Abstract
Further understanding of the associations between personality traits and allostatic load (AL) may be important for predicting, addressing, and optimizing health outcomes. This review synthesized the existing literature reporting the association between the Big Five personality traits and AL in adults to identify the generalizability and robustness of relationships, potential mechanisms underlying the associations, and study characteristics that may be contributing to inconsistencies in the field. Published and unpublished empirical reports were included if at least one of the Big Five traits was examined and an AL index was constructed using at least two biomarkers in a sample of adults. The methodological plan and standardized coding guide were pre-registered and reported (https://osf.io/rxw5a). Based on 11 studies that met eligibility, meta-analysis of correlation coefficients indicated a small but significant positive association between neuroticism and AL, and small but significant inverse associations between both conscientiousness and openness with AL. This review identifies strengths and limitations within the field, as well as several avenues for future research.
Keywords: Personality traits, Big Five, Allostatic Load, Meta-analysis, Systematic review
1 |. Introduction
The interplay between physiological and psychological processes unquestionably adds a layer of complexity to the human condition. While the isolation of these processes is somewhat artificial, we presume that thoughts and emotions can influence and elicit bodily states (e.g., Campbell & Edwards, 2009; Grol & De Raedt, 2020; Sapolsky, 1999) and, likewise, that physiological conditions can influence and elicit cognitive processes and feelings (e.g., Briñol & DeMarree, 2012; Veenstra et al., 2017). At the juncture of physiological and psychological processes, emerging research suggests a link between physiological dysregulation (i.e., allostatic load; AL) and an individual’s enduring behavioral and psychological tendencies (i.e., personality traits). Nuanced understanding of the relationships between AL and personality traits may be important for predicting, addressing, and optimizing health outcomes. Drawing on existing research cataloged in several electronic databases, this review systematically identifies and synthesizes studies reporting associations between AL and the Big Five personality traits.
1.1 |. Allostatic Load.
Originally conceptualized as attributes of the stress response that influence disease processes (McEwen & Stellar, 1993), the AL framework aims to elucidate the mechanisms underlying (co)morbidity to identify individuals who are at greater risk of degeneration and mortality (Seeman et al., 2001). While intermittent upticks across biological systems in the context of true environmental stressors are adaptive, prolonged and cumulative physiological arousal endangers health and optimal aging processes, which is precisely what indices of AL aim to capture. Operational definitions vary between studies, but indices of AL typically include a range of biomarkers that are standardized and composited, whereby higher values indicate higher levels of physiological dysregulation. These biomarkers include, but are not limited to, inflammation markers (e.g., C-reactive protein), as well as assessments of hormonal (e.g., cortisol), cardiovascular (e.g., blood pressure), metabolic (e.g., cholesterol, waist circumference), and lung (e.g., forced vital capacity) functioning. Although AL conceptualizations have received some criticism (e.g., Romero et al., 2009), extensive research finds that AL is associated with many adverse health outcomes, including reduced heart rate variability (Viljoen & Claassen, 2017), as well as increased cognitive and functional decline (Karlamangla et al., 2014; Karlamangla et al., 2002), incidence of conditions such as chronic fatigue syndrome (Maloney et al., 2006), diabetes, hypertension, and cardiovascular disease (Juster et al., 2010; Mattei et al., 2010), and risk of all-cause and cardiovascular mortality (Parker et al., 2022). However, risk stratification based on stressors is complex, as any given social or environmental factor does not predict physiological anomalies with certainty; that is, there are individual differences in maladaptive responses to environmental stressors.
The transactional theory of stress and coping, which views stress as a transactional process between a person and their environment as opposed to a particular environmental stimulus (Lazarus & Folkman, 1984; 1987), seeks to explain the distinction. Notably, the perception or cognitive appraisal of stress, which are distinctly psychological processes, are required to experience stress, while the outcome of stress may manifest physiologically as increased cortisol secretion, heart rate, and blood pressure, which are conspicuously biological processes (also see Cohen et al., 1995; Cohen et al., 2016). Extensive research suggests that the perception of stress is fundamental to AL (for a systematic review, see Beckie, 2012), which leads to consideration of factors involved in patterns of perception and coping responses, such as personality traits. Although cross-context intraindividual variability in perception, emotions, and behaviors exists (e.g., how an individual perceives and subsequently behaves in response to a situation can vary extensively across any given occasion), individuals tend to be highly consistent in their pattern of responding to and interacting with their environment on average, when assessed on several occasions. Specifically, based on two weeks of five daily ecological momentary assessments, individuals tend to demonstrate a unique trend (i.e., central point) in their occasion-to-occasion behavior; at the within-person level, an individual’s average behaviors and comportments are strongly associated across two weeks (r=.90; Fleeson, 2001, 2004).
1.2 |. The Big Five Personality Traits.
A common approach for measuring these enduring patterns of perception, feeling, and behaving is via assessment of the Big Five personality traits (i.e., extraversion, neuroticism, conscientiousness, agreeableness, and openness to experience). Together, these five trait subscales are thought to represent an individual’s unique constellation of behavioral and psychological tendencies, permeating numerous domains of life (McCrae & Costa, 2004). For example, extraversion is positively associated with social interactions (e.g., Argyle & Lu, 1990), neuroticism is positively associated with anxiety symptoms (e.g., Bienvenu et al., 2004), and conscientiousness is positively associated with engagement in physical activity (e.g., Bogg & Roberts, 2004). In addition to correlates of critical health behaviors important for physiological regulation, personality traits influence exposure to both quantity and type of stressors (Bolger & Schilling, 1991; Cimbolic Gunthert et al., 1999; Gartland et al., 2012; Iacovino et al., 2016; Leger et al., 2016), as well as the magnitude of reactivity to stressors (Bolger & Schilling, 1991; Javaras et al., 2012; Mroczek & Almeida, 2004; Penley & Tomaka, 2002; Suls et al., 1998; Suls & Martin, 2005).
An individual’s ongoing tendency to perceive and respond to stress in a maladaptive way may elicit a cascade of physiological reactions across neuroendocrine, immune, cardiovascular, and metabolic regulatory systems, subsequently leading to interindividual variability in dysregulation of biological systems and poorer downstream health outcomes. Indeed, the existing literature suggests that the Big Five personality traits are associated with individual differences in many physiological outcomes, including chronic conditions (Goodwin & Friedman, 2006; Weston et al., 2015; Weston et al., 2020) and mortality (Graham et al., 2017; Jokela et al., 2013; Kern & Friedman, 2008; Wilson et al., 2004). While the mechanisms underlying these associations are not entirely clear, personality traits may contribute to individual differences in the regulation of physiological systems (i.e., differences in AL), subsequently leading to negative health outcomes. Additionally, these enduring psychological tendencies influence behavioral factors (e.g., diet, physical activity, interpersonal interactions), leading to maladaptive physiological dysregulation (e.g., hypertension, systemic inflammation), and thereby contributing to disease and mortality (Milad & Bogg, 2020). However, there is clear between-study variability in study features and researcher decisions across research investigating the associations between AL and each of the Big Five traits. This systematic review aimed to synthesize and critically analyze the outcomes of the existing literature reporting the associations between the Big Five personality traits and AL to assist in identifying which (if any) study characteristics may be contributing to inconsistencies in the field.
2 |. Method
The study justification, search strategy, and methodological approach for this research synthesis were documented and pre-registered through the Open Science Framework (OSF) (https://osf.io/rxw5a). We used PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses; Moher et al., 2009) to create the protocol, and the National Institutes of Health (NIH) Guidance for Assessing the Quality of Observational Cohort and Cross-Sectional Studies was used to assess the quality of articles meeting eligibility.
2.1 |. Search Strategy.
A comprehensive literature search was executed on January 12th, 2022, within Web of Science, PubMed, and Academic Search Complete. The full search strategy applied to each database identified any combination of the following search terms within the title, abstract, or key words: ((“allostatic load”) AND (personality OR extraversion OR neuroticism OR conscientiousness OR agreeableness OR openness)). Due to automatic lemmatization in the PubMed database, which expands the search by adding potentially relevant subject headings to increase the search, the search strategy was modified for this database: ((“allostatic load”) AND (personalit* OR extraver* OR neurotic* OR conscientious* OR agreeable* OR openness)). The search identified any combination of these search terms within the title, abstract, or key words. We also executed forward and backward searches of the studies meeting eligibility. An updated search using the identical search strategy was executed on February 27th, 2023, directly prior to resubmission for publication.
2.2 |. Eligibility Criteria.
Empirical reports met eligibility if i) at least one of the Big Five traits was assessed; ii) an index of AL, including at least two biomarkers, was computed; and iii) the sample included adult humans (i.e., individuals who were defined as ―adults‖ or were 18+ years old). Reports were excluded if the report was not written in English or did not include empirical investigation (e.g., narrative or theoretical papers). While inclusion of only two biomarkers within an AL index would be a very liberal definition of AL, our aim was to identify and critically reflect on all research reporting the association between AL and personality. Published and unpublished reports were eligible for inclusion.
2.3 |. Study Selection.
Of the original 250 citations (159 unique), 64 studies were immediately identified for exclusion, as the focus was children (k=15), or no novel empirical data were reported (k=49; e.g., commentaries). Of the 95 studies that were selected for full text screening, 81 reports did not meet eligibility criteria. This process left 12 published articles, one dissertation, and one conference proceeding that appeared to meet eligibility criteria for synthesis. However, nine of the reports used overlapping datasets: UK’s Understanding Society (Barry et al., 2021; Gallagher et al., 2021); Copenhagen Aging and Midlife Biobank (CAMB; Christensen et al., 2019a; Christensen et al., 2019b); the Lothian Birth Cohort 1936 (LBC; Crook et al., 2018; Lewis et al., 2018); Midlife in the United States (MIDUS; Milad & Bogg, 2020; Turiano et al., 2015; Van Dyke et al., 2020). In four of these cases, priority was given to the report that focused on personality traits and AL, as opposed to simple adjustment for personality traits (Barry et al., 2021; Christensen et al., 2019a; Lewis et al., 2018; Van Dyke et al., 2020). An exception to this rule occurred when two reports arising from the same dataset (MIDUS) both focused on the association between personality and AL. In this case, the full-text manuscript was selected in lieu of the conference proceeding (Turiano et al., 2015). This process resulted in nine reports based on unique data, published between 2011 and 2021. The updated, identical search identified an additional 12 (7 unique) potential records, two of which met study eligibility. There were no disagreements between reviewers regarding study eligibility. A standardized coding guide was used to extract data from the reports; two researchers, T.Y. and T.L., coded each report. The original coding guide was pre-registered, and there were no deviations. See Figure 1 for the PRISMA flow diagram visually depicting the selection process and meta-statistics.
