Abstract
Background:
Repetitive blast-related mild traumatic brain injury (mTBI) caused by exposure to high explosives is increasingly common among warfighters as well as civilians. While women have been serving in military positions with increased risk of blast exposure since 2016, there are few published reports examining sex as a biological variable in models of blast mTBI, greatly limiting diagnosis and treatment capabilities. As such, here we examined outcomes of repetitive blast trauma in female and male mice in relation to potential behavioral, inflammatory, microbiome, and vascular dysfunction at multiple timepoints.
Methods:
In this study we utilized a well-established blast overpressure model to induce repetitive (3x) blast-mTBI in both female and male mice. Acutely following repetitive exposure, we measured serum and brain cytokine levels, blood-brain barrier (BBB) disruption, fecal microbial abundance, and locomotion and anxiety-like behavior in the open field assay. At the one-month timepoint, in female and male mice we assessed behavioral correlates of mTBI and PTSD-related symptoms commonly reported by Veterans with a history of blast-mTBI using the elevated zero maze, acoustic startle, and conditioned odorant aversion paradigms.
Results:
Repetitive blast exposure resulted in both similar (e.g., increased IL-6), and disparate (e.g., IL-10 increase only in females) patterns of acute serum and brain cytokine as well as gut microbiome changes in female and male mice. Acute BBB disruption following repetitive blast exposure was apparent in both sexes. While female and male blast mice both exhibited acute locomotor and anxiety-like deficits in the open field assay, only male mice exhibited adverse behavioral outcomes that lasted at least one-month.
Discussion:
Representing a novel survey of potential sex differences following repetitive blast trauma, our results demonstrate unique similar yet divergent patterns of blast-induced dysfunction in female vs. male mice and highlight novel targets for future diagnosis and therapeutic development.
Keywords: mild traumatic brain injury, sex as a biological variable, neuroinflammation, microbiome, blood brain barrier, blast overpressure, posttraumatic stress disorder
INTRODUCTION
Traumatic brain injury (TBI) is currently a leading cause of death and disability not just in the United States but globally (Johnson & Griswold, 2017; Maas et al., 2017; Taylor, 2017). Affecting every segment of the population, TBI is often associated with significantly decreased quality of life and increased financial burden (Di Battista et al., 2012; Malec et al., 2017; Ozga et al., 2018; Taylor, 2017). Post-TBI symptomatology can be highly variable and comorbid with other diagnoses such as posttraumatic stress disorder (PTSD) and chronic pain, resulting in limited diagnosis and treatment capabilities. The vast majority of preclinical TBI research has focused only on male research animals, which may express different symptom trajectories than female animals, resulting in a critical knowledge gap in the field at a time when women are at increasingly high risk for repetitive TBI exposure (Giordano et al., 2020; Gupte et al., 2019; McCabe & Tucker, 2020; Späni et al., 2018).
Blast overpressure (BOP) waves, such as those caused by improvised explosive devices (IEDs) and industrial accidents, are becoming an increasingly common cause of TBI. Referred to as the “signature injury” of the conflicts in Iraq/Afghanistan (OEF/OIF/OND), repetitive blast exposure is the primary source of mTBI in warfighters, a significant driver of comorbid PTSD, and a major source of morbidity among Veterans enrolled in the VA health care system (Adamson et al., 2008; Hendrickson et al., 2018; O’Neil et al., 2013; Owens et al., 2008; Wenger et al., 2018). In these conflicts, an estimated 75% of all mTBI reported by Servicemembers are a result of blast exposure caused by detonation of high explosives (Adamson et al., 2008; Owens et al., 2008). Furthermore, multiple deployments are common (2.77 million Servicemembers have served on 5.4 million deployments since 2011), resulting in a high potential for repetitive blast exposures (Agoston, 2017; Carr et al., 2016; Ravula et al., 2022; Simmons et al., 2020). Currently, there is a lack of research on how these injuries may differentially impact people identifying as male vs. female, yet females currently represent ~15% of active duty Servicemembers and ~20% of the United States Reserve and Guard (Dye et al., 2016; Iverson et al., 2011; Kamarck, 2015; Street et al., 2013).
Indeed, few preclinical studies have included both male and female animals and the few that have, have exclusively focused on impact TBI, a single TBI exposure, and/or only examined acute timepoints. Models of single moderate to severe impact TBI have demonstrated disparate inflammatory outcomes in male vs. female mice acutely following injury (Bromberg et al., 2020; Doran et al., 2019; Krukowski, 2021; Krukowski et al., 2020; Späni et al., 2018; Villapol et al., 2017). Likewise, a handful of reports have characterized potential sex differences following a single blast exposure (Hubbard et al., 2022; Kawa et al., 2020; McNamara et al., 2022; Russell, Handa, et al., 2018; Russell, Richardson, et al., 2018). Hubbard et al (2022) found increased anxiety-like behavior in male but not female rats in the open field and elevated plus maze following single blast mTBI with body shielding, whereas McNamara et al (2022) found no injury effects in either female or male mice on the elevated plus and zero mazes and Russell et al (2018) found increased anxiety-like behavior in male, and to a lesser extent in females. Critically, very little is known about the effects of repetitive blast exposure in male vs. female rodents.
Given the lack of preclinical research using models of repetitive blast exposure in female rodents, this study was envisioned as a novel survey of adverse outcomes commonly seen following repetitive blast trauma in warfighters, with a specific focus on delineating time-dependent outcomes related to inflammatory, blood-brain barrier (BBB), microbiome, and behavioral pathology. The ‘microbiota-gut-brain-axis’ (MGBA) represents a critical bidirectional communication system between the gut and the brain, involving neural, immune, and endocrine pathways critical for brain health and response to stress and trauma (Cryan et al., 2019; Moloney et al., 2014; Thursby & Juge, 2017; Wiley et al., 2017). While both cytokine and microbiome changes have been reported following impact TBI, the effects of blast (a whole-body injury capable of damaging the gut) on the gut microbiome and how these changes might related to more chronic behavioral outcomes has not been examined in male or female mice. Together, our results highlight new potential targets for diagnosis and treatment development and confirm the need for increased research dedicated to understanding how repetitive blast trauma affects diverse populations.
MATERIALS AND METHODS
Animals
All experiments utilized female and male (as determined by genital appearance at weaning) C57Bl/6J mice aged 9–11 weeks old at time of arrival to VA Puget Sound. All mice were at least 11 weeks of age at the time of sham/blast exposure. Mice were housed one to three per cage by sex on a 12:12 light:dark cycle (lights on at 06:00), and were given ad libitum food and water. Less than 5% of mice in the study were single housed (resulting from animals needing to be separated due to fighting or animal loss from blast exposure) and single housed mice did not significantly differ from group housed mice on experimental outcomes. Room temperature was set to 21 degrees C; room humidity was set to 35%. All animal experiments were carried out in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines and were approved by the VA Puget Sound Institutional Animal Care and Use Committees. Mice were acclimated to the housing room for a week following arrival and subsequently handled for an additional week prior to sham or blast exposure. Three experimental timelines were employed (Figure 1a) in separate sets of mice. To increase rigor and reproducibility, each experimental timeline included at least two cohorts of mice each run at separate times.
Figure 1.
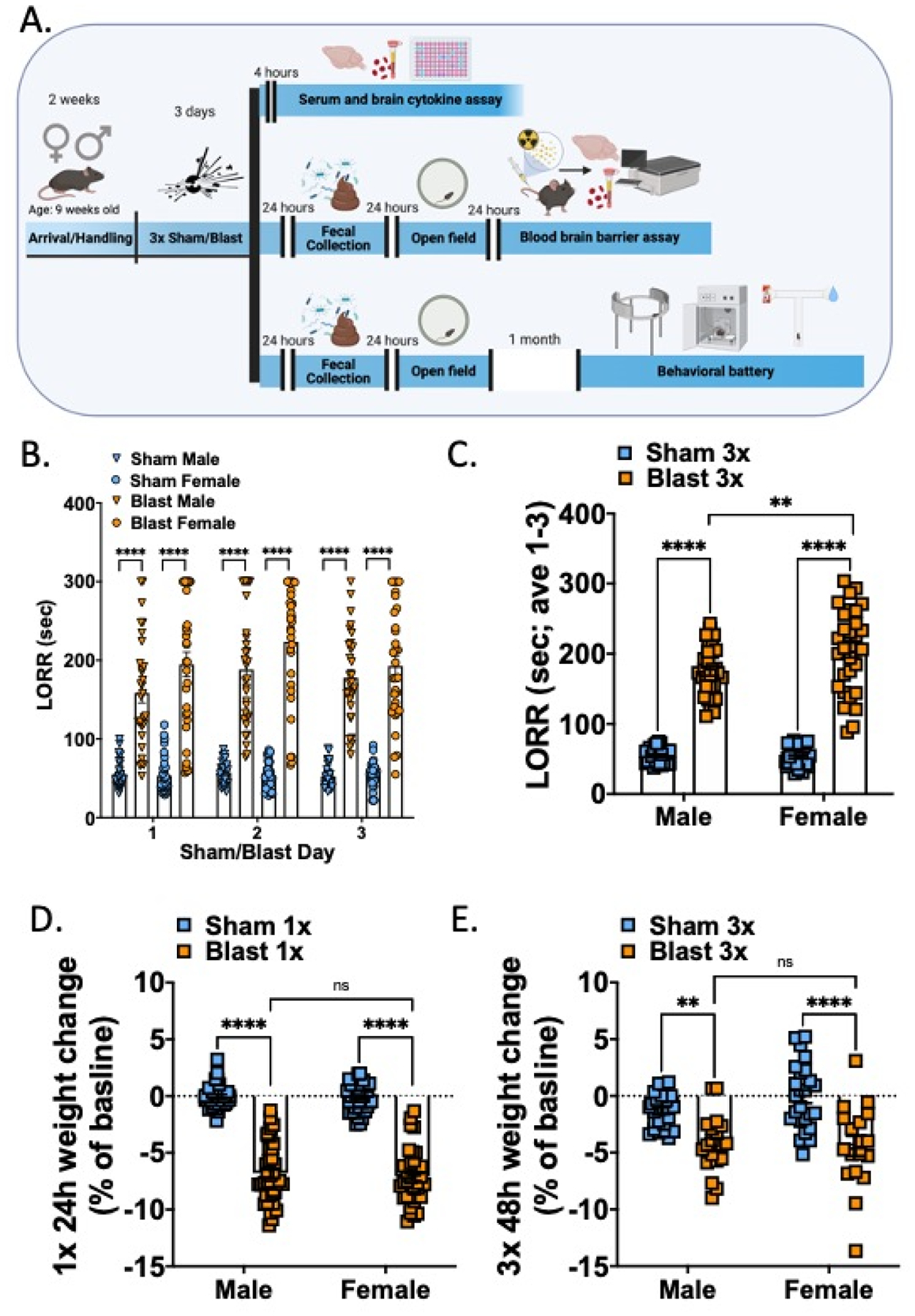
Female and male mice both display increased LORR and weight loss acutely following repetitive blast exposure. (A) Experimental timelines. Three groups of female and male mice were used. (B) LORR is increased acutely following each blast exposure in female and male mice. (C) Mean LORR across days 1–3 of blast exposure is increased in female and male mice and female blast mice are significantly higher than male blast mice. (D) Both female and male mice lose weight to a similar extent 24 hours following a single blast exposure. (E). Both female and male mice lose weight to a similar extent 48 hours following final repetitive (3x) blast exposure. n=19–26 per group. Two-way ANOVA, Šídák’s multiple comparison post-hoc. **p ≤ 0.01, ****p ≤ 0.0001, ns = not significant. Values represent mean ± SEM.
Model of Blast Trauma
The shock tube (Baker Engineering and Risk Consultants, San Antonio, TX) was designed to generate blast overpressures that mimic open field high explosive detonations encountered by military Servicemembers in combat. Design and modeling characteristics have been described in detail elsewhere (Baskin et al., 2021; Huber et al., 2013; Logsdon et al., 2020; Meabon et al., 2016; Schindler et al., 2017; Schindler, Terry, et al., 2021; Schindler, Baskin, et al., 2021). Briefly, mice were weighed and then anesthetized with isoflurane (induced at 5% and maintained at 2–3% for the duration of the blast), secured against a gurney, and placed into the shock tube oriented perpendicular to the oncoming blast wave (ventral body surface towards the blast). Utilizing condensed helium, pressurized air is built up on one end of the tube and released in a way that creates a blast overpressure wave that induces neuropathological and behavioral changes in line with blast mild traumatic brain injuries (mTBI) in warfighters (Ghai et al., 2020; Meabon et al., 2020; Peskind et al., 2011; Petrie et al., 2014; Schindler, Baskin, et al., 2021; Schindler et al., 2017; Wilkinson et al., 2012). Sham (control) animals received anesthesia only for a duration matched to blast animals. Repeated blast/sham exposures occurred successively over the course of three days (one per day). The blast overpressure (BOP) peak intensity (psi), initial pulse duration (ms), and impulse (psi▪ms) used were in keeping with mild blast injury (19.1 +/− 0.09 psi). Under these experimental conditions, the overall survival rate exceeded 97%, with blast-exposed mice presenting comparable to sham-exposed mice by inspection 2–4 hours-post blast exposure as previously reported (Baskin et al., 2021; Huber et al., 2013, 2016; Logsdon, Meabon, et al., 2018; Logsdon et al., 2020; Meabon et al., 2016; Schindler et al., 2017; Schindler, Terry, et al., 2021; Schindler, Baskin, et al., 2021). Following sham/blast exposure and removal from isoflurane, loss of righting reflex (LORR) was recorded as the amount of time it took for animals to right themselves. Once animals were able to regain full sternal recumbency, they were weighed and then returned to their cages for recovery.
