Abstract
Background
SARS-CoV-2 invades mitochondria of infected cells resulting in disordered metabolism, mitophagy, and abnormal levels of mitochondrial proteins in extracellular vesicles. Blood extracellular vesicle SARS-CoV-2 proteins and mitochondrial proteins were quantified in COVID-19 to assess possible roles as biomarkers.
Methods
Total extracellular vesicles were precipitated from blood of age- and gender-matched participants with no infection (n=10), acute COVID-19 (n=16), post-acute sequelae of COVID-19 (PASC or long COVID) (n=30), or post-acute COVID without PASC (n=8) and their extracted proteins quantified by enzyme-linked immunosorbent assays (ELISAs).
Results
Total extracellular vesicle levels of S1 (receptor-binding domain [RBD]) protein were significantly higher in acute infections than in uninfected controls, post-acute infection without PASC, and PASC. Total extracellular vesicle levels of nucleocapsid (N) protein were significantly higher in PASC than in uninfected controls, acute infections, and post-acute infection without PASC. Neither acute levels of S1(RBD) or N proteins predicted progression to PASC. Levels of neither SARS-CoV-2 protein in established PASC correlated with neuropsychiatric manifestations. Significant decreases in total extracellular vesicle levels of the mitochondrial proteins MOTS-c, VDAC-1, and humanin, and elevations of levels of SARM-1 were observed in acutely infected patients who would develop PASC. Significant decreases in total extracellular vesicle levels of MOTS-c and humanin, but not VDAC-1, and elevations of total extracellular vesicle levels of SARM-1 were characteristic of PASC patients with neuropsychiatric manifestations.
Conclusions
Total extracellular vesicle levels of SARS-CoV-2 proteins in COVID-19 indicate intracellular presence of SARS-CoV-2. Abnormal total extracellular vesicles levels of mitochondrial proteins in acute infections predict a high risk of PASC and later in established PASC are indicative of neuropsychiatric manifestations.
Keywords: Long COVID, Mitochondria, Neuropsychiatric effects of COVID-19, SARS-CoV-2 proteins S1 and N
CLINICAL SIGNIFICANCE.
-
•
Intracellular SARS-CoV-2 proteins S1 (receptor-binding domain) and N (nucleoprotein) were detectable in blood extracellular vesicles in acute COVID-19 and long COVID.
-
•
Significantly abnormal levels of several functional mitochondrial proteins were observed in blood extracellular vesicles of acute COVID-19 patients who developed long COVID.
-
•
Persistence of intracellular SARS-CoV-2 in long COVID suggests that their treatment should include cell-permeant agents, such as anti-viral drugs and anti-SARS-CoV-2 nanobodies.
Alt-text: Unlabelled box
Introduction
A range of social isolation methods and acceptance of at least 1 full-course of highly effective vaccines by nearly 70% of the US population have strikingly decreased the prevalence of acute COVID-19.1 , 2 The incidence of severe pulmonary involvement in acute COVID-19 has decreased concurrently as impressively. Unfortunately, during the same time period, there has been a significant increase in prevalence of post-acute sequelae of COVID-19 (PASC) or long COVID, which results in diverse disabling manifestations in multiple organ systems.3 , 4 The incidence of PASC is unrelated to the nature or severity of preceding acute COVID-19 and is especially dangerous in the elderly, who suffer increased frequencies of myocardial infarction, strokes, and new diabetes mellitus in the year after a bout of acute COVID-19.5, 6, 7, 8, 9
Management of PASC is seriously compromised by an absence of laboratory tests that predict in acute infection the risk of subsequent PASC, the likely specific distribution of organ-system involvement, potential pathogenic mechanisms, or the benefit of anti-viral drug therapy. Comprehensive studies of blood immune cell types, cytokines, and metabolites, especially of kynurenic acid and tryptophan, have led to a predictive model that is being tested.10 However, many of these analytes are difficult and expensive to quantify and results do not consistently correlate with distinct symptom profiles.
