Abstract
Background
Colorectal cancer (CRC) patients in early to mid-adulthood (≤50 years) are challenged by high symptom burden (i.e., pain, fatigue, distress) and age-related stressors (e.g., managing family, work). Cognitive behavioral theory (CBT)-based coping skills training interventions reduce symptoms and improve quality of life in cancer patients. However, traditional CBT-based interventions are not accessible to these patients (e.g., in-person sessions, during work day), nor designed to address symptoms within the context of this stage of life. We developed a mobile health (mHealth) coping skills training program for pain, fatigue and distress (mCOPE) for CRC patients in early to mid-adulthood. We utilize a randomized controlled trial to test the extent to which mCOPE reduces pain, fatigue and distress (multiple primary outcomes) and improves quality of life and symptom self-efficacy (secondary outcomes).
Methods/Design
Patients (N = 160) ≤50 years with CRC endorsing pain, fatigue and/or distress are randomized 1:1 to mCOPE or standard care. mCOPE is a five-session CBT-based coping skills training program (e.g., relaxation, activity pacing, cognitive restructuring) that was adapted for CRC patients in early to mid-adulthood. mCOPE utilizes mHealth technology (e.g., videoconference, mobile app) to deliver coping skills training, capture symptom and skills use data, and provide personalized support and feedback. Self-report assessments are completed at baseline, post-treatment (5–8 weeks post-baseline; primary endpoint), and 3- and 6-months later.
Conclusions
mCOPE is innovative and potentially impactful for CRC patients in early to mid-adulthood. Hypothesis confirmation would demonstrate initial efficacy of a mHealth cognitive behavioral intervention to reduce symptom burden in younger CRC patients.
Keywords: Colorectal cancer, Symptom management, Psycho-oncology, mHealth
1. Background
Colorectal cancer (CRC) incidence and mortality rates have risen sharply in adults under age 50 [1,2]. Consistent with other cancer populations, CRC patients frequently endorse symptoms of pain, fatigue and psychological distress (i.e., anxiety, depression). However, CRC patients face a particularly high and challenging symptom burden due to the nature of the disease and its treatment. For example, CRC patients experience stoma-related, abdominal, and neuropathic pain; treatment-related and emotional fatigue; and psychological distress related to body image, bowel dysfunction, stoma management, and fear of cancer progression or recurrence. Research in CRC patients has demonstrated that pain, fatigue and distress are interrelated and contribute to lower overall quality of life [[3], [4], [5], [6], [7], [8]].
CRC in patients in early to mid-adulthood is often surprising and uniquely challenging, as individuals must manage burdensome cancer symptoms and side effects in the context of age-related stress. Adults ages 18 to 50 are in their busiest and most productive years, often balancing work or education, new or less established romantic relationships, family building or raising children, and/or caring for elderly parents. A cancer diagnosis during this time can be highly disruptive, as managing multiple roles and competing demands requires significant time and energy that may place additional strain on patients’ already compromised physical and mental health. Indeed, age-related factors (e.g., being unpartnered, being in school, being employed) place early and mid-adulthood patients at higher risk for cancer-related distress than senior adults [9].
Psychosocial interventions that address multiple, interrelated CRC symptoms (e.g., pain, fatigue, distress) in the context of age-related challenges could improve disease management and quality of life. There is strong evidence to support the use of cognitive behavioral theory (CBT)-based interventions as an efficacious treatment for cancer-related symptoms [[10], [11], [12], [13]]. Traditional analgesic and other medication regimens for symptom management often do not fully relieve symptoms, and cancer patients report significant medication side effects that may interfere with continuance in life roles [14,15]. CBT-based coping skills training protocols may be particularly effective for this population by reducing multiple symptom severity while also improving patients' confidence in their ability to manage symptoms (e.g., symptom coping self-efficacy), thereby enabling participation in important life roles and improving quality of life [[16], [17], [18], [19], [20]]. For example, cognitive and behavioral coping strategies include relaxation training, activity pacing and planning, and cognitive restructuring, which can improve patients’ abilities to manage cancer-related pain, fatigue and distress [16,17,19], as well as physical and social functioning and overall quality of life [16,17,20,21].
Despite the evidence, CBT interventions for cancer symptom management are seldom employed, and traditional face-to-face approaches to delivering CBT do not meet the unique needs of many CRC patients in early to mid-adulthood. Most research trials on CBT-based coping skills training protocols have been time consuming (number and frequency of sessions), delivered in-person, and conducted with patients with an average age above 55 [[22], [23], [24], [25]]. Intervention protocols must be adapted to increase accessibility and acceptability and tailored to meet the specific and unique needs of patients in early to mid-adulthood dealing with age-related stressors and high time demands.
To address these challenges, we designed a CBT-based mHealth Coping Skills Training for Symptom Management (mCOPE) intervention to target pain, fatigue and psychological distress in CRC patients in early to mid-adulthood. mCOPE is delivered using mHealth technology (videoconferencing, adjunctive mobile app). The rationale, design, methods, and analytic plan for a prospective randomized controlled trial (RCT) comparing mCOPE to care as usual are presented. The RCT aims to: 1) test the extent to which mCOPE improves patient-reported symptoms (i.e., pain, fatigue, distress; multiple primary outcomes) and quality of life (secondary outcome) from baseline to post-treatment compared to Standard Care (SC), and 2) examine improved self-efficacy for symptom management as a mediator of the relationship between study arm and symptom severity. It is hypothesized that mCOPE will result in 1) decreased pain, fatigue and distress; 2) increased quality of life; and that 3) the effects of mCOPE on these outcomes will be mediated by self-efficacy.
2. Design and methods
The current study is an ongoing RCT being conducted at Duke Cancer Institute and its satellite sites. This study was approved by the Duke Institutional Review Board and registered with ClinicalTrials.gov (NCT0476317).
2.1. Participants
Participants are recruited from the Duke Cancer Institute, which includes the Duke Cancer Center, Duke Regional Hospital, Duke Cancer Center Raleigh, and Duke Cancer Center Cary. Study enrollment began in September 2021.
Participants will include 160 men and women who meet the following inclusion criteria: 1) ≥18 and ≤ 50 years old, 2) diagnosis of stage I-IV CRC within the past 3 years, 3) self-reported pain, fatigue and/or psychological distress, with a minimum of two of three symptoms reported at ≥3 on 0–10 scale, and 4) English-speaking. Exclusion criteria include: 1) cognitive impairment (e.g., dementia), 2) presence of a severe psychiatric condition (i.e., psychotic disorder/episode, suicidal intent), and 3) participation in a coping skills training protocol in the past 6 months.
