Abstract
Schizophrenia is a major mental disorder that affects approximately 1% of the population worldwide. Cognitive deficits are a key feature of the disorder and a primary cause of long-term disability. Over the past decades, significant literature has accumulated demonstrating impairments in early auditory perceptual processes in schizophrenia. In this review, we first describe early auditory dysfunction in schizophrenia from both a behavioral and neurophysiological perspective and examine their interrelationship with both higher order cognitive constructs and social cognitive processes. Then, we provide insights into underlying pathological processes, especially in relationship to glutamatergic and N-methyl-D-aspartate receptor (NMDAR) dysfunction models. Finally, we discuss the utility of early auditory measures as both treatment targets for precision intervention and as translational biomarkers for etiological investigation. Altogether, this review points out the crucial role of early auditory deficits in the pathophysiology of schizophrenia, in addition to major implications for early intervention and auditory-targeted approaches.
Keywords: schizophrenia, auditory, tone-matching, event-related potentials, NMDA receptor
1. INTRODUCTION
Schizophrenia is a serious and persistent mental disorder and the 3rd leading cause of worldwide disability among mental disorders across age groups (Collaborators, 2022). Although the outcome of schizophrenia is not uniformly negative, only a minority of individuals with first episode psychosis return to work or report being in a relationship (Ajnakina et al., 2021; Cowman et al., 2021). Life expectancy is reduced by 10–20 years of age (Chesney et al., 2014). The direct and indirect annual costs of schizophrenia are estimated in the billions of dollars (Charrier et al., 2013; Cloutier et al., 2016). Although the diagnostic criteria for schizophrenia focus primarily on positive symptoms, such as hallucinations or delusions, outcome in schizophrenia is strongly dependent upon impairments in cognitive processes that as yet are incompletely understood (rev. in Green et al., 2019; Javitt, 2022).
In the National Institute of Mental Health Research Domain Criteria (RDoC) processes, the Cognitive Systems domain is defined to include constructs relating to perception as well as higher order processes such as attention, memory, language, cognitive control and working memory. In addition, reception of non-facial communication, such as auditory information, is a specific subconstruct of the Social Processes domain (Cuthbert, 2014). Over the past decades, significant literature has accumulated demonstrating impairments in auditory perceptual processes in schizophrenia and their interrelationship with both higher order cognitive constructs and social processes (Javitt, 2009; Javitt and Freedman, 2015). Moreover, auditory perceptual processes are highly amenable to translational, cross-species investigation, and thus can be used to gain insights into underlying pathological processes, especially in relationship to glutamatergic and N-methyl-D-aspartate receptor (NMDAR) hypofunction models (Javitt and Freedman, 2015; Javitt et al., 1996; Kantrowitz et al., 2016).
This article reviews the accumulating literature on auditory dysfunction in schizophrenia from a behavioral, neurophysiological and translational perspective and highlights the utility of auditory measures as both treatment targets for precision intervention and as translational biomarkers for etiological investigation. Our goal is both to highlight areas where the field is relatively mature, as reflected in convergent meta-analytic findings across groups, as well as areas of controversy where further study may leader to deeper understanding of the pathophysiology of the disorder.
2. BEHAVIORAL MEASURES OF EARLY AUDITORY DEFICITS IN SCHIZOPHRENIA
2.1. The human auditory system
The human auditory system projects from the outer ear through the cochlea (inner ear), brainstem (superior olivary nucleus, inferior colliculus) and thalamic relays (medial geniculate nucleus, MGN) to primary auditory cortex (A1) and secondary auditory cortex in the belt and parabelt regions. These early auditory (EA) cortical regions include Heschl’s gyrus (HG) and surrounding regions of the superior temporal plane (STP) and correspond mainly to Brodmann's areas BA41 (medial) and BA42 (lateral). In the current Human Connectome Project Multimodal Parcellation atlas (HCP-MMP1.0)(Glasser et al., 2016) the EA region is divided into A1, MBelt, Lbelt, Pbelt and retroinsular (RI) parcels. EA project to auditory association (AA) regions that are located in the anterior portion of the planum temporale and lateral superior temporal sulcus (STS) and correspond primarily to BA22 (Fig. 1A).
Figure 1.
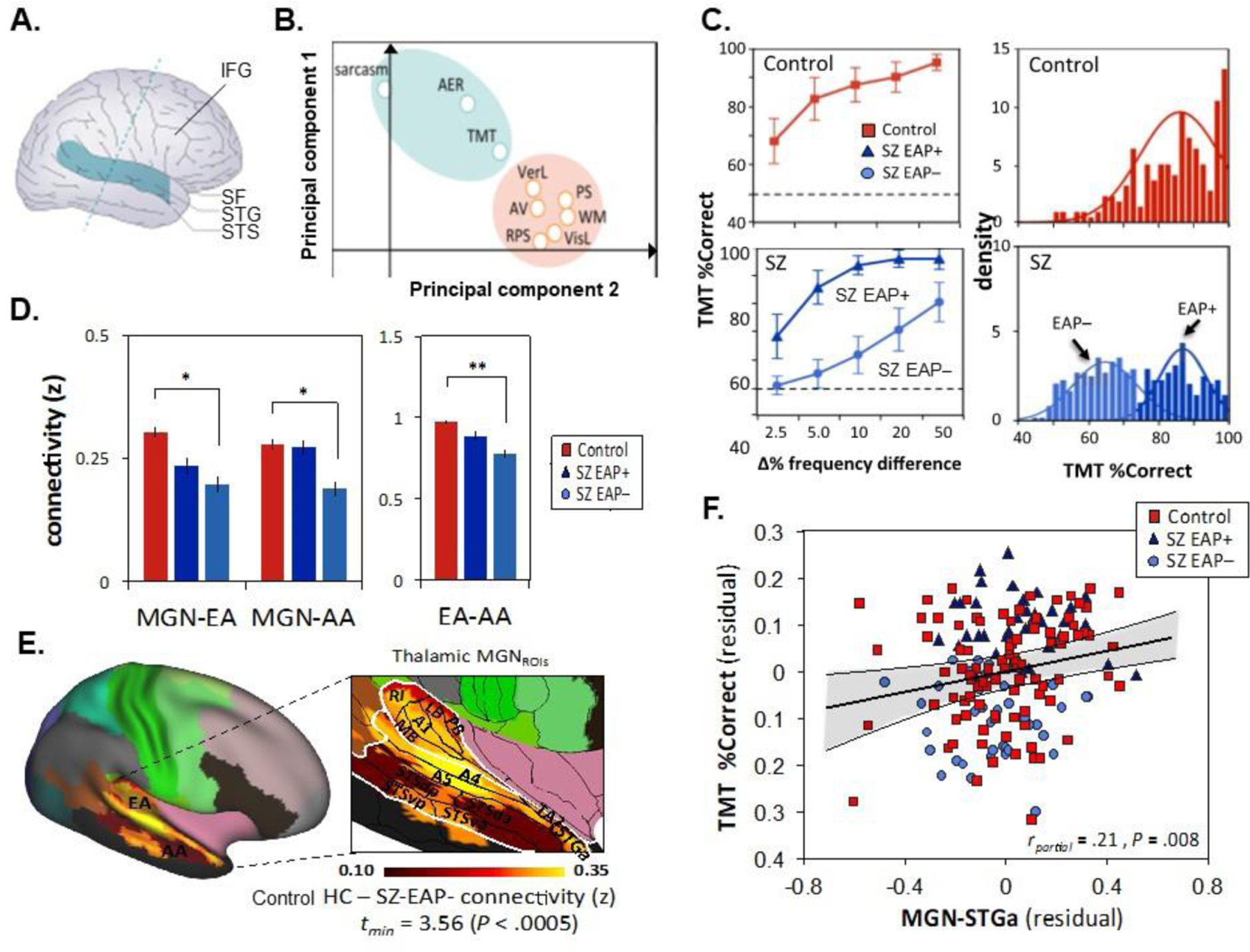
Behavioral approches to early auditory assessment. A. Anatomy of the auditory cortex. The superior temporal gyrus (STG) is bordered superiorly by the lateral sulcus (LS) in monkeys and the Sylvian fissure (SF) in humans. Inferiorly, the STG is bordered by the superior temporal sulcus (STS) in both monkeys and humans. The blue dashed line shows the orientation of electrical currents generated within the auditory cortex. (adapted from Javitt and Sweet, 2015). B. Principal Component Analysis biplot showing two clusters: MCCB domains T-scores (orange) and tone-matching task (TMT), auditory emotion recognition (AER) and sarcasm percent correct (green). The two principal components captured 63.6% of the data variability. PS processing speed, AV attention/vigilence, WM working memory, VerL verbal learning, VisL visual learning, RPS reasoning/problem solving (Adapated from Donde et al., 2019b). C. Left: Line plots of mean percentage correct of tone-matching task for each level of frequency difference. Right: Density histograms of TMT percent correct responses showing unimodal distribution for the Control group only. For the SZ (schizophrenia) group, the bimodal distribution had a substantially lower BIC (Bayesian Information Criterion) than a unimodal model, and the bimodal/unimodal likelihood ratio was highly significant (χ. = 22.23, P < 0.0001) (Adapated from Donde et al., 2019b). D. Bargraph (mean +/− SD) of resting-state functional connecting z-scores between Glasser’s regions across groups. *P<0.05; **P<0.005 (Adapted from Donde et al., 2019b). E. Voxel-wise comparisons between controls and SZ-EAP- with bilateral thalamic (MGN) ROIs based on Glasser’s regions for auditory pathway. AA=Associative Auditory: A4=Brodmann area A4, A5=Brodmann area A5, STGa=anterior superior temporal gyrus, STSda=dorsoanterior superior temporal sulcus, STSdp=dorsoposterior superior temporal sulcus, STSva=ventroanterior superior temporal sulcus, STSvp=dorsoposterior superior temporal sulcus, TA2=anterosuperior temporal area. EA=Early Auditory: A1=primary auditory, LB=lateral belt, MB=medial belt, PB=parabelt, RI=retro-insula. MGN=thalamic Medial Geniculate Nuclei (Adapted from Donde et al., 2019b). F. Scatterplot of total percent correct on tone-matching performance versus rsFC-MRI between MGN and STGa, which belongs to AA. Partial r was computed across two sites (outpatient and inpatient) and two groups (Controls and SZ) (Adapted from Donde et al., 2019b).
In the HCP-MMP1.0 atlas, AA regions are divided into parcels A4, A5, STSdp, STSda, STSvp, STSva, STGa, and TA2. AA, in turn, projects to distributed “higher-order” brain regions in the temporal, parietal and frontal cortices through parallel “what” and “where” pathways (Poeppel et al., 2012). Projections to pars triangularis regions of the left and right inferior frontal gyri (IFG), corresponding to Broca’s area and Broca’s right homolog (BA45), respectively, may be of particular importance as these regions mediate auditory inputs into language (left) and prosodic (right) generation regions (Belyk and Brown, 2014; Kujala and Leminen, 2017)
In canonical network models, EA regions fall primarily within the sensorimotor network (Yeo et al., 2011), emphasizing the close interrelationship between auditory and motor systems (rev. in (Morillon et al., 2019), whereas AA regions fall within the default mode network, emphasizing their relationship to processes such as social cognition (Yeshurun et al., 2021). Deficits in auditory processing in schizophrenia may be mapped against these underlying substrates.
2.2. Historic approaches to auditory sensory processing
As originally conceptualized by Kraepelin, deficits in cognitive function represent a core feature of schizophrenia and contribute significantly to impairments in functional outcome (Donde et al., 2019a; Kraepelin, 1907). Cognitive processes may be subdivided into two broad categories: those involved primarily with social interaction (“social cognition”) and those more broadly involved in higher order cognitive operations such as attention, working memory, reasoning and problem solving, or visual/verbal learning (“neurocognition”). Deficits in both social- and neurocognition have been extensively documented in schizophrenia (Green, 1996; Green et al., 2000; Javitt, 2022) and are evaluated using well-established cognitive batteries, such as the MATRICS consensus cognitive battery (MCCB) (Kern et al., 2008; Nuechterlein et al., 2008). In contrast, sensory processes have been less studied and there are currently no standardized assessment batteries for use in clinical characterization. Nevertheless, there is increasing evidence to document both impairments in early auditory processing and contributions of auditory processing deficits to deficits in both social and neurocognition.
Study of the auditory function in schizophrenia is influenced heavily by the concept of the auditory “echoic” memory system, that was elaborated most completely by Cowan in the early 1980’s (Cowan, 1984). An initial model of human cognitive organization viewed sensory regions as largely passive structures that conveyed sensory information to higher cognitive regions (Broadbent, 1958). This was updated by Baddeley in the 1970’s, who emphasized the existence of additional, “slave” systems, such as the phonological loop, that maintained sensory-specific short-term memories (Baddeley, 1970). In the 1980–90s, Cowan and others identified a specific short-term memory system, termed the auditory sensory or “echoic” memory system, that stored relatively unprocessed sensory information for durations of ~20–30 seconds outside the “attentional spotlight” (Cowan, 1993; Javitt, 2009).
In retrospect, the first published test of echoic memory in schizophrenia was performed in the mid 1960’s even prior to description of the system. In their study, Billingberg & Jonsson sought to investigate mechanisms underlying paranoia by presenting schizophrenia patients with friendly or neutral words recited with friendly, neutral or threatening intonation (the “Intonation test”) (Billingberg and Jonsson, 1965). As a control, an additional auditory test was included in which participants had to identify a short “sound effect” (e.g., a train, a dentist drill).
As predicted, patients demonstrated paranoid misattribution (i.e., “neutral” and “friendly” stimuli considered as “threatening”) in comparison to controls. However, schizophrenia patients were also impaired in the “sound effect” auditory test, suggesting the possibility of a more basic underlying auditory deficit. Consequently, a second study was conducted with an even simpler sensory control task in which individuals were asked to match tones following brief delay. In this study, no misattribution of threat was observed. Nevertheless, patients were significantly impaired in the ability to match tones (Donde et al., 2019a; Jonsson and Sjostedt, 1973).
2.3. Auditory sensory (“echoic”) memory impairment in schizophrenia
2.3.1. Tone-matching paradigm
The first focused tests of early auditory processing (EAP) were performed in the mid-1990’s, based on “echoic memory” concepts (Holcomb et al., 1995; Strous et al., 1995). These studies used a simple tone-matching test (TMT) in which two tones were presented with brief intervening delay (rev. in (Donde et al., 2017; Javitt and Sweet, 2015)). Subsequent studies demonstrated that the elevations in tone-matching thresholds reflected failures in encoding of the initial sensory information, while subsequent retention of information within the echoic memory trace was intact (Javitt et al., 1997). Furthermore, schizophrenia participants were no more affected than controls by same (Rabinowicz et al., 2000) or cross-modality (Javitt et al., 1997) distractors, again emphasizing the “bottom-up” nature of the deficit and encouraging further investigation of the underlying processes. A recent meta-analysis of eighteen separate studies showed a statistically large deficit in schizophrenia (SMD=1.17, p<0.001) (Donde et al., 2017). Significant heterogeneity of the deficit was also observed (p=0.04) suggesting likely cohort effects (Donde et al., 2017). In long-term follow-up studies, tone-matching scores have been found to be extremely stable over time, suggesting that they reflect a trait, rather than state, psychophysiological measure (Donde et al., 2019b).
2.3.2. Categorical analysis
In support of the heterogeneity finding, further statistical analysis of tone-matching data demonstrated a bimodal deficit pattern (Donde et al., 2019b), which permitted groups to be separated into those with impaired (EAP−) vs. intact (EAP+) EAP function (Fig. 1C). EAP− individuals were more likely drawn from chronic inpatient or residential care facilities, whereas EAP+ individuals were more likely drawn from outpatient facilities and to be living independently (Donde et al., 2019b; Lee et al., 2018b; Rabinowicz et al., 2000). EAP− and EAP+ individuals showed similar levels of positive and negative symptoms, although cognitive symptoms were significantly higher in EAP− vs. EAP+ individuals. EAP− vs. EAP+ individuals also had lower estimated premorbid IQ (96.2±11.5 vs. 106.3±8.5, d=.67) although both remained close to the population mean. EAP− individuals also achieved fewer grades of education (13.2±7.2 vs. 16.7±8.9) and were significantly less likely to have pursued education beyond high school (25.5% vs. 50.9%), even though parental socioeconomic status was similar to EAP+.
In neuropsychological testing using the MATRICS battery (Kern et al., 2008; Nuechterlein et al., 2008), EAP− individuals showed an additional deficit of ~1 sd across measures, although with somewhat preserved function in visual learning and reasoning/problem solving. Mean antipsychotic dose was significantly higher for EAP− vs. EAP+ individuals (d=.35, <.005) but did not co-vary with tone-matching test performance. Nevertheless, these results support EAP deficits as a potential marker of treatment-resistance, which may encourage early use of clozapine.
2.3.3. Relationship to social- and neurocognition
Deficits in tone matching performance correlate highly with measures of auditory social cognition including auditory emotion recognition (AER) and sarcasm (“attitudinal prosody”) detection, and with measures of auditory verbal learning across participants (Donde et al., 2019b). In principal components analysis, tone matching, social cognition measures and MCCB domain scores separated along 2 axes which can be conceptualized as auditory vs. visual (PC1) and social vs. neurocognition (PC2) (Fig. 1B). TMT performance segregated most strongly with the auditory social cognition measures, but with limited distance to neurocognitive measures such as verbal learning, emphasizing the importance of EAP deficits to both social and neurocognitive dysfunction.
2.3.4. Additional auditory behavioral paradigm
Although the majority of tone-matching studies have investigated pitch discrimination, similar deficits are observed for detection of intensity (Donde et al., 2020a; Holcomb et al., 1995; Jonsson and Sjostedt, 1973), duration (Donde et al., 2020a; Jonsson and Sjostedt, 1973), or location (Matthews et al., 2013; Olsson and Nielzen, 1999; Perrin et al., 2010; Perrin et al., 2018). In one study that utilized the Montreal Battery for Assessment of Amusia (Kantrowitz et al., 2014b), 45% of participants with schizophrenia were classified as “amusical” vs. 9% of healthy comparison individuals. Large-size statistical effects (d=1.3) were seen across “melody,” “rhythm” and “memory” domains, and remained significant even following statistical control for overall cognitive impairments. A recent study using the Test of Basic Auditory Abilities (TBAC) along with other pitch discrimination tasks found significant, moderate effect sizes (d=.51-.66) across intensity, frequency and duration (Kraus et al., 2019), also supporting prior research.