In our initial investigations of possible COVID-19-relevant blood biomarkers, we focused on viral S1(receptor-binding domain [RBD]) and N (nucleoprotein) proteins, and functional mitochondrial proteins involved in cellular energy (adenosine triphosphate) generation, cytoprotection, redox balance, and immunity.11 Mitochondria are targeted for damage when cells are invaded by SARS-CoV-2 that results in decreased normal functions, disordered mitophagy, and abnormal levels of constituent proteins in extracellular vesicles.12 As we sought specific biomarkers for neuropsychiatric manifestations, plasma extracellular vesicles derived from neurons and astrocytes were isolated by sequential precipitation and immunoabsorption with antibodies specific for surface proteins of the respective cells of origin.13, 14, 15, 16 Neuron-derived extracellular vesicles and astrocyte-derived extracellular vesicle levels of S1(RBD) and N proteins were higher in PASC patients irrespective of neuropsychiatric manifestations than in uninfected controls and late post-acute subjects without PASC.11 In contrast, neuron-derived extracellular vesicle levels of mitochondrial electron chain proteins CI-6 and CIII-10, cytoprotective peptides humanin and MOTS-c, and several calcium channel proteins were decreased significantly in PASC with but not without neuropsychiatric manifestations.11 Application of this same plasma neuron-derived extracellular vesicle protein platform in acute COVID-19 showed that abnormal levels of some mitochondrial proteins, but not those of S1(RBD) and N proteins, have predictive value for risk of later development of PASC.17
The distinctive goals of the current studies were to use plasma total extracellular vesicles, prepared in a single step that can be completed in any certified clinical lab, to determine if simple kit-quantified levels of S1(RBD), N protein, and mitochondrial proteins previously shown to reflect different states of COVID-19 can distinguish between acute COVID-19 that will and will not proceed to PASC and separate PASC without from PASC with neuropsychiatric manifestations.
Methods
Recruitment and Evaluation of Participants
Subjects with nucleic acid assay-documented SARS-CoV-2 infection had been enrolled in 2 University of California San Francisco (UCSF)-based cohorts and donated plasma or serum that was stored in a UCSF Biobank: 1) those acutely infected were in a study of the natural history of SARS-CoV-2 infection (FindCOVID; findcovid19.org) (n=16) and 2) those in a post-acute phase were in a longitudinal cohort (Long-term Impact of Infection with Novel Coronavirus [LIINC], NCT04362150; www.liincstudy.org) (n=38, of whom 8 were post-acute controls without PASC). For these groups, which have been previously described,18 19 participant data and blood were collected between April 2020 and December 2021, when original strains were predominant. Blood from acute cohort participants was collected during the week after symptom onset, and that from post-acute cohort participants was collected monthly for 4 months. Controls for the acute and post-acute cohorts (n=10) were healthy adults matched to cohort participants by age and sex, and who donated blood for Biobank storage at least 3 years before the COVID-19 pandemic.
For both cohorts, pre-existing co-morbidities, current symptoms, and disabilities attributable to COVID-19 were documented at enrollment and between 4 and 6 months after enrollment. This was completed consistently using the Patient Health Questionnaire (PHQ-8), a broad questionnaire, and the General Anxiety Disorder questionnaire (GAD-7), which measures agitation, irritability, anxiety, and depression. Post-acute sequelae of COVID-19 manifestations were defined as symptoms of acute COVID-19 that persisted beyond 4 weeks from onset and those that first appeared later than 4 weeks beyond onset. All studies were reviewed and approved by the UCSF Institutional Review Board, and all participants provided written informed consent.
Six groups of participants were assembled for this study and are cited in each frame of Figures 1 and 2: uninfected controls (n=10), acutely infected who would proceed to PASC (n=8), acutely infected whose COVID-19 would resolve (n=8), post-acute phase without PASC (n=8), PASC with neuropsychiatric manifestations (n=15), and PASC without neuropsychiatric manifestations (n=15).
