Abstract
Purpose:
To examine the relationship between daily fluctuations in symptoms and sedentary behavior (SB) during chemotherapy (CT) for breast cancer.
Methods:
Breast cancer patients (N=68, Mage= 48.5±10.4 years) undergoing CT wore an activity monitor on their hip to assess daily SB and completed prompts assessing symptoms (affect, anxiety, depression, fatigue, pain, and physical and cognitive functioning) for 10 consecutive days (3 days pre-, day of, and 6 days post-CT) at the beginning, middle and end cycles of CT. Mixed models assessed the bi-directional between person (BP) and within person (WP) associations of current day symptoms with minutes of SB measured on 1) the same day and 2) the next day, controlling for relevant covariates.
Results:
WP same day results revealed a significant association between affect, anxiety, fatigue, physical functioning, pain, and cognitive functioning and same day SB. Worse than average symptom ratings on a given day were associated with more SB that day. There was a significant WP relationship between previous day anxiety, depression, and physical function and next day SB (i.e., worse than average symptom ratings the previous day were associated with more SB the next day). WP same day results revealed a significant association between same day SB and affect, anxiety, fatigue, pain, physical functioning, and cognitive functioning. WP relationships were significant for previous day SB and next day affect and pain (i.e. higher than average SB associated with lower ratings). Relationships persisted when controlling for MVPA. There were no significant BP results.
Conclusions:
Higher symptom ratings were associated with increased SB and higher SB was associated with worse symptoms. Future work should identify SB reduction intervention approaches tailoring to daily symptom burden during CT for breast cancer.
Keywords: WOMEN’S HEALTH, INACTIVITY, SYMPTOM REPORTING, LONGITUDINAL
INTRODUCTION
It is estimated that more than 3.8 million women in the United States have been diagnosed with some type of invasive breast cancer (1). The majority (86%) of breast cancer patients will receive chemotherapy as their primary cancer treatment (1). Chemotherapy is a widely employed, systemic treatment option that is used to treat multiple stages of breast cancer (i.e., resurgence of cancer to metastatic disease) (2). Many individuals who undergo chemotherapy experience side effects such as nausea, vomiting, fatigue, bruising, lowered immune system, and changes in taste or smell (2–4). Symptoms vary based on frequency of treatment, dosage of medication, individual health differences, and stage of cancer (4). However, studies consistently show that individuals undergoing chemotherapy experience some level of burdensome side effects regardless of the time, dose, or frequency of their treatment with symptoms persisting well-beyond their treatment period (4). Due to the persistent nature of chemotherapy side effects, it is important that cancer survivors avoid and reduce unhealthy behaviors that could exacerbate chemotherapy side effects further.
One of these unhealthy behaviors is sedentary behavior, defined as any waking behavior in a sitting or lying position with an energy expenditure of less than 1.5 METs (5). Post-treatment breast cancer survivors engage in more sedentary behavior than healthy controls, spending an estimated 9.2 hours of their waking time sedentary almost an hour more than healthy controls (8.3 hours/waking day) (6). Sedentary behavior is associated with many deleterious health outcomes among the general population, including metabolic syndrome and all-cause mortality (7–9). Among the cancer population specifically, sedentary behavior is also associated with poor health outcomes such as weight gain, increased waist circumference, poor quality of life, cancer specific mortality, and higher levels of fatigue, pain, and depression among breast cancer survivors (10–14).
Given these negative effects, it is important to understand whether a temporal relationship exists between chemotherapy symptoms and sedentary behavior. To date, it is known, among post-treatment breast cancer survivors, demographic variables including working part time, being married, higher BMI, greater number of chronic conditions, advanced disease stage, and treatment with radiation are all associated with increased sedentary time (15, 16). Additionally, beliefs regarding the negative effects of sedentary behavior, outcome expectations regarding the benefits of reducing sedentary behavior, and lifestyle self-efficacy are correlated with less time spent sedentary (15).
Most studies utilize retrospective data to examine the relationships between sedentary behavior and symptoms, and few studies have examined this relationship in real time or during chemotherapy. Ecological momentary assessment (EMA) could provide insights into relationships between real time symptoms and sedentary behaviors, while reducing errors associated with recall (17). Through EMA, responders can report real-time symptoms, affect, and behavior statistics close in time to the primary experience being measured (i.e., treatment related side effects) (11). In a study by Pinto et al., post-treatment breast cancer survivors responded to EMA prompts, while wearing an accelerometer. Within-person results indicated an increased state of anxiety, stress, sadness, worry, and fatigue were associated with increased sedentary behavior, while more positive affect was associated with less sedentary behavior (18). Additionally, the effects of fatigue and positive affect were lagged, indicating that current levels of fatigue and positive affect predicted later sedentary behaviors (18). However, this study did not examine the relationship between symptoms and sedentary behavior during chemotherapy. Given that the time of primary treatment like chemotherapy may be a pivotal moment for health behavior change among cancer survivors, understanding the dynamic relationship between symptoms and sedentary behavior during chemotherapy is especially important (19–21). We previously explored these relationships in relation to moderate-to-vigorous and light intensity physical activity, finding a significant within-person effect of patients’ symptoms on their physical activity behaviors within this study (22). The present study sought to examine the relationship between daily changes in symptoms and SB during CT for breast cancer. To do this, we examined the effect of same day symptoms on same day SB and the reciprocal relationship of the effect of same day SB on same day symptoms as it is unknown in which direction these variables may influence one another. Further, we examined the lagged effect of each association, estimating the relationship between previous day symptoms on next day SB and, likewise, previous day SB on next day symptoms.
