Abstract
Purpose:
To investigate whether associations between diabetic retinopathy (DR) and dementia and Alzheimer’s disease (AD) remain significant after controlling for several measures of diabetes severity.
Design:
Retrospective cohort study.
Methods:
ACT is a prospective cohort study of adults ≥ 65 years, randomly selected and recruited from the membership rolls of Kaiser Permanente Washington, who are dementia free at enrollment and followed biennially until incident dementia. ACT participants were included in this study if they had at enrollment or developed type 2 diabetes mellitus during follow-up, and data were collected through September, 2018 (3,516 person-years of follow-up). Diabetes was defined by ≥2 diabetes medication fills in one year. Diagnosis of DR was based on International Classification of Diseases Ninth and Tenth Revision codes. Estimates of microalbuminuria, long-term glycemia, and renal function from longitudinal laboratory records were used as indicators of diabetes severity. AD and dementia were diagnosed using research criteria at expert consensus meetings.
Results:
A total of 536 participants (median baseline age 75 [interquartile range 71–80], 54% women) met inclusion criteria. Significant associations between DR >5 years duration with dementia (hazard ratio 1.81 [95% confidence interval 1.23–2.65]) and AD (1.80 [1.15–2.82]) were not altered by adjustment for estimates of microalbuminuria, long-term glycemia, and renal function (dementia: 1.69 [1.14–2.50]; AD: 1.73 [1.10–2.74]).
Conclusions:
Among people with type 2 diabetes, DR itself appears to be an important biomarker of dementia risk in addition to glycemia and renal complications.
Keywords: diabetic retinopathy, diabetes, Alzheimer disease, all-cause dementia, microvascular disease, hyperglycemia, end-organ damage, diabetic nephropathy
Table of Contents Statement
Analyses of the data from the prospective cohort study, Adult Changes in Thought, revealed that associations between diabetic retinopathy and dementia or Alzheimer’s disease remain significant after controlling for several indicators of diabetes severity. Strong associations between the longer duration diabetic retinopathy (>5 years) and both dementia and Alzheimer’s disease are not altered by adjustments for time-varying modeling estimates of microalbuminuria, long-term glycemia, and renal function.
INTRODUCTION
Alzheimer disease dementia (AD) and all-cause dementia represent a significant public health problem worldwide. More than 46 million older adults are affected by dementia globally, and this number is expected to nearly triple by 2050.1 It has long been appreciated that people with diabetes are at higher risk for AD and dementia than those without diabetes,2–4 prompting some experts to refer to AD as type 3 diabetes.5 Many potential mechanisms have been proposed to explain the high incidence of dementia in people with diabetes, including direct effects of acute and chronic hyperglycemia, cerebral microvascular dysfunction, and brain insulin resistance.3,6–8
In the United States, an estimated 25% percent of people with type 2 diabetes develop diabetic retinopathy (DR) within 4 years of being diagnosed with diabetes, and more than 70% develop DR within 10 years of their diagnosis.9 The presences of DR has been identified as a risk factor for dementia in people with diabetes in a number of prior studies.10–12 However, most of these analyses did not capture DR duration or adjust for apolipoprotein E (APOE) genotype, an important risk factor for dementia, and relied on International Classification of Disease (ICD) codes for dementia diagnoses instead of an expert consensus panel and research criteria.
The underlying factors that account for the relationship between DR and dementia or AD risks have not been well established. It is possible that the DR may simply reflect a greater diabetes severity which incurs higher dementia risk. Another possibility is something specific about DR beyond factors related to diabetes severity, perhaps related to the similar embryonic origin between brain and retina. A distinctive association between DR and dementia or AD after accounting for diabetes severity would suggest that further explorations of DR may be particularly valuable for improving scientific understanding of shared pathophysiology between brain and retina.
Therefore, we used data from a prospective cohort study to assess whether the previously-reported DR and dementia or AD associations are independent of measures of diabetes severity and glycemic control. We hypothesized that the associations between DR and dementia/AD would be unaffected by measures of diabetes severity. We used estimates of long-term glycemia, microalbuminuria representing microvascular disease, and estimated glomerular filtration rate (eGFR) representing end-organ damage as indicators of overall diabetes severity.
MATERIALS AND METHODS
Adult Changes in Thought (ACT) cohort and the study population with medically treated diabetes
We assembled a cohort of participants in the ACT study cohort who had at enrollment or developed diabetes mellitus during follow-up, who were then followed for the development of dementia. The ACT study is a prospective cohort study of adults ≥ 65 years randomly selected and recruited from the membership rolls of Kaiser Permanente Washington (KPW), an established Washington State health care delivery system.13 The study began in 1994, and electronic laboratory and pharmacy data have been available since 1988 and 1977, respectively. All participants were dementia-free at enrollment and followed biennially until incident dementia such as Alzheimer’s disease.13 Detailed study methods have been published previously.13,14 Self-reported, general demographic information were ascertained at recruitment, and APOE genotype was characterized in >80% of participants. At the initial screening and at each biennial visit,14,15 participants underwent cognitive assessment via the Cognitive Abilities Screening Instrument (CASI)16 and completed surveys on medical history and risk factors. This study was approved by the Institutional Review Boards of KPW, and the University of Washington and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent.
Comprehensive ophthalmic care was provided for all members of KPW and was not related to ACT enrollment. For primary analyses, diabetes was defined by a pharmacy record of diabetes medication fills at least twice in one year either prior to or during ACT enrollment as we have done previously.7 A broader composite definition of diabetes not restricted to prescription history was used in sensitivity analyses. (Supplemental Methods 1 in the Appendix) Participants were excluded from analyses if they did not meet the aforementioned criteria for diabetes, had no information on apolipoprotein E (APOE) genotype or race and ethnicity, or did not complete any ACT visits beyond baseline (Supplemental Figure 1).–Results reported were based on all data collected in ACT until completion of the last batch on September 30th, 2018.
