Abstract
Neuroimmune pathways regulate brain function to influence complex behavior and play a role in several neuropsychiatric diseases, including alcohol use disorder (AUD). In particular, the interleukin-1 (IL-1) system has emerged as a key regulator of the brain’s response to ethanol (alcohol). Here we investigated the mechanisms underlying ethanol-induced neuroadaptation of IL-1β signaling at GABAergic synapses in the prelimbic region of the medial prefrontal cortex (mPFC), an area responsible for integrating contextual information to mediate conflicting motivational drives. We exposed C57BL/6J male mice to the chronic intermittent ethanol vapor-2 bottle choice paradigm (CIE-2BC) to induce ethanol dependence, and conducted ex vivo electrophysiology and molecular analyses. We found that the IL-1 system regulates basal mPFC function through its actions at inhibitory synapses on prelimbic layer 2/3 pyramidal neurons. IL-1β can selectively recruit either pro-survival (PI3K/Akt) or pro-inflammatory (MyD88/p38 MAPK) mechanisms to produce opposing synaptic effects. In ethanol naïve conditions, there was a strong PI3K/Akt bias leading to a disinhibition of pyramidal neurons. Ethanol dependence produced opposite IL-1 effects - enhanced local inhibition via a switch in IL-1β signaling to the canonical pro-inflammatory MyD88 pathway. Ethanol dependence also increased mPFC IL-1β levels, while decreasing expression of downstream effectors (Akt, p38 MAPK). Thus, IL-1β may represent a key neural substrate in ethanol-induced cortical dysfunction. As the IL-1 receptor antagonist (kineret) is already FDA-approved, this work underscores the high therapeutic potential of IL-1 signaling/neuroimmune-based treatments for AUD.
Keywords: Akt, alcohol, interleukin 1, IL-1β, IL-1R1, MyD88, neuroimmune, p38 MAPK, PI3K, sIPSC, synaptic transmission
1. Introduction
Alcohol use disorder (AUD) is a chronic, relapsing disease characterized by compulsive alcohol use, loss of control over alcohol intake, and a negative emotional state when not using alcohol. Individuals with AUD have reduced prefrontal cortex volumes and deficits in prefrontal tasks, such as emotional processing, decision-making and inhibitory control (Le Berre et al., 2017; Stavro et al., 2013). The neuroimmune system, including interleukin 1 (IL-1) signaling, influences cognitive function and its dysfunction is implicated in AUD (Crews and Vetreno, 2014; Roberto et al., 2018; Tsai, 2017). Human studies have linked alcohol drinking and AUD risk with functional single nucleotide polymorphisms (SNPs) in the genes encoding the pro-inflammatory cytokine interleukin 1β (IL-1β; IL1B gene) and the IL-1 receptor antagonist (IL-1RA; IL1RN gene) (Liu et al., 2009; Pastor et al., 2005; Saiz et al., 2009; Serretti et al., 2006). Of particular note, the IL1B C-511T SNP (rs16944) identified in several of these AUD studies increases IL-1β gene expression and release and is associated with cognitive decline (Chen et al., 2006; Papiol et al., 2007; Tsai, 2017; Tu et al., 2014). Postmortem studies of individuals with AUD report cortical proliferation of activated microglia and elevated brain and peripheral levels of IL-1β (Coleman et al., 2018; Crews and Vetreno, 2014). This synergistic bidirectional relationship between alcohol and IL-1 likely contributes to the increasingly risky decision-making and impulsivity that drive further cycles of binge drinking as individuals transition to the dependent state.
IL-1β has emerged as a key regulator of the cortical response to ethanol (alcohol). Pro-IL-1β is rapidly cleaved into its active form and released locally from both neurons and glia in response to immune challenge (Nemeth and Quan, 2021; Roberto et al., 2018). IL-1β then binds its cognate IL-1 receptor 1 (IL-1R1) and recruits IL-1R1 accessory protein (IL-1RAcP) to activate the myeloid differentiation primary response 88/p38 mitogen-activated protein kinase (MyD88/p38 MAPK) pathway and trigger an inflammatory response (Davis et al., 2006; Nemeth and Quan, 2021). Importantly, IL-1β/IL-1R1 pro-inflammatory signaling in the central nervous system (CNS) is tightly controlled through several mechanisms; the endogenous antagonist IL-1RA competes with IL-1β for binding to IL-1R1, IL-1R2 is a decoy receptor that sequesters IL-1β, the brain expresses a second IL-1R1 accessory protein (IL-1RAcPb) that cannot activate MyD88, and IL-1β signaling can selectively activate downstream pro-survival pathways including phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) (Davis et al., 2006; Huang et al., 2011; Nemeth and Quan, 2021; Nguyen et al., 2011; Qian et al., 2012; Smith et al., 2009). Gene and protein expression of IL-1β, IL-1R1 and many of these IL-1-related effectors are altered in the cortex of ethanol-exposed rodents and non-human primates (Alfonso-Loeches et al., 2013; Iancu et al., 2018; Pascual et al., 2017; Pradier et al., 2018; Silva-Gotay et al., 2021; Tiwari and Chopra, 2013, 2012; Walter et al., 2020; Warden et al., 2020), and IL-1β-associated neuroinflammatory responses in the cortex and hippocampus of ethanol-dependent rodents are linked to cognitive deficits (Tiwari and Chopra, 2013, 2012). While these data strongly suggest that IL-1 signaling contributes to ethanol-induced cognitive dysfunction, little is known about the underlying cortical mechanisms.
The rodent medial prefrontal cortex (mPFC) is functionally homologous to the human dorsolateral prefrontal cortex, with similar cell types, laminar structure and circuitry to direct motivated behavior and behavioral flexibility (Anastasiades and Carter, 2021). The region is split into subregions; the prelimbic cortex integrates contextual information to mediate conflicting motivational drives (e.g. reward-seeking vs. escaping to safety), whereas the infralimbic cortex plays a more generalized role in active avoidance (Capuzzo and Floresco, 2020; Moorman et al., 2015). Both subregions have superficial input (2/3) and deeper output (5) layers containing glutamatergic pyramidal neurons that are tightly gated by γ-aminobutyric acid (GABA) interneurons (Anastasiades and Carter, 2021). We have previously identified pyramidal neurons in layer 2/3 of the prelimbic cortex as particularly ethanol sensitive (Varodayan et al., 2018), and there is growing interest in understanding how ethanol alters mPFC pyramidal cell excitability and network rhythmicity through its modulation of inhibitory transmission (Hughes et al., 2020, 2019; Joffe et al., 2020; Li et al., 2021; Patel et al., 2022; Pleil et al., 2015). Thus, here we used molecular and cellular physiology approaches in a mouse model of chronic ethanol exposure (Patel et al., 2019) to uncover a dependence-induced pro-inflammatory switch in IL-1’s GABAergic influence at prelimbic layer 2/3 pyramidal neurons.
2. Materials & Methods
All procedures in this study were approved by The Scripps Research Institute (TSRI) Institutional Animal Care and Use Committee, consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.1. Animals
We used adult male C57BL/6J mice (n=202; 29.3±0.24 g) from The Jackson Laboratory (Bar Harbor; ME) and adult male Myd88 knockout mice (n=5; 32.5±1.4 g; provided by Dr. Yuri Blednov from the University of Texas at Austin, Austin, TX; originally from Jackson Laboratories). The mice were housed in a temperature- and humidity-controlled room on a 12 h reverse light cycle, and food and water were available ad libitum.
2.2. Chronic intermittent ethanol – two bottle choice model
We used the chronic intermittent ethanol vapor-2 bottle choice (CIE-2BC) paradigm to generate naïve and ethanol-exposed C57BL/6J mice (Figure 1A) (Patel et al., 2019, 2021b). The paradigm produces three animal groups with varying levels of ethanol exposure: 1) naïve mice that did not receive ethanol, 2) non-dependent mice that only received ethanol through limited access 2BC drinking sessions and have a moderate level of voluntary ethanol consumption, and 3) ethanol-dependent mice that received CIE vapor exposure in addition to 2BC drinking sessions and thus escalated their voluntary ethanol intake.
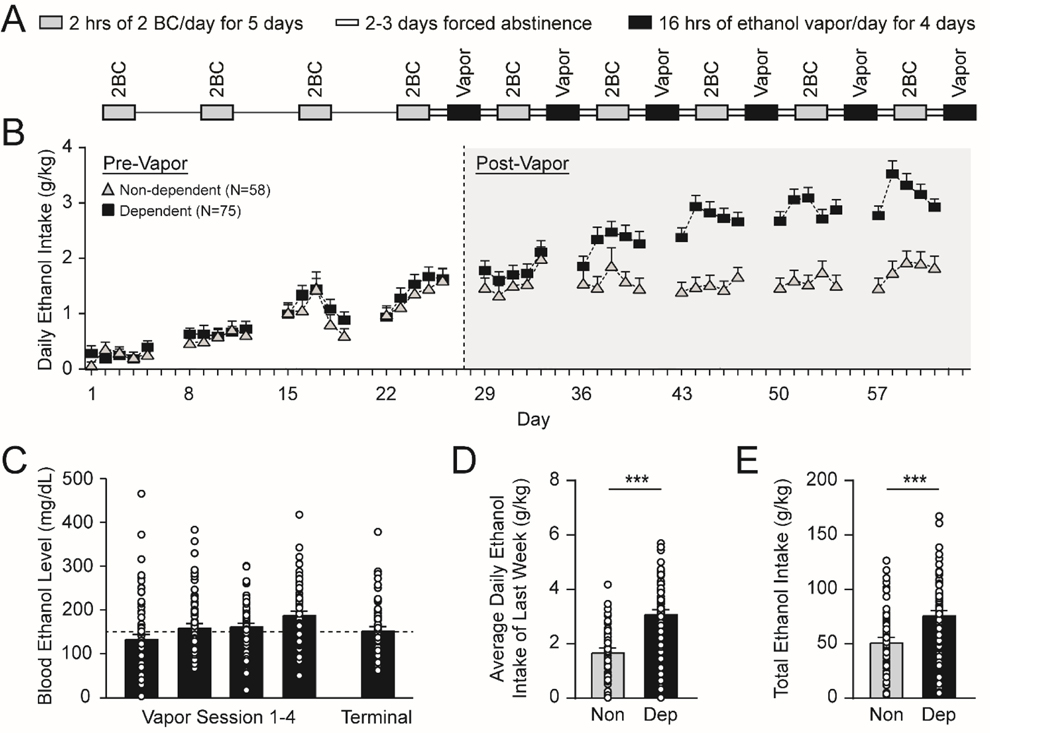
Figure 1.
Chronic-intermittent ethanol (CIE) - two-bottle choice (2BC) paradigm. A. Schematic illustrating the CIE-2BC behavioral paradigm used to induce ethanol dependence in C57BL/6J mice. B. Daily ethanol intake during 2BC drinking sessions escalates in dependent vs. non-dependent mice. C. Average blood ethanol levels (BEL) and terminal BEL achieved by the ethanol dependent mice during weekly CIE vapor exposure. D-E. Dependent mice consume more ethanol (D) during the last week of 2BC, and (E) across all 2BC drinking sessions compared to non-dependent mice. All data are presented as mean±SEM. N=38 non-dependent and 66 dependent mice. ***p<.001 by unpaired t-test.
To establish baseline 2BC ethanol drinking, mice were singly housed 30 min before the lights were turned off and given limited access (2 h) to two drinking tubes (one with 15% w/v ethanol and the other with tap water) for 5 days per week for 4 consecutive weeks. Mice were returned to group housing in their home cages at the end of each drinking session. The mice were then assigned to the non-dependent group or to be made ethanol dependent, with both groups matched for ethanol and water consumption (Figure 1B; water data not shown).
