Abstract
Purpose:
To describe cases of patients with presumable dysimmune small fiber neuropathy-related neuropathic corneal pain (NCP), presenting with IgM auto-antibodies against trisulfated heparin disaccharide (TS-HDS) or fibroblast growth factor receptor-3 (FGFR-3).
Methods:
Case series of three NCP patients with positive anti-TS-HDS and/or anti-FGFR-3 auto-antibodies and systemic small fiber neuropathy (SFN) as confirmed by positive skin biopsy results.
Results:
All three patients were female with a mean age of 34.3± 6.1. They suffered from moderate to severe persistent chronic ocular discomfort (10/10, 10/10 and 9/10 on a visual analogue scale, respectively). While one patient suffered from ocular pain and photophobia alone, the other 2 patients experienced additional non-ocular pain. One of the patients had pain on her face and head, and 1 patient reported neck and lower back pain. Two patients had high anti-TS-HDS IgM titers, whereas 1 patient had both high anti-TS-HDS IgM and anti-FGFR-3 IgG titers. Skin biopsy confirmed presence of SFN in all patients by demonstrating decreased intraepidermal nerve fiber density.
Conclusion:
Presence of anti-TSHDS and anti-FGFR-3 auto-antibodies in NCP patients with positive skin biopsy findings for SFN highlights the potential role of dysimmune SFN in the pathogenesis of this disease.
Keywords: dysimmune neuropathy, fibroblast growth factor receptor 3, neuropathic corneal pain, skin biopsy, non-length dependent small fiber neuropathy, trisulfated heparan disaccharide
1. INTRODUCTION
Small fiber neuropathy (SFN) is a common type of peripheral neuropathy, affecting thinly myelinated Aδ and unmyelinated C nerve fibers.1 Patients typically present with non-specific symptoms, such as numbness, tingling, burning, pain and discomfort, as well as hyperalgesia, and allodynia.1–3 Commonly, the clinical examination is either normal or mildly affected, with deficits in perception of temperature, pinprick, and tactile stimuli, as well as impaired autonomic function. Conventional nerve conduction studies and electromyography are typically normal.1–4
SFN can be classified in two subtypes, according to anatomic localization of initial symptoms: symmetric length dependent SFN (LD-SFN) and non-length dependent SFN (NLD-SFN).1–3 In LD-SFN, symptoms start from the distal limbs, and gradually progress proximally. In contrast, in NLD-SFN symptoms show a patchy and asymmetric pattern and may involve the face, scalp, proximal limbs and the trunk.1–3 Indeed, the distal to proximal ratio of intraepidermal nerve density (IEND) has been proposed as a potential identifier in the classification of SFN.5, 6 Nonetheless, severe distal small fiber loss was reported in the presence of non-length dependent pattern and, therefore, this topic remains controversial.6 Various etiologies, including metabolic, inflammatory, autoimmune, toxic, infectious, and genetic disorders, among others, may cause SFN.1, 7 While metabolic disorders, such as glucose intolerance and diabetes have been shown to result in LD-SFN,1, 8, 9 NLD-SFN is thought to be mostly caused by dysimmune, toxic, and idiopathic etiologies.1, 8, 9 Conceptually, NLD-SFN is considered to be a form of sensory ganglionopathy, rather than a primary axonopathy.1, 10, 11
Dysimmune neuropathies have become of recent interest in both the neurological research field and clinical practice.12, 13 Dysimmune neuropathies are polyneuropathies that have presumed immune mediated or inflammatory etiology and diverse clinical presentations.14 Peripheral nervous system axons and myelin are believed to be strong antigenic targets, and antibodies (Abs) against these targets may result in dysimmune neuropathies.12, 13 Serological Abs against IdoA2S-GlcNS-6S, trisulfated heparin disaccharide (TS-HDS) and fibroblast growth factor receptor-3 (FGFR-3) molecules have recently been studied in patients with SFN.1, 8, 15–17 Although the exact pathophysiological role of these Abs in the peripheral nervous system has not been determined yet, several studies have investigated the presence of anti-TS-HDS and/or anti-FGFR-3 Abs in patients with SFN.1, 8, 15–17 Presence of anti-TS-HDS and/or anti-FGFR-3 Abs in SFN patients was compared with amyotrophic lateral sclerosis patients,8 an heterogenous group of sensorimotor neuropathies, other neurological diseases, systemic auto-immune diseases, and healthy blood donors,10 and Ab positivity was more common in those patients with SFN.8, 10 These studies concluded that anti-TS-HDS and/or anti-FGFR-3 may present in cryptogenic SFN patients and may be predominantly found in a specific subgroup of SFN.8, 10 Several reports comparing patients with elevated titers for these Abs versus those with negative or normal Ab titers, suggested that the elevated levels of these Abs were associated with painful, primarily sensory, non-length dependent polyneuropathy.1, 8, 15–17
The type of nerve fibers involved in the disease, the clinical symptoms, and lack or minimal presence of clinical signs of SFN, in particular with NLD-SFN, is similar to the clinical presentation of neuropathic corneal pain (NCP).1, 18, 19 Although ocular involvement has, to date, not been described as a clinical presentation of NLD-SFN, the involvement of other divisions within the trigeminal nerve, with burning mouth syndrome, refractory facial pain in Heerfordt syndrome, scalp pruritus in dermatomyositis, and anti-FGFR-3 positive sensory neuropathy has previously been reported.10, 20–22 Therefore, we hypothesize that NCP, a pain syndrome related to the ophthalmic branch of trigeminal nerve, can be a clinical sign of NLD-SFN, and that NLD-SFN may be initially present with NCP, and that some cases of NCP may be a localized subtype of NLD-SFN. Herein, we present 3 NCP patients, in whom positive anti-TSHDS and/or anti-FGFR-3 Abs and abnormal distal limb biopsies for small nerve fiber were documented. The presence of elevated Abs is considered suggestive of a dysimmune mechanism. We suggest that identification of dysimmune neuropathies/mechanisms in NCP may add to our knowledge and pathophysiology of the disease, thus guiding both ophthalmologists, neurologists, and scientists to develop new approaches to understand the pathophysiology, diagnosis, and treatment of the disease.