Cytokine Measurement:
Mice (n=13–15) were euthanized via cervical decapitation 4 hours after their last sham or blast exposure. Trunk blood was collected in 1.5-mL capacity serum-separator tubes, allowed to clot at room temperature for 30–40 minutes, and then centrifuged at 3,000 × g for 10 minutes. Serum was then aliquoted and stored at −80°C until analyzed. Whole brains were also collected at the time of euthanasia, hemisectioned, and flash frozen at −80°C. One hemisphere of brain tissue was then lysed in a 0.02% Triton-X homogenization buffer of 10mM HEPES, 1.5mM MgCl2, and 10mM KCl, with fresh protease/phosphatase inhibitor cocktail (Sigma) in a bead homogenizer. The samples were then centrifuged at 4°C at 18,000 × g for 10 minutes and the supernatant was aliquoted and stored at −80°C. Pro- and anti-inflammatory cytokine level were then analyzed using the IDEXX (Columbia, MO) Cytokine Mouse 25-Plex Panel and values were normalized by protein concentration quantified with a BCA Protein Assay Kit.
Fecal Microbiome:
Fresh fecal pellets were collected 24 hours following final sham or blast exposure (n=18–25). Collection occurred in the morning between 09:00 and 11:00 using sterile technique. Fecal pellets were flash frozen in liquid nitrogen and stored at −80°C until shipment to Diversigen for downstream processing and analysis. Briefly, the DNA of fecal pellets were extracted and sequenced by Diversigen Inc. (New Brighton, MN) using their BoosterShot Shallow Shotgun Sequencing. DNA sequences were aligned to a curated database containing all representative genomes in RefSeq for bacteria. Alignments were made at 97% identity against all reference genomes. Every input sequence was compared to every reference sequence in the Diversigen Venti database using fully gapped alignment with BURST. For taxonomy assignment, each input sequence was assigned the lowest common ancestor that was consistent across at least 80% of all reference sequences tied for best hit. Samples with fewer than 10,000 sequences were discarded. Operational taxonomic units (OTUs) accounting for less than one millionth of all strain-level markers and those with less than 0.01% of their unique genome regions covered (and < 0.1% of the whole genome) at the species level were discarded. The number of counts for each OTU was normalized to the OTU’s genome length.
Blood-brain barrier disruption assessment:
Following established procedures (Banks et al., 2015; Logsdon, Meabon, et al., 2018; Logsdon et al., 2020), albumin (Sigma, St. Louis MO), (~66.44 kDa), a blood-borne molecule with minimal penetration into the CNS, was labeled with 99mTc (GE Healthcare, Piscataway, NJ). A mixture of 240 mg/ml stannous tartrate and 1 mg/ml albumin was adjusted to pH 3.0 with HCl and one millicurie of 99mTc-NaOH4 was added to this mixture and incubated for 20 min. The 99mTc-albumin was purified on a column of G-10 Sephadex (GE Healthcare) in 0.1 ml fractions of phosphate buffer (0.25 M). Radioactivity in the purified 99mTc-albumin peak was more than 90% acid precipitable in an equal volume of 1% bovine plasma albumin (BSA) and trichloroacetic acid (30%). 5 × 106 cpm/mouse of purified 99mTc-albumin fraction was prepared in a final volume (0.2 ml/mouse) of lactated Ringer’s solution containing 1% BSA.
In keeping with previous experiments (Logsdon, Meabon, et al., 2018; Logsdon et al., 2020), 72 hours after the final blast/sham exposure, mice (n=8–10) were anesthetized with urethane (4 g/kg; 0.2 ml; ip), the jugular veins were exposed, and 99mTc-albumin (5 × 106 Counts per minute (cpm)) in 0.2 ml of lactated Ringer’s solution with 1% BSA was injected into the jugular vein and allowed to circulate for ten minutes. After ten minutes of circulation, the abdominal aorta was clamped with hemostats, severed, and blood collected in 1.5-mL capacity tubes containing heparin and then centrifuged at 4 degrees C at 3,200 × g for 10 minutes. Plasma was then aliquoted and stored at −80°C until analyzed. Immediately after blood collection, the vascular space of the brain was perfused with 20 ml of lactated Ringer’s solution through the left ventricle of the heart in less than 1 min, thus washing out the vascular contents of the brain so that only 99mTc-albumin which had leaked into the brain remained. The brain was then collected and flash frozen in liquid nitrogen. 99mTc radioactivity in the plasma and brain was measured by a gamma counter. Brain tissue radioactivity was calculated by dividing the cpm in the brain by the weight of the brain to yield cpm/g. Plasma radioactivity was calculated by dividing the cpm in the plasma by the microliters of plasma counted to yield cpm/microliter. The brain tissue radioactivity was then divided by the corresponding plasma radioactivity and the results given in units of microliters/gram of brain tissue.
Behavioral assays:
Acute (48 hours post): To probe potential locomotor deficits and anxiety-like behavior acutely following sham/blast exposure, mice (n=18–25) were tested in an open field assay. Mice were allowed 5 minutes to explore a large circular open space (1 meter diameter) and their movements were recorded from above and analyzed with Anymaze (Wood Dale, IL). On this test, decreases in the amount of time spent in the middle of the environment is indicative of an anxiety-like phenotype. The total distance traveled, the delay to first enter the center of the field, time spent in the center, and entries into the center were recorded and analyzed.
One-month post:
A behavioral battery consisting of three testing paradigms was conducted over 1 week (one test paradigm per day) starting one month after the last sham/blast exposure (n=12–15). The order of behavioral tests was specifically chosen to go from the least stressful to the most stressful task to prevent carryover distress from one behavior to the next and each test was separated by 1–2 days.
Elevated zero maze (EZM):
Animals were allowed to explore an elevated zero maze (Maze Engineers, Skokie, IL) for 5 min. Decreased time spent exploring the open arms is thought to reflect an anxiety-like behavior. Movement was recorded from above and analyzed using Anymaze (Stoelting, Wood Dale, IL).
Acoustic startle (AS):
In accordance with previous reports (Baskin et al., 2021) acoustic startle habituation and prepulse inhibition were measured using SR-LAB acoustic startle boxes (San Diego Instruments, San Diego, CA). Following a 5-min acclimation period, startle habituation testing consisted of 50 trials of 120-dB pulses delivered with an inter-trial interval of 7–23 s. Following a two min break period, prepulse inhibition (PPI) was next assessed and consisted of forty trials of 81-dB prepulse followed by a 120-dB pulse with varying interstimulus interval (ISI) of 2–1,000 ms (five trials each). Due to the established effects of blast exposure on hearing loss, we use these established within-subject analysis approaches of startle habituation and PPI in attempts to mitigate potential confounds of hearing loss on startle outcome measures and interpretation.
Conditioned odorant aversion (COA):
As previously described (Schindler et al., 2021), mice were first exposed to a neutral almond scent starting five minutes prior to sham/blast exposure by placing a mesh tea ball containing a quarter nestlet with 20 ul almond extract into the home cage. The tea ball and the nestlet with almond scent was refreshed daily and remained in the home cage until 24 hours after the final sham/blast exposure. To measure blast-induced aversion, a tea ball with almond extract nestlet was placed in the left arm of a tea maze and a tea ball with a clean nestlet was placed in the right arm of the t-maze. Animals were then placed in the long arm of the T-maze, equidistant away from either tea ball and given 5 min to explore the entire maze. Latency to enter and time spent in each of the two distal ends of the short arms was recorded and analyzed using Anymaze (Stoelting, Wood Dale, IL).
Analysis:
Data are expressed as means ± SEM. Differences between groups were determined using two-way (between-subjects design: sex and exposure factors) analysis of variance (ANOVA) followed by posthoc testing using Sidak’s multiple comparisons test. Microbiome bioinformatics (alpha diversity (Shannon), beta diversity (Bray Curtis), and relative abundance) were performed by Diversigen. Shannon diversity and relative abundance at the taxonomic order level were compared across all groups using Kruskal-Wallis test followed by Mann-Whitney U test for individual group by group comparison if Kruskal-Wallis was significant at false discovery rate (FDR) p<0.1. Bray Curtis dissimilarity was used to estimate beta diversity among samples and represented using PCoA plot. Analysis of similarities (ANOSIM) was used to compare beta diversity across groups (999 permutations). Spearman correlation with FDR correction (p<0.1) was used to examine associations between microbial abundance and behavioral parameters. Hierarchical clustering was performed using Ward agglomeration method. Differentially expressed taxa were identified using linear discriminant analysis effect size (LEfSe) using the Huttenhower Galaxy module (Segata et al., 2011). Reported p values denote two-tailed probabilities of p≤0.05 and non-significance (n.s.) indicates p>0.05. Statistical analysis and visualization were conducted using Graph Pad Prism 4.0 (GraphPad Software, Inc., La Jolla, CA) and with custom Python scripts.
RESULTS
Repetitive blast exposure increases loss of righting reflex and acute weight loss in both female and male mice.
Adult female and male mice were exposed to repetitive BOPs (1 BOP/day for 3 consecutive days) using a pneumatic shock tube as previously described (Huber et al., 2013; Logsdon, Meabon, et al., 2018; Schindler et al., 2017). Loss of righting reflex (LORR; the time it takes for a mouse to right itself following sedation) was measured starting immediately upon removal from isoflurane and consisted of placing the mouse on its back and waiting until it rights itself two times within 15 seconds. Following LORR, mice were weighed and then returned to their home cage to recover.
Repetitive blast exposure resulted in increased LORR in both male and female mice (Fig 1b) immediately following each sham/blast exposure (two-way RM ANOVA: main effect of exposure F(3,124) = 171.5, p<0.0001), but was not affected by sham/blast day (two-way RM ANOVA: main effect of time F(2,217) = 3.19, ns) and there was no interaction effect (two-way RM ANOVA: main effect of time F(6,248) = 1.30, ns). When examining the mean LORR across all three exposure days, there was a significant interaction effect between exposure and sex (two-way ANOVA: interaction effect F(1,124) = 6.674, p=0.01) and main effects of exposure (two-way ANOVA: main effect of exposure F(1,124) = 482.6, p<0.0001) and time (two-way ANOVA: main effect of time F(1,124) = 5.384, p=0.022). Šídák’s multiple comparison post-hoc test revealed that male blast mice (n=32) took longer to right themselves when compared to male sham mice (n=33; p<0.0001) and female blast mice (n=27) had longer LORR than both female shams (n=38; p<0.0001) and male blast mice (p<0.01).
Acute and sub-acute weight loss was also seen in both male and female blast mice (Fig 1d, e). Following one blast (Fig 1d), blast mice lost more weight (represented as a decrease in percent of their total baseline weight) than their sham controls (two-way RM ANOVA: main effect of exposure F(1,144) = 466.5, p<0.0001). There was no significant interaction effect between exposure and sex (two-way ANOVA: interaction effect F(1,144) = 0.07, ns) or main effect of sex (two-way ANOVA: main effect of sex F(1,144) = 0.346, ns). Šídák’s multiple comparison post-hoc found that both male and female blast mice lost weight when compared to their sham controls but there was no difference between male and female blast mice. When examining weight loss at 48 hours post (day of open field testing), there remained a main effect of blast (two-way ANOVA: main effect of exposure F(1,84) = 39.72, p<0.0001) and no significant interaction effect between exposure and sex (two-way ANOVA: interaction effect F(1,84) = 1.45, ns) or main effect of sex (two-way ANOVA: main effect of sex F(1,84) = 0.927, ns) (Fig 1e). Šídák’s multiple comparison post-hoc found that both male and female blast mice lost weight when compared to their sham controls but there was no difference in percent weight loss between male and female blast mice.
Repetitive blast exposure differentially affects acute cytokine changes in serum and brain of female and male mice.
To determine if acute changes in serum and brain cytokine levels were different between female and male mice following repetitive blast, we euthanized a subgroup of mice (Fig 1a) 4 hours following their final sham/blast exposure. Analysis revealed both blast and sex differences dependent on cytokine sample type (Fig 2–3). Results from two-way ANOVA (exposure, sex factors) followed by Šídák’s multiple comparisons tests for each analyze measured in appreciable concentration are reported in Tables 1 (serum) and 2 (brain).
Figure 2.
