilatory responses and rating of perceived exertion during exercise at 40 and 70% VO2peak.
Table S2. Smallest measurable changes for select variables
Figure S1. Minute Ventilation () and /Oxygen Uptake () During Submaximal Fixed Relative Intensity Lower-body Cycling Exercise.