2.4. Regional correlates of auditory echoic memory impairments
Critical substrates for auditory echoic memory assessed by tone-matching paradigms are localized to the auditory cortex in the region of the STP in non-human primates (Colombo et al., 1990; Iversen, 1973), and are unaffected even by large structural lesions in the prefrontal areas (Iversen, 1973; Zatorre and Samson, 1991). In humans, detection of pitch change between individual tones (i.e., simple tone-matching) appears to be specifically associated to the A1 activity, as shown by elevated threshold in case reports of ischemia and edema involving the medial part of the HG and sparing the associative components of the auditory cortex (Habib et al., 1995; Hattiangadi et al., 2005). Congruent with this association, tone-matching abilities remained similar to controls in patients with temporal lobe excisions that encroached the STG while sparing the HG (Johnsrude et al., 2000). Fine-grained analysis of loudness may also depend upon A1, although coarse discrimination is possible even following A1 lesion (Dykstra et al., 2012).
Furthermore, neuroimaging studies showed that the ability to detect pitch-change is related to isolated A1 activations (Griffiths, 2003). In parallel, a slightly more complex component of active frequency analysis of non-verbal tones was identified: the detection of pitch change in pitch patterns such as melodies (i.e., identifying if 3 tones have “same” or “different” pitch and, if “different”, which one differed from the others) (Griffiths, 2001, 2003). By contrast to simple tone-matching (i.e., identifying if 2 tones have “same” or “different” pitch), detection of change in pitch patterns is instead associated with AA regions, as shown by additional activations in associative areas located within the STG (Patterson, 1990), STP (Griffiths et al., 1998; Stewart et al., 2008) or both (Warren and Griffiths, 2003) when healthy participants are exposed to pitch change in a ≥ 3-tones melody.
In schizophrenia, tone-matching deficits have also been evaluated relative to resting-state functional connectivity between structures within the early auditory system, including MGN, EA and AA auditory regions. EAP− individuals showed significant functional connectivity impairments among these structures (Fig. 1D). When analyses were performed relative to pre-defined cortical parcels, between-group differences were prominent both between MGN and A1, and between MGN and parcels A4, A5 and STGa (Fig. 1E) that are known to be activated during auditory language tasks (Glasser et al., 2016). Correlations with tone-matching impairments in patients intercorrelated most strongly with functional connectivity between MGN and STGa, an area that is also activated by tasks involving theory-of-mind (Fig. 1F). Overall, these findings support concepts that tone-matching deficits reflect dysfunction within primary auditory cortex and surrounding regions and encourage further targeted investigation of these regions.
2.5. Auditory (phonological) reading impairments in schizophrenia
EAP deficits similar to those observed in schizophrenia are also reported in developmental dyslexia and are related to the ability to manipulate speech sounds (“phonological processing”). Initial evidence of impaired phonological processing in schizophrenia came from studies of the ability of schizophrenia individuals to distinguish isolated phonemes, such as /da/ or /ba/, that differ only in pitch direction change at stimulus onset (Kugler and Caudrey, 1983). Although the underlying tonal frequencies of individual phonemes may vary considerably both across speakers and across position of the phoneme in a word, there is normally a sharp categorical boundary between phonemes such that these within-phoneme variations are ignored. In schizophrenia, a shallower boundary is observed, consistent with a reduced precision in processing the underlying tonal frequencies (Cienfuegos et al., 1999).
Subsequent studies evaluated phonological processing using tasks such as the Comprehensive Test of Phonological Processing (CTOPP) (Wagner et al., 1999), and showed significant impairment in the ability to manipulate phonemes as indexed by measures such as phonological awareness or memory (Revheim et al., 2006; Revheim et al., 2014). In these studies, deficits in phonological processing correlated significantly with deficits in more basic tone-matching ability and, in turn, predicted impairments in reading fluency and overall functional outcome. In addition, the correlations between phonological processing deficits and functional outcome remained significant even following covariation for other neurocognitive deficits (e.g., working memory). As with tone-matching, phonological processing impairments correlated with resting-state functional connectivity indices within the early auditory system (Donde et al., 2019b). At present, as with tone-matching, phonological processing and reading fluency measures are not incorporated into standard batteries for the assessment of schizophrenia (e.g., MCCB), but might become valuable additions.
3. NEUROPHYSIOLOGICAL BASES OF EARLY AUDITORY PROCESSING DEFICITS
Neurophysiological mechanisms underlying EAP deficits can be efficiently investigated using EEG and event-related potential (ERP) measures, which may be used to trace the flow of information within the early auditory system with high temporal resolution, and fMRI, which may be used to localize ERP generator regions. One ERP measure that has proven particularly useful is mismatch negativity (MMN). MMN indexes processes relevant to the echoic memory system and can be used translationally across species and computationally to investigate underlying mechanisms. MMN has also proven useful in testing neurodevelopmental vs. neurodegenerative mechanisms and in identifying individuals for early intervention. Paradigms for assessment of auditory plasticity have been developed for the study of developmental dyslexia and have also proven sensitive to neurophysiological impairments in schizophrenia. Additional measures including the auditory 40 Hz steady-state response (ASSR) and P1/N1 potentials provide additional insights into mechanisms of early auditory dysfunction in schizophrenia.
3.1. Mismatch negativity (MMN) paradigms
MMN is elicited most commonly in the context of an auditory oddball paradigm in which a sequence of repetitive auditory stimuli is interrupted infrequently by a physically or conceptually different deviant (“oddball”) stimulus (Fig. 2A). In this paradigm, MMN is defined as the difference in response amplitude to deviant minus standard stimuli (Fig. 2B). Using fMRI, generators for MMN have been localized primarily to Heschl’s gyrus and surrounding regions of the STG and planum temporale (Molholm et al., 2006; Opitz et al., 2002; Schonwiesner et al., 2007; Wible et al., 2001). Deviance-related activity is observed as well in inferior frontal gyrus (IFG), particularly on the right hemisphere (Doeller et al., 2003; Giard et al., 1990; Kantrowitz et al., 2015) (Fig. 2C).
Figure 2.

Neurophysiological approaches to early auditory assessment. A. The typical oddball sequence (A, left) utilizes two stimuli that differ in stimulus quality. One of the stimuli is designated the “redundant” and accounts for the majority of the presentations (in this case ~90%). The overabundance of redundant presentations establishes a regular pattern that is violated by “oddball” (or “deviant”) stimuli, which rarely occur (in this case ~10% of presentations) (Adapted from Ross and Hamm, 2020). B. Time-domain waveforms by group (CHR = Clinical High Risk, SZ = Schizophrenia). The peak latency window is shown in yellow (adapted from Sehatpour et al., 2020). C. Activations in response to duration changes in healthy individuals. Statistical parameter maps showing significant responses in yellow (P < 0.05 corrected) shown on an individual gray matter surface [international consortium for brain mapping single subject anatomical template; right (F) and left hemispheres (G)]. HG: Heschl’s gyrus; PFC, mid-ventrolateral prefrontal cortex; PT: planum temporale; STG, superior temporal gyrus; STS, superior temporal sulcus (Adapted from Schonwiesner et al., 2007). D. Dynamic causal model of the response to auditory deviants (frequency). The sources comprising the networks are connected with forward (dark gray), backward (gray), or lateral (light gray) connections, all of which can show condition-specific changes. This is an asymmetrical three-level hierarchical network, comprising five extrinsically interconnected cortical areas (emulating long-range connections between A1, STG, and the right IFG) and has condition-specific intrinsic connections at the level of the left and right A1 (emulating local adaptation) allowing for bothextrinsic and intrinsic connectivity changes. A1: primary auditory cortex; STG: superior temporal gyrus; IFG: inferior temporal gyrus (Adapted from Garrido et al., 2009). E. Time-Frequency plots of location MMN by group (CHR = Clinical High Risk, SZ = Schizophrenia). Box region shows alpha response interval. Inset: Scalp topographies with the alpha integration window (adapted from Sehatpour et al., 2020).
MMN deficits in schizophrenia were first reported in the early 1990’s to both duration (Shelley et al., 1991) and frequency (pitch) deviants (Javitt et al., 1993). Since then, deficits have been extensively replicated across multiple deviant types, including intensity, location, omission, and white noise stimuli, with mean effect sizes (d) in the range of .6–1.2 (rev. in Avissar et al., 2018; Erickson et al., 2017; Umbricht and Krljes, 2005; Xiong et al., 2017)) and large correlations with severity of tone-matching impairments (Javitt et al., 2000b; Lee et al., 2018b; Sehatpour et al., 2020). Moreover, MMN has proven to have high cross-site and test-retest reliability, making it well suited for multicenter investigation (Light et al., 2012).
MMN assessments have also been used to understand the nature of phonemic processing abnormalities in schizophrenia. As with other MMN types, MMN to alterations in the phonetic properties of phonemes have been repeatedly documented in patients, and shown to correlate with illness duration, likelihood to relapse and functional outcome (e.g. (Fisher et al., 2008; Fisher et al., 2019; Kawakubo et al., 2007; Kawakubo et al., 2011; Mi et al., 2021)).
3.2. Time course of auditory dysfunction
Neurodevelopmental studies in children indicate that tone-matching ability becomes adultlike in children aged 6 to 7 years, while the sensitivity to change in pitch-patterns (i.e. ≥ 3-tones melodies) significantly improves later, from 8 to 9 years of age, suggesting that EA inputs to the surrounding AA structures allowing pitch-pattern detection are crucial over the course of brain development (Cooper, 1994; Fancourt, 2013; Trehub et al., 1986). Sequential acquisition of tone-matching and pitch-patterns discrimination ability throughout the neurodevelopmental course indicates that early alterations (Rapoport et al., 2012) or neurodegenerative processes during later adolescence described in schizophrenia could account for present findings (Insel, 2010). However, studies performed to date have not observed deficits in tone-matching ability in individuals at clinical high risk (CHR) for schizophrenia, suggesting that early auditory processes are relatively intact before the onset of the disorder, or that individuals with premorbid EAP deficits are not being captured by existing CHR criteria and current recruitment approaches (Corcoran et al., 2015; Donde et al., 2019b; Kraus et al., 2022). In contrast to the behavioral measures, related neurophysiological indices, such as MMN continue to develop into adulthood (Bishop et al., 2011), and thus may index continued neurodevelopment during ages associated with schizophrenia onset (Corcoran et al., 2018b).
Consistent with this development time course, MMN may be especially useful in predicting conversion and remission in CHR individuals. MMN deficits in individuals with prodromal symptoms of schizophrenia were first reported over 15 years ago (Bodatsch et al., 2011; Brockhaus-Dumke et al., 2005) and have been replicated extensively since with greatest predictive value provided by a combined pitch and duration (“double”) deviant (rev. in Hamilton et al., 2022; Tada et al., 2019). Significant deficits are seen between CHR individuals as a group with an effect size of ~.4 sd depending upon stimulus type (Fig. 2B), and between CHR individuals that convert to schizophrenia vs. those who do not (Perez et al., 2014).
MMN may also assist in prediction of individuals who will remit from the CHR vs. those who remain symptomatic (Hamilton et al., 2021). As in individuals with established schizophrenia, MMN also predicts functional disability in CHR individuals (Hamilton et al., 2018b; Tada et al., 2019). Nevertheless, results have been somewhat variable across studies and may be affected by factors such as medication status (Hamilton et al., 2022). Large-scale studies are presently ongoing and may provide definitive evidence for utility of MMN within the clinical management of early-stage schizophrenia.
In contrast to the meta-analytic deficits reported in CHR, a recent meta-analysis of first-episode schizophrenia reported relatively intact activity at illness onset (Haigh et al., 2017a), followed by degeneration over the initial course of the illness associated with volume reductions in auditory sensory cortex (Curtis et al., 2021; Haigh et al., 2017a; Haigh et al., 2017b; McCarley et al., 2002; Murphy et al., 2020; Salisbury et al., 2020b; Umbricht et al., 2006). At present, the differential findings in CHR and first-episode schizophrenia are somewhat different to reconcile, but may suggest multiple trajectories to schizophrenia, including potentially an acute neurodegenerative pattern that is poorly represented within CHR programs. For instance, it is possible that the present CHR recruitment methods and criteria might not adequately account for individuals with premorbid EAP deficiencies and more severe cognitive deficits.
To the extent that auditory function does degenerate over early stages of the illness, it is possible that it could be rescued by appropriate behavioral, pharmacological or non-invasive-brain stimulation-based interventions. MMN has been extensively characterized in both non-human primate (Lakatos et al., 2020) and rodent (Featherstone et al., 2018) models, permitting its use both translationally and in early stage drug development (see Section 4).
3.3. Stimulus-specific adaptation vs. prediction error
The MMN paradigm is predicated on the concept thatbrain responses to auditory stimuli differ when they are presented in isolation vs. when they are presented intermixed with more frequent intervening standards. Because MMN is expressed as the difference between responses to deviant and standard stimuli during repeated presentation, an early controversy regarding MMN generation focused on the degree to which it reflects a relative reduction in responses to standard stimuli during the course of stimulation – a process termed “stimulus specific adaptation” (SSA) vs. a short-term memory-based increase in response to the deviant stimulus (Naatanen et al., 1978; Winkler and Czigler, 1998) - a process that has increasingly come to be termed “prediction error” (PE) (Friston, 2005; Heilbron and Chait, 2018; Wacongne, 2016).
SSA concepts are based on the observation that auditory ERP to repetitive standard stimuli decrease with increasing stimulation rate, so that stimuli that are presented less often in the oddball sequence (deviants) will elicit larger responses than those presented more frequently (standards) based on refractoriness alone (Harpaz et al., 2021; May, 2021). Nevertheless, early studies argued against this explanation first, by demonstrating that the first stimulus in a sequence does not elicit MMN; second, by showing topography differences between MMN and N1 responses to standard stimuli; and finally, by using paradigmatic control conditions (e.g. multiple standards), which produce equivalent refractoriness but nevertheless prevent MMN generation (Heilbron and Chait, 2018; Naatanen et al., 1978; Winkler and Czigler, 1998).
Mnemonic template models of MMN are based on the concept that excitatory neurons in the brain are under some level of ongoing tonic inhibition and that repetitive standards create a disinhibitory mnemonic template in neurons sensitive to stimulus features dissimilar to those of the standard. When a stimulus differs from the preceding standards, it elicits greater activity than if the same stimulus had been presented in isolation. In this framework, prediction errors are generated within the same neurons that encode the bottom-up predictions (Javitt et al., 1996; Lakatos et al., 2020; Naatanen et al., 1978).
More recent predictive coding (PC) models have proposed instead that predictions are generated primarily top-down, although they converge with mnemonic template models in proposing that the prediction error (PE) signal is generated within lower-level cortical regions (Friston, 2005). Although the assumption of separate prediction PE neurons, and of top-down generation of predictions remains controversial (Denham and Winkler, 2020; Heilbron and Chait, 2018), nevertheless it serves as a heuristic model for ongoing research.
PC-based models of MMN have been tested using dynamic causal modelling (DCM) to evaluate the relative contribution of feedforward and feedback connections in the auditory system. In DCM studies in normative individual, MMN was best modelled by assuming local interactions with A1, as well as reciprocal interactions between A1, STG and inferior frontal gyrus (IFG) in the region of Broca’s right homolog (Garrido et al., 2008; Garrido et al., 2009) (Fig. 2D). Notably, however, the leading phase (e.g. 50–150 ms) of MMN in response to frequency deviants reflected primarily intrinsic alterations within auditory cortex, whereas extrinsic changes between cortical levels were manifest primarily at longer latencies (i.e. >150 ms) (Garrido et al., 2009).
In schizophrenia, between-group deficits are prominent at the earliest phase of MMN generation and remain consistent over time (e.g. Fig. 2B), consistent with the concept that they reflect abnormalities primarily in intrinsic changes within auditory cortex (Garrido et al., 2009). These findings are consistent as well with fMRI studies showing significant reductions in deviance-related activity not only in A1 (Gaebler et al., 2020) but also within subcortical relay regions such as inferior colliculus and thalamic MGN (Gaebler et al., 2020) that propagate in a feed-forward approach to produce impaired activations in schizophrenia.
Although the majority of MMN studies in schizophrenia use a simple oddball paradigm, some studies have used omission (“missing stimulus”) (Kreitschmann-Andermahr et al., 1999; Rudolph et al., 2015; Salisbury and McCathern, 2016), gestalt (Coffman et al., 2017; Haigh et al., 2017b; Salisbury et al., 2020a) or local-global (Kirihara et al., 2020) paradigms that emphasize PE vs. SSA models. To the extent that these have been studied, effect sizes are similar to those in simpler paradigms (Avissar et al., 2018), supporting the importance of mnemonic-based models for underlying basic auditory dysfunction in schizophrenia.
3.4. Time-frequency correlates and local circuit processing
Further information regarding the nature of early auditory processing deficits can be obtained using time-frequency (TF) analyses of ERP data. In TF analyses, the electrophysiological activity is decomposed according to underlying frequency content. Canonical frequency ranges include delta (.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–24 Hz) and gamma (>24 Hz). Critical measures derived from TF analyses include evoked power, which is derived from the average response, and both intertrial coherence (ITC) and total power, which are derived from processing of responses to individual stimuli. TF analyses permit greater insights into the local circuit and cellular processes that give rise to the time-domain ERP waveform findings (Javitt et al., 2020).
In TF analyses, MMN maps primarily to the theta frequency range (Fuentemilla et al., 2008; Javitt et al., 2000b; Jin et al., 2014), as do MMN deficits in schizophrenia (Hochberger et al., 2018; Javitt, 2000; Javitt et al., 2018; Kaser et al., 2013; Lee et al., 2017; Xiong et al., 2019) (Fig. 2E), which strongly implicates the function of circuits involving somatostatin-type interneurons (Hamm and Yuste, 2016; Javitt et al., 2020; Womelsdorf et al., 2014). In contrast, responses to rapidly presented standard stimuli map primarily to the alpha frequency range. Similarly, an early alpha response can also be resolved prior to MMN in the difference waveforms (Fig. 2E). As with the subsequent theta-frequency responses, alpha frequency responses are reduced to both standard and deviant stimuli in schizophrenia (Lee et al., 2018b; Sehatpour et al., 2020), suggesting that inputs into auditory cortex may be impaired as well as local cortical processing. TF analyses are relatively preserved across species, and thus enable parallel preclinical studies to evaluate the cellular and molecular level changes that may underlie auditory processing impairments in schizophrenia (see Section 4).