Figure 1.
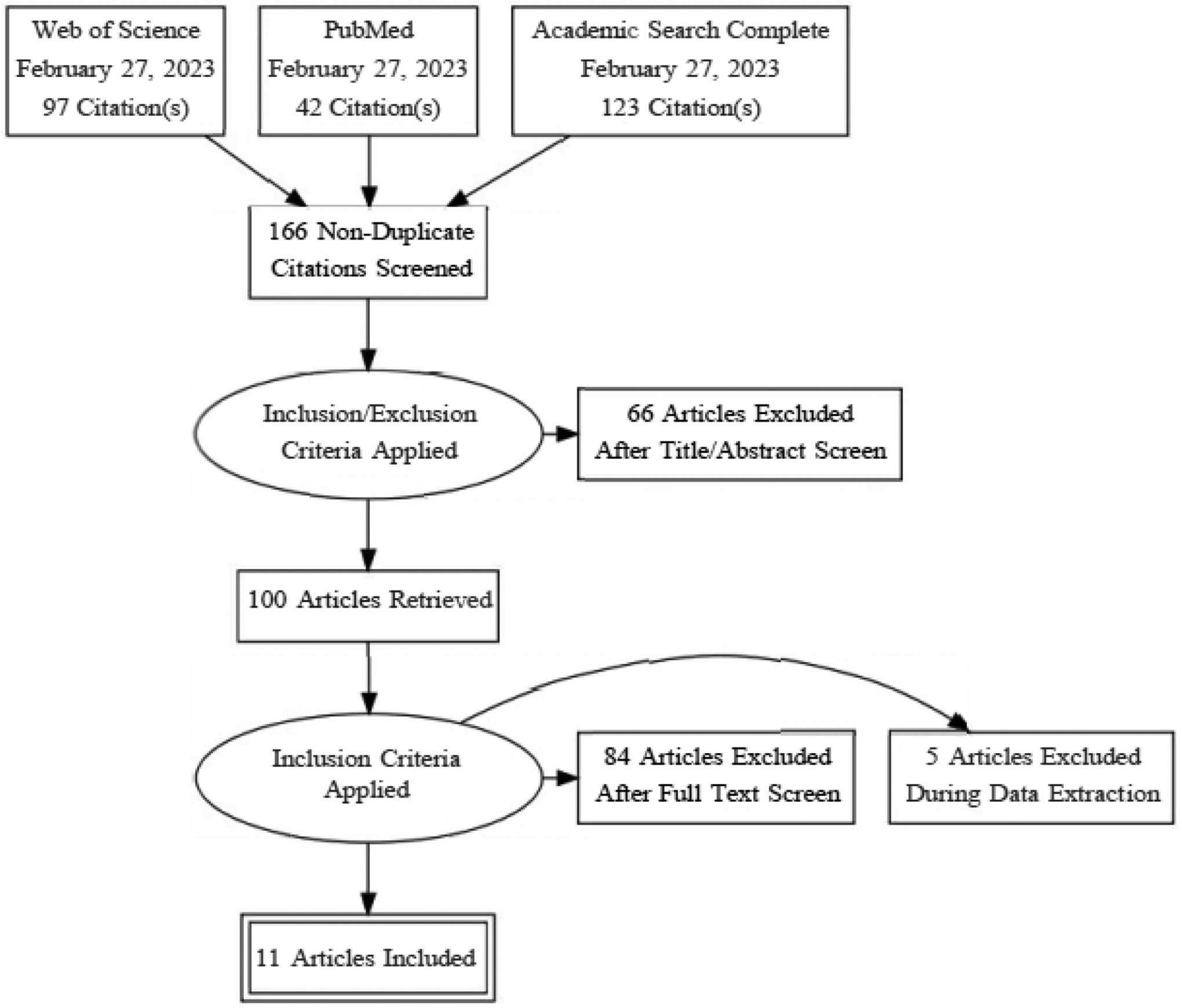
PRISMA Flow Diagram
2.4 |. Meta-Analysis.
A multi-level random effects approach was chosen to meta-analyze the results, as heterogeneity in effect sizes was expected beyond heterogeneity that could be explained by sampling error alone (Borenstein et al., 2010). Further, this approach was selected as the goal was to investigate the average observed effect in the larger population of studies, and multi-level structure for meta-analysis provides the opportunity for several dependent effects to be assessed within the same model (i.e., effects are nested within studies). Meta-analytic procedures were executed in R using the Metafor package (Viechtbauer, 2010) with Maximum Likelihood (ML) applied for variance estimation, and sensitivity analyses using Restricted Maximum Likelihood Estimator (REML). Raw correlation coefficients were corrected for the slight negative bias based on work by Olkin and Pratt (1958). Sampling variances were estimated based on work by Hedges (1983), which provides unbiased estimates of the sampling variances. We report I2 and the Q statistic, which, respectively, provide the proportion of true variability of the effects relative to the total variation in observed effects and the weighted sum of squared differences between the observed effects and the weighted average effect (e.g., a measure of variation around the average). I2 (Higgins et al., 2003) is a measure of relative heterogeneity based on the studies included, and therefore should be interpreted cautiously. Random variance is likely inconsistent when the sample sizes of included studies range extensively (in this case, Nrange=95–5500), and I2 does not track true heterogeneity when there are disparities in sampling precision. A significant Q statistic suggests that variation between effect sizes is due to more than what is expected from sampling variability. Analytic scripts and data files are available on the OSF project page.
2.5 |. Quality Assessment.
The National Institutes of Health (NIH) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, a well-known and established tool, was used. Two reviewers (T.Y. and T.L.) evaluated study quality, and discussions resolved differing opinions.
3 |. Results
3.1. Study Characteristics.
The 11 eligible studies included a total of 18,942 unique participants with a mean baseline age of 59.4 (SD=11.8) years. See Table 1 for an alphabetized list of included studies, report characteristics, and demographics. All studies used an existing dataset, except for two (Kobrosly, 2012; Otto et al., 2021). Apart from Hawkley et al. (2011), all studies included predominantly White participants (though five of these did not specifically report race).
Table 1.
Sample Characteristics for Studies Meeting Systematic Review Eligibility
| Study | Title | Dataset | Country of origin | N | % Female | Mean age (SD) | Age range |
|---|---|---|---|---|---|---|---|
| (Christensen et al., 2018) | Parental socioeconomic position and midlife allostatic load: A study of potential mediators | Copenhagen Perinatal Cohort | Denmark | 361 | 53.5 | 27 (4.4) / 50 (0.8) | NR |
| (Christensen et al., 2019b) | Big Five personality traits and allostatic load in midlife | Copenhagen Aging and Midlife Biobank | Denmark | 5512 | 31.1 | 54.4 (3.6) | 49–63 |
| (Crook et al., 2018) | Apolipoprotein E genotype does not moderate the associations of depressive symptoms, neuroticism and allostatic load with cognitive ability and cognitive aging in the Lothian Birth Cohort 1936 | Lothian Birth Cohort 1936 | UK | 1028 | 49.8 | 69.5 (0.8) | NR |
| (Gallagher et al., 2021) | Allostatic load and mental health during COVID-19: The moderating role of neuroticism | Understanding Society | UK | 956 | 47.1 | 44.9 (13.5) | 16–80 |
| (Hawkley et al., 2011) | Mediators of the relationship between socioeconomic status and allostatic load in the Chicago Health, Aging, and Social Relations Study (CHASRS) | Chicago Health, Aging, and Social Relations | USA | 208 | 52.9 | 58.4 (NR) | 51–69 |
| (Luo et al., 2022) | Personality and health: Disentangling their between-person and within-person relationship in three longitudinal studies | Swedish Adoption/Twin Study of Aging | Sweden | 767* | 59.6* | 66.0 (9.0)* | 45–91* |
| (Kobrosly, 2012) | An epidemiologic investigation into the relationship between stress, allostatic load, and depressive disorder among older adults | Rochester Study of Healthy Minds and Bodies | USA | 125 | 66.4 | 76.1 (5.9) | 67–94 |
| (Milad & Bogg, 2020) | Personality traits, coping, health-related behaviors, and cumulative physiological health in a national sample: 10 year prospective effects of conscientiousness via perceptions of activity on allostatic load | Midlife in the United States | USA | 1054 | 54.7 | 46.2 (11.8) | 25–74 |
| (Otto et al., 2021) | Borderline Personality Disorder in a “life history theory” perspective: Evidence for a fast “pace-of-life-syndrome” | Community clinical sample | Germany | 95 | 100.0 | 25.9 (4.6) | NR |
| (Richards et al., 2023) | Subjective social status and allostatic load among older people in England: A longitudinal analysis | English Longitudinal Study of Ageing | UK | 2926* | 55.0* | 64.0 (8.1)* | 50*–89 |
| (Stephan et al., 2016) | Allostatic load and personality: A 4-year longitudinal study | Health and Retirement | USA | 5200* | 59.5* | 66.9 (8.9)* | 50–99* |
Note. UK=United Kingdom; USA=United States of America; NR=not reported.
Reflects baseline measurement occasion.
3.2 |. Assessment and Computation of AL.
Table 2 reports information regarding the various biomarkers and computation approaches for the AL indices. The quantity of biomarkers included in AL indices ranged from four (Otto et al., 2021) to 14 (Christensen et al., 2018; Christensen et al., 2019b). All studies included biomarkers representing the immune/inflammatory, metabolic, and cardiovascular systems, except for Otto et al. (2021), which only included biomarkers from the metabolic and cardiovascular systems. As four further exceptions, Gallagher et al. (2021) included a biomarker representing the neuroendocrine system (DHEA-s), while Hawkley et al. (2011) included biomarkers representing the sympathetic nervous and adrenomedullary system functioning (urinary norepinephrine and epinephrine). Hawkley et al. (2011) and Kobrosly (2012) also included assessments of cortisol (e.g., diurnal cortisol slope), which reflects hypothalamic-pituitary-adrenal (HPA) axis functioning. Finally, Richards et al. (2023) included three indicators representing lung functioning (forced expiratory volume in one second, forced vital capacity, and peak expiratory flow).
Table 2.
