Abstract
Postmenopausal osteoporosis is a kind of degenerative disease, also described as “invisible killer.” Estrogen is generally considered as the key hormone for women to maintain bone mineral content during their lives. Iron accumulation refers to a state of human serum ferritin that is higher than the normal value but less than 1000 μg/L. It has been found that iron accumulation and osteoporosis could occur simultaneously with the decrease in estrogen level after menopause. In recent years, many studies indicated that iron accumulation plays a vital role in postmenopausal osteoporosis, and a significant correlation has been found between iron accumulation and fragility fractures. In this review, we summarize and analyze the relevant literature including randomized controlled trials, systematic reviews, and meta-analyses between January 1996 and July 2022. We investigate the mechanism of the effect of iron accumulation on bone metabolism and discuss the relationship of iron accumulation, osteoporosis, and postmenopausal fragility fractures, as well as the main clinical treatment strategies. We conclude that it is necessary to pay attention to the phenomenon of iron accumulation in postmenopausal women with osteoporosis and explore the in-depth mechanism of abnormal bone metabolism caused by iron accumulation, in order to facilitate the discovery of effective therapeutic targets for postmenopausal osteoporosis.
Keywords: Iron accumulation, Postmenopausal osteoporosis, Bone metabolism, Osteoporotic fracture
Abstract
绝经后骨质疏松症是一种发生于骨骼的退行性疾病,号称“隐形杀手”。雌激素是女性一生中维持骨矿物质含量的关键激素。铁蓄积是指人血清铁蛋白高于正常值但低于1000 μg/L的状态。研究发现,绝经后随着雌激素水平的降低,铁蓄积和骨质疏松症可同时发生。近年来,很多研究表明铁蓄积在绝经后骨质疏松症发生中起着至关重要的作用,且铁蓄积与骨质疏松性骨折也有显著的相关性。在本文中,我们对1996年1月至2022年7月期间的随机对照试验、系统综述和荟萃分析等相关文献进行了总结和分析,探讨铁蓄积对骨代谢影响的机制,阐明铁蓄积与骨质疏松、绝经后脆性骨折的关系及临床主要治疗策略。我们总结认为,有必要关注女性绝经后骨质疏松铁蓄积现象。深入探讨铁蓄积引起骨代谢异常的机制,有助于发现绝经后骨质疏松有效的治疗靶点。
Keywords: 铁蓄积, 绝经后骨质疏松, 骨代谢, 骨质疏松性骨折
1. Introduction
Clinically, serum ferritin (SF) is commonly used as an indicator of iron homeostasis in the body. One ferritin molecule can bind 4500 iron atoms, and its normal value in females is 12 to 150 μg/L; when the SF is >1000 μg/L, the case is considered to be iron overload; SF levels exceeding the normal value but less than 1000 μg/L are often regarded as iron accumulation (Chen et al., 2015; Yuan et al., 2019). Among the essential trace elements, iron has the highest content and demand in the human body, accounting for 0.0057% of the body mass and measuring a total amount of 4–5 g. A total of 75% of the iron exists in porphyrins, while 25% is found in non-porphyrin iron-containing compounds, mainly including flavin, iron-sulfur protein, ferritin, and transferrin (Tf). The life cycle of intestinal mucosal epithelial cells is 2 to 6 d, and the iron content of these cells stored in ferritin is excreted from the intestinal cavity with their exfoliation, which is the principal means to excrete iron from human body. Women can discharge iron during menstrual blood loss; however, urine, sweat, digestive juice, or bile does not contain iron (Wang and Xu, 2022). Accordingly, iron accumulation often occurs in postmenopausal women for the following reasons: (1) the amount of iron excreted by menstruation is about 36 mg/year; (2) the level of postmenopausal estrogen decreases to 10% of the premenopausal normal value; however, the level of SF can elevate by two to three times. Since menstruation is the principal means for women to discharge excess iron, with the onset of menopause, the main iron-discharging pathway becomes blocked, iron excretion is sharply reduced, and iron can accumulate (Wang and Xu, 2022). The incidence of postmenopausal osteoporosis in women aged over 50 years is approximately 50% (Yuan et al., 2019).
In clinical practice, it is often found that iron accumulation and osteoporosis take place simultaneously in female patients with the decrease in estrogen level after menopause (Chen et al., 2015; Wang and Xu, 2022). As such, iron accumulation may be related to the level of estrogen and postmenopausal osteoporosis. In many rat models of iron accumulation, it has been proved that iron accumulation could decrease bone mineral density and raise the incidence of osteoporosis (Yuan et al., 2019; Hang et al., 2020; Liu LL et al., 2021). All these findings indicate that iron accumulation is an independent risk factor of osteoporosis in postmenopausal women (Chen et al., 2015; Wang and Xu, 2022).
In this review, we firstly describe the impact of iron accumulation on bone metabolism and introduce its mechanism. Next, the relationship between iron accumulation and postmenopausal fragility fracture is discussed. Finally, we introduce clinical treatment strategies for postmenopausal osteoporosis.