Dementia and AD diagnoses
ACT participants who scored ≤85 on their biennial CASI screening underwent further extensive standardized cognitive workup, including physical and neurologic examinations and a neuropsychological test battery.17 (Supplemental Methods 2) When participants received a full dementia evaluation, their medical history and results from clinical laboratory and imaging studies were reviewed at a multidisciplinary consensus conference. Dementia diagnoses were determined using Diagnostic and Statistical Manual of Mental Disorders, fourth edition, criteria.18 Probable and possible AD diagnoses were determined using the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer Disease and Related Disorders Association criteria.19
Study covariates
DR and other eye disease variables.
The primary exposure in the study was DR, defined as a clinical diagnosis based on International Classification of Diseases (ICD)-9 and -10 codes from participants’ electronic medical records. (Supplemental Table 1) DR was categorized by duration of the disease. A priori, we decided to control for age-related macular degeneration (AMD) and glaucoma history (identified using ICD-9/-10 codes) given known associations between these two eye diseases and dementia.20 For all three eye diseases, we determined duration based on years since diagnosis as a time-varying exposure. We constructed a time-varying covariate to describe the duration of each eye disease as disease-free (reference group), disease within 5 years of diagnosis, and disease >5 years since diagnosis at each time point.
Demographic and baseline variables.
Sex, self-reported race and ethnicity (non-Hispanic White vs. other race and ethnicity groups), APOE genotype (characterized as ≥1 ε4 allele vs. 0ε4 alleles), highest education degree (high-school or below vs. degree beyond high-school), ACT recruitment cohort (original cohort enrolled 1994–1996, expansion cohort enrolled 2000–2003, and replacement cohort enrolled 2004 onward), and time-varying smoking status (never vs. current/former smoker) were extracted.
Diabetes severity variables
1). Microalbuminuria.
We chose microalbuminuria as a proxy of microvascular disease severity, an important indicator of diabetes severity. We used the level of albuminuria determined by urine albumin-to-creatinine ratio (ACR) to account for the diabetes-related microvascular disease severity. At any given time point, we retrieved each patient’s highest urine ACR in the previous five years from the KPW laboratory records and categorized it as normoalbuminuria (ACR: 0 - <30 mcg/mg reference level), microalbuminuria (ACR: 0 - <300 mcg/mg), or macroalbuminuria (ACR >300 mcg/mg). Sensitivity analyses using each patient’s mean ACR values over the preceding five year period were also performed. (Supplemental Methods 3)
2). Long-term glycemia.
We estimated long-term glycemia by calculating average glucose values over the previous five years using a previously published Bayesian model approach developed by Crane et al,7 which incorporates measures of fasting and random glucose and HbA1c obtained as part of clinical care.
3). Renal function, based on eGFR constructs.
We used a time-varying marker of renal function, the estimated glomerular filtration rate (eGFR), to understand the overall severity of diabetes-related end organ damage. We derived three constructs from eGFR measures from a five-year observation period in which there were at least 3 eGFR records obtained at least 2 years apart, as previously described by O’Hare et al.21 We fitted a linear model of a participant’s eGFR measures over the past five years to define: 1) the trajectory using the estimated regression slope, 2) the variation using the standard error of residuals, and 3) the mean level of eGFR at 3 years into the 5-year window.
Other covariates.
Self-reported histories of several known dementia-related vascular diseases were ascertained at each ACT biennial visit, including: 1) heart disease (composite variable including myocardial infarct, angina, coronary artery bypass graft [CABG], and angioplasty), 2) cerebrovascular disease (composite variable including stroke, transient ischemic attack, and carotid endarterectomy), 3) congestive heart failure, and 4) hypertension. Sensitivity analyses defined hypertension using a combination of self-reported medical history from ACT and electronic health records. (Supplemental Methods 3).
Statistical Analysis
Our primary outcome was all-cause dementia, and our primary exposure of interest was DR categorized as none, DR ≤5 years, or DR >5 years. We fit three separate Cox proportional hazard regression models for each outcome: Model A, Model B, and Model C. Each subsequent regression model adjusts for all the covariates in the previous model plus additional covariates.
Risk sets were defined by age (rather than calendar time) with time-at-risk for each participant starting at the latter of their baseline ACT visit and the date of their second qualifying diabetes medication fill (both within one year). Follow-up ended upon the earliest of the following events: all-cause dementia or Alzheimer dementia diagnosis, the participant’s final ACT visit, active disenrollment from ACT, death, or the final date of ACT data availability (September 30th, 2018). We used multiple imputation to impute missing values of ACR and eGFR constructs to minimize potential biases and efficiency loss in a complete-case analysis. (Supplemental Methods 4) The 95% confidence intervals (CI) of hazard ratios (HR) were reported along with p-values from a two-sided Wald test in all analyses. A p-value less than 0.05 was considered statistically significant. All analyses were conducted in R 4.0.2.
Primary analyses.
We fit Cox models with all-cause dementia as outcome without (model A) and with (model B) adjustment for microalbuminuria, long-term glycemia, and three eGFR constructs. We repeated the primary analyses using AD dementia as the outcome.
Secondary analyses.
We also built an expanded model adjusting for additional dementia-related vascular diseases (model C) to evaluate any meaningful change in the associations between DR and all-cause/AD dementia from model B. We conducted the following sensitivity analyses detailed in the supplement: 1) using a broader definition of diabetes, 2) adding a history of lower extremity amputation and individual markers of coronary angioplasty and CABG as indicators of macrovascular end organ complications, 3) varying classifications of DR durations, 4) using a more robust definition of hypertension instead of survey-based data, and 5) controlling for insulin use. (Supplemental Methods 1, 3)
Evaluation of variable importance with Akaike weights.
We evaluated the relative dementia and AD prognostic contributions from DR duration, APOE ε4 genotype, and all considered measures of diabetes severity (microalbuminuria long-term glycemia, and eGFR-based measures) using the sum of Akaike weights. (Supplemental Methods 5)
RESULTS
Analytic sample
A total of 711 out of 5,546 dementia-free ACT participants developed diabetes prior to or during follow-up and were potentially eligible participants in this analysis. Of these, 536 (75%) were included in the analyses after removing 91 without ACT follow-up visits, 82 missing information on APOE, and 2 missing race/ethnicity (Supplemental Figure 1). Members of the analytic cohort were at risk for dementia for a median of 5.9 years (IQR 3.2 – 9.0 years), with a total of 3,516 person-years of follow-up. All participants with DR diagnoses were seen by either an ophthalmologist or an optometrist who performs dilated funduscopic examination for routine DR screening, and 95% of them (n=253) were seen on the day of DR diagnosis. By the end of follow-up, 177 participants met criteria of –DR –>5 years–, 92 participants met criteria for –DR– ≤5 years–, and 267 never developed DR.