Allostatic Load Indices and Personality Assessments
| Study | AL # | List of AL biomarkers | Computation of AL | Traits assessed | Trait scale (items) |
|---|---|---|---|---|---|
| (Christensen et al., 2018) | 14 | CRP, IL-6, TNF-α, % body fat, blood glucose, BMI, HbA1c, HDL, LDL, total cholesterol, triglycerides, waist/hip ratio, DBP, SBP | Sex-specific z scores→Cut-off points for each biomarker based on sex-specific upper quartile→Composite score ranging 0–14 | E, N | EPQ (101 total) |
| (Christensen et al., 2019b) | 14 | CRP, IL-6, TNF-α, % body fat, blood glucose, BMI, HbA1c, HDL, LDL, total cholesterol, triglycerides, waist/hip ratio, DBP, SBP | z scores→Cut-off points for each biomarker based on sex-specific upper quartile→Composite score ranging 0–14 | All 5 | NEO-FFI (12/scale) |
| (Crook et al., 2018) | 9 | CRP, fibrinogen, albumin, BMI, HbA1c, HDL ratio, triglycerides, DBP, SBP | Log transformed biomarkers with non-normal distributions→Absolute mean z scores→Summed | N | IPIP (10) |
| (Gallagher et al., 2021) | 12 | DHEA-s, CRP, fibrinogen, IGF-1, albumin, BMI, HbA1c, HDL, LDL, waist circumference, DBP, SBP | z score→Cut-off points for each biomarker based on sex-specific upper quartile or sex-specific risk or established criteria→Composite score ranging 0–12 | N | FFMt (3) |
| (Hawkley et al., 2011) | 9 | Cortisol, epinephrine, norepinephrine, HbA1c, HDL, total cholesterol, waist circumference, DBP, SBP | Computed z scores (sex-specific z scores for some biomarkers)→Average across z scores | E, N, A | Big 5 PI (20/scale) |
| (Luo et al., 2022) | 7 | Blood sugar, HDL, total cholesterol, triglycerides, waist/hip ratio, DBP, SBP | Computed z scores→Average across z scores | E, N | EPI (9/scale) |
| (Kobrosly, 2012) | 7 | Average diurnal cortisol slope, IGF-1, IL-6, waist/hip ratio, resting heart rate, DBP, SBP | Computed z scores | N | NEO-FFI (74 total) |
| (Milad & Bogg, 2020) | 10 | CRP, fibrinogen, IL-6, fasting glucose, HbA1c, HDL, HOMA-IR, triglycerides, waist/hip ratio, pulse | Log transformed some biomarkers→Parceled biomarkers to reflect functioning within inflammation, glucose, and lipid systems | All 5 | Big Five Adjectives (4/scale) |
| (Otto et al., 2021) | 4 | BMI, waist/hip ratio, DBP, SBP | Used cut-off values reported by Seeman et al. (1997) AND sample-specific z score cut-off points based on quartiles→Composite score ranging 0–4 | All 5 | NEO-FFI (12/scale) |
| (Richards et al., 2023) | 13 | CRP, fibrinogen, BMI, fasting blood glucose, HbA1c, HDL/total cholesterol ratio, triglycerides, waist circumference, DBP, SBP, FEV1, FVC, PEF | Coded biomarkers based on clinical cut-offs or high-risk quartiles (and doctor decision)→Separated by system (cardiovascular, inflammation, metabolic, body fat, lung function)→Composite score ranging 0–5 | All 5 | MIDI (25 total) |
| (Stephan et al., 2016) | 8 | CRP, cystatin C, HbA1C, HDL, total cholesterol, waist circumference, DBP, SBP | Log transformed some biomarkers→z scores→Averaged across all z scores | All 5 | MIDI (26 total) |
Note. Biomarkers are listed by order of system and in alphabetical order within the system: neuroendocrine (DHEA-s), hypothalamic-pituitary-adrenal axis functioning (average diurnal cortisol slope/cortisol), sympathetic nervous/adrenomedullary system (epinephrine, norepinephrine), inflammatory (CRP, IGF-1, IL-6, TNF-α), metabolic (BMI, HbA1c, HDL, HOMA-IR, LDL), cardiovascular (DBP, SBP), and lung function (FEV1, FVC, PEF). DHEA-s=dehydroepiandrosterone sulfate; CRP=high sensitivity C-reactive protein; IGF-1=insulin-like growth factor one; IL-6=Interleukin 6; TNF-α=tumor necrosis factor-α; BMI=body mass index; HbA1c=glycated/glycosylated hemoglobin; HDL=high density lipoprotein; HOMA-IR=homeostasis model assessment-estimated insulin resistance; LDL=low density lipoprotein; DBP=diastolic blood pressure; SBP=systolic blood pressure; FEV1=forced expiratory volume in one second; FVC=forced vital capacity; PEF=peak expiratory flow; E=extraversion; N=neuroticism; A=agreeableness; EPQ=Eysenck Personality Questionnaire; NEO-FFI=NEO-Five Factor Inventory; IPIP=International Personality Item Pool; FFMt=Five-Factor Model traits; Big 5 PI=Big Five Personality Inventory; MIDI=Midlife Development Personality Inventory; EPI=Eysenck Personality Inventory.
Prior to calculating z scores, some studies log transformed at least a portion of the included biomarkers to normalize their distributions. Specifically, Crook et al. (2018) log transformed CRP, fibrinogen, HbA1c, triglycerides, BMI, SBP, and DBP. Milad and Bogg (2020) log transformed CRP, IL-6, glycosylated hemoglobin, fasting glucose, insulin resistance, and triglycerides. Stephan et al. (2016) log transformed all blood biomarkers (HbA1C, CRP, HDL, total cholesterol, and cystatin C). Furthermore, some studies selected risk category cut-offs based on sex-specific z scores for some biomarkers (e.g., waist circumference; Gallagher et al., 2021; Hawkley et al., 2011; Kobrosly, 2012) or all biomarkers (Christensen et al., 2018). Half of the studies did not note whether risk cut-offs were based on sex-specific z scores (Christensen et al., 2019b; Crook et al., 2018; Luo et al., 2022; Milad & Bogg, 2020; Stephan et al., 2016). Across studies and biomarkers, higher values typically represented high risk, except for HDL and assessments of lung function, in which low values indicated high risk. Some authors treated both high and low biomarker values as high risk (e.g., IGF-1; Gallagher et al., 2021; see Supplementary Text 1 for all exceptions).
There was extensive variation in AL scoring algorithms across studies, namely: (a) dichotomization of each biomarker based on sample-specific and/or sex-specific z scores (e.g., 0=lower three quartiles; 1=upper quartile), and then summation; (b) dichotomization based on existing cut-off values (Seeman et al., 1997) and/or sample-specific z score cut-off points (depending on biomarker), and then summation; (c) calculation of the within-person average across biomarker z scores (a continuous AL index); (d) parceling of biomarkers to reflect functioning across biological systems; and (e) calculation of mean absolute z scores, and then a latent variable approach using item parcels (sum of values across biomarkers, where values are the mean absolute z scores). See Table 2 and Supplementary Text 2 for further details.
3.3 |. Assessment and Computation of Personality.
Every study used a validated personality assessment (see Table 2). Almost half of the studies reported findings for all of the Big Five personality traits (Christensen et al., 2019b; Milad & Bogg, 2020; Otto et al., 2021; Richards et al., 2023; Stephan et al., 2016), while the remainder of studies either only assessed or reported results regarding one to three traits. Assessment of each trait included as few as three items (Gallagher et al., 2021), and up to 26 items (Stephan et al., 2016). Across studies, items were assessed according to 5-point, 7-point, or 9-point scales, except for the EPQ, in which response options are dichotomous (0=no; 1=yes). Internal reliability estimates for trait scales were acceptable to good across studies and traits (see Supplementary Text 3). Personality variables were computed as the sum (Christensen et al., 2018; Christensen et al., 2019b; Gallagher et al., 2021; Luo et al., 2022; Otto et al., 2021) or average (Hawkley et al., 2011; Kobrosly, 2012; Milad & Bogg, 2020; Richards et al., 2023; Stephan et al., 2016) of subscale items. Richards et al. (2023) further computed z scores for the trait subscales. Kobrosly (2012) assessed neuroticism on two occasions and used the within-person mean neuroticism score for analyses. Finally, Crook et al. (2018) modeled neuroticism as a latent variable using item parcels (sum of different item responses).
3.4 |. Analytic Approach and Qualitative Report of the Results.
Extensive variability in analytic strategies were used to examine the relationships between traits and AL. Personality traits were examined as potential mediators or moderators of the association between AL and an outcome variable. AL was also examined as a potential mediator of the association between personality traits and an outcome variable. Studies additionally examined the direct associations or direct lagged effects between traits and AL, along with several potential mediators or moderators of the associations. The following paragraphs provide a more detailed explanation of the analytic approach across studies, as well as the primary findings reported by each study.
3.4.1 |. Personality Traits as Mediators.
Hawkley et al. (2011) examined traits as potential mediators of the association between socioeconomic status (SES) and AL. Adjusting for gender and ethnicity, partial correlations indicated that none of the personality traits were significantly correlated with SES or AL; therefore, the researchers did not further investigate the role of personality traits in subsequent mediation analyses. Likewise, Christensen et al. (2018) examined potential mediators (including extraversion and neuroticism) on associations between socioeconomic position (SEP) and AL. Although extraversion was positively associated with AL, neither extraversion nor neuroticism mediated the association between SEP and AL (2018). Similarly, Otto et al. (2021) compared differences in psychological constructs (including personality traits) between female participants diagnosed with Borderline Personality Disorder and healthy controls on fast pace-of-life syndrome, as well as the extent to which this syndrome predicted AL. Results suggested no differences in personality traits between groups; as such, no further analyses were executed to examine mediating associations between traits, fast pace-of-life syndrome, and AL. In contrast, Richards et al. (2023) examined several potential mediators (including the Big Five personality traits) of the association between AL and subjective social status. Lower conscientiousness, extraversion, and neuroticism, as well as higher agreeableness and openness, were associated with higher AL. Furthermore, adding the Big Five personality traits to the model attenuated the relationship between AL and subjective social status.
3.4.2 |. AL as a Mediator.