2. Effect of iron accumulation on bone metabolism and its mechanism
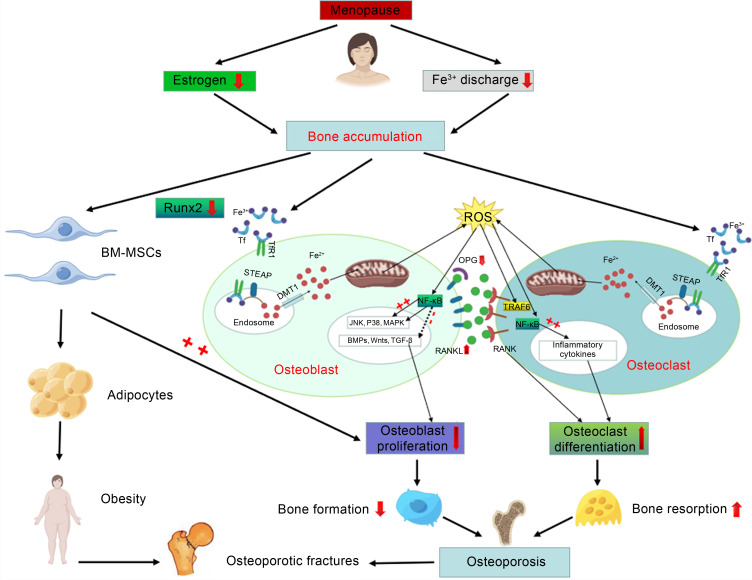
Bone is a hard organ composed of bone tissue and periosteum, which mainly acts as structural support, surface for muscle attachment, source of blood cells, and mineral storage, and protects organs. The internal structure of the bone conforms to the principles of biomechanics and can be adaptively updated and reconstructed. Bone tissue is composed of osteoprogenitor cells, osteoblasts, osteocytes, and osteoclasts. Although these four cell types have different structures and functions, they are interrelated and participate in the occurrence and remodeling of bone tissue. This process involves the formation and absorption of bone tissue. Firstly, osteoprogenitor cells proliferate and differentiate into osteoblasts, which produce osteoids and then transform into osteocytes after being embedded in osteoids. With the calcification of osteoid into bone, hard bone tissue is formed. During this process, some parts of the original bone tissue can be eroded and absorbed by osteoclasts. The formation and absorption of bone tissue occur simultaneously and are in a dynamic equilibrium. It is currently believed that through mutual regulation and cooperation, osteoblasts and osteoclasts can form various specific forms of bone and that bone growth and development can adapt to the growth and development of individuals (Wang and Xu, 2022). Therefore, the main purpose of studying the effect of iron accumulation on bone metabolism is to clarify the effects of iron accumulation on osteoblasts and osteoclasts (Fig. 1).
Fig. 1. Mechanism of iron accumulation and its impact on postmenopausal osteoporotic fractures. BM-MSCs: bone marrow-mesenchymal stem cells; Runx2: Runt-related transcription factor 2; STEAP: six-transmembrane epithelial antigen of the prostate; Tf: transferrin; TfR1: Tf receptor 1; DMT1: divalent metal transporter 1; JNK: Jun-N-terminal kinase; MAPK: mitogen-activated protein kinase; BMP: bone morphogenetic protein; TGF-β: transforming growth factor-β; NF-κB: nuclear factor-κB; RANK: receptor activator of NF-κB; RANKL: RANK ligand; ROS: reactive oxygen species; OPG: osteoprotegerin; TRAF6: tumor necrosis factor receptor-associated factor 6.
2.1. Effect of iron accumulation on osteoblasts
Osteoblasts originate from bone marrow-mesenchymal stem cells (BM-MSCs) and are distributed among the surface of bone tissue. They can produce osteoids and secrete various cytokines, regulate the formation and absorption of bone tissue, and promote bone calcification (Liu et al., 2017; Che et al., 2021; Jorgensen and Khoury, 2021).
A growing number of experiments have indicated that excessive iron can inhibit the growth of osteoblasts (Balogh et al., 2016; Liu et al., 2017; Che et al., 2021). The following views are held on this topic.
(1) Excessive iron can induce osteoblast apoptosis through the signal transduction pathway, leading to apoptosis mediated by mitochondria. Several studies have shown that many apoptosis signals (such as DNA damage and oxidant) can cause mitochondrial damage and membrane permeability changes (Ke et al., 2017; Che et al., 2021; Xu et al., 2021). Several proteins of the B-cell lymphoma-2 (Bcl-2) family, such as Bcl-2, Bcl-2-associated X (Bax) and Bcl-XL, are located on the mitochondrial membrane. Bcl-2 can inhibit apoptosis by preventing the release of cytochrome C (cyto-C) from the mitochondria, while Bax promotes the release of cyto-C and apoptosis via binding to the membrane channels on the mitochondria. Upon entering the cytoplasm, cyto-C can bind to the precursor of caspase-9 together with apoptotic protease-activating-factor 1 (Apaf-1), which leads to the activation of caspase-9. This can activate caspase-3 to induce apoptosis. This view has been confirmed by Tian et al. (2016). It is known that the production of reactive oxygen species (ROS) plays an important role in the process of osteogenesis. Iron produces high-activity hydroxyl oxygen free radicals through the Fenton reaction, which leads to an elevated ROS level. Too much ROS can not only reduce the activity of MSC differentiation into osteoblasts, but also weaken the ability of MSC proliferation, which in turn leads to decreased osteoblast formation (Jorgensen and Khoury, 2021). Xu et al. (2021) found that nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 (NOX4) is an important factor in the production of ROS. When osteoblasts were treated individually with different concentrations of ferric ammonium citrate (FAC), the production of ROS enhanced with increasing concentration. ROS can hinder the production of osteoblasts through the above pathways. It can be inferred that NOX4 is the key enzyme for the iron-induced production of ROS in osteoblasts.
(2) Iron accumulation accelerates the autophagy of osteoblasts. It is known that divalent metal transporter 1 (DMT1) can regulate the autophagy and apoptosis of osteoblasts, and the expression of microtubule-associated protein 1 light chain 3-II (LC3-II) can be used to measure this autophagy level. Liu et al. (2017) cultured osteoblasts in medium containing different concentrations of FAC, and determined the levels of DMT1 after FAC treatment by western blotting and immunofluorescence. The results showed that FAC could increase the contents of DMT1 and LC3-II in osteoblasts and the autophagy of osteoblasts, which confirmed the above speculation.
(3) By downregulating the Runt-related transcription factor 2 (Runx2), excessive iron can inhibit the osteogenic differentiation of BM-MSCs, while the inhibitory effect of iron depends on the upregulation of ferritin (Balogh et al., 2016). BM-MSCs are initially regulated by osteogenic Runx2 in the process of differentiation into active osteoblasts (Komori et al., 1997; Otto et al., 1997). When Runx2 is activated, this process becomes regulated by a variety of secretory factors, and the related signaling pathway is finally activated to complete osteogenic differentiation (Fiedler et al., 2002; Tang et al., 2009). Zarjou et al. (2010) reported that iron accumulation could inhibit osteoblast activity and reduce extracellular matrix mineralization via increasing the activity of ferrous oxidase in ferritin. Further studies have found that excessive iron could inhibit the mineralization of extracellular matrix induced by bone morphogenetic protein-2 (BMP-2) through activating Hedgehog signal transduction (Yang et al., 2011; Doyard et al., 2012).