2.2. Study procedures
Fig. 1 summarizes study flow. Potentially eligible patients are identified by electronic medical record screening and mailed a letter that includes an opt out phone number. Interested patients are screened for symptom severity. Eligible patients complete informed consent. Participants complete a baseline assessment including demographic, medical and psychosocial questionnaires. Immediately following completion of the baseline assessment, participants are randomly assigned with equal allocation (1:1) to: 1) mHealth Coping Skills Training for Symptom Management (mCOPE) or 2) Standard Care (SC). Participants are re-assessed 5–8 weeks post-randomization (i.e., post-treatment for mCOPE; primary time point) and at 3- and 6-month follow-ups. Randomization and assessments are administered by staff not involved in intervention delivery.
Fig. 1.
Study flow.
2.3. Intervention conditions
2.3.1. mCOPE
2.3.1.1. CBT-based protocols for behavioral symptom management
Protocol development was informed by the literature and our group's experience developing, refining and testing the efficacy of mHealth approaches to CBT-based Pain Coping Skills Training (PCST) interventions in patients with chronic diseases [[17], [18], [19],[22], [23], [24], [25], [26]]. CBT posits that cognitions and behaviors can affect how symptoms impact one's daily life and, in turn, how symptoms affect what we do, think and feel [27]. This evidence has led to the belief that targeting thoughts (e.g., negative thinking) and behaviors (e.g., an overly sedentary and restricted lifestyle) can have a positive effect on multiple areas [10,19,[28], [29], [30], [31]]; CBT-based interventions have demonstrated efficacy in improving patients' management of cancer-related pain, fatigue and distress [16,17,19,31]. These interventions have also led to improvements in symptom coping self-efficacy and quality of life [16,17,20,21]. Therefore, cognitive behavioral coping skills training protocols may be particularly beneficial for improving CRC patients' abilities to cope with multiple, interrelated symptoms.
2.3.1.2. mCOPE protocol development
In intervention research, adaptation refers to changes in protocol content and/or delivery to meet the needs of a particular group, while tailoring refers to personalizing content to meet the needs of an individual participant [32,33]. The mCOPE protocol was developed based on a previous pilot randomized trial demonstrating the feasibility, engagement, and acceptability of a coping skills training program for CRC survivors [26]. The mCOPE protocol was adapted from existing mHealth CBT-based coping skills training protocols developed by our team to address the unique symptom needs and stage-of-life challenges faced by CRC patients in early to mid-adulthood, and coping skills examples and applications are tailored to the specific needs and experiences of each participant. Key elements of protocol adaptation and tailoring are described below and in Table 1.
Table 1.
How mCOPE Addresses the Unique Needs and Challenges of Colorectal Cancer Patients in Early to Mid-Adulthood.
| Symptom Needs & Life Challenges | mCOPE Protocol is Designed to Address These Unique Needs |
|---|---|
| High levels of pain, fatigue and distress, and uncertainty about how to control symptoms |
|
| Pain, fatigue and distress specific to colorectal cancer patients (e.g., bowel dysfunction, abdominal pain, treatment-related fatigue and neuropathic pain, stoma -related distress) |
|
| High time demands |
|
| Stress related to managing multiple life roles (e.g., working outside the home, caregiver for young children and/or elderly parents) as well as other stage-of-life challenges (e.g., family planning, concerns about finances and health insurance) |
|
| Negative cognitions related to having cancer as a younger person and the challenges around meeting stage-of-life demands now and in the future. |
|
2.3.1.3. Intervention length and delivery format
Traditional CBT-based symptom coping protocols have been lengthy (e.g., 10–12, 60-minute sessions) and delivered mainly in-person at tertiary medical centers [[29], [30], [31]]. CRC patients in early to mid-adulthood face barriers to participation in such protocols, namely time constraints and the cost and burden of travel (e.g., childcare, time off from work) [[34], [35], [36]]. The mCOPE protocol was adapted to be brief, consisting of five, 45-60-minute sessions. Data suggests that brief behavioral interventions for symptom management in chronic disease populations are efficacious in reducing symptoms and improving quality of life [12,37,38].
Virtual delivery was selected to increase accessibility, feasibility, and effectiveness of mCOPE by expanding patient access and decreasing burden [39]. Recent work conducted by our team suggests that mHealth-delivered protocols are comparable to in-person protocols and may have some distinct advantages, including increased treatment fidelity [39,40]. mHealth technology offers increased convenience and flexibility, as interventions can be delivered to patients in their own environment (e.g., home, work) at convenient times. Videoconference-delivered sessions plus a mobile app was deemed optimal, as this follows the National Institutes of Health recommendation to integrate face-to-face contact with remote strategies to provide balance between technology and human touch when intervening with patients with chronic disease. Our teams' recent experience suggests videoconferencing mirrors in-person sessions and is interactive, personal, and effective [39]. Empirical work also suggests educational and psychosocial content is better communicated through videoconferencing than by phone [41], as videoconference may enhance factors suggested by social cognitive theory to impact self-efficacy for symptom management: mastery, enhanced by practice/feedback in the home environment; vicarious learning, enhanced by visual modeling by the therapist; verbal encouragement, personalized to the patient's home skills practice; and, observation of and addressing negative physiological and affective responses to skills use in the patient's practice environment.
2.3.1.4. Intervention content
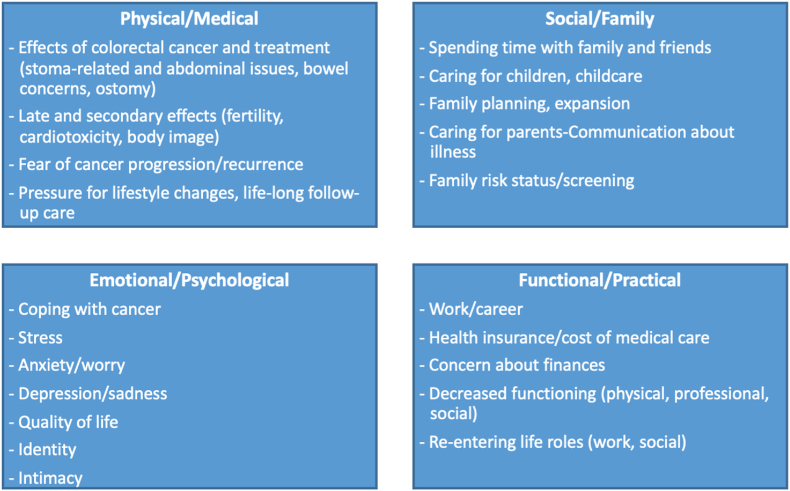
Coping skills training interventions have traditionally delivered a general set of skills using the same examples for all patients. mCOPE Session 1 includes a menu of CRC-specific concerns and stage-of-life challenges for CRC patients in early to mid-adulthood (Fig. 2). Based on patient report, the therapist tailors coping skills applications and examples to the unique and specific symptom needs and stage-of-life challenges of each participant. For example, stoma procedures are common for CRC patients and can cause pain and discomfort, worry, and potentially isolation from normal activities. The therapist works to normalize such concerns and to collaboratively target related pain and distress with relaxation techniques, activity pacing and planning, and cognitive restructuring skills practice. Specifically, patients are taught that relaxation can decrease abdominal tension and pain, as well as distress experienced when in public, thereby decreasing barriers to activities of daily living (e.g., work, dating, social engagements).