Crook et al. (2018) examined whether AL mediated the association between neuroticism and cognitive outcomes, finding that neuroticism and AL had small-to-moderate negative associations with cognitive functioning for individuals with and without an APOE ε4 allele. However, AL did not mediate the associations between neuroticism and cognitive impairment or cognitive decline. Kobrosly (2012) conducted a path analysis to examine the associations among neuroticism, AL, life stress, SEP, social isolation, and depressive symptoms. Results revealed that neuroticism was positively and strongly associated with social isolation and depressive symptoms, but the association between neuroticism and AL was not examined directly in path analyses. Gallagher et al. (2021) examined the extent to which AL prior to the pandemic was predictive of mental health during the pandemic, and whether the associations were moderated by neuroticism. Findings suggested that the association between AL and poor mental health during the pandemic were exacerbated in individuals with moderate and high levels of neuroticism (2021).
3.4.3 |. Associations Between AL and Traits.
Christensen et al. (2019b) examined sex-stratified associations between personality traits and AL, adjusting for all traits and several covariates (e.g., sociodemographic factors, health-related behaviors). Results suggested that conscientiousness and openness were inversely associated with AL for all participants, while extraversion was positively associated with AL in male participants only. However, the AL-extraversion and AL-openness associations were attenuated after adjusting for sociodemographic factors. Milad and Bogg (2020) examined the direct and indirect effects (via coping styles and health behaviors) of personality traits at baseline on a latent measurement model of AL 10 years later. Results suggested that higher extraversion was prospectively associated with higher AL, that higher conscientiousness was prospectively associated with lower AL, and that both associations were mediated by greater perceptions of being active, such that higher perceptions of activity were protective against heightened AL for individuals high in extraversion and conscientiousness. Similarly, Stephan et al. (2016) examined the extent to which sociodemographic variables moderated associations between AL and personality traits, as well as change in personality traits. Results suggested that higher AL was associated with lower extraversion, higher neuroticism, and lower conscientiousness, as well as declines in extraversion, conscientiousness, and agreeableness, over four years (2016). Finally, Luo et al. (2022) examined potential lagged effects between AL and traits (neuroticism and extraversion) over up to five occasions at approximately three-year intervals. Neither extraversion nor neuroticism was linked to AL at the within-person or between-person levels.
3.5 |. Meta-Analytic Results.
We fit a series of five multi-level meta-analyses to synthesize the overall association between AL and each Big Five trait with bivariate correlation coefficients as the unit of effect size. See Supplementary Text 4 for details regarding the identification of effects across studies. The included reports (K=11) yielded a total of 48 effects. Figure 2 depicts funnel plots of the individual and synthesized effects.
Figure 2.
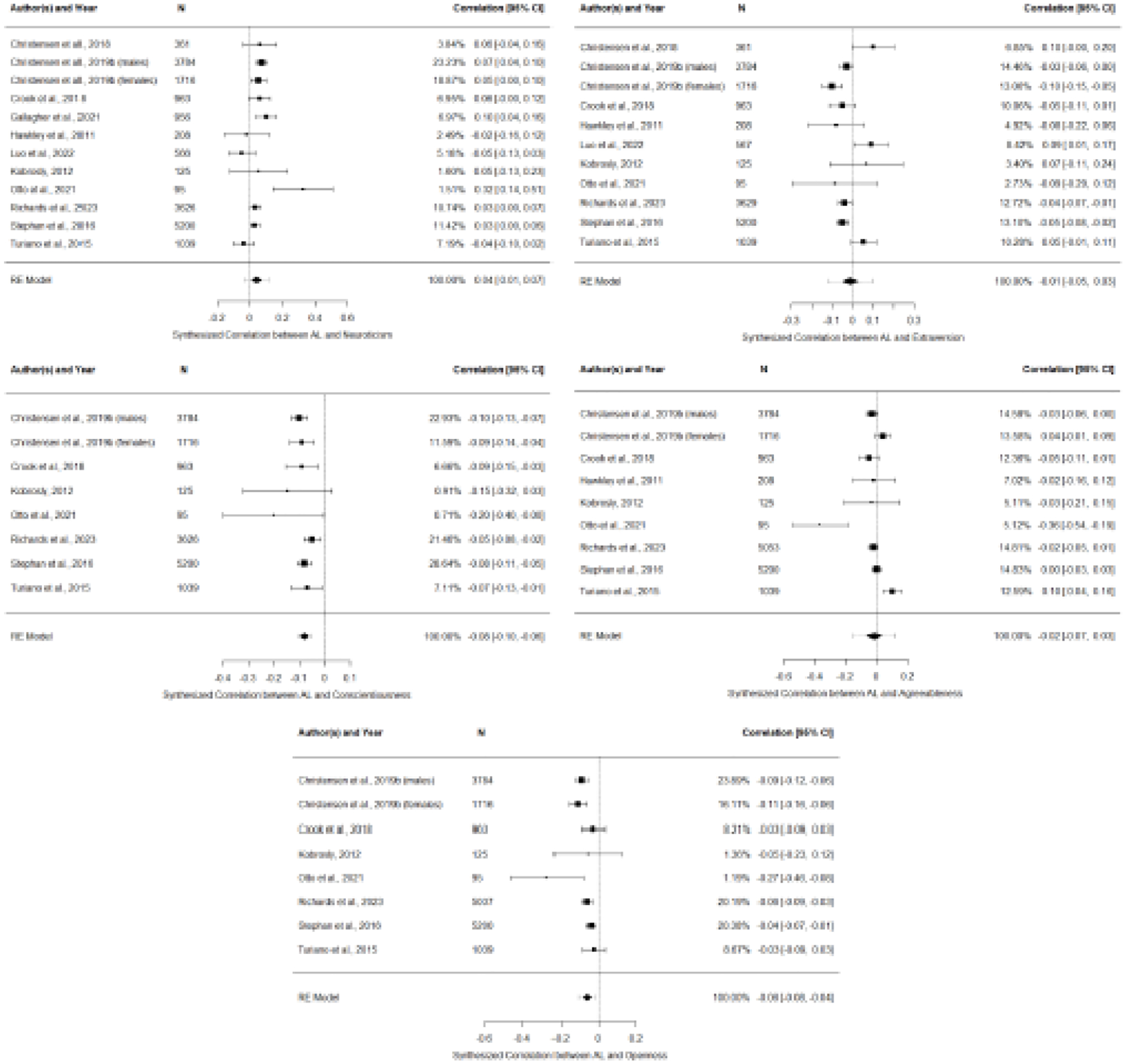
Forest Plots of the Bivariate Correlation Coefficients Between Personality Traits and Allostatic Load Across Studies Meeting Eligibility
The overall association between neuroticism and AL was very small and significant (r=.04, 95%CI[0.01, 0.07], SE=0.02, p=.01; k=11), suggesting that individuals higher in neuroticism tended to have more physiological dysregulation. There was significant heterogeneity in effect sizes (Q11=29.23, p<.01), and the proportion of true variability of the effects relative to the total variation in observed effects was moderate (I2=61.10%). The overall association between conscientiousness and AL was small and significant (r=−.08, 95%CI[−0.10, −0.06], SE=0.01, p<.01; k=7), suggesting that individuals higher in conscientiousness tended to have better physiological regulation, with minimal heterogeneity in effect sizes (Q7=7.21, p=.41; I2=10.56%). The overall association between openness and AL was small and significant (r=−.06, 95%CI[−0.08, −0.04], SE=0.01, p<.01; k=7), suggesting that individuals higher in openness tended to have better physiological regulation, though there was significant heterogeneity in effect sizes (Q7=16.48, p=.02; I2=43.39%).
The overall associations between AL and extraversion (r=−.01, 95%CI[−0.05, 0.03], SE=0.02, p=.52; k=10), as well as AL and agreeableness (r=−.01, 95%CI[−0.7, 0.03], SE=0.03, p=.50; k=8), were not significant, suggesting no consistent relationships between these traits and AL. There was also significant heterogeneity across effect sizes for extraversion (Q10=34.62, p<.01; I2=78.90%) and agreeableness (Q8=36.90, p<.01; I2=87.83%).
Figures depicting funnel plot asymmetry for publication bias are reported for the AL-neuroticism and AL-extraversion correlations (see Supplementary Figure 1); however, these plots should be interpreted cautiously given that at least 10 effect sizes are required to distinguish chance from true asymmetry (Sterne et al., 2011), which is also why funnel plots are not reported for conscientiousness, openness, or agreeableness. Use of REML (as opposed to ML) as the variance estimator did not meaningfully change the effect size or p value for any of the personality trait-AL associations.
3.6 |. Quality Assessment.
A table outlining the quality assessment of included studies is reported on the OSF project page (https://osf.io/rkxas/). Several criteria were fulfilled by all studies, though only one study (Kobrosly, 2012) fulfilled every relevant quality assessment criterion. Nevertheless, the quality of studies was deemed to be quite high overall, with nine reports receiving ―good‖ ratings and only two receiving ―poor‖ ratings. Gallagher et al. (2021) was deemed poor as i) the number of participants that met eligibility for analyses was extremely reduced compared to the total sample (N=10,175 to N=956), resulting in possible bias, ii) blood pressure (BP), which is particularly prone to measurement imprecision (e.g., Juraschek et al., 2020) was only measured once, iii) demographic characteristics of the sample were not clearly reported (e.g., education was dichotomized as ―college education‖ versus ―high school or less‖), and iv) the AL index computation was unclear (i.e., an independent researcher could not replicate the approach). Otto et al. (2021) was deemed poor because i) only four biomarkers in the AL index were used, which likely reflects a narrowly defined metabolic syndrome as opposed to AL, and ii) the Borderline Personality Disorder (BPD) patient group and the control group were not drawn from the same population, and the researchers did not measure any socioeconomic characteristics; as such, any between-group differences in primary predictors (e.g., neuroticism, childhood adversity and trauma, aggression, chronic stress) may not reflect differences due to BPD status.
4 |. Discussion
The current systematic review and meta-analysis details the various approaches researchers have used to investigate the relationships between the Big Five and AL among adults (K=11). Meta-analytic results suggest a positive association between neuroticism and AL, inverse associations between conscientiousness and AL and openness and AL, and no overall significant associations between extraversion or agreeableness and AL. These results should be interpreted with caution, as the meta-analyses were not well-powered.