Baseline characteristics of the analytic sample, both overall and stratified by the duration of DR as of the end of follow-up are shown in Table 1. Our study cohort had slightly more women (54%) than men, with a median age of 75 years at baseline. A total of 84% of cohort members identified as White and 24% had at least one APOE ε4 allele.
Table 1:
| No DR (N=267) |
DR ≤5 years (N=92) |
DR >5 years (N=177) |
Overall (N=536) |
|
|---|---|---|---|---|
| Age at initial entry to risk set (years) | ||||
| Median | 76 | 75 | 74 | 75 |
| Interquartile Range | 71–81 | 72–80 | 71–78 | 71–80 |
| Female sex -- no. (%) | 134 (50%) | 53 (58%) | 100 (56%) | 287 (54%) |
| Race -- no. (%) | ||||
| White | 228 (85%) | 79 (86%) | 141 (80%) | 448 (84%) |
| Black | 17 (6%) | 5 (5%) | 15 (8%) | 37 (7%) |
| Asian | 11 (4%) | 4 (4%) | 10 (6%) | 25 (5%) |
| American Indian or Alaskan Native | 1 (0%) | 1 (1%) | 0 (0%) | 2 (0%) |
| Native Hawaiian or Pacific Islander | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Other, Including Mixed | 10 (4%) | 3 (3%) | 11 (6%) | 24 (4%) |
| Hispanic -- no. (%) | 6 (2%) | 1 (1%) | 3 (2%) | 10 (2%) |
| Degree beyond high school at ACT entry -- no. (%) | 109 (41%) | 35 (38%) | 61 (34%) | 205 (38%) |
| APOE ε4 allele carrier -- no. (%) | 57 (21%) | 27 (29%) | 42 (24%) | 126 (24%) |
| Smoking status at start of follow-up -- no. (%) | ||||
| Current smoker | 5 (2%) | 2 (2%) | 8 (5%) | 15 (3%) |
| Former smoker | 134 (50%) | 47 (51%) | 85 (48%) | 266 (50%) |
| Never smoked | 128 (48%) | 43 (47%) | 84 (47%) | 255 (48%) |
| Cohort -- no. (%) | ||||
| Original: enrolled 1994–1996 | 133 (50%) | 51 (55%) | 85 (48%) | 269 (50%) |
| Expansion: enrolled 2000–2003 | 48 (18%) | 14 (15%) | 39 (22%) | 101 (19%) |
| Replacement: enrolled 2004 onward | 86 (32%) | 27 (29%) | 53 (30%) | 166 (31%) |
| DR duration at start of follow-up – no. (%) | ||||
| None | 267 (100%) | 61 (66%) | 46 (26%) | 374 (70%) |
| DR ≤5 years | 0 (0%) | 31 (34%) | 74 (42%) | 105 (20%) |
| DR >5 years | 0 (0%) | 0 (0%) | 57 (32%) | 57 (11%) |
See Supplemental Figure 1 for counts of individuals removed due to missing data.
Baseline here was defined as either each participant’s baseline ACT study visit or the first time of a second fill of diabetic medications within a year, whichever occurred later.
ACT, Adult Changes in Thought
Associations between DR and dementia/AD, Model A
Over the course of follow-up, 139 (26%) participants developed all-cause dementia, 101 of which (19% of the analytic sample) were Alzheimer dementia. The incidence rates of both all-cause dementia and AD were higher for cohort members with a longer duration of DR. (Supplemental Table 2) Participants with either DR ≤5 years or DR > 5 years had higher hazards of both all-cause dementia and AD compared to those without DR, (Table 2) but only those for DR> 5 years reached statistical significance. (HR for all-cause dementia: 1.81, 95% CI 1.23–2.65, p=0.003; HR for AD: 1.80, 95% CI 1.15–2.82, p=0.01).
Table 2:
Hazard ratios (HRs) by diabetic retinopathy (DR) duration.
| All-cause dementia | Alzheimer dementia | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| DR ≤5 years vs. No DR | ||||
| Model A a | 1.53 (0.97–2.43) | 0.070 | 1.59 (0.93–2.74) | 0.096 |
| Model B b | 1.33 (0.83–2.13) | 0.232 | 1.44 (0.83–2.52) | 0.199 |
| Model C c | 1.35 (0.84–2.16) | 0.214 | 1.44 (0.82–2.52) | 0.204 |
| DR >5 years vs. No DR | ||||
| Model A a | 1.81 (1.23–2.65) | 0.003 | 1.80 (1.15–2.82) | 0.012 |
Model A: adjusted for time-varying diabetic retinopathy duration (diagnosis within past 5 years, diagnosis over 5 years ago), glaucoma and age-related macular degeneration duration, age, gender, self-reported non-Hispanic White (yes/no), carrying at least one APOE ε4 allele, degree beyond high school at ACT entry (yes/no), type of ACT recruitment cohort, and time-varying smoking status (never vs. current/former).
Model B: Model A adjusted for microalbuminuria (worst urine albumin-to-creatinine ratio [ACR] status in past 5 years defined as normal/reference with ACR<30 mcg/mg, microalbuminuria with ACR 30–300 mcg/mg, or macroalbuminuria with ACR >300 mcg/mg), long-term glycemia (average glucose over previous 5 years), and renal function based on eGFR measures (3 eGFR constructs: average eGFR, eGFR standard error, eGFR trajectory)
Model C: Model B adjusted for self-reported history of vascular diseases (heart disease, cerebrovascular disease, congestive heart failure, and hypertension).
CI, confidence interval; eGFR, estimated glomerular filtration rate.
