Abstract
Non-exosomal non-coding RNAs (non-exo-ncRNAs) and exosomal ncRNAs (exo-ncRNAs) have been associated with the pathological development of myocardial infarction (MI). Accordingly, this analytical review provides an overview of current MI studies on the role of plasma non-exo/exo-ncRNAs. We summarize the features and crucial roles of ncRNAs and reveal their novel biological correlations via bioinformatics analysis. The following contributions are made: (1) we comprehensively describe the expression profile, competing endogenous RNA (ceRNA) network, and “pre-necrotic” biomarkers of non-exo/exo-ncRNAs for MI; (2) functional enrichment analysis indicates that the target genes of ncRNAs are enriched in the regulation of apoptotic signaling pathway and cellular response to chemical stress, etc.; (3) we propose an updated and comprehensive view on the mechanisms, pathophysiology, and biomarker roles of non-exo/exo-ncRNAs in MI, thereby providing a theoretical basis for the clinical management of MI.
Keywords: Exosome, Non-exosomal non-coding RNA (ncRNA), Exosomal ncRNA, Long non-coding RNA (lncRNA), MicroRNA (miRNA), Circular RNA (circRNA), Myocardial Infarction (MI)
Abstract
非外泌体/外泌体非编码RNAs与心肌梗死(MI)的病理发展相关。本文对目前血浆中非外泌体/外泌体非编码RNAs在MI中的作用进行了概述,总结了上述非编码RNAs的特点和关键作用,并通过生物信息学分析揭示其新的生物学关联性,内容包括:(1)全面描述了MI中非外泌体/外泌体非编码RNAs的表达、内源竞争RNA网络和“坏死前”的生物标志物;(2)分析证实非编码RNAs的靶基因在调控细胞凋亡信号通路和细胞对化学应激反应等过程中富集;(3)对非外泌体/外泌体非编码RNAs在MI中的调节机制、病理生理学功能和作为生物标志物方面的作用提出了新的视角,为其临床治疗提供理论依据。
Keywords: 外泌体, 非外泌体非编码RNA(ncRNA), 外泌体ncRNA, 长链非编码RNA(lncRNA), 微小RNA(miRNA), 环状RNA(circRNA), 心肌梗死
1. Introduction
Despite significant advances in the prevention, diagnosis, and treatment of cardiovascular diseases over the past 30 years, myocardial infarction (MI) continues to be the leading cause of death in people with cardiovascular illnesses (Roth et al., 2020). The pathogenesis of MI is mainly associated with the autophagy, apoptosis, and necrosis of cardiomyocytes (Koyanagi, 2003). Oxidative stress, pyroptosis, inflammation, and fibrosis are also involved in the occurrence and development of MI. Therefore, exploring the molecular mechanisms and biomarkers of MI, as well as early intervention is essential to reduce MI-associated mortality. After searching published studies, we find that Guo et al. (2017), Kowara et al. (2021), and Wang et al. (2022) have explored the functions of non-coding RNAs (ncRNAs) in MI from the perspectives of ncRNA classification, regulatory mechanisms, and ncRNA crosstalk, respectively, and their results demonstrated the functional diversity of ncRNAs in MI. However, there is no comprehensive report on the mechanisms of both ncRNAs and exosomal ncRNAs in MI. From this perspective, we set out to generalize the regulatory mechanisms of non-exosomal- and exosome-derived long non-coding RNA (lncRNA), microRNA (miRNA), and circular RNA (circRNA) in MI in anticipation of discovering new advances in MI prevention and treatment. A comparison of this study with the studies by Guo et al. (2017), Kowara (2021), and Wang et al. (2022) is shown in Table 1. The workflow of this paper is presented in Fig. 1.
Table 1.
Some novelties of this study compared to published works: exploring the hub genes of myocardial infarction through bioinformatics analysis
| Study | Classification criteria | ceRNA network | Exosome ncRNAs |
|---|---|---|---|
| Our study | Classified by ncRNA category | Included | Included |
| Guo et al. (2017) | Classified by regulatory mechanism | Included, but only briefly described | NA |
| Kowara et al. (2021) | Classified by the period of disease progression | Not presented separately | NA |
| Wang et al. (2022) | Classified by ncRNA crosstalk network | NA | NA |
ceRNA: competing endogenous RNA; ncRNA: non-coding RNA; NA: not applicable.
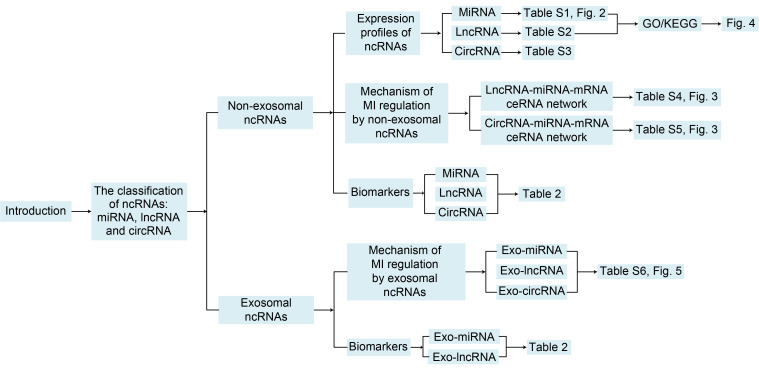
Fig. 1. Overall workflow of this study. ncRNAs: non-coding RNAs; MiRNA: microRNA; LncRNA: long non-coding RNA; CircRNA: circular RNA; GO: Gene Ontology; KEGG: Kyoto Encyclopedia Genes and Genomes; MI: myocardial infarction; mRNA: messenger RNA; ceRNA: competing endogenous RNA; Exo: exosomal.
2. Classification of ncRNAs
Different from transcripts, ncRNAs usually lack the function of encoding proteins. Research increasingly shows that genes that make up more than 90% of what was once called “junk” DNA in the human genome can be transcribed into ncRNAs (Hombach and Kretz, 2016). This means that ncRNAs may be more abundant than messenger RNAs (mRNAs) in terms of quantity and diversity. Although ncRNAs do not possess the ability to produce proteins, this does not mean that they carry no information or functions. With the in-depth study of ncRNAs, scholars have classified ncRNAs into two categories based on their regulatory roles: (1) Housekeeping ncRNAs, such as ribosomal RNA (rRNA), transfer RNA (tRNA), and small nuclear RNA (snRNA). These are ubiquitously expressed in cells and mainly regulate the general cellular functions of eukaryotes. (2) Regulatory ncRNAs, such as miRNA, small interfering RNA (siRNA), PIWI-interacting RNA (piRNA), circRNA, and lncRNA. The distribution of regulatory ncRNAs is regulated by external functional requirements, and they mainly exert regulatory effects on genes’ epigenetic and post-transcriptional levels (Zhang et al., 2019). Therefore, in this study, we focused on regulatory ncRNAs, namely, miRNA, lncRNA, and circRNA, to investigate their association with the occurrence and regulation of MI (Guo et al., 2017).
2.1. MiRNA
MiRNA is an endogenous short non-coding single-stranded RNA with a length of about 22 nucleotides (nt), which is widely expressed in almost all cells and is highly conserved among species. MiRNAs can inhibit mRNA translation or promote mRNA degradation by binding to the 3'-untranslated region (UTR), the coding region, or 5'-UTR of the target mRNA (Kabekkodu et al., 2018). MiRNAs can also interact with RNA-binding proteins (RBPs) to mediate the post-transcriptional silencing of target genes (Bartel, 2004). Numerous studies have shown that miRNAs are involved in regulating the physiological state and pathological processes of the body (Bhaskaran and Mohan, 2014; Lu et al., 2019; Ji et al., 2021). They have also attracted much attention because of the diversity and complexity of their roles in MI (D'Alessandra et al., 2012).
2.2. LncRNA
As a type of ncRNA, lncRNA has longer transcripts than miRNA, with a length of more than 200 nt. Distinct from miRNAs that can only target mRNAs, lncRNAs not only have the ability to directly perform gene modification, but also can “sponge” bound miRNAs and reverse the repressive effect of miRNAs on mRNAs (Quinn and Chang, 2016). Previous studies on genetic imprinting have shown that lncRNAs can perform epigenetic modification of genes by recruiting chromatin remodeling complexes to specific sites (Navarro et al., 2006; Gupta et al., 2010). For example, the lncRNA named HOTAIR in the Hox locus of primary breast tumor is highly expressed in epithelial cancer cells and induces genome-wide transformation of polycomb repressive complex 2 (PRC2), ultimately enhancing gene expression and cancer invasiveness (Gupta et al., 2010). Aberrant lncRNA transcripts were also found in cardiomyocytes with ischemic/hypoxic injury, suggesting the possible regulatory role of lncRNAs in MI at the transcriptional, post-transcriptional, or epigenetic level (Li H et al., 2019; Xie et al., 2021).
2.3. CircRNA
CircRNAs have been considered as a special type of ncRNAs formed by the back splicing of linear pre-mRNA introns, exons, or intergenic regions. The size of circRNAs varies from 100 to 4000 nt. Due to the idiographic covalent closed-loop structure, circRNAs lack a 5',7-methylguanosine (m7G) cap and a 3' poly(A) tail (Kristensen et al., 2019), and have more stable properties than linear RNAs, which are less susceptible to exonuclease or RNase R decomposition (Zhang et al., 2013). This characteristic of circRNAs is also known as “RNase R resistance.”
CircRNAs have different exon-intron splicing and circularization methods, resulting in their different types and corresponding localizations: exonic circRNAs (ecircRNAs) (Chen et al., 2018), exon-intron circRNAs (EIcircRNAs) (Li et al., 2017), and intronic circRNAs (ciRNAs) (Zhang et al., 2013). It is worth emphasizing that ecircRNAs can regulate the expression of mRNAs by “sponge” binding to miRNAs, and ecircRNAs have many binding sites for miRNAs (Sun et al., 2019). For example, Hansen et al. (2013) demonstrated that circRNA, sex-determining region Y (Sry), can inhibit the activity of miRNA-138 (miR-138) through a “sponge” effect, and Feng et al. (2019) demonstrated that Sry functions to block the growth and migration of tumor tissues. Fu et al. (2017) proved that hsa_circ_0005986 acts as a sponge of miR-129-5p to regulate the expression level of Notch1 mRNA. By “sponging” to target miRNAs, circRNAs can prevent interaction between miRNAs and mRNA, thereby regulating gene expression and transducing signals (Aufiero et al., 2019).
3. Introduction to novel biological vesicular exosomes and their biology
Membranous vesicles can be secreted by a variety of cells in an organism. According to their biological mechanism and size, these vesicles are classified as exosomes, microcapsules, or apoptotic bodies. Exosomes are a class of lipid bilayer membrane vesicles secreted by cells, which are about 30‒150 nm in diameter and contain a variety of bioactive molecules such as proteins, lipids, and nucleic acids (Pegtel and Gould, 2019). It has been found that due to their smaller size compared to apoptotic vesicles and their intact lipid bilayer membrane structure, exosomes are ideal carriers of intercellular signaling and integration.
Through the wrapping of the exosome membrane, genetic materials from donor cells, such as exosomal ncRNAs, can act as sponges for endogenous miRNAs, mediating the expression of target genes or activating relevant signaling pathways to produce biological effects (Zhang et al., 2015). Previously, Zheng et al. (2021) described that exosomal miRNAs can affect cardiovascular disease progression by binding to target genes to mediate target mRNA silencing. Moreover, not only exosomes are derived from different cell types, but also their inclusions exhibit similar RNA changes and specificity to donor cells, further demonstrating that they are important carriers of intercellular information transfer and regulation (Zhang et al., 2015). Therefore, based on ncRNAs, we aimed to further investigate how exosomal ncRNAs regulate MI progression, to provide a deeper understanding of the pathological process and clinical treatment of MI.