Ethanol dependent mice underwent 4–5 cycles of a 2-week protocol: 4 days of CIE (16 h ethanol vapor/8 h air) in vapor chambers (La Jolla Alcohol Research, La Jolla, CA), 3 days forced abstinence, 5 days of 2BC (same parameters as in baseline training) and 2 days forced abstinence (Figure 1A–B). Immediately prior to each ethanol vapor exposure, dependent mice were i.p. injected with 1.75 g/kg ethanol + 68.1 mg/kg pyrazole (alcohol dehydrogenase inhibitor from Sigma, St; Louis, MO). Blood ethanol levels (BELs) were obtained weekly on the third or fourth day of vapor exposure; tail bloods were collected with heparinized capillary tubes and centrifuged for 20 min at 13000 rpm at 4oC, and the supernatants processed in a GM7 analyzer (Analox Instruments, London, UK). Ethanol drip rates in the vapor chambers were adjusted to achieve BELs of 150–250 mg/dL (Figure 1C). A total of 78 ethanol-dependent mice were generated, of which 60 were sacrificed immediately after their final vapor session (Dep group) and 6 were withdrawn for 3–5 days (WD group). Non-dep mice (N=58) received 2BC on the same schedule as the dependent mice to control for ethanol intake, but were exposed to air in identical chambers. Naïve mice (N=66) were not exposed to any ethanol. Naïve and non-dependent mice also received 68.1 mg/kg pyrazole in saline i.p. on the same injection schedule as the dependent mice. All mice were sacrificed at the same time-point as Dep mice, with Non-dep mice sacrificed immediately after their final air session (Frank et al., 2013; Haun et al., 2018; Huitron-Resendiz et al., 2018; Lopez et al., 2017; Okhuarobo et al., 2020; Patel et al., 2019, 2021b; Piggott et al., 2020; Schweitzer et al., 2016; Warden et al., 2020). Of note, the route of ethanol exposure (drinking vs. vapor exposure), time period of exposure (2 h vs. 16 h), and the resultant average blood ethanol levels (~50 vs. ~150 mg/dL, data not shown) are different between Non-dep and Dep mice. Therefore, the Non-dep mice form a secondary control group that allow for determination of whether changes observed in Dep mice specifically result from the chronic ethanol vapor (which produces the dependent phenotype) or after ethanol drinking alone. To probe any potential impact that the timing of the last ethanol exposure (3–6 days prior to sacrifice in the Non-dep mice) might have on IL-1 signaling, a group of ethanol vapor-exposed mice were withdrawn for 3–5 days (WD mice).
2.3. Immunohistochemistry and confocal microscopy
We conducted the IL-1β immunostaining as reported recently (Patel et al., 2019). Briefly, the mice (N=4 per group) were perfused with ice-cold PBS followed with Z-Fix fixation solution (Anatech Ltd., Battle Creek, MI). The brains were postfixed in Z-Fix at 4˚C for 72 h, cryoprotected with 30% sucrose at 4˚C for ~72 h (until brains sank), flash frozen with 2-methylbutane on dry ice, and stored at −80˚C. Using a cryostat, we cut sequential coronal sections (35 μm) containing the mPFC (Bregma 1.54–1.98 mm) and stored them in cryoprotective solution (50% v/v phosphate buffer, 30% w/v sucrose, 1% w/v polyvinylpyrrolidone, 30% v/v ethylene glycol) at −20˚C until staining. For immunostaining, we used every third section (n=4 slices per mouse). The sections were blocked in 10% normal donkey serum in PBS followed by incubation in an unconjugated Fab fragment donkey anti-mouse IgG for 2 h at room temperature. Then, we incubated sections in an antibody cocktail of mouse anti-NeuN (1:500, MAB377, Millipore, MA) (Rakela et al., 2018; Zhuo et al., 2001), rabbit anti-IL-1β (1:500, ab9787, Abcam, MA) (Kim et al., 2015; Shin et al., 2014) and goat anti-Iba-1 (1:300, NB1001028, Novus Biologicals, CO) (Aniszewska et al., 2015; Gentry et al., 2022) overnight at 4°C. The next day, sections were incubated in secondary antibody solution containing Alexa Fluor 488-conjugated donkey anti-rabbit and Alexa Fluor 647-conjugated donkey anti-goat. Afterwards sections were incubated in a biotin-conjugated donkey anti-mouse secondary followed by Alexa Fluor 568-conjugated streptavidin. Sections were then incubated in Hoechst 33342 (1:1000) for 1 h at room temperature. The specificity of the anti-IL-1β antibody was previously confirmed by pre-absorption with recombinant mouse IL-1β, which blocked further staining with the IL-1β antibody (Patel et al., 2019).
All 3D tiled and stitched images were acquired with a Zeiss laser scanning confocal microscope (LSCM) 880 Airyscan using a 40x (1.4 na) and 63x (1.4 na) objective and the 32-channel GaAsP-PMT area detector. All 12-bit multi-tiled image stacks (on average 20 slices at 40x and 30 slices at 63x) were acquired with Nyquist resolution parameters using a 0.3 μm step size and optimal frame size of 1932×1932 of the entire PFC region. Images were imported as 3D tiled mega images into Imaris (Bitplane, MA USA) software for image analysis. Within Imaris NeuN-immunolabeled neurons and Iba-1-immunolabeled microglia fluorescent signals were surface rendered as 3D regions of interest (ROIs). Each of these ROIs of both cell types were further filtered by the presence of an intracellular nuclear signal to mark it a viable cell for further analysis. These volume-rendered cell ROIs were then used to create a mask of IL-1β signals that reside within these cell volumes. The surface rendering module was then used to 3D outline fluorescent signals of IL1β per voxel, using intensity thresholds above background based on samples labeled with secondary antibodies alone. These 3D rendered total areas and volumes of intracellular IL1β clusters were exported into excel and scored in accordance with total cell volume per sample. 4 left and right hemispheres of mPFC from 4 different mice were imaged per group, with a total of 2500 neurons and 1000 microglia analyzed per group. We used nested t-tests and one-way ANOVA to determine statistically significant changes in the IL-1β expression between the neurons and microglia, and among the ethanol treatment groups, respectively.
2.4. Western Blotting
Western blot analyses for brain regional changes in protein were conducted in mice as previously described (Franklin and Paxinos, 2008; Pahng et al., 2017). Mice (N=12 per group) were anesthetized with 3–5% isoflurane and decapitated, and the brains were rapidly removed, snap-frozen in isopentane, and stored at −80 ºC until brain region dissection. The night before dissection, brains were moved to −20 ºC. During the dissection, the brains were mounted and sliced using a cryostat at −12 ºC. mPFC brain punches (0.5 mm thick) were taken from frozen tissue using a 16-gauge needle according to (Franklin and Paxinos, 2008). Brain punches were homogenized by sonication in a lysis buffer (320 mm sucrose, 5 mm HEPES, 1 mm EGTA, 1 mm EDTA, 1% SDS, protease inhibitor cocktail (diluted 1:100), and phosphatase inhibitor cocktails II and III (diluted 1:100); Sigma, St. Louis, MO, USA). Tissue homogenates were heated at 95 ºC for 5 min and the total protein concentration was measured using a detergent-compatible Lowry method (Bio-Rad, Hercules, CA, USA). The samples were aliquoted and stored at −80 ºC until Western blotting. Samples of protein (20 μg) were separated by SDS-polyacrylamide gel electrophoresis on 12% acrylamide gels using a Tris/Tricine/SDS buffer system (Bio-Rad). The gels were electrophoretically transferred to polyvinylidene difluoride membranes (GE Healthcare, Piscataway, NJ, USA). Membranes were blocked for 1 h in 5% non-fat milk at room temperature and incubated overnight in 2.5% non-fat milk with primary antibody at 4 ºC. The primary antibodies were phospho-Akt (Thr308) (1:500; Cell Signaling; Cat # 13038), phospho-Akt (Ser473) (1:3000; Cell Signaling; Cat # 4060), phospho-p38 MAPK (Thr180/Tyr182) (1:5000; Cell Signaling; Cat # 4511), and mouse IL-1R1 (1:2000; R&D Systems; Cat # AF771). Membranes were washed and incubated with species-specific peroxidase-conjugated secondary antibody (1:10,000; Bio-Rad) for 1 h at room temperature. Membranes were washed and incubated in a chemiluminescent reagent (Immobilon Crescendo Western HRP Substrate, Millipore Corporation, Billerica, MA, USA), and exposed to film. Following film development, membranes were stripped for 30 min at room temperature (Restore; Thermo Scientific) and reprobed for total Akt (1:3000; Cell Signaling; Cat # 4691), total p38 MAPK (1:5000; Cell Signaling; Cat # 9212), total ERK (1:20000; Cell Signaling; Cat # 9102), or β-tubulin (1:1,000,000; Santa Cruz Biotechnology; Cat # sc-53140) levels. The immunoreactivity of the bands was detected using densitometry (Image J 1.45S; Bethesda, MD, USA). To normalize the data across the blots, the densitometry values were expressed as a percentage of the mean of the naïve controls for each gel.
2.5. Electrophysiology
Mice were anesthetized (3–5% isoflurane) and decapitated, and the brains placed in oxygenated (95% O2/5% CO2), cold high-sucrose solution (pH 7.3–7.4; in mM): 206.0 mM sucrose; 2.5 mM KCl; 0.5 mM CaCl2; 7.0 mM MgCl2; 1.2 mM NaH2PO4; 26.0 mM NaHCO3; 5.0 glucose; 5.0 HEPES, as previously described (Schweitzer et al., 2016; Varodayan et al., 2022, 2018). Coronal slices (300 μm) containing the prelimbic and infralimbic cortices were sectioned (Leica VT1200S; Buffalo Grove, IL, USA) and incubated in oxygenated artificial cerebrospinal fluid: 130 mM NaCl, 3.5 mM KCl, 2 mM CaCl2, 1.25 mM NaH2PO4, 1.5 mM MgSO4, 24 mM NaHCO3, and 10 mM glucose for 30 min at 32 °C and then for 30 min at room temperature.
Cells in the prelimbic cortex were visualized with infrared-differential interference contrast (IR-DIC) optics, a w60 water immersion objective (Olympus BX51WI, Tokyo, Japan) and a CCD camera (EXi Aqua, QImaging, Surrey, BC, Canada), and layer 2/3 pyramidal cells located 100–300 μm from the pial surface were identified by their characteristic size and shape (Salling and Harrison, 2014; Varodayan et al., 2018). Whole-cell voltage-clamp recordings from a total of 277 neurons were performed in gap-free acquisition mode with a sampling rate per signal of 20 kHz and low-pass filtered at 10 kHz, using a Multiclamp 700B amplifier, Digidata 1440A and pClamp 10.2 software (all Molecular Devices, Sunnyvale, CA, USA).
Pipettes (3–6 MΩ resistance) were filled with KCl internal solution: 145 mM KCl, 5 mM EGTA, 2 mM MgCl2, 10 mM HEPES, 2 mM Mg+-ATP, 0.2 mM Na+-GTP; or K-gluconate internal solution: 145 mM K-gluconate, 5 mM EGTA, 2 mM MgCl2, 10 mM HEPES, 2 mM Mg+- ATP, 0.2 mM Na+-GTP. Spontaneous or miniature γ-aminobutyric acid ionotropic receptor (GABAA)-mediated inhibitory postsynaptic currents (s/mIPSCs) were pharmacologically isolated using 20 μM 6,7-dinitroquinoxaline-2,3-dione (DNQX), 30 μM DL-2-amino-5-phosphonovalerate (APV) and 1 μM CGP 55845A, and in the case of mIPSCs, 0.5 μM tetrodotoxin. Spontaneous glutamatergic excitatory postsynaptic currents (sEPSCs) were pharmacologically isolated using 1 μM CGP 55845A and 30 μM bicuculline. All neurons were clamped at −70 mV. Series resistance was monitored with a 10 mV pulse, and recordings with a series resistance > 15 MΏ or a > 20% change in series resistance were excluded from the final data set. Each compound was applied directly to the bath in known concentrations. We purchased Akt inhibitor (MK-2206), AP-5, CGP 55845A, DNQX, p38 MAPK inhibitor (SB202190) and PI3K inhibitor (LY294002) from Tocris Biosciences (Ellisville, MI, USA); recombinant mouse IL-1β from BioLegend (San Diego, CA, USA); recombinant human IL-1RA from Peprotech (Rocky Hill, NJ, USA); bicuculline and tetrodotoxin from Sigma (St. Louis, MO, USA); and alcohol (ethanol) from Remet (La Mirada, CA, USA). Hydrocinnamoyl-L-valyl-pyrrolidine (AS-1) - a small-molecule MyD88 mimetic - was synthesized as previously described (Bajo et al., 2019a; Bartfai et al., 2003; Davis et al., 2006). Importantly, AS-1 is specifically targeted to the IL-1R1/MyD88 interaction as it was modeled on a three amino acid sequence at the BB-loop of the TIR domain (Bartfai et al., 2003; Davis et al., 2006). Other pro-inflammatory members of the Toll-like receptor (TLR) family have different MyD88/TIR domain-mediated interactions due to amino acid variations in their BB-loops, and empirical evidence supports that AS-1 does not alter their recruitment of MyD88.