2. CASE PRESENTATIONS
This retrospective case series of 3 patients visiting the Cornea Service of the New England Eye Center, Tufts Medical Center, Tufts University School of Medicine, Boston, MA, was approved by the Institutional Board Review of Tufts Medical Center/Tufts University Health Sciences. The protocol conformed to the Declaration of Helsinki and adhered to the Health Insurance Portability and Accountability Act (HIPAA). The medical records of patients who were diagnosed clinically with NCP by the same experienced ophthalmologist (PH), and confirmed by in vivo confocal microscopy (IVCM), were reviewed. Diagnosis of NCP was made based on presence of neuropathic ocular symptoms (burning, stinging, light sensitivity, pain), symptom severity out of proportion to ocular surface findings, and corneal nerve abnormalities as detected by IVCM (HRT3/RCM, Heidelberg Engineering GmbH, Heidelberg, Germany). Demographic features of patients, detailed disease history, ocular surface disease index (OSDI) score,23 symptom scores assessed by visual analogue scale (VAS),24 slit-lamp biomicroscopy findings, proparacaine challenge test (PCT) results,18 IVCM findings, anti-TS-HDS and anti-FGFR-3 titers, neurologic examination, and skin biopsy results of lower limbs for small nerve fiber changes were reviewed. Skin biopsy was performed from two distinct anatomic areas, including distal lateral leg and proximal thigh by using a 3 mm punch to evaluate the epidermal nerve fiber density as biomarker of SFN.1, 2 Assessment of skin biopsy results and their clinical relevance were made by experienced neurologists (KF or OS).
2.1. Case 1
A 29-year-old Caucasian female presented with pressure-like stabbing eye pain in both eyes, predominantly on the right eye, after uncomplicated laser-assisted in situ keratomileusis (LASIK) surgery 9 months ago. Her pain started approximately 20 days after surgery and was persistent and consistent afterwards. Her pain started in the right eye and on the right side of her face and head. It worsened over time and spread to her left side of her face. She had no prior ocular risk factors, was not a contact lens wearer, and was not diagnosed with dry eye disease prior to surgery. Her past medical history was not significant. Prior to presentation, she had been seen by at least three ophthalmologist and a neurologist and treated with topical corticosteroids (methylprednisolone and loteprednol), autologous serum tears four times daily, topical lifitegrast 5% twice daily, intravenous corticosteroids, periocular injections, systemic gabapentin 100 mg three times daily, and nortriptyline 10 mg three times daily. Although with some of these treatments her pain levels decreased minimally, it was not consistent and overall, she did not feel relief.
On presentation, her OSDI score was 56.2 (scale 0–100) and she reported her pain frequency as 3/4 (most of the time) on the OSDI questionnaire. The pain severity was graded 10/10 in the right eye, and 8/10 in the left eye assessed by VAS. Demographic features and clinical characteristics of the patient are presented in Table 1.
Table 1.
Demographic and clinical features of cases
| Case 1 | Case 2 | Case 3 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Age (years) | 29 | 41 | 54 | |||
| Gender | Female | Female | Female | |||
| Race | Other | White | White | |||
| Dominant symptom | Pain | Pain | Photophobia | |||
| Symptom onset | Acute | Acute | Acute | |||
| Co-morbid conditions | LASIK | HZO, eosinophilic granulomatosis, CVID, anxiety, depression | DED | |||
| OSDI | 56.2 | 75.0 | 56.2 | |||
| Symptom severity (VAS) | 10/10 | 10/10 | 9/10 | |||
| Clinical Features | OD | OS | OD | OS | OD | OS |
| TBUT (sec) | 10 | 10 | 7 | 8 | 7 | 6 |
| CFS (Oxford Scale) | 0 | 0 | +2 | +3 | +1 | +1 |
| Schirmer I score (mm/5min) | 10 | 15 | 1 | 0 | 2 | 6 |
| Stimulated Jones Schirmer (mm/5min) | N/A | N/A | 10.5 | 8.0 | N/A | N/A |
OSDI: Ocular Surface Disease Index, VAS: Visual analogue scale, TBUT: Tear break-up time, CFS: Corneal Fluorescein staining, LASIK: Laser in situ keratomileusis, HZO: Herpes zoster ophthalmicus, CVID: Common variable immunodeficiency, DED: Dry eye disease, N/A: Not applicable
On examination, her best corrected visual acuity (BCVA) on presentation was 20/20 in both eyes. On slit-lamp biomicroscopy, central LASIK flaps with a superior hinge were observed. Tear break-up time (TBUT) was 10 seconds in both eyes, and no corneal fluorescein staining was detected (Table 1). Schirmer’s I results were 10 mm/5 min for the right eye and 15 mm/5 min for the left eye (Table 1). Intraocular pressures (IOP) were 15 mmHg in both eyes. After instillation of one drop of 0.5% proparacaine in both eyes, pain levels remained the same in both eyes, suggesting non-ocular component of the pain likely either due to ganglionopathy or central sensitization. On IVCM, the central corneal nerve density was significantly decreased compared to healthy individuals (19.1–25.9 mm/mm2, Figure 1A, 2A)25 in both eyes (a mean total nerve density of 8,123.0 μm/mm2 in the right eye and 6,176.8 μm/mm2 in the left eye, Figure 1A). In addition to decreased nerve density, microneuromas were observed. The mean dendritiform cell (DC) density was 12.5 cells/mm2 in the right eye and 33.1 cells/mm2 in the left eye (DC density in healthy eyes 26: 19.6±2.8 cells/mm2) (Figure 3A).
Figure 1.