In addition, Liu H et al. (2021) pointed out that with increasing iron concentration, the expression of messenger RNA (mRNA) of Tf receptor 1 (TfR1) and DMT1 decreases, while the mRNA expression of membrane iron transporter ferroportin 1 (FPN1) shows an upward trend. Iron intake can be mediated by the Tf/TfR1 pathway or DMT1 and proton coupling pathway, while FNP1 is related to iron ion excretion. Therefore, it can be inferred that when iron accumulates, the iron intake of osteoblasts decreases and the rate of iron excretion increases.
2.2. Effect of iron accumulation on osteoclasts
Osteoclasts are a kind of huge multinucleated cells, which are generally considered to form by the fusion of monocytes in small numbers, scattered on the surface of bone tissue, and are rich in cytoplasmic organelles, especially lysosomes and mitochondria. Osteoclasts release a variety of hydrolases and organic acids in bone resorption lacunae, and thereby dissolve bone salts, decompose organic components, and participate in bone resorption (Roodman, 2009; Kodama and Kaito, 2020).
Osteoclast formation is a complex multi-step process. Firstly, the precursor osteoclasts differentiate into mononuclear osteoclasts, then fuse to form multinucleated osteoclasts, and finally further differentiate to maturity (Kodama and Kaito, 2020).
At present, the receptor activator of nuclear factor-κB (RANK) ligand (RANKL)/osteoprotegerin (OPG) system is known as a central regulator of osteoclast differentiation and activation (Udagawa et al., 2021). OPG binds to RANKL on the surface of osteoclasts and competitively prevents RANKL from binding to RANK on the surface of the osteoclast lineage, thereby inhibiting the differentiation of precursor osteoclasts (Simonet et al., 1997; Carrillo-López et al., 2021). There is increasing evidence that excessive iron can affect not only osteoclastogenesis, but also osteoclast activity and bone resorption (Roodman, 2009; Balogh et al., 2018). The process of iron absorption mediated by TfR1 was found to be associated with osteoclast differentiation (Roodman, 2009). When the iron intake increases, the differentiation activity of osteoclasts also rises. In addition, ROS can recognize precursor osteoclasts and osteoclasts to transmit bone resorption signals by stimulating bone formation-related cells to produce a variety of cytokines, such as OPG, macrophage-colony stimulating factor (M-CSF), and RANKL (Zarjou et al., 2010). Tartrate-resistant acid phosphatase (TRAP) is often used clinically as an indicator of bone resorption. It is believed that bone resorption can be prevented by inhibiting the activity of TRAP in osteoclasts. TRAP mRNA contains iron regulatory elements in the 5'-flanking sequence, and hence TRAP expression is regulated by iron at the gene transcription level (Balogh et al., 2018).
In summary, iron accumulation can inhibit osteoblast activity and promote osteoclast differentiation, which destroys bone homeostasis in the body and increases the incidence of fragility fractures arising from the loss of bone mass.
3. Correlation between iron accumulation and other factors affecting bone metabolism
3.1. Effects of estrogen and iron accumulation on bone metabolism
In adolescence, estrogen can promote bone maturation and epiphyseal healing. If the level of estrogen declines, bone maturation will be delayed. In addition, estrogen can stimulate osteoblast activity and inhibit osteoclast activity. The former promotes the deposition of calcium in the bone and increases bone firmness, while the latter inhibits the rate of bone resorption and reduces bone loss. After menopause, due to the reduction in estrogen secretion, calcium in the bone is gradually lost, which increases the prevalence of osteoporosis. These changes are accompanied by increased iron content in females, resulting in a negative correlation between estrogen content and iron content (Gaffney-Stomberg, 2019).
The balance transformation of intracellular iron metabolism affects bone metabolism, and so is the case with estrogen. Therefore, it is necessary to comprehensively consider the relationship between iron, estrogen and bone metabolism on postmenopausal osteoporosis.
Jian et al. (2009) proposed that estrogen has a negative feedback effect on iron content in women. After menopause, the level of estrogen decreases, and the inhibition of iron content is significantly weakened, resulting in iron overload. Hamad et al. (2020) reported that estrogen can regulate intracellular iron metabolism by inhibiting liver-derived hepcidin antimicrobial peptide (HAMP) synthesis which maintains the integrity of ferroportin. Iron and estrogen have different degrees of antagonistic effects during bone resorption: iron ions can promote the progress of bone resorption, while estrogen only inhibits it. Wang et al. (2018) suggested that estrogen deficiency combined with iron accumulation can promote osteoclast activity and accelerate bone loss, which may be related to the nuclear factor-κB (NF-κB) signaling pathway. The promotion effect of iron on osteoclast differentiation may be related to ROS, while estrogen negatively inhibits ROS production. Other literature has shown that hepcidin plays a balancing and feedback role between iron and estrogen, and affects bone metabolism through changes in iron or estrogen level (Xu et al., 2011; Lu et al., 2015; Nemeth and Ganz, 2021). By studying the relationship between estrogen and hepcidin, it was found that estrogen binds to the estrogen response element half-site of the hepcidin gene and can thus downregulate hepcidin. Hepcidin itself is the main regulator of iron homeostasis (Nemeth and Ganz, 2021).
3.2. Relationship between hepcidin and iron accumulation
Hepcidin is a cysteine-rich antibacterial peptide synthesized and secreted by the liver. It plays a negative regulatory role in the iron balance of the body, and has a certain therapeutic effect in disorders of iron metabolism. In the process of iron metabolism, FPN1 is the only channel through which iron is transported from cells to the blood (Liu H et al., 2021). Hepcidin binds to FPN1 to promote its internalization and degradation. When the body is overloaded with iron, the hepcidin gene is highly expressed, causing the liver to synthesize and secrete hepcidin, decelerate the degradation of FPN1, and block the export of iron transport to the blood; that is, the transport of iron from the intestinal epithelium and macrophages to the blood is reduced. In iron deficiency, the above process shows the opposite trend, thereby maintaining the body’s iron homeostasis.
Many studies indicated that hepcidin can promote the differentiation of MSCs into osteoblasts and osteoblast gene expression by activating the BMP-2/small mothers against decapentaplegic protein (Smad) signaling pathway and mitogen-activated protein kinase (MAPK)/P38 signaling pathway; hepcidin can also increase the concentration of Ca2+ in osteoblasts through L-type calcium channels (Xu et al., 2011; Lu et al., 2015). In animal research, Jiang et al. (2019) constructed zebrafish models with ferritin gene knockout, and found that iron accumulated in zebrafish, the osteogenic gene was inhibited, and bone mass was greatly reduced. Zhang YQ et al. (2018) showed that adenine is an activator of hemoglobin, which upregulates hemoglobin by promoting the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling pathway to Smad, thus regulating iron balance and preventing the iron overload effect.