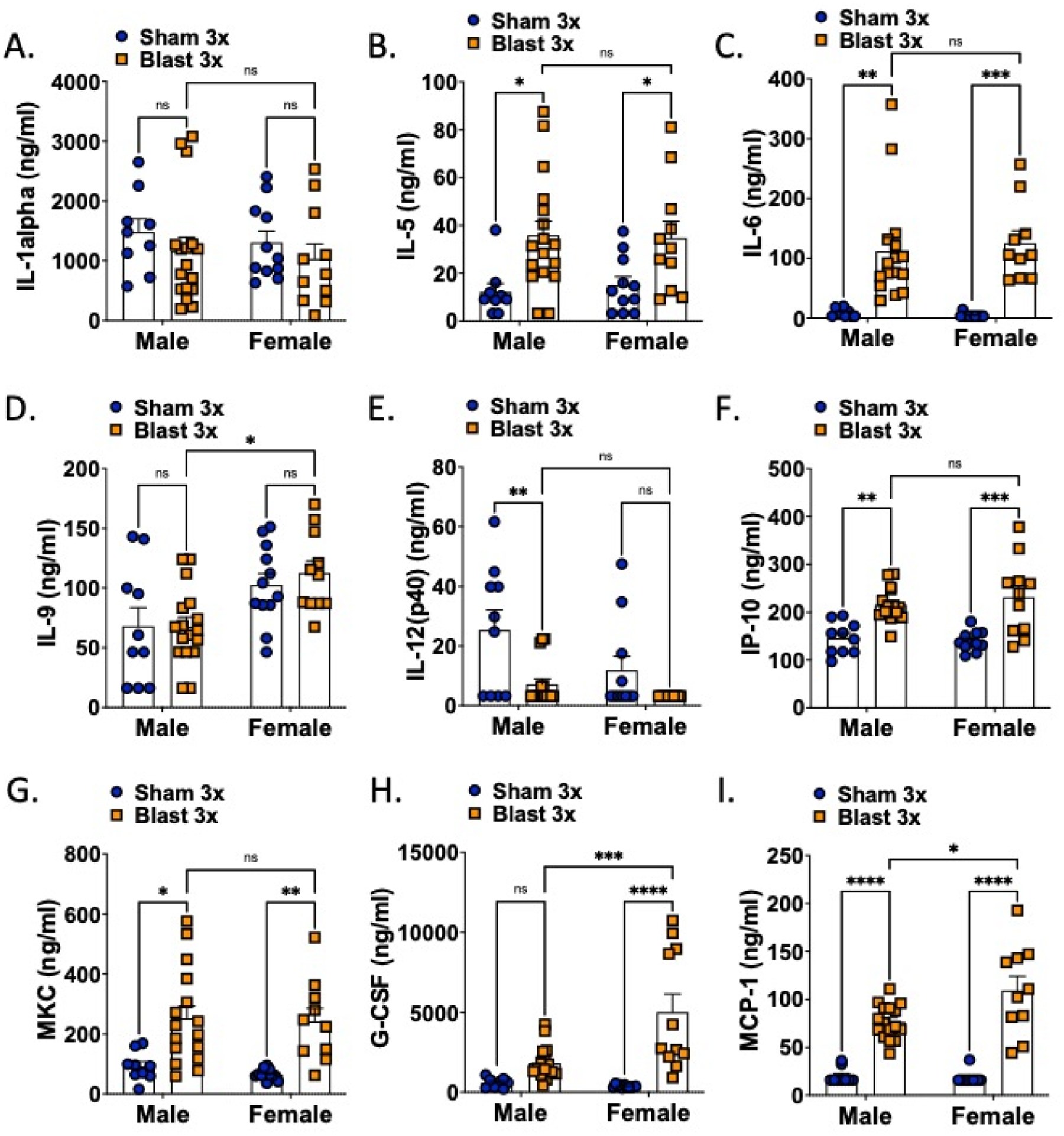
Serum cytokine levels are acutely affected by blast in both female and male mice. n=10–15. Two-way ANOVA Šídák’s multiple comparison post-hoc. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001, ns = not significant. Values represent mean ± SEM.
Figure 3.
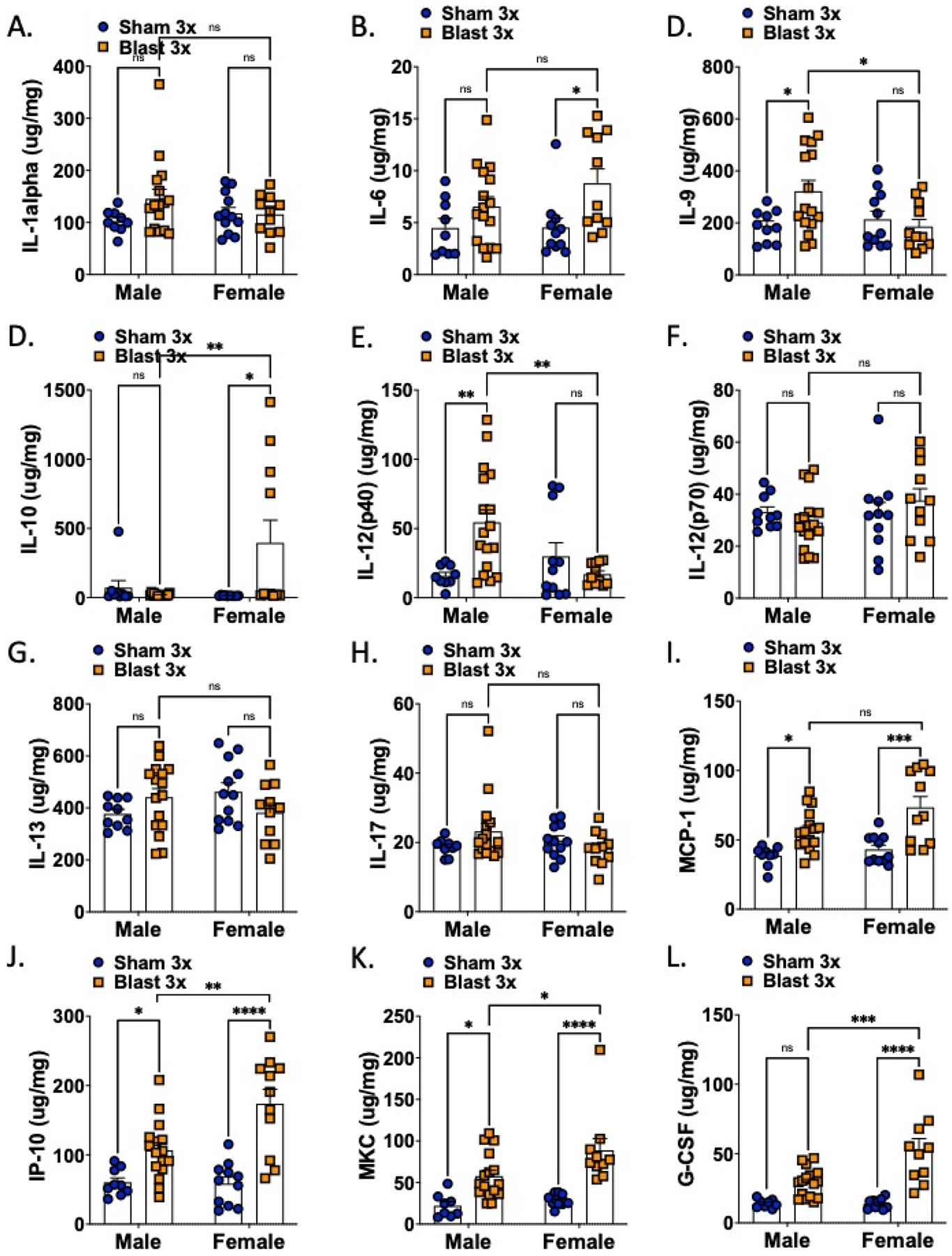
Brain cytokine levels are acutely affected by blast in both female and male mice. n=10–15. Two-way ANOVA Šídák’s multiple comparison post-hoc. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001, ns = not significant. Values represent mean ± SEM.
Table 1.
Statistical analysis of cytokine levels in blood serum four hours after the final blast. All analyses used two-way ANOVA and Šídák post-hoc analysis.
| Analyte | Interaction F(df) | Interaction P | Sex F(df) | Sex P | Exposure F(df) | Exposure P |
|---|---|---|---|---|---|---|
| IL-1α | F[1,44] = 0.008 | 0.927 | F[1,44] = 0.415 | 0.523 | F[1,44] = 1.53 | 0.223 |
| IL-5 | F[1,44] = 0.113 | 0.739 | F[1,44] = 0.019 | 0.89 | F[1,44] = 13.91 | 0.0005 |
| IL-6 | F[1,43] = 0.266 | 0.608 | F[1,43] = 0.06 | 0.807 | F[1,43] = 38.62 | <0.0001 |
| IL-9 | F[1,46] = 0.242 | 0.625 | F[1,46] = 14.28 | 0.0005 | F[1,46] = 0.218 | 0.643 |
| IL-12 (p40) | F[1,44] = 1.611 | 0.211 | F[1,44] = 5.324 | 0.026 | F[1,44] = 12.66 | 0.0009 |
| IP-10 | F[1,43] = 0.637 | 0.429 | F[1,43] = 0.116 | 0.735 | F[1,44] = 34.02 | <0.0001 |
| MKC | F[1,43] = 0.063 | 0.803 | F[1,43] = 0.289 | 0.594 | F[1,43] = 24.29 | <0.0001 |
| G-CSF | F[1,42] = 8.429 | 0.006 | F[1,42] = 6.36 | 0.016 | F[1,42] = 24.99 | <0.0001 |
| MCP-1 | F[1,42] = 5.602 | 0.023 | F[1,42] = 4.302 | 0.044 | F[1,42] = 99.65 | <0.0001 |
Table 2.
Statistical analysis of cytokine levels in brain lysate four hours after the final blast. All analyses used two-way ANOVA and Šídák post-hoc analysis.
| Analyte | Interaction F(df) | Interaction P | Sex F(df) | Sex P | Exposure F(df) | Exposure P |
|---|---|---|---|---|---|---|
| IL-1α | F[1,44] = 2.212 | 0.144 | F[1,44] = 0.249 | 0.62 | F[1,44] = 1.850 | 0.1807 |
| IL-6 | F[1,43]= 1.040 | 0.314 | F[1,43] = 1.107 | 0.299 | F[1,43] = 8.612 | 0.005 |
| IL-9 | F[1,44] = 5.247 | 0.027 | F[1,44] = 2.422 | 0.127 | F[1,44] = 2.256 | 0.14 |
| IL-10 | F[1,42] = 6.909 | 0.012 | F[1,42] = 3.38 | 0.062 | F[1,42] = 3.999 | 0.052 |
| IL-12 (p40) | F[1,44] = 9.873 | 0.003 | F[1,44]= 2.02 | 0.162 | F[1,44] = 2.48 | 0.123 |
| IL-12 (p70) | F[1,45] = 1.706 | 0.198 | F[1,45] = 1.143 | 0.291 | F[1,45] = 0.039 | 0.844 |
| IL-13 | F[1,45] = 4.977 | 0.031 | F[1,45] = 0,157 | 0.694 | F[1,45] = 0.061 | 0,805 |
| IL-17 | F[1,44] = 3.216 | 0.08 | F[1,44]= 0.619 | 0.436 | F[1,44] = 0.363 | 0.55 |
| MCP-1 | F[1,43] = 1.585 | 0.215 | F[1,43] = 4.919 | 0.032 | F[1,43] = 25.01 | <0.0001 |
| IP-10 | F[1,43] = 6.567 | 0.014 | F[1,43] = 5.877 | 0.02 | F[1,43] = 36.15 | <0.0001 |
| MKC | F[1,42] = 2.2 | 0.146 | F[1,42] = 5.502 | 0.031 | F[1,42] = 33.37 | <0.0001 |
| G-CSF | F[1,41] = 8.167 | 0.007 | F[1,41] = 7.932 | 0.007 | F[1,41] = 40.83 | <0.0001 |
In the serum (Fig 2, Table 1), there was a main effect of blast on 1L-5, IL-6, IL-12(p40), G-CSF, IP-10, MKC, and MCP-1. There was main effect of sex on IL-9, IP-12(p40), G-CSF, and MCP-1. Only G-CSF and MCP-1 showed an interaction effect. Blast male and female mice only significantly differed in two cytokines, G-CSF and MCP-1, with blast males expressing significantly lower levels than blast females. In samples taken from whole brain (Fig 3, Table 2), there was a main effect of blast on IL-6, G-CSF, IP-10, MKC, and MCP-1. There were main effects of sex on G-CSF, IP-10, MKC, and MCP-1 and an interaction effect on IL-9, IL-10, IL-12(p40), IL-13, G-CSF, and IP-10. Blast males showed higher levels of IL-9 and IL-12(p40) as compared to blast females whereas blast females showed higher levels of IL-10, G-CSF, IP-10, and MKC as compared to blast males.
Unique gut microbiome signatures in female vs. male mice following repetitive blast exposure.
To determine if repetitive blast exposure differentially affected the gut microbiome in female vs. male mice, fecal samples were collected 24 hours post final sham/blast exposure and analyzed using shallow shotgun sequencing. Alpha diversity measured using the Shannon Index (Figure 4a) was not different when comparing across groups (Kruskal Wallis F(4,94) = 2.47, p=0.57). Conversely, beta diversity measured using Bray Curtis distance (Figure 4b) was significantly different across groups (ANOSIM, 999 permutations, p=0.001). Furthermore, we found significant differences in abundance of nine bacteria orders (Figure 4c). Finally, we examined relative abundance differences at the species level using LeFSe (Huttenhower Lab Galaxy) and found unique species characterizing each of the four experimental groups (Figure 5).
Figure 4.