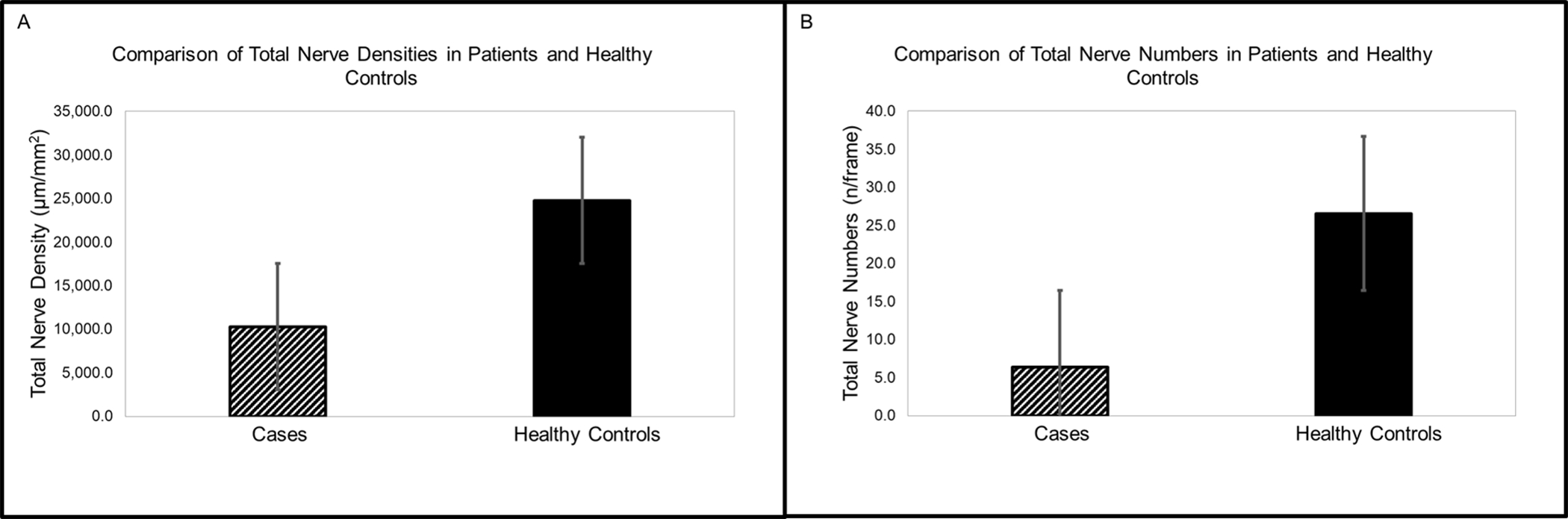
Comparison of central corneal nerve densities and nerve fiber numbers between cases and healthy control group. A) The mean total nerve density was 10,279.0±1,542.9 μm/mm2 in cases and 24,762.3±4,661.3 μm/mm2 in healthy controls. B) The mean nerve fiber number was 5.8±1.0 fiber/frame in cases and 26.6±2.1 fiber/frame in healthy controls.
Figure 2.
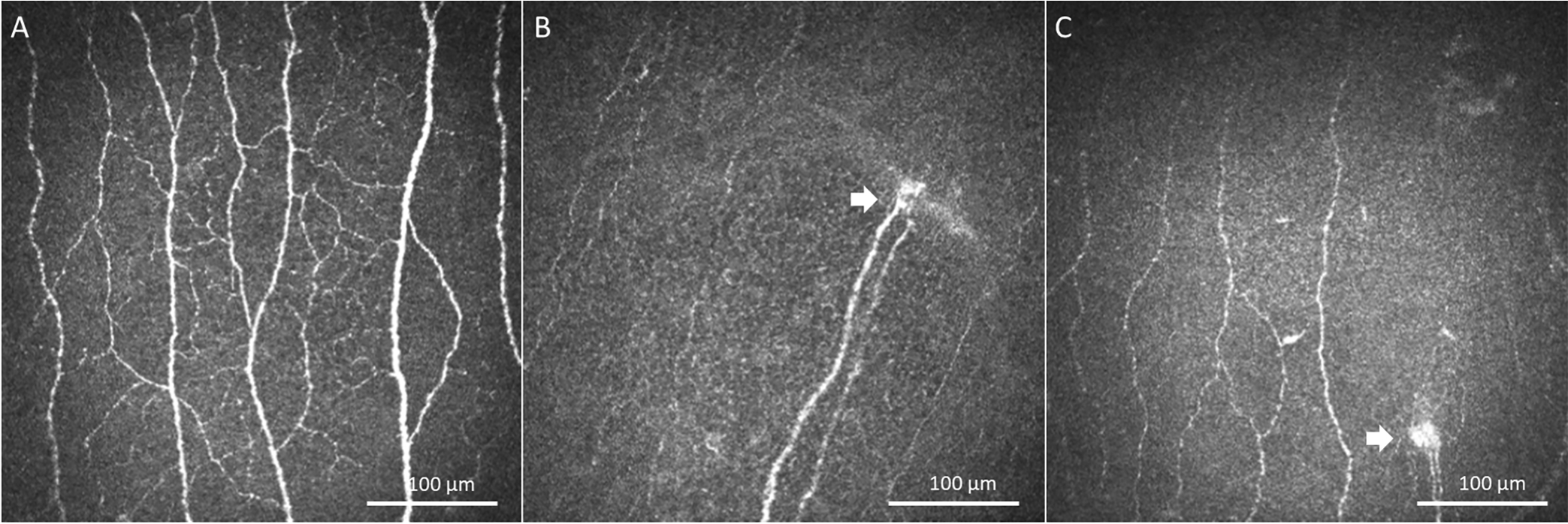
In Vivo Laser Confocal Microscopy Images (HRT3/RCM, Heidelberg Engineering GmbH, Heidelberg, Germany). IVCM images demonstrated corneal nerves in a healthy eye (A) and extremely damaged corneal nerves and presence of microneuromas (white arrows) in, Case 2 (B), and Case 3 (C).
Figure 3.