Adjusting for microalbuminuria, long-term glycemia and eGFR constructs, Model B
The distributions of these covariates stratified by current DR status are shown in Supplemental Table 3 and Supplemental Figures 2–4. Significant and strong associations between DR > 5 years and both all-cause dementia and AD persisted after adjusting for microalbuminuria, long-term glycemia and eGFR measures (HR for all-cause dementia 1.69, 95% CI 1.14–2.50, p=0.01; HR for AD 1.73, 95% CI 1.10–2.74, p=0.02). (Figure 1 and Table 2) Adjustment for these variables had similar effects on associations between DR ≤55 years and both types of dementia.
Figure 1:
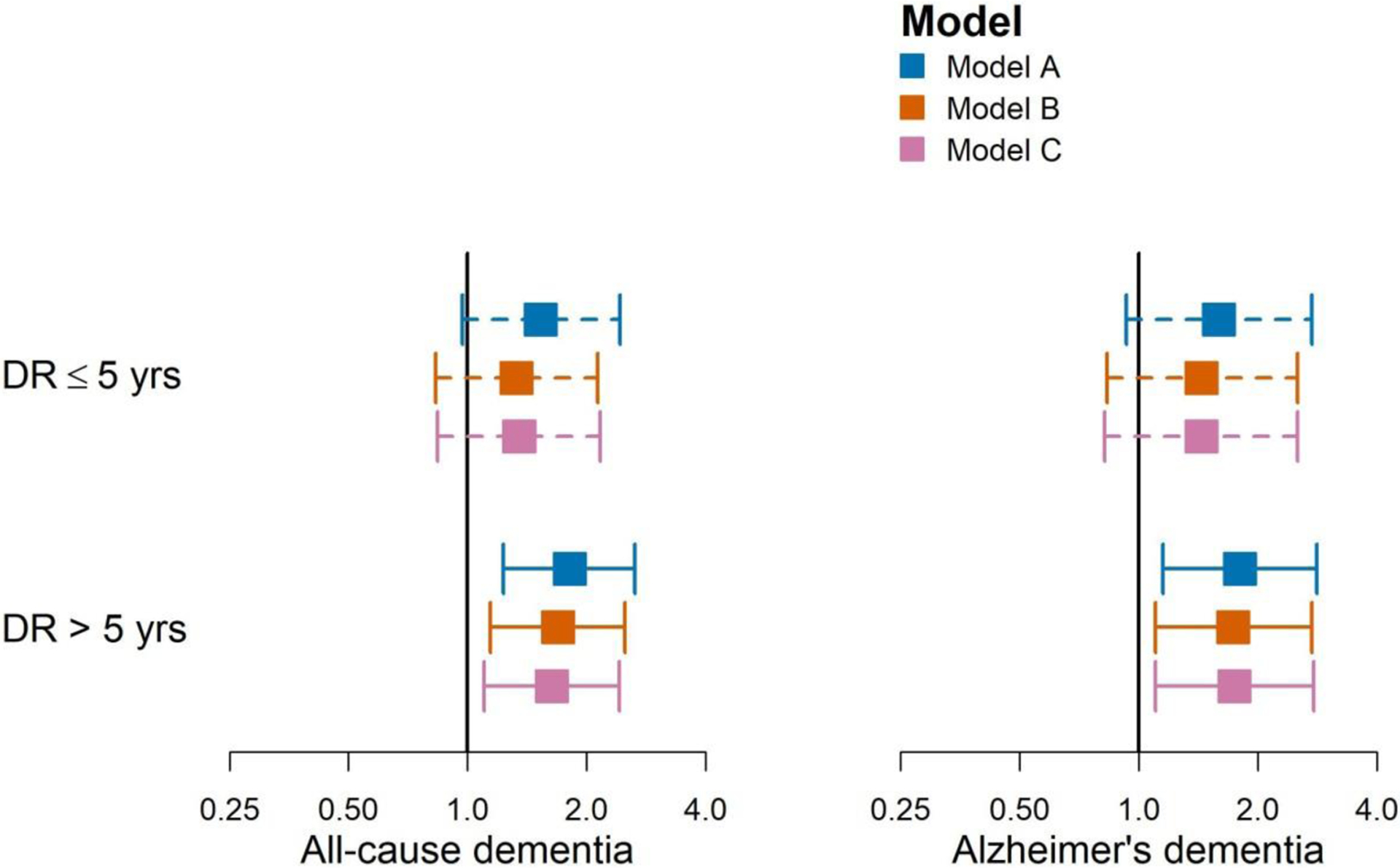
Point estimates (95% CIs) of hazard ratios by diabetic retinopathy (DR) duration. Model A: Model A adjusted for time-varying diabetic retinopathy duration (diagnosis within past 5 years, diagnosis over 5 years ago), glaucoma and age-related macular degeneration duration, age, gender, self-reported non-Hispanic White (yes/no), carrying at least one APOE ε4 allele, degree beyond high school at Adult Changes in Thought (ACT) study entry (yes/no), type of ACT recruitment cohort, and time-varying smoking status (never vs. current/former); Model B: Model A adjusted for microalbuminuria (worst urine albumin-to-creatinine ratio [ACR] status in past 5 years defined as normal/reference with ACR<30 mcg/mg, microalbuminuria with ACR 30–300 mcg/mg, or macroalbuminuria with ACR >300 mcg/mg), long-term glycemia (average glucose over previous 5 years), and renal function based on eGFR measures (3 estimated glomerular filtration rate (eGFR) constructs: average eGFR, eGFR standard error, eGFR trajectory). Model C: Model B adjusted for self-reported history of vascular diseases (heart disease, cerebrovascular disease, congestive heart failure, and hypertension).