4. Data collection and inclusion/exclusion criteria
The main objective of this study was to explore the function and mechanism of action of ncRNAs in MI by reviewing the relevant literature. We searched the PubMed (https://pubmed.ncbi.nlm.nih.gov) database for MI-related papers published in the past five years. The inclusion criteria were: (1) the study disease model was MI or myocardial ischemia/hypoxia; (2) the study target was ncRNA (miRNA, lncRNA, and circRNA); (3) the biological vesicle type was exosome. The exclusion criteria were: (1) non-MI and non-myocardial ischemia/hypoxia models; (2) the study model was MI combined with other diseases; (3) the article type was review; (4) the article was purely bioinformatics analysis. Based on the above criteria, we screened the search results and summarized the results in tables below.
5. Method of enrichment analysis and network construction
Gene Ontology (GO) is a powerful tool to examine the molecular functions (MFs), cellular components (CCs), and biological processes (BPs) of genes. Kyoto Encyclopedia Genes and Genomes (KEGG) pathway enrichment analysis was performed to understand the links among different genes and signaling pathways. GO/KEGG enrichment analyses can map scattered target genes to different functional categories and grasp the effect direction of ncRNAs and target genes at the overall level. This study performed GO and KEGG in Metascape Database (https://metascape.org), an online gene functional annotation tool, to provide a comprehensive set of biological information of genes and proteins, where P<0.05 and minimal overlap >3 were set as the cutoff criteria (Zhou et al., 2019). The visualized regulatory network was constructed using the Cytoscape software, version 3.4.0 (http://chianti.ucsd.edu/cytoscape-3.4.0) (Shannon et al., 2003).
6. Non-exosomal ncRNAs in MI
6.1. Expression and regulation of non-exosomal ncRNAs in MI
6.1.1. Regulatory role of differentially expressed miRNAs in MI
MiRNAs can regulate target genes in the form of direct effects or coordinated/combined modification of genes in the form of regulatory factors, and a single miRNA can regulate multiple transcripts, which greatly increases the breadth of interaction with target genes. Increasing evidence suggests that miRNAs play a major role in the development of biological and pathological processes in MI. Zhao JX et al. (2019) reported that the exosomal miR-182 derived from mesenchymal stromal cells can change the polarization state of mouse macrophages and reduce ischemia-reperfusion myocardial injury. Another study showed that the overexpression of miR-101 can target DNA damage-inducible transcript 4 (DDIT4) mRNA to inhibit the autophagy and apoptosis of cardiomyocytes in mice with MI (Li et al., 2020). Additionally, our search revealed that many miRNAs are capable of targeting multiple messenger RNAs to exert different regulatory abilities during MI.
6.1.1.1. MiR-34
MiR-34 is a class of miRNAs with known regulatory roles in cardiovascular disease. The miR-34 family was found to be encoded by two different genes on chromosome 1 and chromosome 11 and possess three matrices, miR-34a, miR-34b, and miR-34c. Dong et al. (2019) showed that miR-34a expression was elevated after MI and promoted myocardial necrosis, inflammatory cell infiltration, and collagen fibrillation in rats by inhibiting the activity of silent information regulator 1 (SIRT1). Similarly, the inhibition of miR-34a precursor miR-34a-5p levels in post-ischemia-reperfusion cardiomyocytes was able to attenuate ischemia/reperfusion (I/R) injury in rat myocardium (Wang et al., 2019). Furthermore, Wang et al. (2020) reported that miR-34-5p was a downstream target of lncRNA small nuclear RNA host gene 7 (SNHG7) and that miR-34-5p could target Rho-associated, coiled-coil domain-containing protein kinase 1 (ROCK1) to inhibit the fibrosis of cardiac fibroblasts, thus forming a miR-34-5p-centered lncRNA SNHG7/miR-34-5p/ROCK1 regulatory network, which was able to improve post-infarction cardiac function.
6.1.1.2. MiR-155
MiR-155 has been demonstrated to play a role in tumors (Wu and Wang, 2020), blood system diseases (Hawez et al., 2019), and gastrointestinal system diseases (Zhu et al., 2020). Wang CX et al. (2017) revealed that miR-155 is expressed at a high level in infarcted heart tissue and is mainly located in macrophages of the damaged heart. The high level of miR-155 in turn inhibits the proliferation of cardiac fibroblasts and collagen regeneration, which causes the infarcted myocardium to be in a long period of inflammation, causing continuous myocardial damage (Wang CX et al., 2017). Conversely, inhibition of miR-155 can exert myocardial protective effects. For example, research by Guo et al. (2019) showed that silencing miR-155 can reverse the downregulation of B-cell lymphoma-2 (Bcl2) and X-chromosome-linked inhibitor of apoptosis protein (XIAP) and the upregulation of Bcl2-associated X protein (Bax) and cleaved-caspase-3 induced by H2O2, reduce cell apoptosis, and increase the vitality of myocardial cells injured by MI via targeting the RBP Quaking (QKI). In addition, miR-155 could exert anti-ischemia-reperfusion injury effects by targeting hypoxia-inducible factor-1α (HIF-1α) (Chen et al., 2019), Bcl2-associated athanogene 5 (BAG5) and the mitogen-activated protein kinase (MAPK)/c-Jun N-terminal kinase (JNK) (Xi et al., 2020) signaling pathways. All these pieces of evidence suggest that by acting on different target genes, miRNAs function in a variety of pathological processes in cardiomyocytes and are important regulators in MI.
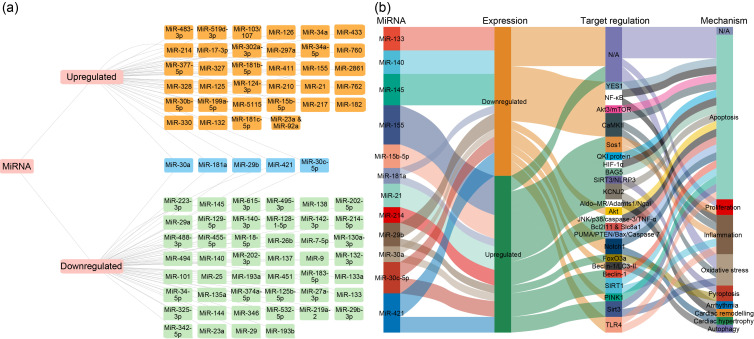
From previously published data, we collected the miRNAs whose functions have been clarified in MI, which yielded a total of 101 miRNAs, including 55 downregulated and 46 upregulated miRNAs (Table S1 and Fig. 2a). In addition, we concluded that 12 miRNAs (miR-133, miR-140, miR-145, miR-155, miR-15b-5p, miR-181a, miR-21, miR-214, miR-29b, miR-30a, miR-30c-5p, and miR-421) have more than one direct mRNA target and play a crucial role in the development and process of MI via apoptosis, inflammation, proliferation, and so on (Fig. 2b). The above data suggest that these differential miRNAs can influence the development and course of MI through various biological processes, such as apoptosis and autophagy.
Fig. 2. Network of microRNA (miRNA)-messenger RNA (mRNA) pairs in myocardial infarction (MI). (a) Network of miRNAs: the orange boxes represent upregulated miRNAs, the green boxes represent downregulated miRNAs, and the blue boxes represent these miRNAs differentially expressed in different studies, with both up- and downregulated expression. (b) Network of miRNA-mRNA pairs: 12 miRNAs (including miR-133, miR-140, miR-145, miR-155, miR-15b-5p, miR-181a, miR-21, miR-214, miR-29b, miR-30a, miR-30c-5p, and miR-421) have more than one direct mRNA targets, which play a crucial role in the development and process of MI via apoptosis, inflammation, proliferation, and so on. N/A: not available; YES1: YES proto-oncogene 1; NF-κB: nuclear factor-κB; Akt: protein kinase B; mTOR: mammalian target of rapamycin; CaMKII: Ca2+/calmodulin-dependent protein kinase II; Sos1: Son of Sevenless 1; KCNJ2: potassium inwardly rectifying channel subfamily J member 2; AIdo-MR: aldosterone-mineralocorticoid receptor; QKI: Quaking; HIF-1α: hypoxia inducible factor-1α; BAG5: B-cell lymphoma-2 (Bcl2)-associated athanogene 5; SIRT3: sirtuin 3; NLRP3: nucleotide-binding domain and leucine-rich repeat protein 3; JNK: c-Jun N-terminal kinase; TNF-α: tumor necrosis factor-α; PINK1: phosphatase and tensin homolog (PTEN)-induced putative kinase 1; TLR4: Toll-like receptor-4.
6.1.2. Regulatory role of differentially expressed lncRNAs in MI
With the global popularity of miRNA research, studies on lncRNAs in MI have also been in the focus of scholars. For example, lncRNA HOTAIR, which is elevated after myocardial ischemia-reperfusion, causes an increase in lactate dehydrogenase (LDH) release and caspase-3 viability by competing with miR-126 for the binding site of the target gene serine/arginine-rich splicing factor 1 (SRSF1), resulting in the decreased viability of cardiomyocytes and myocardial damage (Sun and Hu, 2020). In addition, existing data revealed that zinc finger NFX1-type-containing 1 (ZNFX1) antisense RNA 1 (ZFAS1), a lncRNA with sarcoplasmic reticulum Ca2+-ATPase 2a (SERCA2a) inhibitory effect, is highly expressed after MI, can inhibit the Ca2+ reuptake process of SERCA2a, and causes calcium overload in myocardial cells, leading to myocardial damage and inadequate cardiac contractility (Zhang Y et al., 2018). Furthermore, new evidence supports that antizyme inhibitor 2-sv (AZIN2-sv) (Li XZ et al., 2019), myosin heavy chain-associated RNA transcript (MHRT) (Lang et al., 2021), and taurine upregulated gene 1 (TUG1) (Zhang SL et al., 2021) can also regulate fibrosis and vascular remodeling after MI through different pathways, and play a key role in the initiation and progression of MI.
The lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) was first reported to be related to tumor metastasis, and has since been demonstrated to play a role in controlling epigenetic gene regulation and splicing (Ji et al., 2003). The expression of MALAT1 in the peripheral blood of patients with MI is elevated (Vausort et al., 2014), which is linked to the hypoxia pathway (Choudhry and Mole, 2016). Hu et al. (2018) investigated the likely mechanism of MALAT1 in mice with MI and found that MALAT1 can be used as the competing endogenous RNA (ceRNA) of miR-320 to regulate the level of phosphatase and tensin homolog deleted on chromosome 10 (Pten), thus regulating the level of myocardial apoptosis. Besides, in MI conditions, MALAT1 can also regulate autophagy through miR-144-3p (Gong et al., 2019) and miR-125b-5p/nucleotide-binding and oligomerization domain-like receptor C5 (NLCR5) signaling pathways (Liu ZY et al., 2020). The tuberous sclerosis 2 (TSC2)-mammalian target of rapamycin (mTOR) pathway is another pathway, through which MALAT1 regulates apoptosis in cardiac myocytes (Hu et al., 2019). The strong regulatory ability of MALAT1 in MI suggests that it is an important molecule affecting MI progression.