METHODS
Recruitment
Patients were recruited from a large urban, academic medical center in the U.S. Participants were identified through the electronic medical record and recruited prior to their first or second dose of chemotherapy. Inclusion criteria were as follows: (i) female ≥ 18 years of age; (ii) diagnosed with stage I-III breast cancer; (iii) scheduled to receive chemotherapy at study site and able to complete first data collection time point prior to/during second chemotherapy cycle; (iv) have/had an operable tumor (v) no history of other primary cancer with exception of non-melanoma skin cancer; (vi) own a smartphone; (vii) access to a computer with internet, and (viii) able to read and write in English.
Potential participants were either contacted in person following an oncology appointment, referred by their physician, or sent an email detailing the study endorsed by their physician. After screening, all participants signed an informed consent. Further details on recruitment methods can be viewed in our previous publication (23). Briefly, 318 participants were approached following medical record screening, 104 completed the screening form, 77 were eligible for participation and consented to participate, and 68 participants enrolled in the study completing at least data time point. All study procedures were approved by the university’s Institutional Review Board.
Study Procedures
EMA methodology (24) was used in this prospective, longitudinal study to measure participants’ symptoms and accelerometry was used to assess sedentary behavior. Data were collected for 10 consecutive days at three time points throughout chemotherapy treatment. We define and refer to the three time points as T1 (beginning, cycle 1 or 2 of chemotherapy), T2 (middle, mid-point of chemotherapy), and T3 (end, last cycle of chemotherapy). We combined treatment status into a dichotomous variable, defined as 1) “pre-treatment/day of treatment” or three days pre-chemotherapy plus the day of chemotherapy and 2) “post-treatment” or the six days post chemotherapy. At T1, participants were given a study orientation to introduce them to study procedures and expectations. Additionally, participants received an online questionnaire to report health history and demographics. Participants received an assessment packet at each time point that included the accelerometer, accelerometer log, and procedural instructions. Participants’ assessment period began 3 days prior to their chemotherapy dose, and they were instructed to wear the accelerometer 24/7 and answer EMA prompts on their smartphone four times per day. Following data collection, participants mailed back the accelerometer and accelerometer log using a provided self-addressed and pre-paid envelope. In order to maintain adherence to study protocol, participants were sent reminders/check-in emails at three time points during each data collection period: (1) the day before data collection; (2) mid-period check-in on day 5, and (3) post data-collection to remind to return materials. In addition, participants were contacted by phone and email if they missed 3 consecutive EMA prompts.
Measures
Demographics and Health History
Demographic information including age, race/ethnicity, income, education, and employment status were reported by participants on an online questionnaire sent following study consent. Participants also self-reported health history, disease characteristics (i.e., number of comorbidities and current health status), and height and body weight to calculate body mass index (BMI). BMI and age were confirmed via medical records. Information about disease stage and chemotherapy dose cycle number and date were collected from participants’ medical records.
Symptoms
We wanted to look at a wide range of symptoms to better understand the possible relationship between sedentary behavior and symptom burden during treatment since very little research in this area exists. We chose symptoms that commonly occur during chemotherapy. Current presence and severity of symptoms were assessed using modified single items from the Patient-Reported Outcomes Measurement Information System (PROMIS)(25). EMA symptom burden prompts asked participants to rate their affect, anxiety, depression, fatigue, physical functioning, and pain. These questions are further detailed in Table 1. Participants received four prompts per day on their smartphone via text message. Participants had 60 minutes to complete each prompt, and text message reminders were sent up to 3 times every 15 minutes to participants who had not yet completed the current prompt. Participants self-reported their wake and bedtimes to generate the times they received prompts. Table 2 details the daily symptom burden prompt schedule.
Table 1.
Symptom burden prompt questions from the Patient-Reported Outcomes Measurement Information System (PROMIS)
| Affect (26) | Anxiety (27, 28) | Depression (27, 28) | Fatigue (27, 28) | Physical Function (Walking) (27, 28) | Physical Function (ADL) (27, 28) | Pain (27, 28) | Cognitive Function (27, 28) | |
|---|---|---|---|---|---|---|---|---|
| Question | Estimate how good or bad you feel right now. | My worries overwhelm me right now. | How would you rate your depression right now? | How would you rate your fatigue right now? | Are you physically able to go for a walk for at least 15 minutes right now? | To what extent are you able to carry out your everyday physical activities such as walking, climbing stairs, carrying groceries, or moving a chair right now? | What is your pain level right now? | My mind is as sharp as usual right now. |
| Type of Response | 11-Point Likert Scale | 5-Point Likert Scale | 5-Point Likert Scale | 5-Point Likert Scale | 5-Point Likert Scale | 5-Point Likert Scale | 11-Point Likert Scale | 5-Point Likert Scale |
| Response Choices | 0 (very bad) - 10 (very good)* | 1 (strongly disagree) - 5 (strongly agree) | 1 (none) - 5 (very severe) | 1 (none) - 5 (very severe) | 1 (completely) - 5 (not at all) | 1 (without any difficulty) - 5 (unable to do) | 0 (no pain) - 10 (worst pain imaginable) | 1 (not at all) - 5 (very much)* |
Note:
indicates items that were reversed scored for analyses; higher scores indicate worse symptom ratings.
ADL Activities of Daily Living
Table 2.