The positive association between neuroticism and AL is unsurprising, as AL is thought to reflect maladaptive physiological responding as a result of heightened stress perceptions (McEwen & Stellar, 1993). Characterized by anxiety, depressive symptoms, and a propensity toward negative emotionality (Eysenck & Eysenck, 1985; McCrae & Costa, 2004), neuroticism is associated with heightened vulnerability to stressors, including greater exposure and more intense reactions to perceived stress (Bolger & Schilling, 1991; Craske, 1999; Suls et al., 1998; Suls & Martin, 2005). In combination with the existing research, the current findings highlight opportunities for interventions aiming to decrease physiological dysregulation by addressing stress perceptions and responses, particularly for those high in neuroticism. For instance, associations between neuroticism and negative affect may be mediated by threat appraisals (Schneider, 2004); neuroticism is associated with threat perception, negative affect, and poor task performance, but individuals high in neuroticism may only experience more negative affect and perceptions of stress in the context of threat appraisal (e.g., the perception of inadequate coping resources relative to the demands required by the situation). As such, interventions aiming to decrease AL may target individuals who are high in neuroticism, focusing on developing skills for reappraising situations that are perceived as threatening.
Conversely, individuals higher in openness may be more resilient to stress. Openness is characterized by cognitive flexibility, curiosity, and intellectual engagement (Costa, 2008; McCrae & Sutin, 2007). Individuals high in openness may approach potentially stressful situations with greater flexibility and adaptability; these individuals, who tend to appreciate varied emotional experience, may also evaluate environmental stressors as appealing opportunities to indulge in alternative perspectives. Consistent with these postulations, research suggests that openness is related to greater coping capacity in the face of stress (Penley & Tomaka, 2002), as well as higher average positive affect, which is mediated by lower threat appraisal (Schneider et al., 2012). Notably, openness can change; research suggests that interventions have increased openness to experience, either purposefully (e.g., via openness to action training; Stieger et al., 2020) or unintentionally (e.g., via cognitive training; Jackson et al., 2012). Consistent with Stephen et al.’s (2016) findings that openness declines when individuals exceed a high level of cumulative physiological dysregulation, future research investigating whether increases in openness lead to better physiological regulation would improve our understanding of the causal relationship between openness and AL.
Finally, the significant inverse association between conscientiousness and AL is consistent with both empirical research and theoretical frameworks of conscientiousness, which is characterized by competence, dutifulness, self-discipline, and diligence (Costa et al., 1991). For instance, the Invest-and-Accrue model of conscientiousness proposes that individuals high in conscientiousness devote current resources (e.g., time, energy, assets) to maximize future gains, which subsequently contributes to positive outcomes across various domains, including improved physiological health (Hill & Jackson, 2016). Likewise, the existing literature consistently reports that conscientiousness is positively associated with health-promoting behaviors and inversely associated with risky behaviors (for a meta-analysis, see Bogg & Roberts, 2004). Indeed, Milad and Bogg’s (2020) findings (i.e., higher perceptions of activity were protective against higher levels of AL, but only for individuals high in conscientiousness) exemplify the conscientiousness-AL link: individuals high in conscientiousness tend to engage in healthier behaviors, which leads to better physiological regulation. Notably, our meta-analysis reflecting the relationship between conscientiousness and AL was distinguished by the least amount of heterogeneity in effects, providing more confidence in the reliability of the synthesized effect.
The null effects for extraversion and agreeableness match the individual study estimates well. There was a relatively inconsistent spread of effects for these traits, with positive, negative, and null correlations, which is also reflected in the meta-analytic summaries and heterogeneity statistics.
4.1 |. Variability Across Eligible Studies.
While the reports varied across primary study characteristics (e.g., sample size, study design, country of origin), the most prominent heterogeneity was observed in the AL algorithm approaches. For instance, one researcher stratified all biomarkers by sex prior to computing z scores, others only sex-stratified some biomarkers (e.g., waist circumference), and many did not sex-stratify any biomarkers, despite well-established sex-differences in many of the measured physiological biomarkers, including CRP (Khera et al., 2009), IGF-1 (Austad & Bartke, 2015), BP (Ji et al., 2020), BMI (Flegal et al., 2016), triglyceride synthesis (Lonardo et al., 2019), and cholesterol metabolism (Palmisano et al., 2018). Research also suggests sex-differences in cortisol responses to stress (Kudielka & Kirschbaum, 2005) and between DHEA-s and adverse health outcomes (Goldman & Glei, 2007). Although traditional AL index computations do not include recommendations regarding sex-stratification, the extensive body of research documenting sex-differences in both levels of biomarkers and associated outcomes implies the importance of stratifying biomarker scores by sex prior to computing AL risk scores. Extensive researcher decisions were further evidenced across the various operational definitions of ―high risk‖ and the components of AL indices included across each study. Finally, z score standardization is a limitation, as the definition of risk is inherently sample specific, such that any given individual might be categorized as meeting high risk on a given biomarker in one sample, but not another.
Concern regarding the fidelity of AL indices has been raised by several reviews (e.g., Johnson et al., 2017; Juster et al., 2010). Without an established standardized method, researchers must make extensive decisions in their approach, leading to the observed between-study differences in computed constructs (i.e., Silberzahn et al., 2018) and hindrance of the development of AL as a target for clinical intervention. Together, the widespread variability in algorithm scoring approaches may have resulted in somewhat distinct indices of AL, limiting the cross-study comparative validity of the AL variables. Given this variability, the extent to which the magnitude and/or significance of associations between the Big Five traits and AL are affected by varying operational definitions of AL across studies remains unclear (i.e., similarities in approaches may have facilitated evaluation of differences in outcomes). However, as there is no gold standard in measurement and computation of AL, researchers are forced to make decisions based on best judgment, and all studies synthesized biomarkers based on previous research and theoretical conceptualizations of AL.
Extensive variability was also evident in the analytical approaches used to assess the relationships between personality traits and AL, which highlights the inherent challenges of empirically examining the relationships between physiological and psychological constructs. That is, mediation and moderation models aim to refine, understand, and make causal inferences about constructs (Wu & Zumbo, 2008), and the cross-study heterogeneity observed in analytic approach reflects the complex interplay between physiological and psychological processes, as well as differences in theoretical understanding of how these processes affect one another.
Finally, cross-sectional assessments may not sufficiently capture the interplay of personality traits and AL as people change and develop across the lifespan. Importantly, biological wear-and-tear accumulates over time, leading to changes in physiological dysfunction over time (e.g., van Deurzen & Vanhoutte, 2019). Likewise, the existing literature documents individual differences in intraindividual change and variability in personality across development (e.g., Graham et al., 2020; Stephan et al., 2016). Indeed, if AL-Big Five associations change over time, as was demonstrated by Stephan et al. (2016), findings may differ between the 11 eligible reports due to capturing the relationships at different points across development. Similarly, Luo et al. (2022) reported correlation coefficients representing trait-AL associations across five measurement occasions, which varied over time (e.g., r=.01 to .10 for extraversion). However, these associations did not appear to change systematically, given null between- and within-person associations between AL and either neuroticism and extraversion, though the sample was relatively small (N=566). Future research using repeated measurements may be especially important for elucidating associations between the Big Five and AL.
4.2 |. Limitations Reported by Eligible Studies.
Various limitations were highlighted across the included studies (see Supplementary Table 1). For instance, many authors noted deviations in available biomarker measurements as a limitation (e.g., missing HPA-axis biomarkers). Not including a specific biomarker or an entire category of biomarkers may have implications for the construct validity of AL indices, and authors typically posited that access to more biomarker assessments was ideal for capturing AL. Future work investigating the minimum number and type of biomarkers for predictive validity would enrich the field.
Interestingly, some limitations were rarely highlighted. For instance, several biomarkers are subject to physiological variation and measurement imprecision (McCormack & Holmes, 2020): based on two serial assessments, glucose and HbA1c vary 2–5% based on measurement imprecision alone, and this variation increases to 6–10% when also accounting for natural biological variation; HDL and LDL cholesterol and triglycerides fluctuate 2–5% between two serial measurements due to measurement imprecision, which increases to 11–20% and 31–40%, respectively, when also accounting for natural biological variation (2020). Likewise, BP can often be substantially higher (i.e., white coat hypertension) or lower (i.e., masked hypertension) in the presence of a medical practitioner, compared to typical/resting BP. While most studies recorded BP at least three serial times to improve precision, few studies assessed biomarkers on repeated occasions, and only Kobrosly (2012) repeatedly assessed biomarkers on a short-term scale to improve reliability (e.g., intraindividual means of IL-6 and IGF-1, collected at three occasions within a year). Given potential imprecision and biological variability, AL biomarkers (which were predominately assessed by a single assessment in a single context), may not provide the most accurate representation of physiological dysregulation.
The generalizability of the results is also questionable. Apart from Hawkley et al. (2011), all samples included predominantly European/White participants with relatively high SES, and authors rarely mentioned the broader implications of prevalent sampling bias. For example, studies examining personality and AL among primarily European/White samples often did not account for the inherent differences between individuals of different races, such as differential waist-to-hip ratio cut-offs (Lear et al., 2010) or disparate creatinine production influencing cortisol measures (Hawkley et al., 2011). Furthermore, these homogenous samples likely do not adequately account for differences in the experiences of individuals with lower levels of education and SES, who may experience greater day-to-day stress and therefore be at an increased risk for accumulating AL. Indeed, research indicates that Black individuals (Van Dyke et al., 2020) and those with lower education levels and SES (Hawkley et al., 2011; Merkin et al., 2009) have significantly higher AL. Recruiting more diverse samples is critical for improving our understanding of how personality plays a role in the exacerbated accumulation of AL across diverse individuals and populations.
4.3 |. Current Study Strengths and Limitations.
This is the first systematic review and meta-analysis to synthesize associations between the Big Five personality traits and AL. We adhered to PRISMA protocols to ensure thorough and transparent reporting, which included pre-registering our data collection procedures, research design, and analytic plan. The only deviation was our use of the NIH guidelines for the quality assessment of included articles. We also executed our search within several electronic databases and completed systematic forward and backward searches of the eligible studies. However, alternative terms reflecting AL may exist (e.g., dysregulation burden), potentially limiting identification of all relevant reports. Similarly, we chose to focus on the Big Five model of personality, though additional models or aspects of personality may be relevant to physiological dysregulation. Given our search terms (e.g., personality, neuroticism, extraversion), this review would have identified articles using alternative models of personality (e.g., HEXACO, Eysenck’s Personality Inventory) if they had been published. However, future work should aim to synthesize research reporting the relationships between AL and additional aspects of personality not captured by the Big Five, such as temperament, rumination, aggression, or perceived control.