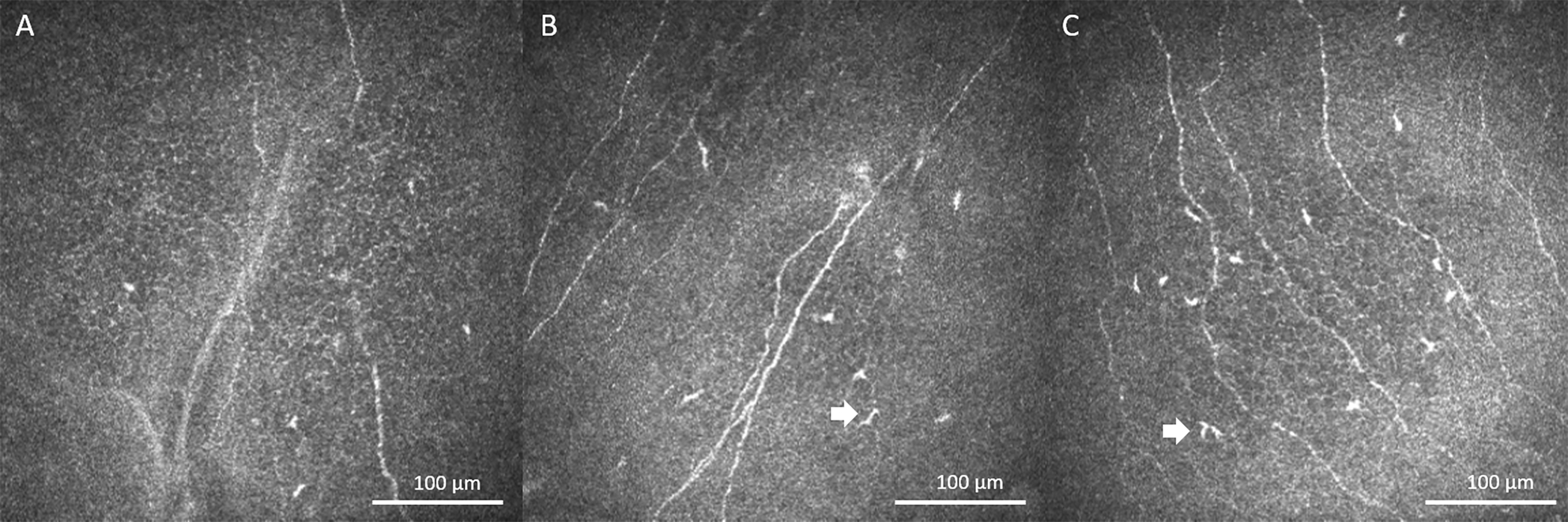
In Vivo Laser Confocal Microscopy Images (HRT3/RCM, Heidelberg Engineering GmbH, Heidelberg, Germany). IVCM images demonstrated dendritiform cells (white arrows) in patient corneas; Case 1 (A), slightly increased DC density in Case 2 (B) and Case 3 (C).
The patient did not report pain other than her eye, face, and head. She reported numbness and tingling sensation on her feet which started 9 months after the development of ocular and facial pain. Considering the clinical findings, detailed serological evaluation was obtained, showing an elevated titer for anti-TS-HDS IgM (15,000; N<10,000). The patient was subsequently referred to the neurology department for further evaluation and skin biopsy of her lower limbs. Neurological evaluation showed reduced pain and temperature sensation in her feet. Other neurological evaluations, including cranial nerves, motor system, reflexes, coordination, and gait were intact. Her skin biopsy showed a mean IEND of 3.5 fiber/mm2 (N>8.4 fiber/mm2) on the distal leg and 9.0 fiber/mm2 (N>9.3 fiber/mm2) on the proximal thigh, which was lower than 5th percentile in her distal leg, confirming SFN. The diagnosis of dysimmune SFN was confirmed with both neurological evaluation, skin biopsy result, and positive TS-HDS Ab titer.
The initial treatment was with topical 0.5% loteprednol etabonate (4 times daily, tapered over 6 weeks), 20% autologous serum tears 8 times daily, and low dose naltrexone treatment starting at 1.5 mg and gradually increased to 4.5 mg daily. At the 3rd month of treatment, the patient reported improvement in the frequency of pain, however, symptom severity remained the same. Her systemic medication was subsequently switched to fluoxetine 30 mg BID daily. Although, the symptoms improved significantly with fluoxetine treatment, she could not tolerate the treatment because of mellowed out mood. Treatment was changed to gabapentin 300 mg 3 times daily and fluoxetine 20 mg BID.
2.2. Case 2
A 41-year-old Caucasian female presented with bilateral sudden onset of eye pain accompanied by light sensitivity and irritation with onset 1 year before her visit. Her pain started as a sharp pain and she reported severe and constant pain which only relieved during sleep. Her pain was described as a sensation of many needles being stuck in her eye. Her past ocular history was significant for recurrent herpes zoster ophthalmicus (HZO) 5 years ago in the right eye, and was diagnosed with Sjögren’s syndrome last year based on clinical findings of dry eye and dry mouth, but with negative serological markers. She reported two episodes of HZO recurrences in her right eye 5 years prior to presentation and remained free of recurrence and stable on oral valacyclovir since then. Her past medical history was significant for eosinophilic granulomatosis, common variable immune deficiency (CVID), anxiety, and depression (Table 1). Previous ocular treatments included topical corticosteroids, autologous serum tears, cyclosporine A, lifitegrast, punctal cautery, PROSE lenses, and systemic gabapentin without improvement. On presentation, she was on valacyclovir 500 mg once daily, and subcutaneous immunoglobulin (scIG) once weekly, for CVID.
Her OSDI score was 75.0/100, showing frequency of pain 4/4 (all of the time). The pain intensity was 10/10 based on VAS. She also reported severe non-ocular pain on the right side of the head and neck, radiating to the right lower back which was graded as 7/10 on VAS. She did not report neuropathic symptoms in her limbs. On examination, her BCVA was 20/20 in both eyes. On slit-lamp biomicroscopy, her conjunctiva and anterior chamber were quite in both eyes. Her TBUT was 7 seconds in the right eye and 8 seconds in the left eye (Table 1). Further, corneal fluorescein staining showed 2+ superficial punctate epitheliopathy, based on the Oxford scale, in the both eyes, respectively (Table 1). Schirmer’s I test showed 1 mm/5 min for the right eye and 0 mm/5 min for the left eye (Table 1). Stimulated Jones Schirmer’s test resulted in 10.5 mm/5 min for the right eye and 8.0 mm/5 min for the left eye. IOP were measured at 11 mmHg in both eyes. After instilling one drop of 0.5% proparacaine eye drop, she reported only 10% of pain relieve in both eyes, suggesting non-ocular component of pain due to either centralization of ganglionopathy. IVCM showed a mean total central corneal nerve density of 12,776.4 μm/mm2 in the right eye, 16,869.8 μm/mm2 in the left eye (Figure 1A), a mean total nerve fiber of 3.6 fiber/frame in the right eye, 11.0 fiber/frame in the left eye (Figure 1B), and presence of microneuromas (Figure 2B). The mean DC density increased in both eyes (56.3 cells/mm2 in the right eye and 25 cells/mm2 in the left eye) (Figure 3B).