Each experiment was conducted using tissue from a minimum of 3 mice, with 1–3 cells per animal contributing to a single treatment group. Group sizes ranged from 5–18 cells from 3–12 mice depending on the study, with the exact numbers for each experiment provided in the figure legends. For all experiments, the analyzer was blinded to the animal treatment group, but not the ex vivo drug applications. E/IPSC properties (frequency, amplitude, rise time and decay time) were analyzed using Mini Analysis (Synaptosoft Inc., Fort Lee, NJ) and visually confirmed. In these experiments, decreased frequencies indicate lower neurotransmitter release probabilities, while changes in amplitude and kinetics are linked to altered postsynaptic receptor composition/expression (Otis et al., 1994). Events <5 pA were excluded from the analysis, and the average properties from a minimum of 60 events during a 3 min interval within 6–15 min of drug application were calculated. To control for cell-to-cell variation in baseline electrophysiology properties, drug effects were normalized to their own neuron’s baseline prior to group analyses. All final values were analyzed for independent significance using one-sample t-tests and compared using paired t-tests or one-way ANOVAs with a post hoc Tukey’s multiple comparisons test where appropriate, using Prism 9.5.0 (GraphPad, San Diego, CA). Generally, increased E/IPSC frequencies are associated with higher release probabilities, while changes in E/IPSC amplitude and kinetics are linked to altered postsynaptic receptor function (Otis et al., 1994).
3. Results
3.1. Dependent mice escalate their alcohol drinking
We used the chronic intermittent ethanol (CIE) - two bottle choice (2BC) paradigm to generate ethanol-naïve and ethanol-exposed C57BL/6J mice (Figure 1A–C). The paradigm produces three animal groups with varying levels of ethanol exposure: 1) naïve mice that did not receive ethanol, 2) non-dependent mice that only received ethanol through limited access 2BC drinking sessions and have a moderate level of voluntary ethanol consumption, and 3) ethanol-dependent mice that received CIE vapor exposure in addition to 2BC drinking sessions and thus escalated their voluntary ethanol intake. The dependent mice had a higher daily ethanol intake during the last week of drinking and consumed a greater total amount of ethanol across all drinking sessions compared to non-dependent mice (Daily intake: t(134)=6.84, p<.001; Total intake: t(134)=4.37, p<.001 by unpaired t-test Figure 1D–E).
3.2. Ethanol dependence increases mPFC IL-1β
Since interleukin-1 (IL-1) signaling is a key mediator of the mPFC’s response to alcohol, we hypothesized that expression of key IL-1 effectors may be altered by dependence. Immunohistochemical analyses revealed that mPFC IL-1β co-labeled to a greater extent with a neuronal marker (NeuN: 13.85±2.72 integrated density/cell; n= 8–9 sections) than a microglial marker (Iba1: 1.89±0.55 ID/cell; n=8–9 sections) in naïve mice, suggesting that neurons are a primary local source of IL-1β under physiological conditions (t(14)=4.31, p=.0007 by nested t-test; Figure 2A–B). IL-1β staining was increased in mPFC neurons of dependent mice compared to naïve and non-dependent animals (F(2,9)=12.13, p=0.0028; Figure 2A, C). Dependence also increased microglial IL-1β staining (F(2,9)=11.03, p=.0038; Figure 2A, D). In contrast, western blot analyses of naïve vs. dependent mice revealed no change in mPFC IL-1 receptor 1 (IL-1R1) protein abundance (Figure 2E) or the structural protein β-tubulin (loading control; Figure 2F). Of note, protein levels were not examined in the non-dependent mice since their IL-1β cellular expression patterns and functional responses (see below) matched those of the ethanol-naïve mice. Thus, IL-1β may represent a key neural substrate in the mPFC dysfunction associated with AUD.
Figure 2.

Ethanol dependence increases mPFC IL-1β expression. A. Representative serial tiled and stitched confocal images (left), 3D rendered 63X confocal images (middle), and two magnified maximum intensity projection confocal images (right) of the mPFC showing IL-1β (green) co-labeling with NeuN (red) and Iba-1 (orange) from naïve, non-dependent and dependent mice. B. Nested t-test comparison of IL-1β expression between neurons and microglia. Histogram bars correspond to average values per animal calculated from 2 sections (individual points). C-D. Quantification of IL-1β co-labeling with (C) NeuN and (D) Iba1 in naïve, non-dependent and dependent mice using nested ANOVA. E-F. Representative images and histograms of western blot analyses of (D) IL-1R1 and (E) β-tubulin in naïve vs. dependent mice. All data are presented as mean±SEM. N=12 mice per group for western blotting and N=4 per group for the immunohistochemistry. ###p<.001 by post hoc Tukey’s test.
3.3. IL-1β signaling decreases mPFC inhibitory tone in naïve mice
We next examined the impact of IL-1 signaling on mPFC activity, specifically focusing on layer 2/3 pyramidal neurons in the prelimbic cortex of naïve mice (basal sIPSC properties reported in Table 1). We found exogenous application of IL-1β [50 ng/mL (Bajo et al., 2015; Patel et al., 2019)] decreased the frequency of action potential-dependent sIPSCs to 81.2±4.1% of baseline (t(17)=4.58, p<.001 by one sample t-test), but had no effect on the sIPSC amplitude or kinetics (Figure 3A–C). Pretreatment with IL-1 receptor antagonist (IL-1RA; 100 ng/mL) prevented IL-1β’s presynaptic actions (t(22)=3.07, p<.001 by unpaired t-test; Figure 3D). Moreover, application of IL-1RA alone increased the sIPSC frequency to 129.7±8.5% (t(10)=3.51, p<.01 by one-sample t-test; Figure 3B, E), indicating that IL-1R1 regulates mPFC interneuron activity under physiological conditions perhaps due to basal IL-1β release.
Table 1.
Basal sIPSC properties in prelimbic cortex layer 2/3 pyramidal neurons
| Treatment | Frequency (Hz) | Amplitude (pA) | Rise Time (ms) | Decay Time (ms) | |
|---|---|---|---|---|---|
| sIPSCs | Naïve (n=79 cells from 50 mice) | 1.99±0.12 | 54.1±1.3 | 2.07±0.04# | 5.11±0.12 |
| Non-dependent (n=42 cells from 34 mice) | 1.77±0.14 | 52.0±1.7 | 2.14±0.04## | 5.39±0.19 | |
| Dependent (n=79 cells from 47 mice) | 1.99±0.13 | 51.9±1.3 | 2.09±0.04# | 5.30±0.27 | |
| Withdrawn (n=12 cells from 6 mice) | 1.69±0.21 | 47.1±2.7 | 1.78±0.05 | 5.83±0.27 |
Baseline sIPSC frequencies, amplitudes, rise times and decay times in prelimbic layer 2/3 pyramidal neurons of naïve, non-dependent, dependent and 3–5 days withdrawn mice. There were significant differences in the sIPSC rise times across animal groups as compared by one-way ANOVA [rise time: F(3,214)=3.0698, p<.001], but not the other sIPSC properties [frequency: F(2,214)=0.69, p=.56; amplitude: F(3,214)=1.46, p=.22; decay time: F(3,214)=1.33, p=.26]. All data are presented as mean±SEM.
p<.05
p<.01 vs. withdrawn by Tukey’s post hoc test.
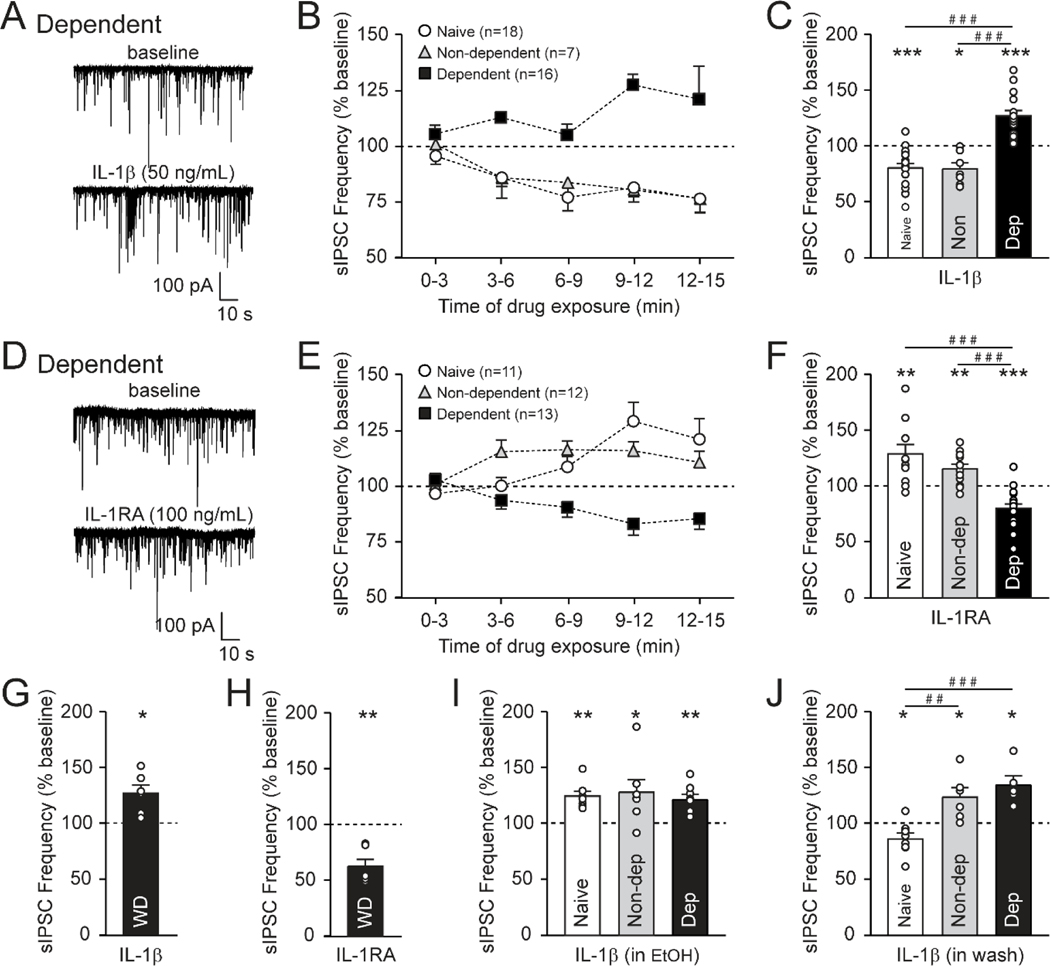
Figure 3.
IL-1β dampens prelimbic cortex layer 2/3 inhibitory tone in naïve mice. A. Schematic illustrating the location of the prelimbic cortex (PrL) (left panel). Pipettes were directed at layer 2/3 (×4, middle panel), and pyramidal neurons were identified by their triangular shape with IR-DIC optics (×60, right panel). B. Representative sIPSC traces from PrL layer 2/3 pyramidal neurons before and during 50 ng/mL IL-1β or 100 ng/mL IL-1RA application in naïve mice. C. 9–12 min of IL-1β decreases the sIPSC frequency, but does not alter its amplitude or kinetics (n=18 cells from 12 mice). D. Pretreatment with IL-1RA blocked IL-1β’s effects on the sIPSC frequency (n=6–18 cells from 4–12 mice). Note, the IL-1β alone data is repeated from panel C. E. 9–12 min of IL-1RA increases the sIPSC frequency, but does not alter its amplitude or kinetics (n=11 cells from 9 mice). F. IL-1β decreases the mIPSC frequency (n=6 cells from 4 mice). G-H. IL-1β has no effect on the (G) sIPSC frequency of PrL layer 5 pyramidal neurons, or (H) the glutamatergic sEPSC frequency of PrL layer 2/3 pyramidal neurons in naïve mice (n=7–8 cells from 4 mice). I. IL-1β increases the sIPSC frequency in the presence of ex vivo ethanol application (EtOH, 44 mM for a 15 min pretreatment) in PrL layer 2/3 pyramidal neurons of naïve mice. A 15 min washout of the EtOH pretreatment restores the IL-1β-induced decrease in sIPSC frequency (n=7–18 cells from 6–12 mice). Note, the IL-1β alone data is repeated from panel C. All data are presented as mean±SEM. *p<.05, **p<.01, ***p<.001 by one-sample t-test or paired t-test; ###p<.001 by post hoc Tukey’s test.
IL-1β’s regulation of GABA synapses was maintained in the presence of the voltage-gated sodium channel blocker TTX (action potential-independent mIPSCs), suggesting that it acts directly on local interneurons to reduce GABA release (t(5)=4.05, p<.01 by one sample t-test; Figure 3F). Moreover, IL-1β’s influence is restricted to prelimbic layer 2/3 inhibitory synapses, as it did not alter prelimbic layer 5 GABA transmission or prelimbic layer 2/3 glutamate transmission (Figure 3G–H). Thus, IL-1β reduces GABA release onto prelimbic layer 2/3 pyramidal neurons in naïve mice, but does not affect postsynaptic GABAA receptor function.