3.3. Relationship between iron accumulation and special blood vessels in the bone (H-type blood vessels), and its impact on osteoporosis
Bone is a connective tissue that is rich in blood vessels, and there is growing evidence that angiogenesis plays an active role in bone formation, bone repair, and bone remodeling. Blood vessels nourish the skeletal system and remove metabolites by transporting oxygen, nutrients, and other essential cytokines, thereby maintaining the vital life functions of bone cells (Filipowska et al., 2017).
Bone appears in the embryonic period and is derived from the mesenchyme by two ways: intramembranous osteogenesis and cartilage osteogenesis. Diomede et al. (2020) confirmed that these two kinds of bone occurrences are dependent on vascular endothelial growth factor (VEGF). VEGF has a strong role in promoting vascular endothelial cell proliferation and angiogenesis by binding to specific receptors on vascular endothelial cells. Since only endothelial cells have VEGF receptors, the promotive effect of VEGF on the proliferation of other cells is indirect. As early as 2004, low-energy pulsed ultrasound irradiation was applied to promote the formation of new blood vessels in osteoporotic fractures and increase blood flow in the early stage of fracture healing, which was conducive to hematoma mechanization, cell proliferation, and osteogenesis, and enhanced the healing process of osteoporotic fractures (Cheung et al., 2012).
Some scholars performed immunofluorescence staining of mouse bone tissue, and confirmed the presence of a special vascular subtype (H-type blood vessel) in the bone of mice, which is mainly distributed in the metaphysics of the bone and can stimulate bone formation (Kusumbe et al., 2014; Wang et al., 2017). Wang et al. (2017) showed that H-type vessels also exist in human bones, and their number gradually decreases with age, while bone mineral density is positively correlated with the number of H-type vessels. However, existing literature indicated that angiogenesis is induced when the intracellular iron content is decreased or under simulated hypoxia, but the reversal of these conditions will have an anti-angiogenic effect (Kir et al., 2016). Iron is known to be an essential factor for the activation of prolyl hydroxylase. When there is iron deficiency in cells, it will cause the inactivation of prolyl hydroxylase, and increase the stability of hypoxia inducible factor-α (HIF-α), which induces angiogenesis (Jandial et al., 2011; Fan et al., 2014; Erber et al., 2022).
Recently, Hang et al. (2020) constructed rat models and divided them into control group and iron intervention group. The rats in the experimental group were injected with FAC, while those in the control group were administered the same amount of normal saline. After nine weeks, micro-computed tomography analysis, hematoxylin-eosin (HE) staining, and microvascular staining analysis on the femur of rats revealed that iron accumulation led to reduced bone mineral density, accompanied by the decrease in bone vascular bed and the formation of bone microthrombosis. These findings suggested that iron accumulation may interfere with bone formation by reducing the bone vascular bed.
3.4. Gender differences in the effects of iron metabolism on bone metabolism
Osteoporosis is a bone metabolic disease characterized by the destruction of bone microstructure, decreased bone density, and elevated fracture risk. It occurs in postmenopausal women and older men, and is accompanied by the serious complication of fragility fractures.
Clinically, SF is the iron storage status index in the human body. Kim et al. (2013) explored the gender differences in iron metabolism and bone metabolism through a cross-sectional study by enrolling 14 017 subjects (6817 males and 7200 females), and each gender was divided into three groups according to age. Bone mineral density and SF were tested, and the results suggested that: (1) the level of SF in women was markedly lower than that in men, and the association between SF and bone mineral density was only significant in women ≥45 years old; (2) the content of SF in postmenopausal women increased with age, while that in old men remained consistently high, which may be related to the special bone metabolism status of elderly male patients with fragility fracture. The above results suggest that the content of SF in postmenopausal women increases with age, and SF is positively correlated with bone resorption index and bone formation index, which further confirms the existence of iron accumulation. Moreover, iron accumulation is closely related to high bone turnover, while in elderly men, SF maintains a high concentration and is positively correlated with bone resorption and negatively associated with bone formation. Thus, the rate of bone resorption increases and the speed of bone formation slows down; that is, the balance of bone metabolism becomes disordered, which leads to osteoporosis.
4. Correlation analysis between iron accumulation and postmenopausal fragility fracture
Fung et al. (2008) conducted a multicenter study using 420 cases of sickle cell disease or thalassemia. They analyzed the clinical data on patients’ gender, SF, and fracture incidence, and found that the increased risk of fracture was associated with iron overload in these patients. Thalassemia is the most common cause of secondary iron overload with ineffective erythropoiesis with hepcidin suppression, which leads to increased intestinal absorption and increased iron circulation (Hsu et al., 2022).
Zhuang et al. (2020) explored related factors of brittle hip fracture by analyzing 252 postmenopausal osteoporosis women, among which 135 were affected by brittle hip fracture. It was found that in the group of brittle hip fracture, the level of serum 25-hydroxyvitamin was (15.9±8.9) ng/mL, which was far below its normal level. Also, those who suffered from fragility fractures were significantly older than those in the non-hip fracture group. The results highlighted that older age and lower level of serum 25-hydroxyvitamin are the main independent factors of brittle hip fracture in postmenopausal women.
