Abstract
The recent emergence of different SARS-CoV-2 variants creates an urgent need to develop more effective therapeutic agents to prevent COVID-19 outbreaks. Among SARS-CoV-2 essential proteases is papain-like protease (SARS-CoV-2 PLpro), which plays multiple roles in regulating SARS-CoV-2 viral spread and innate immunity such as deubiquitinating and deISG15ylating (interferon-induced gene 15) activities. Many studies are currently focused on targeting this protease to tackle SARS-CoV-2 infection. In this context, we performed a phenotypic screening using an in-house pilot compounds collection possessing a diverse skeleta against SARS-CoV-2 PLpro. This screen identified SIMR3030 as a potent inhibitor of SARS-CoV-2. SIMR3030 has been shown to exhibit deubiquitinating activity and inhibition of SARS-CoV-2 specific gene expression (ORF1b and Spike) in infected host cells and possessing virucidal activity. Moreover, SIMR3030 was demonstrated to inhibit the expression of inflammatory markers, including IFN-α, IL-6, and OAS1, which are reported to mediate the development of cytokine storms and aggressive immune responses. In vitro absorption, distribution, metabolism, and excretion (ADME) assessment of the drug-likeness properties of SIMR3030 demonstrated good microsomal stability in liver microsomes. Furthermore, SIMR3030 demonstrated very low potency as an inhibitor of CYP450, CYP3A4, CYP2D6 and CYP2C9 which rules out any potential drug-drug interactions. In addition, SIMR3030 showed moderate permeability in Caco2-cells. Critically, SIMR3030 has maintained a high in vivo safety profile at different concentrations. Molecular modeling studies of SIMR3030 in the active sites of SARS-CoV-2 and MERS-CoV PLpro were performed to shed light on the binding modes of this inhibitor. This study demonstrates that SIMR3030 is a potent inhibitor of SARS-CoV-2 PLpro that forms the foundation for developing new drugs to tackle the COVID-19 pandemic and may pave the way for the development of novel therapeutics for a possible future outbreak of new SARS-CoV-2 variants or other Coronavirus species.
Keywords: COVID-19, SARS-CoV-2, SARS-CoV-2 PLpro, MERS-CoV, Virucidal activity
Graphical abstract
1. Introduction
In March 2020, the World Health Organization (WHO), in response to the outbreak of the infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, declared it a global pandemic [1]. SARS-CoV-2 is a member of the Coronaviridae family with a single-stranded positive-sense enveloped RNA. It has a typical genomic structure of human coronaviruses, such as (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV). It possesses a similar structure and pathogenicity to SARS-CoV [2,3]. Different small-molecule therapeutics have been approved by the Food and Drug Administration of the U.S (FDA) for COVID-19 treatment, such as remdesivir, molnupiravir, and the viral main protease inhibitor, paxlovid [4]. However, due to the rapid emergence of resistant virus variants, there is an urgent need to develop new therapeutic modalities.
Many studies have focused on targeting SARS-CoV-2 with small molecule inhibitors which block viral proteases and polymerases, including RNA-dependent RNA polymerase (RdRp), the main protease (Mpro or 3CLpro), and the papain-like protease (PLpro) [5]. Major progress has been made with RdRp and 3CLpro inhibitors, for which many drugs are either FDA-approved or in clinical investigation [[6], [7], [8], [9]]. Among the RdRp inhibitors is remdesivir, the first FDA-approved drug for treating severe COVID-19 symptoms [6,7]. Similarly, molnupiravir, an RdRp inhibitor, was discovered through a drug repurposing approach. However, molnupiravir exerts antiviral activity through mutation induction into the viral RNA, eventually inhibiting SARS-CoV-2 replication [10]. While paxlovid, a combination of 3CLpro and cytochrome P450 3A4 inhibitors, was granted FDA approval to be administered to adult and children patients with moderate COVID-19 infection [8]. Despite the effectiveness of the mentioned COVID-19 drugs, some studies have shown that the virus acquired resistance to remdesivir, whereas others, such as paxlovid, showed mutagenic risk [4]. Moreover, the emergence of new SARS-CoV-2 variants, including Omicron, raises a concern about developing 3CLpro mutations, which eventually reduce the effectiveness of the approved 3CLpro inhibitor, paxlovid [4]. Several studies also suggested the possible use of a combination therapeutic strategy to tackle the development of SARS-CoV-2 resistance in the population by targeting multiple SARS-CoV-2 proteases such as 3CLpro and PLpro [11].
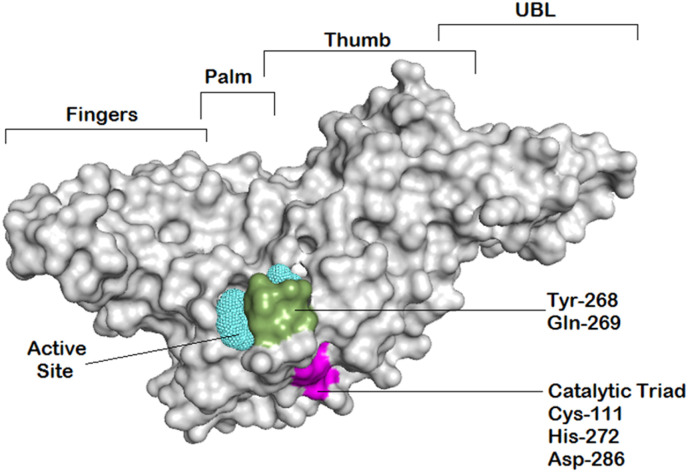
Therefore, SARS-CoV-2 PLpro represents an interesting therapeutic target for the treatment of COVID-19 infection. The structural and functional characteristics of this highly conserved protease were extensively discussed in several articles [[12], [13], [14]]. SARS-CoV-2 PLpro consists of four distinct domains: the thumb, palm, zinc-finger domain, and an N-terminal ubiquitin-like domain (Fig. 1 ) [15]. The PLpro active site contains a catalytic triad composed of Cys111, His272, and Asp286, exclusively targets and cleaves a common motif, LXGG(A/K)X, occurring between nsp1/2, nsp2/3, and nsp3/4, which is involved in viral transcription and replication [16]. It has been reported that the S1 and S2 ubiquitin binding sites of PLpro target the C-terminal domain of ubiquitin and ISG15 with S2, thereby possessing a substrate preference for the distal ubiquitin of K48-linked di-ubiquitin chains [13]. Ubiquitination and ISGylation are post-translational modifications characterized by the addition of ubiquitin and ubiquitin-like protein ISG15 (interferon-induced gene 15) to cellular proteins, leading to modulation of their activity [17]. Many viruses antagonize ubiquitin and ubiquitin-like pathways to induce modification in host cellular signaling and immune evasion. Similarly, studies suggested that SARS-CoV-2 blocks the innate immune responses through PLpro deubiquitinating and deISG15ylating activities [18,19]. Additionally, the zinc-binding domain was found to be central in sustaining PLpro's structural integrity and protease activity [13]. This understanding of PLpro structure and functions aided in the ongoing research for the development of PLpro inhibitors as potential treatment of COVID-19 infection.
Fig. 1.
Overview of the SARS-CoV-2 PLpro enzyme structure illustrating its four domains and the important region for ligand binding. The putative active site is represented by cyan mesh; catalytic triads are highlighted in magenta; while recognition residues are highlighted in green.
To this end, several promising PLpro inhibitors were reported and grouped into covalent or noncovalent inhibitors. Of the known noncovalent potent inhibitors of PLpro is the GRL0617 inhibitor, which is a naphthalene-based molecule [4,5,12]. However, in a cell-based assay, its inhibitory activity was moderate to weak [4]. Therefore, the discovery of first-in-class inhibitors of the SARS-CoV-2 PLpro is highly demanded. With that in mind, we took the initiative to screen an in-house collection of various classes of compounds against SARS-CoV-2 PLpro, and herein we report our findings (Supp. Table 1 ) [[20], [21], [22], [23], [24], [25], [26], [27]]
Table 1.
Primers sequences used for RT-qPCR analysis.
| Gene | Name | Sequence (5` to 3`) | Ref. |
|---|---|---|---|
| ORF1b | HKU-ORF1b-nsp14F | TGGGGYTTTACRGGTAACCT | [61] |
| HKU-ORF1b-nsp14R | AACRCGCTTAACAAAGCACTC | ||
| Spike | Spike-F | CCTACTAAATTAAATGATCTCTGCTTTACT | [68] |
| Spike-R | CAAGCTATAACGCAGCCTGTA | ||
| β-Actin | β-Actin-F | CATGAAGTGTGACGTGGACATCC | [69] |
| β-Actin-R | GCTGATCCACATCTGCTGGAAGG | ||
| IFN-α | IFN-α-F | CTTGTGCCTGGGAGGTTGTC | [70] |
| IFN-α-R | TAGCAGGGGTGAGAGTCTTTG | ||
| IL-6 | IL-6-F | ACCTGAACCTTCCAAAGATG | [71] |
| IL-6-R | GCTTGTTCCTCACTACTCTC | ||
| OAS1 | OAS1_F | TGACTGGCGGCTATAAACC | [72] |
| OAS1_R | TGGGCTGTGTTGAAATGTGT | ||
| CCL-5 | CCL5-F | TACCATGAAGGTCTCCGC | [73] |
| CCL5-R | GACAAAGACGACTGCTGG | ||
| CXCL-10 | CxCL-10_F | CTGCTTTGGGGTTTATCAGA | [74] |
| CxCL-10_R | CCACTGAAAGAATTTGGGC |
2. Results
2.1. In vitro identification of SARS-CoV-2 PLpro inhibitors
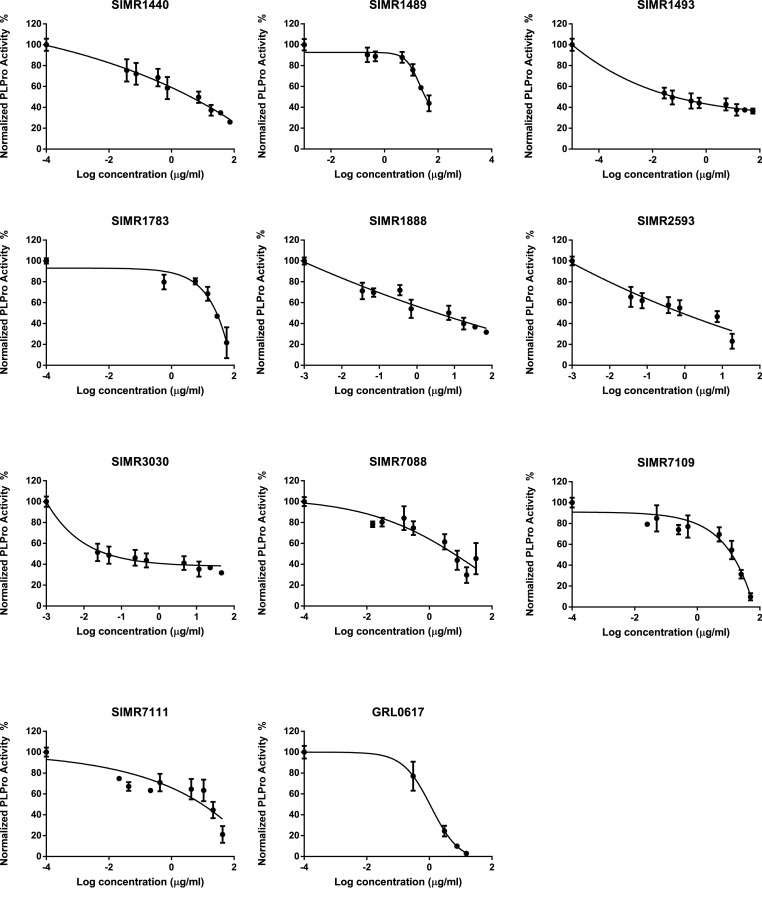
Seventy compounds [[20], [21], [22], [23], [24], [25], [26], [27]] with diverse scaffolds from our in-house pilot compounds library were initially screened to identify potential inhibitors of PLpro at a single concentration of 10 μg/mL using PLpro enzymatic inhibition assay (BPS Bioscience, San Diego, CA, USA). A PLpro inhibitor, GRL-0617, was used as a positive control. The assay revealed that 13 compounds (Supp. Fig. 1) possess more than 50% inhibitory activity at 10 μg/mL concentration. Next, we assessed the concentration required to inhibit 50% of the PLpro enzymatic activity (IC50) in a dose-response manner. As a result, compounds SIMR1440, SIMR1493, SIMR1888, SIMR2593, SIMR3030, SIMR7088, SIMR7109, and SIMR7111 possess IC50 values of 4.12 ± 1.14, 0.0659 ± 0.007, 4.09 ± 2.40, 0.85 ± 0.89, 0.0399 ± 0.081, 6.073 ± 0.65, and 12.83 ± 2.06, 13.95 ± 0.69 (μg/mL), respectively (Table 2 , Fig. 2 , Fig. 3 and Supp. Fig. 1). The inhibitory activity of the positive control GRL-0617 was 1.113 ± 0.175 μg/mL, which is similar to a previously reported value [28]. The most potent activity was observed for SIMR1493, SIMR2593, and SIMR3030. SIMR1489 and SIMR1783 inhibited around 50% of PLpro enzymatic activity at 25 μg/mL concentration (Fig. 3). Compounds SIMR1473, SIMR1911, and SIMR2304 have initially shown very strong inhibitory activity; however, variable results were noticed during the dose-dependent study. Therefore, their IC50 values were not determined. These discrepancies might be due to poor solubility in the assay buffer [29]. In addition, SIMR4560, SIMR4564, SIMR4565, SIMR4621, and SIMR7139 demonstrated high fluorescence readings during the initial screening process compared to DMSO (Supp. Fig. 1, Fig. 2), which is attributed to the autofluorescence of compounds that coincide with excitation and emission detection [30].