As a further strength, we also included both published and unpublished reports and effect sizes, and studies that did not focus predominantly on the association between personality traits and AL, thereby reducing the probability of publication bias. Finally, the current meta-analysis is not well-powered (K=11), as a sample size of 20–25 independent effects sizes is ideal for a random-effects meta-analysis (Valentine et al., 2010). Therefore, the current results should be interpreted with caution.
4.4 |. Future Directions and Conclusions.
Although this review focused on the associations between the Big Five traits and AL, a primary concern regarding the heterogeneity in AL indices appears to be an issue across all AL research. Lack of precision in standard recommendations for AL indices may lead to differences in findings and conclusions due to researcher decisions (as well as the unethical opportunity for cherry picking results). Development and uptake of clear, rigorous guidelines for the assessment of biomarkers and computation of AL indices would unquestionably facilitate comparisons across studies. Yet, the associations that are reported throughout the existing literature are, to some extent, conceptual replication, which may be appreciated as a strength rather than a weakness. That is, the literature is fairly ubiquitous in finding a robust association between AL and adverse outcomes, despite extensive heterogeneity in operational definitions. Similarly, the studies that were excluded due to data redundancy (i.e., using identical samples as studies meeting eligibility) primarily report consistent associations between traits and AL, despite differences in analytic approaches and AL algorithms. Apart from a positive association between agreeableness and AL (which was attenuated to non-significance when adjusting for health behaviors and chronic conditions; Turiano et al., 2015) the findings from the eligible reports align with those from the reports that were removed to avoid redundancy. Specifically, differences in sample characteristics, participant eligibility, or adjusting for specific covariates did not appear to systematically affect associations between traits and AL across the 11 studies.
To prevent bias in results due to researcher decisions, some studies repeated their analyses using various calculations of AL or including additional biomarkers (e.g., Richards et al., 2023). A handful of existing studies have aimed to identify the optimal approach for AL scoring algorithms (Johnson et al., 2017; Li et al., 2019; Seplaki et al., 2005). Despite inconsistencies in efforts to identify the AL computation approach with the best predictive validity, the consensus appears to be Seeman et al.’s (2010) approach for AL scoring algorithm (Johnson et al., 2017; Li et al., 2019). While these recommendations provide critical information for future research, a systematic investigation of differences in quantity and breadth of biological systems included within AL indices may provide further assistance in delineating clear recommendations for the ideal AL index approach. Specifically, investigation of differences in the predictive ability of AL based on the biomarkers or biological systems included within AL indices may provide more nuanced information regarding risk stratification. For instance, the included studies differed in the total number of AL biomarkers measured (i.e., 4–14), as well as represented biological systems (i.e., 2–5). It is unclear whether particular biomarkers are critical for inclusion within an AL index, or whether more biomarkers across multiple systems provides a stronger index of dysregulation. In particular, when using the count of high risk sample-specific cut-offs, the treatment of biomarkers as equal may diminish the predictive validity of the index. Importantly, Hawkley et al. (2011) examined AL as a latent variable, and examined whether risk differed across subsamples for various biological systems. Findings indicated that the effects of SES on AL are specific to certain systems (i.e., cardiovascular and obesity factors) in middle to older adulthood, suggesting that there are likely differences in the importance of dysregulation across particular biological systems across outcomes.
In the absence of precise recommendations, researchers should continue to clearly document procedures for determining high risk categories to ensure transparency and the ability to replicate computations of AL indices. Likewise, stratifying biomarkers by sex prior to determining risk categories may be important, given the wealth of research suggesting sex-differences across many of the biomarkers typically included within AL indices. Finally, AL is theorized to reflect wear and tear on the body by consolidating markers of physiological dysregulation across biological systems (McEwen & Stellar, 1993). However, risk profiles were predominantly based on biomarker levels (i.e., measured at only one occasion), rather than naturally measured dysregulation. Future research may assess within-person variability in biomarkers using intensive measurement designs – to capture day-to-day fluctuations in physiological regulation across various contexts – and subsequently examine whether intensive AL indices overlap with traditional AL indices.
The included studies investigate a wide range of factors that may be involved in the associations between personality traits and AL, particularly SES/SEP. Two studies suggest that traits do not mediate associations between AL and SES/SEP (Christensen et al., 2018; Hawkley et al., 2011); however, other work finds that traits attenuate the association between AL and subjective social status (Richards et al., 2023), suggesting that personality may be important for understanding the relationships between AL and perceived economic circumstances and social standing. Thus, the role of personality traits on relationships between AL and subjective versus objective measures of socioeconomic status warrants further research to investigate the extent to which these represent study-specific findings or true differences. Further, associations between AL and some traits (conscientiousness, openness) may be attenuated by sociodemographic factors (i.e., education, occupational social class; Christensen et al., 2019b). However, Stephan et al. (2016) found that AL was associated with personality change in non-Hispanic individuals and those with higher education, though the sample was 89.6% White and highly educated. The studies meeting eligibility for this review predominantly focus on White/European samples, which provides tremendous rationale for future research drawing on more diverse samples to further investigate the role of ethnicity and SES on associations between personality traits and AL.
Finally, additional mechanisms linking personality traits and AL remain fairly unclear. Interestingly, no study identified by this review focused explicitly on perceived stress as a mediator between personality traits and AL, which is another excellent opportunity for future research. Similarly, and consistent with Milad and Bogg (2020), future research should further explore coping styles and health behaviors as potential mechanisms underlying associations between AL and personality traits.
As the first systematic review and meta-analysis to comprehensively identify and assess studies reporting the association between the Big Five personality traits and AL, we synthesized the existing literature qualitatively and quantitatively. Our meta-analyses suggest that individuals who are higher in neuroticism, or lower in conscientiousness or openness, tend to have more physiological dysregulation. These results suggest that high neuroticism may be a risk factor, while high conscientiousness and openness may be protective factors, for high AL. With only 7–11 independent effects included in each meta-analysis, these results should be interpreted with caution. Further, while Milad and Bogg’s (2020) findings suggest that the perception of leading an active lifestyle was a mediating pathway by which extraversion and conscientiousness were associated with greater physiological resilience, the mechanisms underlying associations between personality traits and AL remain relatively unclear. However, given the robust associations between high AL and several adverse health outcomes (e.g., physical and cognitive impairment, disease, chronic conditions, and mortality), identifying risk factors and correlates of AL, such as personality traits, may facilitate the development of personality-informed interventions aiming to foster optimal aging processes across the lifespan by mitigating physiological dysregulation.
Supplementary Material
Supplementary Figure 1. Funnel Plot Asymmetry for Publication Bias
Highlights:
Meta-analytic results suggest that individuals higher in conscientiousness are lower in allostatic load (AL)
Meta-analytic results suggest that individuals higher in neuroticism are higher in AL
Meta-analytic results suggest that individuals higher in openness are lower in AL
There are extensive between-study differences in AL algorithm computation approaches
Future research should examine perceived stress as a pathway linking traits and AL
Acknowledgments
Data collection procedures, research design, and analytic plan for the current study were pre-registered and reported on the Open Science Framework on December 14th, 2021 (osf.io/rxw5a). Research reported in this publication was financially supported by the National Institute on Aging of the National Institutes of Health under Award Numbers P01AG043362, R01-AG018436, and R01-AG067622. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Dr. Muniz-Terrera acknowledges the support of the Osteopathic Heritage Foundation through funding for the Osteopathic Heritage Foundation Ralph S. Licklider, D.O., Research Endowment in the Heritage College of Osteopathic Medicine. There are no conflicts of interest to disclose among any of the contributing authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argyle M, & Lu L (1990). The happiness of extraverts. Personality and Individual Differences, 11(10), 1011–1017. 10.1016/0191-8869(90)90128-E [DOI] [Google Scholar]
- Austad SN, & Bartke A (2015). Sex differences in longevity and in responses to anti-aging interventions: A mini-review. Gerontology, 62(1), 40–46. 10.1159/000381472 [DOI] [PubMed] [Google Scholar]
- Barry LE, O’Neill S, Heaney LG, & O’Neill C (2021). Stress-related health depreciation: Using allostatic load to predict self-rated health. Social Science & Medicine, 283, 114170. 10.1016/j.socscimed.2021.114170 [DOI] [PubMed] [Google Scholar]
- Beckie TM (2012). A systematic review of allostatic load, health, and health disparities. Biological Research for Nursing, 14(4), 311–346. 10.1177/1099800412455688 [DOI] [PubMed] [Google Scholar]
- Bienvenu OJ, Samuels JF, Costa PT, Reti IM, Eaton WW, & Nestadt G (2004). Anxiety and depressive disorders and the five-factor model of personality: A higher- and lower-order personality trait investigation in a community sample. Depression and Anxiety, 20(2), 92–97. 10.1002/da.20026 [DOI] [PubMed] [Google Scholar]