Considering her clinical findings and the lack of response to the PCT, the patient was diagnosed with concurrent NCP and dry eye disease (DED). Serological evaluation showed elevated titers for anti-TS-HDS IgM (18,000; N<10,000) as well as anti-FGFR-3 IgG (6,000; N<3.000). Her anti-Ro and anti-La antibodies were negative on serology. She was referred for a skin biopsy, which showed decreased intraepidermal nerve fiber density (154 neurites/mm2; below 5th percentile compared to an age-matched database) on the distal leg, whereas, skin biopsy results of her thigh were reported within normal range (354 neurites/mm2). The diagnosis of dysimmune neuropathy was suggestive due to neuropathic pain, not responsive to PCT, presence of pain out of proportion to clinical findings, positive anti-TS-HDS IgM and anti-FGFR-3 IgG by serology, and decreased nerve fibers detected by both IVCM and skin biopsy.
Treatment was initiated with topical 0.5% loteprednol etabonate (4 times daily, tapered over 6 weeks), 20% autologous serum tears 8 times daily, and nortriptyline 70 mg daily, starting from 10 mg and gradually increased. At post-treatment month-3, the patient reported no improvement, and carbamazepine 400 mg daily was added to nortriptyline. However, the patient did not respond to a 3-months combination treatment and intravenous immunoglobulin (IVIG) treatment was initiated.
2.3. Case 3
A 54-year-old Caucasian female presented with history of chronic DED. Punctual plugs were placed in her right lower lid 4 months prior to her visit and she was unable to open her eyelids and was suffering from extreme photophobia since then. The patient was self-diagnosed with blepharospasm and came to our clinic for further evaluation and to get information about Botox treatment. She reported accompanying redness and pain in her eyes when her eyes were open. Her past ocular medical and surgical history were not significant other than DED. She was evaluated for Sjögren’s syndrome and results were reported negative. Prior to presentation, she had been treated with autologous serum tears 20% (8 times daily), doxycycline monohydrate (200 mg/day), artificial tears, topical cyclosporine A, topical corticosteroids (loteprednol), flaxseed oil, humidifier, warm compresses, and acupuncture, but her symptoms persistet.
On presentation, her OSDI score was 56.2/100 with a pain frequency of 2/4 (half of the time), whereas, frequency of light sensitivity was 4/4 (most of the time) (Table 1). The overall symptom intensity at the visit was 9/10 on VAS. The PCT test did not relieve her symptoms, suggesting presence of non-ocular component.
On examination, BCVA was 20/20 in both eyes. Slit-lamp examination showed low tear break-up time, and +1 corneal fluorescein staining based on Oxford scale (Table 1). Her Schirmer’s I results were 2 mm/5 min for the right eye and 6 mm/5 min for the left eye (Table 1). IOP was 15 mmHg in the both eyes. IVCM showed a central corneal nerve density that was decreased (total nerve density; 11,160.3 μm/mm2 in OD and 6,567.7 μm/mm2 in OS) on both eyes in addition to microneuromas (Figure 1 and Figure 2C)) and increased DC density (81.3 cells/mm2 in the right eye and 66.9 cells/mm2 in the left eye) (Figure 3C). Involuntary contractions of periorbital and facial muscles were also noted.
Detailed serologic evaluation showed elevated anti-TS-HDS IgM levels of 32,000 (N<10,000). The patient was subsequently referred to the neurology department for further evaluation and skin biopsy of her lower limbs. Her neurological history revealed occasional numbness in her fingertips and very rarely in her toes. Neurological evaluation evaluations, including cranial nerves, motor system, sensory system, reflexes, coordination, and gait were within normal limits. Her involuntary muscle contractions were diagnosed as Meige syndrome. The skin biopsy result showed a mean IEND of 3.3 fiber/mm2 (N>4.3 fiber/mm2) on the distal leg and 7.6 fiber/mm2 (N>9 fiber/mm2) on the thigh, which was lower than 5th percentile in both sites, confirming SFN.
Treatment was initiated with topical 0.5% loteprednol etabonate (4 times daily, tapered in 6 weeks), 20% autologous serum tears 8 times daily, nortriptyline 75 mg daily, and moisture goggles. She was also referred to the oculoplastic service for blepharospasm and Botox injections. At post-treatment month-3, she reported 50% improvement in her symptoms, however, she was unable to tolerate nortriptyline because of light headedness. Topical treatment was continued with intermittent implantation of self-retained cryopreserved amniotic membrane (PROKERA Slim®, Bio-Tissue, Miami, FL) insertion during flare-ups.
In chronic DED patients, sudden change in symptom character and severity, as well as treatment resistance may be a sign of concurrent NCP and accompanying SFN. In this patient, pain was not the dominant symptom, and sudden onset of extreme photophobia suggested possible presence of an unrevealed etiology. Dysimmune neuropathy related SFN should be kept in mind in patients with non-specific symptoms and/or sudden changes in symptoms, and in the presence of symptom/sign discrepancy.