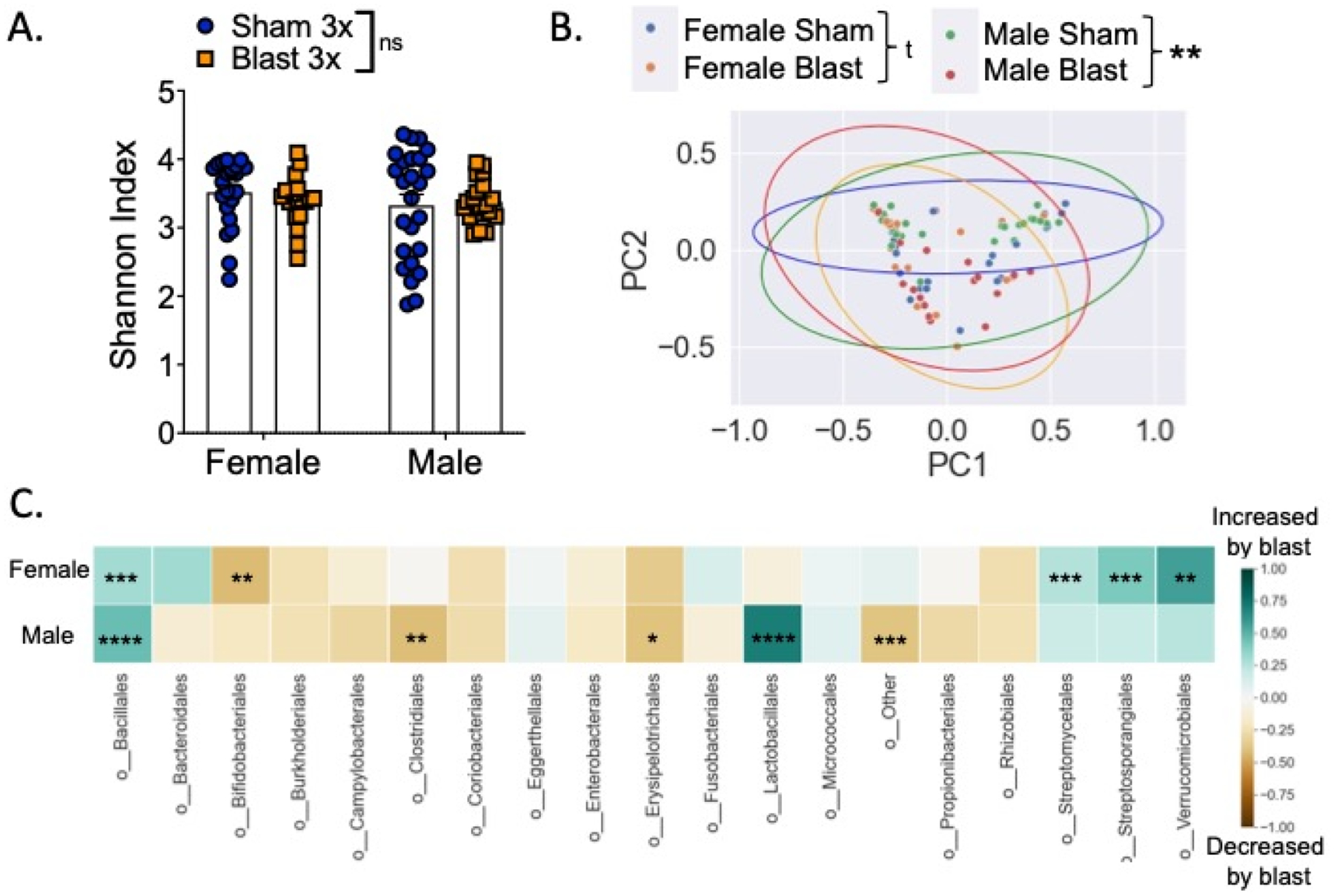
Differential effects of repetitive blast on acute gut microbiome changes in female vs. male mice. (A). No difference in Shannon (alpha) diversity as a function of exposure type or sex. (B) PCoA on Bray-Curtis dissimilarity distances (beta diversity) among the four groups examined. Each point represents an individual sample colored according to group. Principle Component 1 (PC1): 44.6% explained variance; Principle Component 1 (PC2): 19.3% explained variance. Ellipses represent 95% CI around cluster centroid for each experimental group. (C) Differences between female sham vs. blast mice (top row) and male sham vs. blast mice (bottom row) at the order level, expressed as mean relative abundance z score (z score computed separately for female and male mice). n=18–25. Kruskal-Wallis test followed by Mann-Whitney U test for individual group by group comparison if Kruskal-Wallis was significant at FDR p<0.1 (A, C). Analysis of similarities (ANOSIM) (B). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001, ns = not significant. Values represent mean ± SEM.
Figure 5.
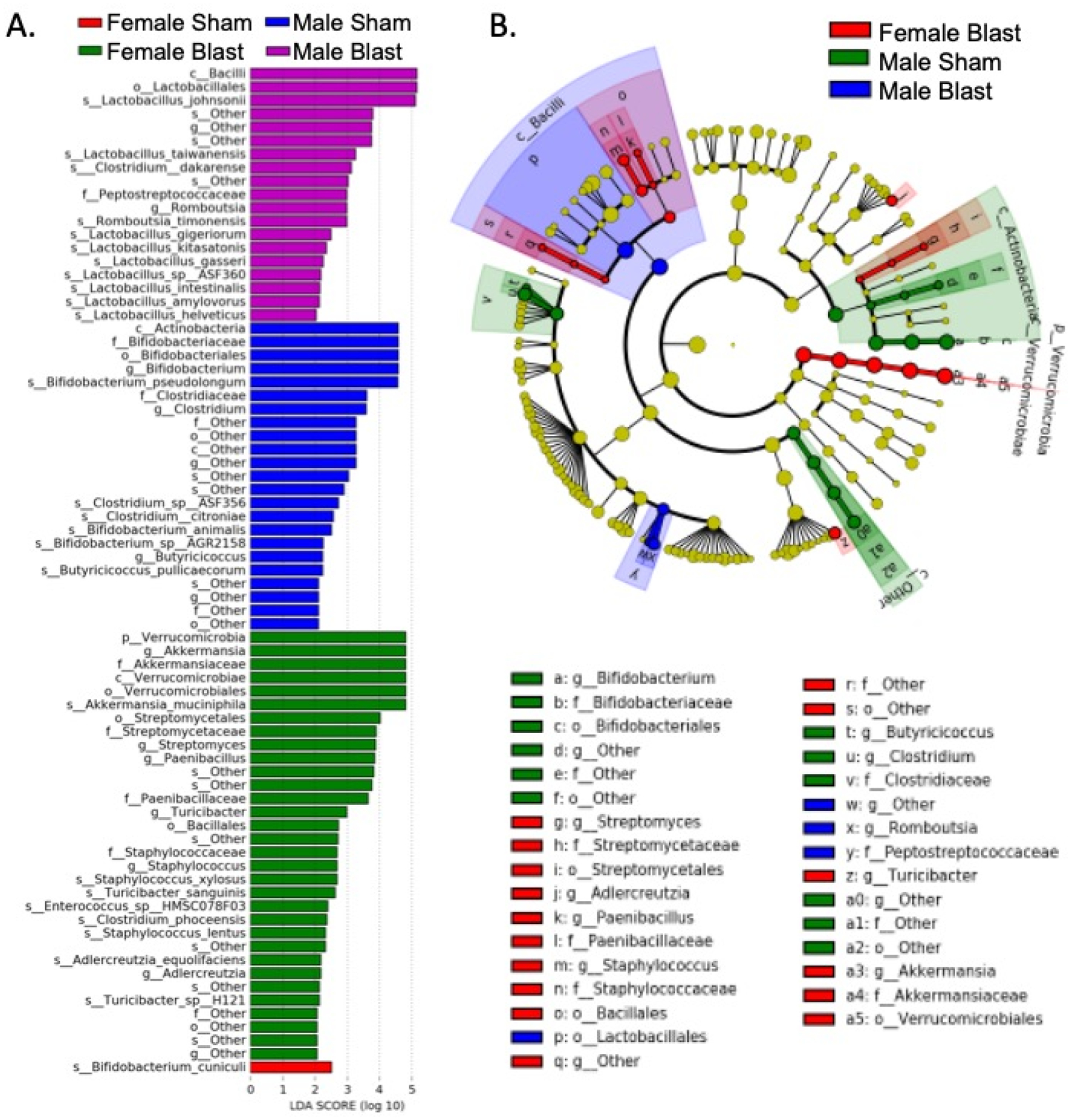
LEfSe analysis of the gut microbial taxonomy. (A). Enriched species (LDA score > 2) in female sham (red), female blast (green), male sham (blue), and male blast (purple). (B) Taxonomic representation of statistically and biologically consistent differences in the four groups. Differences are represented by the color of the most abundant class. Circle diameter is in proportion to that taxon’s abundance.
Repetitive blast exposure decreases locomotion and increases anxiety-like behavior sub-acutely in both male and female mice.
To probe potential locomotor deficits and anxiety-like behavior acutely following blast exposure, we tested male and female mice in a large circular open field 48 hours following their last sham/blast exposure. In distance traveled, mice showed a significant main effect of blast (two-way ANOVA: main effect of exposure F(1,85) = 43.15, p<0.0001) and no significant interaction effect between exposure and sex (two-way ANOVA: interaction effect F(1,85) = 2.573, ns) or main effect of sex (two-way ANOVA: main effect of sex F(1,85) = 0.158, ns) (Fig 6a). In entries to the center of the open field, mice showed a significant main effect of blast (two-way ANOVA: main effect of exposure F(1,85) = 35.71, p=0.0001) and a main effect of sex (two-way ANOVA: main effect of sex F(1,85) = 6.591, ns), but no significant interaction effect between exposure and sex (two-way ANOVA: interaction effect F(1,85) = 0.641, ns) (Fig 6b). In time spent in the center of the open field, mice showed a significant main effect of blast (two-way ANOVA: main effect of exposure F(1,85) = 15.05, p=0.0002) and a main effect of sex (two-way ANOVA: main effect of sex F(1,85) = 8.736, p=0.004), but no significant interaction effect between exposure and sex (two-way ANOVA: interaction effect F(1,85) = 0.081, ns) (Fig 6c). Finally, in delay to first center entry, mice showed a significant main effect of blast (two-way ANOVA: main effect of exposure F(1,85) = 11.57, p=0.001) and no significant interaction effect between exposure and sex (two-way ANOVA: interaction effect F(1,85) = 1.99, ns) or main effect of sex (two-way RM ANOVA: main effect of sex F(1,85) = 0.045, ns) (Fig 6d).
Figure 6.
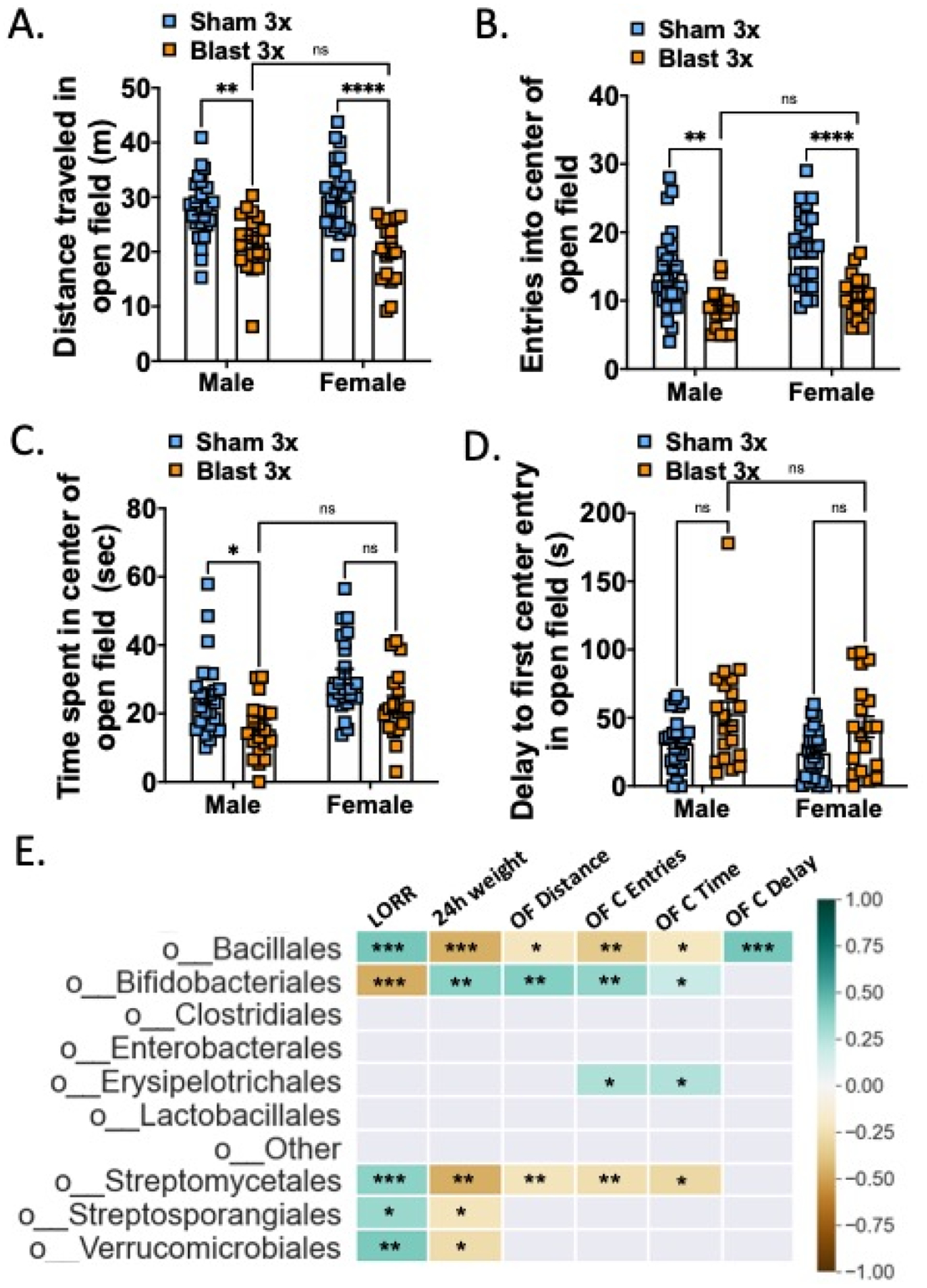
Blast exposure increases acute anxiety-like open field behaviors in both female and male mice. (A) Distance traveled in open field. (B) Entries into center of open field. (C) Time spent in center of open field. (D) Delay to first entry into center of open field. (E) Pearson correlation between bacterial taxa (taxa order that were significantly different between groups from Figure 4) and open field parameters. n=18–25. Two-way ANOVA Šídák’s multiple comparison post-hoc. *p ≤ 0.05, **p ≤ 0.01, ****p ≤ 0.0001, ns = not significant. Values represent mean ± SEM.
To further examine potential interactions between acute behavioral effects and fecal microbiome changes, we computed Pearson correlations and corrected for multiple comparisons using FDR (Figure 6e). The bacterial orders bacillales, bifidobacteriales, and streptomycetales were all correlated with multiple open field parameters as well as LORR and 24-hour weight loss.
Repetitive blast exposure results in comparable delayed-onset BBB disruption in female and male mice.