- Bogg T, & Roberts BW (2004). Conscientiousness and health-related behaviors: A meta-analysis of the leading behavioral contributors to mortality. Psychological Bulletin, 130(6), 887–919. 10.1037/0033-2909.130.6.887 [DOI] [PubMed] [Google Scholar]
- Bolger N, & Schilling EA (1991). Personality and the problems of everyday life: The role of neuroticism in exposure and reactivity to daily stressors. Journal of Personality, 59(3), 355–386. 10.1111/j.1467-6494.1991.tb00253.x [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, & Rothstein HR (2010). A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods, 1(2), 97–111. 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- Briñol P, & DeMarree K (Eds.). (2012). Embodied Validation: Our Bodies Can Change and Also Validate Our Thoughts. In Social Metacognition (Vol. 1–Book, Section, pp. 235–256). Psychology Press. 10.4324/9780203865989-21 [DOI] [Google Scholar]
- Campbell CM, & Edwards RR (2009). Mind–body interactions in pain: The neurophysiology of anxious and catastrophic pain-related thoughts. Translational Research: The Journal of Laboratory and Clinical Medicine, 153(3), 97–101. 10.1016/j.trsl.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DS, Dich N, Flensborg-Madsen T, Garde E, Hansen ÅM, & Mortensen EL (2019a). Objective and subjective stress, personality, and allostatic load. Brain and Behavior, 9(9), e01386. 10.1002/brb3.1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DS, Flensborg-Madsen T, Garde E, Hansen ÅM, & Mortensen EL (2019b). Big Five personality traits and allostatic load in midlife. Psychology & Health, 34(8), 1011–1028. 10.1080/08870446.2019.1585851 [DOI] [PubMed] [Google Scholar]
- Christensen DS, Flensborg-Madsen T, Garde E, Hansen ÅM, Pedersen JM, & Mortensen EL (2018). Parental socioeconomic position and midlife allostatic load: A study of potential mediators. BMC Public Health, 18(1), 1029. 10.1186/s12889-018-5956-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimbolic Gunthert K, Cohen LH, & Armeli S (1999). The role of neuroticism in daily stress and coping. Journal of Personality and Social Psychology, 77(5), 1087–1100. 10.1037/0022-3514.77.5.1087 [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. https://doi.org/2136404 [PubMed] [Google Scholar]
- Cohen S, Kessler RC, & Underwood LG (1995). Measuring stress: A guide for health and social scientists. Oxford University Press. [Google Scholar]
- Costa PT (2008). The Revised NEO Personality Inventory (NEO-PI-R). In The SAGE Handbook of Personality Theory and Assessment: Volume 2—Personality Measurement and Testing (Vol. 1–Book, Section, p. 179). SAGE Publications Ltd. 10.4135/9781849200479.n9 [DOI] [Google Scholar]
- Costa PT Jr., McCrae RR, & Dye DA (1991). Facet scales for agreeableness and conscientiousness: A revision of the NEO Personality Inventory. Personality and Individual Differences, 12(9), 887–898. 10.1016/0191-8869(91)90177-D [DOI] [Google Scholar]
- Craske MG (1999). Anxiety disorders: Psychological approaches to theory and treatment. Westview Press. [Google Scholar]
- Crook Z, Booth T, Cox SR, Corley J, Dykiert D, Redmond P, Pattie A, Taylor AM, Harris SE, Starr JM, & Deary IJ (2018). Apolipoprotein E genotype does not moderate the associations of depressive symptoms, neuroticism and allostatic load with cognitive ability and cognitive aging in the Lothian Birth Cohort 1936. PloS One, 13(2), e0192604. 10.1371/journal.pone.0192604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck HJ 1916–1997, & Eysenck MW (1985). Personality and individual differences: A natural science approach. Plenum Press. [Google Scholar]
- Fleeson W (2001). Toward a structure- and process-integrated view of personality: Traits as density distributions of states. Journal of Personality and Social Psychology, 80(6), 1011–1027. 10.1037/0022-3514.80.6.1011 [DOI] [PubMed] [Google Scholar]
- Fleeson W (2004). Moving personality beyond the person-situation debate: The challenge and the opportunity of within-person variability. Current Directions in Psychological Science: A Journal of the American Psychological Society, 13(2), 83–87. 10.1111/j.0963-7214.2004.00280.x [DOI] [Google Scholar]
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, & Ogden CL (2016). Trends in obesity among adults in the United States, 2005 to 2014. JAMA: The Journal of the American Medical Association, 315(21), 2284–2291. 10.1001/jama.2016.6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S, Sumner R, Creaven AM, O’Súilleabháin PS, & Howard S (2021). Allostatic load and mental health during COVID-19: The moderating role of neuroticism. Brain, Behavior, & Immunity - Health, 16, 100311. 10.1016/j.bbih.2021.100311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartland N, O’Connor DB, & Lawton R (2012). The effects of conscientiousness on the appraisals of daily stressors. Stress and Health, 28(1), 80–86. 10.1002/smi.1404 [DOI] [PubMed] [Google Scholar]
- Goldman N, & Glei DA (2007). Sex differences in the relationship between DHEAS and health. Experimental Gerontology, 42(10), 979–987. 10.1016/j.exger.2007.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, & Friedman HS (2006). Health status and the five-factor personality traits in a nationally representative sample. Journal of Health Psychology, 11(5), 643–654. 10.1177/1359105306066610 [DOI] [PubMed] [Google Scholar]
- Graham EK, Bastarache ED, Milad E, Turiano NA, Cotter KA, & Mroczek DK (2018). Physical activity mediates the association between personality and biomarkers of inflammation. SAGE Open Medicine, 6, 1–10. 10.1177/2050312118774990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham EK, Rutsohn JP, Turiano NA, Bendayan R, Batterham PJ, Gerstorf D, Katz MJ, Reynolds CA, Sharp ES, Yoneda TB, Bastarache ED, Elleman LG, Zelinski EM, Johansson B, Kuh D, Barnes LL, Bennett DA, Deeg DJH, Lipton RB, … Mroczek DK (2017). Personality predicts mortality risk: An integrative data analysis of 15 international longitudinal studies. Journal of Research in Personality, 70, 174–186. 10.1016/j.jrp.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham EK, Weston SJ, Gerstorf D, Yoneda TB, Booth T, Beam CR, Petkus AJ, Drewelies J, Hall AN, Bastarache ED, Estabrook R, Katz MJ, Turiano NA, Lindenberger U, Smith J, Wagner GG, Pedersen NL, Allemand M, Spiro A, … Mroczek DK (2020). Trajectories of Big Five personality traits: A coordinated analysis of 16 longitudinal samples. European Journal of Personality, 34(3), 301–321. 10.1002/per.2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grol M, & De Raedt R (2020). The link between resting heart rate variability and affective flexibility. Cognitive, Affective, & Behavioral Neuroscience, 20(4), 746–756. 10.3758/s13415-020-00800-w [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Lavelle LA, Berntson GG, & Cacioppo JT (2011). Mediators of the relationship between socioeconomic status and allostatic load in the Chicago Health, Aging, and Social Relations Study (CHASRS). Psychophysiology, 48(8), 1134–1145. 10.1111/j.1469-8986.2011.01185.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV (1983). Combining independent estimators in research synthesis. The British Journal of Mathematical & Statistical Psychology, 36(1), 123–131. 10.1111/j.2044-8317.1983.tb00768.x [DOI] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill PL, & Jackson JJ (2016). The invest-and-accrue model of conscientiousness. Review of General Psychology, 20(2), 141–154. 10.1037/gpr0000065 [DOI] [Google Scholar]
- Iacovino JM, Bogdan R, & Oltmanns TF (2016. Personality predicts health declines through stressful life events during late mid-life. Journal of Personality, 84(4), 536–546. 10.1111/jopy.12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JJ, Hill PL, Payne BR, Roberts BW, & Stine-Morrow EA (2012). Can an old dog learn (and want to experience) new tricks? Cognitive training increases openness to experience in older adults. Psychology and Aging, 27(2), 286–292. 10.1037/a0025918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaras KN, Schaefer SM, van Reekum CM, Lapate RC, Greischar LL, Bachhuber DR, Love GD, Ryff CD, & Davidson RJ (2012). Conscientiousness predicts greater recovery from negative emotion. Emotion, 12(5), 875–881. 10.1037/a0028105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN, & Cheng S (2020). Sex differences in blood pressure trajectories over the life course. JAMA Cardiology, 5(3), 255–262. 10.1001/jamacardio.2019.5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Cavallaro FL, & Leon DA (2017). A systematic review of allostatic load in relation to socioeconomic position: Poor fidelity and major inconsistencies in biomarkers employed. Social Science & Medicine, 192, 66–73. 10.1016/j.socscimed.2017.09.025 [DOI] [PubMed] [Google Scholar]
- Jokela M, Batty GD, Nyberg ST, Virtanen M, Nabi H, Singh-Manoux A, & Kivimaeki M (2013). Personality and all-cause mortality: Individual-participant meta-analysis of 3,947 deaths in 76,150 adults. American Journal of Epidemiology, 178(5), 667–675. 10.1093/aje/kwt170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraschek SP, Ishak A, Mukamal KJ, Cohen ML, & Beach JL (2020). Impact of clinic-based blood pressure approaches on blood pressure measurement. American Journal of Hypertension, 33(1), 26–30. 10.1093/ajh/hpz118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, & Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews, 35(1), 2–16. 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Lachman ME, Tun PA, Koretz BK, & Seeman TE (2014). Biological correlates of adult cognition: Midlife in the United States (MIDUS). Neurobiology of Aging, 35(2), 387–394. 10.1016/j.neurobiolaging.2013.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, McEwen BS, Rowe JW, & Seeman TE (2002). Allostatic load as a predictor of functional decline: MacArthur studies of successful aging. Journal of Clinical Epidemiology, 55(7), 696–710. 10.1016/s0895-4356(02)00399-2 [DOI] [PubMed] [Google Scholar]
- Kern ML, & Friedman HS (2008). Do conscientious individuals live longer? A quantitative review. Health Psychology, 27(5), 505–512. 10.1037/0278-6133.27.5.505 [DOI] [PubMed] [Google Scholar]
- Khera A, Vega GL, Das SR, Ayers C, McGuire DK, Grundy SM, & de Lemos JA (2009). Sex differences in the relationship between c-reactive protein and body fat. The Journal of Clinical Endocrinology and Metabolism, 94(9), 3251–3258. 10.1210/jc.2008-2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrosly RW (2012). An epidemiologic investigation into the relationship between stress, allostatic load, and depressive disorder among older adults (Publication No. 3555033) [Doctoral dissertation, University of Rochester]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Kudielka BM, & Kirschbaum C (2005). Sex differences in HPA axis responses to stress: A review. Biological Psychology, 69(1), 113–132. 10.1016/j.biopsycho.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Lazarus RS, & Folkman S (1987). Transactional theory and research on emotions and coping. European Journal of personality, 1(3), 141–169. [Google Scholar]
- Lazarus RS, & Folkman S (1984). Stress, appraisal, and coping. Springer publishing company. [Google Scholar]