Table 2.
Summary of IC50 values of inhibitory in vitro activity against SARS-CoV-2 PLpro of the 13 hit compounds using an enzymatic assay. mean ± SD (n = 3).
| Compound (ug/mL) | SARS-CoV-2 PLpro (0.5 ng/μl) |
|---|---|
| GRL0617 | 1.113 ± 0.175 |
| SIMR1440 | 4.117 + 1.410 |
| SIMR1473 | ND |
| SIMR1489 | 33.224 + 5.78 |
| SIMR1493 | 0.0659 ± 0.0071 |
| SIMR1783 | 27.577 ± 2.27 |
| SIMR1888 | 4.094 ± 2.40 |
| SIMR1911 | ND |
| SIMR2304 | ND |
| SIMR2593 | 0.852 ± 0.89 |
| SIMR3030 | 0.0399 ± 0.081 |
| SIMR7088 | 6.0726 ± 0.65 |
| SIMR7109 | 12.8315 ± 2.057 |
| SIMR7111 | 13.947 ± 0.69 |
ND: Not Determined.
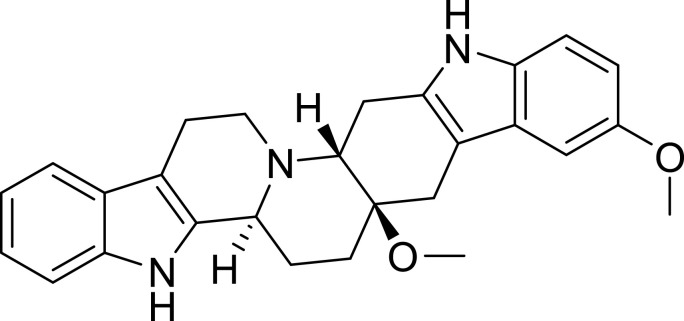
Fig. 2.
Chemical structure of SIMR3030.
Fig. 3.
Dose-dependent inhibition of SARS-CoV-2 PLpro activity by selected candidate compounds. Dose-dependent inhibitory activity and IC50 calculation of selected candidate compounds on SARS-CoV-2 PLpro with different concentrations. To calculate PLpro percent activity, fluorescence intensity was used. DMSO treated control represented as 100% activity and to calculate the percent activity blank readings were subtracted. GRL0617 treated wells served as positive control. Representatives of three experiments (n = 3) with triplicate values were presented graphically. Non-linear regression (curve fit) with dose vs inhibition was used to calculate the IC50 values using GraphPad Prism.
To examine whether the 13 compounds that showed promising SARS-CoV-2 PLpro inhibitory activity exhibit inhibitory activity against other SARS-CoV-2 proteases, their 3CLpro activity was tested using an enzymatic assay. Interestingly, none of these compounds has shown significant inhibition of 3CLpro at 10 μg/mL concentration (Supp. Fig. 3).
2.2. The developed PLpro inhibitors attenuate SARS-CoV-2 viral growth in cell culture
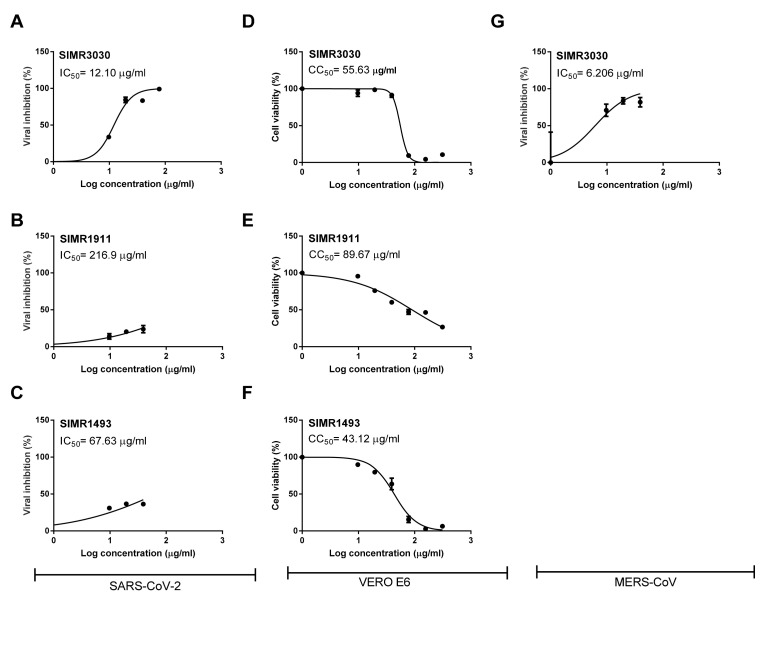
PLpro is one of two cysteine proteases in the SARS-CoV-2 genome, and it is essential in the proteolytic cleavage needed for viral transcription and replication. Thus, three of the most potent SARS-CoV-2 PLpro inhibitors (SIMR1493, SIMR1911, and SIMR3030) were examined for their ability to inhibit viral growth in SARS-CoV-2 infected Vero-E6 cells (Fig. 4 A- C). As a result, SIMR3030 has shown the most potent inhibitory activity with an IC50 value of 12.1 μg/mL. The other two compounds, SIMR1493 and SIMR1911, were less potent, possessing IC50 values of 67.63 and 216.9 μg/mL, respectively. Although SIMR1493 and SIMR1911 have shown potent inhibitory activity in the enzymatic assay, their viral inhibitory activity in the cell-based assay has been sharply reduced, which might be affected by cell permeability [28,29,31].
Fig. 4.
Cytotoxicity and Antiviral Activity of Selected SARS-CoV-2 PLpro inhibitory compounds. Determination of inhibitory concentration 50% (IC50) (A, B,C) and cytotoxic 50% (CC50) (D,E,F) of the tested compounds in Vero-E6 cells against SARS-CoV-2 “Isolate: NRC-03-nhCoV”. Inhibitory concentration 50% (IC50) against MERS-CoV “Isolate: NRCE-HKU270” in Vero-E6 cells (G). The CC50 and IC50 values were calculated using non-linear regression analysis of GraphPad Prism software (version 5.01) by plotting log inhibitor versus normalized response (variable slope).
Interestingly, when SIMR3030 compound was tested against MERS-CoV, it exhibited a potent inhibitory activity with an IC50 value of 6.206 μg/mL (Fig. 4G). To test whether the most active compounds (SIMR3030, SIMR1493 and SIMR1911) are safe, their cellular toxicity was investigated in Vero-E6 cells. As a result, the compounds did not show significant cellular toxicities. They displayed CC50 values of 55.63, 43.12 and 89.67 μg/mL, respectively (Fig. 4D–F and Table 3 ).
Table 3.
Half maximal cytotoxic concentration (CC50), inhibitory concentration 50 (IC50) and selectivity index (CC50/IC50) against SARS-CoV-2 and MERS-CoV for the tested compounds.
| Compound | CC50 (Vero E6) | IC50 (SARS-CoV-2) | SI | IC50 (MERS-CoV) | SI |
|---|---|---|---|---|---|
| SIMR3030 | 55.63 μg/mL | 12.10 μg/mL | 4.60 | 6.206 μg/mL | 8.96 |
| SIMR1911 | 89.67 μg/mL | 216.9 μg/mL | 0.41 | ND | NA |
| SIMR1493 | 43.12 μg/mL | 67.63 μg/mL | 0.64 | ND | NA |
ND: Not Done; NA: Not Applicable.
To further validate these findings, we tested the most potent compound, SIMR3030, using different in vitro cell-based assays infected with different SARS-CoV-2 variants. Thus, we used a viral replication assay to detect SARS-CoV-2 nucleocapsid expression using flow cytometry. In this assay, we used Vero-ACE2 cells, a cell line that highly expresses human angiotensin-converting enzyme 2 (ACE2) for infection by SARS-CoV-2 D614G variant, which is the parent of all variants of concern. Consistently, SIMR3030 demonstrated a potent viral inhibition activity with an IC50 value of 0.0597 μg/mL (Supp. Fig. 5 A and B). These promising results validate our PLpro enzymatic inhibition assay and Vero-E6 cell-based assay findings.
Fig. 5.
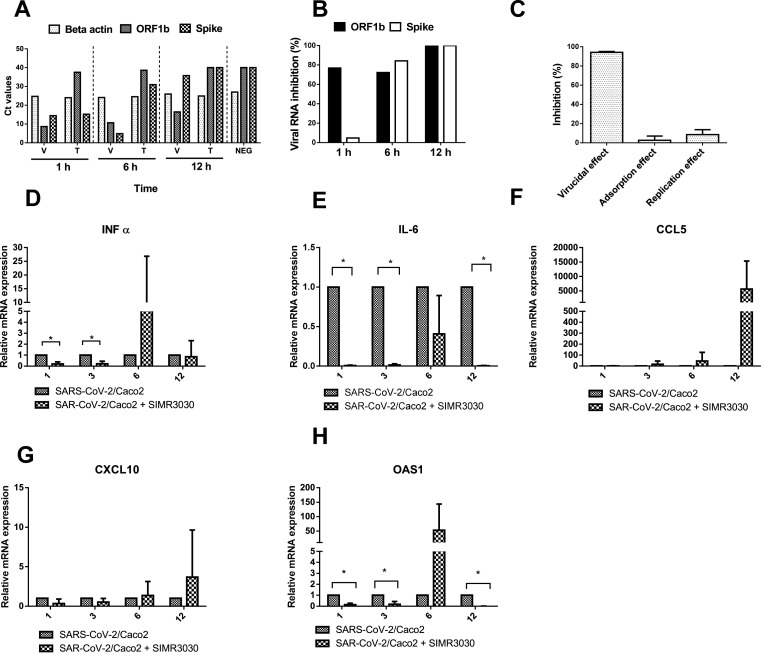
The antiviral and anti-inflammatory in vitro activities of SIMR3030. Virus replication was analyzed by measuring two SARS-CoV-2 genes mRNA expressions (ORF1b and Spike) with the house keeping gene (beta-action) in infected Caco-2 cells (V) and post-infection treated Caco-2 cells (T) at different time intervals (1, 6 and 12 h) were measured by real-time RT-PCR. (A) Ct values of the indicated genes mRNA expression as determined by RT-PCR. (B) Viral RNA percent inhibition (%) between Caco-2 cells infected with SARS-CoV-2 and treated cells at each time point of Fig. 5a (C) Mode of antiviral action for promising SIMR3030 against SARS-CoV-2 as measured by plaque reduction assay. The three major targets including virucidal effect, viral adsorption and viral replication were assessed post treatment. Relative mRNA expression levels of (D) IFNα, (E) IL6, (F) CCL-5, (G) CXCL-10 and (H) QASI in SARS-CoV-2 infected Caco-2 cells with or without treatment with SIMR3030. Data are shown as the mean ± SD. *p < 0.05; **p < 0.01; ***p < 0.001.
2.3. SIMR3030 elicits a weak SARS-CoV-2-PLpro deubiquitinating inhibitory activity
Among the known roles of SARS-CoV-2 PLpro beyond the cleavage of viral polypeptides are deISGylase and deubiquitinase activities with a preference for ubiquitin-like interferon-stimulated gene 15 protein (ISG15) substrates and to a lesser extent ubiquitin chains [18]. Since SIMR3030 indicated a promising inhibitory activity of SARS-CoV-2 viral replication in enzymatic and cell-based assays, it was decided to study its biological effect on SARS-CoV-2-PLpro deubiquitination. Thus, we investigated the role of SIMR3030 in blocking in vitro deubiquitination activity of SARS-CoV-2 PLpro using a commercially available SARS-CoV-2 deubiquitinase assay kit (Supp. Fig. 4). The PLpro inhibitor, GRL0617, which was used as a positive control inhibited 50% of the PLpro deubiquitination activity at a concentration of 3.496 ± 0.257 μg/mL, whereas SIMR3030 showed a considerably lower activity with an IC50 value of 165.21 ± 19.36 μg/mL. This suggests that SIMR3030 might inhibit PLpro activity independent of the deubiquitination activity [12,32].