Fig. 2.
Colorectal cancer-related and stage-of-life challenges of patients in early to mid-adulthood.
2.3.1.5. mCOPE protocol
The developed mCOPE protocol involves two key components: 1) five 45-60-minute videoconference-delivered individual sessions with a study therapist and 2) extension of intervention content into the patient's home environment through an adjunct mobile app. mCOPE participants receive a package of study materials, including a tote bag containing a binder with session handouts and tools to facilitate engagement in skills practice. As needed, participants are provided with an iPhone with data plan to access videoconferencing sessions and the mobile app. A summary of session content and mobile app components is provided in Table 2. Frames of the mobile app are presented in Fig. 3. Detailed descriptions of study components are provided in Table 3 (below).
Table 2.
Overview of mCOPE Intervention Components by Session.
| Intervention Component | Session 1 | Session 2 | Session 3 | Session 4 | Session 5 |
|---|---|---|---|---|---|
| Content by Videoconference Session |
|
|
|
|
|
| Mobile Application Features by Session |
|
|
|
|
|
| Videoconference Elements Across Sessions |
|
||||
| Mobile Application Features Across Sessions |
|
||||
Fig. 3.
Frames of the study mobile app.
Table 3.
Detailed descriptions of study components by session.
| Session 1. Participants introduce themselves and describe their experience with cancer and related symptoms, including pain, fatigue and distress. The rationale for cognitive behavioral coping skills training is presented. Examples of CRC-specific and stage-of-life challenges are reviewed, and challenges specific to the patient are identified to inform tailoring of skill applications (Fig. 2). A simplified theoretical rationale for the program grounded in the Neuromatrix Theory of Pain is presented [[44], [45], [46], [47]]. This introduces the concept that how one thinks about symptoms, what one does, and how one feels can impact the symptom experience, and how these elements are interrelated. A menu of skills to be taught is presented and the first skill, Progressive Muscle Relaxation (PMR), is introduced. Participants are provided with a rationale for the skill, then taught the skill through therapist instruction and video demonstration. The participant and therapist practice the skill together using a guided audio-recording, then discuss the participants' experience. Participants are asked to practice PMR 1–2 times daily until the next session. Problem solving is used to identify time(s) and place(s) where the patient can practice and to identify barriers and solutions. |
| Mobile Application. Participants use the app in session to view a video demonstration of PMR and to access a guided audio-recording. The app is used to facilitate PMR review and home practice. |
| Session 2. Participants learn activity/rest cycling as a strategy for activity pacing. Often in the context of cancer and its treatment, patients find their activity levels fluctuate as a function of their symptoms. Some patients overdo activities when feeling good, which can exacerbate symptoms and require extended rest. Conversely, some patients limit or avoid activity due to fear of exacerbating symptoms, leading to a cycle of avoidance. Activity/rest cycling teaches patients to schedule both activity and rest to improve functioning, productivity and mood. In addition, participants are taught pleasant activity planning. Participants are encouraged to generate a list of activities that may be enjoyable and/or meaningful. Increasing engagement in pleasant activities can help to distract patients from symptoms, increase patients' sense of meaning and purpose, and reduce distress. Participants are encouraged to think creatively about how to adapt activities to be accessible in the context of cancer and stage-of-life challenges. |
| Mobile Application. Participants complete a journal entry in the app, noting what pleasant activities they will engage in during the week. Video demonstration of the activity/rest cycle and pleasant activity planning skills are available for review. |
| Session 3. Participants learn cognitive restructuring to recognize how thoughts can impact feelings and behavior and the ability to cope with symptoms. Participants are introduced to the cognitive behavioral triad to demonstrate how thoughts, feelings and behavior are interrelated and how negative thoughts about symptoms can negatively impact feelings and behavior. Common examples of unhelpful thoughts are described (e.g., “shoulds”, all-or-nothing thinking), and unhelpful thoughts that resonate with the participant are identified. The diagram of stage-of-life challenges (Fig. 2) is used to help identify areas where patients may be prone to unhelpful thinking. Strategies to challenge and shift unhelpful thinking and to generate more balanced thinking are introduced. Participants watch a video demonstration of how to challenge and shift unhelpful cancer-related thoughts, then apply cognitive restructuring to a personal cancer-related thought. Calming self-statements are introduced (e.g., “This too shall pass”; “Let it go”). |
| Mobile Application. Participants complete a journal entry listing a calming self-statement that resonates with them. This unique statement is then sent as a text message the following week. Brief videos reviewing cognitive restructuring are available. |
| Session 4. Participants learn and practice mini-relaxation, a brief, portable relaxation technique that combines muscle relaxation and deep breathing. Participants identify internal (e.g., pain) and external (e.g., work) cues as reminders to engage in mini-relaxation practices. Next, participants learn pleasant imagery, using the mind to create a pleasant scene. Participants identify personally pleasant scenes and are taught to utilize their five senses to vividly imagine the scenes. Pleasant imagery is practiced in session. |
| Mobile Application. Participants practice mini-relaxation and pleasant imagery using app-based audio-recordings. Brief videos describing these skills are available for review. |
| Session 5. All coping skills are reviewed and benefits and challenges associated with practicing skills are discussed. Participants create a coping skills practice schedule and problem solve barriers that might interfere with continued practice. Goal setting is introduced as a final tool to plan for continued coping skills use, and continued use is emphasized as a strategy to help patients manage symptoms and stressors and to engage in meaningful activities. |
| Mobile Application. Participants have access to the app for one year following session completion and continued utilization is encouraged. |
Videoconference Session Overview and Structure. Videoconference sessions are conducted using Zoom. Sessions are scheduled weekly, while allowing flexibility for conflicts and to accommodate the competing demands of participants, and are audio-recorded for quality assurance. The virtual sessions mimic in-person sessions, with study handouts being shared on screen.
Sessions include the therapist teaching cognitive behavioral coping skills to target pain, fatigue and psychological distress. Skill-related examples and discussion of skills application is tailored to the participant. Sessions begin with a review of skills and home practice, including encouragement, problem solving, and planning for continued skills practice. New material is introduced using didactic and experiential components. The therapist models skills, probes for patient reactions, and provides feedback and encouragement. Patients are assigned brief home practice assignments to incorporate session content into daily life to facilitate skill acquisition and generalization [42,43]. Content is summarized in handouts in the study binder and on the app.
Mobile App Overview. The mobile app (Pattern Heath; Durham, NC) provides the following features: 1) accessible coping skills content (audio and video demonstrations), 2) regular assessment of symptoms and coping skills use transmitted in real-time to study staff, and 3) text messaging (3x/week) that includes reminders, encouragement and personalized feedback based on symptoms and coping skills use reports. The app is accessible on Apple iOS and Android devices. Staff assist participants with app download, orientation and technology support.