Expanded model, Model C
Additional adjustment for other dementia-related vascular factors did not substantially alter the inference nor the magnitude of the associations between either DR ≤55 years or DR> 5 years and risks of all-cause dementia and AD. (Table 2 and Figure 1)
Hazard of dementia and relative variable importance
The observed increase in hazard of all-cause dementia among individuals with DR > 5 years relative to no DR (HR 1.69, 95% CI 1.14–2.50) was either comparable to or greater than other risk factors examined, including APOE ε4 genotype (HR for ≥1 vs. no APOE ε4 alleles was 1.54, 95% CI 1.04–2.26), highest vs. lowest quartile of average glucose level (HR 1.85, 95% CI 1.23–2.79), and highest vs. lowest quartile of eGFR variability (HR 1.41, 95% CI 0.90–2.18). Results were similar for AD. (Figure 2 and Supplemental Table 4)
Figure 2:
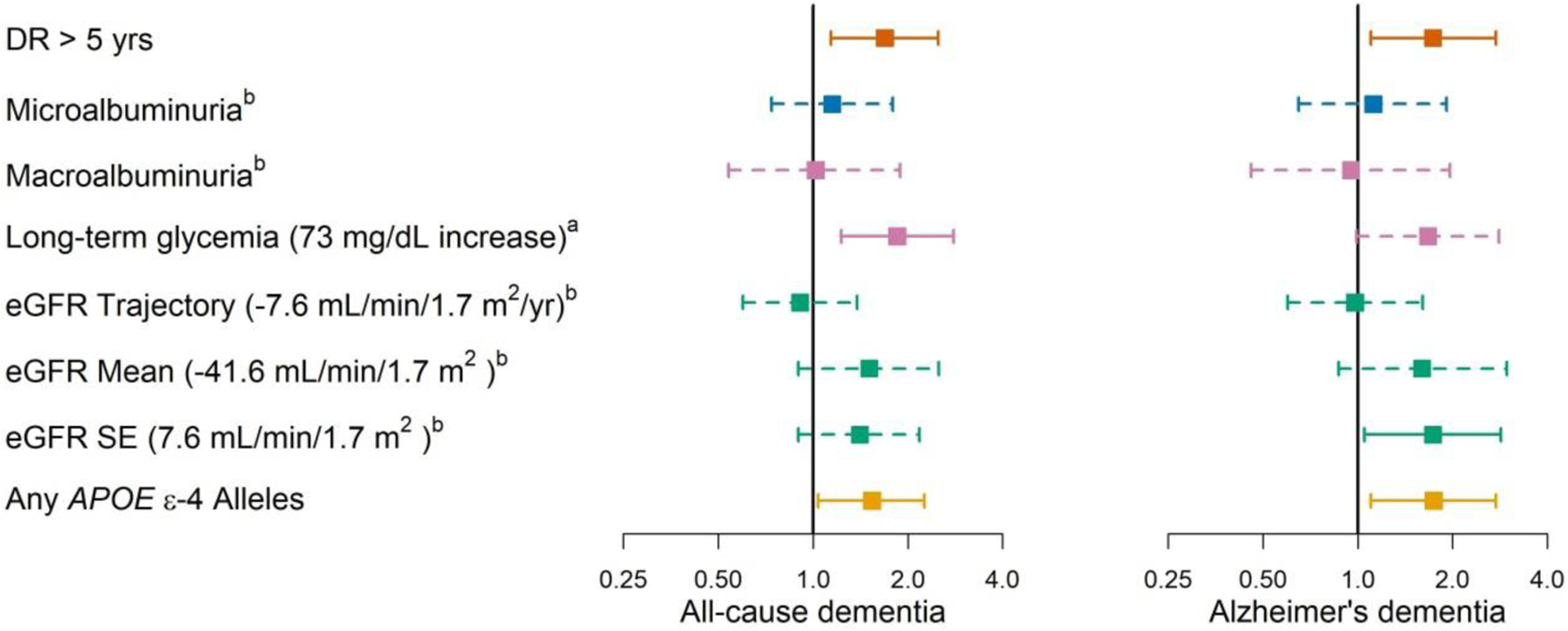
Point estimates (95% confidence intervals) of hazard ratios from Model B. Hazard ratios in continuous covariates, including long-term glycemia levels and the three estimated glomerular filtration rate (eGFR) constructs, are for hazard differences between the average values in the 4th versus 1st quartiles in the analytic sample. Model B adjusted for time-varying diabetic retinopathy duration– (–diagnosis within past 5 years, –diagnosis over 5 years ago), glaucoma and age-related macular degeneration duration, age, gender, self-reported non-Hispanic White (yes/no), carrying at least one APOE ε4 allele, degree beyond high school at Adult Changes in Thought (ACT) study entry (yes/no), type of ACT recruitment cohort, and time-varying smoking status (never vs. current/former), microalbuminuria (worst urine albumin-to-creatinine ratio [ACR] status in past 5 years defined as normal/reference with ACR<30 mcg/mg, microalbuminuria with ACR 30–300 mcg/mg, or macroalbuminuria with ACR >300 mcg/mg), long-term glycemia (average glucose over previous 5 years), and renal function based on eGFR measures (3 eGFR constructs: average eGFR, eGFR standard error, eGFR trajectory).
asee “Long-term glycemia” in “Study Covariates” section of the Methods for details on how the covariate was defined.
bSee “Renal function, based on eGFR constructs” in “Study Covariates” section of the Methods for details on how the covariates were defined.
Based on the sum of Akaike weights (SW) ranking, the categorical DR variable was the most important variable category when prognosing hazards of both all-cause and AD, followed by APOE genotype followed by all measures of diabetes severity. (Supplemental Table 5) Differences between SW values in Supplemental Table 5 indicate that for Alzheimer disease dementia, APOE genotype was nearly as important as DR duration (0.79 vs. 0.80), while indicators of diabetes severity were far less important (0.36). In contrast, APOE genotype and measures of diabetes severity had similar importance for all-cause dementia (0.76 and 0.69, respectively) but were less important than DR (0.86).
Sensitivity Analyses
None of the sensitivity analyses changed our main results. The association between DR > 5 years and both outcomes after adjusting for several measures of diabetes severity persisted when we used a broader definition of diabetes, added a history of lower extremity amputation and individual markers of coronary angioplasty and CABG, varied classifications of DR duration or definitions of variables such as hypertension, and controlled for insulin use (Supplemental Methods 1 and Supplemental Results in the Appendix, and Supplemental Tables 6–10).