We previously reported that repetitive (2X or 3X), but not single (1X) blast exposure results in delayed-onset (72 h) BBB disruption to radiolabeled albumin (Logsdon et al., 2018, 2020). Consistent with these prior reports, we found a blast effect on BBB disruption to radiolabeled albumin 72 hours following the last blast exposure (two-way ANOVA: main effect of exposure F(1,33) = 5.607, p=0.024) but there was no difference between sexes (Figure 7a). Gut microbes and metabolites are capable of modulating BBB function (Logsdon, Erickson, et al., 2018; Parker et al., 2020), thus to further examine potential interactions between BBB disruption and fecal microbiome changes, we computed Pearson correlations and corrected for multiple comparisons using FDR (Figure 7b). Only the bacterial order enterobacterales was correlated with albumin levels after correcting for multiple comparisons.
Figure 7.
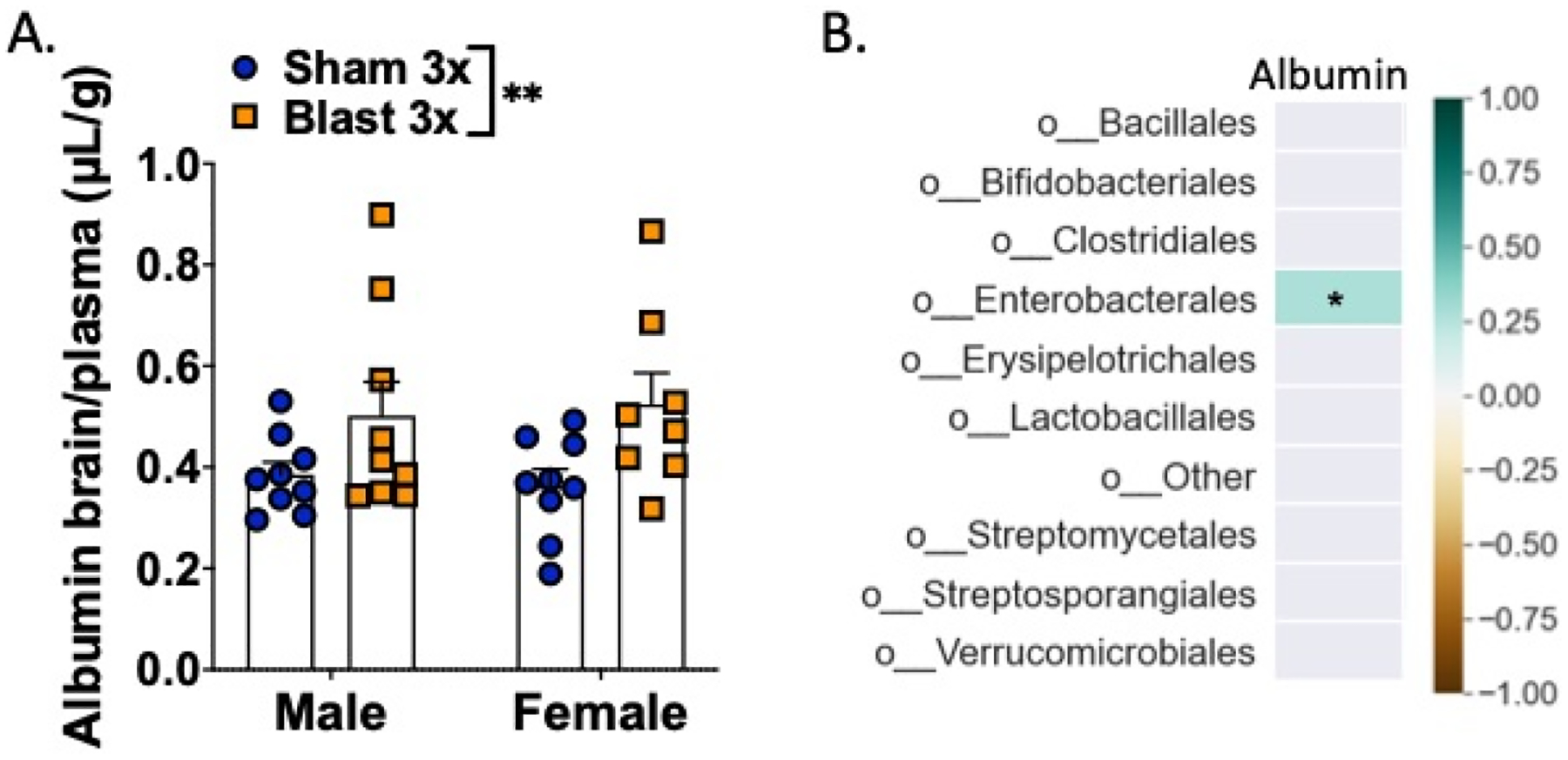
BBB disruption in female and male mice acutely following repetitive blast exposure. (A) Blast increases brain/serum (μl/g) radiolabeled albumin 72 h after final blast. Brain/serum ratios were calculated by dividing the cpm per brain by the cpm per microliter in the corresponding serum and then by the weight of the brain. (B) Pearson correlation between bacterial taxa (taxa order that were significantly different between groups from Figure 4) and albumin BBB permeability. n=8–10. Two-way ANOVA Šídák post-hoc analysis. **p ≤ 0.01, ns = not significant. Values represent means ± SEM and are expressed as microliters per gram of brain tissue.
Male but not female mice exhibit anxious/aversive-like outcomes one month following repetitive blast exposure.
To examine more long-lasting effects of repeated blast exposure on mTBI and PTSD-like outcomes as have been previously reported in male blast mice (Baskin et al., 2021; Elder et al., 2012; Genovese et al., 2013; Perez-Garcia, De Gasperi, et al., 2018; Perez-Garcia, Gama Sosa, et al., 2018; Schindler et al., 2017; Schindler, Terry, et al., 2021; Statz et al., 2019), one month following their last sham/blast exposure, mice were tested in a behavioral battery consisting of elevated zero maze, acoustic startle, and blast-odorant aversion (Fig 8).
Figure 8.
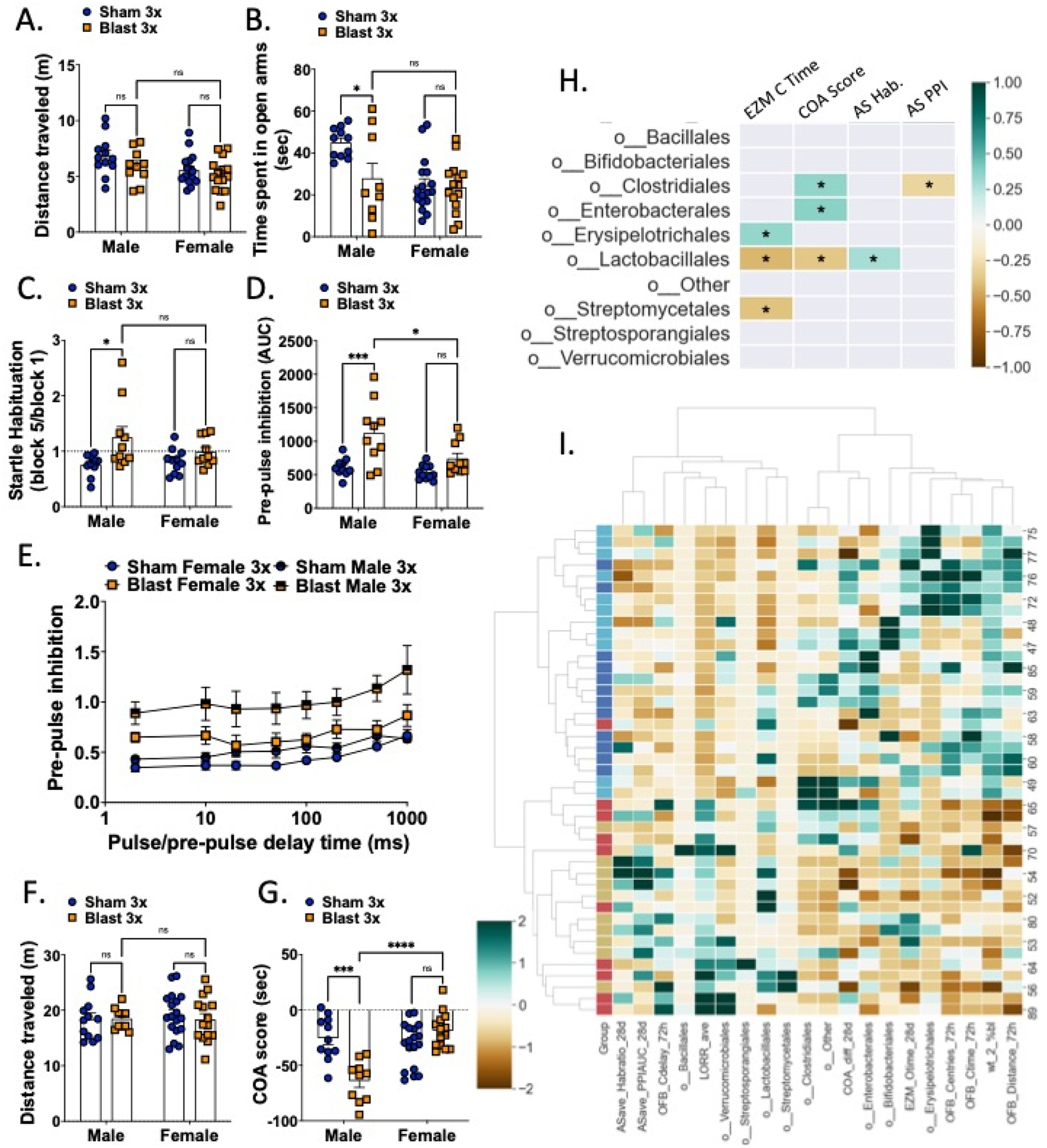
Male but not female mice exhibit blast-induced behavioral deficits at the one-month timepoint. Male and female mice did not differ on total distance traveled on the elevated zero maze 1-month post-blast exposure (A) but male blast mice spent significantly less time than their sham controls in the open arms (B). Only male blast mice showed impaired startle habituation (C) and prepulse inhibition (D, E) on the acoustic startle task. Male and female mice did not differ on total distance traveled in the conditioned odorant aversion posttest (F) but male blast showed an aversion to an odorant previously paired with blast-exposures (G). (H) Pearson correlation between bacterial taxa (taxa order that were significantly different between groups from Figure 4) and one-month behavioral parameters. (I) Heatmap of hierarchical clustering between individual mice vs. behavioral and microbiota composition (taxa order that were significantly different between groups from Figure 4). Each row is a mouse, each column is a parameter. Group column colors: sham female – dark blue; blast female – red; sham male – light blue; blast male yellow. Heatmap colors represent z-score for each parameter computed from all mice. n=12–18. Two-way ANOVA Šídák post-hoc analysis (A-G). *p ≤ 0.05, **p ≤ 0.01, ****p ≤ 0.0001, ns = not significant. Values represent mean ± SEM.
Mice were exposed to an elevated zero maze to probe anxiety-like behaviors with less time spent exploring the open arms indicative of a more anxious phenotype. For distance traveled, there was no significant main effect of exposure (two-way ANOVA F(1,48) = 2.518, ns) and no significant interaction effect between exposure and sex (two-way ANOVA: interaction effect F(1,48) = 0.209, ns), but there was a significant main effect of sex (two-way ANOVA: main effect of sex F(1,48) = 5.95, p=0.018) (Fig 8a). Conversely, for time spent in the open arm of the maze, there was a significant main effect of exposure (two-way ANOVA F(1,48) = 5.354, p=0.025), significant main effect of sex (two-way ANOVA: main effect of sex F(1,48) = 10.10, p=0.003), and a significant interaction effect between exposure and sex (two-way ANOVA: interaction effect F(1,48) = 4.317, p=0.043) (Fig 8b). Post-hoc analysis revealed that blast males spent significantly less time in the open arms than sham males (p<0.05), whereas female sham and blast mice did not differ in open arm time.
To test for a PTSD-like startle response in the mice, mice went through an acoustic startle session which included assessment of startle habituation and prepulse inhibition (PPI). In the startle habituation test, a decrease in the ability to habituate to a repeated presentation of the same stimulus is representative of a deficit in non-associative learning. In the PPI, a decrease in inhibition by the prepulse is indicative of a difficulty in sensory gating (Valsamis & Schmid, 2011). In line with previous reports in male mice (Baskin et al., 2021), we found a main effect of exposure (two-way ANOVA F(1,37) = 8.764, p=0.005), but no significant main effect of sex (two-way ANOVA: main effect of sex F(1,37) = 0.769, ns) or interaction effect between exposure and sex (two-way ANOVA: interaction effect F(1,37) = 2.04, ns) (Fig 8c). PPI was measured following a five-minute rest period after the habituation procedure. The area under the curve of the PPI vs. time delay showed a main effect of exposure (two-way ANOVA F(1,37) = 17.63, p=0.0002) and main effect of sex (two-way ANOVA: main effect of sex F(1,37) = 7.074, p=0.011), but no interaction effect between exposure and delay (two-way ANOVA: interaction effect F(1,37) = 3.218, ns) (Fig 8d). We also examined PPI across time delays and found a main effect of exposure (two-way ANOVA F(3,37) = 9.96, p<0.0001) and main effect of pulse-prepulse delay time (two-way ANOVA: main effect of delay F(5,154) = 13.37, p<0.0001) but no interaction effect between exposure and delay (two-way ANOVA: interaction effect F(21,259) = 0.688, ns) (Fig 8e). Post-hoc analysis revealed that only blast males demonstrated acoustic startle deficits as compared to sham controls but there was no blast effect in females.