3. DISCUSSION
NCP is a new and ill-defined entity, caused by a lesion/injury affecting the corneal somatosensory system.18, 19 Ocular and systemic conditions, such as inflammation, trauma, and iatrogenic factors, such as surgery that may affect the somatosensory system can result in NCP.18, 19 Although, a wide variety of ocular conditions, such as herpetic keratitis, recurrent corneal erosion syndrome, cataract and refractive surgeries, chronic dry eye disease, and systemic conditions, such as diabetes and autoimmune diseases have been reported in the etiology of NCP, not all patients suffering from these conditions develop NCP, indicating the presence of more complex mechanisms in the pathophysiology.18, 19
Clinical and diagnostic features of NCP are notably similar to SFN, including sensory symptoms such as burning, stinging, discomfort, irritation, hyperalgesia, allodynia, type of affected nerve fibers, lack or minimal presence of clinical signs, and a variety of etiological factors. Various systemic conditions have been shown to cause SFN, including metabolic disorders, immune-mediated and inflammatory conditions, vitamin and mineral deficiencies, vitamin B6 toxicity, celiac disease, gluten sensitivity, monoclonal gammopathies, toxins, genetic disorders, infections, cancers, paraneoplastic syndromes, and degenerative causes.1, 3, 27, 28 Metabolic disorders, such as glucose intolerance and diabetes have been reported to cause LD-SFN,1, 8, 9 whereas, dysimmune, toxic, and idiopathic etiologies have been mostly related to NLD-SFN.1, 8, 9 Nevertheless, the etiology of approximately half the cases of SFN etiology still remain idiopathic, despite detailed systemic evaluation.1, 27 Interestingly, Levine et al. have reported that up to 50% of idiopathic SFN patients present with Abs against either TS-HDS or anti-FGFR-3.8 A role of autoimmune factors in the pathogenesis of painful neuropathies is supported by observations in which the transfer of IgG Abs from patients with chronic painful conditions (fibromyalgia and complex regional pain syndrome or CRPS) to mice resulted in increased and prolonged hypersensitivity to noxious mechanical and thermal stimuli,29, 30 as well as increased activity in aδ and C nociceptors.25 Further, it has been shown that transfer of IgG from CPRS patients to mice following small plantar skin incision, created prolonged swelling and persistent hyperalgesia.31
Several previous studies have reported that TS-HDS and FGFR-3 play a role in neuronal maintenance and repair, as well as in programmed neuronal death.32–35 The structure and functions of the TS-HDS epitope and its interaction with FGF family/receptors may provide an insight to potential underlying mechanisms of the associated neuropathy. TS-HDS is the most abundant oligosaccharide domain of heparan sulfate and one of the key regulatory components of extracellular matrix in nervous system.16, 32, 36–38 TS-HDS functions as a regulatory molecule by binding to extracellular growth factors and their receptors. 16, 32, 36 FGF family molecules and their receptors are among the most thoroughly investigated molecules on the regulatory role of TS-HDS in nervous system.16, 32 Mitogenic roles of FGFs for astrocytes and Schwann cells in cell cultures,39 and induction of nerve growth factor production by FGF stimulated astrocytes have been shown.40 Additionally, up-regulation of FGFR-3 expression after peripheral nerve injury has also been reported.30 Interestingly, neuronal apoptosis induced by nerve damage did not occur in FGFR-3 knock-out mice.41 Therefore, FGFR-3, is thought to have role in the maintenance and survival of peripheral neurons 34, 35, 42
The heparan sulfate/FGF pathway related neuropathies have recently been investigated in clinical studies.16, 43 Pestronk et al. claimed that TS-HDS-related sensory neuropathies may originate from dysfunction along this pathway.16, 17 Their group evaluated patients with polyneuropathy who had IgM Abs against TS-HDS.16 All patients in this study had painful, predominantly sensory, distal polyneuropathy.16 Immunohistochemistry evaluation of nerve and muscle biopsies showed IgM deposition around intermediate-sized veins in perimysium and epineurium, while nerve conduction studies revealed axonal loss.16 The same group further reported that persistent discomfort in hands was present in 40% of anti-TS-HDS-positive patients, whereas, it was only present in 7% of anti-TS-HDS-negative neuropathy group, highlighting the possible role of anti-TS-HDS in NLD-SFN.17
The prevalence of anti-TS-HDS Abs and anti-FGFR-3 Abs in SFN has been reported as 37% and 15–19% respectively, in patients with an idiopathic or autoimmunity-related sensorial neuropathy.8, 10, 44 Both anti-TS-HDS and anti-FGFR-3-positive SFN have been reported to be predominantly associated with a female gender and non-length-dependent axonal loss.8 However, involvement of large fibers, motor neurons, and demyelination in the presence of these Abs were also defined in anti-FGFR-3 positive patients.10 Another study indicated that up to 89% of anti-FGFR-3-positive sensory neuropathy patients exhibited a NLD-SFN pattern.10, 44
Antoine et al. later demonstrated that anti-FGFR-3 positivity identifies a sensory neuropathy subgroup with predominant features of younger onset age, progressive course, and trigeminal nerve involvement, which reflects clinical observations in the patients presented herein.10 In their study 5 out of 16 patients presented with trigeminal neuropathy symptoms, including pain, numbness, paresthesia, and allodynia.10 One of these 5 patients had pure trigeminal neuropathy symptoms presenting as right facial pain with allodynia and left paresthesia, whereas, trigeminal symptoms accompanied with symptoms in other body parts in the rest of patients.10 Further, a patient with Sjögren’s syndrome suffered from bilateral trigeminal neuropathy, which was suggested to be related to involvement of trigeminal nerve ganglion.10 In the same study, nerve biopsy results were reported as “nerve fiber loss without regenerating clusters” which were suggested to indicate ganglionopathy rather than axonopathy.10 Additionally, presence of anti-TS-HDS IgM has been found to be more frequent as compared to anti-FGFR-3-positive and Ab-negative patients in the setting of acute onset of neuropathic symptoms in cryptogenic SFN.8 Importantly, 63.5% of anti-FGFR-3 positive sensory neuropathy patients have been shown to exhibit a progressive disease course.10
Although, the association between anti-TS-HDS, anti-FGFR-3 Abs in painful NLD-SFN has been strongly suggested, data on sensitivity and specificity remains limited. The sensitivity, specificity, and positive and negative predictive values of anti-FGFR-3 Abs to differentiate idiopathic or dysimmune neuropathy-related SFN from other causes of SFN and healthy individuals were calculated as 19.0%, 99.6%, 94.1% and 77.3%, respectively.10 In 211 control patients composed of 41 sensory-motor neuropathy, 65 healthy blood donors, 59 other neurological disease, and 46 systemic auto-immune diseases, only 1 patient had positive anti-FGFR-3.10 However, Samara et al. repeated serologic evaluation in 7 patients with anti-FGFR-3 Ab positive neuropathy.15 Three of these patients had lower titers and 2 of the patients had negative results with repeated test suggesting potential inconsistency of anti-FGFR-3 Abs, 15 suggesting serological associations need to be further studied in order to fully understand their clinical relevance.