DISCUSSION
Among 536 ACT participants with diabetes with 3,516 person-years of follow-up, the presence of DR > 5 years was strongly associated with an increased risk of all-cause dementia and Alzheimer disease dementia even after adjusting for time-varying measures of diabetes severity (microalbuminuria, long-term glycemia and eGFR constructs). The strong associations between DR > 5 years and risks of dementia or AD beyond estimates of diabetes severity suggests that the retina may offer useful insights on dementia risk in people with diabetes.
The presences of diabetic retinopathy has been identified as a risk factor for dementia in people with diabetes in a number of prior studies.10–12 However, whether and to what extent this association may reflect the role of DR as an independent risk factor outside factors related to diabetes severity has previously not been made clear. The Edinburgh Type 2 Diabetes Study of 1044 people with diabetes found a significant association between increasing DR severity and decreasing scores on cognitive assessments, with participants with moderate to severe DR demonstrating the lowest performance.11 However, the study design was cross-sectional and relied on measures of cognition rather than definitive dementia diagnoses. Exalto et al. analyzed associations between sight-threatening DR – proliferative retinopathy and macular edema -- and dementia in 29,961 people with type 2 diabetes from the Kaiser Permanente Northern California Diabetes Registry.10 Members of this cohort with sight-threatening DR had an increased risk of incident dementia (HR=1.35; 95%CI 1.21, 1.52) after controlling for vascular risk factors and measures of diabetes severity which included use of diabetic medications, diabetes duration, HbA1c, and hyperglycemia. However, unlike our study, this analysis did not capture DR duration or adjust for APOE genotype, an important risk factor for dementia, and relied on ICD codes for dementia diagnoses instead of an expert consensus panel and research criteria. A recent registry-based study in Denmark compared risk for AD in participants with diabetes with DR and without DR, finding increased risk of AD in those with DR (adjusted HR 1.34, 95% CI 1.18–1.53). In addition, when both groups were compared to age-matched diabetes-free controls, those with diabetes but without DR had a lower risk of developing AD (adjusted HR 0.87, 95% CI 0.81–0.93) while those with DR had a higher risk (adjusted HR 1.24, 95% CI 1.08–1.43), suggesting that DR, and not diabetes alone, may be a specific and important biomarker of AD.12
Our group has previously reported a near two-fold increased risk of developing cerebral microinfarcts in people with DR compared to OR-free individuals with diabetes, suggesting that DR and dementia likely share microvascular pathology during the development of dementia,22 and a recent meta-analysis (which included our study) found a combined odds ratio of 1.75 (95% CI: 1.36, 2.25) for the association between DR and increased risk of cerebral small vessel disease.23 Thus, in the current study, we controlled for microalbuminuria, given that both DR and diabetic nephropathy are widely recognized diabetic microvascular complications that often coexist.24 Diabetic nephropathy is believed to result from endothelial dysfunction in the microvasculature,25 and microalbuminuria, in particular, has been shown to be associated with dementia risk in several prior studies.26–28 Nevertheless, unlike DR, micro/macroalbuminuria levels were not associated with dementia risk in our study, perhaps suggesting a different time course and/or trajectory for renal and retinal microvascular disease as well as different causal factors.
Long-term glycemia and glycemic variability in diabetes have been associated with multiple complications including renal disease, neuropathy, cardiovascular events, DR, and dementia.29,30 Our group has previously shown that long-term glycemia is an important risk factor for dementia in people with diabetes.7 Nevertheless, long-term glycemia did not fully explain the association between DR > 5 years and AD nor between DR > 5 years and dementia, suggesting alternative shared risk factors for DR and AD/dementia. Interestingly, the strong association between DR > 5 years and dementia risk was not impacted when we controlled for insulin use highlighting the importance of DR as a unique risk factor. Severe hypoglycemia and chronic moderate hypoglycemia are also potential risk factors for cognitive impairment, especially in older patients,31 but we did not have the necessary data to assess this relationship in our cohort. Further research is warranted to understand the potential pathways reflected by the association of DR with AD risk outside of the effects of hyperglycemia.32
The strong association between DR > 5 years and dementia risk despite controlling for several markers of diabetes severity suggests that the factors accounting for this association may be specific to the retina and the brain. The hazard of all-cause dementia attributable to DR > 5 years was one of the strongest in our model, and the HR for DR > 5 years was as strong as the one for the APOE ε4 genotype, the strongest genetic risk factor for AD both in literature and many ACT-based studies.33 Despite the known differences between AD and non-AD dementia pathophysiologies, DR > 5 years appears to be an important biomarker associated with increased risks of both AD and all-cause dementia development.
Two recent reviews of the many shared pathological pathways between DR and dementia implicated damage to the neurovascular unit as a key mechanism, including damage to the inner blood-retinal barrier (BRB)34 and the blood-brain barrier (BBB). The authors suggested that T2D patients with retinal neuro- and vasa-degeneration were at higher risk for rapid cognitive decline and AD, while T2D patients without these retinal pathologies exhibited lower rates of cognitive decline.31,35 The breakdown of the BRB is a known feature of DR, and BRB pathology has also been found in AD.36,37 In addition, BBB breakdown has been implicated as an early event in the AD pathology cascade.38–40 Many other potential biomarkers of AD have been identified in the retina, including retinal nerve fiber layer thinning and loss of ganglion cells, neuroinflammation, microvascular changes, functional/metabolic changes, and amyloid beta and hyper-phosphorylated tau pathology.41–43 Although identifying factors that may have incited the loss of BBB and BRB is outside the scope of our study, our study results highlight DR as an important condition that warrants further investigation in understanding dementia risk in people with diabetes.