3.4. Acute ethanol does not alter GABA transmission but transiently switches the IL-1β inhibitory response
Bath application of acute EtOH (44 mM) did not alter any sIPSC properties in prelimbic pyramidal neurons of naïve mice (Supplemental Figure 1A). Despite the lack of effect of acute EtOH alone, we investigated whether mPFC IL-1 signaling is sensitive to acute ethanol (EtOH). We found a significant differences in GABAergic synaptic responses to IL-1β co-application with EtOH, or after EtOH washout (F(2,30)=20.12, p<.001 by one-way ANOVA and post hoc Tukey’s test; Figure 3I). Specifically, an acute EtOH pretreatment (44 mM for 12–15 min) caused IL-1β to increase the sIPSC frequency (125.4±4.6%; t(6)=5.55, p<.01 by one-sample t-test). This functional switch required the presence of EtOH as a 15 min washout of EtOH restored the ability of IL-1β to decrease GABA release (87.0±5.2%; t(7)=2.50, p<.05). Therefore, acute ethanol transiently interferes with IL-1 signaling to cause it to enhance inhibitory input onto prelimbic layer 2/3 pyramidal neurons under ethanol naïve conditions.
3.5. The switch in IL-1β’s synaptic influence persists with ethanol dependence
To investigate whether ethanol dependence produces a long-lasting shift in IL-1 signaling at prelimbic layer 2/3 pyramidal neurons we used the CIE-2BC paradigm. We found that IL-1β and IL-1RA synaptic responses were sensitive to prior chronic ethanol exposure (IL-1β: F(2, 38)=34.13, p<.001; IL-1RA: F(2, 38)=24.05, p<.001 by one-way ANOVA and post hoc Tukey’s test; Figure 4A–F). The effects of IL-1β and IL-1RA in non-dependent mice were similar to the naïve mice (IL-1β: 80.2±5.5%; t(6)=3.61, p<.05; IL-1RA: 116.3±4.1%; t(11)=3.94, p<.01 by one sample t-test). In the dependent mice, however, IL-1β (in the absence of acute ethanol) increased the sIPSC frequency (127.9±4.8%; t(15)=5.79, p<.001; Figure 4C), mimicking the effects of IL-1β (in acute EtOH) in naïve animals (see Figure 3I). Despite this chronic ethanol-induced switch in IL-1β’s influence at GABAergic synapses, its effects continued to be mediated by IL-1R1 (Supplemental Figure 2). Likewise, IL-1RA applied alone significantly decreased the sIPSC frequency in dependent mice (80.5±4.0%; t(17)=4.87, p<0.001; Figure 4F), suggesting an ongoing role for IL-1R1 in facilitating GABA release. This switch in the directionality of the effects of IL-1R1 activation and inhibition on GABA release persisted with 3–5 days of withdrawal after CIE-2BC (IL-1β: 127.1±7.3%; t(5)=3.69, p<.05; IL-1RA: 63.0±6.4%; t(5)=5.78, p<.01; Figure 4G–H). Of note, withdrawal also produced a significant decrease in the basal rise time, indicating altered postsynaptic GABAA receptor function (Table 1). Thus, chronic ethanol produces long-lasting neuroadaptation in IL-1 signaling to enhance mPFC inhibitory tone.
Figure 4.
IL-1β potentiates prelimbic cortex (PrL) layer 2/3 inhibitory tone after ethanol dependence. A. Representative sIPSC traces from PrL layer 2/3 pyramidal neurons before and during 50 ng/mL IL-1β application in dependent mice. B-C. (B) Time course and (C) histogram during 9–12 min of IL-1β application on the sIPSC frequency. IL-1β decreases the sIPSC frequency in naïve and non-dependent mice, but increases it in the dependent animals (n=7–18 cells from 6–12 mice). Note, the histogram naïve data is repeated from panel 3C. D. Representative sIPSC traces before and during 100 ng/mL IL-1RA application in dependent mice. E-F. (E) Time course and (F) histogram during 9–12 min of IL-1RA application on the sIPSC frequency. IL-1RA increases the sIPSC frequency in naïve and non-dependent mice, but decreases it in the dependent animals (n=11–13 cells from 9 mice). Note, the histogram naïve data is repeated from panel 3E. G-H. (G) IL-1β increases and (H) IL-1RA decreases the sIPSC frequency in PrL layer 2/3 pyramidal neurons of mice that experienced 3–5 days of withdrawal after their last ethanol vapor exposure (n=6 cells from 6 mice). I. After EtOH pretreatment, IL-1β increases sIPSC frequencies in naïve, non-dependent and dependent mice (n=7–8 cells from 5–6 mice). Note, the naïve data is repeated from panel 3I. J. A 15 min washout of the EtOH pretreatment does not restore the IL-1β-induced decrease in sIPSC frequency in non-dependent mice (n=5–8 cells from 4–6 mice). Note, the naïve data is repeated from panel 3I. All data are presented as mean±SEM. *p<.05, **p<.01, ***p<.001 by one-sample t-test or paired t-test; ##p<.01, ###p<.001 by post hoc Tukey’s test.
Importantly, the relationship between acute ethanol and the directionality of IL-1’s synaptic influence appears to shift after in vivo ethanol treatment. Acute EtOH pretreatment of mPFC slices caused IL-1β to increase the sIPSC frequency to 128.9±11.2% (t(6)=2.59, p<.05 by one sample t-test) in non-dependent mice similar to naïve mice (Figure 4I). But this functional switch was not restored when IL-1β was applied after a 15 min ACSF washout (124.5±8.5%; t(5)=2.87, p<.05), in contrast to the naïve mice and similar to the dependent mice (F(2,16)=13.56, p<.001; Figure 4J). Moreover, bath application of acute EtOH increased the sIPSC frequency only in the dependent mice, and this increase was blocked by pretreatment with IL-1RA suggesting that acute EtOH may increase IL-1β release in animals with a history of heavy chronic ethanol exposure (Supplemental Figure 1B). In a separate experiment, IL-1RA had no effect after pretreatment with EtOH, consistent with EtOH’s synaptic effects requiring the activation of shared intracellular signaling (Supplemental Figure 1C). Overall, these data suggest that while the effects of a single ethanol exposure on IL-1 synaptic activity are transient, the neuroadaptations that accumulate with repeated exposures fundamentally shift how the neuroimmune system shapes mPFC function in dependence.
3.6. IL-1β regulates GABA synapses via two distinct mechanisms in naïve mice
We next employed a pharmacological approach to identify the intracellular mechanisms that mediate IL-1’s dual effects to either decrease or increase GABA release. Blockade of the MyD88/p38 MAPK pro-inflammatory pathway, using a pretreatment of either 50 μM AS-1 (a mimetic that prevents MyD88 recruitment by IL-1R1 (Bajo et al., 2019b, p. 88; Davis et al., 2006)) or 20 μM SB202190 (a p38 MAPK inhibitor), did not alter IL-1β’s ability to decrease the sIPSC frequency in naïve mice (IL-1β in AS-1: 87.4±4.3%; t(7)=2.90, p<.05; IL-1β in SB202190: 85.5±2.2%; t(6)=6.55, p<.001 by one-sample t-test; Figure 5A). Of note, AS-1 and SB202190 had no per se effects on sIPSC frequencies in this study. Additionally, AS-1 had no effect on prelimbic layer 2/3 GABA synaptic activity in MyD88 knockout mice (Supplemental Figure 3).
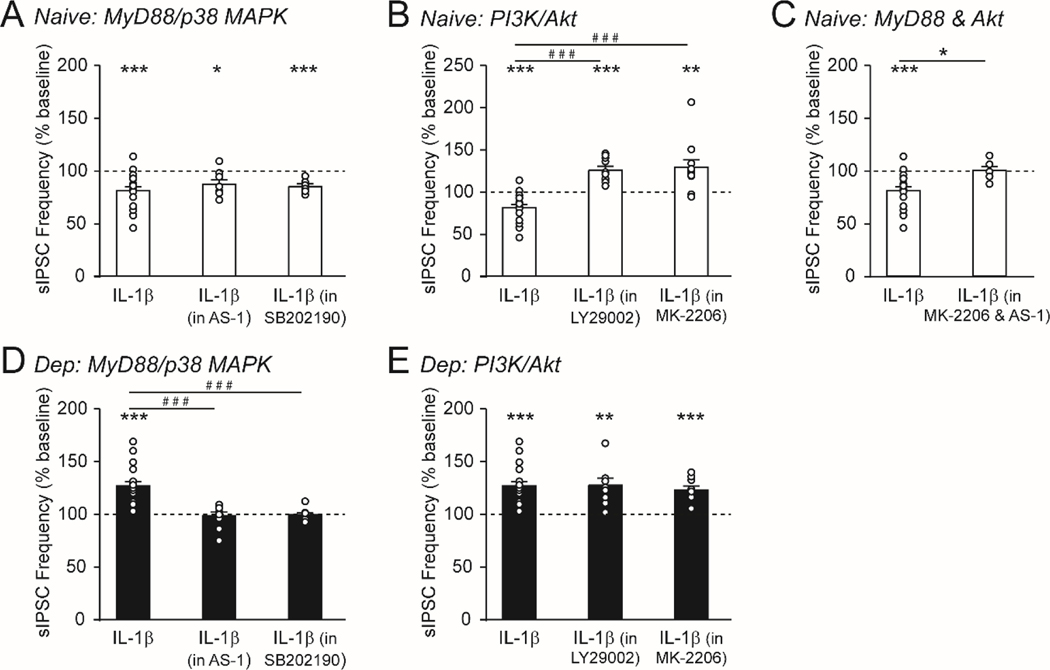
Figure 5.
IL-1β regulates prelimbic cortex layer 2/3 GABA synapses via two distinct downstream signaling mechanisms. A. In naïve mice, pretreatment with the MyD88 mimetic (AS-1, 50 μM) or the p38 MAPK inhibitor (SB202190; 20 μM) did not impact IL-1β’s ability to decrease the sIPSC frequency (n=7–18 cells from 7–12 mice). B. Pretreatment with the PI3K inhibitor (LY294002, 50 μM) or the Akt inhibitor (MK-2206; 200 nM) caused IL-1β to increase the sIPSC frequency in naïve mice (n=10–18 cells from 9–12 mice). C. Pretreatment with both AS-1 + MK-2206 blocked IL-1β’s effects on the sIPSC frequency in naïve mice (n=6–18 cells from 4–12 mice). D. In dependent mice, pretreatment with the MyD88 mimetic or the p38 MAPK inhibitor blocked IL-1β’s effects on the sIPSC frequency (n=9–16 cells from 7–9 mice). E. Pretreatment with the PI3K or Akt inhibitor did not impact IL-1β’s ability to increase the sIPSC frequency in dependent mice (n=10–16 cells from 9 mice). Note, the IL-1β alone data in naïve and dependent mice is repeated from panel 4C. All data are presented as mean±SEM. *p<.05, **p<.01, ***p<.001 by one-sample t-test or paired t-test; #p<.05, ###p<.001 by Tukey’s post hoc test.
IL-1 can also signal through the PI3K/Akt pathway in neurons (Davis et al., 2006, p. 88; Qian et al., 2012), and pretreatment with either 50 μM LY294002 (a PI3K inhibitor) or 200 nM MK-2206 (an Akt inhibitor) prevented the typical IL-1β-induced decrease in sIPSC frequency in naïve mice (F(2,36)=23.75, p<.001 by one-way ANOVA and post hoc Tukey’s test; Figure 5B). Surprisingly, the presence of these inhibitors unmasked a secondary effect of IL-1β to increase the sIPSC frequency (IL-1β in LY294002: 126.2±4.5%; t(9)=5.80, p<.001; IL-1β in MK-2206: 129.3±9.0%; t(10)=3.23, p<.01 by one-sample t-test; Figure 5B), mimicking the effect of IL-1β in dependent animals (see Figure 4C). There were no per se effects of LY294002 or MK-2206 on sIPSC frequencies in this study. Critically, IL-1β had no effect on the sIPSC frequency in naïve mice when both the Akt and MyD88 pathways were blocked (t(22)=2.57, p<.05 by unpaired t-test; Figure 5C), suggesting that IL-1β engages MyD88 signaling in this secondary GABA effect and does not recruit any additional intracellular pathways. Thus, IL-1β/1L-1R1 can regulate mPFC GABA synapses via two distinct mechanisms, though there is a bias towards the pro-survival PI3K/Akt signaling pathway in ethanol naïve conditions.
3.7. Ethanol dependence produces a pro-inflammatory bias in IL-1β signaling
A similar pharmacological approach in dependent mice revealed a shift in IL-1’s recruitment of these intracellular pathways that mirrored the functional switch in its actions at prelimbic layer 2/3 GABA synapses. In the dependent animals, pharmacological blockade of the MyD88/p38 MAPK pathway prevented IL-1β’s actions (F(2,32)=16.71, p<.001 by one-way ANOVA and post hoc Tukey’s; Figure 5D), while PI3K/Akt inhibitors did not alter the potentiating effect of IL-1β (IL-1β in LY294002: 127.7±6.6%; t(10)=4.20, p<.01; IL-1β in MK-2206: 123.1±3.9%; t(9)=5.99, p<.001 by one-sample t-test; Figure 5E).