Daily Prompt Schedule
| Lower Time Boundary | Upper Time Boundary | Random Time within Boundary? | |
|---|---|---|---|
| Prompt 1 | Wake Time | <2 hours after Wake Time | Yes |
| Prompt 2 | >2 hours after Prompt 1 | N/A | Yes |
| Prompt 3 | >2 hours after Prompt 2 | N/A | Yes |
| Prompt 4 | >2 hours after Prompt 3 | <2 hours before Bedtime | Yes |
Sedentary Behavior and Moderate-to-Vigorous Physical Activity
To collect objective data on daily SB, participants wore an Actigraph Accelerometer (model wGT3X-BT; Actigraph Corporation); a valid and reliable measure of free-living activity behavior, (29, 30) for 10 consecutive days (3 day pre-treatment, 1 treatment day, and 6 post-treatment days). Participants wore the monitor on the nondominant hip during waking hours (except when bathing or swimming). Participants recorded their monitor wear times on an activity log and specified when they moved the monitor from their hip, in addition to any nonwear time. Only waking data were analyzed for the present analysis. All sleep data were excluded. Data were collected at 60 second epochs. We used the Choi algorithm to classify nonwear periods, in addition to the log data (31). All data were downloaded and analyzed in ActiLife 6.13.3. Only valid days were included in the analysis. To be considered a valid day of accelerometer wear, the monitor must have been worn during ≥10 waking hours (31, 32). Valid minutes of wear time were categorized by activity intensity (counts/min)(33, 34), using the Troiano cut points (35). Daily sedentary time was classified as minutes spent at <100 counts/min, moderate and vigorous intensity was classified as 2020 counts/min or greater (36). All participants with at least two valid pre-treatment days and three valid post-treatment days were required for inclusion in final analysis.
Statistical Analysis
All analyses were conducted in SPSS version 27 (IBM Corp., Chicago, IL). Means and SD were calculated for SB by time point and treatment status. Due to our nested data structure (observations nested within persons), we used multi-level linear regressions with a random intercept to conduct comparisons by time point and treatment status. First, we examined SB change across time point and treatment status by fitting separate multilevel linear regression models regressing SB on (1) time point (T1, T2, T3), (2) treatment status (prechemotherapy, day of/post-chemotherapy), and (3) time point by treatment status interaction (Model 1). Model 2 repeated the above models controlling for relevant covariates: age, BMI, number of comorbidities, health status, disease stage, chemotherapy type (neoadjuvant, adjuvant), weekend day, accelerometer wear time, treatment cycle number, time point by symptom interaction, and a treatment status by symptom interaction. Model 3 treated time point as a ordinal variable to compare SB outcomes between each time point to better understand SB time course controlling for all covariates. Pairwise comparisons for time point and SB used the Bonferroni correction at the 0.05 level.
Next, we examined the relationship between same day symptoms and same day SB using separate multilevel linear regression models for each symptom. The daily symptom rating was set as the main predictor variable and disaggregated into between subjects and within-subjects versions (37). The between-subjects version represents a participant’s symptom rating average score across all 30 days of study participation. Within-person results represent the deviation of the participant’s daily mean from their overall mean (38), calculated as the difference between their symptom rating that day and their average 30-day symptom rating. Four sets of models were fit to examine the relationship between SB and each symptom. The first set of models fit separate models for each symptom to examine the fixed effects of between- and within-person symptom rating for same day symptoms on same day SB. We included a random intercept and a random time point effect and fixed effect of time point, weekend/weekday, treatment status, BMI, number of comorbidities, health status, disease stage, treatment cycle number, chemotherapy type, accelerometer wear time, time point by treatment status interaction, time point by symptom interaction, and treatment status by symptom interaction on daily SB. We then added minutes of MVPA into the model as a covariate.
The second set of models examined the fixed effects of between- and within-person symptom rating for previous day symptoms on next day SB for each symptom. As with the first set of models, we included a random intercept and a random time point effect and fixed effect of time point, weekend/weekday, treatment status, BMI, number of comorbidities, health status, disease stage, treatment cycle number, chemotherapy type, accelerometer wear time, time point by treatment status interaction, time point by symptom interaction, and treatment status by symptom interaction on daily SB, in addition, we included the next day within-person symptom rating. Again, we then added minutes of MVPA into the model as a covariate. We used the Bonferroni correction to preserve the family-wise error rate at the 0.05 level, this correction corresponds at a <0.002 significance level and 99.8% confidence intervals.
Model sets 3 and 4 were run to examine the reciprocal relationship between SB and each symptom. In the third set of models, separate models were fit to examine the fixed effects of between- and within-person same day SB on same day symptoms. In the fourth set of models, we fit separate models to examine the fixed effects of between- and within-person previous day SB of next day symptom rating. We followed the same procedures as in model sets 1 and 2, controlling for relevant covariates and MVPA, in addition to the next day within-person symptom rating for the lagged model. Bonferroni correction to preserve the family-wise error rate, corresponding to a <0.002 significance level and 99.8% confidence intervals.
Power Analysis
We were powered to detect within-person differences taking into account number of days of observations, number of participants, number of repeated measures per participant, and the intraclass correlation. Assuming an intraclass correlation of 0.4 and an average of ≥8 days of accelerometer wear at all three timepoints [23], we have 80% power to detect effect sizes as small as 0.11. With 67 participants, 10 days of activity data at baseline, and two post-baseline time points, we have adequate power to detect within-person activity changes. Further, to reduce the risk of Type I error we applied the Bonferroni correction to preserve the family-wise error rate at the 0.05 level, this correction corresponds at a <0.002 significance level and 99.8% confidence intervals.
RESULTS
Participants
A total of 75 participants were eligible and consented to participate in the study. All participants with at least one time point of valid sedentary behavior and symptom rating data (n=67) were included in the analyses, with 63 participants having complete data at all three time points. Details regarding recruitment and retention of participants can be found elsewhere [39]. On average, participants were 48.6 years old [range: 31–71], and overweight (27.6 m/kg2 [range: 17.9–52.5]). A majority of participants were White (76.6%) and completed college (67.2%). Eighty percent of participants were diagnosed with Stage I or II disease and 65.7% were receiving adjuvant chemotherapy during the data collection period. Almost half of participants (44.4%) self-reported meeting physical activity guidelines (≥150 minutes per week of MVPA) prior to their cancer diagnosis and on average, participants engaged in 22.7±22.6 minutes of MVPA during the course of their study participation. All participant characteristics are reported in Table 3.