It has been shown that lncRNA TUG1 has multiple roles in MI. For instance, one study revealed that TUG1 is upregulated after MI. It binds to miR-132-3p and upregulates histone deacetylase 3 (HDAC3), thereby reducing the acetylation of histone H3 at lysine 9 (H3K9) and epigenetically inhibiting the expression of antioxidant genes, including Bcl-extra-large (Bcl-xL), peroxiredoxin 2 (Prdx2), and heat shock protein 70 (Hsp70), and provides myocardial protection (Su Q et al., 2020). Furthermore, Su et al. (2019) proposed that downregulating TUG1 can effectively inhibit the autophagy and apoptosis of cardiomyocytes through sponge miR-142-3p, as well as upregulating high mobility group box-1 protein (HMGB1) and Ras-related C3 botulinum toxin substrate 1 (Rac1). Zhang SL et al. (2021) showed that TUG1 has a role in cardiac fibrosis. Highly expressed TUG1 promotes connective tissue growth factor (CTGF) expression through sponging miR-133b, and induces the activation of cardiac fibroblasts and collagen regeneration.
Based on the above data from the published research papers on MI, we summarized and listed 87 lncRNAs whose functions have been clarified in Table S2, including 21 downregulated and 66 upregulated lncRNAs in MI. It was found that these lncRNAs play critical roles in a diversity of biological processes in MI, including apoptosis, proliferation, inflammation, fibrosis, autophagy, oxidative stress, Ca2+ overload, and pyroptosis in MI.
6.1.3. Regulatory role of differentially expressed circRNAs in MI
The classical definition of ncRNAs is transcripts without protein-coding function; however, most ecircRNAs have open reading frames. Consequently, scholars speculated that ecircRNAs may possess the function of translating into peptides/functional proteins, which has been proven on many occasions (Legnini et al., 2017; Zhang ML et al., 2018). For example, Legnini et al. (2017) revealed the translation function of circRNA zinc finger protein 609 (ZNF609)by constructing an artificial circular transcript of circ-ZNF609 and the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) labeling of ZNF609 gene. Moreover, ZNF609 silencing was shown to protect endothelial cells from oxidative stress damage (Liu et al., 2017). CircRNA cerebellar degeneration-related protein 1 transcript (Cdr1as) (Geng et al., 2016), circRNA LAS1L (Sun LY et al., 2020), and circRNA MICRA (Salgado-Somoza et al., 2017) can enhance apoptosis and cause myocardial damage in MI. In the cardiovascular system, knockout of homeodomain-interacting protein kinase 3 (HIPK3) can reduce lipopolysaccharide-induced oxidative stress and inflammatory injury, reduce cardiomyocyte apoptosis, and improve cardiac function (Fan et al., 2020). Garikipati et al. (2019) also validated that CircFndc3b was able to bind to the RBP fused in sarcoma (FUS), which upregulated the level of vascular endothelial growth factor-A (VEGF-A), narrowed the myocardial area after infarction, and improved cardiac function.
Based on the current research data, we could list all the MI-related circRNAs whose functions have been clarified, that is, a total of 33 circRNAs, including 11 downregulated and 22 upregulated circRNAs (Table S3). These circRNAs play crucial roles in various biological processes, including apoptosis, proliferation, inflammation, fibrosis, autophagy, angiogenesis, and pyroptosis in MI.
6.2. ceRNA networks of lncRNAs and circRNAs in MI
Salmena et al. (2011) proposed the concept of ceRNA, which is a special intracellular information integration mechanism. With miRNA as the hub and miRNA response element (MRE) as the binding site, miRNAs are cascaded with protein-coding and non-coding genes, forming a ceRNA network with signaling regulatory functions. In this network, the RNA molecules that can bind to miRNAs are ceRNA molecules (Bartel, 2009). Existing studies have found that mRNA (Zheng et al., 2018), lncRNA (Wang JY et al., 2010), circRNA (Hansen et al., 2013), and pseudogene transcripts (Marques et al., 2012) can all participate in gene regulation as ceRNA. In turn, ceRNAs can inhibit the stability of miRNA-bound target genes, disrupting their translation ability, and are particularly important for disease mechanism studies and interventions, including in MI (Guo et al., 2010). Thus, in the present review, we attempt to summarize the ceRNA network in MI.
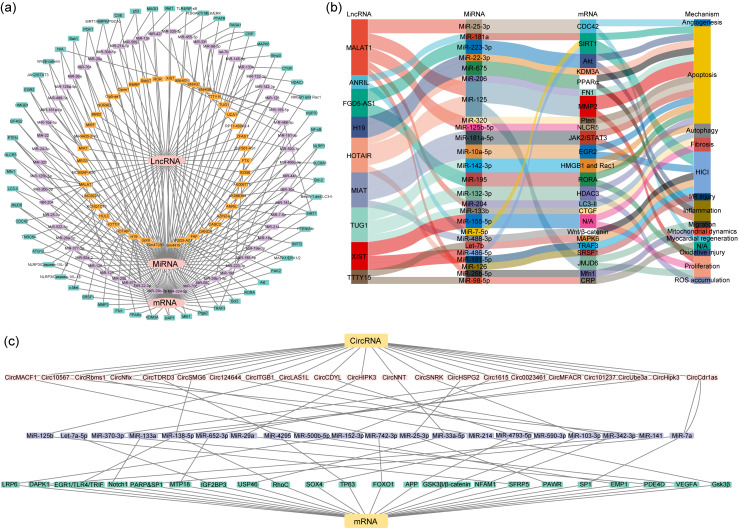
As members of ceRNAs, lncRNAs and circRNAs can bind to miRNAs through competitive adsorption or the sponge effect to form the lncRNA-miRNA-mRNA axis or circRNA-miRNA-mRNA axis, which affects the expression of target genes and regulates disease progression (Ha and Kim, 2014; Liang et al., 2020; Lin et al., 2020; Su and Lv, 2020; Xiao, 2020). A total of 61 lncRNA-miRNA-mRNA axes involved in MI could be summarized from the published results (Table S4 and Fig. 3a). In addition, we highlighted that 9 lncRNAs out of 61 changed lncRNAs (ANRIL, FGD5-AS1, H19, HOTAIR, MALAT1, MIAT, TTTY15, TUG1, and XIST) have more than one directed target miRNAs (Fig. 3b). Regarding circRNA, a total of 22 circRNA-miRNA-mRNA pathways associated with MI could be listed (Table S5 and Fig. 3c). From these results, it was found that lncRNAs and circRNAs regulate the progression of MI through the ceRNA networks related to proliferation, apoptosis, autophagy, migration, oxidative stress, fibrosis, angiogenesis, and inflammation and that the study of key targets in these networks may provide new approaches for MI therapy.
Fig. 3. Competing endogenous RNA (ceRNA) network of long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs) in myocardial infarction (MI). (a) Network of lncRNA-microRNA (miRNA)-messenger RNA (mRNA): the orange box represents lncRNAs; the purple box represents miRNAs; the green box represents mRNAs. (b) Nine lncRNAs (including ANRIL, FGD5-AS1, H19, HOTAIR, MALAT1, MIAT, TTTY15, TUG1, and XIST) have more than one direct target miRNAs, which play crucial roles in the development and process of MI. (c) Network of circRNA-miRNA-mRNA axes in MI: the pink box represents circRNAs; the purple box represents miRNAs; the green box represents mRNAs. N/A: not available; NLRP3: nucleotide-binding domain and leucine-rich repeat protein 3; PTEN: phosphatase and tensin homolog; NLRC5: NLR family CARD domain containing 5; Mfn1: mitofusin 1; EIF4G2: eukaryotic translation initiation factor 4 γ2; HMGB1: high mobility group box 1; EGR2: early growth response 2; JAK2: Janus kinase 2; STAT3: signal transducer and activator of transcription 3; Bcl2: B-cell lymphoma-2; Bak1: Bcl2 antagonist/killer 1; PDK1: pyruvate dehydrogenase kinase 1; SIRT1: sirtuin 1; MAGI3: membrane-associated guanylate kinase, WW and PDZ domain containing 3; Mst1: macrophage stimulating 1; TLR4: Toll-like receptor 4; NF-κB: nuclear factor-κB; PI3K: phosphatidylinositol 3-kinase; AKT: protein kinase B; MEK: MAP kinase-ERK kinase; ERK: extracellular regulated MAP kinase; PTAFR: platelet-activating factor receptor; RASA1: RAS p21 protein activator 1; CRP: C-reactive protein; MAPK6: mitogen-activated protein kinase 6; Binp3: Bcl2-interacting protein 3; CTGF: connective tissue growth factor; HDAC3: histone deacetylase 3; HMGB1: high mobility group box 1; Rac1: Rac family small GTPase 1; HSP70: heat shock protein 70; PAK2: p21 (RAC1)-activated kinase 2; RORA: RAR-related orphan receptor A; TRAF3: tumor necrosis factor (TNF) receptor-associated factor 3; Ptgs2: prostaglandin-endoperoxide synthase 2; Mtfr1: mitochondrial fission regulator 1; KDM3A: lysine demethylase 3A; FN1: fibronectin 1; MMP2: matrix metallopeptidase 2; SRSF1: serine and arginine rich splicing factor 1; ATG12: autophagy related 12; TNRC6A: trinucleotide repeat containing adaptor 6A; CDC42: cell division cycle 42; JMJD6: jumonji domain-containing 6; MALAT1: metastasis-associated lung adenocarcinoma transcript 1; MCM3AP-AS1: MCM3AP antisense RNA 1; MEG3: maternally expressed 3; MIAT: myocardial infarction-associated transcript; MiR4435-2HG: MIR4435-2 host gene; MiRt2: myocardial infraction-associated transcript 2; NORAD: non-coding RNA-activated by DNA damage; Oip5-as1: OIP5 antisense RNA 1; Oprm1: opioid receptor mu 1; RMRP: RNA component of mitochondrial RNA processing endoribonuclease; RMST: rhabdomyosarcoma 2-associated transcript; SNHG7: small nucleolar RNA host gene 7; TTTY15: testis-specific transcript, Y-linked 15; TUG1: taurine up-regulated 1; UCA1: urothelial cancer associated 1; ZFAS1: ZNFX1 antisense RNA 1; XIST: X-inactive specific transcript; CASC2: cancer susceptibility 2; DANCR: differentiation antagonizing non-protein coding RNA; FAF: fundus autofluorescence; FGD5-AS1: FGD5 antisense RNA 1; HOTAIR: HOX transcript antisense RNA; HOTTIP: HOXA transcript at the distal tip; HULC: highly up-regulated in liver cancer; KCNQ1OT1: potassium voltage-gated channel subfamily Q member 1 opposite strand 1.
