Introduction
Porokeratosis is a heritable disorder of atypical clonal expansion of keratinocytes and is defined by the presence of cornoid lamella on histopathology.1 Some of the clinical variants include disseminated superficial actinic porokeratosis (DSAP), disseminated superficial porokeratosis, porokeratosis palmaris et plantaris disseminate, linear porokeratosis, porokeratosis of Mibelli, and punctate porokeratosis. Punctate porokeratosis is a rare, localized subtype of porokeratosis appearing as tender seed-like papules on the palms and soles.2 Although its etiology is unknown, it is not considered a premalignant condition, unlike other variants of porokeratosis, such as linear, disseminated superficial porokeratosis, and giant porokeratosis.3 Lesions are usually destroyed for cosmetic reasons and include treatment options, such as CO2 lasers, 5-fluorouracil, topical imiquimod, photodynamic therapy, topical corticosteroids, topical vitamin D analogs, and systemic and topical retinoids.3, 4, 5 However, current approaches are often ineffective at eradication.6 A newer treatment approach using topical lovastatin/cholesterol to target mevalonate pathway deficiencies in some variants of porokeratosis has been reported with successful outcomes. This article describes a case of punctate porokeratosis in an adult woman treated with lovastatin/cholesterol ointment as an alternative treatment for this uncommon subtype of porokeratosis.
Case report
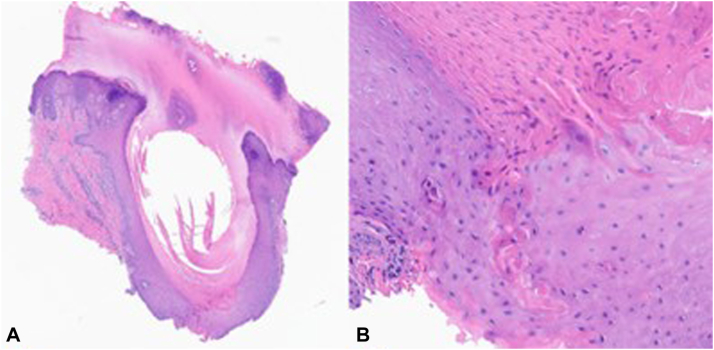
The patient is a 69-year-old woman who presented to the dermatology clinic with numerous scattered 1- to 2-mm round hyperkeratotic papules on the soles of her feet bilaterally, which were painful to the touch. Her first lesion appeared at 26 years of age, with subsequent papules of varying sizes appearing several years later. These lesions only appeared on her feet and required excision every 6 days (Fig 1, A) by the patient with cuticle scissors because of their thickness causing irritation when walking. Her family history was notable for 2 biologic sisters and 1 nephew with similar, but less severe cutaneous findings. Skin biopsy revealed epidermal indentation with disruption of the granular layer by a column of parakeratosis with underlying dyskeratosis (Fig 2, B), which supported the diagnosis of punctate porokeratosis.
Fig 1.
Plantar lesions (A) baseline lesion growth before treatment, requiring excision every 6 days because of patient discomfort. The shadowing does not represent a pigmented (B) photograph showing punctate porokeratosis lesions during a clinic visit after 22 months of treatment. Although the lesions have not resolved and do not differ compared with the initial outward appearance, growth has slowed, and excisions occur every 21 days.
Fig 2.
Punch biopsy of a punctate porokeratosis lesion on the left foot. A, The biopsy shows hyperkeratosis with epidermal indentation. (Hematoxylin-eosin stain; original magnification: ×10.) B, There is a parakeratotic column of cornoid lamella underlying a vertical zone of dyskeratotic cells within the epidermis. (Hematoxylin-eosin stain; original magnification: ×100.)
The initial recommended treatment was a nightly application of topical salicylic acid to the papules. The patient also applied 5% lidocaine cream on her feet because of pain. Because this combination did not ameliorate her symptoms, she began treatment with a compounded topical lovastatin/cholesterol (2% cholesterol/2% lovastatin ointment twice daily) in April 2021 because of its documented safety profile and from previous case reports indicating success in other subtypes of porokeratosis.6 She noted a significant delay in the growth and thickness of the papules base after 19 months of treatment, now requiring lesion excision every 21 days (Fig 1, B) rather than every 6 days. In addition, the lesions gradually became less firm, were easier to excise, and were not as painful when ambulating. The treatment was well-tolerated, and there were no reported adverse effects.