5. Clinical treatments of postmenopausal osteoporosis
At present, the clinical treatments of postmenopausal osteoporosis are mainly divided into two main types: Western medicine treatment and Chinese traditional medicine treatment (Table 1). The Western approach mainly involves treating postmenopausal osteoporosis by inhibiting bone resorption, promoting bone formation, and enhancing bone calcification (Anthamatten and Parish, 2019). Inhibiting bone formation means blocking the activity of osteoclasts and reducing bone calcium loss. Bisphosphonates are mainly used to treat osteoporosis, but are limited in that they can only inhibit bone resorption but cannot produce bone tissue. Therefore, their long-term administration can over-inhibit bone turnover and trigger nephrotoxicity. Promoting bone formation can significantly enhance bone mineral density and improve the symptoms of osteoporosis. Leder (2017) reported that the recombinant human parathyroid hormone 1-34 (PTH 1-34) acts on osteoblasts to stimulate bone formation, thereby increasing bone density and improving bone structure. Its long-term use can reduce the incidence of fractures in postmenopausal osteoporosis patients. Bone mineralization, that is, bone calcification, can promote bone calcification by supplementing calcium preparations or vitamin D drugs to increase blood calcium level. In-depth studies of fragility fractures caused by postmenopausal osteoporosis have yielded a new treatment strategy: vitamin D combined with injectable zoledronic acid. The latter not only has a strong inhibitory effect on osteoclasts, but also significantly increases bone density and bone mineral content. This can greatly benefit postmenopausal women suffering from osteoporosis (Mei et al., 2020). Hormone replacement therapy (HRT) has a consistent favorable effect on postmenopausal osteoporosis, while it carries increased risks of cardiovascular events, thromboembolic disease, stroke, and breast cancer (Rozenberg et al., 2020). As a selective estrogen receptor modulator (SERM), raloxifene can bind tightly to estrogen receptors (ERs), showing the function of estrogen agonist to prevent bone loss and reduce fractures (Ma et al., 2021). Denosumab is a fully human monoclonal antibody that blocks the RANKL binding to RANK, thus inhibiting the activity of osteoclasts and decreasing bone resorption (Kobayakawa et al., 2021). Other than bisphosphonates and denosumab, odanacatib (ODN) is a cathepsin K (CatK) inhibitor that decreases bone resorption with only a transient reduction in serum procollagen type 1 N-terminal propeptide (Papapoulos et al., 2021).
Table 1.
Clinical treatment strategies of postmenopausal osteoporosis
| Treatment method | Therapies | Mechanism | Reference |
|---|---|---|---|
| Western medicine treatments | Recombinant human PTH 1-34: teriparatide | Binds to the PTH receptor of osteoblasts and stimulates osteoblast proliferation and bone formation | Leder, 2017 |
| Bisphosphonates: zoledronic acid | Inhibits the farnesyl diphosphate synthase pathway and promotes osteoclast apoptosis | Mei et al., 2020 | |
| HRT: estradiol | Promotes the Wnt/β-catenin signaling pathway to stimulate osteoblast proliferation and reduce bone resorption by inhibiting the NF-κB signaling pathway | Rozenberg et al., 2020 | |
| SERM: raloxifene | Binds tightly to ER as an estrogen agonist | Ma et al., 2021 | |
| Denosumab | Blocks the RANKL binding to RANK, thus inhibiting the development and activity of osteoclasts | Kobayakawa et al., 2021 | |
| Odanacatib | Inhibits CatK and decreases bone resorption | Papapoulos et al., 2021 | |
| Hepcidin | Regulates iron homeostasis and plays a role in binding to membrane iron transporters on the cell membrane to reduce iron levels | Huang, 2015; Zhang P et al., 2018; Ginzburg, 2019; Camaschella et al., 2020 | |
| Chinese traditional medicine treatments | Icariin | Regulates many signaling pathways, such as anti-osteoporosis, osteogenesis, anti-osteoclast, cartilage formation, angiogenesis, and anti-inflammation | Zhang et al., 2008; Jing et al., 2019; He et al., 2020 |
| APS | Exerts the same estrogen-like effect as icariin | Huo and Sun, 2016; Yang et al., 2016; Ou et al., 2019 | |
| Acupuncture | Upregulates the levels of serum growth hormone and IGF-1 | Chen et al., 2022 |
PTH: parathyroid hormone; HRT: hormone replacement therapy; NF-κB: nuclear factor-κB; SERM: selective estrogen receptor modulator; ER: estrogen receptor; RANK: receptor activator of NF-κB; RANKL: RANK ligand; CatK: cathepsin K; APS: Astragalus polysaccharide; IGF-1: insulin-like growth factor-1.
According to Chinese traditional medicine, kidney deficiency, spleen deficiency, and blood stasis are the main culprits hindering the balance of bone metabolism (Liang et al., 2020). Therefore, the basic principle of Chinese traditional medicine treatment is tonifying kidney essence, invigorating the spleen and stomach, soothing the liver, nourishing the blood, promoting blood circulation, and removing blood stasis. At the same time, acupuncture (Chen et al., 2022), Chinese medicine ironing (Reyan bag), exercise, and other ancillary treatments are used to improve postmenopausal osteoporosis.
5.1. Potential value of reducing the iron level in the clinical treatment of osteoporosis
As introduced above, hepcidin is an endogenous polypeptide hormone, which is an essential signal peptide for iron homeostasis regulation and plays a role in binding to membrane iron transporters on the cell membrane. Zhang P et al. (2018) reported that hepcidin could prevent osteoporosis by reducing iron levels in the body. Huang (2015) first proposed the patent application of “ferritin for the treatment of peri-menopausal and postmenopausal women with osteoporosis.” Ginzburg (2019) established a direct link among iron, iron overload, and osteoporosis, and showed that upregulation of hepcidin can be used as an alternative treatment strategy for osteoporosis. Camaschella et al. (2020) proposed that hepcidin has clinical application value in β-thalassemia, which is mainly caused by iron overload.
Osteoporosis is a systemic disease characterized by reduced bone mass and the microstructural destruction of bone tissue, resulting in an increased risk of bone fragility and fracture. Postmenopausal women are four times more likely to develop osteoporosis than men. On the one hand, this is due to estrogen deficiency after menopause; on the other hand, high iron level is a risk factor of postmenopausal osteoporosis in postmenopausal women. Therefore, reducing iron levels is a promising strategy to prevent and treat osteoporosis.
5.2. Positive effect of icariin on improving postmenopausal osteoporosis
According to the Compendium of Materia Medica, icariin exerts the effect of “reinforcing the essence, strengthening muscles and bones as well as strengthening the heart.” It is currently considered as a natural herb for the treatment of osteoporosis. As a flavonoid, icariin can mimic the effects of estrogen and stimulate the proliferation and differentiation of osteoblasts by binding to ERs.
Icariin can increase the activity of alkaline phosphatase and promote bone regeneration by inducing the expression of Runx2 and the production of BMP-4, and activating BMP signals (Zhang et al., 2008). Jing et al. (2019) confirmed that icariin can prevent osteoporosis caused by iron overload by inhibiting oxidative stress. In 2020, it was proposed that icariin has the potential to induce bone fracture healing by participating in the regulation of a variety of signaling pathways, such as anti-osteoporosis, osteogenesis, anti-osteoclast, cartilage formation, angiogenesis, and anti-inflammation (He et al., 2020). The above data indicate that icariin can be used as a natural development drug to treat osteoporosis.