6.3. Functional enrichment analysis of target mRNAs associated with differentially expressed non-exosomal ncRNAs in MI
6.3.1. Functional enrichment analysis of differently expressed miRNAs-related target mRNA in MI
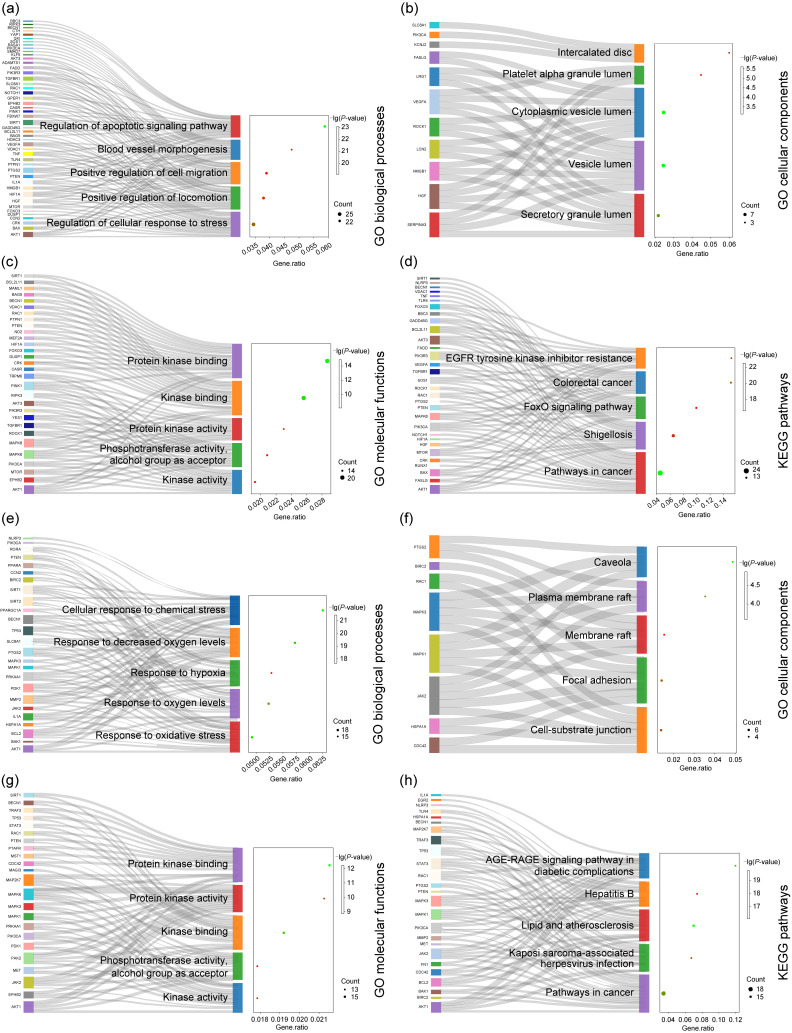
In order to investigate the biological functions and regulatory pathways involved in miRNAs in an organism, miRNA-targeted mRNAs were used for GO/KEGG analysis. The results showed that the major BP, CC, MF, and KEGG pathways enriched by these mRNAs were: regulation of apoptotic signaling pathway (Fig. 4a), intercalated disc (Fig. 4b), protein kinase binding (Fig. 4c), and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor resistance pathway (Fig. 4d), respectively (Table S6). The results of bioinformatics analysis suggested that apoptosis and EGFR tyrosine kinase inhibitor may be the main pathways to mediate the regulation of myocardial injury in MI, laying the foundation for understanding the role and mechanism of miRNAs in MI.
Fig. 4. Gene Ontology (GO) enrichment in biological processes, cellular components, molecular functions, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of microRNA (miRNA) and long non-coding RNA (lncRNA) targets from published experimental data. (a‒d) Bubble diagrams showing that miRNA-messenger RNA (mRNA) could participate in multiple biological processes (a), cellular components (b), molecular functions (c), and KEGG pathways (d) of myocardial infarction (MI); (e‒h) Bubble diagrams showing that the lncRNA-miRNA-mRNA axes could participate in multiple biological processes (e), cellular components (f), molecular functions (g), and KEGG pathways (h) of MI. The circle size represents the gene number, and the color indicates the P-value.
6.3.2. Functional enrichment analysis of lncRNA-miRNA-mRNA axes in MI
Similar to the above, we performed GO and KEGG analyses of lncRNA-miRNA-mRNA genes and visualized the results to find key pathways. The results showed that the main BP, CC, MF, and KEGG pathways enriched by these mRNAs were: cellular response to chemical stress (Fig. 4e), caveola (Fig. 4f), protein kinase binding (Fig. 4g), and advanced glycation end-product (AGE)-receptor for AGE (RAGE) signaling pathway in diabetic complications pathway (Fig. 4h), respectively (Table S7). It is suggested that the cardiomyocyte response to chemical stress may be the main biological process that interferes with myocardial injury in MI.
7. Exosomal ncRNAs in MI
7.1. Exosomal ncRNAs in the pathophysiology of MI
In recent years, research investigating exosomal ncRNAs has become popular. Meanwhile, since the 21st century, the performance of cell therapy in the treatment of myocardial regeneration has been unsatisfactory. The paracrine mechanism that plays a major role in cell therapy has given high hopes regarding the use of exosomes characterized by non-cellular therapy (Huang et al., 2019; Spannbauer et al., 2020; Tan et al., 2020; Saludas et al., 2021). Current studies have shown that exosomal ncRNAs participate in the dynamic evolution of underlying MI through various pathways, involving all aspects of their pathophysiology and potential treatment (Liu J et al., 2020). For example, exosomes derived from mouse embryonic stem cells promoted survival and proliferation in cardiac progenitor cells by delivering miR-294 (Khan et al., 2015). Mao et al. (2019) found that the injection of human mesenchymal stem cell (hMSC)-derived exosomes enriched in lncRNA Krüppel-like factor 3-antisense RNA 1 (KLF3-AS1) in a rat MI model significantly inhibited the expression of pro-inflammatory factors interleukin-1β (IL-1β) and IL-18 in cardiomyocytes and alleviated cardiomyocyte apoptosis. The above studies suggest that, not only stem cell-derived exosomal ncRNAs but also the abundance of exosomal ncRNAs in an organism is expected to form a novel treatment for infarcted myocardium and thus is a promising direction for future MI therapeutic research.
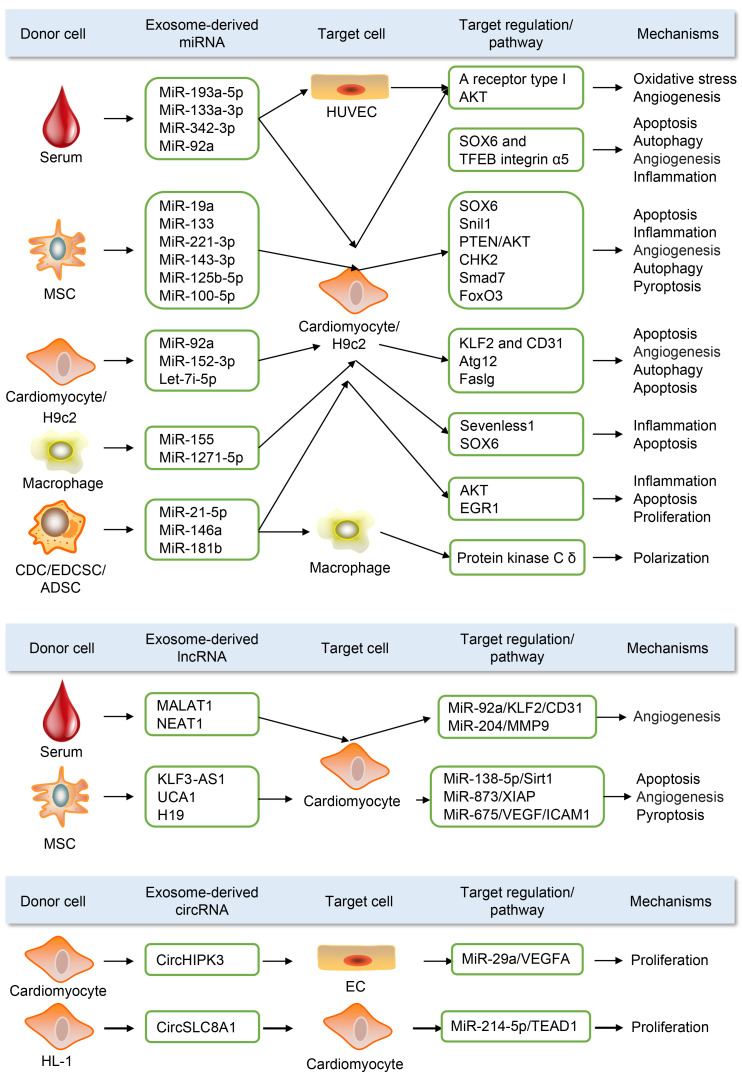
Therefore, this section focuses on the pathophysiological role of exosomal ncRNAs in MI. We highlight 73 exosomal miRNAs, 11 exosomal lncRNAs, and 4 exosomal circRNAs (Table S8) in MI from published papers. In addition, we present a working model of exosomal ncRNA (Fig. 5). Most exosomal ncRNAs, mainly from mesenchymal stem cells and serum, were mainly delivered to cardiomyocytes as the target cells, which play crucial roles in various biological processes, including apoptosis, proliferation, inflammation, fibrosis, autophagy, oxidative stress, and pyroptosis in MI.
Fig. 5. Role of partial exosomal non-coding RNA (ncRNA) in preclinical studies of myocardial infarction (MI). Most exosomal ncRNAs, principally from mesenchymal stem cells and serum, are mainly delivered to cardiomyocytes as the target cells, which play crucial roles in various biological processes, including apoptosis, proliferation, inflammation, fibrosis, autophagy, oxidative stress, and pyroptosis in MI. HUVEC: human umbilical vein endothelial cell; ADSC: adipose-derived stem cell; MSC: mesenchymal stem cell; CDC: cardiosphere-derived cell; EDCSC: explant-derived cardiac stromal cell; HIPK3: homeodomain-interacting protein kinase-3; SLC8A1: solute carrier family 8 member A1; UCA1: urothelial cancer-associated 1; SOX6: sex-determining region Y box 6; TFEB: transcription factor EB; CHK2: checkpoint kinase 2; Smad7: SMAD family member 7; FoxO3: forkhead box O3; KLF2: Kruppel-like transcription factor 2; CD31 (PECAM-1): platelet endothelial cell adhesion molecule-1; Atg12: autophagy-related 12; EGR1: early growth response 1; MMP9: matrix metalloproteinase 9; Sirt1: sirtuin 1; XIAP: X-linked inhibitor of apoptosis protein; ICAM1: intercellular adhesion molecule-1; VEGFA: vascular endothelial growth factor A; EC: endothelial cell.
7.2. Exosomal ncRNAs and non-exosomal ncRNAs as “pre-necrotic” diagnostic biomarkers for MI
Biomarkers are useful tools for disease diagnosis. Currently, the diagnosis of MI is still based on levels of the troponin T and I protein complex. However, the long timescale of troponin changes and its susceptibility to other disease states, such as sepsis or cardiotoxicity, limit the specificity of troponin in the diagnosis of MI (Babuin and Jaffe, 2005). Therefore, it is vital to find a biomarker that is more specific for acute myocardial infarction (AMI). It was found that exosomes in the plasma increase rapidly within 2 d after coronary artery bypass surgery (Emanueli et al., 2016), suggesting that the body is capable of responding to cardiovascular system injury and releasing exosomes. In addition, exosomes carry an abundance of biomolecules. Therefore, exosomal ncRNAs can both meet the early diagnosis of MI and improve the specificity of MI diagnosis. Importantly, as a signaling molecule actively released by the injured heart before the onset of myocardial necrosis, exosomal ncRNAs can also serve as a “pre-necrosis” biomarker for MI.
Chen et al. (2020) showed that the expression of serum exosomes nuclear-enriched abundant transcript 1 (NEAT1) and matrix metalloproteinase 9 (MMP9) was significantly higher in patients with ST-segment elevation MI (STEMI) compared to patients with unstable angina and non-MI. Meanwhile, Zheng et al. (2020) found that circulating exosomes ENST00000556899.1 and ENST00000575985.1 were significantly upregulated in AMI patients compared with control patients. Thus, in addition to contributing to the diagnosis of MI, an additional value of exosomal ncRNAs may be to determine the molecular characteristics of AMI.