Discussions
Heterozygous germline mutations involving the mevalonate metabolic pathway have recently been implicated in some variants of porokeratosis and may contribute to the condition through decreased amounts of cholesterol, an end-product of the mevalonate pathway and an important component of the skin barrier.6,7 In addition, reduced enzymatic activity within this pathway may also contribute to the pathogenesis of porokeratosis through toxic metabolite accumulation, elevated apoptosis activity, and dysmaturation of keratinocytes.6,8,9 Several cases of combined topical lovastatin/cholesterol treatment targeting mevalonate pathway dysfunction have been reported in the literature with good outcomes for various subtypes of porokeratosis, including DSAP, porokeratosis palmaris et plantaris disseminate, and linear porokeratosis.6,9,10 For example, one study noted almost complete clearance of lesions in the DSAP variant after 4 weeks of treatment, with moderate improvement in the porokeratosis palmaris et plantaris disseminate, and linear porokeratosis subtypes.6
To our knowledge, this is the first report of topical lovastatin/cholesterol as a treatment for this rare subtype of porokeratosis. Although the combined topical lovastatin/cholesterol did not lead to complete resolution of the patient’s lesions as noted in other variants of porokeratosis, treatment has steadily reduced the plantar papules' thickness and growth, improving the patient’s quality of life. However, treatment did require 19 months before a significant halt in growth was noted, whereas subtypes, such as DSAP showed dramatic results after 4 weeks.6 Overall, although this pathogenesis-directed treatment may be promising for steady lesion growth reduction with continued application, further studies are needed to fully examine the efficacy of this treatment for punctate porokeratosis.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Sertznig P., von Felbert V., Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26(4):404–412. doi: 10.1111/j.1468-3083.2011.04275.x. [DOI] [PubMed] [Google Scholar]
- 2.Jedlowski P.M., Rainwater G., Paek S.Y. Punctate porokeratosis-pruritic and hyperkeratotic papules on the palms and feet. Proc (Bayl Univ Med Cent) 2020;33(3):415–416. doi: 10.1080/08998280.2020.1755212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasson M., Krain A.D. Porokeratosis and cutaneous malignancy. A review. Dermatol Surg. 1996;22(4):339–342. doi: 10.1111/j.1524-4725.1996.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 4.Weidner T., Illing T., Miguel D., Elsner P. Treatment of porokeratosis: a systematic review. Am J Clin Dermatol. 2017;18(4):435–449. doi: 10.1007/s40257-017-0271-3. [DOI] [PubMed] [Google Scholar]
- 5.Das A., Vasudevan B., Talwar A. Porokeratosis: an enigma beginning to unravel. Indian J Dermatol Venereol Leprol. 2022;88(3):291–299. doi: 10.25259/IJDVL_806_20. [DOI] [PubMed] [Google Scholar]
- 6.Atzmony L., Lim Y.H., Hamilton C., et al. Topical cholesterol/lovastatin for the treatment of porokeratosis: a pathogenesis-directed therapy. J Am Acad Dermatol. 2020;82(1):123–131. doi: 10.1016/j.jaad.2019.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J., Liu Y., Liu F., et al. Loss-of-function mutation in PMVK causes autosomal dominant disseminated superficial porokeratosis. Sci Rep. 2016;6 doi: 10.1038/srep24226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calay D., Vind-Kezunovic D., Frankart A., Lambert S., Poumay Y., Gniadecki R. Inhibition of Akt signaling by exclusion from lipid rafts in normal and transformed epidermal keratinocytes. J Invest Dermatol. 2010;130(4):1136–1145. doi: 10.1038/jid.2009.415. [DOI] [PubMed] [Google Scholar]
- 9.Blue E., Abbott J., Bowen A., Cipriano S.D. Linear porokeratosis with bone abnormalities treated with compounded topical 2% cholesterol/2% lovastatin ointment. Pediatr Dermatol. 2021;38(1):242–245. doi: 10.1111/pde.14447. [DOI] [PubMed] [Google Scholar]
- 10.Diep D., Janitz T., Kannan K.S., et al. Bilateral linear porokeratosis treated with topical cholesterol 2%/lovastatin 2. Cureus. 2022;14(7) doi: 10.7759/cureus.27540. [DOI] [PMC free article] [PubMed] [Google Scholar]