2.4. SIMR3030 decreases SARS-CoV spike and ORF1b mRNA expression levels
To explore the effect of SIMR3030 treatment on the expression levels of SARS-CoV-2 RNA in infected Caco-2 cells, a real-time PCR assay was performed. Infected cells were treated with 20 μg/mL of SIMR3030 at 1, 6, and 12 h time intervals, and then RNA expression levels of ORF-1b and spike viral genes were assessed. Interestingly, a remarkable reduction in viral ORFb RNA expression following treatment with SIMR3030 was observed in comparison with the infected untreated Caco-2 cells at the three indicated time points (Fig. 5A). We also observed a reduction in the expression of Spike RNA after 6 and 12 h of treatment with SIMR3030 (Fig. 5A.) The β-actin values were comparable for all three points, indicating a difference between the infected cells pre- and post-treatment (Fig. 5A). In addition, the percentage of viral RNA inhibition was calculated in infected, and treated cells at each time point. These results showed more than 70% viral ORFb1 RNA inhibition at the three-time points. Spike inhibition was observed only after 6 and 12 h of treatment with SIMR3030. (Fig. 5B). These results further confirm the anti-SARS-CoV-2 activity of SIMR3030.
2.5. SIMR3030 exhibits virucidal activity against SARS-CoV-2
To determine whether SIMR3030 interferes with the viral replication cycle by exerting a direct virucidal effect, indirectly blocking the viral adsorption into the host cell receptors, or impairing intracellular viral replication [33], a plaque infectivity reduction assay was performed. Interestingly, SIMR3030 showed mainly a virucidal effect against SARS-CoV-2 with >90% viral inhibition (Fig. 5C). In addition, a slight viral reduction was observed when interfering with viral adsorption (Average = 2.5%) and replication (average = 8.5%).
2.6. SIMR3030 modulates the mRNA expression levels of pro-inflammatory markers
SARS-CoV-2-PLpro was reported to induce deISGylation and release of ISG15, thereby triggering macrophage differentiation towards pro-inflammatory phenotype [34]. Eventually, the generated pro-inflammatory cytokines and chemokines were tied to developing and progressing SARS-CoV-2-associated cytokine storms. In this context, the effect of SIMR3030 on mRNA expression levels of inflammatory markers was investigated. Thus, treatment of CaCo-2 cells infected with SARS-CoV-2 with SIMR3030 indicated that the expression of various inflammatory markers, including Type 1 Interferon, IFN-α, Cytokines, Il-6, and Interferon stimulated gene OAS1 at different time intervals (Fig. 5D–H) were significantly reduced at 1 and 3 h (for IFN-α) and at 1, 3 and 12 h (for IL-6 and OAS1).
2.7. In vitro administration, distribution, metabolism, and excretion (ADME) studies
To investigate the drug-likeness properties of our most promising compounds (SIMR1493 and SIMR3030), we assessed their maximum aqueous solubility in 2% DMSO/phosphate-buffered saline pH 7.4, their potential for oxidative metabolism in mouse and human liver microsomes, as well as their potential inhibition of the three of the major metabolizing enzymes CYP450, CYP3A4, CYP2D6, and CYP2C9. As shown in Table 4 , SIMR1493 and SIMR3030 demonstrated good aqueous solubility values 56.1 and 166.0 μM. The indolo[3′,2':8,9]quinolizino [4,3-b]carbazole SIMR3030 was more resistant to oxidative metabolism in both mouse and human liver microsomes compared to the benzo [5,6]chromeno [4,3-d]thiazolo [3,2-a]pyrimidine derivative SIMR1493. This microsomal instability of SIMR1493 might be linked to the morpholino sulfonamide group, which is known to undergo chronological oxidative dealkylation catalyzed by CYP3A4, giving rise to nitroso metabolites that can, in turn, inhibit the CYP3A4 enzyme through coordination to the heme iron [35]. This rationale is supported by the inhibition of CYP3A4 with an IC50 value of 134 μM, Table 4 by SIMR1493, which has the morpholino sulfonamide group. The two compounds, however, demonstrated good stability in liver microsomes in the absence of NADPH, indicating that P450-associated oxidation was the primary route of metabolism. Furthermore, SIMR1493 and SIMR3030 demonstrated very low potency as inhibitors of CYP2D6 and CYP2C9. These observations should form the basis to optimize the drug-likeness parameters of these scaffolds for future discovery programs.
Table 4.
In vitro physicochemical ADME data.
| Compound | Max Aq. Sol. (μM) | Permeability (10−6 cm/s) | % Recovery | Stability MLMa t1/2, min | Stability HLMb t1/2, min | Mic. Control Mouse % @ 60 minc | Mic. Control Human % @ 60 minc | CYP3A4 IC50 (μM) | CYP2D6 IC50 (μM) | CYP2C9 IC50 (μM) |
|---|---|---|---|---|---|---|---|---|---|---|
| SIMR-1493 | 56.1 | Not tested | Not tested | 11.2 | 5.7 | 92 | 119 | 134 | >10,000 | >10,000 |
| SIMR-3030 | 166 | 5.90 ± 0.3 | 77 ± 0.7 | >60 | >60 | 108 | 102 | >10,000 | >10,000 | >10,000 |
MLM = Mouse Liver Microsomes.
HLM = Human Liver Microsomes.
Assay carried out without NADPH; percent remaining after 60-min incubation.
2.8. Caco-2 permeability
Caco-2 cell permeability assay was conducted for SIMR3030 at pH 6.5–7.4 to predict its permeability since this in-vitro model exhibits morphological and functional characteristics similar to human intestinal epithelial cells. Accordingly, SIMR3030 cell permeability was found to be 5.90 ± 0.3 × 10−6 cm/s (apical to basolateral, A-B), as shown in Table 4. This moderate permeability across the intestinal barrier predicts an in vivo absorption fraction of 20%–70% [36]. While the recovery of SIMR3030 was 77% ± 0.7% which is within the acceptable range (75–125%).
2.9. SIMR3030 exhibits a satisfactory safety profile in experimental mice model
To investigate the in vivo safety profile of SIMR3030, single- and multiple-dose studies were performed using a Balb/c mice model. Accordingly, no signs of toxicity or body weight loss were detected at single-dose administration of SIMR3030 (25, 50, and 100 mg/kg) (Fig. 6 A). Similarly, no signs of toxicity or weight loss were noticed during 12.5 and 25 mg/kg multiple-dose studies for 14 days (Fig. 6B). Microscopic investigation of organs obtained from SIMR3030 treated mice, including liver, spleen, kidneys, heart, and lungs revealed no histologic variations and no signs of toxic injury (Fig. 6C and D). Similarly, no significant difference was noticed between the untreated and treated mice groups in their hematological and chemistry blood parameters (Supp. Table 2, Table 3). A slight reduction in platelet population in the 25 mg/kg treated mice group was observed in the hematological analysis. All other tested markers were found within the normal range compared to the control group, including the liver function panel (ALT, AST, ALP, and GAMMA GT) and renal function panel (total bilirubin, total protein, albumin, glucose, and urea). In conclusion, SIMR3030 has shown a good in vivo safety profile with no obvious toxicity at different doses observed for 14 days of consecutive treatment, as well as observation for another 14 days after treatment termination.
Fig. 6.
SIMR3030 safety profile showed no toxicity in single and multiple dose studies. (A) Mice weight changes over the indicated period during the treatment in single- and (B) multiple-dose studies. Mean; bars, SD (n = 3–5). (C) Representative figures of H&E staining for SIMR3030-treated (12.5 and 25 mg/kg) and control mice groups. (D) Table displaying the toxicity score for SIMR3030-treated (12.5 and 25 mg/kg) and control mice groups.
2.10. In silico docking studies
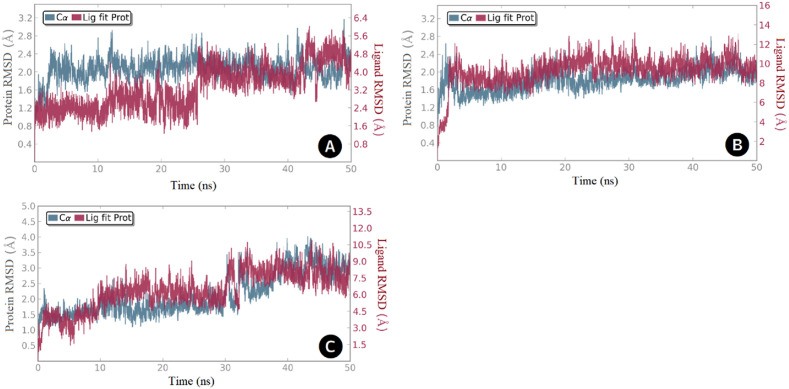
The anatomy of the SARS-CoV-2 PLpro enzyme revealed four major domains, namely, the fingers, palm, thumb, and UBL, in which the active site is situated in a region between the palm and thumb (Fig. 1). The active site contains the catalytic triad Cys-111, His-272, and Asp-286, which is responsible for processing viral polyproteins. Earlier studies have demonstrated the dynamic nature of the active site and suggested the key role played by the amino acid residues Tyr-268 and Gln-269 in recognition of small molecules [[37], [38], [39]]. To study the potential binding modes of the most active compounds, we docked these compounds in the PLpro active site using Autodock Vina. Then the best-docked poses served as templates for subsequent molecular dynamic simulation runs. For each enzyme-ligand complex, system stability was assessed by means of root-mean-squared deviations (RMSD) of protein Cα-atoms and the ligand heavy atoms (Fig. 7 ). In the case of compound SIMR1493, system equilibration (1–3 Å change) was achieved after 28 ns (Fig. 7A), and for compound SIMR2304 was achieved after 4 ns (Fig. 7B), while for compound SIMR3030 equilibration occurred after 35 ns (Fig. 7C).
Fig. 7.
RMSD values over the 50 ns simulation time for the PLpro complexed with; (A) Compound SIMR1493, (B) Compound SIMR2304, and (C) Compound SIMR3030. Enzyme Cα-atoms are in blue, while compound heavy atoms are in red.
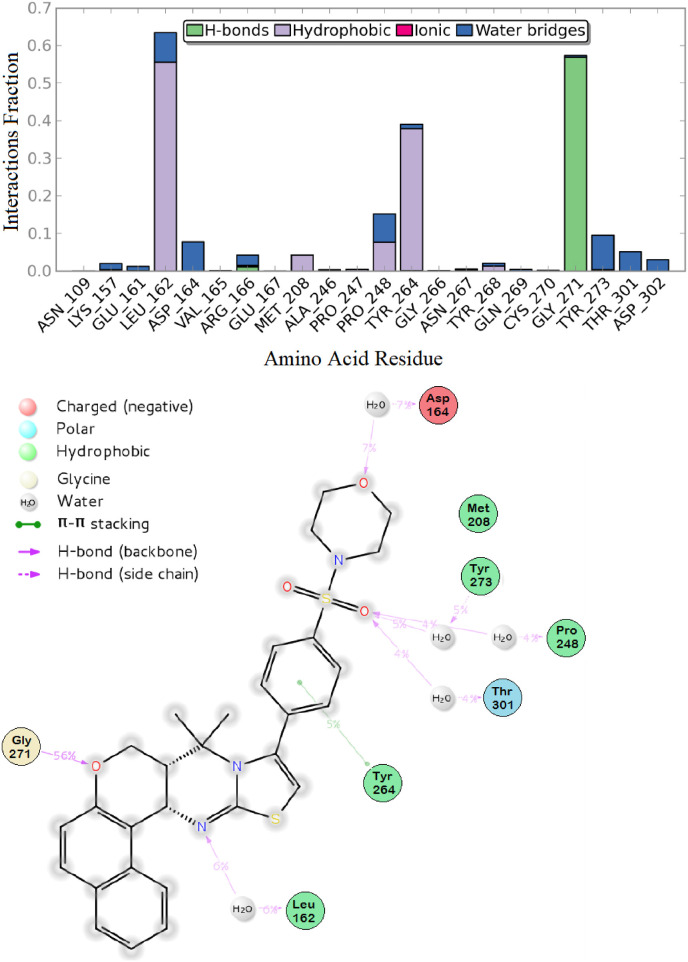
According to these results, the network of interactions that involves SIMR1493 in the PLpro active site was as follows: The morpholino group oxygen was engaged in backbone hydrogen bond (HB) interaction with the corresponding amino acid residue Asp-164 for a period of 7% of the simulation time, while the sulfonyl group was able to water-bridge the amino acids Pro-248, Tyr-273, and Thr-301 for a total of 13% of simulation time. π-π Stacking interaction between the central phenyl ring and the Tyr-264 residue was observed. Furthermore, the thiazolo-pyrimidine nitrogen could form HB with the corresponding residue Leu-162 for about 6% of the time. The benzo-chromen oxygen was involved in significant hydrogen bonding interaction with the corresponding Gly-271 amino acid for a significant simulation time of 56% (Fig. 8 A and B).
