Summary:
Clinical data with enfortumab vedotin suggests that most bladder cancers overexpress NECTIN-4. A recent article shows that NECTIN-4 membranous expression changes with progression to metastatic disease and that low NECTIN-4 expression in metastatic biopsies is potentially associated with EV resistance. These data argue for incorporation of NECTIN-4 expression into future biomarker strategies.
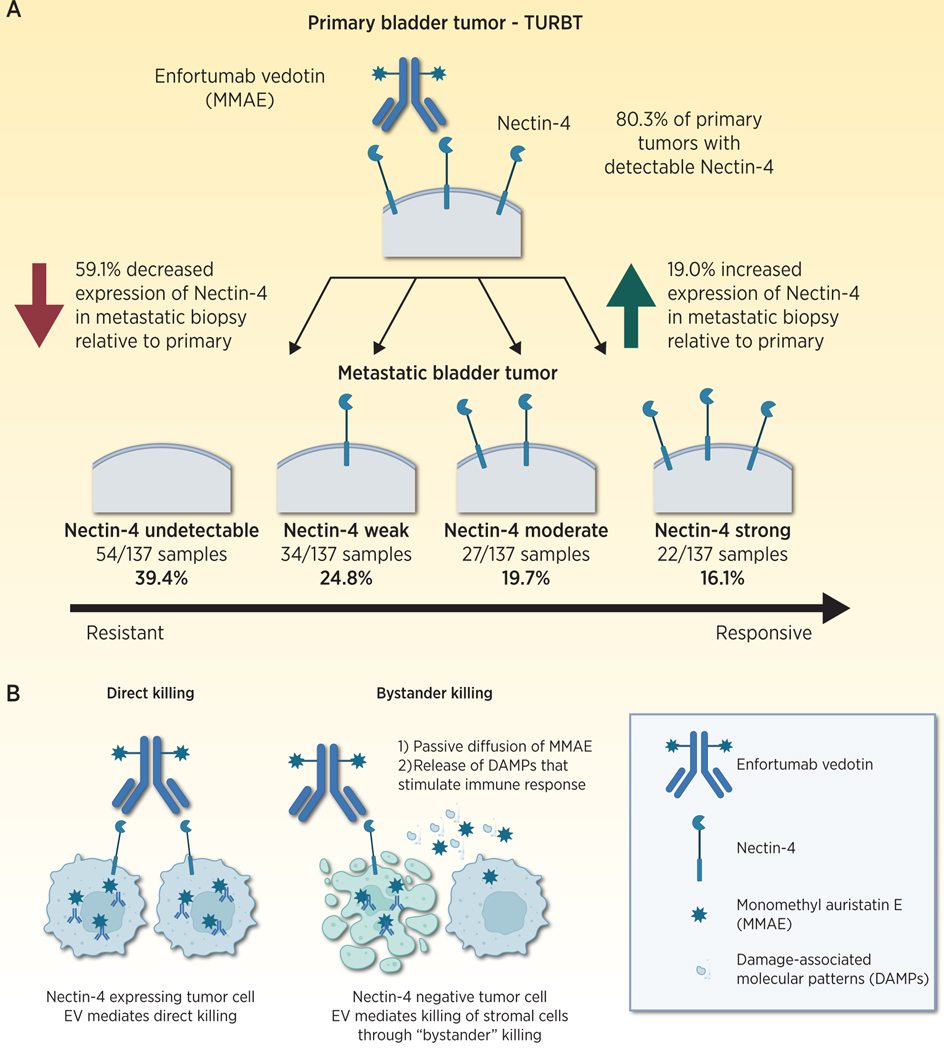
In this issue of Clinical Cancer Research, Klümper and colleagues (1) quantified NECTIN-4 expression from paired primary and metastatic urothelial cancer biopsies to understand changes in NECTIN-4 expression with tumor evolution and potential association with resistance to the antibody drug conjugate (ADC) enfortumab vedotin (EV). The authors show that NECTIN-4 expression can be dynamic with progression from primary to metastatic tumor sites, with loss of NECTIN-4 expression in some metastatic samples. Further, they demonstrate that metastatic tumor samples with low NECTIN-4 expression are associated with shorter progression free survival among patients treated with EV, suggesting that NECTIN-4 expression could be further explored prospectively as a biomarker.