3.5. Auditory plasticity
The auditory cortex was traditionally thought to be functionally and structurally “fixed” after critical periods early in life (Hensch, 2005). However, along with other cortical regions, the auditory cortex remains highly plastic into adulthood, leading to the ability to refine discrimination ability with repeated exposure (rev. in Froemke et al., 2013; Irvine, 2018). Auditory plasticity is commonly assessed using an auditory remediation (AudRem) paradigm originally developed for dyslexia (Ahissar et al., 2006). In the AudRem approach, participants are presented with paired tones [e.g., S1 (“reference”) and S2 (“test”) separated by 1800 ms and indicate which tone is higher in pitch. In the first pair the ratio between tones (Δf) is 50% (e.g., 1000 vs. 500 Hz). Feedback is then given after each trial, and %Δf values are modulated using a staircase procedure to maintain a constant level of accuracy. Two conditions are used. In the random condition (Fig. 3A), the pitch of the S1 varies randomly across trials, preventing learning of specific pitch differences. Conversely, in the fixed condition, the pitch of the S1 tone remains constant, leading to improved performance (i.e., auditory learning) over repeated trials (Fig. 3B).
Figure 3. Behavioural assessment of auditory plasticity.

A,B. Line graph of plasticity for schizophrenia and controls for the random (A) and fixed (B) conditions. C. Bar graph for final JND threshold (%f) from trials 70–80. D. Bar graph for plasticity (%f, mean trial 20–30 to 70–80). E-G. Scatter plot for just-noticeable difference (JND) thresholds versus working memory (E), auditory emotion recognition (F) or C-TOPP composite (reading) (G). Text in scatter plot shows correlations after control for group (partial r). Error bars represent standard error of the mean. ***P<0.001. {Adapted from \Kantrowitz, 2016 #97).
In dyslexia, individuals show intact performance in the random condition, but impaired performance in the fixed condition, which has been associated with a faster decay rate of the implicit memory trace in primary and secondary auditory regions (Ahissar et al., 2006; Jaffe-Dax et al., 2018). In contrast, schizophrenia is associated with impaired performance in both the random condition, reflecting ongoing EAP disturbance, and the fixed condition, representing additional deficits in auditory plasticity (Fig. 3A–D). Deficits, as in dyslexia, correlate with impairments in working memory, auditory emotion recognition and reading ability (Fig. 3E–G) (Kantrowitz et al., 2016). In concurrent ERP recordings, presentation of both the S1 and S2 potentials were associated with sensory responses that mapped primarily into the theta-frequency range and were significantly reduced in schizophrenia. In single-trial analyses, the impairments were associated with reductions in ITC and single-trial power across both alpha and theta frequency ranges, supporting the concept that the behavioral deficits in schizophrenia are associated with impaired processing of the sensory stimuli (Kantrowitz et al., 2016). The ERP version of this paradigm thus provides additional neurophysiological targets for treatment development.
3.6. Additional auditory neurophysiological paradigms
Additional auditory neurophysiological paradigms in schizophrenia include the auditory P300, P1/N1 and ASSR responses, which provide complementary information with regard to task-dependent versus passive auditory processing.
3.6.1. Auditory P300
The auditory P300 (P3) potential is elicited in an “active” oddball paradigm, in which participants are asked to attend and respond (e.g., by button press) to the deviant stimulus. The P3 response is conventionally divided into a P3a component that has primarily a frontal distribution and relates primarily to novelty processing, and a P3b component that shows a distributed frontal, temporal and parietal distribution and may reflect “context updating” following correct detection of the deviant stimulus (Polich, 2007).
Auditory P3 deficits in schizophrenia were first demonstrated the early 1970’s (Roth and Cannon, 1972) and have been replicated repeatedly since (e.g. (Jeon and Polich, 2003; Wang et al., 2022)). Unlike MMN, however, P3 reductions may not be associated with functional disability (Hamilton et al., 2018b). In CHR individuals, P3, like MMN, may reflect resilience as reflected in greater likelihood to remit and reduced likelihood to convert (Hamilton et al., 2019; Hamilton et al., 2021; Lepock et al., 2018). In biotyping studies, reduced P3 amplitude is a hallmark of biotype I psychosis, which overwhelmingly includes individuals with schizophrenia and schizoaffective disorder (Clementz et al., 2022). Reduced P3 is observed as well in unaffected relatives of people with schizophrenia, suggesting that it may serve as a useful endophenotype (Earls et al., 2016).
More recently, P3 deficits in schizophrenia have been shown to correlate with impaired delta frequency entrainment to the stimulus sequence, suggesting potential low-level mechanisms underlying the widespread cortical activation impairment (Lakatos et al., 2013). As compared to MMN, which is sensitive primarily to effects of NMDAR antagonists (Heekeren et al., 2008; Umbricht et al., 2000; Umbricht et al., 2003). P3 amplitude is sensitive as well to effects of 5-HT2A antagonists such as psilocybin (Bravermanova et al., 2018), or LSD (Murray et al., 2021), suggesting that key circuitry is under both glutamatergic and serotonergic control.
3.6.2. Auditory P1/P50 and N1
Both the P1 and N1 (N100) potentials index obligatory brain responses to isolated auditory stimuli. Auditory P1 (also termed P50) shows refractoriness intervals of ~1 sec, and thus is frequently studied using sensory gating approaches in which pairs of tones are presented following brief intervening delay. P50 gating is best defined as the amplitude of the response to the first stimulus (S1) covaried for the response to the second stimulus (S2). Deficits in P50 gating are well established in schizophrenia and have been studied most extensively in relationship to nicotinic cholinergic-mediated inhibitory dysfunction (Martin and Freedman, 2007) and hippocampal theories (Freedman et al., 2020; Javitt and Freedman, 2015) of schizophrenia, but may be sensitive as well to NMDAR antagonists (de la Salle et al., 2022).
Auditory N1 shows a characteristic refractoriness function such that N1 is reduced exponentially at interstimulus interval (ISIs) of <6 sec. Reductions in the auditory N1 are well established in schizophrenia, particularly at long ISI (Javitt, 2015; Javitt et al., 2000a; Salisbury et al., 2010), and correlate with behavioral impairments such as impaired phonetic processing (Senkowski and Moran, 2022). In healthy volunteers, N1 amplitude is reduced during vocalization or self-triggered tone delivery. These effects are both attenuated in schizophrenia (Ford et al., 2013a; Ford et al., 2013b). As with P3, reduced N1 amplitudes are selectively associated with biotype 1 psychosis (i.e. schizophrenia and schizoaffective disorder), representing a low neural response phenotype (Clementz et al., 2022). As opposed to generators for MMN, primary generators for the auditory N1 are located primarily within infragranular layers, supporting the concept that differential neuronal populations underlie auditory N1 refractoriness and MMN generation (Javitt, 2015; Javitt et al., 1996; Lakatos et al., 2020).
3.6.3. Auditory steady-state response (ASSR)
The ASSR is elicited by stimulation within the gamma frequency band, and optimally at 40 Hz. Since the original report over 20 years ago (Kwon et al., 1999), deficits in ASSR have been replicated repeatedly (rev. in Koshiyama et al., 2021a; Onitsuka et al., 2022; Parciauskaite et al., 2021; Tada et al., 2021; Thune et al., 2016) and tied to auditory cortical dysfunction (e.g. Hirano et al., 2020). As with other auditory measures, ASSR is reported to correlate with cognitive impairment and poor outcome in schizophrenia (Koshiyama et al., 2021a) and to predict conversion in CHR individuals (Grent-ť-Jong et al., 2021). ASSR abnormalities have also been correlated with the severity of persistent auditory hallucinations in schizophrenia (Coffman et al., 2022; Mulert et al., 2011; Spencer et al., 2009), suggesting that it may be informative regarding underlying pathology.
4. NEURAL MECHANISMS OF EARLY AUDITORY PROCESSING DEFICITS
The localization of MMN generators to A1, coupled with the fact that A1 structure and function has been extensively characterized across humans and animals and evaluated in post-mortem histological studies of schizophrenia (Fig. 4A), permits the development of strong hypotheses regarding potential underlying mechanisms.
Figure 4 :

Neural mechanisms of early auditory processing deficits. A. Cytoarchitecture of feedforward and feedback auditory circuits. Thalamic projections from the medial geniculate nucleus (MGN) synapse onto pyramidal cells (PCs, dark blue) and interneurons (5HT3-a-R+ including Neuron-derived neurotrophic factor NDNF+ and Vasointestinal peptide VIP+, light blue; Somatostatin SST+, green; Parvalbumin PV+, red). Layer III PCs in primary auditory cortex (A1) send local intralaminar projections to other layer III PCs in this region and longer-range feedforward projections to PCs in layer III of auditory association cortex (A2). Layer V PCs in A2 in turn send excitatory feedback projections to neurons in layer I in A1. Structural alterations are indicated. {Adapted from \Parker, 2017 #123}. B. A schematic illustration of a proposed model of mismatch negativity (MMN) process related to N-methyl-D-aspartate receptor (NMDAR) function. Surface-recorded MMN activity reflects current flow through open, unblocked NMDA receptors on pyramidal neurons in the auditory cortex. In panel 1, NMDAR are normally blocked by Mg2+ at resting membrane potential. In panel 2, when a deviant stimulus is presented without prior standard stimuli, even though the channels open, the blockade prevents current flow. Brain responses therefore occur only through non-NMDAR glutamate receptors. In panel 3, when repetitive standard stimuli are presented, they lead to subthreshold membrane depolarization by inhibition of inhibition , leading to unblocking of the channel. In panel 4, once channels are unblocked, presentation of the deviant stimulus leads to NMDAR-mediated current flow (adapted from Javitt and Sweet, 2015). B. A schematic illustration of the MMN process in the NMDA receptor. Surface-recorded MMN activity reflects current flow through open, unblocked NMDA receptors on pyramidal neurons in the auditory cortex. 1. NMDA receptors are normally blocked by Mg2+ at resting membrane potential. 2. When a deviant stimulus is presented without prior standard stimuli, even though the channels open, the blockade prevents current flow. Brain responses therefore occur only through non-NMDA-type glutamate receptors. 3. When repetitive standard stimuli are presented, they depolarize the membrane, leading to unblocking of the channel. 4. Once channels are unblocked, presentation of the deviant stimulus leads to NMDA-receptor-mediated current flow (rev. in Javitt and Freedman, 2015). C. Auditory ERP curves from human studies (top) showing responses to standard and deviants and difference (MMN) curves from healthy control (HC) and schizophrenia (Sz) groups and from supragranular layer of auditory cortex (adapted from Sehatpour et al., 2020) in non-human primate (NHP) intracranial recording (bottom) showing responses pre and post ketamine administration (adapted from Lakatos et al., 2020). D. Time-frequency evoked power analyses of rodent MMN responses prior to and during treatment with vehicle alone (Control), phencyclidine (PCP, 15 mg/kg/d by minipump) or combined PCP and glycine (by diet) treatment. Open arrows show changes in α-band response showing a reduction during PCP treatment and prevention of this reduction by concurrent glycine treatment (Adapted from Lee et al., 2018a).
4.1. Thalamo-cortical contributions
Human thalamic MGN is divided into a matrix subregion that projects widely across auditory and motor regions and targets supragranular layers of auditory cortex and a core subregion that projects more specifically to the granular layers of auditory cortex. A1 afferents deriving from the MGN matrix are more sensitive to rhythm and intensity (Jones, 1998, 2002, 2009), while inputs deriving from the MGN core have a stronger tonotopic organization (Griffiths, 2003; Viaene et al., 2011). The EA cortex has a tonotopic organization, in which neurophysiological responses to simple auditory stimuli are elicited in a lateral to medial gradient with increasing frequency (Ehret and Romand, 1997; Poeppel et al., 2012). Thalamic inputs into auditory cortex derive from both the core (lemniscal) and matrix (non-lemniscal) pathways. In schizophrenia, spine density is reduced in thalamocortical recipient layers of auditory cortex (Sweet et al., 2009). The distribution of synaptic sizes, moreover, suggests that reductions may be due to long-term depression processes rather than pruning (McKinney et al., 2019), and thus could potentially be rescued during early phases of the illness by appropriate behavioral or pharmacological intervention.
The pattern of neurophysiological dysfunction observed in schizophrenia may provide additional information regarding potential contributions of thalamo-cortical dysfunction. For example, a recent meta-analysis of MMN studies in schizophrenia observed that all simple deviant tones (frequency, duration, intensity) demonstrate deficit with moderate to large effect sizes ranking in the following order: duration, pitch, intensity (Avissar et al., 2018), suggesting differential neural encoding deficits across features in echoic memory. A potential mechanism underlying this pattern in human is that “non-lemniscal” auditory inputs to the auditory cortex deriving from the thalamic matrix may be preferentially involved in MMN duration deficits, while “lemniscal” inputs deriving from the thalamic core may be additionally involved in MMN pitch impairments (Lee et al., 2018b).
This can potentially be explained by the stronger sensitivity to rhythm shown by non-lemniscal-, in comparison to lemniscal-, inputs (Jones, 1998, 2002, 2009), and the stronger tonotopic organization of the lemniscal vs. non-lemniscal pathway (Hu, 2003; Viaene et al., 2011). Therefore, in schizophrenia, it is possible that the frequency impairment relates to more advanced stages of thalamic alteration. Accordingly, this impairment may add to the duration deficit in only a subset of individuals, thereby explaining lower pitch vs. duration mean deficits (Lee et al., 2018b). Alternatively, it has been suggested that duration MMN is more affected by illness duration than frequency MMN, so that differential impairments may be most evidence in more chronic individuals (Umbricht and Krljes, 2005). Regarding intensity, it has been shown that a clear reduction in the event-related potential MMN to intensity deviants is observed early in the illness but not in later stages of evolution (Todd et al., 2008).
Reduced MGN volume has been reported in schizophrenia, especially in individuals with persistent auditory hallucinations (Perez-Rando et al., 2022), but no information is available regarding differential MGN projection systems. Intact thalamocortical boutons are observed in post-mortem auditory cortex in schizophrenia. Neveretheless, a consistent reduction in dendritic spine density is observed in lower layer 3, which mediates feedforward processing (McKinney et al., 2019) (Fig. 4A). In addition, reduced mitochondrial levels are observed, suggestive of reduced pre-synaptic activity (MacDonald et al., 2020).
These findings are consistent with several studies involving different clinical pictures across the psychotic continuum that show global grey matter loss and abnormal functional connectivity encompassing all areas of the auditory cortices in at-risk individuals (Bhojraj et al., 2011; McKechanie et al., 2016), first-episode (Kubicki et al., 2002; McCarley et al., 2002) and established schizophrenia participants (Fusar-Poli et al., 2011). In addition, these findings are thus consistent with impaired inputs into auditory cortex in schizophrenia.
4.2. NMDAR dysfunction
One of the best-replicated features of MMN is its dependence on NMDAR-mediated processes. The ability of NMDAR antagonists to inhibit MMN generation was first demonstrated in monkeys in 1996 (Javitt et al., 1996) and subsequently confirmed in humans (Hamilton et al., 2018a; Rosburg and Kreitschmann-Andermahr, 2016; Umbricht et al., 2000), additional monkey (Gil-da-Costa et al., 2013; Lakatos et al., 2020) and rodents studies (Ehrlichman et al., 2008; Featherstone et al., 2018; Featherstone et al., 2014; Harms, 2016; Lee et al., 2018a; Ross and Hamm, 2020; Schuelert et al., 2018). NMDAR dysfunction has also been shown to be consistent with changes observed in predictive coding models of MMN dysfunction (Schmidt et al., 2013; Wacongne, 2016). Conversely, MMN deficits in schizophrenia and animal models may be ameliorated by NMDAR-targeted treatments including glycine (Greenwood et al., 2018; Lee et al., 2018a) , D-serine (Kantrowitz et al., 2016; Kantrowitz et al., 2018), Luvadaxistat (O'Donnell et al., 2021), and N-acetylcysteine (Lavoie et al., 2008).
The involvement of NMDAR in MMN generation converges with conceptualizations of MMN as a “Hebbian” process, in which information must be integrated from two distinct information streams: one that integrates the recent auditory history and general state variables, and one that represents the most recent auditory stimulus (Javitt, 2000; Javitt and Sweet, 2015). NMDAR are well suited to perform Hebbian integration due to their dual voltage- and ligand-dependence, such that current flow through NMDAR occurs only if local subthreshold depolarization coincides with presynaptic glutamate release (Cotman and Monaghan, 1988; Matsuzaki et al., 2004).
In the case of MMN, a parsimonious model is that the recent auditory history is maintained as a pattern of distributed subthreshold neuronal depolarizations, and that MMN is then triggered when input to the neuron coincides with the ongoing depolarization (Fig. 4B). In addition, current flow through open, unblocked NMDAR triggers plasticity processes that may either decrease (long-term depression) and increase (long-term potentiation) of local synaptic strength (Park et al., 2022). Thus, NMDAR activation may underlie both the MMN generation (Javitt and Freedman, 2015; Javitt et al., 1996; Javitt and Sweet, 2015) and the plasticity events (Schmidt et al., 2013; Wacongne, 2016) that are thought to contribute to MMN dysfunction in schizophrenia.
In monkeys, MMN has been localized to supragranular layers of auditory cortex (Javitt et al., 1992; Javitt et al., 1996; Lakatos et al., 2020), which are known to be under tonic inhibitory control by several classes of spontaneously active inhibitory interneurons. Moreover, monkey MMN may be inhibited by ketamine administration, providing a pattern of deficit similar to that observed in schizophrenia (Fig. 4C). Moreover, in both monkeys and rodents, MMN is associated with increased theta frequency activity to deviant vs. standard stimuli that is inhibited by NMDAR antagonist administration (Fig. 4D). This inhibition is prevented by simultaneous administration of the NMDAR modulator glycine (Lee et al., 2018a).