Quality Assurance. mCOPE sessions are conducted by doctoral-level clinical psychologists with experience delivering CBT-based interventions for cancer symptom management. Interventionists are trained in the manualized protocol by the study PI (Kelleher, PhD). Training includes intensive review of the study manual and recorded session role-plays with a confederate patient, which are reviewed by the PI and evaluated for adherence and competence using a fidelity checklist.
Several steps are taken to ensure consistency with the intervention protocol: 1) the therapist follows a detailed manual, 2) weekly supervision occurs, 3) treatment sessions are audio-recorded, 4) cases are reviewed during bi-weekly supervision and feedback is provided, and 5) ratings of treatment adherence and competence will be conducted and reported back to the supervisor and reviewed with the therapist in bi-weekly supervision sessions. Ten percent of sessions will be randomly selected and reviewed by a senior investigator (not involved in the study) to ensure protocol adherence. Protocol adherence criteria will be developed for each session with satisfactory adherence defined as ≥90% of the maximum possible score on the adherence rating scale [48,49].
2.4. Sandard care
SC participants continue to receive their usual medical care, but not the mCOPE protocol and mobile app. SC participants complete assessments at the same time points as mCOPE participants. SC participants who require or request support services during the study period are provided with an appropriate referral. Participants are not asked to change or decline support services while enrolled.
2.5. Measures
Demographic and Clinical Variables. Demographic and clinical data are collected through medical record review and patient self-report.
Pain. Pain severity is assessed with the four-item Brief Pain Inventory (BPI) [50]. Patients rate their “average pain,” “worst pain,” and “least pain” over the last 7 days, and “pain right now” on a scale from 0 = no pain to 10 = worst pain imaginable. A pain severity score is created as the average of responses.
Fatigue. Fatigue is measured with the Patient-Reported Outcomes Measurement Information System (PROMIS) Adult Fatigue Profile 6-item Short Form [51,52]. Participants evaluate symptoms from mild feelings of tiredness to a debilitating and sustained sense of exhaustion.
Psychological Distress. The Brief Symptom Inventory (BSI) is used to measure psychological distress [[53], [54], [55], [56], [57], [58]]. Example statements include “feeling not interested in things” and “feeling hopeless about the future.” Responses are rated on a 5-point scale from 0 = “not at all” to 4 = “extremely”.
Quality of Life. The Functional Assessment of Cancer Therapy - General (FACT-G), version 4.0, is a 27-item self-report measure that assesses quality of life concerns specific to cancer patients [[59], [60], [61], [62]]. Responses are measured on a 5-point Likert scale and summed to calculate a total score, with higher scores indicating better quality of life.
Self-Efficacy for Symptom Management. The PROMIS Self-Efficacy for Managing Symptoms 8-Item Short Form [63] assesses participants’ confidence in their ability to manage symptoms in different settings and to keep symptoms from interfering with work, sleep, relationship or recreational activities. Items are summed to form a total score, with higher scores indicating greater self-efficacy.
Psychological Services. At each assessment, participants are asked one question about whether they are currently receiving or have received in the past 6 months any type of psychological service (e.g., supportive counseling).
mCOPE Acceptability. The 10-item Client Satisfaction Questionnaire (CSQ) is administered at the post-treatment assessment to measure participants’ satisfaction with treatment [64,65].
Mobile App Process Data. Pattern Health collects process data to describe patient interaction with the app, including number of logins, video views and minutes spent in the app, and usage of specific pages and applications.
2.6. Statistical analyses
Sample Size Calculation and Statistical Power. A total of 160 men and women will be enrolled; 15% attrition is expected, so complete data is expected for 136 participants [40,66]. We will take multiple steps to ensure strong retention. For example, study procedures (e.g., intervention, assessments) are brief and designed to be convenient and flexible for participants. Participants will complete every aspect of this trial remotely; we do not require any in-person appointments.
Assuming a sample size of 68 per group (80/group minus 15% attrition), we have greater than 80% power (alpha = 0.05, 2-tailed) to detect a standardized mean difference of approximately d = 0.50 for pain, fatigue and psychological distress (primary outcomes), which is considered a moderate effect size [23,[67], [68], [69], [70], [71]]. The estimated effect size is similar to what has been found in other symptom coping skills studies [23,70,71].
Preliminary Analyses. Internal consistency of the measures and scale totals will be calculated (Cronbach's alpha). We will examine each measure for sufficient range, outlying values, and highly skewed distributions. Based on data from the pilot study, the outcome measures appear to have a Gaussian distribution and therefore a linear mixed model will be the model of choice. If any outcome measures exhibit non-Gaussian behavior, residuals will be examined and a generalized linear mixed model will be used, with an appropriate distribution chosen based on examination of the residuals. Analyses will utilize an intent-to-treat framework; patients will be analyzed as part of the group to which they are randomized, regardless of treatment compliance. We will compare baseline characteristics of participants who are lost to follow-up or dropout with active participants. We will summarize demographic, clinical, and psychosocial characteristics by intervention arm. Descriptive statistics will describe change in pain, fatigue, distress and quality of life, measured as absolute and percentage change from baseline, separately for each condition.
Aim 1. Primary analysis will examine treatment group differences in pain severity, fatigue, psychological distress (multiple primary outcomes) and quality of life (secondary outcome) from baseline to post-treatment using linear mixed models. We will evaluate each outcome independently and apply a multiplicity adjustment (e.g., Bonferroni) [72]. Given the study design (i.e., repeated measures with the potential for missing values at follow-up), we will use a random effects model. Model parameters will be estimated using the SAS procedure MIXED. An unstructured covariance matrix will be used to account for the within-subject correlation due to repeated measurement. The primary analysis will treat time as categorical, as we will compare differences from baseline to post-treatment across treatment groups (Time 0, 1). Each model will include a fixed effect for treatment time, and a treatment-by-time interaction. A fixed effect for group is not included, since it should be zero due to randomization and omitting it will lead to increased power [73]. A treatment effect will be evidenced by a significant treatment-by-time interaction. We do not anticipate there will be differences in outcomes by study therapist. However, we will test to see if there are any differences in outcomes at post-treatment by therapist. If there are differences, we will use a random effect structure that nests individuals within therapist. Appropriate sensitivity analysis will be carried out to evaluate data missingness and, depending on the extent of missingness, methods will be applied to minimize bias due to missingness. We will conduct supplemental, longitudinal analyses treating time as continuous, evaluating the slope of treatments over time. We will test to see if the slopes are different over time between groups.