Isolation of Blood Total Extracellular Vesicles and Quantification of Their Cargo Proteins
Portions of 0.25 ml of plasma were incubated with thromboplastin D (ThermoFisher Scientific, Waltham, Mass), followed by addition of protease inhibitor cocktail (Roche, Indianapolis, Ind) and phosphatase inhibitor cocktail (ThermoFisher Scientific) as previously described.13, 14, 15, 16 After centrifugation at 3000xg for 30 min at 4°C, supernatants were transferred to new tubes and each received 126 uL of ExoQuick (System Biosciences, Mountainview, Calif), was incubated for 60 min at room temperature and centrifuged at 1500xg for 30 min at 4°C to pellet total extracellular vesicles. Total extracellular vesicle pellets were resuspended in 100 uL of Dulbecco's balanced salt solution, 10 uL was removed for extracellular vesicle counts, sizing and determination of exosome marker proteins as previously described,13 and then 400 uL of mammalian protein extraction reagent (M-PER, ThermoFisher Scientific) were added to each tube to solubilize proteins.
Solubilized total extracellular vesicle proteins were quantified by enzyme-linked immunosorbent assay (ELISA) kits for SARS-CoV-2 protein S1 (RBD), SARS-CoV-2 nucleoprotein N (Abcam, Inc, Waltham, Mass, and Raybiotech Life, Inc, Peachtree Corners, Ga), the tetraspanin exosome marker CD81, 16S mitochondrial rRNA-encoded cytoprotective humanin, subunit 6 of NADH-ubiquinone oxidoreductase (respiratory complex I) (CI-6) (CUSABIO, American Research Products [ARP], Waltham, Mass), mitochondrial open reading frame of the 12S rRNA-c (cytoprotective MOTS-c) (Cloud-Clone Corp, ARP), Sterile Alpha and TIR motif-containing protein 1 (NADH-ase SARM-1), leucine zipper EF-hand containing transmembrane 1 protein (LETM1), and voltage-dependent anion-selective channel protein 1 (VDAC1) (Wuhan FineTest Biotech Co, ARP).
Statistics
The mean value for all determinations of the exosome marker CD81 in each assay group was set at 1.00, and relative values of CD81 for each sample were used to normalize their recovery. The significance of differences between COVID-19 patient and control levels, and between patient group levels that were established to be normally distributed were calculated by an unpaired t test (Graphpad Holdings, LLC, San Diego, Calif). There were no adjustments for potential confounding variables.
Results
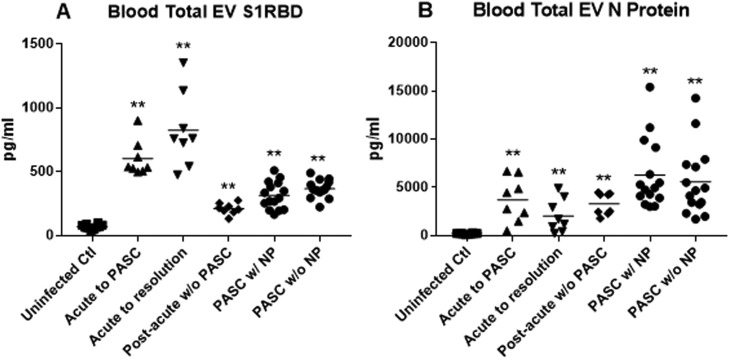
Extracts of blood total extracellular vesicles from patients in the first week of acute infection with SARS-CoV-2 had significantly higher levels of S1 (RBD) protein, irrespective of outcome than those of uninfected controls and patients with post-acute sequelae of COVID-19 (PASC) (Figure 1 A). Extracts of blood total extracellular vesicles from patients with PASC had significantly higher levels of N protein than their late course controls without PASC and patients with acute COVID-19 (Figure 1B). However, neither levels of S1(RBD) protein or nucleoprotein (N) reliably distinguished between patients with acute COVID-19 who would and would not develop PASC nor between patients with established PASC who had and did not have neuropsychiatric manifestations.