NMDAR dysfunction may also contribute to disturbances in auditory N1, ASSR and P3 deficits in schizophrenia (Balla et al., 2020; de la Salle et al., 2021; Schwertner et al., 2018). As with MMN, auditory N1 can be modelled in rodents (Lee et al., 2018a; Schuelert et al., 2018) and primates (Holliday et al., 2018; Javitt and Sweet, 2015; Teichert, 2017) and is sensitive to the effects of both NMDAR antagonists such as ketamine, PCP or MK-801(Lee et al., 2018a; Schuelert et al., 2018; Teichert, 2017) and agonists such as glycine (Lee et al., 2018a) or iclepertin (BI425809) (Rosenbrock et al., 2022). Administration of ketamine to healthy volunteers also reproduces the pattern of corollary discharge deficits observed on N1 generation in schizophrenia (Kort et al., 2017), further supporting the relevance of NMDAR to predictive coding deficits in schizophrenia.
4.3. Excitatory/inhibitory balance
Scalp-recorded ERP such as MMN or N1 reflect current flow primarily within the dendritic trees of cortical pyramidal neurons. Nevertheless, activity is regulated by multiple populations of inhibitory interneurons that exert both local inhibition and local disinhibition.
The best studied interneuron types are differentiated based on expression of parvalbumin (PV) or somatostatin (SOM, SST). These neurons show differential circuit motifs, such that PV interneurons are preferentially involved in generation of high-frequency (beta/gamma) activity, whereas SOM interneurons are preferentially involved in imposing lower-frequency firing patterns on local pyramidal neurons (Javitt et al., 2020; Womelsdorf et al., 2014). Thus, PV interneurons may be differentially important in mediating ASSR deficits in schizophrenia (e.g. Gonzalez-Burgos et al., 2010; Hashimoto et al., 2003; Metzner et al., 2019), whereas SOM interneurons may contribute preferentially to deficits in the generation of lower-frequency components such as MMN (Javitt et al., 2018; Van Derveer et al., 2021). In schizophrenia, reductions in expression of glutamate decarboxylase (GAD67) levels within PV interneurons are observed throughout cortex (Gonzalez-Burgos et al., 2010; Hashimoto et al., 2003), as are reductions in SOM interneurons and SOM mRNA expression (rev.in Zhang et al., 2021). Reductions in PV interneurons are observed as well as in subcortical regions such as inferior colliculus (Kilonzo et al., 2020). Consistent with this, reductions in auditory cortex GABA levels have been reported based upon MR spectroscopy (Atagun et al., 2017).
Both PV and SOM interneurons may be divided into functional distinct subcategories. PV interneurons may be divided into basket cells, which target the cell body and dendrites of excitatory interneurons, and chandelier cells, which target the axon initial segment (Studer and Barkat, 2021). Both populations have been implicated in cortical dysfunction in schizophrenia, along with surrounding perineuronal nets (Glausier and Lewis, 2018; Metzner et al., 2019). SOM interneurons may be divided into non-Martinotti and Martinotti subtypes. Non-Martinotti cells are localized primarily with granular and low supragranular cortical layers and mediate inhibition primarily within a cortical column (Riedemann, 2019).
Conversely, Martinotti cells send ascending axons that ramify in layer 1 and project widely across cortical columns, and may additionally express neuronal nitric oxide synthase (nNOS) (Riedemann, 2019; Smiley et al., 2000). Both PV and SOM interneurons target primarily cortical pyramidal neurons. Layer 1 of auditory cortex is also a zone of convergence for many projection systems and interneuron types that may be of relevance to MMN. These include long-range GABAergic projects from Martinotti cells that uniquely cross isofrequency bands in auditory cortex and thus permit synthesis of cross-frequency information, and multiple local interneuron types (rev. in Javitt, 2022; Studer and Barkat, 2021).
A third class of interneurons is characterized by expression of 5-HT3 receptors (5-HT3R), and includes subtypes that can be characterized by expression of vasoactive intestinal peptide (VIP) or Neuron-Derived Neurotrophic Factor (NDNF) (Studer and Barkat, 2021). VIP interneurons primarily receive input from distant brain regions, project widely across cortical layers and preferentially disinhibits local inhibitory neurons. These neurons may thus play a critical role in top-down regulation of auditory cortex (Kullander and Topolnik, 2021) and thus may participate in microcircuits related to auditory impairments in schizophrenia (Ross and Hamm, 2020).
NDNF interneurons are highly localized within layer 1 and mediate prolonged inhibition of cortical pyramidal interneurons. NDNF interneurons in auditory cortex receive primary input from auditory, somatosensory and surrounding temporal lobe regions, and are inhibited by local Martinotti cells, providing a potential local disinhibitory mechanism within supragranular auditory cortex (Abs et al., 2018) The potential relevance of 5-HT3R-expressing interneurons is supported by the recent demonstration that the 5-HT3R antagonist CVN058 significant enhances MMN generation in schizophrenia (Sehatpour et al., 2022).
Nevertheless, characterization of E/I processes in schizophrenia remains challenging because of the significant heterogeneity of function even within the broader cell-type divisions (Field et al., 2021), and the potential differential properties of GABA interneurons in humans vs. rodents (Hodge et al., 2019). The role of NMDAR in modulation of different GABA interneuron types is also incompletely understood. Nevertheless, large NMDAR-mediated responses have been documented in multiple interneuron classes including VIP- and neurogliaform cells as well as the more widely studied PV and SOM interactions (Booker and Wyllie, 2021), suggesting that NMDAR dysfunction may lead to complex changes in E/I balance.
4.4. Nicotinic receptors
Acetylcholine (ACh) has been implicated in the pathophysiology of schizophrenia based on both genetics and the high-rate of smoking observed in schizophrenia (rev. in Donde et al., 2020b; Featherstone and Siegel, 2015; Freedman, 2014; Tregellas and Wylie, 2019). Furthermore, nicotine has been shown to enhance MMN in healthy individuals (e.g. Baldeweg et al., 2006; Martin et al., 2009), although results have been conflicting across studies (e.g. Donde et al., 2020b; Inami et al., 2007; Mathalon et al., 2014; Smith et al., 2015). In one study that evaluated the interactive effects of ketamine and nicotine in healthy volunteers, ketamine reduced and nicotine enhanced MMN when given separately, but nicotine did not reverse the effects of ketamine, arguing against a potential therapeutic effect in schizophrenia (Hamilton et al., 2018a).
In schizophrenia, the effects of nicotine have also been mixed (rev. in Donde et al., 2020b). In one study, no significant effects of nicotine were observed on MMN latency in a group of 10 non-smoking individuals with schizophrenia (Inami et al., 2007), although a subsequent study showed nicotine-induced enhancement of duration but not frequency MMN in a group of 12 smokers with schizophrenia (Dulude et al., 2010). In a study of 12 schizophrenia outpatients with persistent auditory hallucinations, nicotine did not significantly affect MMN amplitude to frequency, duration or intensity deviants, although nicotine-related changes in MMN correlated with nicotine-induced changes in hallucination severity (Fisher et al., 2012).
Mixed results have also been reported with high-affinity α7 nicotinic ligands. Several studies have reported improvement in P50 gating with nicotinic compounds. In addition one study found a significant dose-dependent improvement in MMN and other auditory ERP measures following 21-day treatment with the EVP-6124 (Encenicline),an α7 positive allosteric modulator (Preskorn et al., 2014), A phase II study with the compound showed significant beneficial effects against persistent cognitive and negative symptoms in schizophrenia (Keefe et al., 2015), although subsequent phase III studies showed no significant benefits (Brannan, 2019). Most recently, no significant change in MMN, symptoms or cognition was observed in a study of AVL-3288, an α7 nicotinic positive allosteric modulator, in 24 non-smoking schizophrenia individuals (Kantrowitz et al., 2020).
In general, studies with nicotine in both schizophrenia and control individuals have been small, limiting statistical power. Furthermore, desensitization effects may limit efficacy of nicotinic compounds during chronic administration (rev. in Donde et al., 2020b). Although the present literature does not support the use of α7 agonists in schizophrenia overall, it remains an open question as to whether individuals with EAP deficits (e.g. impaired MMN) may show selective benefit.
5. IMPLICATIONS FOR PATHOPHYSIOLOGY AND TREATMENT
In addition to serving as translational biomarkers, measures of EAP dysfunction may also help refine overall theories of cognitive dysfunction in schizophrenia and may serve as both stratification biomarkers to assist in defining etiologically distinct subpopulations, and as target-engagement measures to assist in identification of potential plasticity-enhancing agents in schizophrenia.
5.1. Distributed hierarchical dysfunction
Although the overall architecture of cognitive dysfunction in schizophrenia is still under investigation, the field is increasingly moving from regional to distributed models of dysfunction. In the distributed hierarchical concept, the basic molecular and cellular substrates of cognitive dysfunction such as NMDAR dysfunction are considered to be distributed throughout cortex, leading to disturbances in specific types of processing (e.g., long-term potentiation, non-linear gain) that are regionally diffuse but process-specific (Fig 5A). Such models are supported both by genetic studies, in which risk genes for schizophrenia (e.g., GluN2a, setd1a) are widely distributed across cortical and subcortical regions, and postmortem studies showing similar histological abnormalities in sensory and cognitive brain regions. In distributed hierarchical models, disturbances in higher order processing are considered to reflect both impaired local dysfunction within specific higher order brain regions and impaired bottom-up input to those regions.
Figure 5: Implications for pathophysiology and treatment.

A. Proposed model for the contribution of auditory cortex related dysfunction in the pathophysiology of schizophrenia. B. Relationships (Pearson’s r) between tone-matching, reading and functional outcome in SZ (top) (Adapted from Donde et al., 2019c). Relationships (Pearson’s r) between tone-matching, auditory emotion recognition and functional outcome in SZ (bottom). (Adapted from Javitt, 2009). C. Change in early auditory processing (tone-matching task performance) and change in cognition (MCCB composite change score) induced by cognitive remediation in schizophrenia from baseline to follow-up. MCCB: Matrics Consensus Cognitive Battery (Adapted from Medalia et al., 2019). D. Framework for sensory-targeted cognitive training impact in schizophrenia. Sensory training images are extracted from the posit science corporation programs (auditory frequency and visual gratings discrimination exercises). BDNF picture is from ‘Brain-derived neurotrophic factor’ by Microswitch and licensed under CC BY-SA 3.0 (Adapted from Donde et al., 2019d).
Studies of auditory function strongly support such models both within auditory regions and across cortical areas. For instance, within the auditory system pitch discrimination impairments as indexed by TMT were found to significantly predict impairment on a more complex serial pitch-pattern detection task, which, in turn, predicted impaired AER, with increasing effect size across processes. Moreover, the effects of tone matching on AER were fully mediated by the pitch pattern discrimination task (Donde et al., 2019f). Similarly, deficits in simple tone matching may contribute to incorrect sourcing of perceptual material or source-monitoring, which in turn may contribute to the pathogenesis of auditory hallucinations (Donde et al., 2019e). MMN impairments are reported as well in children with specific language impairment (Kujala and Leminen, 2017), suggesting that impairments in these circuits may contribute as well to NMDAR-related language disturbances in schizophrenia (Adler et al., 1999; Corcoran et al., 2018a) as well.
Specific processes that may mediate the interrelationships between impairments in early auditory processing and functional outcome include detection of either emotional (Donde et al., 2019b; Donde et al., 2019f; Gold et al., 2012; Kantrowitz et al., 2015; Kantrowitz et al., 2014a; Leitman et al., 2005; Leitman et al., 2010a; Leitman et al., 2011; Leitman et al., 2006) or attitudinal (Kantrowitz et al., 2014a; Leitman et al., 2006) prosody (“tone of voice”), which will affect social cognition; or impairment in phonological language processing, which affects educational and occupational function (Carrion et al., 2015; Donde et al., 2019b; Friedman et al., 2012; Revheim et al., 2006; Revheim et al., 2014) (Fig. 5A,B). In tonal languages, such as Mandarin, deficits in tonal processing may lead to impaired word identification, which, in turn, undermines occupational outcome in patients (Yang et al., 2012). At the neurophysiological level, deficits in MMN generation account for ~50% of the variance in generation of the attention-dependent auditory P300 potential (Leitman et al., 2010b), with the remaining variance potentially mediated by impairments in other components of auditory processing such as active auditory entrainment (Leitman et al., 2010b).
Similar hierarchical disturbances are also observed in larger scale studies that incorporate a larger range of measures. For example, in one study involving 1415 individuals with schizophrenia, deficits in EAP, as reflected primarily by impaired MMN generation, had a direct effect on overall cognition, which in turn contributed significantly to negative symptoms. Both cognition and experiential negative symptoms, in turn, predicted functional outcome, as reflected in social and occupational function (Thomas et al., 2017). These findings were replicated in a more recent study of 695 schizophrenia individuals and 503 healthy comparators in which sensory discrimination, as operationalized by MMN and P3a, and gamma-band dysfunction, as operationalized using ASSR, contributed in parallel to cognition, which along with sensory discrimination, contributed significantly to negative symptoms. In this latter study, no direct link between cognition and functional outcome was observed (Koshiyama et al., 2021b).
Finally, although the large majority of auditory studies in schizophrenia focus only on the cortical level, those studies that incorporate indices of subcortical function find similar hierarchical relationships. For example, in invasive electrophysiological (Lakatos et al., 2020) and fMRI studies of MMN, deviance-related activity is observed in subcortical structures including inferior colliculus and thalamic MGN (Cacciaglia et al., 2015) and is impaired in schizophrenia (Gaebler et al., 2020). To the extent that location deficits are larger for inter-aural time delay vs. inter-aural intensity cues (Matthews et al., 2013), brainstem processes related to delay detection may also be impaired.
5.2. EAP-targeted therapy
Given the relationship between EAP deficits and functional outcome, there has been increasing interest in EAP-targeted interventions (e.g. Biagianti et al., 2020; Medalia et al., 2019; Vinogradov et al., 2012). For example, based upon hierarchical models, it has been estimated that a 1 sd improvement in EAP measures (~ 1 μV) would produce an improvement of .78 sd for cognition and .28 sd for psychosocial function. At present, the main approaches for intervention include cognitive remediation either alone or combined with pharmacological manipulation. For example, a recent meta-analysis of EAP-targeted remediation studies performed either with the Posit Science Brain Fitness Program or the Phoenix Software Audiolog system calculated mean effect sizes in the range of .5-.9 sd for improvement of auditory function, as well as significant improvements in higher-order cognitive function, although concerns were raised about the durability of the findings (Donde et al., 2019d) (Fig. 5D).
Studies since then (e.g. (Biagianti et al., 2020; Dale et al., 2020; Scoriels et al., 2020) further support these conclusions (see also Kantrowitz, 2019; Mondino et al., 2021). In one informative study, participants were first separated into EAP- and EAP+ groups based on tone-matching ability, and then assigned to cognitive remediation that either did (Brain Basics) or did not (Brain Training) include an auditory component. Only individuals with baseline auditory deficits benefited from auditory-training (Fig. 5C), reinforcing the need to personalize remediation for specific individuals (Medalia et al., 2019).
5.3. Combined treatment approaches
Given the limited plasticity of auditory cortical function in schizophrenia (Fig. 3), several studies are also underway to evaluate the ability of combined treatments to facilitate EAP-targeted interventions. Current studies are being conducted with the tool compounds D-serine and memantine. In addition, non-invasive brain stimulation (NIBS) approaches such as transcranial direct current stimulation (tDCS) may increase plasticity and improve benefit.
5.3.1. D-serine
D-serine is an allosteric modulator of NMDAR that acts through the glycine/D-serine recognition site. In an initial study, sequential administration of acute D-serine led to significant reduction in tone-discrimination thresholds to near-control levels for the trained tones. The improvement was associated as well with an increase in theta-band response (theta-ITC) to the S2 stimulus in the tone pair that correlated with clinical improvement. A significant increase was also observed in MMN response to the trained, but not untrained, tone frequency that also correlated with the behavioral improvement (Kantrowitz et al., 2016). A more recent study also observed significant dose-dependent D-serine effects on both tone-matching and MMN following acute administration (de la Garrigue et al., 2020; Sehatpour et al.). Studies assessing the effects of repeated D-serine administration combined with auditory remediation are presently underway (NCT05046353).
5.3.2. Memantine
Memantine is an NMDAR antagonist that acts preferentially at extrasynaptic NMDAR, which in general work in opposition to intrasynaptic receptors (Javitt, 2022). Thus, as opposed to other NMDAR antagonists (e.g. ketamine), memantine is neither psychotomimetic nor anti-depressant and functions to improve, rather than reduce, plasticity (rev. in Javitt, 2022). Although memantine is used most commonly in Alzheimer disease, it is also reported to have pro-cognitive effects in schizophrenia and to improve MMN generation (Swerdlow et al., 2016), oscillatory dynamics (Light et al., 2017) and E/I balance (Molina et al., 2020). Most recently, memantine has been reported to enhance effects of auditory training (Swerdlow et al., 2020). As with D-serine, effects of memantine as an adjunct to repeated auditory training remain ongoing (NCT04857983)
5.3.3. Non-invasive brain stimulation
Augmentation using transcranial electrical stimulation (tES) or other non-invasive brain stimulation (NIBS)-based approaches may also be possible (e.g. (Dunn et al., 2017)). For example, cathodal tDCS applied to auditory cortex has been observed to reduce auditory hallucinations (Brunelin et al., 2012; Kantrowitz et al., 2019; Kim et al., 2019; Mondino et al., 2021) and improve insight (Adam et al., 2022), supporting concepts that auditory cortical function may be amenable to intervention using locally targeted approaches.
Nevertheless, to date the effects of tDCS on EAP has been investigated to a limited degree. In an initial study of 12 individuals, increases in MMN were observed following anodal stimulation in individuals with reduced pre-stimulation MMN amplitudes (Impey and Knott, 2015). Subsequent studies showed differential effects of anodal and cathodal stimulation (Impey et al., 2016) and interaction with NMDAR antagonists (Impey et al., 2017b), auditory verbal hallucinations and working memory (Impey et al., 2017a). Recent MR-guided high-definition tDCS approaches have proven superior to more traditional low-density stimulation (Bikson et al., 2018; Brunoni et al., 2014; Preskorn et al., 2014; Sehatpour et al., 2021). Future studies using more personalized and focal approaches might therefore demonstrate even further benefit.