Aim 2. Analyses will examine whether changes in self-efficacy mediate the impact of treatment on pain severity, fatigue and psychological distress. This hypothesis will be addressed using parameters from three linear mixed models examining: 1) the effect of treatment arm (x) on the outcome measure; 2) the effect of treatment arm on the potential mediator, self-efficacy; and 3) the effect of self-efficacy on the outcome measure (y). Since we are collecting longitudinal data, we will include a random effect for patient. The use of multilevel structural equation modeling is a mechanism for accounting for the fact that there is dependency between outcomes belonging to the same patient over time. We will use the Monte Carlo approach to estimate the confidence intervals for the mediation effects (i.e., the indirect effect of self-efficacy) in the presence of repeated outcomes [74].
3. Discussion
While CRC incidence rates are dropping overall, incidence in adults aged ≤50 have been rising since the mid-1990's [75]. This led the American Cancer Society and US Preventative Services Task force to revise its CRC screening guidelines to start earlier. Patients in early to mid-adulthood face CRC-related pain, fatigue and distress, which are often compounded by additional stress due to their phase of life. Together, symptoms and stage-of-life stressors can negatively impact patient functioning and quality of life during and well after cancer treatment. Non-pharmacologic cognitive behavioral symptom management interventions are effective, but may not be applicable for or accessible to CRC patients in early to mid-adulthood in their traditional face-to-face formats. mHealth has the potential to overcome barriers to access.
3.1. Innovative features of mCOPE
We designed mCOPE to address key limitations of existing behavioral intervention protocols and delivery models. We utilize mHealth technology and intervention protocol adaptation and tailoring to meet the unique symptom needs and stage-of-life challenges facing CRC patients in early to mid-adulthood. Patients in early to mid-adulthood are at particular risk for decreased functioning (e.g., physical, professional, social) and difficulty re-entering social circles, work, and other life roles during and after cancer treatment [[76], [77], [78]]. In addition, a cancer diagnosis in early to mid-adulthood introduces long-term medical and psychosocial concerns, such as risk for subsequent cancers, infertility, cardiotoxicity, body image distress, insurance coverage and the cost of ongoing care [2,76,[79], [80], [81]]. Providing younger patients with CBT-based coping skills to manage symptoms and stressors has the potential for lifelong benefit as they continue to navigate physical symptoms and emotional stressors associated with their cancer history. Adapting and tailoring the intervention to the needs of younger CRC patients has the potential to increase patient engagement in and satisfaction with the intervention, increase intervention efficacy and impact, and provide substantial relief from debilitating symptom burden.
mCOPE is innovative in its approach to addressing multiple, interrelated symptoms that commonly co-occur in CRC patients. By leveraging CBT-based coping skills that effectively target multiple symptoms, mCOPE has the potential to significantly reduce patients’ symptom burden, improve overall quality of life, and ultimately reduce long-term suffering caused by cancer. If efficacious, the mCOPE intervention could be used as a model to develop similar interventions to address comorbid symptoms in patients with other types of cancer (e.g., lung cancer patients with pain, anxiety and dyspnea) and chronic disease populations who also experience comorbid pain, fatigue and psychological distress (e.g., arthritis).
mCOPE leverages mHealth technology to increase convenience and flexibility in delivering the protocol and addresses common barriers to intervention access (e.g., time, travel burden) for cancer patients in early to mid-adulthood. The use of telehealth to deliver intervention sessions is expected to improve patient adherence to the protocol while maintaining the efficacy of face-to-face delivery. The adjunct mobile app is an innovative tool to increase ease of applied practice, improve patient adherence to home practice, and collect real-time patient self-report symptom and skills data to inform personalized messaging to patients. While many models have adapted singular technologies to facilitate intervention delivery, the combination of the two is less explored and holds great promise to increase intervention access and dissemination potential.
3.2. Anticipated outcomes and future directions
We anticipate this trial will demonstrate initial evidence of mCOPE's efficacy, setting a foundation for future trials and wider intervention dissemination and implementation. We plan to use the information provided by this study to move the field forward in several important ways. First, development and refinement of the manualized protocol, mHealth delivery procedures, and the mobile app will allow for replication and broader dissemination of mCOPE. This will allow for broader reach to CRC patients in early and mid-adulthood, many of whom are treated in the community. This work will include implementation in smaller and rural community-based clinics without psychosocial services. Second, this type of mHealth behavioral intervention may be extended to patients in early and mid-adulthood with other cancer types and/or chronic diseases who experience a similar comorbid symptom presentation and stage-of-life challenges. Third, the mHealth system for assessment of symptoms and coping skills use will provide critical data to move toward further tailoring and personalization of the intervention to further increase efficacy. Fourth, as the number of patients in early to mid-adulthood living with and beyond cancer continues to increase, future work may more specifically address how chronic symptoms interfere with adherence to cancer self-management recommendations (e.g., physical activity, nutrition). In sum, this project will lead to future work that increases intervention efficacy and reach, improves patient engagement and satisfaction, and provides substantial relief from a debilitating symptom burden. This work has the potential to significantly improve symptom management and health-related quality of life for CRC patients in early to mid-adulthood, immediately and into the future.
3.3. Conclusions
This trial tests the efficacy of mCOPE, a novel mHealth behavioral symptom management intervention adapted for and tailored to the unique symptom needs and stage-of-life challenges faced by CRC patients in early to mid-adulthood. Results are expected to demonstrate that mCOPE decreases pain, fatigue and psychological distress and improves quality of life and symptom self-efficacy compared to standard care. Findings will inform future testing and broader dissemination and implementation of mCOPE, which may significantly improve health outcomes in younger CRC patients.
Funding
This work is supported by a grant awarded to the senior author, Sarah A. Kelleher, PhD, from the American Cancer Society RSG-20-134-01-PCSM.
Clinical trial registration number
ClinicalTrials.gov, NCT0476317.
Funding statement
This study is funded through an American Cancer Society Research Scholar grant (RSG-20-134-01-PCSM) awarded to senior author, Sarah A. Kelleher, PhD.