First, the study highlights the importance of tumor heterogeneity in matched primary/metastatic samples. The discordance between primary and metastatic urothelial tumors has been recently characterized by Clinton et al, who found early branched evolution and lesion-to-lesion genomic heterogeneity between primary and metastatic sites, and 23% discordance in actionable genomic alterations (2). Thus, immunohistochemistry (IHC) of primary tumor specimens may not predict target expression at metastatic sites, particularly among patients who progress after multiple lines of therapy. Although the precise function of NECTIN-4 in urothelial cancer remains undefined, the NECTIN family of cell surface proteins appear to be involved in cell-cell adhesion (3,4). Downregulation of NECTIN-4 may result in loss of cell polarity, which may promote or be associated with metastatic spread. Although this remains a hypothesis, the findings by Klümper et al suggest a possible underlying mechanism of loss of nectin-4 expression at metastatic progression. The expression patterns of NECTIN-4 presented in this study are discordant with the relatively high NECTIN-4 H-scores noted with clinical samples in the EV-101 (5) and EV-201 (6) trials. The somewhat lower prevalence in metastatic samples in the current study may reflect differences in sensitivity and specificity of IHC antibody clone used, where a highly sensitive IHC antibody as used in clinical trials of EV-101 and 102 might overestimate NECTIN-4 expression. Alternatively, this discordance may reflect greater heterogeneity in clinical biopsies relative to preclinical models. Indeed, expression of NECTIN-4 in cell lines and patient- and cell-derived xenografts has previously been reported with less ubiquitous expression of NECTIN-4 noted in bladder cancer preclinical models (7,8).
Multiple mechanisms of primary resistance to ADCs and tumor plasticity likely exist. A recent study of metastatic biopsies in 3 patients who progressed on EV demonstrated that NECTIN-4 membrane protein expression remained high despite disease progression, suggesting that other resistance mechanisms may be important (9). Interruption of endosomal trafficking, epitope mutations, and payload drug efflux pumps have all been described as possible resistance mechanisms to ADCs as well (10,11). Furthermore, treatment with neoadjuvant chemotherapy has been shown to drive a chemorefractory “luminal” phenotype (12), which remains enriched in NECTIN-4 (8). Whether these tumors can be more sensitive to subsequent EV remains hypothetical, but points to the importance of tumor plasticity and, potentially, to treatment order.
In this study, NECTIN-4 expression changed with progression to metastatic disease, with some tumors losing NECTIN-4 expression and other increasing expression at metastatic progression (Fig 1A). This new finding suggests that decreased NECTIN-4 expression may be associated with primary resistance and may identify patients more likely to require combination therapy approaches. Low expression of NECTIN-4 in primary tumor samples in this study was concordant with prior retrospective data showing that NECTIN-4 expression was absent in 17% of primary tumors. Klümper et al show that upon progression to metastatic disease, NECTIN-4 expression levels decrease in 59.1% of cases (Figure 1A) This novel finding suggests that NECTIN-4 expression is dynamic with progression of disease, and that antigen escape (loss) may be one potential mechanism for primary resistance.
Figure 1.
Nectin-4 Cell Surface Expression Changes With Progression to Metastatic Disease. A) Quantification of nectin-4 expression in primary tumor samples by Klümper et al. (1) showed that 80.3% of primary tumors express detectable nectin-4. In paired metastatic biopsies, most tumors decrease nectin04 expression by H-Score (59.1%), while others increase nectin-4 expression (19.0%). Quantification of nectin-4 in metastatic biopsies by quartile is shown (Fig 1A, bottom). B) Nectin-4 expression permits binding of enfortumab vedotin and deliver of a Monomethyl auristatin E (MMAE) payload (left). Stromal cell expression of nectin-4 may permit bystander killing of nectin-4 negative tumors cells (right). (Image created with BioRender.com.)
The authors suggest that NECTIN-4 expression by IHC should be used to select patients for treatment with EV. For this to occur, prospective evaluation is needed. Furthermore, NECTIN-4 expression alone, however, may not completely identify the universe of patients who may benefit from EV therapy. Like PD-L1 expression by IHC, NECTIN-4 is potentially a dynamic biomarker subject to intralesional heterogeneity and temporal expression changes that may not be adequately captured by IHC from a single biopsy. As noted by the authors, NECTIN-4 directed PET imaging may be able to overcome this limitation inherent to IHC (13). It is possible that some NECTIN-4 low tumors by IHC are still EV responsive, and there has not yet been a defined minimum threshold of NECTIN-4 expression that is required to induce a treatment response. Perhaps most important, low expressing NECTIN-4 tumors might still respond to enfortumab vedotin alone or in combination therapies through bystander killing, whereby ADC mediated killing on neighboring stromal cells eliminates NECTIN-4 low tumor cells through either passive drug diffusion, or release of damage associated molecular patterns that promote immunogenic cell death (Fig 1B). It also remains unknown whether cytoplasmic or membranous localization of NECTIN-4 plays a significant role in treatment response.