Table 3.
Participant Characteristics (mean [range] or n (%))
| Characteristic | mean [range] or n (%) |
|---|---|
| Age (years) | 48.6 [31–71] |
| Body Mass Index (kg/m2) | 27.6 [17.9–52.5] |
| Race | |
| White | 51 (76.6%) |
| African American | 8 (10.9%) |
| Asian/Pacific Islander | 4 (6.3%) |
| Other | 4 (6.3%) |
| Hispanic/Latino | 9 (12.9%) |
| College degree or greater | 52 (78.1%) |
| Working at least part time | 45 (67.2%) |
| Annual household income ≥$100,000 | 31 (46.3%) |
| Marital Status | |
| Married/Partnered | 45 (67.2%) |
| Single | 11 (16.4%) |
| Divorced/Separated | 5 (7.5%) |
| Widowed | 3 (4.5%) |
| Unknown | 3 (4.5%) |
| Chronic disease | |
| Asthma | 10 (16.9%) |
| Depression | 9 (12.9%) |
| Arthritis | 8 (10.9%) |
| Obesity | 7 (12.1%) |
| Upper gastrointestinal disease | 5 (7.5%) |
| Osteoporosis | 4 (6.3%) |
| Anxiety or panic disorders | 4 (6.3%) |
| Visual impairment | 4 (6.3%) |
| Diabetes | 3 (4.5%) |
| Degenerative disc disease | 3 (4.5%) |
| Hearing impairment | 2 (3.4%) |
| Chronic obstructive pulmonary disease | 1 (1.7%) |
| Congestive heart failure | 1 (1.7%) |
| Overall health status at baseline | |
| Fair/Poor | 5 (7.5%) |
| Good | 25 (37.3%) |
| Excellent/Very Good | 35 (52.2%) |
| Did not answer | 2 (3.0%) |
| Meeting physical activity guidelines | 38 (44.4%) |
| (pre-cancer diagnosis) | |
| Breast cancer stage | |
| Stage I/II | 17 (80%) |
| Stage III | 13 (20%) |
| Chemotherapy type | |
| Neoadjuvant | 23 (34.3) |
| Adjuvant | 44 (65.7%) |
Sedentary Behavior Descriptives
Over the course of the entire study period, participants engaged in over 10 hours/day, or an average of 651.9±171.8 minutes, of time spent sedentary. Controlling for wear time, sedentary behavior increased as time point increased (β=9.2, (95% CI: 2.6, 15.8). Day of/post-treatment days were significantly associated with 20.5 (95% CI: 31.2, 9.7) more minutes of SB compared to pre-treatment days. Finally, there was a significant time by treatment interaction (β=10.6, (95% CI:5.1, 13.8); SB on day of/post-treatment days increased over time. All significant relationships remained after controlling for relevant covariates in model 2.
Symptoms predicting sedentary behavior
Figure 1 provides a graphical representation of the regression coefficients and 99.8% confidence intervals of sedentary behavior on symptom ratings for between-person same day effects, within-person same day effects, and previous day within-person effects.
Figure 1.
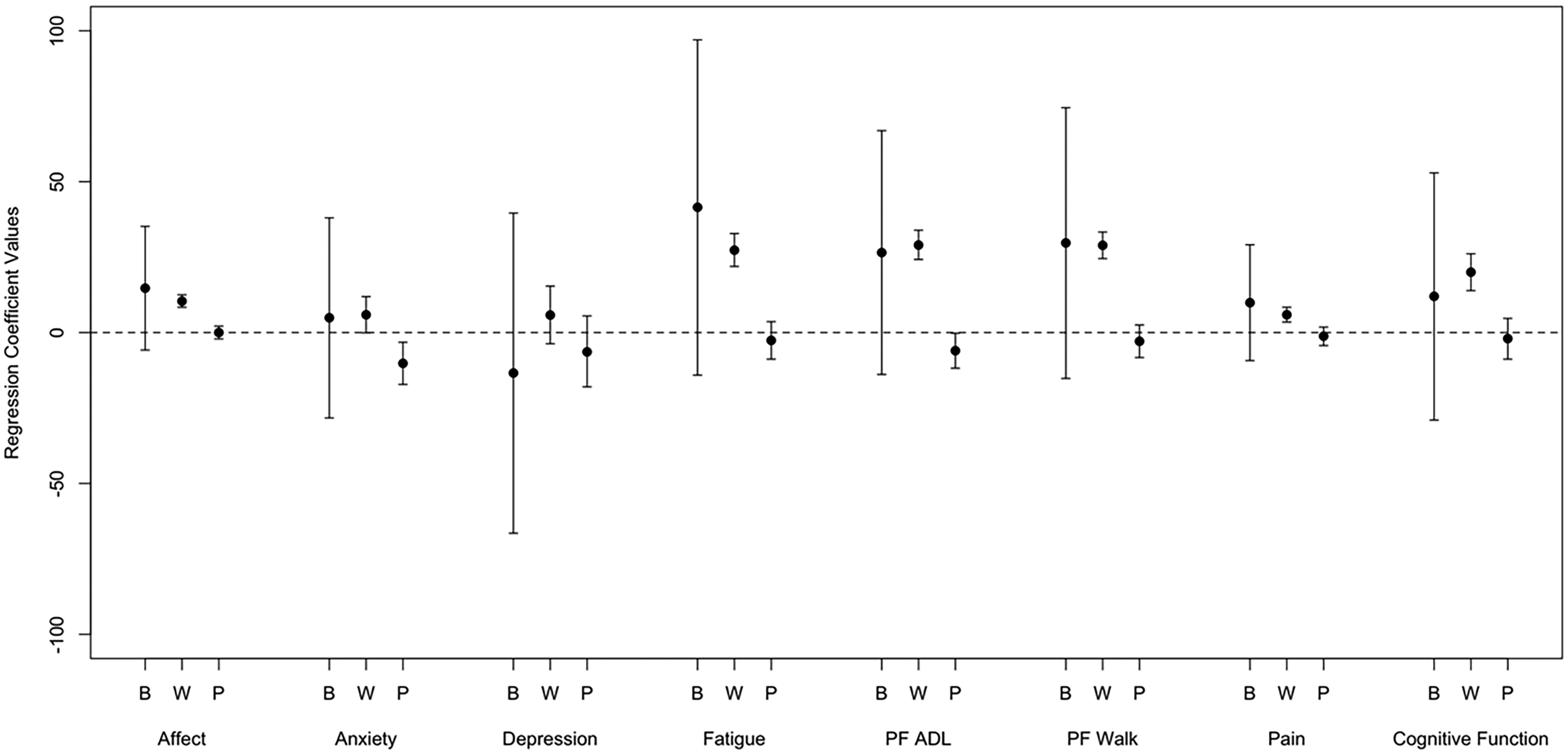
Regression coefficients and 99.8% confidence intervals of sedentary behavior on symptom ratings. Coefficients with confidence intervals that do not include zero are significant. B, between-person, same day effects; W, within-person, same day effects; P, previous day, within-person effects; PF, physical function; ADL, activities of daily living
Same Day Models.