In this study, we could list 16 miRNAs (12 highly expressed and 4 lowly expressed), 5 lncRNAs (3 highly expressed and 2 lowly expressed), 7 circRNAs (3 highly expressed and 4 lowly expressed), 37 exosomal miRNAs (24 highly expressed and 13 lowly expressed), and 3 exosomal lncRNAs (3 highly expressed) (Table 2) in the serum of MI patients from published experimental data.
Table 2.
Potential biomarker roles of serum exosomal and non-exosomal non-coding RNAs (ncRNAs) in myocardial infarction
| Type | Name | Expression | Species | Function | Reference |
|---|---|---|---|---|---|
| MiRNA | MiR-23a | Low | Human | Diagnostic | Li et al. (2018) |
| MiR-21 | High | Human | Diagnostic | Wang ZH et al. (2017) | |
| MiR-143 | Low | Human | Diagnostic | Geng et al. (2020) | |
| MiR-214 | High | Human | Diagnostic | Yin et al. (2019) | |
| MiR-152-5p | Low | Human | Diagnostic | Chen et al. (2022) | |
| MiR-3681-5p | Low | Human | Diagnostic | Chen et al. (2022) | |
| MiR-203 | High | Human | Diagnostic | Li et al. (2022) | |
| MiR-21-5p | High | Human | Diagnostic | Mi et al. (2022) | |
| MiR-126 | High | Human | Diagnostic | Mi et al. (2022) | |
| MiR-223-3p | High | Human | Diagnostic | Scărlătescu et al. (2022) | |
| MiR-142-3p | High | Human | Diagnostic | Scărlătescu et al. (2022) | |
| MiR-146a-5p | High | Human | Diagnostic | Scărlătescu et al. (2022) | |
| MiR-486-5p | High | Human | Diagnostic | Xu et al. (2023) | |
| MiR-451a | High | Human | Diagnostic | Xu et al. (2023) | |
| MiR-21-5p | High | Human | Diagnostic | Xu et al. (2023) | |
| MiR-221/222 | High | Human | Diagnostic | Yu et al. (2022) | |
| LncRNA | ANRIL | High | Human | Diagnostic | Zhang and Wang (2019) |
| TTTY15 | High | Human | Diagnostic | Ma et al. (2021) | |
| LUCAT1 | Low | Human | Diagnostic | Xiao et al. (2021) | |
| SENCR | Low | Human | Diagnostic | Chen MH et al. (2021) | |
| AZIN2-sv | High | Human | Diagnostic | Li XZ et al. (2019) | |
| CircRNA | LAS1L | Low | Human | Diagnostic | Sun et al. (2020) |
| 0124644 | High | Human | Diagnostic | Tan et al. (2021) | |
| MICRA | Low | Human | Diagnostic | Salgado-Somoza et al. (2017) | |
| Fndc3b | Low | Human | Diagnostic | Garikipati et al. (2019) | |
| Hipk3 | Low | Human | Diagnostic | Si et al. (2020) | |
| ACAP2 | High | Human | Diagnostic | Zhang J et al. (2021) | |
| MFACR | High | Human | Diagnostic | Wang SJ et al. (2021) | |
| Exo-miRNA | MiR-21-5p | Low | Human | Diagnostic | Qiao et al. (2019) |
| MiR-204 | Low | Human | Diagnostic | Chen et al. (2020) | |
| MiR-1915-3p | Low | Human | Diagnostic | Su J et al. (2020) | |
| MiR-4 | Low | Human | Diagnostic | Su J et al. (2020) | |
| MiR-3 | Low | Human | Diagnostic | Su J et al. (2020) | |
| MiR-507 | Low | Human | Diagnostic | Su J et al. (2020) | |
| MiR-656 | Low | Human | Diagnostic | Su J et al. (2020) | |
| MiR-340 | Low | Human | Diagnostic | Otero-Ortega et al. (2021) | |
| MiR-424 | Low | Human | Diagnostic | Otero-Ortega et al. (2021) | |
| MiR-29b | High | Human | Diagnostic | Otero-Ortega et al. (2021) | |
| MiR-6718-5p | Low | Human | Diagnostic | Chen SY et al. (2021) | |
| MiR-4329 | Low | Human | Diagnostic | Chen SY et al. (2021) | |
| MiR-126 | High | Human | Diagnostic | Ling et al. (2020a) | |
| MiR-21 | High | Human | Diagnostic | Ling et al. (2020a) | |
| Hsa-let-7i-5p | High | Human | Diagnostic | Guo et al. (2021) | |
| Hsa-miR-143-3p | High | Human | Diagnostic | Guo et al. (2021) | |
| Hsa-miR-1180-3p | High | Human | Diagnostic | Guo et al. (2021) | |
| Hsa-miR-3615 | High | Human | Diagnostic | Guo et al. (2021) | |
| MiR-193a-5p | High | Human | Diagnostic | Cao et al. (2021) | |
| MiR-19a-3p | High | Human | Diagnostic | Wernly et al. (2020) | |
| MiR-19b-3p | High | Human | Diagnostic | Wernly et al. (2020) | |
| Exo-miRNA | MiR-26b-5p | High | Human | Diagnostic | Wernly et al. (2020) |
| MiR-30e-5p | High | Human | Diagnostic | Wernly et al. (2020) | |
| MiR-186-5p | High | Human | Diagnostic | Wernly et al. (2020) | |
| MiR-181d-5p | High | Human | Diagnostic | Wernly et al. (2020) | |
| MiR-125a-5p | High | Human | Diagnostic | Wernly et al. (2020) | |
| MiR-301a-3p | High | Human | Diagnostic | Wernly et al. (2020) | |
| MiR-335-5p | High | Human | Diagnostic | Wernly et al. (2020) | |
| MiR-122-5p | High | Human | Diagnostic | Ling et al. (2020b) | |
| MiR-1-1 | High | Human | Diagnostic | Crouser et al. (2021) | |
| MiR-133a | High | Human | Diagnostic | Crouser et al. (2021) | |
| MiR-208b | High | Human | Diagnostic | Crouser et al. (2021) | |
| MiR-423 | High | Human | Diagnostic | Crouser et al. (2021) | |
| MiR-499 | High | Human | Diagnostic | Crouser et al. (2021) | |
| MiR-342-3p | Low | Human | Diagnostic | Wang B et al. (2021) | |
| MiR-6718 and miR-4329 | Low | Human peripheral blood samples | Diagnostic | Chen SY et al. (2021) | |
| MiR-183 | High | Human | Diagnostic | Zhao XX et al. (2019) | |
| Exo-lncRNA | NEAT1 | High | human | Diagnostic | Chen et al. (2020) |
| ENST00000556899.1 | High | human | Diagnostic | Zheng et al. (2020) | |
| ENST00000575985.1 | High | human | Diagnostic | Zheng et al. (2020) |
To be continued
MiRNA: microRNA; LncRNA: long non-coding RNA; CircRNA: circular RNA; Exo: exosomal; ANRIL: antisense non-coding RNA in the INK4 locus; LAS1L: LAS1-like ribosome biogenesis factor; MICRA: microstructural image compilation with repeated acquisitions; Fndc3b: fibronectin type III domain containing 3b; Hipk3: homeodomain interacting protein kinase 3; ACAP2: centaurin-β2; MFACR: mitochondrial fission and apoptosis-related circRNA; NEAT1: nuclear paraspeckle assembly transcript 1.
Exosomal-derived ncRNAs are a better source for biomarker studies due to their higher quantity, quality, and stability advantages. For example, Kamal and Shahidan (2020) summarized 32 studies involving both exosomal and non-exosomal miRNAs, and concluded that, in 18 of them, exosomal miRNAs were a better source as biomarkers. In addition, 75% of all articles on miRNAs suggested the use of exosomal-derived miRNAs over non-exosomal miRNAs. Thus, exosomal-derived ncRNAs will largely facilitate disease screening and monitoring, and have a promising outlook in disease diagnosis and treatment. However, whether there are functional differences between exosomal ncRNAs and free ncRNAs, and whether exosomal ncRNAs and free ncRNAs are regulated differently under the same stimulus need to be further explored (D'Souza et al., 2018). In addition, there is a lack of uniformity in the dose and sensitivity of non-exosomal ncRNAs and exosomal ncRNAs in the diagnosis of cardiovascular diseases. For example, in STEMI patients, plasma miR-499-5p increased abruptly from 70-fold to 3000-fold (Gidlöf et al., 2011), whereas miR-499 was elevated by only 2-fold in patients with acute heart failure (Corsten et al., 2010). MiR-208a, a miRNA elevated in the plasma of AMI patients, remained undetectable in non-AMI patients, but was readily detectable in 90.9% of AMI patients and 100.0% of AMI patients within 4 h after symptom onset (Wang GK et al., 2010). These data demonstrate the specificity of ncRNAs in disease diagnosis. Therefore, the relationships between the dose, sensitivity, and specificity of ncRNAs and cardiovascular disease diagnosis need to be established depending on the type of ncRNAs and the disease.
8. Conclusions and future perspectives
In this analytical review, we summarized the expression profiles of non-exosomal ncRNAs (including 101 miRNAs, 87 lncRNAs, and 33 circRNAs) and exosomal ncRNAs (including 73 exosomal miRNAs, 11 exosomal lncRNAs, and 4 exosomal circRNAs). Furthermore, among these RNAs, we highlighted 61 lncRNA-miRNA-mRNA axes and 22 circRNA-miRNA-mRNA axes and their important roles in the development of MI through various biological processes, such as apoptosis, inflammation, and autophagy. In addition, we presented that, as indicated by GO and KEGG in published experimental data, these mRNAs in MI are primarily enriched in biological processes such as regulation of apoptotic signaling pathway and cellular response to chemical stress. Finally, we described 16 miRNAs, 5 lncRNAs, 7 circRNAs, 37 exosomal miRNAs, and 3 exosomal lncRNAs that can play a role of “pre-necrotic” diagnostic biomarkers for MI. In summary, we proposed an updated and comprehensive guideline for the mechanisms, pathophysiology, and “pre-necrotic” diagnostic biomarker roles of non-exosomal ncRNAs and exosomal ncRNAs in MI (Fig. 6).
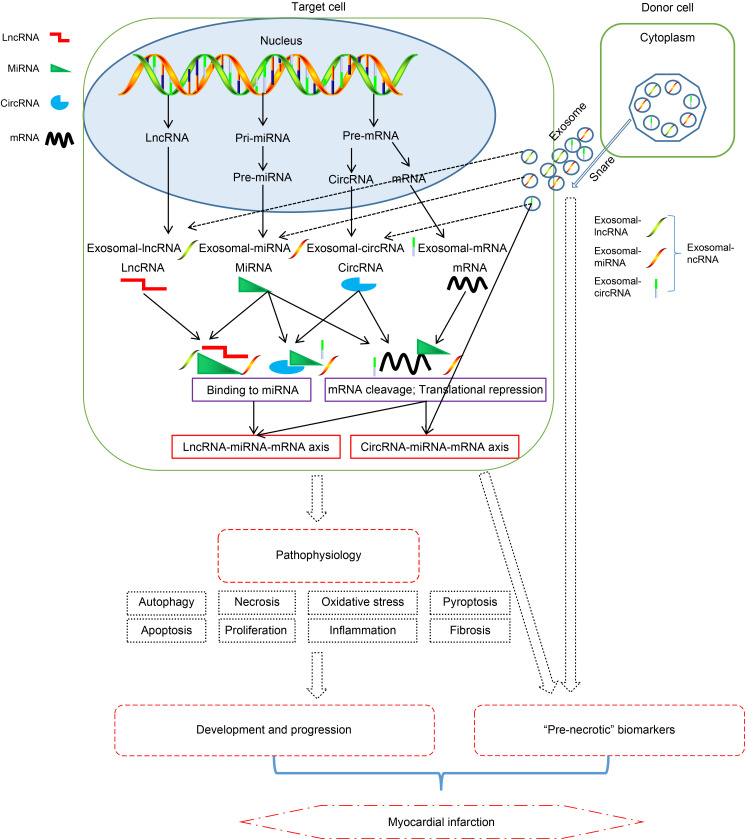
Fig. 6. Updated and comprehensive summary of the roles of non-exosomal non-coding RNA (ncRNAs) and exosomal ncRNAs in myocardial infarction (MI). The potential roles played by long non-coding RNA (lncRNAs), circular RNAs (circRNAs), and microRNAs (miRNAs) in MI. Both lncRNAs and circRNAs can act as sponges for miRNAs. The miRNAs interact with target genes by degrading or inhibiting their messenger RNAs (mRNAs), repressing gene translation, and stabilizing mRNAs. The solid lines represent known information, while the dotted lines represent novel findings.