We then performed Western blot analyses to examine potential dependence-induced changes in the expression of key proteins in these intracellular pathways. We found that total protein levels of Akt and p38 MAPK were significantly decreased in the mPFC of dependent mice compared to naïve mice (Akt: t(22)=6.28, p<.001; p38 MAPK: t(22)=2.40, p<.05 by unpaired t-test), though the phosphorylation ratios of both these proteins remained stable (Figure 6A–E). In contrast, the protein abundance of ERK, a secondary MyD88 signaling pathway that does not mediate IL-1 signaling, was not altered by dependence (Figure 6F). Of note, since protein levels were not examined in the non-dependent mice, downregulation of these intracellular pathways may also occur after the relatively lower doses of ethanol achieved with 2BC alone. Thus, here we used a combination of molecular and neurophysiological approaches to: 1) identify two discrete mechanisms by which IL-1β can influence cortical synaptic activity and 2) establish that ethanol dependence increases IL-1β levels, decreases the expression of key IL-1 downstream effectors and produces a pro-inflammatory bias in IL-1β-induced intracellular signaling to enhance mPFC inhibition.
Figure 6.
Ethanol dependence decreases mPFC expression of downstream IL-1 signaling effectors. A-F. Representative images and histograms of western blot analyses of (A) total Akt, (B) the ratio of phosphorylation at T308 of Akt/total Akt, (C) the ratio of phosphorylation at S473 of Akt/total Akt, (D) total p38 MAPK, (E) the ratio of phosphorylation of p38 MAPK/total p38 MAPK, and (F) total ERK in naïve vs. dependent mice. All data are presented as mean±SEM. N=12 mice per group. *p<.05, ***p<.001 by paired t-test.
4. Discussion
Here we report that the IL-1 system regulates basal mPFC function through its actions at inhibitory synapses on prelimbic layer 2/3 pyramidal neurons in naïve mice. IL-1β can selectively recruit either pro-survival (PI3K/Akt) or pro-inflammatory (MyD88/p38 MAPK) mechanisms to produce opposing synaptic effects (Figure 7A). In ethanol-naïve conditions, there is a strong PI3K/Akt preference leading to a disinhibition of pyramidal neurons (Figure 7B). If this pro-survival pathway is pharmacologically blocked, IL-1β instead engages the MyD88/p38 MAPK pathway to enhance cortical inhibition. Interestingly, with both acute (ex vivo) and chronic (in vivo) ethanol exposure, a pro-inflammatory bias in IL-1 signaling occurs and IL-1β/IL-1R1 enhances GABA release by engaging MyD88/p38 MAPK (Figure 7C). This functional synaptic switch is transient in the case of acute ethanol, but persists with dependence such that the PI3K/Akt pathway remains inaccessible when the pro-inflammatory pathway is blocked. Ethanol dependence also increased IL-1β in mPFC neurons and microglia, while decreasing expression of downstream effectors (Akt, p38 MAPK). This molecular and functional switch in IL-1 signaling may reflect the complex interactions between alcohol and the neuroimmune system, and suggests that IL-1β may represent a key neural substrate in the cortical dysfunction associated with AUD.
Figure 7.
A. Schematic illustrating the two discrete neuroprotective and pro-inflammatory mechanisms by which IL-1β can influence GABAergic transmission in the medial prefrontal cortex (mPFC). B. IL-1R1 may be expressed either pre- or postsynaptically. (1) Its presynaptic activation can selectively recruit either PI3K/Akt or MyD88/p38 MAPK signaling to decrease or increase GABA release, respectively. In naïve mice, there is a strong preference for PI3K/Akt recruitment, leading to a disinhibition of mPFC pyramidal neurons. (2) Postsynaptic IL-1R1 would likely engage a retrograde signal to bidirectionally regulate GABA release via PI3K/Akt or MyD88/p38 MAPK signaling. C. Ethanol dependence increases IL-1β levels and produces a pro-inflammatory bias (MyD88/p38 MAPK) in IL-1R1 signaling. Created with BioRender.com.
4.1. IL-1β modulates basal mPFC function
There is growing evidence that the IL-1 system modulates basal brain activity. We identified IL-1β expression in both mPFC neurons and microglia. On a functional level, ex vivo application of the endogenous competitive antagonist (IL-1RA) increased GABA release onto prelimbic layer 2/3 pyramidal neurons. Thus, at least a subset of mPFC IL-1R1 are constitutively active under physiological conditions, likely due to tonic IL-1β release. Conversely, exogenous 50 ng/mL IL-1β decreased GABA release in naïve mice. Rodent in vivo microdialysis studies report undetectable to low brain levels of IL-1β (0–65 pg/mL) under basal conditions (Folkersma et al., 2008; Gano et al., 2019b; Vasicek et al., 2013), while a low dose ethanol challenge (1.5 g/kg i.p. injection) caused IL-1β to peak at ~300 pg/mL in the rat hippocampus (Gano et al., 2019b). It is important to note that two of these studies measured microdialysis extraction efficiencies of 2–3% at a sampling flow rate of 1 μL/min, suggesting that ethanol may cause brain extracellular levels of IL-1β to rise to a similar concentration as used in our ex vivo study (Folkersma et al., 2008; Vasicek et al., 2013). Regardless, one limitation of our electrophysiological study is that we only tested a single drug concentration. However, similar concentrations of IL-1β (2–50 ng/mL) modulate GABA synapses in multiple brain regions [e.g., cerebellum, hippocampus and the central amygdala (CeA)] (Bajo et al., 2015; Hellstrom et al., 2005; Patel et al., 2021a, 2019; Pringle et al., 1996; Vezzani and Viviani, 2015; Yu and Shinnick-Gallagher, 1994) and have cell type- and brain region-specific effects on glutamate transmission as well as on other neuronal properties (Nemeth and Quan, 2021; Roberto et al., 2018; Vezzani and Viviani, 2015).
IL-1R1 is primarily expressed in neurons and some endothelial cells under normal conditions, but can be induced in astrocytes as part of the inflammatory response (Friedman, 2001; Liu et al., 2019; Moynagh, 2005). The study by Liu et al. also confirmed that IL-1R1 is not expressed in microglia under either normal or inflammatory conditions, and instead endothelial IL-1R1 mediate IL-1β’s indirect activation of microglia (Liu et al., 2019). Notably, neurons are up to one thousand times more sensitive to IL-1β compared to astrocytes or endothelial cells due to neuronal expression of a second isoform of IL-1R1 accessory protein (IL-1RAcPb) that recruits the PI3K/Akt pathway instead of MyD88 (Huang et al., 2011; Liu et al., 2019; Nguyen et al., 2011; Qian et al., 2012; Smith et al., 2009). We found that IL-1β’s disinhibitory effects are action potential-independent ruling out contributions of the wider neural network and suggesting that IL-1R1 is expressed within local synapses (see Figure 7B–C) (Gardoni et al., 2011; Holló et al., 2017). Presynaptic IL-1R1 could bidirectionally modulate GABA release through changes in calcium signaling, or synaptic protein synthesis or posttranslational modification (Neasta et al., 2014; Weston et al., 2012). However, evidence suggests that IL-1R1 is enriched in the postsynaptic terminal (Gardoni et al., 2011; Holló et al., 2017) where its activity could trigger the release of retrograde signals that limit presynaptic GABA release. In particular, endocannabinoids are ethanol-sensitive retrograde signals, and their activity at presynaptic CB1 receptors reduces GABA release via the PI3K/Akt pathway (Neasta et al., 2014; Puighermanal et al., 2009; Varodayan et al., 2017, 2016).
Similar non-immunological IL-1 mechanisms impact a variety of behaviors, including sleep, circadian rhythms, learning and memory, aggression, and pain sensation (Donzis and Tronson, 2014; Krueger, 2008; Shao et al., 2015; Zalcman and Siegel, 2006). For example, physiological concentrations of IL-1β in the hippocampus promote spatial learning and working memory while higher pathological levels produce impairment, likely due to the cytokine’s bidirectional ability to modulate synaptic strength (i.e. long-term potentiation) (Donzis and Tronson, 2014). To the best of our knowledge, there have been no studies directly investigating the cognitive effects of mPFC IL-1 signaling under basal conditions. However, chronic i.c.v. IL-1Ra treatment reversed microglial activation and glutamate/GABA receptor depletion in the prelimbic cortex, and restored non-spatial memory function in hyperammonemic rats (Taoro-González et al., 2019). There are also several human genetic neuroimaging studies that support a link between the IL-1 system in the prefrontal cortex and cognitive function (Papiol et al., 2007; Tu et al., 2014). Of note, several polymorphism in the genes encoding IL-1β and IL-1Ra are associated with alcohol drinking and greater susceptibility to AUD (Liu et al., 2009; Pastor et al., 2005; Saiz et al., 2009; Serretti et al., 2006), suggesting that future studies should examine potential relationships between IL-1 genetic polymorphisms, cortical activity and cognitive function in individuals with AUD.
4.2. Ethanol dependence produces an IL-1β pro-inflammatory bias
Ethanol is a potent activator of IL-1 signaling (Crews and Vetreno, 2014; Roberto et al., 2018). Here, we found that ethanol dependence dramatically increased mPFC IL-1β (but had no effect on IL-1R1) in line with other studies reporting higher cortical and brain IL-1β expression after chronic ethanol exposure in rodents and non-human primates across a variety of ethanol treatment paradigms (Alfonso-Loeches et al., 2013; Iancu et al., 2018; Pascual et al., 2017; Pradier et al., 2018; Silva-Gotay et al., 2021; Tiwari and Chopra, 2013, 2012; Walter et al., 2020; Warden et al., 2020). Of note, a few of these studies identified sex differences in ethanol’s induction of IL-1β, but the effects are inconsistent; one reported an increase in only male PFC (Pascual et al., 2017), another an increase in the PFC of both sexes (Alfonso-Loeches et al., 2013), and the final one a greater increase in female mPFC (Silva-Gotay et al., 2021). These mixed effects might stem from the age of the animals (adolescent vs. adult), route of ethanol administration (experimenter administered i.p. injection vs. voluntary consumption vs. self-administration), and/or length of exposure (2 weeks - 5 months). Thus, a key limitation of the present study is our use of only male subjects, and future studies should explore the impact of mPFC IL-1 signaling in both sexes. Additionally, the majority of these previous studies attribute increased cortical IL-1β production to proliferating microglia (Pradier et al., 2018; Silva-Gotay et al., 2021; Warden et al., 2020), while we observed large increases in both neurons and microglia. These discrepancies may reflect brain region and cell-type differences, as well as dynamic changes in IL-1β signaling during intoxication and withdrawal (Doremus-Fitzwater et al., 2014; Gano et al., 2019a, 2016). While we did not observe any concomitant changes in mPFC IL-1R1 protein levels, dependence could shift synaptic IL-1R1 expression away from neurons to astrocytes (Friedman, 2001; Liu et al., 2019; Moynagh, 2005). In support of this possibility, recent studies have identified several mechanisms of astrocyte-neuronal crosstalk that modulate GABAergic synaptic transmission (Haydon et al., 2009; Lia et al., 2019). Finally, it is important to note that ethanol may also indirectly influence IL-1 signaling through its actions on other neuroimmune factors, such as HMGB1 which can co-release with and then complex to IL-1β to potentiate neuroinflammatory responses (Coleman et al., 2018; Crews and Vetreno, 2014). Given these complexities, it is important that future studies examine the dynamic impact of chronic ethanol exposure and protracted withdrawal in the wider context of the various endogenous mechanisms that tightly control IL-1 signaling (e.g. HMGB1, decoy receptor IL-1R2, etc.).
On a functional level, we found that both ex vivo acute ethanol and in vivo chronic ethanol caused IL-1β to enhance prelimbic layer 2/3 GABA release instead of its usual effect to reduce it. We have previously observed bidirectional synaptic effects of IL-1β in the amygdala, though in that case both GABAergic responses occurred under ethanol naïve conditions (Patel et al., 2019). Here, the switch in IL-1β response was transient with acute ethanol but persisted with dependence even after 3–5 days withdrawal, suggesting that rapid changes after a single exposure may underlie some of the more enduring effects of chronic ethanol. In dependence, this functional switch is accompanied by a synaptic shift to IL-1β recruitment of the MyD88/p38 MAPK pathway. While AS-1 does not disrupt MyD88 recruitment to other members of the TLR/IL-1 receptor superfamily (Bartfai et al., 2003; Davis et al., 2006), future studies should assess whether chronic ethanol produces similar biases in other pro-inflammatory signals.