Same-day within-person associations were significant for affect, fatigue, ADL physical function, walk physical function, pain, and cognitive functioning and daily sedentary behavior (p<0.05 for all). Every 1-point worse symptom rating than an individual’s 30 day average was associated with between 5.9 (pain) and 29.0 (ADL physical function) more minutes of SB on that day (see Figure 1). The within person-effects were larger (i.e. worse than average symptom rating associated with larger increase in SB) on post-treatment days compared to pre-treatment days for ADL-physical functioning, anxiety, pain, and cognitive functioning. When we controlled for MVPA, anxiety was no longer a significant predictor, however, all other relationships between symptoms and SB remained. Within-person effects of symptom ratings on SB were larger (i.e. worse than participant’s average symptom rating was associated with greater time spent sedentary) as time point increased for ADL-physical function, walk physical function, and affect. There were no significant between-person effects.
Lagged models.
Lagged models showed a significant effect of previous day ratings for anxiety and physical function-ADL on next day SB. Every one-point worse rating from a person’s 30-day average rating was associated with 10.2 (anxiety) and-6.0 (ADL physical function) minutes more SB the next day. Effects remained significant following controlling for MVPA.
Sedentary behavior predicting symptoms
Same Day.
Significant within-person effects were observed for all symptoms (Figure 2). On days participants engaged in more sedentary behavior than average, they reported worse ratings on their symptoms that day. There was a significant between-person association between same day SB and affect [β=0.005, (95% CI: 0.00, 0.001) or 0.3 worse affect rating for every 1-hour increase in SB]. For affect, anxiety, ADL physical function, and walk physical function, the within-person effects of SB were significantly larger (i.e. more than average SB associated with worse symptoms) for post-treatment days compared to pre-treatment days. For anxiety, depression, and cognitive functioning, within-person effects of SB were significantly larger as time increased. When MVPA was added into the model, results persisted.
Figure 2.
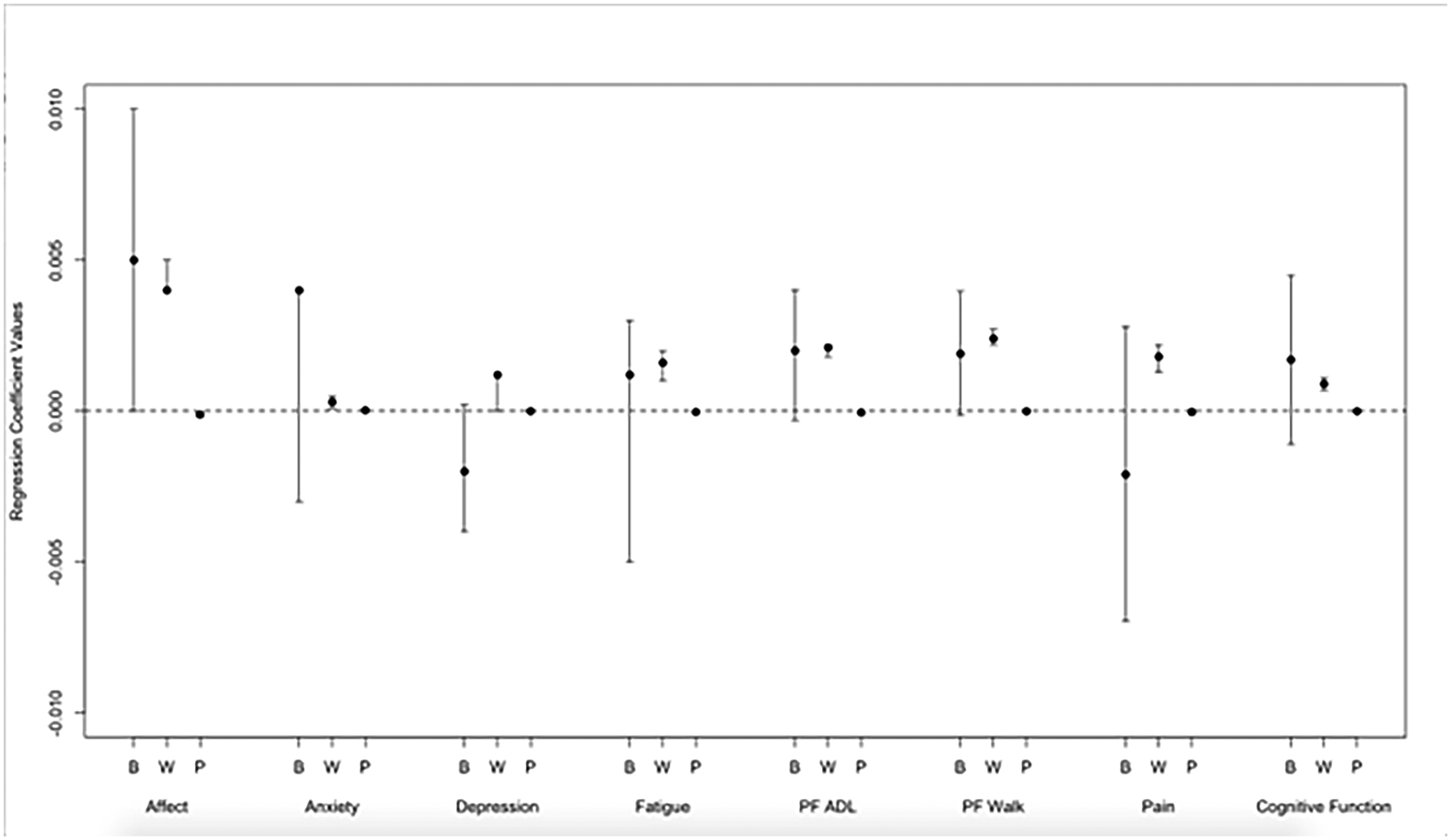
Regression coefficients and 99.8% confidence intervals of symptom ratings on sedentary behavior. Coefficients with confidence intervals that do not include zero are significant. B, between-person, same day effects; W, within-person, same day effects; P, previous day, within-person effects; PF, physical function; ADL, activities of daily living
Previous Day.
Significant within-person effects for previous day SB and next day symptoms were found for affect and ADL physical function. When participants engaged in more SB on a given day, they self-reported worse affect [β=0.006, 95% CI: 0.002, 0.0061] and ADL physical function [β=0.0005, 95% CI: 0.0004, 0.0008] the next day even when controlling for MVPA.
DISCUSSION
The purpose of this study was to examine the relationship between daily changes in symptoms and SB during CT for breast cancer. Overall, there was a significant relationship between later treatment cycles and day of/post treatment days on greater time spent sedentary. Six [affect, anxiety, fatigue, physical functioning (ADL and walk), pain, and cognitive functioning] of the eight symptoms examined showed a significant same-day within-person effect on SB. However, only anxiety, depression, and ADL physical functioning demonstrated a lagged effect on SB and effects were much smaller compared to same day effects. Additionally, time spent in SB demonstrated a significant, same-day, within-person effect on all same day symptoms ratings, except depression. Lagged models showed a significant effect of time spent in previous day SB on next day affect and physical function. In all analyses, results remained consistent with the addition of MVPA into the model.