Fig. 8.
Protein-ligand interaction over the 50 ns simulation period. (A) The fractions of interaction occurred between compound SIMR1493 with the corresponding SARS-CoV-2 PLpro active site residues, (B) 2D-ligand interaction diagram of compound SIMR1493 within the SARS-CoV-2 PLpro active site.
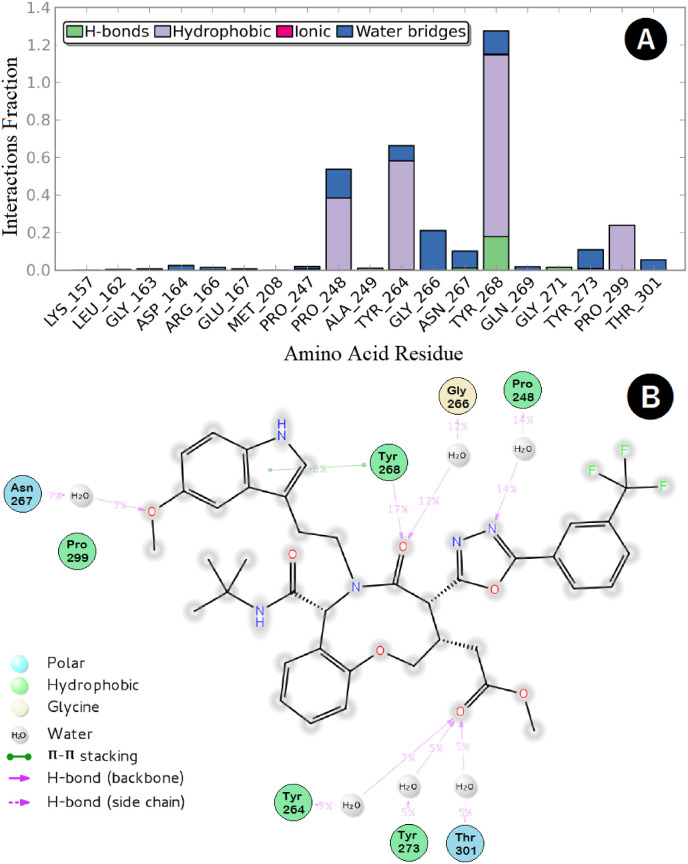
On the other hand, the interaction network between SIMR2304 and the PLpro active site residues could be summarized as follows: the oxadiazole nitrogen was engaged through H-bonding (HB) interaction with the corresponding Pro-248 residue for a period of 14% of the simulation time. In comparison, the methoxy-indole oxygen was able to water-bridge Asn-267 for about 7% of the time. Significant multiple interactions, including HB, hydrophobic, and water bridges, were observed between the oxazoninone carbonyl oxygen and the corresponding Tyr-268 amino acid residue responsible for small-molecules recognition lasted for the period for about 124% of the simulation time (Fig. 9 A and B). Finally, the oxazoninone-acetate carbonyl oxygen was engaged in multiple HB interactions with the corresponding residues Tyr-264, Tyr-273 and Thr-301 for a total of 17% of the simulation time.
Fig. 9.
Protein-ligand interaction over the 50 ns simulation period. (A) The fractions of interaction occurred between compound SIMR2304 with the corresponding SARS-CoV-2 PLpro active site residues, (B) 2D-ligand interaction diagram of compound SIMR2304 within the SARS-CoV-2 PLpro active site.
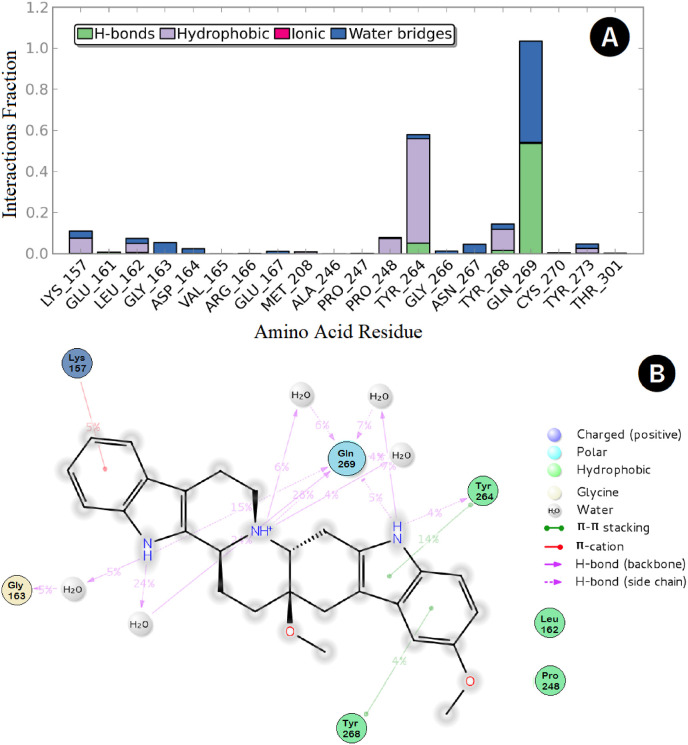
However, the most potent compound, SIMR3030, showed an interesting network of interactions with the PLpro active site that involves the two amino acids Tyr-268 and Gln-269, responsible for small-molecule recognition. In this respect, multiple interactions, such as HB and water bridges, were observed between the compound nitrogen atoms, and the corresponding Gln-269 amino acid residue lasted for about 100% of the simulation time (Fig. 10 A and B). Furthermore, two π-π stacking interactions were observed; one between the Tyr-268 and the corresponding benzo-carbazole terminal ring and another one between the residue Tyr-264 and the carbazole central ring. Additionally, a π-cation interaction was possible between the terminal ring of the indolo-quinolizine moiety and the corresponding Lys-157 residue, while the residue Gly-163 could water-bridge the indolo-quinolizine nitrogen for about 5% of the simulation time.
Fig. 10.
Protein-ligand interaction over the 50 ns simulation period. (A) The fractions of interaction occurred between compound SIMR3030 with the corresponding SARS-CoV-2 PLpro active site residues, (B) 2D-ligand interaction diagram of compound SIMR3030 within the SARS-CoV-2 PLpro active site.
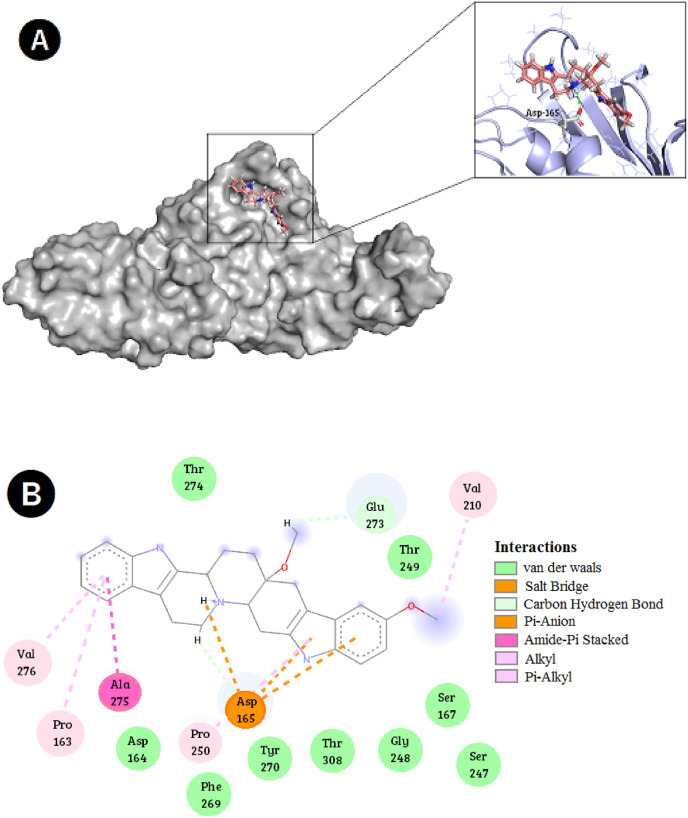
The antiviral activity of SIMR3030 against the MERS-CoV-infected cells encouraged us to perform molecular modeling studies of this compound in the active site of MERS-CoV-2 PLpro. Results showed a perfect fit of SIMR3030 in the enzyme active site (Fig. 11 A). Detailed interactions showed that SIMR3030 is superior in achieving numerous types of hydrophobic interactions, including Van der Waals, alkyl and π-stacking in the enzyme active site. Many other important ionic interactions were observed, especially with the corresponding amino acid residue Asp-165 (Fig. 11B). These observations further confirm the antiviral activity of SIMR3030.
Fig. 11.
Best-docked pose of compound SIMR3030 within the MERS-CoV PLpro active site; (A) Binding mode and hydrogen bond/ionic interactions occurred between compound SIMR3030 with the corresponding MERS-CoV PLpro active site residues, (B) 2D-ligand interaction diagram of compound SIMR3030 within the MERS-CoV PLpro active site.
3. Discussion
The COVID-19 pandemic is a global crisis that is not completely eradicated despite the enormous efforts and extreme measures taken globally. Both preventive and therapeutic approaches were developed for managing COVID-19 through the development of vaccines and small-molecule modalities. Vaccines and anti-COVID drugs have demonstrated remarkable effectiveness within a very short timeframe. The prompt application of therapies was attributed to previous studies against other coronaviruses, such as SARS-CoV and MERS-CoV, which considerably reduced the duration of initial discovery phases [14,40]. In addition, new technologies, such as gene-based vaccines, which depend on DNA, mRNA, or viral vectors, such as recombinant adenoviruses (rAds) exploited in the production of the current vaccines [41]. Nevertheless, SARS-CoV-2 immune evasive strains such as Omicron subvariants (BA.2.75.2, BQ.1.1, and XBB) are still emerging and are currently considered the most dominant worldwide [42]. The Omicron variant and its subvariants are characterized by a high replicative rate in the bronchi and high resistance to the available therapeutic monoclonal antibodies (mAbs) and vaccines [[42], [43], [44], [45], [46], [47], [48]]. As a result, lower success rates have been observed when treating new SARS-CoV-2 variants. Thus, it is crucial to develop novel antiviral modalities [42].
One of the promising therapeutic targets of the SARS-CoV-2 virus is PLpro, a conserved protease between MERS-CoV, SARS-CoV, and SARS-CoV-2. SARS-CoV-2 PLpro possesses a similar sequence of 82% homology to SARS-CoV PLpro. This similarity greatly aided in accelerating the process of identifying and designing novel inhibitors of SARS-CoV-2 PLpro.
In this study, a phenotypic screen was performed using in-house developed compound collection [[20], [21], [22], [23], [24], [25], [26], [27]] to identify PLpro inhibitors using fluorescence resonance energy transfer (FRET)-based assay. The screening revealed that out of the 70 tested compounds, 13 exhibited over 50% inhibitory activity at a concentration of 10 μg/mL (Supp. Fig. 1). Of the best 13 hits, compounds showed poor solubility and auto-fluorescence properties were excluded. The IC50 values of the remaining compounds (SIMR1440, SMR1489, SIMR1493, SIMR1783, SIMR1888, SIMR2593, SIMR3030, SIMR7088, SIMR7109 and SIMR7111) were identified to be within the range 0.0399 ± 0.081 to 27.059 ± 2.27 μg/mL. The most potent compounds, SIMR3030, SIMR1911, and SIMR1493, were then studied for their antiviral effect on SARS-CoV-2 infected cells. Of which, the octahydroindolo [2,3-a]quinolizine SIMR3030 exhibited the most potent activity with an IC50 value of 0.0399 ± 0.081 μg/mL in the enzymatic assay and 12.10 μg/mL in SARS-CoV-2 infected Vero-E6 cells. The activity of SIMR3030 was further evaluated in Vero-ACE2 cells infected with D614G mutated SARS-CoV-2 variant. SIMR3030 exhibited potent antiviral activity in this established model with an IC50 value of 0.0596 μg/mL. The potent antiviral activity of SIMR3030 in Vero-ACE2 cells compared to parental Vero-E6 cells could be mediated through the increased drug efflux through the high expression of P-gp (P-glycoprotein) in Vero-E6 cells [49]. Interestingly, despite the significant difference between SARS-CoV-2 PLpro and MERS-CoV -PLpro, SIMR3030 inhibited MERS-CoV-infected Vero-E6 cell replication with an IC50 of 6.206 μg/mL. Specifically, in the BL-2 region, MERS-CoV PLpro contains a single extra residue and lacks Y268 found in the SARS-CoV-2 PLpro [12]. Moreover, these structural differences might explain the inactivity of previously identified PLpro inhibitors against MERS-CoV PLpro, such as GRL0617 [12]. Molecular modeling studies revealed many interesting interactions of SIMR3030 in the SARS-CoV-2 PLpro active site, which involve the two amino acids responsible for small-molecule recognition (Tyr-268 and Gln-269). On the other hand, the interactions between SIMR3030 and MERS-CoV -PLpro active site involved Asp-165, a key amino acid residue, and several other hydrophobic interactions such as Van der Waals, alkyl, and π-stacking.