Declaration of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- 1.Loomans-Kropp H.A., Umar A. Increasing incidence of colorectal cancer in young adults. J Cancer Epidemiol. 2019;2019 doi: 10.1155/2019/9841295. 9841295-9841295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R.L., Fedewa S.A., Anderson W.F., et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J. Natl. Cancer Inst. 2017;109(8) doi: 10.1093/jnci/djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh P.J., Cho J.R. Changes in fatigue, psychological distress, and quality of life after chemotherapy in women with breast cancer: a prospective study. Cancer Nurs. 2020;43(1):E54–e60. doi: 10.1097/ncc.0000000000000689. [DOI] [PubMed] [Google Scholar]
- 4.Somers T., Keefe F.J., Abernethy A. Vol. 43. 2012. (Electronic Patient Reported Outcomes (ePRO) to Guide the Implementation of Behavioral Cancer Pain Interventions). [Google Scholar]
- 5.Green C.R., Hart-Johnson T., Loeffler D.R. Cancer-related chronic pain: examining quality of life in diverse cancer survivors. Cancer. 2011;117(9):1994–2003. doi: 10.1002/cncr.25761. [DOI] [PubMed] [Google Scholar]
- 6.Shi Q., Smith T.G., Michonski J.D., Stein K.D., Kaw C., Cleeland C.S. Symptom burden in cancer survivors 1 year after diagnosis: a report from the American Cancer Society's Studies of Cancer Survivors. Cancer. 2011;117(12):2779–2790. doi: 10.1002/cncr.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowery A.E., Starr T., Dhingra L.K., et al. Frequency, characteristics, and correlates of pain in a pilot study of colorectal cancer survivors 1-10 years post-treatment. Pain Med. 2013;14(11):1673–1680. doi: 10.1111/pme.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith T.G., Troeschel A.N., Castro K.M., et al. Perceptions of patients with breast and colon cancer of the management of cancer-related pain, fatigue, and emotional distress in community oncology. J. Clin. Oncol. 2019;37(19):1666–1676. doi: 10.1200/JCO.18.01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgoyne M.J., Bingen K., Leuck J., Dasgupta M., Ryan P., Hoffmann R.G. Cancer-related distress in young adults compared to middle-aged and senior adults. J. Adolesc. Young Adult Oncol. 2015;4(2):56–63. doi: 10.1089/jayao.2014.0005. [DOI] [PubMed] [Google Scholar]
- 10.Blumenstein K.G., Brose A., Kemp C., et al. Effectiveness of cognitive behavioral therapy in improving functional health in cancer survivors: a systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2022;175 doi: 10.1016/j.critrevonc.2022.103709. [DOI] [PubMed] [Google Scholar]
- 11.Daniels S. Cognitive behavior therapy for patients with cancer. J. Advert. Pract. Oncol. 2015;6(1):54–56. [PMC free article] [PubMed] [Google Scholar]
- 12.Greer J.A., Traeger L., Bemis H., et al. A pilot randomized controlled trial of brief cognitive-behavioral therapy for anxiety in patients with terminal cancer. Oncol. 2012;17(10):1337–1345. doi: 10.1634/theoncologist.2012-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poort H., Peters M., van der Graaf W.T.A., et al. Cognitive behavioral therapy or graded exercise therapy compared with usual care for severe fatigue in patients with advanced cancer during treatment: a randomized controlled trial. Ann. Oncol. 2020;31(1):115–122. doi: 10.1016/j.annonc.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Kelleher S.A., Fisher H.M., Winger J.G., et al. Virtual reality for improving pain and pain-related symptoms in patients with advanced stage colorectal cancer: a pilot trial to test feasibility and acceptability. Palliat. Support Care. 2022;20(4):471–481. doi: 10.1017/s1478951521002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martel M.O., Finan P.H., Dolman A.J., et al. Self-reports of medication side effects and pain-related activity interference in patients with chronic pain: a longitudinal cohort study. Pain. 2015;156(6):1092–1100. doi: 10.1097/j.pain.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carson J.W., Carson K.M., Jones K.D., Mist S.D., Bennett R.M. Follow-up of yoga of awareness for fibromyalgia: results at 3 months and replication in the wait-list group. Clin. J. Pain. 2012;28(9):804–813. doi: 10.1097/AJP.0b013e31824549b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelleher S.A., Somers T.J., Locklear T., Crosswell A.D., Abernethy A.P. Using patient reported outcomes in oncology clinical practice. Scand J Pain. 2016;13:6–11. doi: 10.1016/j.sjpain.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Sullivan M.L., Shelby R.A., Dorfman C.S., et al. The effect of pre-transplant pain and chronic disease self-efficacy on quality of life domains in the year following hematopoietic stem cell transplantation. Support. Care Cancer. 2018;26(4):1243–1252. doi: 10.1007/s00520-017-3947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Somers T.J., Abernethy A.P., Edmond S.N., et al. A pilot study of a mobile health pain coping skills training protocol for patients with persistent cancer pain. J. Pain Symptom Manag. 2015;50(4):553–558. doi: 10.1016/j.jpainsymman.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White L.L., Cohen M.Z., Berger A.M., Kupzyk K.A., Bierman P.J. Self-efficacy for management of symptoms and symptom distress in adults with cancer: an integrative review. Oncol. Nurs. Forum. 2019;46(1):113–128. doi: 10.1188/19.Onf.113-128. [DOI] [PubMed] [Google Scholar]
- 21.Hart S.L., Hoyt M.A., Diefenbach M., et al. Meta-analysis of efficacy of interventions for elevated depressive symptoms in adults diagnosed with cancer. J. Natl. Cancer Inst. 2012;104(13):990–1004. doi: 10.1093/jnci/djs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbasi M., Dehghani M., Keefe F.J., Jafari H., Behtash H., Shams J. Spouse-assisted training in pain coping skills and the outcome of multidisciplinary pain management for chronic low back pain treatment: a 1-year randomized controlled trial. Eur. J. Pain. 2012;16(7):1033–1043. doi: 10.1002/j.1532-2149.2011.00097.x. [DOI] [PubMed] [Google Scholar]
- 23.Dorfman C.S., Kelleher S.A., Winger J.G., et al. Development and pilot testing of an mHealth behavioral cancer pain protocol for medically underserved communities. J. Psychosoc. Oncol. 2019;37(3):335–349. doi: 10.1080/07347332.2018.1479327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porter L.S., Keefe F.J., Garst J., et al. Caregiver-assisted coping skills training for lung cancer: results of a randomized clinical trial. J. Pain Symptom Manag. 2011;41(1):1–13. doi: 10.1016/j.jpainsymman.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somers T.J., Kelleher S.A., Dorfman C.S., et al. An mHealth pain coping skills training intervention for hematopoietic stem cell transplantation patients: development and pilot randomized controlled trial. JMIR Mhealth Uhealth. 2018;6(3):e66. doi: 10.2196/mhealth.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelleher S.A., Fisher H.M., Winger J.G., Somers T.J., Uronis H.E., Wright A.N., Keefe F.J. Feasibility, engagement, and acceptability of a behavioral pain management intervention for colorectal cancer survivors with pain and psychological distress: data from a pilot randomized controlled trial. Support. Care Cancer: Off. J. Multinatl. Assoc. Support. Care Cancer. 2021;29(9):5361–5369. doi: 10.1007/s00520-021-06126-8. [DOI] [PubMed] [Google Scholar]