- Lear SA, James PT, Ko GT, & Kumanyika S (2010). Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. European Journal of Clinical Nutrition, 64(1), 42–61. 10.1038/ejcn.2009.70 [DOI] [PubMed] [Google Scholar]
- Leger KA, Charles ST, Turiano NA, & Almeida DM (2016). Personality and stressor-related affect. Journal of Personality and Social Psychology, 111(6), 917–928. 10.1037/pspp0000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GJ, Dickie DA, Cox SR, Karama S, Evans AC, Starr JM, Bastin ME, Wardlaw JM, & Deary IJ (2018). Widespread associations between trait conscientiousness and thickness of brain cortical regions. NeuroImage, 176, 22–28. 10.1016/j.neuroimage.2018.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Rosemberg M-AS, Dalton VK, Lee SJ, & Seng JS (2019). Exploring the optimal allostatic load scoring method in women of reproductive age. Journal of Advanced Nursing, 75(11), 2548–2558. 10.1111/jan.14014 [DOI] [PubMed] [Google Scholar]
- Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, Abdelmalek MF, & Suzuki A (2019). Sex differences in nonalcoholic fatty liver disease: State of the art and identification of research gaps. Hepatology, 70(4), 1457–1469. 10.1002/hep.30626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Zhang B, Estabrook R, Graham EK, Driver CC, Schalet BD, … & Mroczek DK (2022). Personality and health: Disentangling their between-person and within-person relationship in three longitudinal studies. Journal of personality and social psychology, 122(3), 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney EM, Gurbaxani BM, Jones JF, de Souza Coelho L, Pennachin C, & Goertzel BN (2006). Chronic fatigue syndrome and high allostatic load. Pharmacogenomics, 7(3), 467–473. 10.2217/14622416.7.3.467 [DOI] [PubMed] [Google Scholar]
- Mattei J, Demissie S, Falcon LM, Ordovas JM, & Tucker K (2010). Allostatic load is associated with chronic conditions in the Boston Puerto Rican Health Study. Social Science & Medicine, 70(12), 1988–1996. 10.1016/j.socscimed.2010.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack JP, & Holmes DT (2020). Your results may vary: The imprecision of medical measurements. BMJ, 368, m149. 10.1136/bmj.m149 [DOI] [PubMed] [Google Scholar]
- McCrae RR, & Costa PT (2004). A contemplated revision of the NEO Five-Factor Inventory. Personality and Individual Differences, 36(3), 587–596. 10.1016/S0191-8869(03)00118-1 [DOI] [Google Scholar]
- McCrae RR, & Sutin AR (2007). New frontiers for the five-factor model: A preview of the literature. Social and Personality Psychology Compass, 1(1), 423–440. 10.1111/j.1751-9004.2007.00021.x [DOI] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840(1), 33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Stellar E (1993). Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine, 153(18), 2093–2101. 10.1001/archinte.1993.00410180039004 [DOI] [PubMed] [Google Scholar]
- Merkin SS, Basurto-Dávila R, Karlamangla A, Bird CE, Lurie N, Escarce J, & Seeman T (2009). Neighborhoods and cumulative biological risk profiles by race/ethnicity in a national sample of U.S. adults: NHANES III. Annals of Epidemiology, 19(3), 194–201. 10.1016/j.annepidem.2008.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad E, & Bogg T (2020). Personality traits, coping, health-related behaviors, and cumulative physiological health in a national sample: 10 year prospective effects of conscientiousness via perceptions of activity on allostatic load. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine, 54(11), 880–892. 10.1093/abm/kaaa024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman D, & The PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLOS MEDICINE, 6(7), e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczek DK, & Almeida DM (2004). The effect of daily stress, personality, and age on daily negative affect. Journal of Personality, 72(2), 355–378. 10.1111/j.0022-3506.2004.00265.x [DOI] [PubMed] [Google Scholar]
- Olkin I, & Pratt JW (1958). Unbiased estimation of certain correlation coefficients. The Annals of Mathematical Statistics, 29(1), 201–211. 10.1214/aoms/1177706717 [DOI] [Google Scholar]
- Otto B, Kokkelink L, & Brüne M (2021). Borderline Personality Disorder in a ―life history theory‖ perspective: Evidence for a fast ―pace-of-life-syndrome.‖ Frontiers in Psychology, 12, 715153. 10.3389/fpsyg.2021.715153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmisano BT, Zhu L, Eckel RH, & Stafford JM (2018). Sex differences in lipid and lipoprotein metabolism. Molecular Metabolism, 15, 45–55. 10.1016/j.molmet.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker HW, Abreu AM, Sullivan MC, & Vadiveloo MK (2022). Allostatic load and mortality: A systematic review and meta-analysis. American Journal of Preventive Medicine, 63(1), 131–140. 10.1016/j.amepre.2022.02.003 [DOI] [PubMed] [Google Scholar]
- Penley JA, & Tomaka J (2002). Associations among the Big Five, emotional responses, and coping with acute stress. Personality and Individual Differences, 32(7), 1215–1228. 10.1016/S0191-8869(01)00087-3 [DOI] [Google Scholar]
- Richards L, Maharani A, & Präg P (2023). Subjective social status and allostatic load among older people in England: A longitudinal analysis. Social Science & Medicine, 320, 115749. [DOI] [PubMed] [Google Scholar]
- Romero LM, Dickens MJ, & Cyr NE (2009). The reactive scope model—A new model integrating homeostasis, allostasis, and stress. Hormones and Behavior, 55(3), 375–389. 10.1016/j.yhbeh.2008.12.009 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM (1999). Glucocorticoids, stress, and their adverse neurological effects: Relevance to aging. Experimental Gerontology, 34(6), 721–732. 10.1016/s0531-5565(99)00047-9 [DOI] [PubMed] [Google Scholar]
- Schneider TR (2004). The role of neuroticism on psychological and physiological stress responses. Journal of Experimental Social Psychology, 40(6), 795–804. 10.1016/j.jesp.2004.04.005 [DOI] [Google Scholar]
- Schneider TR, Rench TA, Lyons JB, & Riffle RR (2012). The influence of neuroticism, extraversion and openness on stress responses. Stress and Health, 28(2), 102–110. 10.1002/smi.1409 [DOI] [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, & McEwen BS (1997). Price of adaptation—Allostatic load and its health consequences: MacArthur studies of successful aging. Archives of Internal Medicine, 157(19), 2259–2268. 10.1001/archinte.1997.00440400111013 [DOI] [PubMed] [Google Scholar]
- Seeman T, Gruenewald T, Karlamangla A, Sidney S, Liu K, Mcewen B, & Schwartz J (2010). Modeling multisystem biological risk in young adults: The Coronary Artery Risk Development in Young Adults Study. American Journal of Human Biology: The Official Journal of the Human Biology Association, 22(4), 463–472. 10.1002/ajhb.21018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T, McEwen B, Rowe J, & Singer B (2001). Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences - PNAS, 98(8), 4770–4775. 10.1073/pnas.081072698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seplaki CL, Goldman N, Glei D, & Weinstein M (2005). A comparative analysis of measurement approaches for physiological dysregulation in an older population. Experimental Gerontology, 40(5), 438–449. 10.1016/j.exger.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Silberzahn R, Uhlmann EL, Martin DP, Anselmi P, Aust F, Awtrey E, Bahník Š, Bai F, Bannard C, & Bonnier E (2018). Many analysts, one data set: Making transparent how variations in analytic choices affect results. Advances in Methods and Practices in Psychological Science, 1(3), 337–356. 10.1177/2515245917747646 [DOI] [Google Scholar]
- Stephan Y, Sutin AR, Luchetti M, & Terracciano A (2016). Allostatic load and personality: A 4-year longitudinal study. Psychosomatic Medicine, 78(3), 302–310. 10.1097/PSY.0000000000000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, … & Higgins JP (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. The BMJ, 343, 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- Stieger M, Wepfer S, Rüegger D, Kowatsch T, Roberts BW, & Allemand M (2020). Becoming more conscientious or more open to experience? Effects of a two-week smartphone-based intervention for personality change. European Journal of Personality, 34(3), 345–366. 10.1002/per.2267 [DOI] [Google Scholar]
- Suls J, & Martin R (2005). The daily life of the garden-variety neurotic: Reactivity, stressor exposure, mood spillover, and maladaptive coping. Journal of Personality, 73(6), 1485–1510. 10.1111/j.1467-6494.2005.00356.x [DOI] [PubMed] [Google Scholar]
- Suls J, Martin R, & David JP (1998). Person-environment fit and its limits: Agreeableness, neuroticism, and emotional reactivity to interpersonal conflict. Personality & Social Psychology Bulletin, 24(1), 88–98. 10.1177/0146167298241007 [DOI] [Google Scholar]
- Turiano N, Bastarache E, Graham E, Mroczek D, Gruenewald T, & Ong A (2015). The Big 5 personality traits and allostatic load in the Midlife in The Us Study (MIDUS). Psychosomatic Medicine, 77(3), A9. [Google Scholar]
- Valentine JC, Pigott TD, & Rothstein HR (2010). Tutorial: How Many Studies Do You Need? A Primer on Statistical Power for Meta-Analysis. Journal of Educational and Behavioral Statistics, 35(2), 215–247. http://www.jstor.org/stable/40785162 [Google Scholar]
- van Deurzen I, & Vanhoutte B (2019). A longitudinal study of allostatic load in later life: The role of sex, birth cohorts, and risk accumulation. Research on Aging, 41(5), 419–442. 10.1177/0164027518813839 [DOI] [PubMed] [Google Scholar]
- Van Dyke ME, Baumhofer NK, Slopen N, Mujahid MS, Clark CR, Williams DR, Lewis TT, & Kau’i Baumhofer N (2020). Pervasive discrimination and allostatic load in African American and White adults. Psychosomatic Medicine, 82(3), 316–323. 10.1097/PSY.0000000000000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra L, Schneider IK, & Koole SL (2017). Embodied mood regulation: The impact of body posture on mood recovery, negative thoughts, and mood-congruent recall. Cognition and Emotion, 31(7), 1361–1376. 10.1080/02699931.2016.1225003 [DOI] [PubMed] [Google Scholar]
- Viechtbauer W (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- Viljoen M, & Claassen N (2017). Allostatic load and heart rate variability as health risk indicators. African Health Sciences, 17(2), 428–435. 10.4314/ahs.v17i2.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston SJ, Graham EK, Turiano NA, Aschwanden D, Booth T, Harrison F, James BD, Lewis NA, Makkar SR, & Mueller S (2020). Is healthy neuroticism associated with chronic conditions? A coordinated integrative data analysis. Collabra: Psychology, 6(1), 42. 10.1525/collabra.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston SJ, Hill PL, & Jackson JJ (2015). Personality traits predict the onset of disease. Social Psychological and Personality Science, 6(3), 309317. 10.1177/1948550614553248 [DOI] [Google Scholar]
- Wilson R, de Leon C, Bienias J, Evans D, & Bennett D (2004). Personality and mortality in old age. The Journals of Gerontology: Series B, 59(3), P110–P116. 10.1093/geronb/59.3.P110 [DOI] [PubMed] [Google Scholar]
- Wu AD, & Zumbo BD (2008). Understanding and using mediators and moderators. Social Indicators Research, 87(3), 367–392. 10.1007/s11205-007-9143-1 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Funnel Plot Asymmetry for Publication Bias