Herein, we report 3 patients with NCP who had probable dysimmune neuropathy related Abs and SFN confirmed with skin biopsy.All our patients were young females as reported in previous studies evaluating dysimmune neuropathy related NLD-SFN. Two of our patients showed acute onset pain, whereas, the other one presented with chronic, progressive pain with acute exacerbation. Regarding ocular surface findings, while Case 1 and Case 3 met conventional definition of NCP, ocular surface symptoms without clinical findings, Case 2 was complicated with clinical DED. Further, patients suffered from different co-morbid diseases, which may contribute nerve injury including refractive surgery, HZO, Lyme disease, and migraine. Case 1 highlights that ocular pain can be the first clinical presentation of SFN, especially after iatrogenic injury to corneal nerves, such as after refractive surgery. Thus, asymptomatic/undiagnosed SFN may be a risk factor in the development of ocular pain following ocular surgeries. Furthermore, in patients with post-LASIK neuralgia dysimmune neuropathy-related SFN may be a previously undetected etiological cause. Moreover, the lack of response to anesthetic drops suggested the need for systemic pharmacotherapy, which in this patient resulted in significant improvement of her ocular and non-ocular symptoms. Case 2 demonstrates sudden onset of severe ocular pain development without direct trauma to the nerves. The history of HZO and presumable Sjögren’s syndrome may have contributed to prior nerve damage. However, the acute development or exacerbation of pain that emerged should raise suspicion for additional causes, in this case SFN. Dysimmune SFN may be the underlying cause of SFN and ocular pain or discomfort in such cases.
Patients with SFN may present with variety of pain features, ocular surface findings, and co-morbid diseases, suggesting that we may encounter dysimmune Ab-related NCP with diverse presentations. Therefore, in our clinical practice, we aim to screen NCP patients for well-defined etiologies of small fiber neuropathy including diabetes, immune-mediated and inflammatory conditions, vitamin and mineral deficiencies, monoclonal gammopathies and Celiac disease per the suggestion of current neurology literature on SFN.1, 4 However, the diagnosis of SFN is challenging and the etiology may remain idiopathic despite detailed serological assessment.1, 4 Considering the broad variety of the clinical presentation of the disease and potential underlying causes, multidisciplinary approach plays significant role in the diagnosis and treatment of SFN and NCP patients. Especially, in the presence of systemic neurological symptoms on multiple body regions, treatment resistance and/or worsening of symptoms despite proper treatment, consultations with other specialties, especially with neurology and rheumatology, are critical.
Although NCP has to date been considered a regional pain disorder, given our observations, we can postulate that systemic dysimmune mechanisms may play an important role in the development of NCP as well. Positive serology for dysimmune markers shows that one of the potential etiologies for NCP can be dysimmune SFN. However, SFN can be present with other etiologies, in which the serology for the presented auto-antibodies would be negative or positive for other markers. Although trigeminal nerve involvement has been reported in dysimmune neuropathies, our patients presented with bilateral ocular pain, suggesting that NLD-SFN may in fact present with localized symptoms. It is therefore conceivable that that NCP may be a novel subgroup of NLD-SFN or that NCP patients represent an initial stage of NLD-SFN. This is similar to Sjögren’s syndrome, in which ocular findings can be the initial presentation.45 Based on our knowledge from well-known auto-/dysimmune disorders, the initial clinical presentation may exhibit a wide variety; symptoms may start from one region and progress to other body parts by time and asymmetric involvement may occur.1, 46 Moreover, the presence or absence of dysimmunity, may be implicated in the observations that while most patients demonstrate physiological nerve regeneration after nerve injury such as ocular surgery, some patients do not. Therefore, further investigation is warranted on whether subclinical or declared SFN represents a risk factor for NCP following ocular procedures.
To best of our knowledge this is the first case series demonstrating the presence of anti-TS-HDS and/or anti-FGFR-3 in patients with NCP, suggesting serological evaluation should be performed in the assessment of NCP patients. Considering the overlapping features of NCP and NLD-SFN, and the relatively well-established association of NLD-SFN and dysimmunity, the role of anti-TS-HDS anti-FGFR-3 Abs deserves further study in NCP or patients with ocular pain and discomfort.
Acknowledgement:
We would like to thank Dr. Vinny Keshav for his meticulous attention to follow the patients’ serologic evaluation and skin biopsy results and Dr. Leyla Yavuz-Saricay for her contribution to preparation of dataset for serology results.
Funding:
NIH R61-NS113341 (PH), Massachusetts Lions Eye Research Fund Inc. (PH), Bettingen Foundation (PH), Lions Club International Foundation (PH), Tufts Medical Center Institutional Support (PH), Research to Prevent Blindness Challenge Grant to the Department of Ophthalmology
Footnotes
Dısclosure: Authors do not have conflict of interest to disclose relevant to this article.