- 27.Keefe F.J. Cognitive behavioral therapy for managing pain. Clin. Psychol. 1996;49(3):4–5. [Google Scholar]
- 28.Keefe F.J., Caldwell D.S., Williams D.A., et al. Pain coping skills training in the management of osteoarthritic knee pain: a comparative study. Behav. Ther. 1990;21(1):49–62. doi: 10.1016/S0005-7894(05)80188-1. [DOI] [Google Scholar]
- 29.Vujanovic A.A., Meyer T.D., Heads A.M., Stotts A.L., Villarreal Y.R., Schmitz J.M. Cognitive-behavioral therapies for depression and substance use disorders: an overview of traditional, third-wave, and transdiagnostic approaches. Am. J. Drug Alcohol Abuse. 2017;43(4):402–415. doi: 10.1080/00952990.2016.1199697. [DOI] [PubMed] [Google Scholar]
- 30.Chalder T., Patel M., James K., et al. Persistent physical symptoms reduction intervention: a system change and evaluation in secondary care (PRINCE secondary) - a CBT-based transdiagnostic approach: study protocol for a randomised controlled trial. BMC Psychiatr. 2019;19(1):307. doi: 10.1186/s12888-019-2297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carson J.W., Carson K.M., Jones K.D., Bennett R.M., Wright C.L., Mist S.D. A pilot randomized controlled trial of the Yoga of Awareness program in the management of fibromyalgia. Pain. 2010;151(2):530–539. doi: 10.1016/j.pain.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castro F.G., Barrera M., Jr., Martinez C.R., Jr. The cultural adaptation of prevention interventions: resolving tensions between fidelity and fit. Prev. Sci. 2004;5(1):41–45. doi: 10.1023/b:prev.0000013980.12412.cd. [DOI] [PubMed] [Google Scholar]
- 33.Chen E.K., Reid M.C., Parker S.J., Pillemer K. Tailoring evidence-based interventions for new populations: a method for program adaptation through community engagement. Eval. Health Prof. 2013;36(1):73–92. doi: 10.1177/0163278712442536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abrahão R., Alvarez E.M., Waters A.R., et al. A qualitative study of barriers and facilitators to adolescents and young adults' participation in cancer clinical trials: oncologist and patient perspectives. Pediatr. Blood Cancer. 2022;69(4) doi: 10.1002/pbc.29479. [DOI] [PubMed] [Google Scholar]
- 35.Hendren S., Chin N., Fisher S., et al. Patients' barriers to receipt of cancer care, and factors associated with needing more assistance from a patient navigator. J. Natl. Med. Assoc. 2011;103(8):701–710. doi: 10.1016/s0027-9684(15)30409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selove R., Kroll T., Coppes M., Cheng Y. Psychosocial services in the first 30 days after diagnosis: results of a web-based survey of Children's Oncology Group (COG) member institutions. Pediatr. Blood Cancer. 2012;58(3):435–440. doi: 10.1002/pbc.23235. [DOI] [PubMed] [Google Scholar]
- 37.Sutanto Y.S., Ibrahim D., Septiawan D., Sudiyanto A., Kurniawan H. Effect of cognitive behavioral therapy on improving anxiety, depression, and quality of life in pre-diagnosed lung cancer patients. Asian Pac. J. Cancer Prev. APJCP. 2021;22(11):3455–3460. doi: 10.31557/apjcp.2021.22.11.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Cancer Society of Clinical Oncology Colorectal cancer: risk factors and prevention. 2022. https://www.cancer.net/cancer-types/colorectal-cancer/risk-factors-and-prevention Accessed 2022.
- 39.Somers T.J., Kelleher S.A., Westbrook K.W., et al. A small randomized controlled pilot trial comparing mobile and traditional pain coping skills training protocols for cancer patients with pain. Pain Res. Treat. 2016;2016 doi: 10.1155/2016/2473629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelleher S.A., Winger J.G., Dorfman C.S., et al. A behavioral cancer pain intervention: a randomized noninferiority trial comparing in-person with videoconference delivery. Psycho Oncol. 2019;28(8):1671–1678. doi: 10.1002/pon.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price E.L., Pérez-Stable E.J., Nickleach D., López M., Karliner L.S. Interpreter perspectives of in-person, telephonic, and videoconferencing medical interpretation in clinical encounters. Patient Educ. Counsel. 2012;87(2):226–232. doi: 10.1016/j.pec.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kazantzis N., Brownfield N.R., Mosely L., Usatoff A.S., Flighty A.J. Homework in cognitive behavioral therapy: a systematic review of adherence assessment in anxiety and depression (2011-2016) Psychiatr. Clin. 2017;40(4):625–639. doi: 10.1016/j.psc.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Tang W., Kreindler D. Supporting homework compliance in cognitive behavioural therapy: essential features of mobile apps. JMIR Ment. Health. 2017;4(2) doi: 10.2196/mental.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burmistr I. Theories of pain, up to Descartes and after neuromatrix: what role do they have to develop future paradigms? Pain Med. 2018;3(1):6–12. doi: 10.31636/pmjua.v3i1.81. [DOI] [Google Scholar]
- 45.Melzack R. Pain - an overview. Acta Anaesthesiol. Scand. 2003;43(9):880–884. doi: 10.1034/j.1399-6576.1999.430903.x. [DOI] [PubMed] [Google Scholar]
- 46.Melzack R. Evolution of the neuromatrix theory of pain. The prithvi raj lecture: presented at the third world congress of world Institute of pain, barcelona 2004. Pain Pract. 2005;5(2):85–94. doi: 10.1111/j.1533-2500.2005.05203.x. [DOI] [PubMed] [Google Scholar]
- 47.Roy M., Wager T.D. Routledge; 2017. Neuromatrix Theory of Pain. The Routledge Handbook of Philosophy of Pain; p. 11. [Google Scholar]
- 48.Perepletchikova F. On the topic of treatment integrity. Clin. Psychol. 2011;18(2):148–153. doi: 10.1111/j.1468-2850.2011.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waltz J., Addis M.E., Koerner K., Jacobson N.S. Testing the integrity of a psychotherapy protocol: assessment of adherence and competence. J. Consult. Clin. Psychol. 1993;61(4):620–630. doi: 10.1037//0022-006x.61.4.620. [DOI] [PubMed] [Google Scholar]
- 50.Cleeland C.S., Ryan K.M. Pain assessment: global use of the brief pain inventory. Ann. Acad. Med. Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 51.Lai J.S., Cella D., Choi S., et al. How item banks and their application can influence measurement practice in rehabilitation medicine: a PROMIS fatigue item bank example. Arch. Phys. Med. Rehabil. 2011;92(10 Suppl):S20–S27. doi: 10.1016/j.apmr.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riley W.T., Rothrock N., Bruce B., et al. Patient-reported outcomes measurement information system (PROMIS) domain names and definitions revisions: further evaluation of content validity in IRT-derived item banks. Qual. Life Res. 2010;19(9):1311–1321. doi: 10.1007/s11136-010-9694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brief Symptom Inventory (BSI)-18: Administration, scoring, and procedures manual . NCS Pearson; 2000. Brief Symptom Inventory (BSI)-18: Administration, Scoring, and Procedures Manual. [Google Scholar]