6. CONCLUSION
EAP deficits are a core feature of schizophrenia and have been shown to contribute to deficits in both social- and neuro-cognition (Fig. 5D). Nevertheless, assessment of these processes has not yet been incorporated into available neuropsychological batteries such as the MCCB. The strong correlation between EAP deficits and outcome argues for the need of a MATRICS-like process to develop a validated, normed EAP assessment battery that could be used along batteries such as the MCCB to evaluate EAP processing, as supported by recent studies using tone matching to personalize cognitive remediation in both clinical and research settings (Medalia et al., 2019; Smith et al., 2021). The degree to which EAP deficits are confined to schizophrenia also requires further investigation.
At the neurophysiological level, recent findings of impairments in the initial , alpha-frequency response to auditory stimulation in cortex converge with recent findings of reduced dendritic size within post-mortem tissue in schizophrenia. It has further been argued that the pattern of dendritic change is more consistent with LTD-like processes than pruning. To the extent that this is correct, both behavioral and pharmacological approaches to enhance LTP over LTD may provide improved therapeutic outcomes.
The ontogeny of EAP deficits in schizophrenia also remains controversial. For example, although one set of studies has documented MMN deficits even prior to illness onset, another set documents normal MMN amplitudes in first-episode patients, with decline over the initial years of illness. These findings raise the possibility that there may be several paths to development of schizophrenia that are captured by MMN and potentially other EAP measures. Nevertheless, further research is needed to reconcile the two sets of findings. To the extent that EAP deficits emerge in the years prior to illness onset in a specific subgroup, early screening, intervention and sensory training remediation strategies are warranted.
At the local circuit level, a strength of auditory ERP measures is their translational utility across rodents, monkeys, and humans, which is aided through the use of TF analysis approaches. Neurophysiological deficits in schizophrenia, in general, are considered to arise from E/I imbalance, but the degree to which this involves dysfunction of excitatory vs. inhibitory mechanisms in any individual remains unknown. The clinical interest in auditory ERP measures converges, at present, with advanced conceptualizations of how different GABA-interneuron subtypes contribute to neuro-oscillatory function.
Nevertheless, as recently reviewed (Booker and Wyllie, 2021; Studer and Barkat, 2021), there remain multiple unanswered questions regarding how GABA interneurons in auditory cortex are best categorized, how they interact to permit complex stimulus processing, and how they are modulated by different glutamatergic processes. For example, recent findings with the 5-HT3R antagonist CVN-058(Sehatpour et al., 2022) argue for greater research into the role of 5-HT3R expressing interneurons as a therapeutic target. At present, there are few therapeutic strategies in schizophrenia targeting specific interneuron populations. An open question is whether EAP measures can be used to guide future research.
Finally, there is a desperate need for biomarkers in psychiatry that can be used to support new treatment development. The ability of NMDAR antagonists and agonists to bidirectionality modulate MMN argues for its use as a pharmacodynamic/target engagement in translational drug development programs. Whether or not MMN or other EAP measures can also be used for diagnostic, predictive, prognostic or risk-assessment purposes remains an open question.
Highlights.
Early auditory processing (EAP) deficits are a core feature of schizophrenia with critical contributions to both neuro- and social cognition
EAP deficits involve impairments at both subcortical and cortical stages of processing, particularly involving glutamatergic and N-methyl-D-aspartate receptor (NMDAR)-mediated processes.
Mismatch negativity (MMN) serves as a neurophysiological biomarker for EAP function and may be used both clinically and translationally.
Undetected EAP deficits may be a critical barrier to functional recovery in schizophrenia
Funding
Preparation of this manuscript was funded in part by NIMH grants R01MH49334 and R01MH123142 to DCJ
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest statement
DCJ holds equity in Glytech, AASI and NRX pharma. He has served as a consultant for SK Life Sciences, Biogen, Boehringer Ingelheim, Autifony and Anavex. He has received research support from Cerevance. He holds intellectual property for use of NMDAR agonists in treatment of schizophrenia; NMDAR antagonists for treatment of depression; neurophysiological measures for detection of amyloid deposition; and parcel-guided approaches to brain stimulation.
JTK reports having received consulting payments within the last 24 months from Alphasights, Medscape, Putnam, techspert.io, Health Monitor, Third Bridge, MEDACorp, Trinity, Globaldata, GKA, Clearview, Clarivate, Health Advances, ECRI Institute, ExpertConnect, Acsel Health, Slingshot, Antheum, Guidepoint, L.E.K., SmartAnalyst, First Thought, Wedbush, Jefferies, Otsuka, Vox Neuro and Reckner. He has served on the MedinCell Psychiatry, Tolmar, Merck, Leal and the Karuna Advisory Boards. He has conducted clinical research supported by the NIMH, Sunovion, Roche, Cerevance, Click, Neurocrine, Corcept, Taisho and Boehringer Ingelheim within the last 24 months. He owns a small number of shares of common stock from GSK.
AM serves as a consultant at Boehringer Ingelheim and Sumitomo and receives royalties from Oxford University Press.
Other authors report no potential COI
References
- Abs E, Poorthuis RB, Apelblat D, Muhammad K, Pardi MB, Enke L, Kushinsky D, Pu DL, Eizinger MF, Conzelmann KK, Spiegel I, Letzkus JJ, 2018. Learning-Related Plasticity in Dendrite-Targeting Layer 1 Interneurons. Neuron 100, 684–699 e686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam O, Blay M, Brunoni AR, Chang HA, Gomes JS, Javitt DC, Jung DU, Kantrowitz JT, Koops S, Lindenmayer JP, Palm U, Smith RC, Sommer IE, Valiengo L, Weickert TW, Brunelin J, Mondino M, 2022. Efficacy of Transcranial Direct Current Stimulation to Improve Insight in Patients With Schizophrenia: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Schizophr Bull [DOI] [PMC free article] [PubMed]
- Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A, 1999. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry 156, 1646–1649. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Lubin Y, Putter-Katz H, Banai K, 2006. Dyslexia and the failure to form a perceptual anchor. Nat Neurosci 9, 1558–1564. [DOI] [PubMed] [Google Scholar]
- Ajnakina O, Stubbs B, Francis E, Gaughran F, David AS, Murray RM, Lally J, 2021. Employment and relationship outcomes in first-episode psychosis: A systematic review and meta-analysis of longitudinal studies. Schizophr Res 231, 122–133. [DOI] [PubMed] [Google Scholar]
- Atagun MI, Sikoglu EM, Soykan C, Serdar Suleyman C, Ulusoy-Kaymak S, Caykoylu A, Algin O, Phillips ML, Ongur D, Moore CM, 2017. Perisylvian GABA levels in schizophrenia and bipolar disorder. Neurosci Lett 637, 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar M, Xie S, Vail B, Lopez-Calderon J, Wang Y, Javitt DC, 2018. Meta-analysis of mismatch negativity to simple versus complex deviants in schizophrenia. Schizophr Res 191, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD, 1970. Estimating the short-term component in free recall. Br J Psychol 61, 13–15. [Google Scholar]
- Baldeweg T, Wong D, Stephan KE, 2006. Nicotinic modulation of human auditory sensory memory: Evidence from mismatch negativity potentials. Int J Psychophysiol 59, 49–58. [DOI] [PubMed] [Google Scholar]
- Balla A, Ginsberg SD, Abbas AI, Sershen H, Javitt DC, 2020. Translational neurophysiological biomarkers of N-methyl-d-aspartate receptor dysfunction in serine racemase knockout mice. Biomark Neuropsychiatry 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyk M, Brown S, 2014. Perception of affective and linguistic prosody: an ALE meta-analysis of neuroimaging studies. Soc Cogn Affect Neurosci 9, 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojraj TS, Sweeney JA, Prasad KM, Eack SM, Francis AN, Miewald JM, Montrose DM, Keshavan MS, 2011. Gray matter loss in young relatives at risk for schizophrenia: relation with prodromal psychopathology. Neuroimage 54 Suppl 1, S272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagianti B, Fisher M, Loewy R, Brandrett B, Ordorica C, LaCross K, Schermitzler B, McDonald M, Ramsay I, Vinogradov S, 2020. Specificity and Durability of Changes in Auditory Processing Efficiency After Targeted Cognitive Training in Individuals With Recent-Onset Psychosis. Front Psychiatry 11, 857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Brunoni AR, Charvet LE, Clark VP, Cohen LG, Deng ZD, Dmochowski J, Edwards DJ, Frohlich F, Kappenman ES, Lim KO, Loo C, Mantovani A, McMullen DP, Parra LC, Pearson M, Richardson JD, Rumsey JM, Sehatpour P, Sommers D, Unal G, Wassermann EM, Woods AJ, Lisanby SH, 2018. Rigor and reproducibility in research with transcranial electrical stimulation: An NIMH-sponsored workshop. Brain Stimul 11, 465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingberg O, Jonsson CO, 1965. The ability of schizophrenic patients to interpret intonation. Acta Psychiatr Scand 41, 218–226. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Hardiman MJ, Barry JG, 2011. Is auditory discrimination mature by middle childhood? A study using time-frequency analysis of mismatch responses from 7 years to adulthood. Developmental science 14, 402–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodatsch M, Ruhrmann S, Wagner M, Muller R, Schultze-Lutter F, Frommann I, Brinkmeyer J, Gaebel W, Maier W, Klosterkotter J, Brockhaus-Dumke A, 2011. Prediction of psychosis by mismatch negativity. Biol Psychiatry 69, 959–966. [DOI] [PubMed] [Google Scholar]
- Booker SA, Wyllie DJA, 2021. NMDA receptor function in inhibitory neurons. Neuropharmacology 196, 108609. [DOI] [PubMed] [Google Scholar]
- Brannan S, 2019. Two global phase III trials of encenicline for cognitive impairments in chronic schizophreina patients: red flags and lessons learned. Schizophr Bull 45, S141–142. [Google Scholar]
- Bravermanova A, Viktorinova M, Tyls F, Novak T, Androvicova R, Korcak J, Horacek J, Balikova M, Griskova-Bulanova I, Danielova D, Vlcek P, Mohr P, Brunovsky M, Koudelka V, Palenicek T, 2018. Psilocybin disrupts sensory and higher order cognitive processing but not preattentive cognitive processing-study on P300 and mismatch negativity in healthy volunteers. Psychopharmacology (Berl) 235, 491–503. [DOI] [PubMed] [Google Scholar]
- Broadbent DE, 1958. Perception and communication
- Brockhaus-Dumke A, Tendolkar I, Pukrop R, Schultze-Lutter F, Klosterkotter J, Ruhrmann S, 2005. Impaired mismatch negativity generation in prodromal subjects and patients with schizophrenia. Schizophr Res 73, 297–310. [DOI] [PubMed] [Google Scholar]
- Brunelin J, Mondino M, Gassab L, Haesebaert F, Gaha L, Suaud-Chagny MF, Saoud M, Mechri A, Poulet E, 2012. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry 169, 719–724. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Shiozawa P, Truong D, Javitt DC, Elkis H, Fregni F, Bikson M, 2014. Understanding tDCS effects in schizophrenia: a systematic review of clinical data and an integrated computation modeling analysis. Expert Rev Med Devices 11, 383–394. [DOI] [PubMed] [Google Scholar]
- Cacciaglia R, Escera C, Slabu L, Grimm S, Sanjuan A, Ventura-Campos N, Avila C, 2015. Involvement of the human midbrain and thalamus in auditory deviance detection. Neuropsychologia 68, 51–58. [DOI] [PubMed] [Google Scholar]
- Carrion RE, Cornblatt BA, McLaughlin D, Chang J, Auther AM, Olsen RH, Javitt DC, 2015. Contributions of early cortical processing and reading ability to functional status in individuals at clinical high risk for psychosis. Schizophr Res 164, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier N, Chevreul K, Durand-Zaleski I, 2013. [The cost of schizophrenia: a literature review]. Encephale 39 Suppl 1, S49–56. [DOI] [PubMed] [Google Scholar]
- Chesney E, Goodwin GM, Fazel S, 2014. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry 13, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cienfuegos A, March L, Shelley AM, Javitt DC, 1999. Impaired categorical perception of synthetic speech sounds in schizophrenia. Biol Psychiatry 45, 82–88. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Parker DA, Trotti RL, McDowell JE, Keedy SK, Keshavan MS, Pearlson GD, Gershon ES, Ivleva EI, Huang LY, Hill SK, Sweeney JA, Thomas O, Hudgens-Haney M, Gibbons RD, Tamminga CA, 2022. Psychosis Biotypes: Replication and Validation from the B-SNIP Consortium. Schizophr Bull 48, 56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier M, Aigbogun MS, Guerin A, Nitulescu R, Ramanakumar AV, Kamat SA, DeLucia M, Duffy R, Legacy SN, Henderson C, Francois C, Wu E, 2016. The Economic Burden of Schizophrenia in the United States in 2013. J Clin Psychiatry 77, 764–771. [DOI] [PubMed] [Google Scholar]
- Coffman BA, Haigh SM, Murphy TK, Salisbury DF, 2017. Impairment in Mismatch Negativity but not Repetition Suppression in Schizophrenia. Brain Topogr 30, 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman BA, Ren X, Longenecker J, Torrence N, Fishel V, Seebold D, Wang Y, Curtis M, Salisbury DF, 2022. Aberrant attentional modulation of the auditory steady state response (ASSR) is related to auditory hallucination severity in the first-episode schizophrenia-spectrum. Journal of psychiatric research 151, 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators GBDMD, 2022. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 9, 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, D'Amato MR, Rodman HR, Gross CG, 1990. Auditory association cortex lesions impair auditory short-term memory in monkeys. Science 247, 336–338. [DOI] [PubMed] [Google Scholar]
- Cooper N, 1994. An exploratory study in themeasurement of children's pitch discrimination ability. Psychol. Music 22, 56–62. [Google Scholar]
- Corcoran CM, Carrillo F, Fernandez-Slezak D, Bedi G, Klim C, Javitt DC, Bearden CE, Cecchi GA, 2018a. Prediction of psychosis across protocols and risk cohorts using automated language analysis. World Psychiatry 17, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran CM, Keilp JG, Kayser J, Klim C, Butler PD, Bruder GE, Gur RC, Javitt DC, 2015. Emotion recognition deficits as predictors of transition in individuals at clinical high risk for schizophrenia: a neurodevelopmental perspective. Psychol Med 45, 2959–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran CM, Stoops A, Lee M, Martinez A, Sehatpour P, Dias EC, Javitt DC, 2018b. Developmental trajectory of mismatch negativity and visual event-related potentials in healthy controls: Implications for neurodevelopmental vs. neurodegenerative models of schizophrenia. Schizophr Res 191, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Monaghan DT, 1988. Excitatory amino acids and neurotransmission: NMDA receptor and Hebb-type synaptic plasticity. Ann. Rev. Neurosci 11, 61–80. [DOI] [PubMed] [Google Scholar]
- Cowan N, 1984. On short and long auditory stores. Psychol Bull 96, 341–370. [PubMed] [Google Scholar]
- Cowan N, 1993. Activation, attention, and short-term memory. Mem Cognit 21, 162–167. [DOI] [PubMed] [Google Scholar]
- Cowman M, Holleran L, Lonergan E, O'Connor K, Birchwood M, Donohoe G, 2021. Cognitive Predictors of Social and Occupational Functioning in Early Psychosis: A Systematic Review and Meta-analysis of Cross-Sectional and Longitudinal Data. Schizophr Bull 47, 1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MT, Coffman BA, Salisbury DF, 2021. Pitch and Duration Mismatch Negativity are Associated With Distinct Auditory Cortex and Inferior Frontal Cortex Volumes in the First-Episode Schizophrenia Spectrum. Schizophr Bull Open 2, sgab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, 2014. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry 13, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CL, Brown EG, Herman AB, Hinkley LBN, Subramaniam K, Fisher M, Vinogradov S, Nagarajan SS, 2020. Intervention-specific patterns of cortical function plasticity during auditory encoding in people with schizophrenia. Schizophr Res 215, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Garrigue N, Glasser J, Sehatpour P, Iosifescu DV, Dias E, Carlson M, Shope C, Sobeih T, Choo TH, Wall MM, Kegeles LS, Gangwisch J, Mayer M, Brazis S, De Baun HM, Wolfer S, Bermudez D, Arnold M, Rette D, Meftah AM, Conant M, Lieberman JA, Kantrowitz JT, 2020. Grant Report on d-Serine Augmentation of Neuroplasticity-Based Auditory Learning in Schizophrenia (dagger). J Psychiatr Brain Sci 5. [PMC free article] [PubMed] [Google Scholar]
- de la Salle S, Choueiry J, McIntosh J, Bowers H, Ilivitsky V, Knott V, 2022. N-methyl-D-aspartate receptor antagonism impairs sensory gating in the auditory cortex in response to speech stimuli. Psychopharmacology (Berl) 239, 2155–2169. [DOI] [PubMed] [Google Scholar]
- de la Salle S, Shah D, Choueiry J, Bowers H, McIntosh J, Carroll B, Ilivitsky V, Knott V, 2021. N-methyl-d-aspartate receptor antagonism modulates P300 event-related potentials and associated activity in salience and central executive networks. Pharmacol Biochem Behav 211, 173287. [DOI] [PubMed] [Google Scholar]
- Denham SL, Winkler I, 2020. Predictive coding in auditory perception: challenges and unresolved questions. The European journal of neuroscience 51, 1151–1160. [DOI] [PubMed] [Google Scholar]
- Doeller CF, Opitz B, Mecklinger A, Krick C, Reith W, Schroger E, 2003. Prefrontal cortex involvement in preattentive auditory deviance detection: neuroimaging and electrophysiological evidence. Neuroimage 20, 1270–1282. [DOI] [PubMed] [Google Scholar]
- Donde C, Avissar M, Weber MM, Javitt DC, 2019a. A century of sensory processing dysfunction in schizophrenia. Eur Psychiatry 59, 77–79. [DOI] [PubMed] [Google Scholar]