With the advancement of medicine, clinicians have a deeper understanding of MI; however, this does not preclude the occurrence of MI. Given their active expression and complex ceRNA network, ncRNAs play an important role in the progression of MI. In this review, we introduced the regulatory role of different types of ncRNAs in the progression of MI, which includes the following aspects: (1) miRNA essentially binds directly to mRNA to inhibit the expression of transcripts, thereby regulating the apoptosis, autophagy, proliferation, fibrosis, and other processes of cardiomyocytes to promote or inhibit MI; (2) lncRNA mainly regulates transcription by acting as miRNA’s “ceRNA” and “sponge” and cooperating with related signaling pathways; (3) we also found that ncRNAs can be packaged, secreted, and then transported to target cells to play a role, and this effect is achieved through exosomes. All these functions show the major advantages of ncRNAs and exosomal ncRNAs with rich variety and diverse regulatory functions in the early diagnosis and treatment of MI.
Furthermore, based on the unique biofilm characteristics of exosomes, people have started to explore whether they can carry clinical drug molecules into target tissues in the body, which possibly has been confirmed by experiments. However, the widespread use of ncRNAs in the treatment of clinical MI is still a distant goal; it is still challenging to explore the use of tissue ncRNAs and exosomal ncRNAs for the diagnosis and treatment of MI. First of all, the current research on ncRNAs is only for scientific research purposes, and it will take time to integrate complex ncRNAs data into a disease-related surveillance network that can be effectively used. Secondly, ncRNAs are rich in functions, and the current understanding is not yet comprehensive. Furthermore, the phenotypic recognition and directional transport of exosomes are formidable challenges. After all, there are abundant cell types in the human body, and cells of the same species in different locations may have different recognition sites. Based on the above, the diverse regulatory functions of ncRNAs and the low clinical interest at present should remind us that we still need a lot of research and technological innovation in this field.
Supplementary information
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81860073, 81760074, and 82160439), the Yunnan Provincial Department of Science and Technology (No. 202001AT070039), the Yunnan Health Training Project of High-Level Talents (No. H-2018032), the 100 Young and Middle-aged Academic and Technical Backbones of Kunming Medical University (No. 60118260106), the Young Talents of Yunnan Thousand Talents Plan (Nos. YNQR-QNRC-2019-006 and RLQN20200002), the Graduate Student Innovation Fund of Kunming Medical University (No. 2022S035), and the Clinical Medical Center for Cardiovascular and Cerebrovascular Disease of Yunnan Province (No. ZX2019-03-01), China.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Nos. 81860073, 81760074, and 82160439), the Yunnan Provincial Department of Science and Technology (No. 202001AT070039), the Yunnan Health Training Project of High-Level Talents (No. H-2018032), the 100 Young and Middle-aged Academic and Technical Backbones of Kunming Medical University (No. 60118260106), the Young Talents of Yunnan Thousand Talents Plan (Nos. YNQR-QNRC-2019-006 and RLQN20200002), the Graduate Student Innovation Fund of Kunming Medical University (No. 2022S035), and the Clinical Medical Center for Cardiovascular and Cerebrovascular Disease of Yunnan Province (No. ZX2019-03-01), China.
Author contributions
Jingru LI, Haocheng MA, and Xinyu WU compiled the data, plotted the figures, and wrote the manuscript. Guihu SUN and Ping YANG performed the data collection. Yunzhu PENG, Qixian WANG, and Luqiao WANG were involved in the manuscript’s study design and initial review. All authors have read and approved the final manuscript, and therefore, have full access to all data in the study and are responsible for the integrity and security of the data.
Compliance with ethics guidelines
Jingru LI, Haocheng MA, Xinyu WU, Guihu SUN, Ping YANG, Yunzhu PENG, Qixian WANG, and Luqiao WANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Aufiero S, Reckman YJ, Pinto YM, et al. , 2019. Circular RNAs open a new chapter in cardiovascular biology. Nat Rev Cardiol, 16(8): 503-514. 10.1038/s41569-019-0185-2 [DOI] [PubMed] [Google Scholar]
- Babuin L, Jaffe AS, 2005. Troponin: the biomarker of choice for the detection of cardiac injury. CMAJ, 173(10): 1191-1202. 10.1503/cmaj/051291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP, 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 116(2): 281-297. 10.1016/s0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- Bartel DP, 2009. MicroRNAs: target recognition and regulatory functions. Cell, 136(2): 215-233. 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran M, Mohan M, 2014. MicroRNAs: history, biogenesis, and their evolving role in animal development and disease. Vet Pathol, 51(4): 759-774. 10.1177/0300985813502820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Wang B, Tang JN, et al. , 2021. Circulating exosomes repair endothelial cell damage by delivering miR-193a-5p. J Cell Mol Med, 25(4): 2176-2189. 10.1111/jcmm.16202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JG, Xu XM, Ji H, et al. , 2019. Inhibiting miR-155 protects against myocardial ischemia/reperfusion injury via targeted regulation of HIF-1α in rats. Iran J Basic Med Sci, 22(9): 1050-1058. 10.22038/ijbms.2019.34853.8270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Guo YN, Sun ZL, et al. , 2021. Long non-coding RNA SENCR alleviates hypoxia/reoxygenation-induced cardiomyocyte apoptosis and inflammatory response by sponging miR-1. Cardiovasc Diagn Ther, 11(3): 707-715. 10.21037/cdt-20-1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NF, Zhao G, Yan X, et al. , 2018. A novel FLI1 exonic circular RNA promotes metastasis in breast cancer by coordinately regulating TET1 and DNMT1. Genome Biol, 19: 218. 10.1186/s13059-018-1594-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Fang HC, Liu RZ, et al. , 2021. miR-6718-5p and miR-4329 can be used as potential biomarkers for acute myocardial infarction. J Card Surg, 36(10): 3721-3728. 10.1111/jocs.15868 [DOI] [PubMed] [Google Scholar]
- Chen XZ, Huang FR, Liu YH, et al. , 2022. Exosomal miR-152-5p and miR-3681-5p function as potential biomarkers for ST-segment elevation myocardial infarction. Clinics, 77: 100038. 10.1016/j.clinsp.2022.100038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZZ, Yan YY, Wu JD, et al. , 2020. Expression level and diagnostic value of exosomal NEAT1/miR-204/MMP-9 in acute ST-segment elevation myocardial infarction. IUBMB Life, 72(11): 2499-2507. 10.1002/iub.2376 [DOI] [PubMed] [Google Scholar]
- Choudhry H, Mole DR, 2016. Hypoxic regulation of the noncoding genome and NEAT1. Brief Funct Genomics, 15(3): 174-185. 10.1093/bfgp/elv050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsten MF, Dennert R, Jochems S, et al. , 2010. Circulating microRNA-208b and microRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet, 3(6): 499-506. 10.1161/circgenetics.110.957415 [DOI] [PubMed] [Google Scholar]
- Crouser ED, Julian MW, Bicer S, et al. , 2021. Circulating exosomal microRNA expression patterns distinguish cardiac sarcoidosis from myocardial ischemia. PLoS ONE, 16(1): e0246083. 10.1371/journal.pone.0246083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessandra Y, Pompilio G, Capogrossi MC, 2012. MicroRNAs and myocardial infarction. Curr Opin Cardiol, 27(3): 228-235. 10.1097/HCO.0b013e3283522052 [DOI] [PubMed] [Google Scholar]
- D'Souza RF, Woodhead JST, Zeng NN, et al. , 2018. Circulatory exosomal miRNA following intense exercise is unrelated to muscle and plasma miRNA abundances. Am J Physiol Endocrinol Metab, 315(4): E723-E733. 10.1152/ajpendo.00138.2018 [DOI] [PubMed] [Google Scholar]
- Dong FF, Dong SH, Liang Y, et al. , 2019. miR-34a promotes myocardial infarction in rats by inhibiting the activity of SIRT1. Eur Rev Med Pharmacol Sci, 23(16): 7059-7065. 10.26355/eurrev_201908_18750 [DOI] [PubMed] [Google Scholar]
- Emanueli C, Shearn AIU, Laftah A, et al. , 2016. Coronary artery-bypass-graft surgery increases the plasma concentration of exosomes carrying a cargo of cardiac microRNAs: an example of exosome trafficking out of the human heart with potential for cardiac biomarker discovery. PLoS ONE, 11(4): e0154274. 10.1371/journal.pone.0154274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan SY, Hu KL, Zhang DY, et al. , 2020. Interference of circRNA HIPK3 alleviates cardiac dysfunction in lipopolysaccharide-induced mice models and apoptosis in H9C2 cardiomyocytes. Ann Transl Med, 8(18): 1147. 10.21037/atm-20-5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Wang QS, Shi C, et al. , 2019. Does circular RNA exert significant effects in ovarian cancer? Crit Rev Eukaryot Gene Expr, 29(2): 161-170. 10.1615/CritRevEukaryotGeneExpr.2019025941 [DOI] [PubMed] [Google Scholar]
- Fu LY, Chen QQ, Yao T, et al. , 2017. Hsa_circ_0005986 inhibits carcinogenesis by acting as a miR-129-5p sponge and is used as a novel biomarker for hepatocellular carcinoma. Oncotarget, 8(27): 43878-43888. 10.18632/oncotarget.16709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garikipati VNS, Verma SK, Cheng ZJ, et al. , 2019. Circular RNA CircFndc3b modulates cardiac repair after myocardial infarction via FUS/VEGF-A axis. Nat Commun, 10: 4317. 10.1038/s41467-019-11777-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng HH, Li R, Su YM, et al. , 2016. The circular RNA Cdr1as promotes myocardial infarction by mediating the regulation of miR-7a on its target genes expression. PLoS ONE, 11(3): e0151753. 10.1371/journal.pone.0151753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng T, Song ZY, Xing JX, et al. , 2020. Exosome derived from coronary serum of patients with myocardial infarction promotes angiogenesis through the miRNA-143/IGF-IR pathway. Int J Nanomedicine, 15: 2647-2658. 10.2147/ijn.S242908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidlöf O, Andersson P, van der Pals J, et al. , 2011. Cardiospecific microRNA plasma levels correlate with troponin and cardiac function in patients with ST elevation myocardial infarction, are selectively dependent on renal elimination, and can be detected in urine samples. Cardiology, 118(4): 217-226. 10.1159/000328869 [DOI] [PubMed] [Google Scholar]