The cellular toxicity of SIMR3030 was investigated in Vero-E6 cells, revealing a half-maximal cytotoxic concentration (CC50) value of 55.63 μg/mL. The promising IC50 value of SIMR3030 encouraged us to understand its antiviral impact. Accordingly, it was found that SIMR3030 blocks the deubiquitination activity of SARS-CoV-2 PLpro (Supp. Fig. 4) and inhibits SARS-CoV-2 replication as measured by its inhibition of mRNA expression of two different SARS-CoV-2 genes, spike (S) and open reading frame (ORF1b) (Fig. 5A and B) [50]. Different studies were conducted to gain more insight into the viral inhibitory mechanism of SIMR3030. These studies indicated that the virucidal activity of SIMR3030 is mediated through direct virion targeting (Fig. 5C).
PLpro inhibitors antagonize deubiquitination and deISG15ylation activities, affecting the host immune response [18,51]. In this context, the effect of SIMR3030 on the expression of pro-inflammatory cytokines and chemokines was examined. As a result, SIMR3030 significantly reduced the mRNA expression levels of IFN-α, IL-6, and OAS1 (Fig. 5D–H). Usually, COVID-19 infection is associated with a weak IFN-I response and hyperactivation of pro-inflammatory cytokines and chemokines such as IL-6, IL-1β, and CXCL-10, which contribute to the immunopathology of COVID-19 [52,53]. In addition, studies showed that macrophages secrete ISG15 via the deISGylation activity of the virus PLpro [34]. The released ISG15 outside the cells further triggers macrophages to release more pro-inflammatory cytokines and chemokines. Eventually, these inflammatory mediators intermediate the immune response shift towards the development of cytokines storm [[54], [55], [56]]. Indeed, WHO recently recommended immunotherapeutic strategies using monoclonal antibodies to target these pro-inflammatory cytokines, such as IL-6 blockers, to treat COVID-19 [[57], [58], [59]]. In this respect, SIMR3030 represents a promising agent since it is not only inhibits SARS-CoV-2 replication but also ameliorates the inflammatory storm generated by COVID-19 infection. In vitro ADME assessment of drug-likeness properties of SIMR3030 demonstrated that it possesses a good microsomal stability in liver microsomes. Furthermore, SIMR3030 demonstrated a very low potency as an inhibitor of CYP450, CYP3A4, CYP2D6, and CYP2C9, which rules out any potential drug-drug interactions. In addition, SIMR3030 exhibited moderate permeability and a good recovery. These findings should pave the way to optimize the drug-likeness properties of this scaffold for future drug discovery campaigns. Moreover, a high safety level of SIMR3030 was determined by the in vivo single- and multiple-dose studies using female Balb/c mice (Fig. 6). These promising findings support the notion for further development of SIMR3030 as a lead drug candidate for the treatment of COVID infections.
4. Conclusion
In conclusion, this study identified a first-in-class molecule, SIMR3030, as a potent inhibitor of SARS-CoV-2 PLpro. This finding was confirmed through enzymatic, cell-based, and molecular modeling studies. The compound possesses an appreciable activity against PLpro of MERS-CoV as well. In addition, SIMR3030 blocks the replication of SARS-CoV-2, reduces SARS-CoV-2 specific gene expression (ORF1b and Spike) and exerts a virucidal effect on SARS-CoV-2. Interestingly, SIMR3030 protects the host's innate immune system by alleviating the expression of pro-inflammatory markers IFN-α, IL-6, and OAS1. In vitro and in vivo safety studies of SIMR3030 indicate its safety profile as a potential antiviral lead drug candidate. In summary, SIMR3030 is a novel inhibitor of SARS-CoV-2 PLpro and forms the foundation for developing a new modality to treat SARS-CoV-2 infection.
4.1. Chemistry
The tested compounds were synthesized and characterized as described before [[20], [21], [22], [23], [24], [25], [26], [27]].
4.2. Papain-like protease (PLpro) enzymatic assay
PLpro (SARS-CoV-2) assay kit (cat no.79995-2 BPS Bioscience, San Diego, CA, USA) was done according to the manufacturer's instructions. Briefly, 10 μL of the recombinant SARS-CoV-2 PLpro (0.5 ng/μl) were added in triplicates to a 384-well plate. Then, 2.5 μL of the tested compounds were added to the enzyme. The plate was sealed and incubated for 2 h at 37 °C. When the incubation period ended, 12.5 μL of the provided PLpro substrate was added to each well to give a final concentration of 21 μM. The plate was sealed again and incubated at 37 °C for an additional hour. Finally, the fluorescence intensity was measured using Synergy™ HTX microplate reader (BioTek, Winooski, VT, USA) at excitation of 360 nm and emission of 460 nm. GRL0617 was used as a positive control. The blank reading was subtracted from all values.
4.3. 3CL protease enzymatic assay
This assay was performed using 3CL Protease, Untagged (SARS-CoV-2) Assay Kit (cat no.78042-2 - BPS bioscience, San Diego, CA, USA). Per the manufacturer's instructions, 10 μL of 3CL Protease (1.5 ng/μl) were added in triplicates to a 384-well plate. Then, 2.5 μL of test compounds were pre-incubated with the enzyme for 30 min at room temperature (RT). The reaction was started by adding 12.5 μL 3CL protease substrate to the wells. The final concentration of the substrate per well was 40 μM. The plate was sealed and incubated at RT for 4 h. To measure the fluorescence intensity, Synergy™ HTX microplate reader (BioTek, Winooski, VT, USA) was used at excitation 360 nm and emission 460 nm. GC376 was used as a positive control. The blank reading was subtracted from all values.
4.4. SARS-CoV-2 PLpro deubiquitinase activity assay
PLpro deubiquitinase activity was detected using an enzymatic activity kit (cat no.79996 - BP Bioscience, San Diego, CA, USA). The experiment was performed similarly to PLpro enzymatic assay, except that PLpro Ubiquitinated fluorogenic substrate was used. Fluorescence was measured at an excitation wavelength of 360 nm and an emission wavelength of 460 nm. GRL-0617 was used as a positive control. Readings were made using a Synergy™ HTX microplate reader (BioTek, Winooski, VT, USA). Where appropriate, the % PLpro deubiquitinase activity was calculated as the ratio of activity in the presence of inhibitor and total activity, considering background readings.
4.5. Cells
4.5.1. Vero-E6 cells
Cells were cultured at 37 °C in Dulbecco's Modified Eagle's medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 1% Penicillin/Streptomycin mixture in 5% CO2 incubator (Invitrogen).
4.5.2. Vero-ACE2 cells
Vero-E6 expressing endogenous ACE2 (BEI, NR-53726) cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, USA) and 1% penicillin/streptomycin (Thermo Fisher Scientific, Waltham, USA).
4.5.3. Caco-2 cells
Human colorectal adenocarcinoma cells were maintained in DMEM (Gibco, Invitrogen) supplemented with 1% Penicillin/Streptomycin (100 IU/mL penicillin, 100 μg/mL streptomycin; Invitrogen) and 10% FBS (FBS; PAA Laboratories).
4.6. Viruses
hCoV-19/Egypt/NRC-3/2020 SARS-CoV-2 “NRC-03-nhCoV” virus and the MERS-CoV isolate NRCE-HKU270 were cultured in Vero-E6 cells, as described previously, with minor modifications [33]. Virus NRC-03-nhCoV or NRCE-HKU270 titers were later determined as described previously [33]. While SARS-CoV-2 D614G (B.1.5, NR-53944) was obtained from BEI resources (NR-52512).
4.7. Antiviral activity of PLpro inhibitors on SARS-CoV-2 D614G using flow cytometry
Vero-ACE2 cells were plated in 24-well plates at a density of 4 × 105 overnight; then, the cells were incubated with candidate inhibitors at different concentrations in CO2 incubators for 1 h. Cells were subjected to virus adsorption with authentic SARS-CoV-2 D614G (MOI = 0.01). After 24 h of cell co-incubation with the virus, cells were fixed with 3.7% formaldehyde for 1 h, followed by a wash with phosphate buffer saline (PBS) three times. Next, cells were permeabilized with 1xIC permeabilization buffer (Thermo Fisher Scientific, Waltham, USA, Cat#00-8333-56) for 10 min on ice, followed by a wash with PBS. Finally, cells were incubated with primary antibody (SARS-CoV-2 anti-Nucleocapsid) followed with anti-Mouse IgG-FITC and subjected to flow cytometric analysis using Attune NxT flow cytometer (Thermo Fisher Scientific, Waltham, USA), and FlowJo software (https://www.flowjo.com/).
4.8. Determination of half-maximal cytotoxic concentration (CC50)
To determine the half-maximal cytotoxic concentration (CC50) in Vero-E6 cells, crystal violet assay method was used as previously described [60]. First, stock preparation was done by dissolving compounds in 10% DMSO in DMEM and further diluting them in the recommended media to obtain different working concentrations. Cells were cultured in 96-well plates at 3 × 105 cells/mL density in 100 μL media per well. Following incubation for 24 h at 37 °C in 5% CO2, cells were treated with various concentrations of the candidate compounds in triplicates. After 72 h treatment, cells were washed once with PBS and fixed with 10% formaldehyde for 1 h at RT after discarding supernatant media. On a bench rocker, 0.1% crystal violet was added to the plates for 20 min and later washed and dried. Next, 200 μL of methanol was added to dissolve crystal violet in each well. Finally, a multi-well plate reader was used to measure absorbance at λ max 570 nm using the Anthos Zenyth 200rt plate reader (Anthos Labtec Instruments, Heerhugowaard, Netherlands). The cytotoxicity of various concentrations compared to the untreated cells was determined using non-linear regression analysis by plotting log inhibitor versus normalized response.
4.9. Determination of half-maximal inhibitory concentration
To assess the half-maximal inhibitory concentration for candidate compounds previously determined method was used with minor modification [33]. Vero-E6 cells were seeded in 96-well tissue culture plates and incubated for 24 h at a humidified 37 °C incubator under 5% CO2. The attached cells were washed with PBS and exposed to virus adsorption (NRC-03-nhCoV” virus “TCID50 = 100) for 1 h at RT. The cell monolayers were treated with 100 μL of media containing varying concentrations of the candidate compounds for 1 h. Later, cells were maintained at 37 °C in 5% CO2 incubator for 72 h. Cells were then fixed with 100 μL of 10% paraformaldehyde for 20 min and stained with 0.1% crystal violet in distilled water for 15 min at RT. Later, 100 μL of absolute methanol was used to dissolve crystal violet dye, and absorbance was measured at 570 nm using Anthos Zenyth 200rt plate reader (Anthos Labtec instruments, Heerhugowaard, Netherlands). The IC50 values were calculated using non-linear regression analysis by plotting log inhibitor versus normalized response.
4.10. Evaluation of the antiviral activity of SIMR3030: Viral quantification with real-time RT-PCR
Caco-2 cell suspension was cultured in 12-well tissue culture plates and incubated overnight to get confluent monolayers. Next, a 100 μL of virus HCoV-19/Egypt/NRC-1/2020 with a dilution of MOI = 0.05 was pre-incubated for 1 h at 37 °C in 5% CO2 incubator. 100 μL of 20 μg/mL of SIMR3030 was prepared separately. After washing the cells twice, the compounds/virus mixtures were added to the corresponding wells. Treated wells, cell control, and virus control were all incubated at 37 °C under 5% CO2 for 1 h with rocking every 15 min to ensure cells were homogeneously exposed to infection and avoid drying. Following 1 h, the inoculum was removed, and 1000 μL of DMEM media containing 1% Penicillin-Streptomycin and 0.2% bovine serum albumin (BSA) was added. Then, infected cells were incubated at 37 °C with 5% CO2 for 48 h. An aliquot of 300 μL was collected at 1, 6, and 12 h from each dilution in duplicate for viral titration using qRT-PCR. qRT-PCR was performed as described previously [61]. The sequences of the used primers (ORF1b and spike) are found in Table 1.