- Donde C, Brunelin J, Haesebaert F, 2020a. Duration, pitch and intensity features reveal different magnitudes of tone-matching deficit in schizophrenia. Schizophr Res 215, 460–462. [DOI] [PubMed] [Google Scholar]
- Donde C, Brunelin J, Mondino M, Cellard C, Rolland B, Haesebaert F, 2020b. The effects of acute nicotine administration on cognitive and early sensory processes in schizophrenia: a systematic review. Neurosci Biobehav Rev 118, 121–133. [DOI] [PubMed] [Google Scholar]
- Donde C, Luck D, Grot S, Leitman DI, Brunelin J, Haesebaert F, 2017. Tone-matching ability in patients with schizophrenia: A systematic review and meta-analysis. Schizophr Res 181, 94–99. [DOI] [PubMed] [Google Scholar]
- Donde C, Martinez A, Kantrowitz JT, Silipo G, Dias EC, Patel GH, Sanchez-Pena J, Corcoran CM, Medalia A, Saperstein A, Vail B, Javitt DC, 2019b. Bimodal distribution of tone-matching deficits indicates discrete pathophysiological entities within the syndrome of schizophrenia. Transl Psychiatry 9, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donde C, Martinez A, Sehatpour P, Patel GH, Kraut R, Kantrowitz JT, Javitt DC, 2019c. Neural and functional correlates of impaired reading ability in schizophrenia. Sci Rep 9, 16022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donde C, Mondino M, Brunelin J, Haesebaert F, 2019d. Sensory-targeted cognitive training for schizophrenia. Expert Rev Neurother 19, 211–225. [DOI] [PubMed] [Google Scholar]
- Donde C, Mondino M, Leitman DI, Javitt DC, Suaud-Chagny MF, D'Amato T, Brunelin J, Haesebaert F, 2019e. Are basic auditory processes involved in source-monitoring deficits in patients with schizophrenia? Schizophr Res 210, 135–142. [DOI] [PubMed] [Google Scholar]
- Donde C, Silipo G, Dias EC, Javitt DC, 2019f. Hierarchical deficits in auditory information processing in schizophrenia. Schizophr Res 206, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulude L, Labelle A, Knott VJ, 2010. Acute nicotine alteration of sensory memory impairment in smokers with schizophrenia. J Clin Psychopharmacol 30, 541–548. [DOI] [PubMed] [Google Scholar]
- Dunn W, Rassovsky Y, Wynn J, Wu AD, Iacoboni M, Hellemann G, Green MF, 2017. The effect of bilateral transcranial direct current stimulation on early auditory processing in schizophrenia: a preliminary study. J Neural Transm (Vienna) 124, 1145–1149. [DOI] [PubMed] [Google Scholar]
- Dykstra AR, Koh CK, Braida LD, Tramo MJ, 2012. Dissociation of detection and discrimination of pure tones following bilateral lesions of auditory cortex. PLoS ONE 7, e44602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls HA, Curran T, Mittal V, 2016. A Meta-analytic Review of Auditory Event-Related Potential Components as Endophenotypes for Schizophrenia: Perspectives From First-Degree Relatives. Schizophr Bull 42, 1504–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G, Romand R, 1997. The central auditory system Oxford University Press, New York. [Google Scholar]
- Ehrlichman RS, Maxwell CR, Majumdar S, Siegel SJ, 2008. Deviance-elicited changes in event-related potentials are attenuated by ketamine in mice. Journal of cognitive neuroscience 20, 1403–1414. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Albrecht M, Ruffle A, Fleming L, Corlett P, Gold J, 2017. No association between symptom severity and MMN impairment in schizophrenia: A meta-analytic approach. Schizophr Res Cogn 9, 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancourt A, Dick F, Stewart L, 2013. Pitch-change detection and pitch-direction discrimination in children. Psychomusicology 23, 73–81. [Google Scholar]
- Featherstone RE, Melnychenko O, Siegel SJ, 2018. Mismatch negativity in preclinical models of schizophrenia. Schizophr Res 191, 35–42. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Shin R, Kogan JH, Liang Y, Matsumoto M, Siegel SJ, 2014. Mice with subtle reduction of NMDA NR1 receptor subunit expression have a selective decrease in mismatch negativity: Implications for schizophrenia prodromal population. Neurobiology of disease 73C, 289–295. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Siegel SJ, 2015. The Role of Nicotine in Schizophrenia. Int Rev Neurobiol 124, 23–78. [DOI] [PubMed] [Google Scholar]
- Field M, Lukacs IP, Hunter E, Stacey R, Plaha P, Livermore L, Ansorge O, Somogyi P, 2021. Tonic GABAA Receptor-Mediated Currents of Human Cortical GABAergic Interneurons Vary Amongst Cell Types. J Neurosci 41, 9702–9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DJ, Grant B, Smith DM, Borracci G, Labelle A, Knott VJ, 2012. Nicotine and the hallucinating brain: effects on mismatch negativity (MMN) in schizophrenia. Psychiatry Res 196, 181–187. [DOI] [PubMed] [Google Scholar]
- Fisher DJ, Labelle A, Knott VJ, 2008. Auditory hallucinations and the mismatch negativity: processing speech and non-speech sounds in schizophrenia. Int J Psychophysiol 70, 3–15. [DOI] [PubMed] [Google Scholar]
- Fisher DJ, Rudolph ED, Ells EML, Knott VJ, Labelle A, Tibbo PG, 2019. Mismatch negativity-indexed auditory change detection of speech sounds in early and chronic schizophrenia. Psychiatry Res Neuroimaging 287, 1–9. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Roach BJ, Keedy SK, Reilly JL, Gershon ES, Sweeney JA, 2013a. Neurophysiological evidence of corollary discharge function during vocalization in psychotic patients and their nonpsychotic first-degree relatives. Schizophr Bull 39, 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Palzes VA, Roach BJ, Mathalon DH, 2013b. Did I Do That? Abnormal Predictive Processes in Schizophrenia When Button Pressing to Deliver a Tone. Schizophr Bull [DOI] [PMC free article] [PubMed]
- Freedman R, 2014. alpha7-nicotinic acetylcholine receptor agonists for cognitive enhancement in schizophrenia. Annu Rev Med 65, 245–261. [DOI] [PubMed] [Google Scholar]
- Freedman R, Olsen-Dufour AM, Olincy A, Consortium on the Genetics of, S., 2020. P50 inhibitory sensory gating in schizophrenia: analysis of recent studies. Schizophr Res 218, 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman T, Sehatpour P, Dias E, Perrin M, Javitt DC, 2012. Differential relationships of mismatch negativity and visual p1 deficits to premorbid characteristics and functional outcome in schizophrenia. Biol Psychiatry 71, 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, 2005. A theory of cortical responses. Philosophical transactions of the Royal Society of London 360, 815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Carcea I, Barker AJ, Yuan K, Seybold BA, Martins AR, Zaika N, Bernstein H, Wachs M, Levis PA, Polley DB, Merzenich MM, Schreiner CE, 2013. Long-term modification of cortical synapses improves sensory perception. Nat Neurosci 16, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentemilla L, Marco-Pallares J, Munte TF, Grau C, 2008. Theta EEG oscillatory activity and auditory change detection. Brain Res 1220, 93–101. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton MJ, Lawrie S, Mc Guire P, Sacchetti E, 2011. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev 35, 1175–1185. [DOI] [PubMed] [Google Scholar]
- Gaebler AJ, Zweerings J, Koten JW, Konig AA, Turetsky BI, Zvyagintsev M, Mathiak K, 2020. Impaired Subcortical Detection of Auditory Changes in Schizophrenia but Not in Major Depression. Schizophr Bull 46, 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MI, Friston KJ, Kiebel SJ, Stephan KE, Baldeweg T, Kilner JM, 2008. The functional anatomy of the MMN: a DCM study of the roving paradigm. Neuroimage 42, 936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Kiebel SJ, Friston KJ, 2009. Dynamic causal modeling of the response to frequency deviants. Journal of neurophysiology 101, 2620–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard MH, Perrin F, Pernier J, Bouchet P, 1990. Brain generators implicated in the processing of auditory stimulus deviance: a topographic event-related potential study. Psychophysiology 27, 627–640. [DOI] [PubMed] [Google Scholar]
- Gil-da-Costa R, Stoner GR, Fung R, Albright TD, 2013. Nonhuman primate model of schizophrenia using a noninvasive EEG method. Proc Natl Acad Sci U S A 110, 15425–15430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, Van Essen DC, 2016. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA, 2018. Mapping pathologic circuitry in schizophrenia. Handb Clin Neurol 150, 389–417. [DOI] [PubMed] [Google Scholar]
- Gold R, Butler P, Revheim N, Leitman DI, Hansen JA, Gur RC, Kantrowitz JT, Laukka P, Juslin PN, Silipo GS, Javitt DC, 2012. Auditory emotion recognition impairments in schizophrenia: relationship to acoustic features and cognition. Am J Psychiatry 169, 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, Lewis DA, 2010. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep 12, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, 1996. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 153, 321–330. [DOI] [PubMed] [Google Scholar]
- Green MF, Horan WP, Lee J, 2019. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry 18, 146–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J, 2000. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the "right stuff"? Schizophr Bull 26, 119–136. [DOI] [PubMed] [Google Scholar]
- Greenwood LM, Leung S, Michie PT, Green A, Nathan PJ, Fitzgerald P, Johnston P, Solowij N, Kulkarni J, Croft RJ, 2018. The effects of glycine on auditory mismatch negativity in schizophrenia. Schizophr Res 191, 61–69. [DOI] [PubMed] [Google Scholar]
- Grent-ť-Jong T, Gajwani R, Gross J, Gumley AI, Krishnadas R, Lawrie SM, Schwannauer M, Schultze-Lutter F, Uhlhaas PJ, 2021. 40-Hz Auditory Steady-State Responses Characterize Circuit Dysfunctions and Predict Clinical Outcomes in Clinical High-Risk for Psychosis Participants: A Magnetoencephalography Study. Biol Psychiatry 90, 419–429. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, 2001. The neural processing of complex sounds. Ann N Y Acad Sci 930, 133–142. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, 2003. Functional imaging of pitch analysis. Ann N Y Acad Sci 999, 40–49. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Buchel C, Frackowiak RS, Patterson RD, 1998. Analysis of temporal structure in sound by the human brain. Nat Neurosci 1, 422–427. [DOI] [PubMed] [Google Scholar]
- Habib M, Daquin G, Milandre L, Royere ML, Rey M, Lanteri A, Salamon G, Khalil R, 1995. Mutism and auditory agnosia due to bilateral insular damage--role of the insula in human communication. Neuropsychologia 33, 327–339. [DOI] [PubMed] [Google Scholar]
- Haigh SM, Coffman BA, Salisbury DF, 2017a. Mismatch Negativity in First-Episode Schizophrenia: A Meta-Analysis. Clin EEG Neurosci 48, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh SM, Matteis M, Coffman BA, Murphy TK, Butera CD, Ward KL, Leiter-McBeth JR, Salisbury DF, 2017b. Mismatch negativity to pitch pattern deviants in schizophrenia. The European journal of neuroscience 46, 2229–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton HK, D'Souza DC, Ford JM, Roach BJ, Kort NS, Ahn KH, Bhakta S, Ranganathan M, Mathalon DH, 2018a. Interactive effects of an N-methyl-d-aspartate receptor antagonist and a nicotinic acetylcholine receptor agonist on mismatch negativity: Implications for schizophrenia. Schizophr Res 191, 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton HK, Perez VB, Ford JM, Roach BJ, Jaeger J, Mathalon DH, 2018b. Mismatch Negativity But Not P300 Is Associated With Functional Disability in Schizophrenia. Schizophr Bull 44, 492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton HK, Roach BJ, Bachman PM, Belger A, Carrion RE, Duncan E, Johannesen JK, Light GA, Niznikiewicz MA, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Cannon TD, Mathalon DH, 2019. Association Between P300 Responses to Auditory Oddball Stimuli and Clinical Outcomes in the Psychosis Risk Syndrome. JAMA psychiatry 76, 1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton HK, Roach BJ, Bachman PM, Belger A, Carrion RE, Duncan E, Johannesen JK, Light GA, Niznikiewicz MA, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, Cannon TD, Mathalon DH, 2022. Mismatch Negativity in Response to Auditory Deviance and Risk for Future Psychosis in Youth at Clinical High Risk for Psychosis. JAMA psychiatry [DOI] [PMC free article] [PubMed]
- Hamilton HK, Roach BJ, Mathalon DH, 2021. Forecasting Remission From the Psychosis Risk Syndrome With Mismatch Negativity and P300: Potentials and Pitfalls. Biol Psychiatry Cogn Neurosci Neuroimaging 6, 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Yuste R, 2016. Somatostatin Interneurons Control a Key Component of Mismatch Negativity in Mouse Visual Cortex. Cell Rep 16, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms L, 2016. Mismatch responses and deviance detection in N-methyl-D-aspartate (NMDA) receptor hypofunction and developmental models of schizophrenia. Biol Psychol 116, 75–81. [DOI] [PubMed] [Google Scholar]
- Harpaz M, Jankowski MM, Khouri L, Nelken I, 2021. Emergence of abstract sound representations in the ascending auditory system. Prog Neurobiol 202, 102049. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA, 2003. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci 23, 6315–6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangadi N, Pillion JP, Slomine B, Christensen J, Trovato MK, Speedie LJ, 2005. Characteristics of auditory agnosia in a child with severe traumatic brain injury: a case report. Brain Lang 92, 12–25. [DOI] [PubMed] [Google Scholar]
- Heekeren K, Daumann J, Neukirch A, Stock C, Kawohl W, Norra C, Waberski TD, Gouzoulis-Mayfrank E, 2008. Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berl) 199, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbron M, Chait M, 2018. Great Expectations: Is there Evidence for Predictive Coding in Auditory Cortex? Neuroscience 389, 54–73. [DOI] [PubMed] [Google Scholar]
- Hensch TK, 2005. Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6, 877–888. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Oribe N, Onitsuka T, Kanba S, Nestor PG, Hosokawa T, Levin M, Shenton ME, McCarley RW, Spencer KM, 2020. Auditory Cortex Volume and Gamma Oscillation Abnormalities in Schizophrenia. Clin EEG Neurosci, 1550059420914201. [DOI] [PubMed]
- Hochberger WC, Joshi YB, Zhang W, Thomas ML, Consortium of Genomics in Schizophrenia, i., Braff DL, Swerdlow NR, Light GA, 2018. Decomposing the constituent oscillatory dynamics underlying mismatch negativity generation in schizophrenia: Distinct relationships to clinical and cognitive functioning. Int J Psychophysiol [DOI] [PMC free article] [PubMed]
- Hodge RD, Bakken TE, Miller JA, Smith KA, Barkan ER, Graybuck LT, Close JL, Long B, Johansen N, Penn O, Yao Z, Eggermont J, Hollt T, Levi BP, Shehata SI, Aevermann B, Beller A, Bertagnolli D, Brouner K, Casper T, Cobbs C, Dalley R, Dee N, Ding SL, Ellenbogen RG, Fong O, Garren E, Goldy J, Gwinn RP, Hirschstein D, Keene CD, Keshk M, Ko AL, Lathia K, Mahfouz A, Maltzer Z, McGraw M, Nguyen TN, Nyhus J, Ojemann JG, Oldre A, Parry S, Reynolds S, Rimorin C, Shapovalova NV, Somasundaram S, Szafer A, Thomsen ER, Tieu M, Quon G, Scheuermann RH, Yuste R, Sunkin SM, Lelieveldt B, Feng D, Ng L, Bernard A, Hawrylycz M, Phillips JW, Tasic B, Zeng H, Jones AR, Koch C, Lein ES, 2019. Conserved cell types with divergent features in human versus mouse cortex. Nature 573, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb HH, Ritzl EK, Medoff DR, Nevitt J, Gordon B, Tamminga CA, 1995. Tone discrimination performance in schizophrenic patients and normal volunteers: impact of stimulus presentation levels and frequency differences. Psychiatry Res 57, 75–82. [DOI] [PubMed] [Google Scholar]
- Holliday WB, Gurnsey K, Sweet RA, Teichert T, 2018. A putative electrophysiological biomarker of auditory sensory memory encoding is sensitive to pharmacological alterations of excitatory/inhibitory balance in male macaque monkeys. J Psychiatry Neurosci 43, 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, 2003. Functional organization of lemniscal and nonlemniscal auditory thalamus. Exp Brain Res 153, 543–549. [DOI] [PubMed] [Google Scholar]
- Impey D, Baddeley A, Nelson R, Labelle A, Knott V, 2017a. Effects of transcranial direct current stimulation on the auditory mismatch negativity response and working memory performance in schizophrenia: a pilot study. J Neural Transm (Vienna) 124, 1489–1501. [DOI] [PubMed] [Google Scholar]
- Impey D, de la Salle S, Baddeley A, Knott V, 2017b. Effects of an NMDA antagonist on the auditory mismatch negativity response to transcranial direct current stimulation. J Psychopharmacol 31, 614–624. [DOI] [PubMed] [Google Scholar]
- Impey D, de la Salle S, Knott V, 2016. Assessment of anodal and cathodal transcranial direct current stimulation (tDCS) on MMN-indexed auditory sensory processing. Brain Cogn 105, 46–54. [DOI] [PubMed] [Google Scholar]
- Impey D, Knott V, 2015. Effect of transcranial direct current stimulation (tDCS) on MMN-indexed auditory discrimination: a pilot study. J Neural Transm (Vienna) 122, 1175–1185. [DOI] [PubMed] [Google Scholar]
- Inami R, Kirino E, Inoue R, Suzuki T, Arai H, 2007. Nicotine effects on mismatch negativity in nonsmoking schizophrenic patients. Neuropsychobiology 56, 64–72. [DOI] [PubMed] [Google Scholar]
- Insel TR, 2010. Rethinking schizophrenia. Nature 468, 187–193. [DOI] [PubMed] [Google Scholar]
- Irvine DRF, 2018. Plasticity in the auditory system. Hearing research 362, 61–73. [DOI] [PubMed] [Google Scholar]
- Iversen SD, 1973. Comparison of superior temporal and inferior prefrontal lesions on auditory and non-auditory tasks in rhesus monkeys. Brain Res 55, 355–367. [DOI] [PubMed] [Google Scholar]
- Jaffe-Dax S, Kimel E, Ahissar M, 2018. Shorter cortical adaptation in dyslexia is broadly distributed in the superior temporal lobe and includes the primary auditory cortex. eLife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, 2000. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiology & neuro-otology 5, 207–215. [DOI] [PubMed] [Google Scholar]
- Javitt DC, 2009. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol 5, 249–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, 2015. Neurophysiological models for new treatment development in schizophrenia: early sensory approaches. Ann N Y Acad Sci 1344, 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, 2022. Cognitive Impairment Associated with Schizophrenia: Toward Novel Therapeutics. Annu Rev Pharmacol Toxicol [DOI] [PubMed]