Fatigue and physical functioning (ADL and walking) had the strongest association with SB such that a one-point poorer symptom rating was related to an increase in SB of between 20–25 minutes that day. This is important because extended time spent in sedentary positions has shown to be associated with obesity, metabolic disorders, and all-cause mortality, even in healthy populations (40). Pinto and colleagues examined the relationship between symptoms predicting SB in breast cancer survivors within 5 years of diagnosis. Their results indicated higher scores for sadness, anxiety, stress, worry, and fatigue were significantly associated with more time spent sedentary (18). Taken together, these studies indicate there is a significant relationship between worsening daily symptoms and higher daily SB across the cancer treatment continuum and that time spent sedentary is also high across the cancer care continuum. Likewise, our results indicate that when the reciprocal relationship is examined (i.e. time spent in SB predicting symptom ratings), there is a significant association for all examined symptoms suggesting a reciprocal loop between time spent sedentary and treatment symptoms. This relationship provides further evidence of the importance of breaking the cycle of time spent sedentary in breast cancer patients undergoing chemotherapy. Future research should investigate behavioral interventions to reduce overall sedentary time in breast cancer patients, especially those undergoing chemotherapy as sedentary behavior is significantly increased during this time period and increases may persist even after treatment ends. Breaking up long bouts of sedentary time with short, low-intensity physical activity bouts may present a viable, achievable intervention for patients undergoing chemotherapy and survivors (41).
Three symptoms, anxiety, depression, and ADL physical function, showed a lagged effect, also significantly impacting time spent sedentary on the following day. These results indicate there may be some benefit to implementing a SB reduction intervention the night before for the following day, however the greatest benefit still seems to be in the current day. These results are consistent with what our group found when we examined the lagged effect of symptoms on physical activity behaviors with results indicating symptoms did not significantly impact next day MVPA, with only anxiety showing a significant impact on LPA (22). Collectively, results indicate future interventions should primarily focus on same day symptoms and SB reduction, but some specific previous day symptoms may also be important.
Finally, our results indicated there was a significant effect of time and treatment day on time spent in SB and symptom ratings. We hypothesized this relationship given the nature of chemotherapy. In our other study using these data, we reported a significant within person effect of symptoms on reducing time spent in MVPA (22). Even so, participants still reported about 22 minutes of MVPA per day, providing encouragement that BC patients undergoing chemotherapy are able to engage in some physical activity and indicating SB is an important behavior to target independent of MVPA intervention. These themes came out in our qualitative work with breast cancer patients undergoing chemotherapy citing preferences for a highly personalized and tailored intervention (42). Patients were interested in receiving education on both safely reducing SB and increasing PA during chemotherapy considering their current symptoms. Taken together, these results indicate first, chemotherapy is an important time-period to target for a SB intervention, given the symptoms associated with treatments. Further, SB intervention will need to take into consideration chemotherapy cycle and treatment day, as symptoms worsen, participants may need additional support for SB reduction during post-treatment and later chemotherapy cycles. Future research should elucidate the effect of the specific chemotherapy regimen and the relationship with treatment-related symptoms to further tailor future interventions.