Finally, to test for aversion-like behaviors in mice, prior to every sham/blast exposure, a fresh almond-scented nestlet in a tea diffuser was placed in the home cages of the mice to associate the scent with the experience of the sham or blast trauma. We measured aversion to the almond-scented nestlet in a posttest conducted in a T-maze where the almond scent was placed in the left arm of the T and a clean nestlet with no scent was placed in the right arm of the T. When examining distance traveled, there was no significant main effect of exposure (two-way ANOVA F(1,48) = 0.0178, ns), no significant main effect of sex (two-way ANOVA: main effect of sex F(1,48) = 0.117, ns), and no significant interaction effect between exposure and sex (two-way ANOVA: interaction effect F(1,48) = 0.209, ns) (Fig 8f). Conversely, in the difference between time spent with the odor vs. without the odor (COA), there was a significant main effect of exposure (two-way ANOVA F(1,48) = 6.148, p=0.017), significant main effect of sex (two-way ANOVA: main effect of sex F(1,48) = 14.11, p=0.0005), and a significant interaction effect between exposure and sex (two-way ANOVA: interaction effect F(1,48) = 24.00, p<0.0001) (Fig 8g). Post-hoc analysis revealed that blast males spent significantly less time with the almond odor than sham males (p<0.05), whereas females did not differ between sham and blast groups.
To further examine potential interactions between behavioral effects at the one-month timepoint and fecal microbiome changes, we computed Pearson correlations and corrected for multiple comparisons using FDR (Figure 8h). The bacterial orders clostridales and lactobacillales were each correlated with distinct behavioral parameters. Finally, hierarchical clustering across timepoints and experimental endpoints revealed distinct clusters of sham male vs. sham female mice but more mixed clusters consisting of male and female blast mice.
DISCUSSION
While the effects of repetitive blast mTBI have been documented using rodent models, these studies almost exclusively used male research subjects. Thus, almost nothing is known regarding potential adverse outcomes of repetitive blast exposure in female rodents, resulting in a critical knowledge gap at a time when women are increasingly seen in combat roles with a high probability of repetitive blast exposure. Here we present novel data demonstrating behavioral and inflammatory changes in male and female mice at multiple timepoints; intriguingly, while sub-acute behavioral outcomes were relatively consistent between male and female blast mice, adverse behavioral outcomes at the one-month timepoint were only found in male mice. These results highlight the importance of examining potential blast effects across sexes and at multiple time points. Furthermore, differential effects of blast in male vs. female mice in cytokine and gut microbiome changes identify potential diagnostic and therapeutic targets for future development and precision medicine efforts.
Understanding the effects of repetitive blast exposure across sexes is critical for diagnostic and treatment development (Agoston, 2017; Giordano et al., 2020; Gupte et al., 2019; McCabe & Tucker, 2020; Ravula et al., 2022; Simmons et al., 2020; Späni et al., 2018). Increased body armor and improved battlefield medicine make multiple deployments are exceedingly common, greatly increasing the potential for repetitive blast exposure resulting in mTBI comorbid with PTSD (Adamson et al., 2008; Carr et al., 2016; O’Neil et al., 2013; Owens et al., 2008; Simmons et al., 2020; Wenger et al., 2018). Indeed, following blast exposure, mTBI-related persistent post-concussive symptoms (e.g., cognitive difficulties, auditory and vestibular dysfunction, sleep disturbances, negative affect) appreciably overlap with PTSD symptoms (e.g., hypervigilance, aversion to trauma cues, cognitive difficulties, sleep disturbances, negative affect) (Adamson et al., 2008; Elder & Cristian, 2009; Owens et al., 2008; Petrie et al., 2014; Schindler, Baskin, et al., 2021; Schindler et al., 2017). Likewise, both mTBI and PTSD can worsen pre-existing psychiatric disorders such as depression and increase and/or exacerbate substance misuse/addiction and other health risk behaviors (e.g., sensation/novelty seeking, impulsivity, risk taking, irritability/aggression) (María-Ríos & Morrow, 2020; McFarlane, 2010; Miller et al., 2013; Olson-Madden et al., 2012; Schindler, Baskin, et al., 2021; Schindler et al., 2017), potentially compounding negative outcomes following injury and trauma. Indeed, in Veterans we previously reported worse outcomes in relation to ‘risky’ drinking behavior as a function of combat exposure and blast mTBI number (Schindler, Baskin, et al., 2021) and a significant correlation between blast mTBI number and findings on neuroimaging (Meabon et al., 2016; Piantino et al., 2021; Schindler, Baskin, et al., 2021). Results from animal models further highlight the importance of studying repetitive blast exposure (Ahmed et al., 2013; Arun et al., 2020; Heyburn et al., 2019; Kamnaksh et al., 2012; Kawa et al., 2018; Logsdon, Meabon, et al., 2018; Logsdon et al., 2020; Meabon et al., 2016; Schindler et al., 2017; Schindler, Terry, et al., 2021; Schindler, Baskin, et al., 2021); we previously demonstrated PTSD-like outcomes in male mice only following repetitive but not single blast mTBI (Schindler et al., 2021) and more recently demonstrated a blast-dose effect in relation to ethanol sensitivity and ‘binge’-like intake patterns in male mice (Schindler, Baskin, et al., 2021). Likewise, we and others have demonstrated blast-dose effects in relation to biochemical (BBB disruption, cytokine expression) and anxiety-like measures following single vs. repetitive blast exposure (Ahmed et al., 2013; Arun et al., 2020; Heyburn et al., 2019; Kamnaksh et al., 2012; Kawa et al., 2018; Logsdon, Meabon, et al., 2018; Logsdon et al., 2020). Critically, to date there are no reports of repetitive blast TBI research in female rodent models.
Acute increase in pro-inflammatory cytokines and BBB disruption have been well documented in male TBI rodents but have yet to be investigated in females following repetitive blast trauma. Here we demonstrate robust blast-induced changes in serum and brain cytokine levels that display similar and disparate patterns in male vs. female mice. In line with these results, models of single moderate to severe impact TBI have demonstrated disparate inflammatory outcomes in male vs. female mice acutely following injury (Bromberg et al., 2020; Doran et al., 2019; Krukowski, 2021; Krukowski et al., 2020; Späni et al., 2018; Villapol et al., 2017). Likewise, a recent study using a single mild blast exposure with body shielding reported worse acute and sub-acute neuroinflammatory and BBB outcomes in male vs. female rats (Hubbard et al., 2022). The discrepancy between this study and our current results of similar BBB disruption in male vs. female blast mice is likely due to the repetitive nature of our blast model, suggesting that while female rodents are protected from acute single TBI effects, repetitive blast exposure is sufficient to result in cytokine changes and BBB disruption in females as well as males.
To more fully understand potential differences in adverse outcomes as an effect of sex, we analyzed fecal microbial abundance acutely following repetitive blast exposure. Intestinal (gut) microbiota and the genes they produce (collectively referred to as the gut metagenome) help regulate homeostasis and benefit the host through a range of physiological functions (e.g., protection against pathogens, digestion/nutrient assimilation, regulation of the immune system) (Moloney et al., 2014; Thursby & Juge, 2017). Likewise, the ‘microbiota-gut-brain-axis’ (MGBA) represents a critical bidirectional communication system between the gut and the brain, involving metabolic, endocrine, neural, and immune pathways critical for brain health and cognition (Cryan et al., 2019; Moloney et al., 2014; Thursby & Juge, 2017; Wiley et al., 2017). Conversely, altered microbial abundance and composition (i.e., dysbiosis) can have detrimental effects on health and wellness, including implications for cognitive functioning, mental health, and neurodegeneration (Ceppa et al., 2020; Liu et al., 2020; Ticinesi et al., 2018; Tremlett et al., 2017). Importantly, alterations in gut microbiota have been documented years post injury in individuals with a history of moderate/severe TBI (Brenner et al., 2020; Urban et al., 2020), and preclinical work using animal models also supports the microbiome as playing a mechanistic role in adverse outcomes following stress and trauma (Angoa-Pérez et al., 2020; Matharu et al., 2019; Opeyemi et al., 2021), but no studies thus far have specifically utilized animal models of blast injury (either single or repetitive). In line with our cytokine results, we find similar and disparate effects of repetitive blast on gut microbiome in male vs. female mice. Linear discriminant analysis of fecal samples demonstrated bifidobacteria as significantly increased in sham vs. blast mice, which is already being tested as a potential probiotic for PTSD and depression, raising the possibility of this bacteria as a potential treatment target for further development. Gut microbes and metabolites are also capable of modulating BBB function (Braniste et al., 2014; Logsdon, Erickson, et al., 2018; Parker et al., 2020), and we found the bacterial order enterobacterales correlated with levels of BBB disruption. Enterobacterales belong to the phylum of Proteobacteria, which are potentially involved in both intestinal and extraintestinal disease (Rizzatti et al., 2017).
In relation to potential sex differences in adverse behavioral outcomes following blast exposure, only three studies thus far have been reported (Hubbard et al., 2022; McNamara et al., 2022; Russell, Handa, et al., 2018). Hubbard et al (2022) found increased anxiety-like behavior in male but not female rats in the open field (at 2 days post) and elevated plus maze (at 14 days post) following single blast mTBI with body shielding. Conversely, McNamara et al (2022) found no injury effects in either female or male mice on the elevated plus and zero mazes when tested at 2–4 weeks post single blast exposure. Finally, Russell et al (2018) found increased anxiety-like behavior in male, and to a lesser extent in female, mice at 6 days post single blast exposure. Differences in reported results are likely due to differences in injury severity and specifically the repetitive nature of our injury model. Furthermore, anxiety tests such as the open field must also be interpreted in relation to potential injury-induced effects on locomotion (i.e., decreased locomotion, and not anxiety-like outcomes, results in more time spent in the center of the open field). Critically, here we report that both male and female mice exposed to repetitive blast exposure exhibit decreased entries into the center of an open field in addition to decreased time spent in the center, suggesting anxiety-like outcomes that are not solely dependent on confounding locomotor effects.
Our behavioral outcomes at the one-month time point are also in line with recent preclinical research looking at animal models of PTSD. One preclinical model of PTSD based on the ability of rats to extinguish fear conditioning, found that female mice were less likely to show long-term fear and anxiety-like behaviors on a variety of behaviors when compared to males with similar deficits in fear extinguishing (Emtyazi et al., 2022). One possible explanation for our disparate results in behavioral outcomes at the one-month timepoint in male vs. female blast mice stem from differences in acute cytokine and microbiome changes; for example, IL-10, an anti-inflammatory cytokine is only increased post-blast in female mice, which could engender protective mechanisms specifically in females that are reflected by decreased behavioral effects following blast at the one-month timepoint.
There are several drawbacks and limitations to our current study, including the lack of brain region specific cytokine measurement and/or biochemical/histochemical assays aimed at understanding potential chances in microglia and astrocytes. Likewise, the behavioral tests conducted were relatively simple and warrant future investigation focused on using more sophisticated operant behavioral paradigms as previously reported in male mice following repetitive blast (Baskin et al., 2021). Finally, we did not measure estrus cycle or gonadal hormone levels, but it is important to note that we consistently find less variability in our female mice as compared to their male counterparts. Likewise, research on sex differences in preclinical models of PTSD repeatedly have indicated no association between estrous stage and development of PTSD-like phenotypes (Emtyazi et al., 2022; Zoladz et al., 2019). An additional future direction is examination of blast effects across age groups, the current study utilized mice aged 11 weeks which is early in adulthood and results may differ for adolescent or geriatric mice.
Despite TBI being a leading cause of morbidity and mortality worldwide, as well as a common outcome of modern-day warfare, understanding sex as a biological variable in TBI is still in its infancy (Cogan et al., 2020; Gupte et al., 2019). Results from existing human literature are mixed, and overwhelmingly these reports were not powered to examine potential sex differences. Here we report on a series of translationally relevant outcome measures known to be impacted by TBI in humans, with the goal of providing a survey comparison of male and female mice at multiple timepoints following repetitive blast mTBI. Together, our results demonstrate that adverse effects of repetitive blast are dependent on interactions between gonadal sex, time from injury, biological sample type, and/or behavioral test, and highlight new targets (e.g., increasing IL-10 in male mice; sex-specific targeted probiotic formulation) for diagnosis and treatment development aimed at understanding how repetitive blast trauma affects diverse populations.
Female mice have increased loss of righting time compared to male mice acutely following blast exposure but do not differ in acute weight loss
Effects of repetitive blast exposure on brain and serum cytokine levels and gut microbial abundance differ in male vs. female mice
Repetitive blast exposure increases blood brain barrier permeability in both male and female mice
Sub-acute locomotor and anxiety deficits are similar in male and female mice, but only male mice exhibit adverse behavioral effects at the one-month timepoint.
Acknowledgements
The authors would like to thank Traci J. Webber, Cindy Pekow, DVM, and Lena Strait-Bodey for their considerable veterinary assistance and care. Timelines were created with BioRender.com. Finally, the authors honor the mice, without which this research would not have been possible.
Funding
This work was supported by grants from NIDA Training Grant 2T32DA007278-26 (BMB) a Department of Veteran Affairs (VA) Basic Laboratory Research and Development (BLR&D) Career Development Award 1IK2BX003258 (AGS), a VA BLR&D Merit Review Award 5I01BX002311 (DGC), University of Washington Friends of Alzheimer’s Research (DGC), the UW Royalty Research Fund (DGC)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This article was submitted as a preprint to Bioarchive (BIORXIV/2022/511013)
Disclaimers
The authors have no conflict of interest to disclose. The views expressed in this scientific presentation are those of the author(s) and do not reflect the official policy or position of the U.S. government or Department of Veteran Affairs.
Ethics approval and consent to participate
All animal experiments were conducted in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines and were approved by the VA Puget Sound Institutional Animal Care and Use Committee.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Availability of data and materials
The data in this study are available from the corresponding author upon reasonable request.