4.11. Determination of anti-SARS-CoV-2 mechanism of SIMR3030
To determine if the most potent compound SIMR3030 hits the viral particle “virucidal effect” and/or interferes with viral adsorption and/or viral replication during the virus replication cycle, plaque infectivity reduction assay was performed as described previously [33].
4.11.1. Viral adsorption mechanism
The viral adsorption mechanism was performed by culturing Vero-E6 cells in a 6-well plate (105 cells/mL) for 24 h at 37 °C. SIMR3030 was co-incubated with the cells without supplements in 200 μL medium at 4 °C. The non-absorbed SIMR3030 was removed through three successive washing with a supplement-free medium. Pretreated cells were co-incubated for 1 h with SARS-CoV-2 virus diluted to 104 PFU/well with the addition of 2% agarose in DMEM medium. Plates were solidified and incubated at 37 °C till the development of viral plaques. 10% formalin solution was used for cell monolayer fixation for 1 h and stained with crystal violet. The relative percentage of plaque formation reduction was calculated compared to control wells, which consisted of untreated Vero-E6 cells directly infected with NRC-03-nhCoV.
4.11.2. Viral replication mechanism
Vero-E6 cells seeded in a 6-well plate at a concentration of (105 cell/mL) were infected for a 1 h contact period with virus post-incubation of 24 h at 37 °C. The non-absorbed viral particles were washed through three successive washes with a supplement-free medium. SIMR3030 was added at different concentrations to infected cells for another 1 h. Inoculate containing SIMR3030 was removed and replaced with 3 mL of DMEM containing 2% agarose. The soft agar was left to solidify until viral plaques were formed. Next, the plaques were fixed and stained as described above. Relative viral replication was measured, and Vero-E6 cells incubated with the virus were considered Control wells. The percentage reduction in plaque formation was calculated compared to the control wells.
4.11.3. Virucidal mechanism
Vero-E6 cells (105 cells/mL) were cultured for 24 h at 37 °C in a 6-well plate. Next, 200 μL of serum-free DMEM containing SARS-CoV-2 was added to each sample with SIMR3030. This mixture was diluted three times each for 10-folds using serum-free medium after 1 h incubation. This allowed the growth of viral particles on Vero-E6 cells. Later, 100 μL of each dilution was added to the Vero-E6 cell monolayer. A DMEM medium was then added after 1 h of contact period to the cells. The soft agar was left to solidify until the formation of viral plaques. The plaques were fixed and stained as described above to calculate the percentage reduction in plaque formation. This was calculated by comparing control wells of cells infected with the virus and not pretreated with the SIMR3030 versus cells infected with the virus and treated with SIMR3030.
4.12. Gene expression by real-time PCR assay of selected pro-inflammatory markers
Caco-2 cells were seeded in 6-well plates and incubated at 37 °C in the presence of 5% CO2. After 24 h, a 20 μg/mL dose of the compound was added to the cells, followed by SARS-CoV-2 infection (MOI = 3). After incubation at 1, 3, 6, and 12 h, cells were harvested for the mRNA expression test. Cultures were done in triplicates. Viral RNA was extracted from the final mixture of samples using a QIAamp Viral RNA Mini kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions. Briefly, RNA was extracted from 140 μL of each sample to obtain 50 μL of RNA extraction solution. NanoDrop Spectrophotometer (A260/280 ratio) was used to determine the concentration and quality of the isolated RNAs. The RNA was immediately subjected to a cDNA assay or kept at −80 °C until needed.
Five RNA microliters were reverse transcribed using the ReverAid RT Kit (ThermoFisher Scientific, Waltham, USA) according to the manufacturer's protocol. RNAs were reverse transcribed by incubation for 60 min at 42 °C, followed by 5 min at 85 °C to inactivate the reverse transcriptase. In a final volume of 20 μL, the reaction conditions were 1 × RT-RevertAid buffer, 0.5 mM concentrations of each deoxynucleoside triphosphate, 1 μM random primer, 10 U of RNase inhibitor, 1 μl of RT-Sensiscript, and 5 μl of template-RNA. Real-Time PCR Amplification Rotor Gene Q Real-time PCR (Qiagen, Hilden, Germany) was used to assess the expression of IL-6, IFN-α, CCL-5, CXCL-10, and OAS-1 using the primer sets as in Table 1. The endogenous control was the housekeeping mRNA β-actin. The cDNA template was combined with SYBER GreenMaster Mix (Qiagen, Hilden, Germany) in a final volume of 20 μl. Real-time PCR reactions were carried out at 94 °C for 5 min, followed by 40 cycles at 94 °C for 30 s, 55 °C for 30 s, and 70 °C for 30 s. All experiments were done in triplicates.
The cycle threshold (CT) values were determined. The ΔCt value, determined by subtracting the CT values of mRNA β-actin from the CT values of the target mRNAs, was used to report mRNA expression. The relative quantitative levels of various mRNAs were determined using the 2-ΔΔ (Ct) technique. GraphPad Prism® 5 software was then used to determine the relative expression of all mRNAs in control and treated samples.
4.13. In vitro ADME studies
Aqueous Solubility, Microsomal Stability, Human CYP 3A4 Inhibition Compound Screening Assay, Human CYP2D6 Inhibition Assay, and Human CYP2C9 Inhibition Assay were performed as previously detailed [62].
4.14. Caco-2 permeability
4.14.1. Permeability assay
The assay was performed as previously described in Ref. [63]. Briefly, Caco-2 cells were seeded at a density of 0.5 × 106 cells/well on collagen-coated polycarbonated filter transwell inserts (Costar, 0.4 μM pore size, 1.12 cm2 surface area). Media was changed every two days and maintained for 7 days. SIMR 3030 was diluted as needed in 1% v/v DMSO/HBSS buffer at pH 6.5 or 7.4. The volume on the apical side was maintained at 450 μL, and the volume on the basolateral side was 1200 μL. The absorption screening protocol used a 50, 75 and 100 μM input concentration, an apical pH of 6.5, and a basolateral pH of 7.4. Samples were taken from the receiver and donor side every 30 min for 2 h and run in duplicate. Concentrations of SIMR3030 was measured by LC/MS/MS with standards of the compound at a range concentration of (10–2500 nM) prepared in 1% v/v DMSO/HBSS, pH 7.4 buffer.
4.14.2. Data analysis and calculations
The apparent permeability coefficient (Papp) of the test compound across the Caco-2 monolayer was calculated using the following equation.
| Papp(cm/s) = | VR*CR,end | * | 1 |
| Δt | A*(CD,mid-CR,mid) |
VR is the volume at the receiver compartment, and CR,end is the concentration of the test compound in the receiver compartment at the end time point. Δt is the incubation time, and A is the surface area of the cell monolayer. CD,mid is a mid-point concentration of the test compound in the donor compartment, which is calculated as the mean value of the donor concentration at time point zero and the donor concentration at the end time point. CR,mid is the mid-point concentration of the test compound in the receiver side, which is half the concentration at the receiver compartment at the end time point. Concentrations of the test compound were expressed as peak areas of the test compound.
The recovery of the test compound was calculated using the following equation.
| Recovery (%) = | VD*CD,end+ Σ (C s(t) VS(t)) +VR*CR,end | *100 |
| VD*CD0 |
CD and CR are the concentrations at donor (D) and receiver (R) compartment at the start (0) or end (end) of the experiment, C S(t) is the concentrations of the samples withdrawn at different time points, t, and V is used for each of the respective volumes.
4.14.3. Fluorescein permeability
Fluorescein was used as the quantitative integrity control for Caco-2 cell monolayers. At the end of the experiment, fluorescein permeability assessment (in the A-B direction at pH 7.4 on both sides) was performed. The cell monolayer with a fluorescein permeability of less than 1.5 × 10−6 cm/s for Caco-2 cells was defined as reflecting an intact monolayer, and the permeability result of the test compound from an intact cell monolayer is reported.
4.15. In vivo safety study
Adult female Balb/c mice weighting 18–25 gm were obtained from The Jackson Laboratory (Bar Harbor, ME) and housed in Sharjah Institute for Medical Research, the University of Sharjah at constant temperature (25 ± 2 °C), humidity (60 ± 10%) and a 12/12 h light/dark cycle. Mice were provided with standard chow and water ad libitum. Additionally, animals were acclimatized for 7 days before starting the experimental work. All animal handling and procedures were approved by the University of Sharjah Animal Care and Use Committee (Reference number: ACUC-08-02-2022). The study was composed of single and multiple-dose phases to identify the highest dose of SIMR3030 that does not cause undesirable side effects or overt toxicity. In the single-dose toxicity study, SIMR3030 (25, 50, and 100 mg/kg) or vehicle (0.5% DMSO in PBS) was administered through intraperitoneal injection to single adult Balb/c mice. A period of at least 24 h was allowed between the testing of each animal. When the selected dose did not cause acute death or a significant change in body weight within 48 h of compound administration, two more mice were introduced to that group and were observed daily for 16 days. A total of three animals were used for each dose level investigated. While in the multiple-dose toxicity study, animals were divided into 3 groups randomly, which received daily doses of the vehicle or test substance SIMR3030 (12.5, 25 mg/kg) through intraperitoneal injection for 14 days. Animals were then subjected to follow-up observations for 12 days post-treatment to detect any possible reversibility, persistence, or delayed occurrence of toxic effects.
4.16. Hematoxylin and eosin staining
To observe microscopic histological changes in collected organs (liver, kidney, spleen, heart, and lung), hematoxylin and eosin (H&E) staining was performed. Following collection, tissues were immediately fixed in 10% neutral-buffered formalin for 24 h. Next, tissues were dehydrated and paraffin-embedded through different stepwise phases, as detailed before [64]. Finally, 4-μm-thick tissue sections were prepared for hematoxylin and eosin stain staining. The stained slides were evaluated through an electric light microscope and used the following histological scoring Nil = 0; mild = 1; moderate = 2 and severe = 3.
4.17. Computational study
4.17.1. Preparation of protease structures
Compounds under investigation were drawn using the software ChemDraw Ultra®, and for each compound, the lowest energy conformation was obtained using MOPAC2012 program embedded within VEGA-ZZ® [65]. The accurate energy minimization was achieved by Austin Model-1 (AM1) semi-empirical force-field within MOPAC.
4.17.2. Preparation of protease structure
The X-ray crystal structures of the wild-type SARS-CoV-2 papain-like protease (PDB-ID: 7JRN) and for the MERS-CoV papain-like protease (PDB-ID: 4RF1) were retrieved from the RCSB Protein Data Bank [66]. Complexed inhibitors and water molecules were extracted from the initial structures. Autodock Tools (MGL Tools 1.5.6rc2) were utilized for adding polar hydrogens and generating the proper Gastieger charges.
4.17.3. Molecular docking
Molecular modeling was performed using AutoDock Vina software [67]. The compounds under investigation were treated by adding polar hydrogens and Gastieger charges. Then, grid boxes of size 20 Å3 were established to cover the active sites of the SARS-CoV-2 PLpro and MERS-CoV PLpro, with a spacing of 1.0 Å between the grid points and centered towards the coordinates of 9.82 (x), −11.83 (y), 32.76 (z) for the SARS-CoV-2 and towards the coordinates of 10.66 (x), 45.42 (y), 29.59 (z) for MERS-CoV PLpro. The exhaustiveness and the number of poses were set to 12 and 10, respectively. Furthermore, the best-docked pose from each docking experiment was considered for further analysis.
4.17.4. Molecular dynamic
Molecular dynamic simulations for the investigated compounds (SIMR1493, SIMR2304 and SIMR3030) concerning SARS-CoV-2 papain-like protease were started from the earlier docked complexes. Dynamic simulation studies were carried out employing Desmond® software embedded within Maestro environment. OPLS_2005 force field parameters were used during all calculations. Each complex was subjected to the same dynamic protocol; the system was solvated using TIP3P explicit water model and 0.15 M NaCl to resemble the physiological ionic strength. Later, system relaxation was achieved by performing a series of short (2000 iterations) restrained and non-restrained solute minimization steps followed by short 12 ps simulation steps using NVT and NPT ensembles. Subsequently, the production run was carried out for a period of 50 ns using the NPT ensemble class, integrating the equation of motion every 2 fs and setting the temperature and pressure to 300 K° and 1 atm, respectively. The short-range interactions (van-der Waals) cut-off was set to 9 Å, while the long-range electrostatic interactions were calculated employing the particle mesh Ewald (PME) method. Trajectories were visualized within Maestro environment, and the results were analyzed using the Desmond interaction diagram panel.