Figure 1.
Plasma total extracellular vesicle (EV) levels of SARS-CoV-2 proteins. (A) S1 (receptor-binding domain) protein and (B) N protein. Each point represents the CD81-normalized value for 1 participant, and the horizontal line in each cluster of points is the mean value for that group. “Uninfected Ctl” are the controls and statistical comparators for “Acute to PASC,” “Acute to resolution,” and “Post-acute w/o PASC” groups. “Post-acute w/o PASC” are the controls and statistical comparators for “PASC w/NP” and “PASC w/o NP groups". P values from unpaired t tests are *P < .01 and **P < .001.
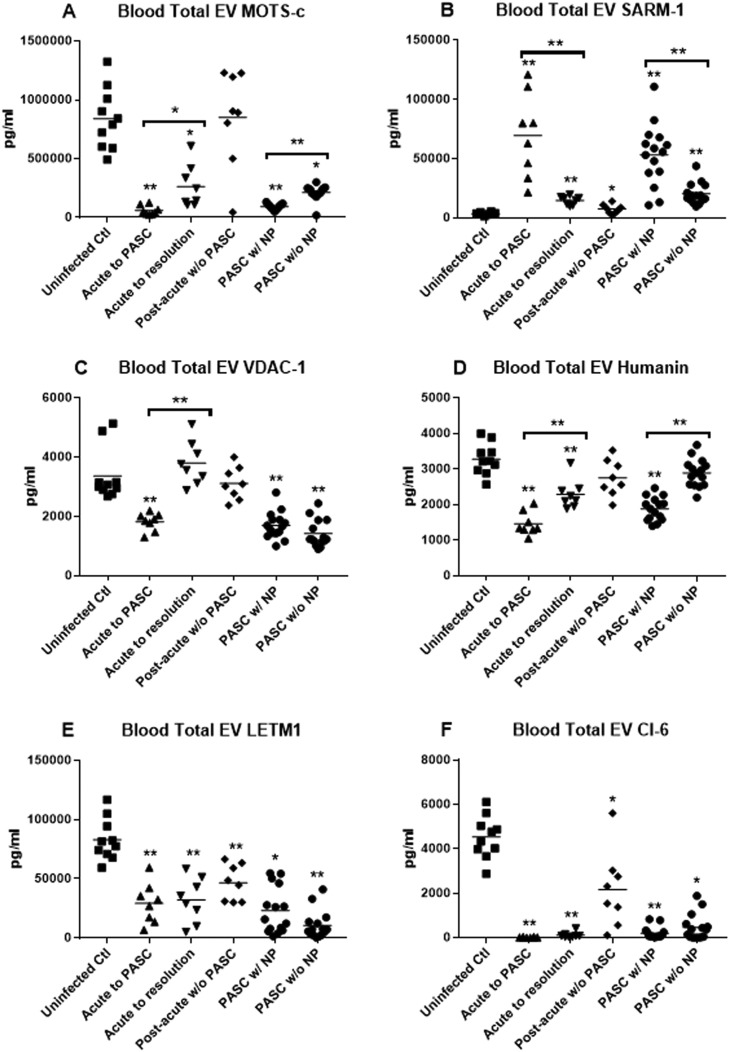
Blood total extracellular vesicle levels of several functionally relevant mitochondrial proteins that are altered when SARS-CoV-2 invades and damages cells11 identify those acutely infected who are at high risk of developing PASC and those with PASC who have resultant neuropsychiatric manifestations (Figure 2 ). Significant differential decreases in blood total extracellular vesicle levels of MOTS-c, VDAC-1, and humanin, and elevations of blood total extracellular vesicle levels of SARM-1 were observed for acutely infected patients who would proceed to PASC but not in those whose acute infection resolved without PASC (Figure 2A-D). Further, similarly significant differential decreases in blood total extracellular vesicle levels of MOTS-c and humanin, but not VDAC-1, and elevations of blood total extracellular vesicle levels of SARM-1 were characteristic of PASC patients with NP. In contrast, blood total extracellular vesicle levels of LETM1 and CI-6 were similarly decreased for both groups of acutely infected patients and both groups with established PASC relative to their respective controls and had no value in predicting risk of developing PASC after acute infection or identifying neuropsychiatric manifestations in those with established PASC (Figure 2E and F).