REFERENCES
- Adamson DM, Burnam MA, Burns RM, Caldarone LB, Cox RA, D’Amico EJ, Diaz C, Eibner C, Fisher G, Helmus TC, Karney B, Kilmer B, Marshall GN, Martin LT, Meredith LS, Metscher KN, Osilla KC, Pacula RL, Ramchand R, … Jaycox LH (2008). Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. RAND Corporation. https://www.rand.org/pubs/monographs/MG720.html
- Agoston DV (2017). Modeling the Long-Term Consequences of Repeated Blast-Induced Mild Traumatic Brain Injuries. Journal of Neurotrauma, 34(S1), S44–S52. 10.1089/neu.2017.5317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed FA, Kamnaksh A, Kovesdi E, Long JB, & Agoston DV (2013). Long-term consequences of single and multiple mild blast exposure on select physiological parameters and blood-based biomarkers. Electrophoresis, 34(15), 2229–2233. 10.1002/elps.201300077 [DOI] [PubMed] [Google Scholar]
- Angoa-Pérez M, Zagorac B, Anneken JH, Briggs DI, Winters AD, Greenberg JM, Ahmad M, Theis KR, & Kuhn DM (2020). Repetitive, mild traumatic brain injury results in a progressive white matter pathology, cognitive deterioration, and a transient gut microbiota dysbiosis. Scientific Reports, 10(1), 8949. 10.1038/s41598-020-65972-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun P, Wilder DM, Eken O, Urioste R, Batuure A, Sajja S, Van Albert S, Wang Y, Gist ID, & Long JB (2020). Long-Term Effects of Blast Exposure: A Functional Study in Rats Using an Advanced Blast Simulator. Journal of Neurotrauma, 37(4), 647–655. 10.1089/neu.2019.6591 [DOI] [PubMed] [Google Scholar]
- Banks WA, Gray AM, Erickson MA, Salameh TS, Damodarasamy M, Sheibani N, Meabon JS, Wing EE, Morofuji Y, Cook DG, & Reed MJ (2015). Lipopolysaccharide-induced blood-brain barrier disruption: Roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. Journal of Neuroinflammation, 12, 223. 10.1186/s12974-015-0434-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin B, Lee SJ, Skillen E, Wong K, Rau H, Hendrickson RC, Pagulayan K, Raskind MA, Peskind ER, Phillips PEM, Cook DG, & Schindler AG (2021). Repetitive Blast Exposure Increases Appetitive Motivation and Behavioral Inflexibility in Male Mice. Frontiers in Behavioral Neuroscience, 15, 792648. 10.3389/fnbeh.2021.792648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Guan NL, Kundu P, Gulyás B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, & Pettersson S (2014). The gut microbiota influences blood-brain barrier permeability in mice. Science Translational Medicine, 6(263), 263ra158. 10.1126/scitranslmed.3009759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner LA, Stamper CE, Hoisington AJ, Stearns-Yoder KA, Stanislawksi MA, Brostow DP, Hoffmire CA, Forster JE, Schneider AL, Postolache TT, & Lowry CA (2020). Microbial Diversity and Community Structures Among Those With Moderate to Severe TBI: A United States-Veteran Microbiome Project Study. The Journal of Head Trauma Rehabilitation, 35(5), 332–341. 10.1097/HTR.0000000000000615 [DOI] [PubMed] [Google Scholar]
- Bromberg CE, Condon AM, Ridgway SW, Krishna G, Garcia-Filion PC, Adelson PD, Rowe RK, & Thomas TC (2020). Sex-Dependent Pathology in the HPA Axis at a Sub-acute Period After Experimental Traumatic Brain Injury. Frontiers in Neurology, 11, 946. 10.3389/fneur.2020.00946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr W, Stone JR, Walilko T, Young LA, Snook TL, Paggi ME, Tsao JW, Jankosky CJ, Parish RV, & Ahlers ST (2016). Repeated Low-Level Blast Exposure: A Descriptive Human Subjects Study. Military Medicine, 181(suppl_5), 28–39. 10.7205/MILMED-D-15-00137 [DOI] [PubMed] [Google Scholar]
- Ceppa FA, Izzo L, Sardelli L, Raimondi I, Tunesi M, Albani D, & Giordano C (2020). Human Gut-Microbiota Interaction in Neurodegenerative Disorders and Current Engineered Tools for Its Modeling. Frontiers in Cellular and Infection Microbiology, 10, 297. 10.3389/fcimb.2020.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan AM, McCaughey VK, & Scholten J (2020). Gender Differences in Outcomes after Traumatic Brain Injury among Service Members and Veterans. PM & R: The Journal of Injury, Function, and Rehabilitation, 12(3), 301–314. 10.1002/pmrj.12237 [DOI] [PubMed] [Google Scholar]
- Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, … Dinan TG (2019). The Microbiota-Gut-Brain Axis. Physiological Reviews, 99(4), 1877–2013. 10.1152/physrev.00018.2018 [DOI] [PubMed] [Google Scholar]
- Di Battista A, Soo C, Catroppa C, & Anderson V (2012). Quality of life in children and adolescents post-TBI: A systematic review and meta-analysis. Journal of Neurotrauma, 29(9), 1717–1727. 10.1089/neu.2011.2157 [DOI] [PubMed] [Google Scholar]
- Doran SJ, Ritzel RM, Glaser EP, Henry RJ, Faden AI, & Loane DJ (2019). Sex Differences in Acute Neuroinflammation after Experimental Traumatic Brain Injury Are Mediated by Infiltrating Myeloid Cells. Journal of Neurotrauma, 36(7), 1040–1053. 10.1089/neu.2018.6019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye JL, Eskridge SL, Tepe V, Clouser MC, & Galarneau M (2016). Characterization and Comparison of Combat-Related Injuries in Women During OIF and OEF. Military Medicine, 181(1 Suppl), 92–98. 10.7205/MILMED-D-15-00237 [DOI] [PubMed] [Google Scholar]
- Elder GA, & Cristian A (2009). Blast-related mild traumatic brain injury: Mechanisms of injury and impact on clinical care. The Mount Sinai Journal of Medicine, New York, 76(2), 111–118. 10.1002/msj.20098 [DOI] [PubMed] [Google Scholar]
- Elder GA, Dorr NP, De Gasperi R, Gama Sosa MA, Shaughness MC, Maudlin-Jeronimo E, Hall AA, McCarron RM, & Ahlers ST (2012). Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. Journal of Neurotrauma, 29(16), 2564–2575. 10.1089/neu.2012.2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emtyazi D, Rabelo TK, Katzman H, Campos AC, Diwan M, Gidyk D, Rabelo Dos Santos P, Giacobbe P, Lipsman N, Aubert I, & Hamani C (2022). Sex differences in long-term fear and anxiety-like responses in a preclinical model of PTSD. Journal of Psychiatric Research, 151, 619–625. 10.1016/j.jpsychires.2022.05.015 [DOI] [PubMed] [Google Scholar]
- Genovese RF, Simmons LP, Ahlers ST, Maudlin-Jeronimo E, Dave JR, & Boutte AM (2013). Effects of mild TBI from repeated blast overpressure on the expression and extinction of conditioned fear in rats. Neuroscience, 254, 120–129. 10.1016/j.neuroscience.2013.09.021 [DOI] [PubMed] [Google Scholar]
- Ghai V, Fallen S, Baxter D, Scherler K, Kim T-K, Zhou Y, Meabon JS, Logsdon AF, Banks WA, Schindler AG, Cook DG, Peskind ER, Lee I, & Wang K (2020). Alterations in Plasma microRNA and Protein Levels in War Veterans with Chronic Mild Traumatic Brain Injury. Journal of Neurotrauma, 37(12), 1418–1430. 10.1089/neu.2019.6826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano KR, Rojas-Valencia LM, Bhargava V, & Lifshitz J (2020). Beyond Binary: Influence of Sex and Gender on Outcome after Traumatic Brain Injury. Journal of Neurotrauma, 37(23), 2454–2459. 10.1089/neu.2020.7230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte R, Brooks W, Vukas R, Pierce J, & Harris J (2019). Sex Differences in Traumatic Brain Injury: What We Know and What We Should Know. Journal of Neurotrauma, 36(22), 3063–3091. 10.1089/neu.2018.6171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson RC, Schindler AG, & Pagulayan KF (2018). Untangling PTSD and TBI: Challenges and Strategies in Clinical Care and Research. Current Neurology and Neuroscience Reports, 18(12), 106. 10.1007/s11910-018-0908-5 [DOI] [PubMed] [Google Scholar]
- Heyburn L, Abutarboush R, Goodrich S, Urioste R, Batuure A, Statz J, Wilder D, Ahlers ST, Long JB, & Sajja VSSS (2019). Repeated Low-Level Blast Overpressure Leads to Endovascular Disruption and Alterations in TDP-43 and Piezo2 in a Rat Model of Blast TBI. Frontiers in Neurology, 10, 766. 10.3389/fneur.2019.00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard WB, Velmurugan GV, Brown EP, & Sullivan PG (2022). Resilience of females to acute blood-brain barrier damage and anxiety behavior following mild blast traumatic brain injury. Acta Neuropathologica Communications, 10(1), 93. 10.1186/s40478-022-01395-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber BR, Meabon JS, Hoffer ZS, Zhang J, Hoekstra JG, Pagulayan KF, McMillan PJ, Mayer CL, Banks WA, Kraemer BC, Raskind MA, McGavern DB, Peskind ER, & Cook DG (2016). Blast exposure causes dynamic microglial/macrophage responses and microdomains of brain microvessel dysfunction. Neuroscience, 319, 206–220. 10.1016/j.neuroscience.2016.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber BR, Meabon JS, Martin TJ, Mourad PD, Bennett R, Kraemer BC, Cernak I, Petrie EC, Emery MJ, Swenson ER, Mayer C, Mehic E, Peskind ER, & Cook DG (2013). Blast exposure causes early and persistent aberrant phospho- and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury. Journal of Alzheimer’s Disease: JAD, 37(2), 309–323. 10.3233/JAD-130182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson KM, Hendricks AM, Kimerling R, Krengel M, Meterko M, Stolzmann KL, Baker E, Pogoda TK, Vasterling JJ, & Lew HL (2011). Psychiatric diagnoses and neurobehavioral symptom severity among OEF/OIF VA patients with deployment-related traumatic brain injury: A gender comparison. Women’s Health Issues: Official Publication of the Jacobs Institute of Women’s Health, 21(4 Suppl), S210–217. 10.1016/j.whi.2011.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WD, & Griswold DP (2017). Traumatic brain injury: A global challenge. The Lancet. Neurology, 16(12), 949–950. 10.1016/S1474-4422(17)30362-9 [DOI] [PubMed] [Google Scholar]
- Kamarck KN (2015, September 1). Women in Combat: Issues for Congress (United States) [Report]. UNT Digital Library; Library of Congress. Congressional Research Service. https://digital.library.unt.edu/ark:/67531/metadc743572/
- Kamnaksh A, Kwon S-K, Kovesdi E, Ahmed F, Barry ES, Grunberg NE, Long J, & Agoston D (2012). Neurobehavioral, cellular, and molecular consequences of single and multiple mild blast exposure. Electrophoresis, 33(24), 3680–3692. 10.1002/elps.201200319 [DOI] [PubMed] [Google Scholar]
- Kawa L, Arborelius UP, Hökfelt T, & Risling M (2020). Sex-Specific Differences in Rodents Following a Single Primary Blast Exposure: Focus on the Monoamine and Galanin Systems. Frontiers in Neurology, 11, 540144. 10.3389/fneur.2020.540144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa L, Kamnaksh A, Long JB, Arborelius UP, Hökfelt T, Agoston DV, & Risling M (2018). A Comparative Study of Two Blast-Induced Traumatic Brain Injury Models: Changes in Monoamine and Galanin Systems Following Single and Repeated Exposure. Frontiers in Neurology, 9, 479. 10.3389/fneur.2018.00479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukowski K (2021). Short review: The impact of sex on neuroimmune and cognitive outcomes after traumatic brain injury. Brain, Behavior, & Immunity - Health, 16, 100327. 10.1016/j.bbih.2021.100327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukowski K, Nolan A, Frias ES, Grue K, Becker M, Ureta G, Delgado L, Bernales S, Sohal VS, Walter P, & Rosi S (2020). Integrated Stress Response Inhibitor Reverses Sex-Dependent Behavioral and Cell-Specific Deficits after Mild Repetitive Head Trauma. Journal of Neurotrauma, 37(11), 1370–1380. 10.1089/neu.2019.6827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Gao J, Zhu M, Liu K, & Zhang H-L (2020). Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Molecular Neurobiology, 57(12), 5026–5043. 10.1007/s12035-020-02073-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon AF, Erickson MA, Rhea EM, Salameh TS, & Banks WA (2018). Gut reactions: How the blood-brain barrier connects the microbiome and the brain. Experimental Biology and Medicine (Maywood, N.J.), 243(2), 159–165. 10.1177/1535370217743766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon AF, Meabon JS, Cline MM, Bullock KM, Raskind MA, Peskind ER, Banks WA, & Cook DG (2018). Blast exposure elicits blood-brain barrier disruption and repair mediated by tight junction integrity and nitric oxide dependent processes. Scientific Reports, 8(1), 11344. 10.1038/s41598-018-29341-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon AF, Schindler AG, Meabon JS, Yagi M, Herbert MJ, Banks WA, Raskind MA, Marshall DA, Keene CD, Perl DP, Peskind ER, & Cook DG (2020). Nitric oxide synthase mediates cerebellar dysfunction in mice exposed to repetitive blast-induced mild traumatic brain injury. Scientific Reports, 10(1), 9420. 10.1038/s41598-020-66113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Büki A, Chesnut RM, Citerio G, Coburn M, Cooper DJ, Crowder AT, Czeiter E, Czosnyka M, Diaz-Arrastia R, Dreier JP, Duhaime A-C, … InTBIR Participants and Investigators. (2017). Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. The Lancet. Neurology, 16(12), 987–1048. 10.1016/S1474-4422(17)30371-X [DOI] [PubMed] [Google Scholar]