Ethics approval and consent to participate
This study was approved by the University of Sharjah Animal Care and Use Committee (Approval number ACUC-08-02-2022).
Authors’ contributions
T.H.A. designed the compound library with help from A.S and V.S. A.S and V.S synthesized the compounds. H.A.O., designed and supervised the experiments. F.H., A.S.,V.S., A.M., A.K.A.,C.Z, performed the experiments, analyzed the data. F.H., D.M.Z., H.A.O. and T.H.A. wrote the manuscript. F.H., D.M.Z., performed animal experiments. F.H., H.A.O., I.Y.H., studied and analyzed the histopathology of animal organs. H.T. performed and analyzed the molecular modeling studies. T.H.A. and H.A.O. conceived the project. H.A.O. and T.H.A. supervised the work and wrote and edited the manuscript. D.M.Z, H.A.O., T.H.A., S.-L. L and A.M. reviewed the data and manuscript. All authors discussed the results and commented on the manuscript. All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Taleb H. Al-Tel reports financial support was provided by Al Jalila Foundation.
Acknowledgments
This work was supported by grants from Al Jalila Foundation (grant number AJF202058), the Research Funding Department at the University of Sharjah (grant number CoV19-0306), and the American University of Sharjah (grants numbers FRG21-M-S26 and FRG21-M-S27) granted to A.F.M. and I.A.A.Work in S.-L. Liu's lab was supported by funds provided by a private donor to The Ohio State University. The authors highly acknowledge the ADME studies carried out in the research group of Professors Magid Abou-Gharbia and Wayne Childers at Moulder Center for Drug Discovery Research, School of Pharmacy, Temple University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejmech.2023.115380.
Abbreviations
- Covid-19
Coronavirus Disease 2019
- CCL-5
Chemokine (C–C motif) ligand 5
- CXCL-10
CXC motif chemokine ligand 10
- IFN
interferon, IL-6: Interleukin 6
- ISG15
ubiquitin-like interferon-stimulated gene 15 protein
- MERS-CoV
Middle East respiratory syndrome coronavirus
- OAS1
2′-5′-oligoadenylate synthetase 1
- ORF1b
open reading frames
- P-gp:
P-glycoprotein
- PLpro
papain-like protease
- RdRp:
RNA-dependent RNA polymerase
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- 3CLpro
; Main protease
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Adil M.T., Rahman R., Whitelaw D., Jain V., Al-Taan O., Rashid F., Munasinghe A., Jambulingam P. SARS-CoV-2 and the pandemic of COVID-19, Postgrad. Med. J. 2021;97:110–116. doi: 10.1136/postgradmedj-2020-138386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.V'Kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Z., Lian X., Su X., Wu W., Marraro G.A., Zeng Y. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir. Res. 2020;21:224. doi: 10.1186/s12931-020-01479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan H., Hu Y., Jadhav P., Tan B., Wang J. Progress and challenges in targeting the SARS-CoV-2 papain-like protease. J. Med. Chem. 2022;65:7561–7580. doi: 10.1021/acs.jmedchem.2c00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannalire R., Cerchia C., Beccari A.R., Di Leva F.S., Summa V. Targeting SARS-CoV-2 proteases and polymerase for COVID-19 treatment: state of the art and future opportunities. J. Med. Chem. 2022;65:2716–2746. doi: 10.1021/acs.jmedchem.0c01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fatkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C., Members A.-S.G. Remdesivir for the treatment of covid-19 - final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chera A., Tanca A. Remdesivir: the first FDA-approved anti-COVID-19 treatment for young children. Discoveries. 2022;10:e151. doi: 10.15190/d.2022.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanne J.H. Covid-19: FDA authorizes pharmacists to prescribe Paxlovid, B.M. J. 2022;378:o1695. doi: 10.1136/bmj.o1695. [DOI] [PubMed] [Google Scholar]
- 9.Menendez J.C. Approaches to the potential therapy of COVID-19: a general overview from the medicinal chemistry perspective. Molecules. 2022;27 doi: 10.3390/molecules27030658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabinger F., Stiller C., Schmitzova J., Dienemann C., Kokic G., Hillen H.S., Hobartner C., Cramer P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 2021;28:740–746. doi: 10.1038/s41594-021-00651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ton A.T., Pandey M., Smith J.R., Ban F., Fernandez M., Cherkasov A. Targeting SARS-CoV-2 papain-like protease in the postvaccine era. Trends Pharmacol. Sci. 2022;43:906–919. doi: 10.1016/j.tips.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Z., Huang B., Tang J., Liu S., Liu M., Ye Y., Liu Z., Xiong Y., Zhu W., Cao D., Li J., Niu X., Zhou H., Zhao Y.J., Zhang G., Huang H. The complex structure of GRL0617 and SARS-CoV-2 PLpro reveals a hot spot for antiviral drug discovery. Nat. Commun. 2021;12:488. doi: 10.1038/s41467-020-20718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang H., Yang P., Zhang J. Potential inhibitors targeting papain-like protease of SARS-CoV-2: two birds with one stone. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.822785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calleja D.J., Lessene G., Komander D. Inhibitors of SARS-CoV-2 PLpro. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.876212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ton A.T., Pandey M., Smith J.R., Ban F., Fernandez M., Cherkasov A. Targeting SARS-CoV-2 papain-like protease in the postvaccine era. Trends Pharmacol. Sci. 2022 doi: 10.1016/j.tips.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maiti B.K. Can papain-like protease inhibitors halt SARS-CoV-2 replication? A.C.S. Pharmacol. Transl. Sci. 2020;3:1017–1019. doi: 10.1021/acsptsci.0c00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rut W., Lv Z., Zmudzinski M., Patchett S., Nayak D., Snipas S.J., El Oualid F., Huang T.T., Bekes M., Drag M., Olsen S.K. Activity profiling and crystal structures of inhibitor-bound SARS-CoV-2 papain-like protease: a framework for anti-COVID-19 drug design. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abd4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin D., Mukherjee R., Grewe D., Bojkova D., Baek K., Bhattacharya A., Schulz L., Widera M., Mehdipour A.R., Tascher G., Geurink P.P., Wilhelm A., van der Heden van Noort G.J., Ovaa H., Muller S., Knobeloch K.P., Rajalingam K., Schulman B.A., Cinatl J., Hummer G., Ciesek S., Dikic I. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020;587:657–662. doi: 10.1038/s41586-020-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malakhova O.A., Zhang D.E. ISG15 inhibits Nedd4 ubiquitin E3 activity and enhances the innate antiviral response. J. Biol. Chem. 2008;283:8783–8787. doi: 10.1074/jbc.C800030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivasulu V., Khanfar M., Omar H.A., ElAwady R., Sieburth S.M., Sebastian A., Zaher D.M., Al-Marzooq F., Hersi F., Al-Tel T.H. Sequencing [4 + 1]-cycloaddition and aza-michael addition reactions: a diastereoselective cascade for the rapid access of pyrido[2',1':2,3]/thiazolo[2',3':2,3]imidazo[1,5-a]quinolone scaffolds as potential antibacterial and anticancer motifs. J. Org. Chem. 2019;84:14476–14486. doi: 10.1021/acs.joc.9b01919. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasulu V., Schilf P., Ibrahim S., Shehadi I.A., Malik O.G., Sieburth S., Khanfar M.A., Hamad M., Abu-Yousef I.A., Majdalawieh A.F., Al-Tel T.H. Divergent strategy for diastereocontrolled synthesis of small- and medium-ring architectures. J. Org. Chem. 2020;85:10695–10708. doi: 10.1021/acs.joc.0c01244. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasulu V., Sieburth S.M., Khanfar M.A., Abu-Yousef I.A., Majdalawieh A., Ramanathan M., Sebastian A., Al-Tel T.H. Stereoselective late-stage transformations of indolo[2,3-a]quinolizines skeleta to nature-inspired scaffolds. J. Org. Chem. 2021;86:12872–12885. doi: 10.1021/acs.joc.1c01523. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasulu V., Srikanth G., Khanfar M.A., Abu-Yousef I.A., Majdalawieh A.F., Mazitschek R., Setty S.C., Sebastian A., Al-Tel T.H. Stereodivergent Complexity-to-Diversity Strategy en Route to the Synthesis of Nature-Inspired Skeleta. J. Org. Chem. 2022;87:1377–1397. doi: 10.1021/acs.joc.1c02698. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasulu V., Mazitschek R., Kariem N.M., Reddy A., Rabeh W.M., Li L., O'Connor M.J., Al-Tel T.H. Modular Bi-directional one-pot strategies for the diastereoselective synthesis of structurally diverse collections of constrained beta-carboline-benzoxazepines. Chemistry. 2017;23:14182–14192. doi: 10.1002/chem.201702495. [DOI] [PubMed] [Google Scholar]
- 25.Srinivasulu V., Schilf P., Ibrahim S., Khanfar M.A., Sieburth S.M., Omar H., Sebastian A., AlQawasmeh R.A., O'Connor M.J., Al-Tel T.H. Multidirectional desymmetrization of pluripotent building block en route to diastereoselective synthesis of complex nature-inspired scaffolds. Nat. Commun. 2018;9:4989. doi: 10.1038/s41467-018-07521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivasulu V., Shehadeh I., Khanfar M.A., Malik O.G., Tarazi H., Abu-Yousef I.A., Sebastian A., Baniowda N., O'Connor M.J., Al-Tel T.H. One-pot synthesis of diverse collections of benzoxazepine and indolopyrazine fused to heterocyclic systems. J. Org. Chem. 2019;84:934–948. doi: 10.1021/acs.joc.8b02878. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasulu V., Sieburth S.M., El-Awady R., Kariem N.M., Tarazi H., O'Connor M.J., Al-Tel T.H. Post-ugi cascade transformations for accessing diverse chromenopyrrole collections. Org. Lett. 2018;20:836–839. doi: 10.1021/acs.orglett.7b03986. [DOI] [PubMed] [Google Scholar]
- 28.Osipiuk J., Azizi S.A., Dvorkin S., Endres M., Jedrzejczak R., Jones K.A., Kang S., Kathayat R.S., Kim Y., Lisnyak V.G., Maki S.L., Nicolaescu V., Taylor C.A., Tesar C., Zhang Y.A., Zhou Z., Randall G., Michalska K., Snyder S.A., Dickinson B.C., Joachimiak A. Structure of papain-like protease from SARS-CoV-2 and its complexes with non-covalent inhibitors. Nat. Commun. 2021;12:743. doi: 10.1038/s41467-021-21060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di L., Kerns E.H. Biological assay challenges from compound solubility: strategies for bioassay optimization. Drug Discov. Today. 2006;11:446–451. doi: 10.1016/j.drudis.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Ibanez G., Calder P.A., Radu C., Bhinder B., Shum D., Antczak C., Djaballah H. Evaluation of compound optical interference in high-content screening. S.L.A.S. Discov. 2018;23:321–329. doi: 10.1177/2472555217707725. [DOI] [PubMed] [Google Scholar]
- 31.Ma C., Wang J. Validation and invalidation of SARS-CoV-2 papain-like protease inhibitors. ACS Pharmacol Transl Sci. 2022;5:102–109. doi: 10.1021/acsptsci.1c00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freitas B.T., Durie I.A., Murray J., Longo J.E., Miller H.C., Crich D., Hogan R.J., Tripp R.A., Pegan S.D. Characterization and noncovalent inhibition of the deubiquitinase and deISGylase activity of SARS-CoV-2 papain-like protease. ACS Infect. Dis. 2020;6:2099–2109. doi: 10.1021/acsinfecdis.0c00168. [DOI] [PubMed] [Google Scholar]