This study has several limitations. We relied on various indicators of renal function to estimate the diabetes severity; however, adding additional variables did not alter our results. We used multiple imputation to minimize potential biases in the longitudinal dataset but potential bias due to non-missing-at-random data cannot be ruled out. Furthermore, the diabetes severity metric in our study may not comprehensively capture other aspects of diabetes severity that might confound the associations between DR and both dementia and AD risks; hence residual confounding may remain as is expected from any observational studies. We focused on clinical identification of AD dementia as opposed to AD defined by biomarker profiles, and it is uncertain whether results would have been similar with biomarker-defined AD. We relied on ICD codes for eye diseases, however, several validation studies have shown strong agreement with medical records data,44,45 and multiple large ophthalmic epidemiologic studies have relied on ICD codes alone.44–47 We performed additional review and found that 98% of ICD-9/-10 codes were confirmed by eye specialists. A significant portion of ICD codes did not specify DR severity. Thus, we were unable to analyze the association between DR severity and dementia risk. Our study cohort was overwhelmingly White so our results may not be generalizable to other populations.
Among ACT cohort members with diabetes included in this study, there was a strong association between DR > 5 years and both dementia and AD that persisted after adjustment for several measures of diabetes severity. These findings suggest that the course of DR in people with diabetes could provide useful information on dementia risk. Further, a deeper understanding of the causal pathways underlying the association between DR > 5 years and dementia could offer useful insights on dementia.
Supplementary Material
Highlights.
Diabetic retinopathy is associated with increased risk of Alzheimer disease/dementia.
Mechanisms for diabetic retinopathy (DR) and dementia associations are unknown.
Estimates of glycemia and renal function were used as diabetes severity indicators.
Associations between DR & dementia remained after adjusting for diabetes severity.
Diabetic retinopathy itself appears to be an important biomarker of dementia risk.
ACKNOWLEDGEMENTS
a. Funding/Support:
This research has been funded by National Institutes of Health grants NIH OT2OD032644, K23EY029246 (National Eye Institute), and R01AG060942, U01AG006781, and U19AG066567 (National Institute on Aging), the Latham Vision Research Innovation Award (Seattle, WA), the Klorfine Family Endowed Chair, the C. Dan and Irene Hunter Endowed Professorship, and by an unrestricted grant from Research to Prevent Blindness. The sponsors or funding organizations had no role in the design or conduct of this research. All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the National Institute on Aging or the National Institutes of Health.
b. Financial Disclosures:
The authors declare the following competing interests: Dr. C. Lee reports a grant from the Alzheimer’s disease Drug Discovery Foundation (ADDF). Dr. Su reports a grant from the Patient-Centered Outcomes Research Institute. Dr. A. Lee reports support from the US Food and Drug Administration, grants from Santen, Carl Zeiss Meditec, Microsoft, NVIDIA, and Novartis, and personal fees from Genentech, Verana Health, Gyroscope, Topcon, and Johnson and Johnson, outside of the submitted work; this article does not reflect the opinions of the Food and Drug Administration. Dr. O’Hare reports grants from the National Institute of Diabetes and Digestive and Kidney Disease and Veteran’s Affairs Health Services Research and Development. Dr. Larson reports royalties from UpToDate.
c. Other Acknowledgements:
We would like to thank Timothy-Paul Kung (University of Washington) for his expert assistance with the ICD codes. We thank the participants of the Adult Changes in Thought (ACT) study for the data they have provided and the many ACT investigators and staff who steward that data. You can learn more about ACT at: https://actagingstudy.org/.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting presentation: These results were presented at the recent Association for Research in Vision and Ophthalmology annual meeting in Denver, CO (May 4, 2022).
Supplemental Material available at AJO.com.
REFERENCES
- 1.Prince MJ. World Alzheimer Report 2015: The Global Impact of Dementia | Alzheimer’s Disease International. Published August 25, 2015. Accessed April 6, 2020. https://www.alz.co.uk/research/world-report-2015
- 2.World Health Organization. Global Report on Diabetes. World Health Organization; 2016. [Google Scholar]
- 3.Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4(6):640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42(5):484–491. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen TT, Ta QTH, Nguyen TTD, Le TT, Vo VG. Role of Insulin Resistance in the Alzheimer’s Disease Progression. Neurochem Res. 2020;45(7):1481–1491. [DOI] [PubMed] [Google Scholar]
- 6.Umemura T, Kawamura T, Hotta N. Pathogenesis and neuroimaging of cerebral large and small vessel disease in type 2 diabetes: A possible link between cerebral and retinal microvascular abnormalities. J Diabetes Investig. 2017;8(2):134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crane PK, Walker R, Hubbard RA, et al. Glucose levels and risk of dementia. N Engl J Med. 2013;369(6):540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bello-Chavolla OY, Antonio-Villa NE, Vargas-Vázquez A, Ávila-Funes JA, Aguilar-Salinas CA. Pathophysiological Mechanisms Linking Type 2 Diabetes and Dementia: Review of Evidence from Clinical, Translational and Epidemiological Research. Curr Diabetes Rev. 2019;15(6):456–470. [DOI] [PubMed] [Google Scholar]
- 9.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Exalto LG, Biessels GJ, Karter AJ, Huang ES, Quesenberry CP Jr, Whitmer RA. Severe diabetic retinal disease and dementia risk in type 2 diabetes. J Alzheimers Dis. 2014;42 Suppl 3:S109–S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding J, Strachan MWJ, Reynolds RM, et al. Diabetic retinopathy and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes. 2010;59(11):2883–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedersen FN, Stokholm L, Pouwer F, et al. Diabetic Retinopathy Predicts Risk of Alzheimer’s Disease: A Danish Registry-Based Nationwide Cohort Study. J Alzheimers Dis. 2022;86(1):451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737–1746. [DOI] [PubMed] [Google Scholar]