- Gong XH, Zhu Y, Chang HX, et al. , 2019. Long noncoding RNA MALAT1 promotes cardiomyocyte apoptosis after myocardial infarction via targeting miR-144-3p. Biosci Rep, 39(8): BSR20191103. 10.1042/bsr20191103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HL, Ingolia NT, Weissman JS, et al. , 2010. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature, 466(7308): 835-840. 10.1038/nature09267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Liu HB, Sun C, et al. , 2019. MicroRNA-155 promotes myocardial infarction-induced apoptosis by targeting RNA-binding protein QKI. Oxid Med Cell Longev, 2019: 4579806. 10.1155/2019/4579806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Li R, Yang LF, et al. , 2021. Evaluation of exosomal miRNAs as potential diagnostic biomarkers for acute myocardial infarction using next-generation sequencing. Ann Transl Med, 9(3): 219. 10.21037/atm-20-2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Luo F, Liu Q, et al. , 2017. Regulatory non-coding RNAs in acute myocardial infarction. J Cell Mol Med, 21(5): 1013-1023. 10.1111/jcmm.13032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RA, Shah N, Wang KC, et al. , 2010. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature, 464(7291): 1071-1076. 10.1038/nature08975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha MJ, Kim VN, 2014. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol, 15(8): 509-524. 10.1038/nrm3838 [DOI] [PubMed] [Google Scholar]
- Hansen TB, Jensen TI, Clausen BH, et al. , 2013. Natural RNA circles function as efficient microRNA sponges. Nature, 495(7441): 384-388. 10.1038/nature11993 [DOI] [PubMed] [Google Scholar]
- Hawez A, Al-Haidari A, Madhi R, et al. , 2019. MiR-155 regulates PAD4-dependent formation of neutrophil extracellular traps. Front Immunol, 10: 2462. 10.3389/fimmu.2019.02462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach S, Kretz M, 2016. Non-coding RNAs: classification, biology and functioning. Adv Exp Med Biol, 937: 3-17. 10.1007/978-3-319-42059-2_1 [DOI] [PubMed] [Google Scholar]
- Hu H, Wu JW, Li D, et al. , 2018. Knockdown of lncRNA MALAT1 attenuates acute myocardial infarction through miR-320-Pten axis. Biomed Pharmacother, 106: 738-746. 10.1016/j.biopha.2018.06.122 [DOI] [PubMed] [Google Scholar]
- Hu H, Wu JW, Yu XF, et al. , 2019. Long non-coding RNA MALAT1 enhances the apoptosis of cardiomyocytes through autophagy inhibition by regulating TSC2-mTOR signaling. Biol Res, 52: 58. 10.1186/s40659-019-0265-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Hu SQ, Cheng K, 2019. A new era of cardiac cell therapy: opportunities and challenges. Adv Healthc Mater, 8(2): 1801011. 10.1002/adhm.201801011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji ML, Jiang H, Wu F, et al. , 2021. Precise targeting of miR-141/200c cluster in chondrocytes attenuates osteoarthritis development. Ann Rheum Dis, 80(3): 356-366. 10.1136/annrheumdis-2020-218469 [DOI] [PubMed] [Google Scholar]
- Ji P, Diederichs S, Wang WB, et al. , 2003. MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene, 22(39): 8031-8041. 10.1038/sj.onc.1206928 [DOI] [PubMed] [Google Scholar]
- Kabekkodu SP, Shukla V, Varghese VK, et al. , 2018. Clustered miRNAs and their role in biological functions and diseases. Biol Rev Camb Philos Soc, 93(4): 1955-1986. 10.1111/brv.12428 [DOI] [PubMed] [Google Scholar]
- Kamal NNSBNM, Shahidan WNS, 2020. Non-exosomal and exosomal circulatory microRNAs: which are more valid as biomarkers? Front Pharmacol, 10: 1500. 10.3389/fphar.2019.01500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Nickoloff E, Abramova T, et al. , 2015. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res, 117(1): 52-64. 10.1161/circresaha.117.305990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowara M, Borodzicz-Jazdzyk S, Rybak K, et al. , 2021. Therapies targeted at non-coding RNAs in prevention and limitation of myocardial infarction and subsequent cardiac remodeling-current experience and perspectives. Int J Mol Sci, 22(11): 5718. 10.3390/ijms22115718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi S, 2003. The pathogenesis of myocardial infarction and risk factors. Nihon Rinsho, 61(Suppl 5): 333-338. [PubMed] [Google Scholar]
- Kristensen LS, Andersen MS, Stagsted LVW, et al. , 2019. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet, 20(11): 675-691. 10.1038/s41576-019-0158-7 [DOI] [PubMed] [Google Scholar]
- Lang MJ, Ou DK, Liu ZH, et al. , 2021. LncRNA MHRT promotes cardiac fibrosis via miR-3185 pathway following myocardial infarction. Int Heart J, 62(4): 891-899. 10.1536/ihj.20-298 [DOI] [PubMed] [Google Scholar]
- Legnini I, di Timoteo G, Rossi F, et al. , 2017. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell, 66(1): 22-37.e29. 10.1016/j.molcel.2017.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Cheng ZJ, Tang YY, et al. , 2019. Expression profile of long non‑coding RNAs in cardiomyocytes exposed to acute ischemic hypoxia. Mol Med Rep, 19(1): 302-308. 10.3892/mmr.2018.9658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang N, Wen X, et al. , 2022. Serum miRNA-203 as a novel biomarker for the early prediction of acute ST-elevation myocardial infarction. J Cardiovasc Transl Res, 15(6): 1406-1413. 10.1007/s12265-022-10269-2 [DOI] [PubMed] [Google Scholar]
- Li QL, Gao YP, Zhu J, et al. , 2020. MiR-101 attenuates myocardial infarction-induced injury by targeting DDIT4 to regulate autophagy. Curr Neurovasc Res, 17(2): 123-130. 10.2174/1567202617666200211113016 [DOI] [PubMed] [Google Scholar]
- Li SL, Ren J, Sun QM, 2018. The expression of microRNA-23a regulates acute myocardial infarction in patients and in vitro through targeting PTEN. Mol Med Rep, 17(5): 6866-6872. 10.3892/mmr.2018.8640 [DOI] [PubMed] [Google Scholar]
- Li XZ, Sun YL, Huang SL, et al. , 2019. Inhibition of AZIN2-sv induces neovascularization and improves prognosis after myocardial infarction by blocking ubiquitin-dependent talin1 degradation and activating the Akt pathway. eBioMedicine, 39: 69-82. 10.1016/j.ebiom.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZY, Huang C, Bao C, et al. , 2017. Correction: corrigendum: exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol, 24(2): 194. 10.1038/nsmb0217-194a [DOI] [PubMed] [Google Scholar]
- Liang ZZ, Guo C, Zou MM, et al. , 2020. CircRNA-miRNA-mRNA regulatory network in human lung cancer: an update. Cancer Cell Int, 20: 173. 10.1186/s12935-020-01245-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Chen HW, Zhao GA, et al. , 2020. Advances in research on the circRNA-miRNA-mRNA network in coronary heart disease treated with traditional Chinese medicine. Evid Based Complement Alternat Med, 2020: 8048691. 10.1155/2020/8048691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H, Guo ZY, Shi YF, et al. , 2020a. Serum exosomal microRNA-21, microRNA-126, and PTEN are novel biomarkers for diagnosis of acute coronary syndrome. Front Physiol, 11: 654. 10.3389/fphys.2020.00654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H, Guo ZY, Du SS, et al. , 2020b. Serum exosomal miR-122-5p is a new biomarker for both acute coronary syndrome and underlying coronary artery stenosis. Biomarkers, 25(7): 539-547. 10.1080/1354750X.2020.1803963 [DOI] [PubMed] [Google Scholar]
- Liu C, Yao MD, Li CP, et al. , 2017. Silencing of circular RNA-ZNF609 ameliorates vascular endothelial dysfunction. Theranostics, 7(11): 2863-2877. 10.7150/thno.19353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wu JD, Li LB, et al. , 2020. The role of exosomal non-coding RNAs in coronary artery disease. Front Pharmacol, 11: 603104. 10.3389/fphar.2020.603104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZY, Liu J, Wei Y, et al. , 2020. LncRNA MALAT1 prevents the protective effects of miR-125b-5p against acute myocardial infarction through positive regulation of NLRC5. Exp Ther Med, 19(2): 990-998. 10.3892/etm.2019.8309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QJ, Wu RF, Zhao M, et al. , 2019. miRNAs as therapeutic targets in inflammatory disease. Trends Pharmacol Sci, 40(11): 853-865. 10.1016/j.tips.2019.09.007 [DOI] [PubMed] [Google Scholar]
- Ma RF, Gao L, Liu YH, et al. , 2021. LncRNA TTTY15 knockdown alleviates H2O2-stimulated myocardial cell injury by regulating the miR-98-5p/CRP pathway. Mol Cell Biochem, 476(1): 81-92. 10.1007/s11010-020-03887-4 [DOI] [PubMed] [Google Scholar]
- Mao Q, Liang XL, Zhang CL, et al. , 2019. LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Res Ther, 10: 393. 10.1186/s13287-019-1522-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AC, Tan J, Lee S, et al. , 2012. Evidence for conserved post-transcriptional roles of unitary pseudogenes and for frequent bifunctionality of mRNAs. Genome Biol, 13(11): R102. 10.1186/gb-2012-13-11-r102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi XL, Gao YP, Hao DJ, et al. , 2022. Prognostic value of circulating microRNA-21-5p and microRNA-126 in patients with acute myocardial infarction and infarct-related artery total occlusion. Front Cardiovasc Med, 9: 947721. 10.3389/fcvm.2022.947721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro P, Page DR, Avner P, et al. , 2006. Tsix-mediated epigenetic switch of a CTCF-flanked region of the Xist promoter determines the Xist transcription program. Genes Dev, 20(20): 2787-2792. 10.1101/gad.389006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Ortega L, Alonso-Lopez E, Pérez-Mato M, et al. , 2021. Similarities and differences in extracellular vesicle profiles between ischaemic stroke and myocardial infarction. Biomedicines, 9(1): 8. 10.3390/biomedicines9010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel DM, Gould SJ, 2019. Exosomes. Annu Rev Biochem, 88: 487-514. 10.1146/annurev-biochem-013118-111902 [DOI] [PubMed] [Google Scholar]
- Qiao L, Hu SQ, Liu SY, et al. , 2019. MicroRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J Clin Invest, 129(6): 2237-2250. 10.1172/JCI123135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Chang HY, 2016. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet, 17(1): 47-62. 10.1038/nrg.2015.10 [DOI] [PubMed] [Google Scholar]
- Roth GA, Mensah GA, Johnson CO, et al. , 2020. Global burden of cardiovascular diseases and risk factors, 1990‒2019: update from the GBD 2019 Study. J Am Coll Cardiol, 76(25): 2982-3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado-Somoza A, Zhang L, Vausort M, et al. , 2017. The circular RNA MICRA for risk stratification after myocardial infarction. IJC Heart Vasc, 17: 33-36. 10.1016/j.ijcha.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L, Poliseno L, Tay Y, et al. , 2011. A ceRNA hypothesis: the Rosetta stone of a hidden RNA language? Cell, 146(3): 353-358. 10.1016/j.cell.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saludas L, Oliveira CC, Roncal C, et al. , 2021. Extracellular vesicle-based therapeutics for heart repair. Nanomaterials, 11(3): 570. 10.3390/nano11030570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scărlătescu AI, Barbălată T, Sima AV, et al. , 2022. miR-146a-5p, miR-223-3p and miR-142-3p as potential predictors of major adverse cardiac events in young patients with acute ST elevation myocardial infarction-added value over left ventricular myocardial work indices. Diagnostics, 12(8): 1946. 10.3390/diagnostics12081946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, et al. , 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res, 13(11): 2498-2504. 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si XY, Zheng H, Wei GQ, et al. , 2020. CircRNA hipk3 induces cardiac regeneration after myocardial infarction in mice by binding to notch1 and miR-133a. Mol Ther Nucleic Acids, 21: 636-655. 10.1016/j.omtn.2020.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spannbauer A, Mester-Tonczar J, Traxler D, et al. , 2020. Large animal models of cell-free cardiac regeneration. Biomolecules, 10(10): 1392. 10.3390/biom10101392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Li JY, Yu QL, et al. , 2020. Exosomal miRNAs as potential biomarkers for acute myocardial infarction. IUBMB Life, 72(3): 384-400. 10.1002/iub.2189 [DOI] [PubMed] [Google Scholar]