Capturing the universe of patients who may benefit from EV therapy may require transcription-based biomarker approaches to identify treatment responsive tumors. For example, specific transcriptional signatures may capture tumors with low levels of NECTIN-4 expression by IHC that are still able to respond to enfortumab vedotin. Preclinical studies demonstrate an association between luminal transcriptional signatures and response to EV [7], and these and other immune based transcriptional signatures (14,15) warrant further investigation as potential biomarkers of treatment response.
These data have direct implications for future biomarker development strategies and clinical use of ADC s. As ADCs and other targeted therapies emerge as options across disease states, (16) a growing understanding of the association of NECTIN-4 baseline expression and treatment resistance will be key to patient selection. While this retrospective analysis suggests that lower baseline NECTIN-4 expression is associated with shorter progression free survival, these findings need to be validated in larger prospective studies. There is also the potential to combine NECTIN-4 expression with other composite biomarkers that may predict response to EV monotherapy or combination therapy incorporating other intratumoral factors including tumor mutational burden (17), PD-L1 expression, FGFR3 mutations, or HER2 expression status. From a therapeutic standpoint, combinatorial therapies may be able to “rescue” treatment responses in NECTIN-4 low tumors, as is suggested by encouraging data with enfortumab vedotin with the anti-PD-1 antibody pembrolizumab. The emerging data from EV-103 cohort A and cohort K suggests that combining enfortumab vedotin with pembrolizumab may have a synergistic effect, and the immune microenvironment may be a critical component of sensitivity and/or resistance to treatment (18). In the EV-103 cohort A study, responses with combination therapy were noted independent of PD-L1 expression with activity noted in NECTIN-4 low tumors (5/12 patients with H-score < 150 with CR/PR) (19). We are simply “scratching the surface” of predictive biomarkers for ADC therapy, and the data presented by Klümper and colleagues suggest that low NECTIN-4 expression may be associated with treatment resistance and argue for further evaluation of NECTIN-4 expression by IHC in future biomarker development approaches.
Acknowledgments:
The authors are supported by the National Institutes of Health MSKCC Support Grant/Core Grant (P30 CA008748). D. H. Aggen is supported by the American Society of Clinical Oncology (2021CDA-7419879885). C. E. Chu is supported by a Ruth L. Kirschstein National Research Service Award (T32CA082088), American Society of Clinical Oncology Young Investigator Award, and a Ferdinand C. Valentine Fellowship Award.
Footnotes
COI:
D. Aggen: Consulting Fees and Trial Funding: Seattle Genetics, Astellas. Consulting Fees: BMS, 2Seventy bio. CME: MJH LifeSciences, Curio Sciences.
J. Rosenberg: Consulting Fees and Trial Funding: Bayer, Seattle Genetics, AstraZeneca, Roche/Genentech, Astellas, QED Therapeutics. Consulting Fees: BMS, Merck, Pfizer, Pharmacyclics, Boehringer Ingelheim, GSK, Infinity, Janssen, Mirati, EMD-Serono, Gilead, BioClin, Lilly, Tyra Biosciences, IMVax, Aadi, Hengrui. CME: Research to Practice, MJH LifeSciences, Medscape, UpToDate, Clinical Care Options, OncLive. Honoraria: EMD-Serono, Pfizer, MashupMedia, Astellas
References
- 1.Klümper N, Ralser DJ, Ellinger J, Roghmann F, Albrecht J, Below E, et al. Membranous NECTIN-4 expression frequently decreases during metastatic spread of urothelial carcinoma and is associated with enfortumab vedotin resistance. Clinical Cancer Research 2022. doi 10.1158/1078-0432.Ccr-22-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinton TN, Chen Z, Wise H, Lenis AT, Chavan S, Donoghue MTA, et al. Genomic heterogeneity as a barrier to precision oncology in urothelial cancer. Cell Reports 2022;41(12) doi 10.1016/j.celrep.2022.111859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brancati F, Fortugno P, Bottillo I, Lopez M, Josselin E, Boudghene-Stambouli O, et al. Mutations in PVRL4, encoding cell adhesion molecule nectin-4, cause ectodermal dysplasia-syndactyly syndrome. Am J Hum Genet 2010;87(2):265–73 doi 10.1016/j.ajhg.