Limitations and Strengths
This study presents limitations related to the sample and recruitment strategies. The sample was relatively small, but adequately powered for within-person analyses. Study participants were recruited from a single academic medical center, which may contribute to the homogeneity of the sample. Additionally, this study excluded women with metastatic (stage IV) breast cancer. Women diagnosed with stage IV breast cancer may experience additional symptoms or symptoms of differing intensity that influence sedentary behavior. Therefore, it is important to note our findings may not be generalizable to other more heterogeneous samples. Future research should examine findings in a more diverse group of patients (e.g., race, ethnicity, income, geography, diagnosis, treatment type). We used Actigraph accelerometers worn at the hip, analyzed using counts, to assess sedentary behavior. The limitations to this movement-based approach have been well documented but indicate this approach still provides a valid and reliable measurement of time spent in sedentary pursuits (33, 43). We acknowledge the limitations in our analysis of the physical activity data by intensity category. An important future research question should investigate the effect of activity type (i.e. aerobic versus strength training) on symptoms and other survivorship outcomes. Finally, although our EMA prompts were adapted from validated questionnaires, the clinical significance of their change, as measured, is not known. Further, we assessed a wide range of chemotherapy symptoms, however, there are other symptoms that we did not measure such as chemotherapy-induced neuropathy, that could also influence participants sedentary behavior. Given that most symptoms improved significantly with reduced time spent sedentary, these results suggest that generally, same day reduction in sedentary time improves cancer symptoms at the day level.
Despite these limitations, this is the first study to utilize EMA methodology to investigate the relation between common symptoms experienced by breast cancer patients undergoing chemotherapy and sedentary behavior. Physical activity was measured objectively, and symptoms were reported in real-time in participants’ natural environments. These methods increase data validity. Additionally, the completely remote nature of the study decreased participant burden. Given daily data collection at multiple time points, this study provides insights into the acute effect of symptoms on sedentary behavior.
CONCLUSIONS
Overall, our results showed there was a significant effect of time point and treatment status on time spent sedentary. Additionally, there were significant within-person effects on SB for affect, anxiety, fatigue, physical functioning (ADL and walk), pain, and cognitive functioning. Further, our results indicate a significant reciprocal relationship with a significant within-person relationship between greater time spent in SB predicting worse individual symptoms ratings for all variables, except depression. Taken together these results indicate there is a significant temporal, treatment, and daily symptom effect on time spent sedentary in breast cancer patients undergoing chemotherapy. Given survivors undergoing treatment for breast cancer show a significant increase in their time spent sedentary, increasing their risk of negative symptoms, future research should target this time period to develop ways to reduce sedentary behaviors taking into account daily fluctuations in treatment-related symptoms.
Acknowledgements, Conflicts of Interest, and Source of Funding
This work was supported by the Northwestern Memorial Hospital Lynn Sage Cancer Research Foundation Grants Initiative and the NCI K07CA196840 awarded to S. Phillips. Whitney Welch is supported by R37CA225877 01A1S1 and P30AG059988. The authors have no conflicts of interest. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate manipulation. The results of the study do not constitute endorsement by the American College of Sports Medicine.
REFERENCES
- 1.Hayat MJ, Howlader N, Reichman M, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12(1): 20–37. [DOI] [PubMed] [Google Scholar]
- 2.Anderson M, Matey L. Overview of cancer and cancer treatment. In: Olsen M, LeFebvre K, Brassil K, editors. Chemotherapy and Immunotherapy Guidelines and Recommendations for Practice. Pittsburgh, PA: Oncology Nursing Society; 2019. p. 25–50. [Google Scholar]
- 3.Olsen M, Naseman R. Chemotherapy. In: Olsen M, LeFebvre K, Brassil K, editors. Chemotherapy and Immunotherapy Guidelines and Recommendations for Practice. Pittsburgh, PA: Oncology Nursing Society; 2019. p. 61–90. [Google Scholar]
- 4.Chu E, DeVita V. Physician’s Cancer Chemotherapy Drug Manual. 2019, Burlington, MA: Jones & Bartlett Learning. [Google Scholar]
- 5.Thivel D, Tremblay A, Genin PM, Panahi S, Riviere D, Duclos M. Physical activity, inactivity, and sedentary behaviors: definitions and implications in occupational health. Front Public Health. 2018;6:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips SM, Dodd KW, Steeves J, McClain J, Alfano CM, McAuley E. Physical activity and sedentary behavior in breast cancer survivors: new insight into activity patterns and potential intervention targets. Gynecol Oncol. 2015;138(2):398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katzmarzyk PT, Lee IM. Sedentary behaviour and life expectancy in the USA: a cause-deleted life table analysis. BMJ Open. 2012;2(4):e000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews CE, George SM, Dallal CM, et al. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am J Clin Nutr. 2012;95(2):437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwardson CL, Gorley T, Davies MJ, et al. Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLoS One. 2012;7(4):e34916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch BM, Dunstan DW, Healy G, Winkler E, Eakin E, Owen N. Objectively measured physical activity and sedentary time of breast cancer survivors, and associations with adiposity: findings from NHANES (2003–2006). Cancer Causes Control. 2010;21(2):283–8. [DOI] [PubMed] [Google Scholar]
- 11.Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2691–709. [DOI] [PubMed] [Google Scholar]