- Javitt DC, Doneshka P, Zylberman I, Ritter W, Vaughan HG Jr., 1993. Impairment of early cortical processing in schizophrenia: an event-related potential confirmation study. Biol Psychiatry 33, 513–519. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Freedman R, 2015. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry 172, 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Jayachandra M, Lindsley RW, Specht CM, Schroeder CE, 2000a. Schizophrenia-like deficits in auditory P1 and N1 refractoriness induced by the psychomimetic agent phencyclidine (PCP). Clin Neurophysiol 111, 833–836. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Lee M, Kantrowitz JT, Martinez A, 2018. Mismatch negativity as a biomarker of theta band oscillatory dysfunction in schizophrenia. Schizophr Res 191, 51–60. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Schroeder CE, Steinschneider M, Arezzo JC, Vaughan HG Jr., 1992. Demonstration of mismatch negativity in the monkey. Electroencephalography and clinical neurophysiology 83, 87–90. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley A, Ritter W, 2000b. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol 111, 1733–1737. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Siegel SJ, Spencer KM, Mathalon DH, Hong LE, Martinez A, Ehlers CL, Abbas AI, Teichert T, Lakatos P, Womelsdorf T, 2020. A roadmap for development of neuro-oscillations as translational biomarkers for treatment development in neuropsychopharmacology. Neuropsychopharmacology 45, 1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC, 1996. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A 93, 11962–11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Strous RD, Grochowski S, Ritter W, Cowan N, 1997. Impaired precision, but normal retention, of auditory sensory ("echoic") memory information in schizophrenia. J Abnorm Psychol 106, 315–324. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Sweet RA, 2015. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat Rev Neurosci 16, 535–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YW, Polich J, 2003. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology 40, 684–701. [DOI] [PubMed] [Google Scholar]
- Jin Y, Diaz B, Colomer M, Sebastian-Galles N, 2014. Oscillation encoding of individual differences in speech perception. PLoS ONE 9, e100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsrude IS, Penhune VB, Zatorre RJ, 2000. Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain 123 ( Pt 1), 155–163. [DOI] [PubMed] [Google Scholar]
- Jones EG, 1998. A new view of specific and nonspecific thalamocortical connections. Adv Neurol 77, 49–71; discussion 72–43. [PubMed] [Google Scholar]
- Jones EG, 2002. Thalamic organization and function after Cajal. Prog Brain Res 136, 333–357. [DOI] [PubMed] [Google Scholar]
- Jones EG, 2009. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Ann N Y Acad Sci 1157, 10–23. [DOI] [PubMed] [Google Scholar]
- Jonsson CO, Sjostedt A, 1973. Auditory perception in schizophrenia: a second study of the Intonation test. Acta Psychiatr Scand 49, 588–600. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, 2019. N-methyl-d-aspartate-type glutamate receptor modulators and related medications for the enhancement of auditory system plasticity in schizophrenia. Schizophr Res 207, 70–79. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Epstein ML, Beggel O, Rohrig S, Lehrfeld JM, Revheim N, Lehrfeld NP, Reep J, Parker E, Silipo G, Ahissar M, Javitt DC, 2016. Neurophysiological mechanisms of cortical plasticity impairments in schizophrenia and modulation by the NMDA receptor agonist D-serine. Brain 139, 3281–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Epstein ML, Lee M, Lehrfeld N, Nolan KA, Shope C, Petkova E, Silipo G, Javitt DC, 2018. Improvement in mismatch negativity generation during d-serine treatment in schizophrenia: Correlation with symptoms. Schizophr Res 191, 70–79. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Hoptman MJ, Leitman DI, Moreno-Ortega M, Lehrfeld JM, Dias E, Sehatpour P, Laukka P, Silipo G, Javitt DC, 2015. Neural Substrates of Auditory Emotion Recognition Deficits in Schizophrenia. J Neurosci 35, 14909–14921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Hoptman MJ, Leitman DI, Silipo G, Javitt DC, 2014a. The 5% difference: early sensory processing predicts sarcasm perception in schizophrenia and schizo-affective disorder. Psychol Med 44, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC, Freedman R, Sehatpour P, Kegeles LS, Carlson M, Sobeih T, Wall MM, Choo TH, Vail B, Grinband J, Lieberman JA, 2020. Double blind, two dose, randomized, placebo-controlled, cross-over clinical trial of the positive allosteric modulator at the alpha7 nicotinic cholinergic receptor AVL-3288 in schizophrenia patients. Neuropsychopharmacology 45, 1339–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Scaramello N, Jakubovitz A, Lehrfeld JM, Laukka P, Elfenbein HA, Silipo G, Javitt DC, 2014b. Amusia and protolanguage impairments in schizophrenia. Psychol Med 44, 2739–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Sehatpour P, Avissar M, Horga G, Gwak A, Hoptman MJ, Beggel O, Girgis RR, Vail B, Silipo G, Carlson M, Javitt DC, 2019. Significant improvement in treatment resistant auditory verbal hallucinations after 5 days of double-blind, randomized, sham controlled, fronto-temporal, transcranial direct current stimulation (tDCS): A replication/extension study. Brain Stimul [DOI] [PMC free article] [PubMed]
- Kaser M, Soltesz F, Lawrence P, Miller S, Dodds C, Croft R, Dudas RB, Zaman R, Fernandez-Egea E, Muller U, Dean A, Bullmore ET, Nathan PJ, 2013. Oscillatory underpinnings of mismatch negativity and their relationship with cognitive function in patients with schizophrenia. PLoS ONE 8, e83255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakubo Y, Kamio S, Nose T, Iwanami A, Nakagome K, Fukuda M, Kato N, Rogers MA, Kasai K, 2007. Phonetic mismatch negativity predicts social skills acquisition in schizophrenia. Psychiatry Res 152, 261–265. [DOI] [PubMed] [Google Scholar]
- Kawakubo Y, Suga M, Tochigi M, Yumoto M, Itoh K, Sasaki T, Kano Y, Kasai K, 2011. Effects of metabotropic glutamate receptor 3 genotype on phonetic mismatch negativity. PLoS One 6, e24929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Meltzer HA, Dgetluck N, Gawryl M, Koenig G, Moebius HJ, Lombardo I, Hilt DC, 2015. Randomized, Double-Blind, Placebo-Controlled Study of Encenicline, an alpha7 Nicotinic Acetylcholine Receptor Agonist, as a Treatment for Cognitive Impairment in Schizophrenia. Neuropsychopharmacology 40, 3053–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Mintz J, Seidman LJ, Stover E, Marder SR, 2008. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry 165, 214–220. [DOI] [PubMed] [Google Scholar]
- Kilonzo VW, Sweet RA, Glausier JR, Pitts MW, 2020. Deficits in Glutamic Acid Decarboxylase 67 Immunoreactivity, Parvalbumin Interneurons, and Perineuronal Nets in the Inferior Colliculus of Subjects With Schizophrenia. Schizophr Bull [DOI] [PMC free article] [PubMed]
- Kim J, Iwata Y, Plitman E, Caravaggio F, Chung JK, Shah P, Blumberger DM, Pollock BG, Remington G, Graff-Guerrero A, Gerretsen P, 2019. A meta-analysis of transcranial direct current stimulation for schizophrenia: "Is more better?". Journal of psychiatric research 110, 117–126. [DOI] [PubMed] [Google Scholar]
- Kirihara K, Tada M, Koshiyama D, Fujioka M, Usui K, Araki T, Kasai K, 2020. A Predictive Coding Perspective on Mismatch Negativity Impairment in Schizophrenia. Front Psychiatry 11, 660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort NS, Ford JM, Roach BJ, Gunduz-Bruce H, Krystal JH, Jaeger J, Reinhart RM, Mathalon DH, 2017. Role of N-Methyl-D-Aspartate Receptors in Action-Based Predictive Coding Deficits in Schizophrenia. Biol Psychiatry 81, 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama D, Miyakoshi M, Thomas ML, Joshi YB, Molina JL, Tanaka-Koshiyama K, Sprock J, Braff DL, Swerdlow NR, Light GA, 2021a. Unique contributions of sensory discrimination and gamma synchronization deficits to cognitive, clinical, and psychosocial functional impairments in schizophrenia. Schizophr Res 228, 280–287. [DOI] [PubMed] [Google Scholar]
- Koshiyama D, Thomas ML, Miyakoshi M, Joshi YB, Molina JL, Tanaka-Koshiyama K, Sprock J, Braff DL, Swerdlow NR, Light GA, 2021b. Hierarchical Pathways from Sensory Processing to Cognitive, Clinical, and Functional Impairments in Schizophrenia. Schizophr Bull 47, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E, 1907. Clinical Psychiatry Macmillan, New York. [Google Scholar]
- Kraus MS, Walker TM, Jarskog LF, Millet RA, Keefe RSE, 2019. Basic auditory processing deficits and their association with auditory emotion recognition in schizophrenia. Schizophr Res 204, 155–161. [DOI] [PubMed] [Google Scholar]
- Kraus MS, Walker TM, Perkins D, Keefe RSE, 2022. Basic auditory processing and emotion recognition in individuals at clinical high risk for psychosis. Schizophr Res Cogn 27, 100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitschmann-Andermahr I, Rosburg T, Meier T, Volz HP, Nowak H, Sauer H, 1999. Impaired sensory processing in male patients with schizophrenia: a magnetoencephalographic study of auditory mismatch detection. Schizophr Res 35, 121–129. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW, 2002. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage 17, 1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler BT, Caudrey DJ, 1983. Phoneme discrimination in schizophrenia. Br J Psychiatry 142, 53–59. [DOI] [PubMed] [Google Scholar]
- Kujala T, Leminen M, 2017. Low-level neural auditory discrimination dysfunctions in specific language impairment-A review on mismatch negativity findings. Dev Cogn Neurosci 28, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Topolnik L, 2021. Cortical disinhibitory circuits: cell types, connectivity and function. Trends Neurosci 44, 643–657. [DOI] [PubMed] [Google Scholar]
- Kwon JS, O'Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW, 1999. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry 56, 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, O'Connell MN, Barczak A, McGinnis T, Neymotin S, Schroeder CE, Smiley JF, Javitt DC, 2020. The Thalamocortical Circuit of Auditory Mismatch Negativity. Biol Psychiatry 87, 770–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Schroeder CE, Leitman DI, Javitt DC, 2013. Predictive suppression of cortical excitability and its deficit in schizophrenia. J Neurosci 33, 11692–11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, Bovet P, Bush AI, Conus P, Copolov D, Fornari E, Meuli R, Solida A, Vianin P, Cuenod M, Buclin T, Do KQ, 2008. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology 33, 2187–2199. [DOI] [PubMed] [Google Scholar]
- Lee M, Balla A, Sershen H, Sehatpour P, Lakatos P, Javitt DC, 2018a. Rodent Mismatch Negativity/theta Neuro-Oscillatory Response as a Translational Neurophysiological Biomarker for N-Methyl-D-Aspartate Receptor-Based New Treatment Development in Schizophrenia. Neuropsychopharmacology 43, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Sehatpour P, Dias EC, Silipo GS, Kantrowitz JT, Martinez AM, Javitt DC, 2018b. A tale of two sites: Differential impairment of frequency and duration mismatch negativity across a primarily inpatient versus a primarily outpatient site in schizophrenia. Schizophr Res 191, 10–17. [DOI] [PubMed] [Google Scholar]
- Lee M, Sehatpour P, Hoptman MJ, Lakatos P, Dias EC, Kantrowitz JT, Martinez AM, Javitt DC, 2017. Neural mechanisms of mismatch negativity dysfunction in schizophrenia. Mol Psychiatry 22, 1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC, 2005. Sensory contributions to impaired prosodic processing in schizophrenia. Biol Psychiatry 58, 56–61. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Laukka P, Juslin PN, Saccente E, Butler P, Javitt DC, 2010a. Getting the cue: sensory contributions to auditory emotion recognition impairments in schizophrenia. Schizophr Bull 36, 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G, Javitt DC, 2010b. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. Am J Psychiatry 167, 818–827. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Wolf DH, Laukka P, Ragland JD, Valdez JN, Turetsky BI, Gur RE, Gur RC, 2011. Not pitch perfect: sensory contributions to affective communication impairment in schizophrenia. Biol Psychiatry 70, 611–618. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Ziwich R, Pasternak R, Javitt DC, 2006. Theory of Mind (ToM) and counterfactuality deficits in schizophrenia: misperception or misinterpretation? Psychol Med 36, 1075–1083. [DOI] [PubMed] [Google Scholar]
- Lepock JR, Mizrahi R, Korostil M, Bagby RM, Pang EW, Kiang M, 2018. Event-Related Potentials in the Clinical High-Risk (CHR) State for Psychosis: A Systematic Review. Clin EEG Neurosci 49, 215–225. [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Rissling AJ, Radant A, Sugar CA, Sprock J, Pela M, Geyer MA, Braff DL, 2012. Characterization of neurophysiologic and neurocognitive biomarkers for use in genomic and clinical outcome studies of schizophrenia. PLoS ONE 7, e39434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Zhang W, Joshi YB, Bhakta S, Talledo JA, Swerdlow NR, 2017. Single-Dose Memantine Improves Cortical Oscillatory Response Dynamics in Patients with Schizophrenia. Neuropsychopharmacology 42, 2633–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald ML, Garver M, Newman J, Sun Z, Kannarkat J, Salisbury R, Glausier J, Ding Y, Lewis DA, Yates N, Sweet RA, 2020. Synaptic Proteome Alterations in the Primary Auditory Cortex of Individuals With Schizophrenia. JAMA psychiatry 77, 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LF, Davalos DB, Kisley MA, 2009. Nicotine enhances automatic temporal processing as measured by the mismatch negativity waveform. Nicotine Tob Res 11, 698–706. [DOI] [PubMed] [Google Scholar]
- Martin LF, Freedman R, 2007. Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol 78, 225–246. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Ahn KH, Perry EB Jr., Cho HS, Roach BJ, Blais RK, Bhakta S, Ranganathan M, Ford JM, D'Souza DC, 2014. Effects of nicotine on the neurophysiological and behavioral effects of ketamine in humans. Front Psychiatry 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H, 2004. Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N, Todd J, Mannion DJ, Finnigan S, Catts S, Michie PT, 2013. Impaired processing of binaural temporal cues to auditory scene analysis in schizophrenia. Schizophr Res 146, 344–348. [DOI] [PubMed] [Google Scholar]
- May PJC, 2021. The Adaptation Model Offers a Challenge for the Predictive Coding Account of Mismatch Negativity. Front Hum Neurosci 15, 721574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW, Salisbury DF, Hirayasu Y, Yurgelun-Todd DA, Tohen M, Zarate C, Kikinis R, Jolesz FA, Shenton ME, 2002. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch Gen Psychiatry 59, 321–331. [DOI] [PubMed] [Google Scholar]
- McKechanie AG, Moorhead TW, Stanfield AC, Whalley HC, Johnstone EC, Lawrie SM, Owens DG, 2016. Negative symptoms and longitudinal grey matter tissue loss in adolescents at risk of psychosis: preliminary findings from a 6-year follow-up study. Br J Psychiatry 208, 565–570. [DOI] [PubMed] [Google Scholar]
- McKinney BC, MacDonald ML, Newman JT, Shelton MA, DeGiosio RA, Kelly RM, Fish KN, Sampson AR, Lewis DA, Sweet RA, 2019. Density of small dendritic spines and microtubule-associated-protein-2 immunoreactivity in the primary auditory cortex of subjects with schizophrenia. Neuropsychopharmacology 44, 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia A, Saperstein AM, Qian M, Javitt DC, 2019. Impact of baseline early auditory processing on response to cognitive remediation for schizophrenia. Schizophr Res [DOI] [PMC free article] [PubMed]
- Metzner C, Zurowski B, Steuber V, 2019. The Role of Parvalbumin-positive Interneurons in Auditory Steady-State Response Deficits in Schizophrenia. Sci Rep 9, 18525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi L, Wang L, Li X, She S, Li H, Huang H, Zhang J, Liu Y, Zhao J, Ning Y, Zheng Y, 2021. Reduction of phonetic mismatch negativity may depict illness course and predict functional outcomes in schizophrenia. Journal of psychiatric research 137, 290–297. [DOI] [PubMed] [Google Scholar]
- Molholm S, Sehatpour P, Mehta AD, Shpaner M, Gomez-Ramirez M, Ortigue S, Dyke JP, Schwartz TH, Foxe JJ, 2006. Audio-visual multisensory integration in superior parietal lobule revealed by human intracranial recordings. Journal of neurophysiology 96, 721–729. [DOI] [PubMed] [Google Scholar]
- Molina JL, Voytek B, Thomas ML, Joshi YB, Bhakta SG, Talledo JA, Swerdlow NR, Light GA, 2020. Memantine Effects on Electroencephalographic Measures of Putative Excitatory/Inhibitory Balance in Schizophrenia. Biol Psychiatry Cogn Neurosci Neuroimaging 5, 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondino M, Fonteneau C, Simon L, Donde C, Haesebaert F, Poulet E, Brunelin J, 2021. Advancing clinical response characterization to frontotemporal transcranial direct current stimulation with electric field distribution in patients with schizophrenia and auditory hallucinations: a pilot study. Eur Arch Psychiatry Clin Neurosci 271, 85–92. [DOI] [PubMed] [Google Scholar]
- Morillon B, Arnal LH, Schroeder CE, Keitel A, 2019. Prominence of delta oscillatory rhythms in the motor cortex and their relevance for auditory and speech perception. Neurosci Biobehav Rev 107, 136–142. [DOI] [PubMed] [Google Scholar]
- Mulert C, Kirsch V, Pascual-Marqui R, McCarley RW, Spencer KM, 2011. Long-range synchrony of gamma oscillations and auditory hallucination symptoms in schizophrenia. Int J Psychophysiol 79, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy N, Ramakrishnan N, Walker CP, Polizzotto NR, Cho RY, 2020. Intact Auditory Cortical Cross-Frequency Coupling in Early and Chronic Schizophrenia. Front Psychiatry 11, 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CH, Tare I, Perry CM, Malina M, Lee R, de Wit H, 2021. Low doses of LSD reduce broadband oscillatory power and modulate event-related potentials in healthy adults. Psychopharmacology (Berl) [DOI] [PMC free article] [PubMed]