5.3. Astragalus polysaccharides as potential candidates for the prevention and treatment of postmenopausal osteoporosis
Astragalus polysaccharide (APS) is the main active ingredient of the traditional Chinese medicine Astragalus, which has the same estrogen-like effect as icariin. In recent years, it has been found that APS can attenuate iron overload-induced MSC dysfunction by inhibiting ROS production by mitochondria (Huo and Sun, 2016; Yang et al., 2016). The mouse model induced by ovariectomy (OVX) showed that APS had great anti-osteoporotic activity and could significantly increase bone mineral density, which was achieved by regulating the forkhead box O3 (FoxO3a)/Wnt signaling pathway (Ou et al., 2019). These findings suggest that APS may be a potential strategy for the prevention and treatment of postmenopausal osteoporosis.
6. Conclusions and prospects
Osteoporosis, known as the “invisible killer” in postmenopausal women, has no obvious early symptoms. Iron accumulation is not only a risk factor of postmenopausal osteoporosis in elderly women, but also affects the treatment outcomes of osteoporosis, although the relevant mechanism remains to be further investigated. Therefore, in postmenopausal women with osteoporosis, studies on the phenomenon of iron accumulation and its influence on abnormal bone metabolism are of great value for the clinical treatment of postmenopausal osteoporosis.
Acknowledgments
This work was supported by the Outstanding Youth Science Fund Project of the First Affiliated Hospital of Bengbu Medical College (No. 2021byyfyyq04), China.
Funding Statement
This work was supported by the Outstanding Youth Science Fund Project of the First Affiliated Hospital of Bengbu Medical College (No. 2021byyfyyq04), China.
Author contributions
Hui CAI and Huimei ZHANG prepared the manuscript. Heng ZHANG drew the figure and table. Weiting HE completed literature collection. Heng ZHANG provided the proofreading of the manuscript and revised the article. All authors have read and approved the final version.
Compliance with ethics guidelines
Hui CAI, Huimei ZHANG, Weiting HE, and Heng ZHANG declare that they have no conflicts of interest.
This review does not contain any studies with human or animal subjects performed by any of the authors.
References
- Anthamatten A, Parish A, 2019. Clinical update on osteoporosis. J Midwifery Womens Health, 64(3): 265-275. 10.1111/jmwh.12954 [DOI] [PubMed] [Google Scholar]
- Balogh E, Tolnai E, Nagy B, et al. , 2016. Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim Biophys Acta (BBA) Mol Basis Dis, 1862(9): 1640-1649. 10.1016/j.bbadis.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Balogh E, Paragh G, Jeney V, 2018. Influence of iron on bone homeostasis. Pharmaceuticals, 11(4): 107. 10.3390/ph11040107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camaschella C, Nai A, Silvestri L, 2020. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica, 105(2): 260-272. 10.3324/haematol.2019.232124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-López N, Martínez-Arias L, Fernández-Villabrille S, et al. , 2021. Role of the RANK/RANKL/OPG and Wnt/β-catenin systems in CKD bone and cardiovascular disorders. Calcif Tissue Int, 108(4): 439-451. 10.1007/s00223-020-00803-2 [DOI] [PubMed] [Google Scholar]
- Che JM, Lv HH, Yang JC, et al. , 2021. Iron overload induces apoptosis of osteoblast cells via eliciting ER stress-mediated mitochondrial dysfunction and p-eIF2α/ATF4/CHOP pathway in vitro. Cell Signal, 84: 110024. 10.1016/j.cellsig.2021.110024 [DOI] [PubMed] [Google Scholar]
- Chen B, Li GF, Shen Y, et al. , 2015. Reducing iron accumulation: a potential approach for the prevention and treatment of postmenopausal osteoporosis. Exp Ther Med, 10(1): 7-11. 10.3892/etm.2015.2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Lin LM, Wang XD, et al. , 2022. Effect of Lingnan Chen’s acupuncture on postmenopausal osteoporosis and serum GH and IGF-1. Chin Acupunct Moxibust, 42(9): 979-984 (in Chinese). 10.13703/j.0255-2930.20211010-0001 [DOI] [PubMed] [Google Scholar]
- Cheung WH, Sun MH, Zheng YP, et al. , 2012. Stimulated angiogenesis for fracture healing augmented by low-magnitude, high-frequency vibration in a rat model-evaluation of pulsed-wave Doppler, 3-D power Doppler ultrasonography and micro-CT microangiography. Ultrasound Med Biol, 38(12): 2120-2129. 10.1016/j.ultrasmedbio.2012.07.025 [DOI] [PubMed] [Google Scholar]
- Diomede F, Marconi GD, Fonticoli L, et al. , 2020. Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int J Mol Sci, 21(9): 3242. 10.3390/ijms21093242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyard M, Fatih N, Monnier A, et al. , 2012. Iron excess limits HHIPL-2 gene expression and decreases osteoblastic activity in human MG-63 cells. Osteoporos Int, 23(10): 2435-2445. 10.1007/s00198-011-1871-z [DOI] [PubMed] [Google Scholar]
- Erber L, Liu S, Gong Y, et al. , 2022. Quantitative proteome and transcriptome dynamics analysis reveals iron deficiency response networks and signature in neuronal cells. Molecules, 27(2): 484. 10.3390/molecules27020484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LH, Li J, Yu ZF, et al. , 2014. The hypoxia-inducible factor pathway, prolyl hydroxylase domain protein inhibitors, and their roles in bone repair and regeneration. Biomed Res Int, 2014: 239356. 10.1155/2014/239356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler J, Röderer G, Günther KP, et al. , 2002. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem, 87(3): 305-312. 10.1002/jcb.10309 [DOI] [PubMed] [Google Scholar]