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reymond N, Fabre S, Lecocq E, Adelaïde J, Dubreuil P, Lopez M. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J Biol Chem 2001;276(46):43205–15 doi 10.1074/jbc.M103810200. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg J, Sridhar SS, Zhang J, Smith D, Ruether D, Flaig TW, et al. EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients With Nectin-4-Positive Solid Tumors, Including Metastatic Urothelial Carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2020;38(10):1041–9 doi 10.1200/JCO.19.02044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu EY, Petrylak DP, O’Donnell PH, Lee JL, van der Heijden MS, Loriot Y, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2021;22(6):872–82 doi 10.1016/S1470-2045(21)00094-2. [DOI] [PubMed] [Google Scholar]
- 7.Challita-Eid PM, Satpayev D, Yang P, An Z, Morrison K, Shostak Y, et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Research 2016;76(10):3003–13 doi 10.1158/0008-5472.CAN-15-1313. [DOI] [PubMed] [Google Scholar]
- 8.Chu CE, Sjöström M, Egusa EA, Gibb EA, Badura ML, Zhu J, et al. Heterogeneity in NECTIN4 Expression Across Molecular Subtypes of Urothelial Cancer Mediates Sensitivity to Enfortumab VedotinNECTIN4 Expression Mediates Sensitivity to EV. Clinical Cancer Research 2021;27(18):5123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman-Censits J, Lombardo K, McConkey D, Hahn NM, Bashir B, Kelly WK, et al. New and topics: enfortumab vedotin mechanisms of response and resistance in urothelial cancer - What do we understand so far? Urol Oncol 2021;39(10):619–22 doi 10.1016/j.urolonc.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Coates JT, Sun S, Leshchiner I, Thimmiah N, Martin EE, McLoughlin D, et al. Parallel Genomic Alterations of Antigen and Payload Targets Mediate Polyclonal Acquired Clinical Resistance to Sacituzumab Govitecan in Triple-Negative Breast Cancer. Cancer discovery 2021. doi 10.1158/2159-8290.CD-21-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabaud O, Berger L, Crompot E, Adélaide J, Finetti P, Garnier S, et al. Overcoming Resistance to Anti-Nectin-4 Antibody-Drug Conjugate. Mol Cancer Ther 2022;21(7):1227–35 doi 10.1158/1535-7163.Mct-22-0013. [DOI] [PubMed] [Google Scholar]
- 12.Seiler R, Gibb EA, Wang NQ, Oo HZ, Lam HM, van Kessel KE, et al. Divergent Biological Response to Neoadjuvant Chemotherapy in Muscle-invasive Bladder Cancer. Clin Cancer Res 2019;25(16):5082–93 doi 10.1158/1078-0432.CCR-18-1106. [DOI] [PubMed] [Google Scholar]
- 13.Campbell DO, Noda A, Verlinsky A, Snyder J, Fujita Y, Murakami Y, et al. Preclinical Evaluation of an Anti-Nectin-4 ImmunoPET Reagent in Tumor-Bearing Mice and Biodistribution Studies in Cynomolgus Monkeys. Mol Imaging Biol 2016;18(5):768–75 doi 10.1007/s11307-016-0953-x. [DOI] [PubMed] [Google Scholar]
- 14.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554(7693):544–8 doi 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aggen DH, Drake CG. Biomarkers for immunotherapy in bladder cancer: a moving target. J Immunother Cancer 2017;5(1):94 doi 10.1186/s40425-017-0299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrylak DP, Flaig TW, Mar N, Gourdin TS, Srinivas S, Rosenberg JE, et al. Study EV-103 Cohort H: Antitumor activity of neoadjuvant treatment with enfortumab vedotin monotherapy in patients (pts) with muscle invasive bladder cancer (MIBC) who are cisplatin-ineligible. Journal of Clinical Oncology 2022;40(6_suppl):435- doi 10.1200/JCO.2022.40.6_suppl.435. [DOI] [Google Scholar]
- 17.Samstein RM, Lee C-H, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nature genetics 2019;51(2):202–6 doi 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoimes CJ, Flaig TW, Milowsky MI, Friedlander TW, Bilen MA, Gupta S, et al. Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer. J Clin Oncol 2022:Jco2201643 doi 10.1200/jco.22.01643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg JE, Milowsky M, Ramamurthy C, Mar N, McKay RR, Friedlander T, et al. LBA73 Study EV-103 Cohort K: Antitumor activity of enfortumab vedotin (EV) monotherapy or in combination with pembrolizumab (P) in previously untreated cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (la/mUC). Annals of Oncology 2022;33:S1441 doi 10.1016/j.annonc.2022.08.079. [DOI] [Google Scholar]