- 14.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144(2):73–81. [DOI] [PubMed] [Google Scholar]
- 15.Crane PK, Gibbons LE, McCurry SM, et al. Importance of home study visit capacity in dementia studies. Alzheimers Dement. 2016;12(4):419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6(1):45–58; discussion 62. [DOI] [PubMed] [Google Scholar]
- 17.Crane PK, Trittschuh E, Mukherjee S, et al. Incidence of cognitively defined late-onset Alzheimer’s dementia subgroups from a prospective cohort study. Alzheimers Dement. 2017;13(12):1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Menta Disorders (4th Ed.). American Psychiatric Publishing, Inc.; 1994. [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–939. [DOI] [PubMed] [Google Scholar]
- 20.Lee CS, Larson EB, Gibbons LE, et al. Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimers Dement. 2019;15(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Hare A, Walker R, Haneuse S, Crane P, Larson E. O2- 04- 08: Variability in trajectory of estimated glomerular filtration rate is associated with risk of dementia. Alzheimers Dement. 2011;7(4S_Part_9):S297–S297. [Google Scholar]
- 22.Lee CS, Larson EB, Gibbons LE, et al. Ophthalmology-Based Neuropathology Risk Factors: Diabetic Retinopathy is Associated with Deep Microinfarcts in a Community-Based Autopsy Study. J Alzheimers Dis. 2019;68(2):647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chai YH, Zhang YP, Qiao YS, et al. Association Between Diabetic Retinopathy, Brain Structural Abnormalities, and Cognitive Impairment for Accumulated Evidence in Observational Studies. Am J Ophthalmol. 2022;239:37–53. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Wang Y, Li L, et al. Diabetic retinopathy may predict the renal outcomes of patients with diabetic nephropathy. Ren Fail. 2018;40(1):243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng H, Harris RC. Renal endothelial dysfunction in diabetic nephropathy Cardiovasc Hematol Disord Drug Targets. 2014;14(1):22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Etgen T, Chonchol M, Förstl H, Sander D. Chronic kidney disease and cognitive impairment: a systematic review and meta-analysis. Am J Nephrol. 2012;35(5):474–482. [DOI] [PubMed] [Google Scholar]
- 27.Barzilay JI, Fitzpatrick AL, Luchsinger J, et al. Albuminuria and dementia in the elderly: a community study. Am J Kidney Dis. 2008;52(2):216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheppach JB, Coresh J, Wu A, et al. Albuminuria and Estimated GFR as Risk Factors for Dementia in Midlife and Older Age: Findings From the ARIC Study. Am J Kidney Dis. 2020;76(6):775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaster B, Hirsch IB. The effects of improved glycemic control on complications in type 2 diabetes. Arch Intern Med. 1998;158(2):134–140. [DOI] [PubMed] [Google Scholar]
- 30.Gorst C, Kwok CS, Aslam S, et al. Long-term Glycemic Variability and Risk of Adverse Outcomes: A Systematic Review and Meta-analysis. Diabetes Care. 2015;38(12):2354–2369. [DOI] [PubMed] [Google Scholar]
- 31.Simó R, Ciudin A, Simó-Servat O, Hernández C. Cognitive impairment and dementia: a new emerging complication of type 2 diabetes-The diabetologist’s perspective. Acta Diabetol. 2017;54(5):417–424. [DOI] [PubMed] [Google Scholar]
- 32.Stehouwer CDA. Microvascular Dysfunction and Hyperglycemia: A Vicious Cycle With Widespread Consequences. Diabetes. 2018;67(9):1729–1741. [DOI] [PubMed] [Google Scholar]
- 33.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. [DOI] [PubMed] [Google Scholar]
- 34.Campbell M, Humphries P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol. 2012;763:70–84. [PubMed] [Google Scholar]
- 35.Little K, Llorián-Salvador M, Scullion S, et al. Common pathways in dementia and diabetic retinopathy: understanding the mechanisms of diabetes-related cognitive decline. Trends Endocrinol Metab. 2022;33(1):50–71. [DOI] [PubMed] [Google Scholar]
- 36.van de Haar HJ, Burgmans S, Jansen JFA, et al. Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease. Radiology. 2017;282(2):615. [DOI] [PubMed] [Google Scholar]
- 37.Shi H, Koronyo Y, Rentsendorj A, et al. Identification of early pericyte loss and vascular amyloidosis in Alzheimer’s disease retina. Acta Neuropathologica. 2020;139(5):813–836. doi: 10.1007/s00401-020-02134-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur J Ophthalmol. 2011;21 Suppl 6:S3–S9. [DOI] [PubMed] [Google Scholar]
- 39.Erickson MA, Banks WA. Blood-Brain Barrier Dysfunction as a Cause and Consequence of Alzheimer’s Disease. J Cereb Blood Flow Metab. 2013;33(10):1500–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai Z, Qiao PF, Wan CQ, Cai M, Zhou NK, Li Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. J Alzheimers Dis. 2018;63(4):1223–1234. [DOI] [PubMed] [Google Scholar]
- 41.Blazes M, Lee CS. Understanding the Brain through Aging Eyes. Adv Geriatr Med Res. 2021;3(2). doi: 10.20900/agmr20210008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alber J, Goldfarb D, Thompson LI, et al. Developing retinal biomarkers for the earliest stages of Alzheimer’s disease: What we know, what we don’t, and how to move forward. Alzheimer’s & Dementia. 2020;16(1):229–243. doi: 10.1002/alz.12006 [DOI] [PubMed] [Google Scholar]
- 43.Sivak JM. The aging eye: common degenerative mechanisms between the Alzheimer’s brain and retinal disease. Invest Ophthalmol Vis Sci. 2013;54(1):871–880. [DOI] [PubMed] [Google Scholar]
- 44.Muir KW, Gupta C, Gill P, Stein JD. Accuracy of international classification of diseases, ninth revision, clinical modification billing codes for common ophthalmic conditions. JAMA Ophthalmol. 2013;131(1):119–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lau M, Prenner JL, Brucker AJ, VanderBeek BL. Accuracy of Billing Codes Used in the Therapeutic Care of Diabetic Retinopathy. JAMA Ophthalmol. 2017;135(7):791–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee CS, Gibbons LE, Lee AY, et al. Association Between Cataract Extraction and Development of Dementia. JAMA Intern Med. Published online December 6, 2021. doi: 10.1001/jamainternmed.2021.6990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olivier MMG, Smith O, Croteau-Chonka CC, et al. Demographic and Clinical Characteristics Associated with Minimally Invasive Glaucoma Surgery Use: An IRIS® Registry Retrospective Cohort Analysis. Ophthalmology. Published online February 15, 2021. doi: 10.1016/j.ophtha.2021.02.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