It is also possible that chronic ethanol completely disrupts PI3K/Akt signaling. However, this is unlikely as PI3K/Akt signaling is essential for neuronal health (Davis et al., 2006; Diem et al., 2003; Qian et al., 2012; Smith et al., 2009) and we observed similar dependence-induced decreases in mPFC Akt and p38 MAPK protein levels with no change in their respective phosphorylation ratios indicative of an intracellular compensatory response to elevated IL-1β levels. Alternatively, ethanol activation of PI3K/Akt induces mammalian target of rapamycin (mTOR) complex 1 activity, leading to enhanced rates of synaptic protein synthesis, altered pre- and postsynaptic transmission, and higher ethanol seeking and consumption (Neasta et al., 2014; Weston et al., 2012). Finally, ethanol interacts with several other cortical mechanisms that may also contribute to this pro-inflammatory switch in mPFC IL-1 synaptic signaling, including hyperpolarization-activated cyclic nucleotide–gated (HCN) channels, anaplastic lymphoma kinases (Alk), TLR 3/TIR-domain-containing adapter-inducing interferon-β signaling, TRAF family member-associated NF-κB activator (TANK), endostatin, corticotropin releasing factor receptor 1 (CRF1) and heat shock factor 1 (HSF1) (Avchalumov et al., 2021; Hughes et al., 2020; McCarthy et al., 2018; Müller et al., 2019; Patel et al., 2022; Pignataro et al., 2007; Salling et al., 2018; Schweitzer et al., 2016; Toledo Nunes et al., 2019; Varodayan et al., 2011; Varodayan and Harrison, 2013).
Given the novelty of our current findings, it is difficult to speculate on the behavioral consequences, particularly regarding how mPFC IL-1 pro-inflammatory bias contributes to alcohol-related cognitive impairment. However, human and preclinical studies report strong associations among chronic alcohol exposure, pro-inflammatory signaling (including IL-1β), and cognitive decline (Coppens et al., 2019; Crews and Vetreno, 2014; Müller et al., 2019; Nunes et al., 2019). Furthermore, recent and ongoing clinical trials of multiple anti-inflammatory treatments for AUD (e.g. ibudilast, apremilast, and minocycline) (Grodin et al., 2021; Meredith et al., 2021; Petrakis et al., 2019), generally report suppression of IL-1β and other pro-inflammatory factors associated with reduced heavy drinking and alcohol craving (Grodin et al., 2021). Interestingly, ibudilast’s positive drinking outcome is associated with reduced functional connectivity in prefrontal cortex-striatum circuits in response to alcohol cues (Burnette et al., 2021). In contrast, minocycline (which inhibits p38 MAPK and pro-inflammatory cytokine production) did not significantly reduce pro-inflammatory cytokine serum levels, alcohol craving or cognitive deficits in individuals with AUD (Petrakis et al., 2019). Collectively, these studies provide strong evidence that pharmacotherapies that target the neuroimmune system may benefit individuals with certain AUD symptoms and/or patient subtypes.
4.3. Conclusions
Developing individualized, highly specific treatment requires a precise neurobiological understanding of the complex interactions between alcohol and neuroimmune function. In this study, we have identified the mPFC IL-1 system as a potential neural substrate of AUD. Specifically, we found that ethanol dependence dramatically increased mPFC IL-1β and triggered a pro-inflammatory bias in IL-1 signaling that enhances local inhibition. It is currently unclear whether this mechanistic switch is a cause or consequence of the pathophysiology of AUD. Regardless, this finding is particularly significant as the IL-1 receptor antagonist (kineret) is FDA approved and so could potentially be repurposed to treat the hypofrontality associated with AUD.
Supplementary Material
Supplemental Figure 1. Acute ethanol increases GABA release in dependent mice via IL-1 signaling. A. Ex vivo ethanol application (EtOH, 44 mM for 15 min) increases the sIPSC frequency in prelimbic cortex layer 2/3 pyramidal neurons of dependent mice, but has no effect in naïve or non-dependent mice (n=13–21 cells from 7–13 mice). B. Pretreatment with IL-1RA (100 ng/mL for 15 min) blocked EtOH’s potentiation of the sIPSC frequency in dependent mice (n=5 cells from 3 mice). C. IL-1RA had no effect after pretreatment with EtOH in dependent mice (n=7 cells from 3 mice). All data are presented as mean±SEM. *p<.05, **p<.01 by one-sample t-test.
Supplemental Figure 2. IL-1β’s presynaptic effects are mediated by IL-1R1 in non-dependent and dependent mice. A-B. Pretreatment with IL-1RA blocked IL-1β’s effects on prelimbic cortex layer 2/3 sIPSC frequencies in (A) non-dependent (n=5–7 cells from 3–6 mice) and (B) dependent mice (n=5–16 cells from 4–9 mice). Note, the IL-1β alone data is repeated from panel 4D. All data are presented as mean±SEM. **p<.01, ***p<.001 by one-sample t-test or paired t-test.
Supplemental Figure 3. AS-1 had no effect on sIPSC properties in prelimbic cortex layer 2/3 pyramidal neurons of ethanol-naïve MyD88 knockout mice (n=10 cells from 5 mice). All data are presented as mean±SEM.
Highlights:
-
5.
IL-1β decreases GABA release via a pro-survival pathway in naïve mice
-
6.
Ethanol dependence increases IL-1β
-
7.
Ethanol dependence causes a synaptic switch in IL-1β signaling
-
8.
IL-1β increases GABA release via a pro-inflammatory pathway in dependent mice
Acknowledgements:
This is manuscript number 30198 from The Scripps Research Institute. This study was supported by grants from the National Institutes of Health: AA013498 (MR), AA027700 (MR), AA021491 (MR), AA017447 (MR), AA006420 (MR), T32-AA007456 (MQS), AA029841 (MR), AA025408 (FPV), AA017823 (FPV), AA025996 (SE), GM129454 (TDD), AA013520 (YAB), The Schimmel Family Chair, The Pearson Center for Alcoholism and Addiction Research, and The Scripps Research Institute’s Animal Models Core Facility.
Footnotes
Declarations of interest: None
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfonso-Loeches S, Pascual M, Guerri C, 2013. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology 311, 27–34. 10.1016/j.tox.2013.03.001 [DOI] [PubMed] [Google Scholar]
- Anastasiades PG, Carter AG, 2021. Circuit organization of the rodent medial prefrontal cortex. Trends Neurosci 44, 550–563. 10.1016/j.tins.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniszewska A, Chłodzińska N, Bartkowska K, Winnicka MM, Turlejski K, Djavadian RL, 2015. The expression of interleukin-6 and its receptor in various brain regions and their roles in exploratory behavior and stress responses. J Neuroimmunol 284, 1–9. 10.1016/j.jneuroim.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Avchalumov Y, Kreisler AD, Xing N, Shayan AA, Bharadwaj T, Watson JR, Sibley B, Somkuwar SS, Trenet W, Olia S, Piña-Crespo JC, Roberto M, Mandyam CD, 2021. Sexually dimorphic prelimbic cortex mechanisms play a role in alcohol dependence: protection by endostatin. Neuropsychopharmacology. 10.1038/s41386-021-01075-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Patel RR, Hedges DM, Varodayan FP, Vlkolinsky R, Davis TD, Burkart MD, Blednov YA, Roberto M, 2019a. Role of MyD88 in IL-1β and Ethanol Modulation of GABAergic Transmission in the Central Amygdala. Brain Sci 9, E361. 10.3390/brainsci9120361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Patel RR, Hedges DM, Varodayan FP, Vlkolinsky R, Davis TD, Burkart MD, Blednov YA, Roberto M, 2019b. Role of MyD88 in IL-1β and Ethanol Modulation of GABAergic Transmission in the Central Amygdala. Brain Sci 9. 10.3390/brainsci9120361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Varodayan FP, Madamba SG, Robert AJ, Casal LM, Oleata CS, Siggins GR, Roberto M, 2015. IL-1 interacts with ethanol effects on GABAergic transmission in the mouse central amygdala. Front Pharmacol 6, 49. 10.3389/fphar.2015.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfai T, Behrens MM, Gaidarova S, Pemberton J, Shivanyuk A, Rebek J, 2003. A low molecular weight mimic of the Toll/IL-1 receptor/resistance domain inhibits IL-1 receptor-mediated responses. Proc Natl Acad Sci U S A 100, 7971–7976. 10.1073/pnas.0932746100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette EM, Ray LA, Irwin MR, Grodin EN, 2021. Ibudilast attenuates alcohol cue-elicited frontostriatal functional connectivity in alcohol use disorder. Alcohol Clin Exp Res 45, 2017–2028. 10.1111/acer.14696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuzzo G, Floresco SB, 2020. Prelimbic and Infralimbic Prefrontal Regulation of Active and Inhibitory Avoidance and Reward-Seeking. J Neurosci 40, 4773–4787. 10.1523/JNEUROSCI.0414-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wilkins LM, Aziz N, Cannings C, Wyllie DH, Bingle C, Rogus J, Beck JD, Offenbacher S, Cork MJ, Rafie-Kolpin M, Hsieh C-M, Kornman KS, Duff GW, 2006. Single nucleotide polymorphisms in the human interleukin-1B gene affect transcription according to haplotype context. Hum Mol Genet 15, 519–529. 10.1093/hmg/ddi469 [DOI] [PubMed] [Google Scholar]
- Coleman LG, Zou J, Qin L, Crews FT, 2018. HMGB1/IL-1β complexes regulate neuroimmune responses in alcoholism. Brain Behav Immun 72, 61–77. 10.1016/j.bbi.2017.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens V, Morrens M, Destoop M, Dom G, 2019. The Interplay of Inflammatory Processes and Cognition in Alcohol Use Disorders-A Systematic Review. Front Psychiatry 10, 632. 10.3389/fpsyt.2019.00632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, 2014. Neuroimmune basis of alcoholic brain damage. Int Rev Neurobiol 118, 315–357. 10.1016/B978-0-12-801284-0.00010-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CN, Mann E, Behrens MM, Gaidarova S, Rebek M, Rebek J, Bartfai T, 2006. MyD88-dependent and -independent signaling by IL-1 in neurons probed by bifunctional Toll/IL-1 receptor domain/BB-loop mimetics. Proceedings of the National Academy of Sciences 103, 2953–2958. 10.1073/pnas.0510802103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diem R, Hobom M, Grötsch P, Kramer B, Bähr M, 2003. Interleukin-1β protects neurons via the interleukin-1 (IL-1) receptor-mediated Akt pathway and by IL-1 receptor-independent decrease of transmembrane currents in vivo. Molecular and Cellular Neuroscience 22, 487–500. 10.1016/S1044-7431(02)00042-8 [DOI] [PubMed] [Google Scholar]
- Donzis EJ, Tronson NC, 2014. Modulation of learning and memory by cytokines: signaling mechanisms and long term consequences. Neurobiol Learn Mem 115, 68–77. 10.1016/j.nlm.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Buck HM, Bordner K, Richey L, Jones ME, Deak T, 2014. Intoxication-and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcohol Clin Exp Res 38, 2186–2198. 10.1111/acer.12481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkersma H, Brevé JJP, Tilders FJH, Cherian L, Robertson CS, Vandertop WP, 2008. Cerebral microdialysis of interleukin (IL)-1beta and IL-6: extraction efficiency and production in the acute phase after severe traumatic brain injury in rats. Acta Neurochir (Wien) 150, 1277–1284; discussion 1284. 10.1007/s00701-008-0151-y [DOI] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF, 2013. Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain Behav Immun 33, 1–6. 10.1016/j.bbi.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K, Paxinos G, 2008. The Mouse Brain in Stereotaxic Coordinates, The coronal plates and diagrams Compact, 3rd Edition. [Google Scholar]
- Friedman WJ, 2001. Cytokines regulate expression of the type 1 interleukin-1 receptor in rat hippocampal neurons and glia. Exp Neurol 168, 23–31. 10.1006/exnr.2000.7595 [DOI] [PubMed] [Google Scholar]