Figure 2.
Plasma total extracellular vesicle (EV) levels of mitochondrial proteins. (A) MOTS-c, (B) SARM-1, (C) VDAC-1, (D) humanin, (E) LETM-1, and (F) CI-6. Each point represents the CD81-normalized value for 1 participant, and the horizontal line in each cluster of points is the mean value for that group. “Uninfected Ctl” are the controls and statistical comparators for “Acute to PASC,” “Acute to resolution,” and “Post-acute w/o PASC” groups. “Post-acute w/o PASC” are the controls and statistical comparators for “PASC w/NP” and “PASC w/o NP groups”. The P values from unpaired t tests are *P < .01 and **P < .001.
Discussion
Preparation of plasma total extracellular vesicles by a simple 1-step precipitation permits accurate quantification of their cargo proteins by standard ELISA kits in any certified clinical laboratory. Determination of meaningfully elevated total extracellular vesicle levels of S1(RBD) protein and N protein supports the presence of intracellular SARS-CoV-2 in acute infection and PASC (Figure 1) and provides sufficient viral S1 (RBD) protein for determination of the SARS-CoV-2 strain (Table ). Using anti-viral drugs for acute infections may be advisable if total extracellular vesicle protein tests reveal some virulent strains and if a patient is elderly and has other risks or co-morbidities. The indications for using anti-viral drugs for PASC remain to be determined.
Table.
Clinical Applications of Measurements of Plasma TEV Proteins in COVID-19
| A. SARS-CoV-2 S1 (Receptor-Binding Domain) and N (Nucleoprotein) |
|
| B. Functional mitochondrial proteins altered by SARS-CoV-2 |
|
Total extracellular vesicle levels of many functional mitochondrial proteins are abnormal in acute SARS-CoV-2 infections and PASC, including MOTS-c, VDAC-1, humanin, SARM-1, the ion channel LETM1, and subunit 6 of NADH-ubiquinone oxidoreductase (respiratory complex I) (CI-6) (Figure 2). LETM1 and CI-6 are significantly decreased in acute COVID-19 and PASC relative to their respective controls whether or not patients have a high risk acutely of proceeding to PASC or later have neuropsychiatric manifestations as part of their PASC. In contrast, total extracellular vesicle levels of MOTS-c, VDAC-1, humanin, and SARM-1 are abnormal acutely and in PASC (except for VDAC-1) only in patients who have a high risk acutely of proceeding to PASC or later have neuropsychiatric manifestations as part of their PASC (Figure 2). Previously we developed a profile of neuron-derived extracellular vesicle functional mitochondrial proteins that were abnormal in patients with PASC who had neuropsychiatric manifestations, but were not found in others of this PASC cohort without neuropsychiatric manifestations.11 These included humanin, MOTS-c, CI-6, subunit 10 of electron chain respiratory complex III, mitochondrial uniporter, and sodium-calcium exchanger. The neuron-derived extracellular vesicle functional mitochondrial proteins that were abnormal in patients with acute COVID-19 who would develop PASC but not in those whose infection would resolve were MOTS-c, VDAC-1, humanin, and SARM-1.17 Thus, there is very prominent overlap of the MPs in total extracellular vesicles and neuron-derived extracellular vesicles that are predictive of PASC and its specific characteristics.