- 12.Nurnazahiah A, Shahril MR, Nor Syamimi Z, Ahmad A., Sulaiman S, Lua PL. Correction to: Relationship of objectively measured physical activity and sedentary behaviour with health-related quality of life among breast cancer survivors. Health Qual Life Outcomes. 2020;18(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nurnazahiah A, Shahril MR, Nor Syamimi Z, Ahmad A, Sulaiman S, Lua PL. Relationship of objectively measured physical activity and sedentary behaviour with health-related quality of life among breast cancer survivors. Health Qual Life Outcomes. 2020;18(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trinh L, Amireault S, Lacombe J, Sabiston CM. Physical and psychological health among breast cancer survivors: interactions with sedentary behavior and physical activity. Psychooncology. 2015;24(10):1279–85. [DOI] [PubMed] [Google Scholar]
- 15.Gavin KL, Welch WA, Conroy D. Sedentary behavior after breast cancer: motivational, demographic, disease, and health status correlates of sitting time in breast cancer survivors. Cancer Causes Control. 2019;30(6):569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips SM, Lloyd GR, Awick EA, McAuley E. Correlates of objectively measured sedentary behavior in breast cancer survivors. Cancer Causes Control. 2016;27(6):787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marszalek J, Morgulec-Adamowicz N, Rutkowska I, Kosmol A. Using Ecological Momentary Assessment to Evaluate Current Physical Activity. Biomed Res Int. 2014;2014:915172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinto BM, Kindred MD, Dunsiger SI, Williams DM. Sedentary behavior among breast cancer survivors: a longitudinal study using ecological momentary assessments. J Cancer Surviv. 2021;15(4):546–53. [DOI] [PubMed] [Google Scholar]
- 19.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23(24):5814–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabiston CM, Brunet J, Vallance JK, Meterissian S. Prospective examination of objectively assessed physical activity and sedentary time after breast cancer treatment: sitting on the crest of the teachable moment. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1324–30. [DOI] [PubMed] [Google Scholar]
- 21.Rabin C Promoting lifestyle change among cancer survivors: when is the teachable moment? Am J Lifestyle Med. 2009;3(5):369–78. [Google Scholar]
- 22.Phillips SM, Welch WA, Fanning J, et al. Daily physical activity and symptom reporting in breast cancer patients undergoing chemotherapy: an intensive longitudinal examination. Cancer Epidemiol Biomarkers Prev. 2020;29(12):2608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solk P, Gavin K, Fanning J, et al. Feasibility and acceptability of intensive longitudinal data collection of activity and patient-reported outcomes during chemotherapy for breast cancer. Qual Life Res. 2019;28(12):3333–46. [DOI] [PubMed] [Google Scholar]
- 24.Shiffman S, Stone AA. Ecological momentary assessment: a new tool for behavioral medicine research. In: Krantz David S., Baum Andrew, editors. Technology and Methods in Behavioral Medicine. Mahwah, NJ: Lawrence Erlbaum Associates; 1998. p. 117–31. [Google Scholar]
- 25.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardy CJ, Rejeski WJ. Not what, but how one feels: the measurement of affect during exercise. J Sport Exerc Psychol. 1989;11(3):304–17. [Google Scholar]
- 27.Garcia SF, Cella D, Clauser S, et al. Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. J Clin Oncol. 2007;27(32):5106–12. [DOI] [PubMed] [Google Scholar]
- 28.Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18(3):263–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sport Exerc. 1998;30(5):777–81. [DOI] [PubMed] [Google Scholar]
- 30.Welk GJ, McClain J, Eisenmann JC, Wickel EE. Field validation of the MTI Actigraph and BodyMedia armband monitor using the IDEEA monitor. Obesity (Silver Spring). 2007;15(4):918–28. [DOI] [PubMed] [Google Scholar]
- 31.Bassett DR. Validity of four motion sensors in measuring moderate intensity physical activity. Med Sci Sport Exerc. 2000;32(9):S471–80. [DOI] [PubMed] [Google Scholar]
- 32.Tudor-Locke C, Swift DL, Schuna JM, et al. WalkMore: a randomized controlled trial of pedometer-based interventions differing on intensity messages. BMC Public Health. 2014;14:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson P. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011;43(8):1561–7. [DOI] [PubMed] [Google Scholar]
- 35.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. [DOI] [PubMed] [Google Scholar]
- 36.Phillips SM, Welch WA, Fanning J, et al. Daily physical activity and symptom reporting in breast cancer patients undergoing chemotherapy: an intensive longitudinal examination. Cancer Epidemiol Biomarkers Prev. 2020;29(12):2608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedeker D, Mermelstein RJ, Demirtas H. Modeling between-subject and within-subject variances in ecological momentary assessment data using mixed-effects location scale models. Stat Med. 2012;31(27):3328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curran PJ, Bauer DJ. The disaggregation of within-person and between-person effects in longitudinal models of change. Annu Rev Psychol. 2011;62:583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solk P, Gavin K, Fanning J, et al. Feasibility and acceptability of intensive longitudinal data collection of activity and patient-reported outcomes during chemotherapy for breast cancer. Qual Life Res. 2019;28(12):3333–46. [DOI] [PubMed] [Google Scholar]
- 40.Dallal CM, Brinton LA, Matthews CE, et al. Accelerometer-based measures of active and sedentary behavior in relation to breast cancer risk. Breast Cancer Res Treat. 2012;134(3):1279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blair CK. Harding E, Wiggins C, et al. A home-based mobile health intervention to replace sedentary time with light physical activity in older cancer survivors: randomized controlled pilot trial. JMIR Cancer. 2021;7(2):e18819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen AM, Welch WA, Gavin K, et al. Preferences for mHealth physical activity interventions during chemotherapy for breast cancer: a qualitative evaluation. Support Care Cancer. 2020;28(4):1919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart TL, Ainsworth BE, Tudor-Locke C. Objective and subjective measures of sedentary behavior and physical activity. Med Sci Sports Exerc. 2011;43(3):449–51. [DOI] [PubMed] [Google Scholar]