- Gano A, Doremus-Fitzwater TL, Deak T, 2016. Sustained alterations in neuroimmune gene expression after daily, but not intermittent, alcohol exposure. Brain Res 1646, 62–72. 10.1016/j.brainres.2016.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano A, Mondello JE, Doremus-Fitzwater TL, Deak T, 2019a. Rapid alterations in neuroimmune gene expression after acute ethanol: Timecourse, sex differences and sensitivity to cranial surgery. J Neuroimmunol 337, 577083. 10.1016/j.jneuroim.2019.577083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano A, Vore AS, Sammakia MN, Deak T, 2019b. Assessment of Extracellular Cytokines in the Hippocampus of the Awake Behaving Rat Using Large-Molecule Microdialysis Combined with Multiplex Arrays After Acute and Chronic Ethanol Exposure. Alcohol Clin Exp Res 43, 640–654. 10.1111/acer.13963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardoni F, Boraso M, Zianni E, Corsini E, Galli CL, Cattabeni F, Marinovich M, Di Luca M, Viviani B, 2011. Distribution of interleukin-1 receptor complex at the synaptic membrane driven by interleukin-1β and NMDA stimulation. J Neuroinflammation 8, 14. 10.1186/1742-2094-8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry NW, McMahon T, Yamazaki M, Webb J, Arnold TD, Rosi S, Ptáček LJ, Fu Y-H, 2022.Microglia are involved in the protection of memories formed during sleep deprivation. Neurobiol Sleep Circadian Rhythms 12, 100073. 10.1016/j.nbscr.2021.100073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Bujarski S, Towns B, Burnette E, Nieto S, Lim A, Lin J, Miotto K, Gillis A, Irwin MR, Evans C, Ray LA, 2021. Ibudilast, a neuroimmune modulator, reduces heavy drinking and alcohol cue-elicited neural activation: a randomized trial. Transl Psychiatry 11, 355. 10.1038/s41398-021-01478-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun HL, Griffin WC, Lopez MF, Solomon MG, Mulholland PJ, Woodward JJ, McGinty JF, Ron D, Becker HC, 2018. Increasing Brain-Derived Neurotrophic Factor (BDNF) in medial prefrontal cortex selectively reduces excessive drinking in ethanol dependent mice. Neuropharmacology 140, 35–42. 10.1016/j.neuropharm.2018.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Blendy J, Moss SJ, Rob Jackson F, 2009. Astrocytic control of synaptic transmission and plasticity: a target for drugs of abuse? Neuropharmacology 56 Suppl 1, 83–90. 10.1016/j.neuropharm.2008.06.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom IC, Danik M, Luheshi GN, Williams S, 2005. Chronic LPS exposure produces changes in intrinsic membrane properties and a sustained IL-beta-dependent increase in GABAergic inhibition in hippocampal CA1 pyramidal neurons. Hippocampus 15, 656–664. 10.1002/hipo.20086 [DOI] [PubMed] [Google Scholar]
- Holló K, Ducza L, Hegyi Z, Dócs K, Hegedűs K, Bakk E, Papp I, Kis G, Mészár Z, Bardóczi Z, Antal M, 2017. Interleukin-1 receptor type 1 is overexpressed in neurons but not in glial cells within the rat superficial spinal dorsal horn in complete Freund adjuvant-induced inflammatory pain. J Neuroinflammation 14, 125. 10.1186/s12974-017-0902-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Smith DE, Ibanez-Sandoval O, Sims JE, Friedman WJ, 2011. Neuron-Specific Effects of Interleukin-1 Are Mediated by a Novel Isoform of the IL-1 Receptor Accessory Protein. Journal of Neuroscience 31, 18048–18059. 10.1523/JNEUROSCI.4067-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes BA, Bohnsack JP, O’Buckley TK, Herman MA, Morrow AL, 2019. Chronic Ethanol Exposure and Withdrawal Impair Synaptic GABAA Receptor-Mediated Neurotransmission in Deep-Layer Prefrontal Cortex. Alcohol Clin Exp Res 43, 822–832. 10.1111/acer.14015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes BA, Crofton EJ, O’Buckley TK, Herman MA, Morrow AL, 2020. Chronic ethanol exposure alters prelimbic prefrontal cortical Fast-Spiking and Martinotti interneuron function with differential sex specificity in rat brain. Neuropharmacology 162, 107805. 10.1016/j.neuropharm.2019.107805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitron-Resendiz S, Nadav T, Krause S, Cates-Gatto C, Polis I, Roberts AJ, 2018. Effects of Withdrawal from Chronic Intermittent Ethanol Exposure on Sleep Characteristics of Female and Male Mice. Alcohol Clin Exp Res 42, 540–550. 10.1111/acer.13584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iancu OD, Colville A, Walter NAR, Darakjian P, Oberbeck DL, Daunais JB, Zheng CL, Searles RP, McWeeney SK, Grant KA, Hitzemann R, 2018. On the relationships in rhesus macaques between chronic ethanol consumption and the brain transcriptome. Addict Biol 23, 196–205. 10.1111/adb.12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Winder DG, Conn PJ, 2020. Contrasting sex-dependent adaptations to synaptic physiology and membrane properties of prefrontal cortex interneuron subtypes in a mouse model of binge drinking. Neuropharmacology 178, 108126. 10.1016/j.neuropharm.2020.108126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Ojo-Amaize E, Spencer B, Rockenstein E, Mante M, Desplats P, Wrasidlo W, Adame A, Nchekwube E, Oyemade O, Okogun J, Chan M, Cottam H, Masliah E, 2015. Hypoestoxide reduces neuroinflammation and α-synuclein accumulation in a mouse model of Parkinson’s disease. J Neuroinflammation 12, 236. 10.1186/s12974-015-0455-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, 2008. The role of cytokines in sleep regulation. Curr Pharm Des 14, 3408–3416. 10.2174/138161208786549281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre A-P, Fama R, Sullivan EV, 2017. Executive Functions, Memory, and Social Cognitive Deficits and Recovery in Chronic Alcoholism: A Critical Review to Inform Future Research. Alcohol Clin Exp Res 41, 1432–1443. 10.1111/acer.13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Cabrera-Garcia D, Salling MC, Au E, Yang G, Harrison NL, 2021. Alcohol reduces the activity of somatostatin interneurons in the mouse prefrontal cortex: A neural basis for its disinhibitory effect? Neuropharmacology 188, 108501. 10.1016/j.neuropharm.2021.108501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lia A, Zonta M, Requie LM, Carmignoto G, 2019. Dynamic interactions between GABAergic and astrocytic networks. Neurosci Lett 689, 14–20. 10.1016/j.neulet.2018.06.026 [DOI] [PubMed] [Google Scholar]
- Liu L, Hutchinson MR, White JM, Somogyi AA, Coller JK, 2009. Association of IL-1B genetic polymorphisms with an increased risk of opioid and alcohol dependence. Pharmacogenet Genomics 19, 869–876. 10.1097/FPC.0b013e328331e68f [DOI] [PubMed] [Google Scholar]
- Liu X, Nemeth DP, McKim DB, Zhu L, DiSabato DJ, Berdysz O, Gorantla G, Oliver B, Witcher KG, Wang Y, Negray CE, Vegesna RS, Sheridan JF, Godbout JP, Robson MJ, Blakely RD, Popovich PG, Bilbo SD, Quan N, 2019. Cell-Type-Specific Interleukin 1 Receptor 1 Signaling in the Brain Regulates Distinct Neuroimmune Activities. Immunity 50, 317–333.e6. 10.1016/j.immuni.2018.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Miles MF, Williams RW, Becker HC, 2017. Variable effects of chronic intermittent ethanol exposure on ethanol drinking in a genetically diverse mouse cohort. Alcohol 58, 73–82. 10.1016/j.alcohol.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy GM, Warden AS, Bridges CR, Blednov YA, Harris RA, 2018. Chronic ethanol consumption: role of TLR3/TRIF-dependent signaling. Addict Biol 23, 889–903. 10.1111/adb.12539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith LR, Burnette EM, Grodin EN, Irwin MR, Ray LA, 2021. Immune treatments for alcohol use disorder: A translational framework. Brain Behav Immun 97, 349–364. 10.1016/j.bbi.2021.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, Aston-Jones G, 2015. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res 1628, 130–146. 10.1016/j.brainres.2014.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynagh PN, 2005. The interleukin-1 signalling pathway in astrocytes: a key contributor to inflammation in the brain. J Anat 207, 265–269. 10.1111/j.1469-7580.2005.00445.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller CP, Chu C, Qin L, Liu C, Xu B, Gao H, Ruggeri B, Hieber S, Schneider J, Jia T, Tay N, Akira S, Satoh T, Banaschewski T, Bokde ALW, Bromberg U, Büchel C, Quinlan EB, Flor H, Frouin V, Garavan H, Gowland P, Heinz A, Ittermann B, Martinot J-L, Martinot M-LP, Artiges E, Lemaitre H, Nees F, Papadopoulos Orfanos D, Paus T, Poustka L, Millenet S, Fröhner JH, Smolka MN, Walter H, Whelan R, Bakalkin G, Liu Y, Desrivières S, Elliott P, Eulenburg V, Levy D, Crews F, Schumann G, 2019. The Cortical Neuroimmune Regulator TANK Affects Emotional Processing and Enhances Alcohol Drinking: A Translational Study. Cereb Cortex 29, 1736–1751. 10.1093/cercor/bhy341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Barak S, Hamida SB, Ron D, 2014. mTOR complex 1: a key player in neuroadaptations induced by drugs of abuse. J Neurochem 130, 172–184. 10.1111/jnc.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth DP, Quan N, 2021. Modulation of Neural Networks by Interleukin-1. Brain Plast 7, 17–32. 10.3233/BPL-200109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Rothwell NJ, Pinteaux E, Boutin H, 2011. Contribution of interleukin-1 receptor accessory protein B to interleukin-1 actions in neuronal cells. Neurosignals 19, 222–230. 10.1159/000330803 [DOI] [PubMed] [Google Scholar]
- Nunes PT, Kipp BT, Reitz NL, Savage LM, 2019. Aging with alcohol-related brain damage: Critical brain circuits associated with cognitive dysfunction. Int Rev Neurobiol 148, 101–168. 10.1016/bs.irn.2019.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okhuarobo A, Bolton JL, Igbe I, Zorrilla EP, Baram TZ, Contet C, 2020. A novel mouse model for vulnerability to alcohol dependence induced by early-life adversity. Neurobiol Stress 13, 100269. 10.1016/j.ynstr.2020.100269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I, 1994. Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci U S A 91, 7698–7702. 10.1073/pnas.91.16.7698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahng AR, Paulsen RI, McGinn MA, Edwards KN, Edwards S, 2017. Neurobiological Correlates of Pain Avoidance-Like Behavior in Morphine-Dependent and Non-Dependent Rats. Neuroscience 366, 1–14. 10.1016/j.neuroscience.2017.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papiol S, Molina V, Rosa A, Sanz J, Palomo T, Fañanás L, 2007. Effect of interleukin-1beta gene functional polymorphism on dorsolateral prefrontal cortex activity in schizophrenic patients. Am J Med Genet B Neuropsychiatr Genet 144B, 1090–1093. 10.1002/ajmg.b.30542 [DOI] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Marcos M, Torres J-L, Costa-Alba P, García-García F, Laso F-J, Guerri C, 2017. Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict Biol 22, 1829–1841. 10.1111/adb.12461 [DOI] [PubMed] [Google Scholar]
- Pastor IJ, Laso FJ, Romero A, González-Sarmiento R, 2005. Interleukin-1 gene cluster polymorphisms and alcoholism in Spanish men. Alcohol Alcohol 40, 181–186. 10.1093/alcalc/agh153 [DOI] [PubMed] [Google Scholar]
- Patel RR, Khom S, Steinman MQ, Varodayan FP, Kiosses WB, Hedges DM, Vlkolinsky R, Nadav T, Polis I, Bajo M, Roberts AJ, Roberto M, 2019. IL-1β expression is increased and regulates GABA transmission following chronic ethanol in mouse central amygdala. Brain Behav. Immun. 75, 208–219. 10.1016/j.bbi.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RR, Varodayan FP, Herman MA, Jimenez V, Agnore R, Gao L, Bajo M, Cuzon Carlson VC, Walter NA, Fei SS, Grant KA, Roberto M, 2021a. Synaptic effects of IL-1β and CRF in the central amygdala after protracted alcohol abstinence in male rhesus macaques. Neuropsychopharmacology. 10.1038/s41386-021-01231-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RR, Wolfe SA, Bajo M, Abeynaike S, Pahng A, Borgonetti V, D’Ambrosio S, Nikzad R, Edwards S, Paust S, Roberts AJ, Roberto M, 2021b. IL-10 normalizes aberrant amygdala GABA transmission and reverses anxiety-like behavior and dependence-induced escalation of alcohol intake. Prog Neurobiol 199, 101952. 10.1016/j.pneurobio.2020.101952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RR, Wolfe SA, Borgonetti V, Gandhi PJ, Rodriguez L, Snyder AE, D’Ambrosio S, Bajo M, Domissy A, Head S, Contet C, Dayne Mayfield R, Roberts AJ, Roberto M, 2022. Ethanol withdrawal-induced adaptations in prefrontal corticotropin releasing factor receptor 1-expressing neurons regulate anxiety and conditioned rewarding effects of ethanol. Mol Psychiatry. 10.1038/s41380-022-01642-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Ralevski E, Gueorguieva R, Sloan ME, Devine L, Yoon G, Arias AJ, Sofuoglu M, 2019. Targeting neuroinflammation with minocycline in heavy drinkers. Psychopharmacology (Berl) 236, 3013–3021. 10.1007/s00213-019-05205-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott VM, Lloyd SC, Perrine SA, Conti AC, 2020. Chronic Intermittent Ethanol Exposure Increases Ethanol Consumption Following Traumatic Stress Exposure in Mice. Front Behav Neurosci 14, 114. 10.3389/fnbeh.2020.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro L, Miller AN, Ma L, Midha S, Protiva P, Herrera DG, Harrison NL, 2007. Alcohol regulates gene expression in neurons via activation of heat shock factor 1. J Neurosci 27, 12957–12966. 10.1523/JNEUROSCI.4142-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, McCall NM, Maldonado-Devincci AM, Morrow AL, Jones SR, Kash TL, 2015. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology 99, 735–749. 10.1016/j.neuropharm.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradier B, Erxlebe E, Markert A, Rácz I, 2018. Microglial IL-1β progressively increases with duration of alcohol consumption. Naunyn Schmiedebergs Arch Pharmacol 391, 455–461. 10.1007/s00210-018-1475-7 [DOI] [PubMed] [Google Scholar]