- Naatanen R, Gaillard AW, Mantysalo S, 1978. Early selective-attention effect on evoked potential reinterpreted. Acta. Psychol. (Amst) 42, 313–329. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR, 2008. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 165, 203–213. [DOI] [PubMed] [Google Scholar]
- O'Donnell P, Dong C, Murty VP, Asgharnejad M, Du X, Summerfelt A, Wendland JR, Dunayevich E, Litman R, Hetrick W, Hong LE, Rosen LB, 2021. Luvadaxistat, a D-amino acid oxidase inhibitor, improves mismatch negativity in patients with schizophrenia, ACNP 2021 Annual Meeting, San Juan, Puerto Rico [DOI] [PMC free article] [PubMed]
- Olsson O, Nielzen S, 1999. Contralateral induction by frequency spectrum in hallucinating schizophrenics. Psychiatry Res 87, 65–75. [DOI] [PubMed] [Google Scholar]
- Onitsuka T, Tsuchimoto R, Oribe N, Spencer KM, Hirano Y, 2022. Neuronal imbalance of excitation and inhibition in schizophrenia: a scoping review of gamma-band ASSR findings. Psychiatry Clin Neurosci [DOI] [PubMed]
- Opitz B, Rinne T, Mecklinger A, von Cramon DY, Schroger E, 2002. Differential contribution of frontal and temporal cortices to auditory change detection: fMRI and ERP results. Neuroimage 15, 167–174. [DOI] [PubMed] [Google Scholar]
- Parciauskaite V, Bjekic J, Griskova-Bulanova I, 2021. Gamma-Range Auditory Steady-State Responses and Cognitive Performance: A Systematic Review. Brain Sci 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DK, Stein IS, Zito K, 2022. Ion flux-independent NMDA receptor signaling. Neuropharmacology 210, 109019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RD, 1990. The tone height of multiharmonic sounds, Music Perception 8, 203–214. [Google Scholar]
- Perez-Rando M, Elvira UKA, Garcia-Marti G, Gadea M, Aguilar EJ, Escarti MJ, Ahullo-Fuster MA, Grasa E, Corripio I, Sanjuan J, Nacher J, 2022. Alterations in the volume of thalamic nuclei in patients with schizophrenia and persistent auditory hallucinations. Neuroimage Clin 35, 103070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez VB, Woods SW, Roach BJ, Ford JM, McGlashan TH, Srihari VH, Mathalon DH, 2014. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol Psychiatry 75, 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin MA, Butler PD, DiCostanzo J, Forchelli G, Silipo G, Javitt DC, 2010. Spatial localization deficits and auditory cortical dysfunction in schizophrenia. Schizophr Res 124, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin MA, Kantrowitz JT, Silipo G, Dias E, Jabado O, Javitt DC, 2018. Mismatch negativity (MMN) to spatial deviants and behavioral spatial discrimination ability in the etiology of auditory verbal hallucinations and thought disorder in schizophrenia. Schizophr Res 191, 140–147. [DOI] [PubMed] [Google Scholar]
- Poeppel D, Overath T, Popper AN, Fay RR, 2012. The Human Auditory Cortex. Springer Science & Business Media
- Polich J, 2007. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol 118, 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH, Gawryl M, Dgetluck N, Palfreyman M, Bauer LO, Hilt DC, 2014. Normalizing effects of EVP-6124, an alpha-7 nicotinic partial agonist, on event-related potentials and cognition: a proof of concept, randomized trial in patients with schizophrenia. J Psychiatr Pract 20, 12–24. [DOI] [PubMed] [Google Scholar]
- Rabinowicz EF, Silipo G, Goldman R, Javitt DC, 2000. Auditory sensory dysfunction in schizophrenia: imprecision or distractibility? Arch Gen Psychiatry 57, 1149–1155. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Gogtay N, 2012. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry 17, 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revheim N, Butler PD, Schechter I, Jalbrzikowski M, Silipo G, Javitt DC, 2006. Reading impairment and visual processing deficits in schizophrenia. Schizophr Res 87, 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revheim N, Corcoran CM, Dias E, Hellmann E, Martinez A, Butler PD, Lehrfeld JM, DiCostanzo J, Albert J, Javitt DC, 2014. Reading deficits in schizophrenia and individuals at high clinical risk: relationship to sensory function, course of illness, and psychosocial outcome. Am J Psychiatry 171, 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedemann T, 2019. Diversity and Function of Somatostatin-Expressing Interneurons in the Cerebral Cortex. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosburg T, Kreitschmann-Andermahr I, 2016. The effects of ketamine on the mismatch negativity (MMN) in humans - A meta-analysis. Clin Neurophysiol 127, 1387–1394. [DOI] [PubMed] [Google Scholar]
- Rosenbrock H, Dorner-Ciossek C, Giovannini R, Schmid B, Schuelert N, 2022. Effects of the Glycine Transporter-1 Inhibitor Iclepertin (BI 425809) on Sensory Processing, Neural Network Function, and Cognition in Animal Models Related to Schizophrenia. J Pharmacol Exp Ther 382, 223–232. [DOI] [PubMed] [Google Scholar]
- Ross JM, Hamm JP, 2020. Cortical Microcircuit Mechanisms of Mismatch Negativity and Its Underlying Subcomponents. Front Neural Circuits 14, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth WT, Cannon EH, 1972. Some features of the auditory evoked response in schizophrenics. Arch Gen Psychiatry 27, 466–471. [DOI] [PubMed] [Google Scholar]
- Rudolph ED, Ells EM, Campbell DJ, Abriel SC, Tibbo PG, Salisbury DF, Fisher DJ, 2015. Finding the missing-stimulus mismatch negativity (MMN) in early psychosis: altered MMN to violations of an auditory gestalt. Schizophr Res 166, 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Coffman BA, Haigh SM, 2020a. Reductions in Complex Mismatch Negativity to Extra Tone Gestalt Pattern Deviance in First-Episode Schizophrenia. Front Psychiatry 11, 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Collins KC, McCarley RW, 2010. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Schizophr Bull 36, 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, McCathern AG, 2016. Abnormal Complex Auditory Pattern Analysis in Schizophrenia Reflected in an Absent Missing Stimulus Mismatch Negativity. Brain Topogr 29, 867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Shafer AR, Murphy TK, Haigh SM, Coffman BA, 2020b. Pitch and Duration Mismatch Negativity and Heschl's Gyrus Volume in First-Episode Schizophrenia-Spectrum Individuals. Clin EEG Neurosci 51, 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Diaconescu AO, Kometer M, Friston KJ, Stephan KE, Vollenweider FX, 2013. Modeling ketamine effects on synaptic plasticity during the mismatch negativity. Cerebral cortex (New York, N.Y. : 1991) 23, 2394–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonwiesner M, Novitski N, Pakarinen S, Carlson S, Tervaniemi M, Naatanen R, 2007. Heschl's gyrus, posterior superior temporal gyrus, and mid-ventrolateral prefrontal cortex have different roles in the detection of acoustic changes. Journal of neurophysiology 97, 2075–2082. [DOI] [PubMed] [Google Scholar]
- Schuelert N, Dorner-Ciossek C, Brendel M, Rosenbrock H, 2018. A comprehensive analysis of auditory event-related potentials and network oscillations in an NMDA receptor antagonist mouse model using a novel wireless recording technology. Physiol Rep 6, e13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertner A, Zortea M, Torres FV, Caumo W, 2018. Effects of Subanesthetic Ketamine Administration on Visual and Auditory Event-Related Potentials (ERP) in Humans: A Systematic Review. Front Behav Neurosci 12, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoriels L, Genaro LT, Mororo LGC, Keffer S, Guimaraes A, Ribeiro PVS, Tannos FM, Novaes C, Franca AI, Goldenstein N, Sahakian BJ, Cavalcanti MT, Fisher M, Vinogradov S, Panizzutti R, 2020. Auditory versus visual neuroscience-informed cognitive training in schizophrenia: Effects on cognition, symptoms and quality of life. Schizophr Res 222, 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehatpour P, Avissar M, Kantrowitz JT, Corcoran CM, De Baun HM, Patel GH, Girgis RR, Brucato G, Lopez-Calderon J, Silipo G, Dias E, Martinez A, Javitt DC, 2020. Deficits in Preattentive Processing of Spatial Location and Negative Symptoms in Subjects at Clinical High Risk for Schizophrenia. Front Psychiatry 11, 629144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehatpour P, Donde C, Adair D, Kreither J, Lopez-Calderon J, Avissar M, Bikson M, Javitt DC, 2021. Comparison of cortical network effects of high-definition and conventional tDCS during visuomotor processing. Brain Stimul 14, 33–35. [DOI] [PubMed] [Google Scholar]
- Sehatpour P, Iosifescu DV, de Baun HMS,C, Mayer M, Gangwisch J, Dias E, Sobeih T, Choo TH, Wall MW, Medalia A, Saperstein AM, Kegeles LS, Girgis RR, Carlson M, Kantrowitz JT, in revision. Dose dependent augmentation of neuroplasticity-based auditory learning in schizophrenia: a double-blind, placebo-controlled, randomized, target engagement clinical trial of the N-methyl-D-aspartate-type glutamate receptor (NMDAR) agonist D-serine. Biol. Psychiatry in revision [DOI] [PMC free article] [PubMed]
- Sehatpour P, Javitt DC, De Baun HM, Carlson M, Beloborodova A, Margolin DH, Carlton MBL, Brice NL, Kantrowitz JT, 2022. Mismatch negativity as an index of target engagement for excitation/inhibition-based treatment development: a double-blind, placebo-controlled, randomized, single-dose cross-over study of the serotonin type-3 receptor antagonist CVN058. Neuropsychopharmacology 47, 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkowski D, Moran JK, 2022. Early evoked brain activity underlies auditory and audiovisual speech recognition deficits in schizophrenia. Neuroimage Clin 33, 102909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N, 1991. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry 30, 1059–1062. [DOI] [PubMed] [Google Scholar]
- Smiley JF, McGinnis JP, Javitt DC, 2000. Nitric oxide synthase interneurons in the monkey cerebral cortex are subsets of the somatostatin, neuropeptide Y, and calbindin cells. Brain Res 863, 205–212. [DOI] [PubMed] [Google Scholar]
- Smith DM, Fisher D, Blier P, Ilivitsky V, Knott V, 2015. The separate and combined effects of monoamine oxidase A inhibition and nicotine on the mismatch negativity event related potential. Pharmacol Biochem Behav 137, 44–52. [DOI] [PubMed] [Google Scholar]
- Smith M, Saperstein A, Meyler s., Styke S, Medalia A, 2021. Feasibility and clinical utility of early auditory processing assessment for CR personalization in community mental health settings, The 24th Annual Cognitive Remediation in Psychiatry Conference, New York , NY. [Google Scholar]
- Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW, 2009. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC neuroscience 10, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart L, Overath T, Warren JD, Foxton JM, Griffiths TD, 2008. fMRI evidence for a cortical hierarchy of pitch pattern processing. PLoS One 3, e1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous RD, Cowan N, Ritter W, Javitt DC, 1995. Auditory sensory ("echoic") memory dysfunction in schizophrenia. Am J Psychiatry 152, 1517–1519. [DOI] [PubMed] [Google Scholar]
- Studer F, Barkat TR, 2021. Inhibition in the auditory cortex. Neurosci Biobehav Rev 132, 61–75. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA, 2009. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology 34, 374–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Bhakta S, Chou HH, Talledo JA, Balvaneda B, Light GA, 2016. Memantine Effects On Sensorimotor Gating and Mismatch Negativity in Patients with Chronic Psychosis. Neuropsychopharmacology 41, 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Bhakta SG, Talledo J, Kotz J, Roberts BZ, Clifford RE, Thomas ML, Joshi YB, Molina JL, Light GA, 2020. Memantine effects on auditory discrimination and training in schizophrenia patients. Neuropsychopharmacology 45, 2180–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Kirihara K, Ishishita Y, Takasago M, Kunii N, Uka T, Shimada S, Ibayashi K, Kawai K, Saito N, Koshiyama D, Fujioka M, Araki T, Kasai K, 2021. Global and Parallel Cortical Processing Based on Auditory Gamma Oscillatory Responses in Humans. Cerebral cortex (New York, N.Y. : 1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Kirihara K, Mizutani S, Uka T, Kunii N, Koshiyama D, Fujioka M, Usui K, Nagai T, Araki T, Kasai K, 2019. Mismatch negativity (MMN) as a tool for translational investigations into early psychosis: A review. Int J Psychophysiol 145, 5–14. [DOI] [PubMed] [Google Scholar]
- Teichert T, 2017. Loudness- and time-dependence of auditory evoked potentials is blunted by the NMDA channel blocker MK-801. Psychiatry Res 256, 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ML, Green MF, Hellemann G, Sugar CA, Tarasenko M, Calkins ME, Greenwood TA, Gur RE, Gur RC, Lazzeroni LC, Nuechterlein KH, Radant AD, Seidman LJ, Shiluk AL, Siever LJ, Silverman JM, Sprock J, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL, Light GA, 2017. Modeling Deficits From Early Auditory Information Processing to Psychosocial Functioning in Schizophrenia. JAMA psychiatry 74, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thune H, Recasens M, Uhlhaas PJ, 2016. The 40-Hz Auditory Steady-State Response in Patients With Schizophrenia: A Meta-analysis. JAMA psychiatry 73, 1145–1153. [DOI] [PubMed] [Google Scholar]
- Todd J, Michie PT, Schall U, Karayanidis F, Yabe H, Naatanen R, 2008. Deviant matters: duration, frequency, and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Biol Psychiatry 63, 58–64. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Wylie KP, 2019. Alpha7 Nicotinic Receptors as Therapeutic Targets in Schizophrenia. Nicotine Tob Res 21, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trehub SE, Cohen AJ, Thorpe LA, Morrongiello BA, 1986. Development of the perception of musical relations: semitone and diatonic structure. J Exp Psychol Hum Percept Perform 12, 295–301. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Krljes S, 2005. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res 76, 1–23. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC, 2000. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry 57, 1139–1147. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Vollenweider FX, Schmid L, Grubel C, Skrabo A, Huber T, Koller R, 2003. Effects of the 5-HT2A agonist psilocybin on mismatch negativity generation and AX-continuous performance task: implications for the neuropharmacology of cognitive deficits in schizophrenia. Neuropsychopharmacology 28, 170–181. [DOI] [PubMed] [Google Scholar]
- Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC, 2006. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol Psychiatry 59, 762–772. [DOI] [PubMed] [Google Scholar]
- Van Derveer AB, Bastos G, Ferrell AD, Gallimore CG, Greene ML, Holmes JT, Kubricka V, Ross JM, Hamm JP, 2021. A Role for Somatostatin-Positive Interneurons in Neuro-Oscillatory and Information Processing Deficits in Schizophrenia. Schizophr Bull 47, 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM, 2011. Synaptic properties of thalamic input to the subgranular layers of primary somatosensory and auditory cortices in the mouse. J Neurosci 31, 12738–12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, de Villers-Sidani E, 2012. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology 37, 43–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacongne C, 2016. A predictive coding account of MMN reduction in schizophrenia. Biol Psychol 116, 68–74. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA, 1999. CTOPP: Comprehensive Test of Phonological Processing (Examiner's Manual.). Pro-Ed, Austin, TX. [Google Scholar]
- Wang B, Zartaloudi E, Linden JF, Bramon E, 2022. Neurophysiology in psychosis: The quest for disease biomarkers. Transl Psychiatry 12, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JD, Griffiths TD, 2003. Distinct mechanisms for processing spatial sequences and pitch sequences in the human auditory brain. J Neurosci 23, 5799–5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible CG, Kubicki M, Yoo SS, Kacher DF, Salisbury DF, Anderson MC, Shenton ME, Hirayasu Y, Kikinis R, Jolesz FA, McCarley RW, 2001. A functional magnetic resonance imaging study of auditory mismatch in schizophrenia. Am J Psychiatry 158, 938–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I, Czigler I, 1998. Mismatch negativity: deviance detection or the maintenance of the 'standard'. Neuroreport 9, 3809–3813. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Valiante TA, Sahin NT, Miller KJ, Tiesinga P, 2014. Dynamic circuit motifs underlying rhythmic gain control, gating and integration. Nat Neurosci 17, 1031–1039. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Ll X, Zhao L, Wang C, 2017. Mismatch Negativity in Han Chinese Patients with Schizophrenia: A Meta-Analysis. Shanghai Arch Psychiatry 29, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong YB, Bo QJ, Wang CM, Tian Q, Liu Y, Wang CY, 2019. Differential of Frequency and Duration Mismatch Negativity and Theta Power Deficits in First-Episode and Chronic Schizophrenia. Front Behav Neurosci 13, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Chen S, Chen CM, Khan F, Forchelli G, Javitt DC, 2012. Schizophrenia, culture and neuropsychology: sensory deficits, language impairments and social functioning in Chinese-speaking schizophrenia patients. Psychol Med 42, 1485–1494. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL, 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y, Nguyen M, Hasson U, 2021. The default mode network: where the idiosyncratic self meets the shared social world. Nat Rev Neurosci 22, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Samson S, 1991. Role of the right temporal neocortex in retention of pitch in auditory short-term memory. Brain 114 ( Pt 6), 2403–2417. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xiong BR, Zhang LQ, Huang X, Yuan X, Tian YK, Tian XB, 2021. The Role of the GABAergic System in Diseases of the Central Nervous System. Neuroscience 470, 88–99. [DOI] [PubMed] [Google Scholar]