The isolation of plasma total extracellular vesicles by a 1-step precipitation and standard kit ELISA quantification of their constituent SARS-CoV-2 proteins and mitochondrial proteins permits demonstration of acute and long-term (PASC) intracellular infection and consequent mitochondrial damage (Table). It also allows determination of the strain(s) of SARS-CoV-2 and serial assessment of the effects of anti-viral drugs and other treatments. The levels of SARS-CoV-2 proteins do not predict risk of acute infection progressing to PASC or the specific manifestations of PASC. The levels of several mitochondrial proteins in acute infections do predict the relative risk of progressing to PASC and their levels in PASC predict the likelihood of long-term neuropsychiatric manifestations (Table).
Acknowledgments
The authors are grateful to the following biomedical investigators for their thoughtful and careful recruitment and evaluation of study participants:
J. Daniel Kelly, MD, PhD, Khamal Anglin, MD, Matthew So, MD, Scott Lu, MBBS, Sarah A. Goldberg, BA, Jeffrey N. Martin, MD (Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, Calif), Michael J. Peluso, MD, Rebecca Hoh, RD, Steven G. Deeks, MD (Division of HIV, Infectious Diseases and Global Medicine, Department of Medicine, University of California San Francisco, San Francisco, Calif), Timothy J. Henrich, MD (Division of Experimental Medicine, Department of Medicine, University of California San Francisco, San Francisco, Calif).
Footnotes
Funding: This research was supported by the Intramural Program of the National Institute on Aging.
Conflict of Interest: EJG declares a potential conflict due to his pending application with the US Office of Patents and Trademarks that covers the methods of plasma extracellular vesicle isolation and analyses. All other authors declare no conflicts of interests.
Authorship: All authors had access to the data and a role in writing this manuscript.
References
- 1.Clark A, Jit M, Warren-Gash C, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8:e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goetzl EJ, Kapogiannis D. Long-COVID: Phase 2 of the COVID-19 pandemic. Am J Med. 2022;135(11):1277–1279. doi: 10.1016/j.amjmed.2022.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu YH, Chen Y, Wang QH, et al. One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: a longitudinal cohort study. JAMA Neurol. 2022;79:509–517. doi: 10.1001/jamaneurol.2022.0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10:311–321. doi: 10.1016/S2213-8587(22)00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puntmann VO, Martin S, Shchendrygina A, et al. Long-term cardiac pathology in individuals with mild initial COVID-19 illness. Nat Med. 2022;28:2117–2123. doi: 10.1038/s41591-022-02000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruffieux H, Hanson AL, Lodge S, et al. A patient-centric modeling framework captures recovery from SARS-CoV-2 infection. Nat Immunol. 2023;24:349–358. doi: 10.1038/s41590-022-01380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peluso MJ, Deeks SG, Mustapic M, et al. SARS-CoV-2 and mitochondrial proteins in neural-derived exosomes of COVID-19. Ann Neurol. 2022;91:772–781. doi: 10.1002/ana.26350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shang C, Liu Z, Zhu Y, et al. SARS-CoV-2 causes mitochondrial dysfunction and mitophagy impairment. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.780768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goetzl EJ, Schwartz JB, Abner EL, Jicha GA, Kapogiannis D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann Neurol. 2018;83:544–552. doi: 10.1002/ana.25172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mustapic M, Eitan E, Werner JK, Jr., et al. Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front Neurosci. 2017;11:278. doi: 10.3389/fnins.2017.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetzl EJ, Boxer A, Schwartz JB, et al. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology. 2015;85:40–47. doi: 10.1212/WNL.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goetzl EJ, Kapogiannis D, Schwartz JB, et al. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer's disease. FASEB J. 2016;30:4141–4148. doi: 10.1096/fj.201600816R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly JD, Yao PJ, Peluso MJ, et al. Abnormal levels of mitochondrial proteins in neuron-derived extracellular vesicles in acute COVID-19 predict long-COVID2023.
- 18.Peluso MJ, Lu S, Tang AF, et al. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2021;224:1839–1848. doi: 10.1093/infdis/jiab490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peluso MJ, Deitchman AN, Torres L, et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109518. [DOI] [PMC free article] [PubMed] [Google Scholar]