- Pringle AK, Gardner CR, Walker RJ, 1996. Reduction of cerebellar GABAA responses by interleukin-1 (IL-1) through an indomethacin insensitive mechanism. Neuropharmacology 35, 147–152. 10.1016/0028-3908(95)00161-1 [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A, 2009. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci 12, 1152–1158. 10.1038/nn.2369 [DOI] [PubMed] [Google Scholar]
- Qian J, Zhu L, Li Q, Belevych N, Chen Q, Zhao F, Herness S, Quan N, 2012. Interleukin-1R3 mediates interleukin-1–induced potassium current increase through fast activation of Akt kinase. Proc Natl Acad Sci USA 109, 12189–12194. 10.1073/pnas.1205207109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakela B, Brehm P, Mandel G, 2018. Astrocytic modulation of excitatory synaptic signaling in a mouse model of Rett syndrome. Elife 7, e31629. 10.7554/eLife.31629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Patel RR, Bajo M, 2018. Ethanol and Cytokines in the Central Nervous System. Handb Exp Pharmacol 248, 397–431. 10.1007/164_2017_77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiz PA, Garcia-Portilla MP, Florez G, Corcoran P, Arango C, Morales B, Leza JC, Alvarez S, Díaz EM, Alvarez V, Coto E, Nogueiras L, Bobes J, 2009. Polymorphisms of the IL-1 gene complex are associated with alcohol dependence in Spanish Caucasians: data from an association study. Alcohol Clin Exp Res 33, 2147–2153. 10.1111/j.1530-0277.2009.01058.x [DOI] [PubMed] [Google Scholar]
- Salling MC, Harrison NL, 2014. Strychnine-sensitive glycine receptors on pyramidal neurons in layers II/III of the mouse prefrontal cortex are tonically activated. J Neurophysiol 112, 1169–1178. 10.1152/jn.00714.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, Skelly MJ, Avegno E, Regan S, Zeric T, Nichols E, Harrison NL, 2018. Alcohol Consumption during Adolescence in a Mouse Model of Binge Drinking Alters the Intrinsic Excitability and Function of the Prefrontal Cortex through a Reduction in the Hyperpolarization-Activated Cation Current. J Neurosci 38, 6207–6222. 10.1523/JNEUROSCI.0550-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer P, Cates-Gatto C, Varodayan FP, Nadav T, Roberto M, Lasek AW, Roberts AJ, 2016. Dependence-induced ethanol drinking and GABA neurotransmission are altered in Alk deficient mice. Neuropharmacology 107, 1–8. 10.1016/j.neuropharm.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A, Liappas I, Mandelli L, Albani D, Forloni G, Malitas P, Piperi C, Zisaki A, Tzavellas EO, Papadopoulou-Daifoti Z, Prato F, Batelli S, Pesaresi M, Kalofoutis A, 2006. Interleukin-1 alpha and beta, TNF-alpha and HTTLPR gene variants study on alcohol toxicity and detoxification outcome. Neurosci Lett 406, 107–112. 10.1016/j.neulet.2006.07.003 [DOI] [PubMed] [Google Scholar]
- Shao Q, Li Y, Wang Q, Zhao J, 2015. IL-10 and IL-1β mediate neuropathic-pain like behavior in the ventrolateral orbital cortex. Neurochem Res 40, 733–739. 10.1007/s11064-015-1521-5 [DOI] [PubMed] [Google Scholar]
- Shin J-W, Cheong Y-J, Koo Y-M, Kim S, Noh C-K, Son Y-H, Kang C, Sohn N-W, 2014. α-Asarone Ameliorates Memory Deficit in Lipopolysaccharide-Treated Mice via Suppression of Pro-Inflammatory Cytokines and Microglial Activation. Biomol Ther (Seoul) 22, 17–26. 10.4062/biomolther.2013.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Gotay A, Davis J, Tavares ER, Richardson HN, 2021. Alcohol drinking during early adolescence activates microglial cells and increases frontolimbic Interleukin-1 beta and Toll-like receptor 4 gene expression, with heightened sensitivity in male rats compared to females. Neuropharmacology 197, 108698. 10.1016/j.neuropharm.2021.108698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Lipsky BP, Russell C, Ketchem RR, Kirchner J, Hensley K, Huang Y, Friedman WJ, Boissonneault V, Plante M-M, Rivest S, Sims JE, 2009. A Central Nervous System-Restricted Isoform of the Interleukin-1 Receptor Accessory Protein Modulates Neuronal Responses to Interleukin-1. Immunity 30, 817–831. 10.1016/j.immuni.2009.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S, 2013. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol 18, 203–213. 10.1111/j.1369-1600.2011.00418.x [DOI] [PubMed] [Google Scholar]
- Taoro-González L, Cabrera-Pastor A, Sancho-Alonso M, Arenas YM, Meseguer-Estornell F, Balzano T, ElMlili N, Felipo V, 2019. Differential role of interleukin-1β in neuroinflammation-induced impairment of spatial and nonspatial memory in hyperammonemic rats. FASEB J 33, 9913–9928. 10.1096/fj.201900230RR [DOI] [PubMed] [Google Scholar]
- Tiwari V, Chopra K, 2013. Resveratrol abrogates alcohol-induced cognitive deficits by attenuating oxidative-nitrosative stress and inflammatory cascade in the adult rat brain. Neurochem Int 62, 861–869. 10.1016/j.neuint.2013.02.012 [DOI] [PubMed] [Google Scholar]
- Tiwari V, Chopra K, 2012. Attenuation of oxidative stress, neuroinflammation, and apoptosis by curcumin prevents cognitive deficits in rats postnatally exposed to ethanol. Psychopharmacology (Berl) 224, 519–535. 10.1007/s00213-012-2779-9 [DOI] [PubMed] [Google Scholar]
- Toledo Nunes P, Vedder LC, Deak T, Savage LM, 2019. A Pivotal Role for Thiamine Deficiency in the Expression of Neuroinflammation Markers in Models of Alcohol-Related Brain Damage. Alcohol Clin Exp Res 43, 425–438. 10.1111/acer.13946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S-J, 2017. Effects of interleukin-1beta polymorphisms on brain function and behavior in healthy and psychiatric disease conditions. Cytokine Growth Factor Rev 37, 89–97. 10.1016/j.cytogfr.2017.06.001 [DOI] [PubMed] [Google Scholar]
- Tu P-C, Su T-P, Huang C-C, Yang AC, Yeh H-L, Hong C-J, Liou Y-J, Liu M-E, Lin C-P, Tsai S-J, 2014. Interleukin-1 beta C-511T polymorphism modulates functional connectivity of anterior midcingulate cortex in non-demented elderly Han males. Brain Struct Funct 219, 61–69. 10.1007/s00429-012-0484-4 [DOI] [PubMed] [Google Scholar]
- Varodayan FP, Bajo M, Soni N, Luu G, Madamba SG, Schweitzer P, Roberto M, 2017. Chronic alcohol exposure disrupts CB1 regulation of GABAergic transmission in the rat basolateral amygdala. Addict Biol 22, 766–778. 10.1111/adb.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Harrison NL, 2013. HSF1 transcriptional activity mediates alcohol induction of Vamp2 expression and GABA release. Front Integr Neurosci 7, 89. 10.3389/fnint.2013.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Patel RR, Matzeu A, Wolfe SA, Curley DE, Khom S, Gandhi PJ, Rodriguez L, Bajo M, D’Ambrosio S, Sun H, Kerr TM, Gonzales RA, Leggio L, Natividad LA, Haass-Koffler CL, Martin-Fardon R, Roberto M, 2022. The Amygdala Noradrenergic System Is Compromised With Alcohol Use Disorder. Biol Psychiatry 91, 1008–1018. 10.1016/j.biopsych.2022.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Pignataro L, Harrison NL, 2011. Alcohol induces synaptotagmin 1 expression in neurons via activation of heat shock factor 1. Neuroscience 193, 63–71. 10.1016/j.neuroscience.2011.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Sidhu H, Kreifeldt M, Roberto M, Contet C, 2018. Morphological and functional evidence of increased excitatory signaling in the prelimbic cortex during ethanol withdrawal. Neuropharmacology 133, 470–480. 10.1016/j.neuropharm.2018.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Soni N, Bajo M, Luu G, Madamba SG, Schweitzer P, Parsons LH, Roberto M, 2016. Chronic ethanol exposure decreases CB1 receptor function at GABAergic synapses in the rat central amygdala. Addict Biol 21, 788–801. 10.1111/adb.12256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasicek TW, Jackson MR, Poseno TM, Stenken JA, 2013. In vivo microdialysis sampling of cytokines from rat hippocampus: comparison of cannula implantation procedures. ACS Chem Neurosci 4, 737–746. 10.1021/cn400025m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Viviani B, 2015. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96, 70–82. 10.1016/j.neuropharm.2014.10.027 [DOI] [PubMed] [Google Scholar]
- Walter NAR, Zheng CL, Searles RP, McWeeney SK, Grant KA, Hitzemann R, 2020. Chronic Voluntary Ethanol Drinking in Cynomolgus Macaques Elicits Gene Expression Changes in Prefrontal Cortical Area 46. Alcohol Clin Exp Res 44, 470–478. 10.1111/acer.14259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden AS, Wolfe SA, Khom S, Varodayan FP, Patel RR, Steinman MQ, Bajo M, Montgomery SE, Vlkolinsky R, Nadav T, Polis I, Roberts AJ, Mayfield RD, Harris RA, Roberto M, 2020. Microglia Control Escalation of Drinking in Alcohol-Dependent Mice: Genomic and Synaptic Drivers. Biological Psychiatry 88, 910–921. 10.1016/j.biopsych.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston MC, Chen H, Swann JW, 2012. Multiple roles for mammalian target of rapamycin signaling in both glutamatergic and GABAergic synaptic transmission. J Neurosci 32, 11441–11452. 10.1523/JNEUROSCI.1283-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Shinnick-Gallagher P, 1994. Interleukin-1 beta inhibits synaptic transmission and induces membrane hyperpolarization in amygdala neurons. J Pharmacol Exp Ther 271, 590–600. [PubMed] [Google Scholar]
- Zalcman SS, Siegel A, 2006. The neurobiology of aggression and rage: role of cytokines. Brain Behav Immun 20, 507–514. 10.1016/j.bbi.2006.05.002 [DOI] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A, 2001. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 31, 85–94. 10.1002/gene.10008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Acute ethanol increases GABA release in dependent mice via IL-1 signaling. A. Ex vivo ethanol application (EtOH, 44 mM for 15 min) increases the sIPSC frequency in prelimbic cortex layer 2/3 pyramidal neurons of dependent mice, but has no effect in naïve or non-dependent mice (n=13–21 cells from 7–13 mice). B. Pretreatment with IL-1RA (100 ng/mL for 15 min) blocked EtOH’s potentiation of the sIPSC frequency in dependent mice (n=5 cells from 3 mice). C. IL-1RA had no effect after pretreatment with EtOH in dependent mice (n=7 cells from 3 mice). All data are presented as mean±SEM. *p<.05, **p<.01 by one-sample t-test.
Supplemental Figure 2. IL-1β’s presynaptic effects are mediated by IL-1R1 in non-dependent and dependent mice. A-B. Pretreatment with IL-1RA blocked IL-1β’s effects on prelimbic cortex layer 2/3 sIPSC frequencies in (A) non-dependent (n=5–7 cells from 3–6 mice) and (B) dependent mice (n=5–16 cells from 4–9 mice). Note, the IL-1β alone data is repeated from panel 4D. All data are presented as mean±SEM. **p<.01, ***p<.001 by one-sample t-test or paired t-test.
Supplemental Figure 3. AS-1 had no effect on sIPSC properties in prelimbic cortex layer 2/3 pyramidal neurons of ethanol-naïve MyD88 knockout mice (n=10 cells from 5 mice). All data are presented as mean±SEM.