- 54.Derogatis L.R., Fitzpatrick M. In: The Use of Psychological Testing for Treatment Planning and Outcomes Assessment: Vol 3 Instruments for Adults. Maruish M.E., editor. Erlbaum; 2004. The SCL-90-R, the brief symptom inventory (BSI), and the BSI-18; pp. 1–41. [Google Scholar]
- 55.Peshkin B.N., Demarco T.A., Graves K.D., et al. Telephone genetic counseling for high-risk women undergoing BRCA1 and BRCA2 testing: rationale and development of a randomized controlled trial. Genet. Test. 2008;12(1):37–52. doi: 10.1089/gte.2006.0525. [DOI] [PubMed] [Google Scholar]
- 56.Derogatis L.R. NCS Pearson; 2000. Brief Symptom Inventory (BSI)-18: Administration, Scoring, and Procedures Manual. [Google Scholar]
- 57.López P.M., Peiró A.C., Vaillo Y.A., Galdón M.J. Psychometric properties of the Brief Symptom Inventory-18 in a heterogeneous sample of adult cancer patients. Rev. Latinoam. Psicol. 2019;51(1):1–8. doi: 10.14349/rlp.2019.v51.n1.1. [DOI] [Google Scholar]
- 58.Derogatis L.R., Fitzpatrick M. In: Maruish M.E., editor. Vol. 3. Erlbaum; 2004. The use of psychological testing for treatment planning and outcomes assessment; pp. 1–41. (Instruments for Adults. The SCL-90-R, the Brief Symptom Inventory (BSI), and the BSI-18). [Google Scholar]
- 59.Cella D.F., Tulsky D.S., Gray G., et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J. Clin. Oncol. 1993;11(3):570–579. doi: 10.1200/jco.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 60.Costet N., Lapierre V., Benhamou E., Le Galès C. Reliability and validity of the functional assessment of cancer therapy general (FACT-G) in French cancer patients. Qual. Life Res. 2005;14(5):1427–1432. doi: 10.1007/s11136-004-5531-z. [DOI] [PubMed] [Google Scholar]
- 61.Galdón M.J., Durá E., Andreu Y., et al. Psychometric properties of the Brief Symptom Inventory-18 in a Spanish breast cancer sample. J. Psychosom. Res. 2008;65(6):533–539. doi: 10.1016/j.jpsychores.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 62.Yost K.J., Thompson C.A., Eton D.T., et al. The Functional Assessment of Cancer Therapy - general (FACT-G) is valid for monitoring quality of life in patients with non-Hodgkin lymphoma. Leuk. Lymphoma. 2013;54(2):290–297. doi: 10.3109/10428194.2012.711830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gruber-Baldini A.L., Velozo C., Romero S., Shulman L.M. Validation of the PROMIS(®) measures of self-efficacy for managing chronic conditions. Qual. Life Res. 2017;26(7):1915–1924. doi: 10.1007/s11136-017-1527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelly P.J., Kyngdon F., Ingram I., Deane F.P., Baker A.L., Osborne B.A. The Client Satisfaction Questionnaire-8: psychometric properties in a cross-sectional survey of people attending residential substance abuse treatment. Drug Alcohol Rev. 2018;37(1):79–86. doi: 10.1111/dar.12522. [DOI] [PubMed] [Google Scholar]
- 65.Larsen D.L., Attkisson C.C., Hargreaves W.A., Nguyen T.D. Assessment of client/patient satisfaction: development of a general scale. Eval. Progr. Plann. 1979;2(3):197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- 66.Teo I., Krishnan A., Lee G.L. Psychosocial interventions for advanced cancer patients: a systematic review. Psycho Oncol. 2019;28:1394–1407. doi: 10.1002/pon.5103. [DOI] [PubMed] [Google Scholar]
- 67.Cohen J. second ed. L. Erlbaum Associates; 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 68.Sullivan G.M., Feinn R. Using effect size-or why the p value is not enough. J Grad Med Edu. 2012;4(3):279–282. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winger J.G., Ramos K., Kelleher S.A., et al. Meaning-centered pain coping skills training: a pilot feasibility trial of a psychosocial pain management intervention for patients with advanced cancer. J. Palliat. Med. 2022;25(1):60–69. doi: 10.1089/jpm.2021.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gorin S.S., Krebs P., Badr H., et al. Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J. Clin. Oncol. 2012;30(5):539–547. doi: 10.1200/JCO.2011.37.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vickerstaff V., Ambler G., King M., Nazareth I., Omar R.Z. Are multiple primary outcomes analysed appropriately in randomised controlled trials? A review. Contemp. Clin. Trials. 2015;45(Pt A):8–12. doi: 10.1016/j.cct.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 73.Van Breukelen G.J. ANCOVA versus change from baseline: more power in randomized studies, more bias in nonrandomized studies [corrected] J. Clin. Epidemiol. 2006 Sep;59(9):920–925. doi: 10.1016/j.jclinepi.2006.02.007. Epub 2006 Jun 23. Erratum in: J Clin Epidemiol. 2006 Dec;59(12):1334. [DOI] [PubMed] [Google Scholar]
- 74.Preacher K.J., Zyphur M.J., Zhang Z. A general multilevel SEM framework for assessing multilevel mediation. Psychol. Methods. 2010;15(3):209–233. doi: 10.1037/a0020141. [DOI] [PubMed] [Google Scholar]
- 75.American Cancer Society Cancer facts & figures. 2022. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html 2022. Accessed 2022.
- 76.Banegas M.P., Schneider J.L., Firemark A.J., et al. The social and economic toll of cancer survivorship: a complex web of financial sacrifice. J Cancer Surviv. 2019;13(3):406–417. doi: 10.1007/s11764-019-00761-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Husson O., Zebrack B.J. Perceived impact of cancer among adolescents and young adults: relationship with health-related quality of life and distress. Psycho Oncol. 2017;26(9):1307–1315. doi: 10.1002/pon.4300. [DOI] [PubMed] [Google Scholar]
- 78.Vetsch J., Wakefield C.E., McGill B.C., et al. Educational and vocational goal disruption in adolescent and young adult cancer survivors. Psycho Oncol. 2018;27(2):532–538. doi: 10.1002/pon.4525. [DOI] [PubMed] [Google Scholar]
- 79.Anaka M., Abdel-Rahman O. Managing 5FU cardiotoxicity in colorectal cancer treatment. Cancer Manag. Res. 2022;14:273–285. doi: 10.2147/CMAR.S273544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharpe L., Patel D., Clarke S. The relationship between body image disturbance and distress in colorectal cancer patients with and without stomas. J. Psychosom. Res. 2011;70(5):395–402. doi: 10.1016/j.jpsychores.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 81.Stupart D., Win A.K., Winship I.M., Jenkins M. Fertility after young-onset colorectal cancer: a study of subjects with Lynch syndrome. Colorectal Dis. 2015;17(9):787–793. doi: 10.1111/codi.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.