- Su Q, Lv XW, 2020. Revealing new landscape of cardiovascular disease through circular RNA-miRNA-mRNA axis. Genomics, 112(2): 1680-1685. 10.1016/j.ygeno.2019.10.006 [DOI] [PubMed] [Google Scholar]
- Su Q, Liu Y, Lv XW, et al. , 2019. Inhibition of lncRNA TUG1 upregulates miR-142-3p to ameliorate myocardial injury during ischemia and reperfusion via targeting HMGB1- and Rac1-induced autophagy. J Mol Cell Cardiol, 133: 12-25. 10.1016/j.yjmcc.2019.05.021 [DOI] [PubMed] [Google Scholar]
- Su Q, Liu Y, Lv XW, et al. , 2020. LncRNA TUG1 mediates ischemic myocardial injury by targeting miR-132-3p/HDAC3 axis. Am J Physiol Heart Circ Physiol, 318(2): H332-H344. 10.1152/ajpheart.00444.2019 [DOI] [PubMed] [Google Scholar]
- Sun LY, Zhao JC, Ge XM, et al. , 2020. Circ_LAS1L regulates cardiac fibroblast activation, growth, and migration through miR-125b/SFRP5 pathway. Cell Biochem Funct, 38(4): 443-450. 10.1002/cbf.3486 [DOI] [PubMed] [Google Scholar]
- Sun Y, Hu ZQ, 2020. LncRNA HOTAIR aggravates myocardial ischemia-reperfusion injury by sponging microRNA-126 to upregulate SRSF1. Eur Rev Med Pharmacol Sci, 24(17): 9046-9054. 10.26355/eurrev_202009_22850 [DOI] [PubMed] [Google Scholar]
- Sun ZQ, Chen C, Su YF, et al. , 2019. Regulatory mechanisms and clinical perspectives of circRNA in digestive system neoplasms. J Cancer, 10(13): 2885-2891. 10.7150/jca.31167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Pan WN, Chen HL, et al. , 2021. Circ_0124644 serves as a ceRNA for miR-590-3p to promote hypoxia-induced cardiomyocytes injury via regulating SOX4. Front Genet, 12: 667724. 10.3389/fgene.2021.667724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SJO, Floriano JF, Nicastro L, et al. , 2020. Novel applications of mesenchymal stem cell-derived exosomes for myocardial infarction therapeutics. Biomolecules, 10(5): 707. 10.3390/biom10050707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vausort M, Wagner DR, Devaux Y, 2014. Long noncoding RNAs in patients with acute myocardial infarction. Circ Res, 115(7): 668-677. 10.1161/circresaha.115.303836 [DOI] [PubMed] [Google Scholar]
- Wang B, Cao C, Han DJ, et al. , 2021. Dysregulation of miR-342-3p in plasma exosomes derived from convalescent AMI patients and its consequences on cardiac repair. Biomed Pharmacother, 142: 112056. 10.1016/j.biopha.2021.112056 [DOI] [PubMed] [Google Scholar]
- Wang CX, Zhang CC, Liu LX, et al. , 2017. Macrophage-derived miR-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol Ther, 25(1): 192-204. 10.1016/j.ymthe.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GK, Zhu JQ, Zhang JT, et al. , 2010. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J, 31(6): 659-666. 10.1093/eurheartj/ehq013 [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang SW, Li XH, et al. , 2020. LncRNA SNHG7 promotes cardiac remodeling by upregulating ROCK1 via sponging miR-34-5p. Aging, 12(11): 10441-10456. 10.18632/aging.103269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Liu XF, Wu HC, et al. , 2010. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res, 38(16): 5366-5383. 10.1093/nar/gkq285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SJ, Li L, Deng WJ, et al. , 2021. CircRNA MFACR is upregulated in myocardial infarction and downregulates miR-125b to promote cardiomyocyte apoptosis induced by hypoxia. J Cardiovasc Pharmacol, 78(6): 802-808. 10.1097/FJC.0000000000001123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SQ, Wang Y, Cheng HX, et al. , 2022. The networks of noncoding RNAs and their direct molecular targets in myocardial infarction. Int J Biol Sci, 18(8): 3194-3208. 10.7150/ijbs.69671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang Z, Wang TZ, et al. , 2019. Inhibition of miR-34a-5p protected myocardial ischemia reperfusion injury-induced apoptosis and reactive oxygen species accumulation through regulation of Notch Receptor 1 signaling. Rev Cardiovasc Med, 20(3): 187-197. 10.31083/j.rcm.2019.03.545 [DOI] [PubMed] [Google Scholar]
- Wang ZH, Sun XY, Li CL, et al. , 2017. MiRNA-21 expression in the serum of elderly patients with acute myocardial infarction. Med Sci Monit, 23: 5728-5734. 10.12659/msm.904933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernly B, Paar V, Aigner A, et al. , 2020. Anti-CD3 antibody treatment reduces scar formation in a rat model of myocardial infarction. Cells, 9(2): 295. 10.3390/cells9020295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DH, Wang CZ, 2020. miR-155 regulates the proliferation of glioma cells through PI3K/AKT signaling. Front Neurol, 11: 297. 10.3389/fneur.2020.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J, Li QQ, Li BQ, et al. , 2020. miR-155 inhibition represents a potential valuable regulator in mitigating myocardial hypoxia/reoxygenation injury through targeting BAG5 and MAPK/JNK signaling. Mol Med Rep, 21(3): 1011-1020. 10.3892/mmr.2020.10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao SH, Wang Y, Cao XC, et al. , 2021. Long non-coding RNA LUCAT1 inhibits myocardial oxidative stress and apoptosis after myocardial infarction via targeting microRNA-181a-5p. Bioengineered, 12(1): 4546-4555. 10.1080/21655979.2021.1966351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YW, 2020. Construction of a circRNA-miRNA-mRNA network to explore the pathogenesis and treatment of pancreatic ductal adenocarcinoma. J Cell Biochem, 121(1): 394-406. 10.1002/jcb.29194 [DOI] [PubMed] [Google Scholar]
- Xie LH, Zhang QQ, Mao J, et al. , 2021. The roles of lncRNA in myocardial infarction: molecular mechanisms, diagnosis biomarkers, and therapeutic perspectives. Front Cell Dev Biol, 9: 680713. 10.3389/fcell.2021.680713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LWJ, Tian L, Yan ZR, et al. , 2023. Diagnostic and prognostic value of miR-486-5p, miR-451a, miR-21-5p and monocyte to high-density lipoprotein cholesterol ratio in patients with acute myocardial infarction. Heart Vessels, 38(3): 318-331. 10.1007/s00380-022-02172-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin YG, Lv L, Wang WN, 2019. Expression of miRNA-214 in the sera of elderly patients with acute myocardial infarction and its effect on cardiomyocyte apoptosis. Exp Ther Med, 17(6): 4657-4662. 10.3892/etm.2019.7464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Xu JF, Song M, et al. , 2022. Associations of circulating microRNA-221 and 222 with the severity of coronary artery lesions in acute coronary syndrome patients. Angiology, 73(6): 579-587. 10.1177/00033197211034286 [DOI] [PubMed] [Google Scholar]
- Zhang J, Li S, Li L, et al. , 2015. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics, 13(1): 17-24. 10.1016/j.gpb.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tang YR, Zhang J, et al. , 2021. CircRNA ACAP2 is overexpressed in myocardial infarction and promotes the maturation of miR-532 to induce the apoptosis of cardiomyocyte. J Cardiovasc Pharmacol, 78(2): 247-252. 10.1097/FJC.0000000000001065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang YM, 2019. Expression and function of lncRNA ANRIL in a mouse model of acute myocardial infarction combined with type 2 diabetes mellitus. J Chin Med Assoc, 82(9): 685-692. 10.1097/JCMA.0000000000000182 [DOI] [PubMed] [Google Scholar]
- Zhang ML, Zhao K, Xu XP, et al. , 2018. A peptide encoded by circular form of LINC-PINT suppresses oncogenic transcriptional elongation in glioblastoma. Nat Commun, 9: 4475. 10.1038/s41467-018-06862-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PJ, Wu WY, Chen Q, et al. , 2019. Non-coding RNAs and their integrated networks. J Integr Bioinform, 16(3): 20190027. 10.1515/jib-2019-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Wang NB, Ma QY, et al. , 2021. LncRNA TUG1 acts as a competing endogenous RNA to mediate CTGF expression by sponging miR-133b in myocardial fibrosis after myocardial infarction. Cell Biol Int, 45(12): 2534-2543. 10.1002/cbin.11707 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang XO, Chen T, et al. , 2013. Circular intronic long noncoding RNAs. Mol Cell, 51(6): 792-806. 10.1016/j.molcel.2013.08.017 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jiao L, Sun LH, et al. , 2018. LncRNA ZFAS1 as a SERCA2a inhibitor to cause intracellular Ca2+ overload and contractile dysfunction in a mouse model of myocardial infarction. Circ Res, 122(10): 1354-1368. 10.1161/circresaha.117.312117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JX, Li XL, Hu JX, et al. , 2019. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res, 115(7): 1205-1216. 10.1093/cvr/cvz040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XX, Jia YP, Chen HZ, et al. , 2019. Plasma-derived exosomal miR-183 associates with protein kinase activity and may serve as a novel predictive biomarker of myocardial ischemic injury. Exp Ther Med, 18(1): 179-187. 10.3892/etm.2019.7555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng DD, Huo M, Li B, et al. , 2021. The role of exosomes and exosomal microRNA in cardiovascular disease. Front Cell Dev Biol, 8: 616161. 10.3389/fcell.2020.616161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng LF, Li XM, Chou JJ, et al. , 2018. Stard13 3'-untranslated region functions as a ceRNA for TP53INP1 in prohibiting migration and invasion of breast cancer cells by regulating miR-125b activity. Eur J Cell Biol, 97(1): 23-31. 10.1016/j.ejcb.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Zheng ML, Liu XY, Han RJ, et al. , 2020. Circulating exosomal long non-coding RNAs in patients with acute myocardial infarction. J Cell Mol Med, 24(16): 9388-9396. 10.1111/jcmm.15589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YY, Zhou B, Pache L, et al. , 2019. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun, 10: 1523. 10.1038/s41467-019-09234-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Li HR, Liu YJ, et al. , 2020. miR-155 antagomir protect against DSS-induced colitis in mice through regulating Th17/Treg cell balance by Jarid2/Wnt/β-catenin. Biomed Pharmacother, 126: 109909. 10.1016/j.biopha.2020.109909 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.