- 33.Mostafa A., Kandeil A., Y A.M.M.E., Kutkat O., Moatasim Y., Rashad A.A., Shehata M., Gomaa M.R., Mahrous N., Mahmoud S.H., GabAllah M., Abbas H., Taweel A.E., Kayed A.E., Kamel M.N., Sayes M.E., Mahmoud D.B., El-Shesheny R., Kayali G., Ali M.A. FDA-approved drugs with potent in vitro antiviral activity against severe acute respiratory syndrome coronavirus 2. Pharmaceuticals. 2020;13 doi: 10.3390/ph13120443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munnur D., Teo Q., Eggermont D., Lee H.H.Y., Thery F., Ho J., van Leur S.W., Ng W.W.S., Siu L.Y.L., Beling A., Ploegh H., Pinto-Fernandez A., Damianou A., Kessler B., Impens F., Mok C.K.P., Sanyal S. Altered ISGylation drives aberrant macrophage-dependent immune responses during SARS-CoV-2 infection. Nat. Immunol. 2021;22:1416–1427. doi: 10.1038/s41590-021-01035-8. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y.J., Davis C.D., Dworetzky S., Fitzpatrick W.C., Harden D., He H., Knox R.J., Newton A.E., Philip T., Polson C., Sivarao D.V., Sun L.Q., Tertyshnikova S., Weaver D., Yeola S., Zoeckler M., Sinz M.W. Fluorine substitution can block CYP3A4 metabolism-dependent inhibition: identification of (S)-N-[1-(4-fluoro-3- morpholin-4-ylphenyl)ethyl]-3- (4-fluorophenyl)acrylamide as an orally bioavailable KCNQ2 opener devoid of CYP3A4 metabolism-dependent inhibition. J. Med. Chem. 2003;46:3778–3781. doi: 10.1021/jm034111v. [DOI] [PubMed] [Google Scholar]
- 36.Press B., Di Grandi D. Permeability for intestinal absorption: Caco-2 assay and related issues. Curr. Drug Metabol. 2008;9:893–900. doi: 10.2174/138920008786485119. [DOI] [PubMed] [Google Scholar]
- 37.Bosken Y.K., Cholko T., Lou Y.C., Wu K.P., Chang C.A. Insights into dynamics of inhibitor and ubiquitin-like protein binding in SARS-CoV-2 papain-like protease. Front. Mol. Biosci. 2020;7:174. doi: 10.3389/fmolb.2020.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ibrahim T.M., Ismail M.I., Bauer M.R., Bekhit A.A., Boeckler F.M. Supporting SARS-CoV-2 papain-like protease drug discovery: in silico methods and benchmarking. Front. Chem. 2020;8 doi: 10.3389/fchem.2020.592289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ismail M.I., Ragab H.M., Bekhit A.A., Ibrahim T.M. Targeting multiple conformations of SARS-CoV2 Papain-Like Protease for drug repositioning: an in-silico study. Comput. Biol. Med. 2021;131 doi: 10.1016/j.compbiomed.2021.104295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 41.Bok K., Sitar S., Graham B.S., Mascola J.R. Accelerated COVID-19 vaccine development: milestones, lessons, and prospects. Immunity. 2021;54:1636–1651. doi: 10.1016/j.immuni.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Focosi D., McConnell S., Casadevall A. The Omicron variant of concern: diversification and convergent evolution in spike protein, and escape from anti-Spike monoclonal antibodies. Drug Resist. Updates. 2022;65 doi: 10.1016/j.drup.2022.100882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui K.P.Y., Ho J.C.W., Cheung M.C., Ng K.C., Ching R.H.H., Lai K.L., Kam T.T., Gu H., Sit K.Y., Hsin M.K.Y., Au T.W.K., Poon L.L.M., Peiris M., Nicholls J.M., Chan M.C.W. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603:715–720. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- 44.Wilhelm A., Widera M., Grikscheit K., Toptan T., Schenk B., Pallas C., Metzler M., Kohmer N., Hoehl S., Marschalek R., Herrmann E., Helfritz F.A., Wolf T., Goetsch U., Ciesek S. Limited neutralization of the SARS-CoV-2 Omicron subvariants BA.1 and BA.2 by convalescent and vaccine serum and monoclonal antibodies. EBioMedicine. 2022;82 doi: 10.1016/j.ebiom.2022.104158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans J.P., Zeng C., Qu P., Faraone J., Zheng Y.M., Carlin C., Bednash J.S., Zhou T., Lozanski G., Mallampalli R., Saif L.J., Oltz E.M., Mohler P.J., Xu K., Gumina R.J., Liu S.L. Neutralization of SARS-CoV-2 Omicron sub-lineages BA.1, BA.1.1, and BA.2. Cell Host Microbe. 2022;30:1093–1102 e1093. doi: 10.1016/j.chom.2022.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qu P., Evans J.P., Zheng Y.M., Carlin C., Saif L.J., Oltz E.M., Xu K., Gumina R.J., Liu S.L. Evasion of neutralizing antibody responses by the SARS-CoV-2 BA.2.75 variant. Cell Host Microbe. 2022;30:1518–1526 e1514. doi: 10.1016/j.chom.2022.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qu P., Faraone J.N., Evans J.P., Zheng Y.M., Yu L., Ma Q., Carlin C., Lozanski G., Saif L.J., Oltz E.M., Gumina R.J., Liu S.L. Durability of booster mRNA vaccine against SARS-CoV-2 BA.2.12.1, BA.4, and BA.5 subvariants. N. Engl. J. Med. 2022;387:1329–1331. doi: 10.1056/NEJMc2210546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qu P., Evans J.P., Faraone J.N., Zheng Y.M., Carlin C., Anghelina M., Stevens P., Fernandez S., Jones D., Lozanski G., Panchal A., Saif L.J., Oltz E.M., Xu K., Gumina R.J., Liu S.L. Cell Host Microbe; 2022. Enhanced Neutralization Resistance of SARS-CoV-2 Omicron Subvariants BQ.1. BQ.1.1, BA.4.6, BF.7, and BA.2.75.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y., Binder J., Yurgelonis I., Rai D.K., Lazarro S., Costales C., Kobylarz K., McMonagle P., Steppan C.M., Aschenbrenner L., Anderson A.S., Cardin R.D. Generation of a VeroE6 Pgp gene knock out cell line and its use in SARS-CoV-2 antiviral study. Antivir. Res. 2022;208 doi: 10.1016/j.antiviral.2022.105429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zito Marino F., De Cristofaro T., Varriale M., Zannini G., Ronchi A., La Mantia E., Campobasso C.P., De Micco F., Mascolo P., Municino M., Municino E., Vestini F., Pinto O., Moccia M., De Stefano N., Nappi O., Sementa C., Zotti G., Pianese L., Giordano C., Franco R. Variable levels of spike and ORF1ab RNA in post-mortem lung samples of SARS-CoV-2-positive subjects: comparison between ISH and RT-PCR. Virchows Arch. 2022;480:597–607. doi: 10.1007/s00428-021-03262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis D.S.M., Ho J., Wills S., Kawall A., Sharma A., Chavada K., Ebert M., Evoli S., Singh A., Rayalam S., Mody V., Taval S. Aloin isoforms (A and B) selectively inhibits proteolytic and deubiquitinating activity of papain like protease (PLpro) of SARS-CoV-2 in vitro. Sci. Rep. 2022;12:2145. doi: 10.1038/s41598-022-06104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodriguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sa Ribero M., Jouvenet N., Dreux M., Nisole S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tripathy A.S., Vishwakarma S., Trimbake D., Gurav Y.K., Potdar V.A., Mokashi N.D., Patsute S.D., Kaushal H., Choudhary M.L., Tilekar B.N., Sarje P., Dange V.S., Abraham P. Pro-inflammatory CXCL-10, TNF-alpha, IL-1beta, and IL-6: biomarkers of SARS-CoV-2 infection. Arch. Virol. 2021;166:3301–3310. doi: 10.1007/s00705-021-05247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masood K.I., Yameen M., Ashraf J., Shahid S., Mahmood S.F., Nasir A., Nasir N., Jamil B., Ghanchi N.K., Khanum I., Razzak S.A., Kanji A., Hussain R., M E.R., Hasan Z. Upregulated type I interferon responses in asymptomatic COVID-19 infection are associated with improved clinical outcome. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-02489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kimura A., Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur. J. Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 57.Schaper F., Rose-John S. Interleukin-6: biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015;26:475–487. doi: 10.1016/j.cytogfr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Masia M., Fernandez-Gonzalez M., Garcia J.A., Padilla S., Garcia-Abellan J., Botella A., Mascarell P., Agullo V., Gutierrez F. Robust long-term immunity to SARS-CoV-2 in patients recovered from severe COVID-19 after interleukin-6 blockade. EBioMedicine. 2022;82 doi: 10.1016/j.ebiom.2022.104153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matthay M.A., Luetkemeyer A.F. IL-6 receptor antagonist therapy for patients hospitalized for COVID-19: who, when, and how? JAMA. 2021;326:483–485. doi: 10.1001/jama.2021.11121. [DOI] [PubMed] [Google Scholar]
- 60.Feoktistova M., Geserick P., Leverkus M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016 doi: 10.1101/pdb.prot087379. 2016) pdb prot087379. [DOI] [PubMed] [Google Scholar]
- 61.Kutkat O., Moatasim Y., Al-Karmalawy A.A., Abulkhair H.S., Gomaa M.R., El-Taweel A.N., Abo Shama N.M., GabAllah M., Mahmoud D.B., Kayali G., Ali M.A., Kandeil A., Mostafa A. Robust antiviral activity of commonly prescribed antidepressants against emerging coronaviruses: in vitro and in silico drug repurposing studies. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-17082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manandhar A., Srinivasulu V., Hamad M., Tarazi H., Omar H., Colussi D.J., Gordon J., Childers W., Klein M.L., Al-Tel T.H., Abou-Gharbia M., Elokely K.M. Discovery of novel small-molecule inhibitors of SARS-CoV-2 main protease as potential leads for COVID-19 treatment. J. Chem. Inf. Model. 2021;61:4745–4757. doi: 10.1021/acs.jcim.1c00684. [DOI] [PubMed] [Google Scholar]
- 63.Peng Y., Yadava P., Heikkinen A.T., Parrott N., Railkar A. Applications of a 7-day Caco-2 cell model in drug discovery and development. Eur. J. Pharmaceut. Sci. 2014;56:120–130. doi: 10.1016/j.ejps.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Tolba M.F., El-Serafi A.T., Omar H.A. Caffeic acid phenethyl ester protects against glucocorticoid-induced osteoporosis in vivo: impact on oxidative stress and RANKL/OPG signals. Toxicol. Appl. Pharmacol. 2017;324:26–35. doi: 10.1016/j.taap.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 65.Pedretti A., Villa L., Vistoli G. VEGA--an open platform to develop chemo-bio-informatics applications, using plug-in architecture and script programming. J. Comput. Aided Mol. Des. 2004;18:167–173. doi: 10.1023/b:jcam.0000035186.90683.f2. [DOI] [PubMed] [Google Scholar]
- 66.Lasek W., Janyst M., Wolny R., Zapala L., Bocian K., Drela N. Immunomodulatory effects of inosine pranobex on cytokine production by human lymphocytes. Acta Pharm. 2015;65:171–180. doi: 10.1515/acph-2015-0015. [DOI] [PubMed] [Google Scholar]
- 67.Trott O., Olson A.J., Vina AutoDock. Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H.W., Lo S.K., Chan K.H., Poon V.K., Chan W.M., Ip J.D., Cai J.P., Cheng V.C., Chen H., Hui C.K., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petersen H., Mostafa A., Tantawy M.A., Iqbal A.A., Hoffmann D., Tallam A., Selvakumar B., Pessler F., Beer M., Rautenschlein S., Pleschka S. NS segment of a 1918 influenza A virus-descendent enhances replication of H1N1pdm09 and virus-induced cellular immune response in mammalian and avian systems. Front. Microbiol. 2018;9:526. doi: 10.3389/fmicb.2018.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Du Y., Cui R., Tian N., Chen M., Zhang X.L., Dai S.M. Regulation of type I interferon signature by VGLL3 in the fibroblast-like synoviocytes of rheumatoid arthritis patients via targeting the Hippo pathway. Arthritis Res. Ther. 2022;24:188. doi: 10.1186/s13075-022-02880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin L., Lenz L.L., Cambier J.C. Cellular reactive oxygen species inhibit MPYS induction of IFNbeta. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li G., Ju J., Weyand C.M., Goronzy J.J. Age-associated failure to adjust type I IFN receptor signaling thresholds after T cell activation. J. Immunol. 2015;195:865–874. doi: 10.4049/jimmunol.1402389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chakrabarti A.K., Vipat V.C., Mukherjee S., Singh R., Pawar S.D., Mishra A.C. Host gene expression profiling in influenza A virus-infected lung epithelial (A549) cells: a comparative analysis between highly pathogenic and modified H5N1 viruses. Virol. J. 2010;7:219. doi: 10.1186/1743-422X-7-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bibert S., Roger T., Calandra T., Bochud M., Cerny A., Semmo N., Duong F.H., Gerlach T., Malinverni R., Moradpour D., Negro F., Mullhaupt B., Bochud P.Y., Swiss Hepatitis C.C.S. IL28B expression depends on a novel TT/-G polymorphism which improves HCV clearance prediction. J. Exp. Med. 2013;210:1109–1116. doi: 10.1084/jem.20130012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.