- Filipowska J, Tomaszewski KA, Niedźwiedzki Ł, et al. , 2017. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis, 20(3): 291-302. 10.1007/s10456-017-9541-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung EB, Harmatz PR, Milet M, et al. , 2008. Fracture prevalence and relationship to endocrinopathy in iron overloaded patients with sickle cell disease and thalassemia. Bone, 43(1): 162-168. 10.1016/j.bone.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney-Stomberg E, 2019. The impact of trace minerals on bone metabolism. Biol Trace Elem Res, 188: 26-34. 10.1007/s12011-018-1583-8 [DOI] [PubMed] [Google Scholar]
- Ginzburg YZ, 2019. Hepcidin-ferroportin axis in health and disease. Vitam Horm, 110: 17-45. 10.1016/bs.vh.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad M, Bajbouj K, Taneera J, 2020. The case for an estrogen-iron axis in health and disease. Exp Clin Endocrinol Diabetes, 128(4): 270-277. 10.1055/a-0885-1677 [DOI] [PubMed] [Google Scholar]
- Hang HF, Dong LJ, Tang XB, et al. , 2020. Bone microthrombus promotes bone loss in iron accumulation rats. Curr Med Sci, 40(5): 943-950. 10.1007/s11596-020-2251-8 [DOI] [PubMed] [Google Scholar]
- He CY, Wang Z, Shi JS, 2020. Pharmacological effects of icariin. Adv Pharmacol, 87: 179-203. 10.1016/bs.apha.2019.10.004 [DOI] [PubMed] [Google Scholar]
- Hsu CC, Senussi NH, Fertrin KY, et al. , 2022. Iron overload disorders. Hepatol Commun, 6(8): 1842-1854. 10.1002/hep4.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, 2015. Treatment of osteoporosis in peri-and post-menopausal women with hepcidin. US Patent 8999935. [Google Scholar]
- Huo J, Sun X, 2016. Effect of Astragalus polysaccharides on ovariectomy-induced osteoporosis in mice. Genet Mol Res, 15(4): gmr15049169. 10.4238/gmr15049169 [DOI] [PubMed] [Google Scholar]
- Jandial R, Chen MY, Ciacci J, 2011. HIF-1α potentiates mesenchymal stem cell mediated osteogenesis by coupling to angiogenesis. Neurosurgery, 69(4): N13-N14. 10.1227/01.neu.0000405591.47966.a1 [DOI] [PubMed] [Google Scholar]
- Jian JL, Pelle E, Huang X, 2009. Iron and menopause: does increased iron affect the health of postmenopausal women? Antioxid Redox Signal, 11(12): 2939-2943. 10.1089/ars.2009.2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Chen B, Yan YL, et al. , 2019. Hepcidin protects against iron overload-induced inhibition of bone formation in zebrafish. Fish Physiol Biochem, 45(1): 365-374. 10.1007/s10695-018-0568-z [DOI] [PubMed] [Google Scholar]
- Jing XZ, Du T, Chen K, et al. , 2019. Icariin protects against iron overload-induced bone loss via suppressing oxidative stress. J Cell Physiol, 234(7): 10123-10137. 10.1002/jcp.27678 [DOI] [PubMed] [Google Scholar]
- Jorgensen C, Khoury M, 2021. Musculoskeletal progenitor/stromal cell-derived mitochondria modulate cell differentiation and therapeutical function. Front Immunol, 12: 606781. 10.3389/fimmu.2021.606781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke JY, Cen WJ, Zhou XZ, et al. , 2017. Iron overload induces apoptosis of murine preosteoblast cells via ROS and inhibition of AKT pathway. Oral Dis, 23(6): 784-794. 10.1111/odi.12662 [DOI] [PubMed] [Google Scholar]
- Kim BJ, Lee SH, Koh JM, et al. , 2013. The association between higher serum ferritin level and lower bone mineral density is prominent in women ≥45 years of age (KNHANES 2008-2010). Osteoporos Int, 24(10): 2627-2637. 10.1007/s00198-013-2363-0 [DOI] [PubMed] [Google Scholar]
- Kir D, Saluja M, Modi S, et al. , 2016. Cell-permeable iron inhibits vascular endothelial growth factor receptor-2 signaling and tumor angiogenesis. Oncotarget, 7(40): 65348-65363. 10.18632/oncotarget.11689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayakawa T, Miyazaki A, Saito M, et al. , 2021. Denosumab versus romosozumab for postmenopausal osteoporosis treatment. Sci Rep, 11: 11801. 10.1038/s41598-021-91248-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama J, Kaito T, 2020. Osteoclast multinucleation: review of current literature. Int J Mol Sci, 21(16): 5685. 10.3390/ijms21165685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, et al. , 1997. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell, 89(5): 755-764. 10.1016/s0092-8674(00)80258-5 [DOI] [PubMed] [Google Scholar]
- Kusumbe AP, Ramasamy SK, Adams RH, 2014. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature, 507(7492): 323-328. 10.1038/nature13145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder BZ, 2017. Parathyroid hormone and parathyroid hormone-related protein analogs in osteoporosis therapy. Curr Osteoporos Rep, 15(2): 110-119. 10.1007/s11914-017-0353-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang WQ, Zhong C, Li YM, 2020. Overview of etiology and pathogenesis and advance in the treatment of osteoporosis in Chinese medicine. Chin J Osteoporos, 26(1): 135-139 (in Chinese). 10.3969/j.issn.1006-7108.2020.01.028 [DOI] [Google Scholar]
- Liu F, Zhang WL, Meng HZ, et al. , 2017. Regulation of DMT1 on autophagy and apoptosis in osteoblast. Int J Med Sci, 14(3): 275-283. 10.7150/ijms.17860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wang YW, Chen WD, et al. , 2021. Iron accumulation regulates osteoblast apoptosis through lncRNA XIST/miR-758-3p/caspase 3 axis leading to osteoporosis. IUBMB Life, 73(2): 432-443. 10.1002/iub.2440 [DOI] [PubMed] [Google Scholar]
- Liu LL, Liu GW, Liu H, et al. , 2021. Iron accumulation deteriorated bone loss in estrogen-deficient rats. J Orthop Surg Res, 16: 525. 10.1186/s13018-021-02663-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HD, Lian LY, Shi DH, et al. , 2015. Hepcidin promotes osteogenic differentiation through the bone morphogenetic protein 2/small mothers against decapentaplegic and mitogen-activated protein kinase/P38 signaling pathways in mesenchymal stem cells. Mol Med Rep, 11(1): 143-150. 10.3892/mmr.2014.2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HY, Chen S, Lu LL, et al. , 2021. Raloxifene in the treatment of osteoporosis in postmenopausal women with end-stage renal disease: a systematic review and meta-analysis. Horm Metab Res, 53(11): 730-737. 10.1055/a-1655-4362 [DOI] [PubMed] [Google Scholar]