- Malec JF, Van Houtven CH, Tanielian T, Atizado A, & Dorn MC (2017). Impact of TBI on caregivers of veterans with TBI: Burden and interventions. Brain Injury, 31(9), 1235–1245. 10.1080/02699052.2016.1274778 [DOI] [PubMed] [Google Scholar]
- María-Ríos CE, & Morrow JD (2020). Mechanisms of Shared Vulnerability to Post-traumatic Stress Disorder and Substance Use Disorders. Frontiers in Behavioral Neuroscience, 14, 6. 10.3389/fnbeh.2020.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matharu D, Dhotre D, Balasubramanian N, Pawar N, Sagarkar S, & Sakharkar A (2019). Repeated mild traumatic brain injury affects microbial diversity in rat jejunum. Journal of Biosciences, 44(5), 120. [PubMed] [Google Scholar]
- McCabe JT, & Tucker LB (2020). Sex as a Biological Variable in Preclinical Modeling of Blast-Related Traumatic Brain Injury. Frontiers in Neurology, 11, 541050. 10.3389/fneur.2020.541050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane AC (2010). The long-term costs of traumatic stress: Intertwined physical and psychological consequences. World Psychiatry: Official Journal of the World Psychiatric Association (WPA), 9(1), 3–10. 10.1002/j.2051-5545.2010.tb00254.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara EH, Tucker LB, Liu J, Fu AH, Kim Y, Vu PA, & McCabe JT (2022). Limbic Responses Following Shock Wave Exposure in Male and Female Mice. Frontiers in Behavioral Neuroscience, 16, 863195. 10.3389/fnbeh.2022.863195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meabon JS, Cook DG, Yagi M, Terry GE, Cross DJ, Muzi M, Pagulayan KF, Logsdon AF, Schindler AG, Ghai V, Wang K, Fallen S, Zhou Y, Kim T-K, Lee I, Banks WA, Carlson ES, Mayer C, Hendrickson RC, … Peskind ER (2020). Chronic elevation of plasma vascular endothelial growth factor-A (VEGF-A) is associated with a history of blast exposure. Journal of the Neurological Sciences, 417, 117049. 10.1016/j.jns.2020.117049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meabon JS, Huber BR, Cross DJ, Richards TL, Minoshima S, Pagulayan KF, Li G, Meeker KD, Kraemer BC, Petrie EC, Raskind MA, Peskind ER, & Cook DG (2016). Repetitive blast exposure in mice and combat veterans causes persistent cerebellar dysfunction. Science Translational Medicine, 8(321), 321ra6. 10.1126/scitranslmed.aaa9585 [DOI] [PubMed] [Google Scholar]
- Miller SC, Baktash SH, Webb TS, Whitehead CR, Maynard C, Wells TS, Otte CN, & Gore RK (2013). Risk for addiction-related disorders following mild traumatic brain injury in a large cohort of active-duty U.S. airmen. The American Journal of Psychiatry, 170(4), 383–390. 10.1176/appi.ajp.2012.12010126 [DOI] [PubMed] [Google Scholar]
- Moloney RD, Desbonnet L, Clarke G, Dinan TG, & Cryan JF (2014). The microbiome: Stress, health and disease. Mammalian Genome: Official Journal of the International Mammalian Genome Society, 25(1–2), 49–74. 10.1007/s00335-013-9488-5 [DOI] [PubMed] [Google Scholar]
- Olson-Madden JH, Forster JE, Huggins J, & Schneider A (2012). Psychiatric diagnoses, mental health utilization, high-risk behaviors, and self-directed violence among veterans with comorbid history of traumatic brain injury and substance use disorders. The Journal of Head Trauma Rehabilitation, 27(5), 370–378. 10.1097/HTR.0b013e318268d496 [DOI] [PubMed] [Google Scholar]
- O’Neil ME, Carlson K, Storzbach D, Brenner L, Freeman M, Quiñones A, Motu’apuaka M, Ensley M, & Kansagara D (2013). Complications of Mild Traumatic Brain Injury in Veterans and Military Personnel: A Systematic Review. Department of Veterans Affairs (US). http://www.ncbi.nlm.nih.gov/books/NBK189785/ [PubMed]
- Opeyemi OM, Rogers MB, Firek BA, Janesko-Feldman K, Vagni V, Mullett SJ, Wendell SG, Nelson BP, New LA, Mariño E, Kochanek PM, Bayır H, Clark RSB, Morowitz MJ, & Simon DW (2021). Sustained Dysbiosis and Decreased Fecal Short-Chain Fatty Acids after Traumatic Brain Injury and Impact on Neurologic Outcome. Journal of Neurotrauma, 38(18), 2610–2621. 10.1089/neu.2020.7506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens BD, Kragh JF, Wenke JC, Macaitis J, Wade CE, & Holcomb JB (2008). Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. The Journal of Trauma, 64(2), 295–299. 10.1097/TA.0b013e318163b875 [DOI] [PubMed] [Google Scholar]
- Ozga JE, Povroznik JM, Engler-Chiurazzi EB, & Vonder Haar C (2018). Executive (dys)function after traumatic brain injury: Special considerations for behavioral pharmacology. Behavioural Pharmacology, 29(7), 617–637. 10.1097/FBP.0000000000000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A, Fonseca S, & Carding SR (2020). Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes, 11(2), 135–157. 10.1080/19490976.2019.1638722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garcia G, De Gasperi R, Gama Sosa MA, Perez GM, Otero-Pagan A, Tschiffely A, McCarron RM, Ahlers ST, Elder GA, & Gandy S (2018). PTSD-Related Behavioral Traits in a Rat Model of Blast-Induced mTBI Are Reversed by the mGluR2/3 Receptor Antagonist BCI-838. ENeuro, 5(1), ENEURO.0357–17.2018. 10.1523/ENEURO.0357-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garcia G, Gama Sosa MA, De Gasperi R, Lashof-Sullivan M, Maudlin-Jeronimo E, Stone JR, Haghighi F, Ahlers ST, & Elder GA (2018). Chronic post-traumatic stress disorder-related traits in a rat model of low-level blast exposure. Behavioural Brain Research, 340, 117–125. 10.1016/j.bbr.2016.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskind ER, Petrie EC, Cross DJ, Pagulayan K, McCraw K, Hoff D, Hart K, Yu C-E, Raskind MA, Cook DG, & Minoshima S (2011). Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent post-concussive symptoms. NeuroImage, 54 Suppl 1, S76–82. 10.1016/j.neuroimage.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie EC, Cross DJ, Yarnykh VL, Richards T, Martin NM, Pagulayan K, Hoff D, Hart K, Mayer C, Tarabochia M, Raskind MA, Minoshima S, & Peskind ER (2014). Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. Journal of Neurotrauma, 31(5), 425–436. 10.1089/neu.2013.2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantino J, Schwartz DL, Luther M, Newgard C, Silbert L, Raskind M, Pagulayan K, Kleinhans N, Iliff J, & Peskind E (2021). Link between Mild Traumatic Brain Injury, Poor Sleep, and Magnetic Resonance Imaging: Visible Perivascular Spaces in Veterans. Journal of Neurotrauma, 38(17), 2391–2399. 10.1089/neu.2020.7447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravula AR, Das T, Gosain A, Dolalas T, Padhi S, Chandra N, & Pfister BJ (2022). An update on repeated blast traumatic brain injury. Current Opinion in Biomedical Engineering, 24, 100409. 10.1016/j.cobme.2022.100409 [DOI] [Google Scholar]
- Rizzatti G, Lopetuso LR, Gibiino G, Binda C, & Gasbarrini A (2017). Proteobacteria: A Common Factor in Human Diseases. BioMed Research International, 2017, 9351507. 10.1155/2017/9351507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AL, Handa RJ, & Wu TJ (2018). Sex-Dependent Effects of Mild Blast-induced Traumatic Brain Injury on Corticotropin-releasing Factor Receptor Gene Expression: Potential Link to Anxiety-like Behaviors. Neuroscience, 392, 1–12. 10.1016/j.neuroscience.2018.09.014 [DOI] [PubMed] [Google Scholar]
- Russell AL, Richardson MR, Bauman BM, Hernandez IM, Saperstein S, Handa RJ, & Wu TJ (2018). Differential Responses of the HPA Axis to Mild Blast Traumatic Brain Injury in Male and Female Mice. Endocrinology, 159(6), 2363–2375. 10.1210/en.2018-00203 [DOI] [PubMed] [Google Scholar]
- Schindler AG, Baskin B, Juarez B, Janet Lee S, Hendrickson R, Pagulayan K, Zweifel LS, Raskind MA, Phillips PEM, Peskind ER, & Cook DG (2021). Repetitive blast mild traumatic brain injury increases ethanol sensitivity in male mice and risky drinking behavior in male combat veterans. Alcoholism, Clinical and Experimental Research, 45(5), 1051–1064. 10.1111/acer.14605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Meabon JS, Pagulayan KF, Hendrickson RC, Meeker KD, Cline M, Li G, Sikkema C, Wilkinson CW, Perl DP, Raskind MR, Peskind ER, Clark JJ, & Cook DG (2017). Blast-related disinhibition and risk seeking in mice and combat Veterans: Potential role for dysfunctional phasic dopamine release. Neurobiology of Disease, 106, 23–34. 10.1016/j.nbd.2017.06.004 [DOI] [PubMed] [Google Scholar]
- Schindler AG, Terry GE, Wolden-Hanson T, Cline M, Park M, Lee J, Yagi M, Meabon JS, Peskind ER, Raskind MM, Phillips PEM, & Cook DG (2021). Repetitive Blast Promotes Chronic Aversion to Neutral Cues Encountered in the Peri-Blast Environment. Journal of Neurotrauma, 38(7), 940–948. 10.1089/neu.2020.7061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, & Huttenhower C (2011). Metagenomic biomarker discovery and explanation. Genome Biology, 12(6), R60. 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons MM, Engel CC, Hoch E, Orr P, Anderson B, & Azhar GS (2020). Neurological Effects of Repeated Exposure to Military Occupational Levels of Blast: A Review of Scientific Literature. RAND Corporation. https://www.rand.org/pubs/research_reports/RR2350.html
- Späni CB, Braun DJ, & Van Eldik LJ (2018). Sex-related responses after traumatic brain injury: Considerations for preclinical modeling. Frontiers in Neuroendocrinology, 50, 52–66. 10.1016/j.yfrne.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statz JK, Ciarlone SL, Goodrich JA, McCarron RM, Walker PB, Norris JN, Ahlers ST, & Tschiffely AE (2019). Affective profiling for anxiety-like behavior in a rodent model of mTBI. Behavioural Brain Research, 368, 111895. 10.1016/j.bbr.2019.04.009 [DOI] [PubMed] [Google Scholar]
- Street AE, Gradus JL, Giasson HL, Vogt D, & Resick PA (2013). Gender differences among veterans deployed in support of the wars in Afghanistan and Iraq. Journal of General Internal Medicine, 28 Suppl 2, S556–562. 10.1007/s11606-013-2333-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CA (2017). Traumatic Brain Injury–Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2007 and 2013. MMWR. Surveillance Summaries, 66. 10.15585/mmwr.ss6609a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thursby E, & Juge N (2017). Introduction to the human gut microbiota. The Biochemical Journal, 474(11), 1823–1836. 10.1042/BCJ20160510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticinesi A, Tana C, Nouvenne A, Prati B, Lauretani F, & Meschi T (2018). Gut microbiota, cognitive frailty and dementia in older individuals: A systematic review. Clinical Interventions in Aging, 13, 1497–1511. 10.2147/CIA.S139163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremlett H, Bauer KC, Appel-Cresswell S, Finlay BB, & Waubant E (2017). The gut microbiome in human neurological disease: A review. Annals of Neurology, 81(3), 369–382. 10.1002/ana.24901 [DOI] [PubMed] [Google Scholar]
- Urban RJ, Pyles RB, Stewart CJ, Ajami N, Randolph KM, Durham WJ, Danesi CP, Dillon EL, Summons JR, Singh CK, Morrison M, Kreber LA, Masel B, Miller AL, Wright TJ, & Sheffield-Moore M (2020). Altered Fecal Microbiome Years after Traumatic Brain Injury. Journal of Neurotrauma, 37(8), 1037–1051. 10.1089/neu.2019.6688 [DOI] [PubMed] [Google Scholar]
- Valsamis B, & Schmid S (2011). Habituation and prepulse inhibition of acoustic startle in rodents. Journal of Visualized Experiments: JoVE, 55, e3446. 10.3791/3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S, Loane DJ, & Burns MP (2017). Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia, 65(9), 1423–1438. 10.1002/glia.23171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger JW, O’Connell C, & Cottrell L (2018). Examination of Recent Deployment Experience Across the Services and Components. RAND Corporation. https://www.rand.org/pubs/research_reports/RR1928.html
- Wiley NC, Dinan TG, Ross RP, Stanton C, Clarke G, & Cryan JF (2017). The microbiota-gut-brain axis as a key regulator of neural function and the stress response: Implications for human and animal health. Journal of Animal Science, 95(7), 3225–3246. 10.2527/jas.2016.1256 [DOI] [PubMed] [Google Scholar]
- Wilkinson CW, Pagulayan KF, Petrie EC, Mayer CL, Colasurdo EA, Shofer JB, Hart KL, Hoff D, Tarabochia MA, & Peskind ER (2012). High prevalence of chronic pituitary and target-organ hormone abnormalities after blast-related mild traumatic brain injury. Frontiers in Neurology, 3, 11. 10.3389/fneur.2012.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz PR, D’Alessio PA, Seeley SL, Kasler CD, Goodman CS, Mucher KE, Allison AS, Smith IF, Dodson JL, Stoops TS, & Rorabaugh BR (2019). A predator-based psychosocial stress animal model of PTSD in females: Influence of estrous phase and ovarian hormones. Hormones and Behavior, 115, 104564. 10.1016/j.yhbeh.2019.104564 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this study are available from the corresponding author upon reasonable request.