- Mei M, Xiang ZJ, Yang JH, et al. , 2020. Efficacy of zoledronic acid for prevention of bone loss in early-stage breast cancer patients receiving adjuvant therapy: a meta-analysis of 13 randomized controlled trials. Curr Probl Cancer, 44(2): 100507. 10.1016/j.currproblcancer.2019.100507 [DOI] [PubMed] [Google Scholar]
- Nemeth E, Ganz T, 2021. Hepcidin-ferroportin interaction controls systemic iron homeostasis. Int J Mol Sci, 22(12): 6493. 10.3390/ijms22126493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, et al. , 1997. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell, 89(5): 765-771. 10.1016/s0092-8674(00)80259-7 [DOI] [PubMed] [Google Scholar]
- Ou L, Wei PF, Li M, et al. , 2019. Inhibitory effect of Astragalus polysaccharide on osteoporosis in ovariectomized rats by regulating FoxO3a /Wnt signaling pathway. Acta Cir Bras, 34(5): e201900502. 10.1590/s0102-865020190050000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapoulos S, Bone H, Cosman F, et al. , 2021. Incidence of hip and subtrochanteric/femoral shaft fractures in postmenopausal women with osteoporosis in the phase 3 long-term odanacatib fracture trial. J Bone Miner Res, 36(7): 1225-1234. 10.1002/jbmr.4284 [DOI] [PubMed] [Google Scholar]
- Roodman GD, 2009. Osteoclasts pump iron. Cell Metab, 9(5): 405-406. 10.1016/j.cmet.2009.04.005 [DOI] [PubMed] [Google Scholar]
- Rozenberg S, Al-Daghri N, Aubertin-Leheudre M, et al. , 2020. Is there a role for menopausal hormone therapy in the management of postmenopausal osteoporosis? Osteoporos Int, 31(12): 2271-2286. 10.1007/s00198-020-05497-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, et al. , 1997. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell, 89(2): 309-319. 10.1016/s0092-8674(00)80209-3 [DOI] [PubMed] [Google Scholar]
- Tang Y, Wu XW, Lei WQ, et al. , 2009. TGF-β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med, 15(7): 757-765. 10.1038/nm.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Wu SL, Dai ZP, et al. , 2016. Iron overload induced death of osteoblasts in vitro: involvement of the mitochondrial apoptotic pathway. PeerJ, 4: e2611. 10.7717/peerj.2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa N, Koide M, Nakamura M, et al. , 2021. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab, 39(1): 19-26. 10.1007/s00774-020-01162-6 [DOI] [PubMed] [Google Scholar]
- Wang AF, Xu YJ, 2022. Influence of iron accumulation on postmenopausal osteoporosis. Chin J Osteoporos Bone Miner Res, 15(3): 225-231 (in Chinese). 10.3969/j.issn.1674-2591.2022.03.001 [DOI] [Google Scholar]
- Wang L, Zhou F, Zhang P, et al. , 2017. Human type H vessels are a sensitive biomarker of bone mass. Cell Death Dis, 8(5): e2760. 10.1038/cddis.2017.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen B, Sun JY, et al. , 2018. Iron-induced oxidative stress stimulates osteoclast differentiation via NF-κB signaling pathway in mouse model. Metabolism, 83: 167-176. 10.1016/j.metabol.2018.01.005 [DOI] [PubMed] [Google Scholar]
- Xu GP, Li X, Zhu ZY, et al. , 2021. Iron overload induces apoptosis and cytoprotective autophagy regulated by ROS generation in Mc3t3-E1 cells. Biol Trace Elem Res, 199(10): 3781-3792. 10.1007/s12011-020-02508-x [DOI] [PubMed] [Google Scholar]
- Xu YJ, Li GF, Du BC, et al. , 2011. Hepcidin increases intracellular Ca2+ of osteoblast hFOB1.19 through L-type Ca2+ channels. Regul Pept, 172(1-3): 58-61. 10.1016/j.regpep.2011.08.009 [DOI] [PubMed] [Google Scholar]
- Yang F, Yan GG, Li Y, et al. , 2016. Astragalus polysaccharide attenuated iron overload-induced dysfunction of mesenchymal stem cells via suppressing mitochondrial ROS. Cell Physiol Biochem, 39(4): 1369-1379. 10.1159/000447841 [DOI] [PubMed] [Google Scholar]
- Yang Q, Jian JL, Abramson SB, et al. , 2011. Inhibitory effects of iron on bone morphogenetic protein 2-induced osteoblastogenesis. J Bone Miner Res, 26(6): 1188-1196. 10.1002/jbmr.337 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Xu F, Cao Y, et al. , 2019. Iron accumulation leads to bone loss by inducing mesenchymal stem cell apoptosis through the activation of caspase3. Biol Trace Elem Res, 187(2): 434-441. 10.1007/s12011-018-1388-9 [DOI] [PubMed] [Google Scholar]
- Zarjou A, Jeney V, Arosio P, et al. , 2010. Ferritin ferroxidase activity: a potent inhibitor of osteogenesis. J Bone Miner Res, 25(1): 164-172. 10.1359/jbmr.091002 [DOI] [PubMed] [Google Scholar]
- Zhang DW, Cheng Y, Wang NL, et al. , 2008. Effects of total flavonoids and flavonol glycosides from Epimedium koreanum Nakai on the proliferation and differentiation of primary osteoblasts. Phytomedicine, 15(1-2): 55-61. 10.1016/j.phymed.2007.04.002 [DOI] [PubMed] [Google Scholar]
- Zhang P, Wang S, Wang L, et al. , 2018. Hepcidin is an endogenous protective factor for osteoporosis by reducing iron levels. J Mol Endocrinol, 60(4): 299-308. 10.1530/jme-17-0301 [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Wang XD, Wu Q, et al. , 2018. Adenine alleviates iron overload by cAMP/PKA mediated hepatic hepcidin in mice. J Cell Physiol, 233(9): 7268-7278. 10.1002/jcp.26559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang HF, Wang PW, Li YZ, et al. , 2020. Analysis of related factors of brittle hip fracture in postmenopausal women with osteoporosis. Orthop Surg, 12(1): 194-198. 10.1111/os.12605 [DOI] [PMC free article] [PubMed] [Google Scholar]