REFERENCES
- 1.Farhad K Current Diagnosis and Treatment of Painful Small Fiber Neuropathy. Curr Neurol Neurosci Rep. 2019;19(12):103. [DOI] [PubMed] [Google Scholar]
- 2.Sopacua M, Hoeijmakers JGJ, Merkies ISJ, Lauria G, Waxman SG, Faber CG. Small-fiber neuropathy: Expanding the clinical pain universe. J Peripher Nerv Syst. 2019;24(1):19–33. [DOI] [PubMed] [Google Scholar]
- 3.Oaklander AL, Nolano M. Scientific Advances in and Clinical Approaches to Small-Fiber Polyneuropathy: A Review. JAMA Neurol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basantsova NY, Starshinova AA, Dori A, Zinchenko YS, Yablonskiy PK, Shoenfeld Y. Small-fiber neuropathy definition, diagnosis, and treatment. Neurol Sci. 2019;40(7):1343–50. [DOI] [PubMed] [Google Scholar]
- 5.Provitera V, Gibbons CH, Wendelschafer-Crabb G, Donadio V, Vitale DF, Loavenbruck A, et al. The role of skin biopsy in differentiating small-fiber neuropathy from ganglionopathy. Eur J Neurol. 2018;25(6):848–53. [DOI] [PubMed] [Google Scholar]
- 6.Gemignani F, Bellanova MF, Saccani E, Pavesi G. Non-length-dependent small fiber neuropathy: Not a matter of stockings and gloves. Muscle & Nerve. 2022;65(1):10–28. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L Small Fiber Neuropathy. Semin Neurol. 2019;39(5):570–7. [DOI] [PubMed] [Google Scholar]
- 8.Levine TD, Kafaie J, Zeidman LA, Saperstein DS, Massaquoi R, Bland RJ, et al. Cryptogenic small-fiber neuropathies: Serum autoantibody binding to trisulfated heparan disaccharide and fibroblast growth factor receptor-3. Muscle Nerve. 2020;61(4):512–5. [DOI] [PubMed] [Google Scholar]
- 9.Khan S, Zhou L. Characterization of non-length-dependent small-fiber sensory neuropathy. Muscle Nerve. 2012;45(1):86–91. [DOI] [PubMed] [Google Scholar]
- 10.Antoine JC, Boutahar N, Lassablière F, Reynaud E, Ferraud K, Rogemond V, et al. Antifibroblast growth factor receptor 3 antibodies identify a subgroup of patients with sensory neuropathy. J Neurol Neurosurg Psychiatry. 2015;86(12):1347–55. [DOI] [PubMed] [Google Scholar]
- 11.Gorson KC, Herrmann DN, Thiagarajan R, Brannagan TH, Chin RL, Kinsella LJ, et al. Non-length dependent small fibre neuropathy/ganglionopathy. J Neurol Neurosurg Psychiatry. 2008;79(2):163–9. [DOI] [PubMed] [Google Scholar]
- 12.Steck A, Yuki N, Graus F. Antibody testing in peripheral nerve disorders. Handb Clin Neurol. 2013;115:189–212. [DOI] [PubMed] [Google Scholar]
- 13.Kieseier BC, Mathey EK, Sommer C, Hartung HP. Immune-mediated neuropathies. Nat Rev Dis Primers. 2018;4(1):31. [DOI] [PubMed] [Google Scholar]
- 14.Rajabally YA. Chapter 1 - Dysimmune neuropathies. In: Rajabally YA, editor. Dysimmune Neuropathies: Academic Press; 2020. p. 1–3. [Google Scholar]
- 15.Samara V, Sampson J, Muppidi S. FGFR3 Antibodies in Neuropathy: What to Do With Them? J Clin Neuromuscul Dis. 2018;20(1):35–40. [DOI] [PubMed] [Google Scholar]
- 16.Pestronk A, Choksi R, Logigian E, Al-Lozi MT. Sensory neuropathy with monoclonal IgM binding to a trisulfated heparin disaccharide. Muscle Nerve. 2003;27(2):188–95. [DOI] [PubMed] [Google Scholar]
- 17.Pestronk A, Schmidt RE, Choksi RM, Sommerville RB, Al-Lozi MT. Clinical and laboratory features of neuropathies with serum IgM binding to TS-HDS. Muscle Nerve. 2012;45(6):866–72. [DOI] [PubMed] [Google Scholar]
- 18.Dieckmann G, Goyal S, Hamrah P. Neuropathic Corneal Pain: Approaches for Management. Ophthalmology. 2017;124(11s):S34–s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goyal S, Hamrah P. Understanding Neuropathic Corneal Pain--Gaps and Current Therapeutic Approaches. Semin Ophthalmol. 2016;31(1–2):59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauria G, Majorana A, Borgna M, Lombardi R, Penza P, Padovani A, et al. Trigeminal small-fiber sensory neuropathy causes burning mouth syndrome. Pain. 2005;115(3):332–7. [DOI] [PubMed] [Google Scholar]
- 21.Hirai T, Miyagawa S, Matsui K, Kurita A. [Small fiber neuropathy in a patient with complete Heerfordt syndrome manifesting as refractory facial pain]. Rinsho Shinkeigaku. 2014;54(7):585–8. [DOI] [PubMed] [Google Scholar]
- 22.Hurliman E, Groth D, Wendelschafer-Crabb G, Kennedy W, Kavand S, Ericson M, et al. Small-fibre neuropathy in a patient with dermatomyositis and severe scalp pruritus. Br J Dermatol. 2017;176(1):209–11. [DOI] [PubMed] [Google Scholar]
- 23.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–21. [DOI] [PubMed] [Google Scholar]
- 24.Reed MD, Van Nostran W. Assessing pain intensity with the visual analog scale: a plea for uniformity. J Clin Pharmacol. 2014;54(3):241–4. [DOI] [PubMed] [Google Scholar]
- 25.Cruzat A, Qazi Y, Hamrah P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul Surf. 2017;15(1):15–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kheirkhah A, Rahimi Darabad R, Cruzat A, Hajrasouliha AR, Witkin D, Wong N, et al. Corneal Epithelial Immune Dendritic Cell Alterations in Subtypes of Dry Eye Disease: A Pilot In Vivo Confocal Microscopic Study. Invest Ophthalmol Vis Sci. 2015;56(12):7179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDonald S, Sharma TL, Li J, Polston D, Li Y. Longitudinal follow-up of biopsy-proven small fiber neuropathy. Muscle Nerve. 2019;60(4):376–81. [DOI] [PubMed] [Google Scholar]
- 28.Terkelsen AJ, Karlsson P, Lauria G, Freeman R, Finnerup NB, Jensen TS. The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. Lancet Neurol. 2017;16(11):934–44. [DOI] [PubMed] [Google Scholar]
- 29.Cuhadar U, Gentry C, Vastani N, Sensi S, Bevan S, Goebel A, et al. Autoantibodies produce pain in complex regional pain syndrome by sensitizing nociceptors. Pain. 2019;160(12):2855–65. [DOI] [PubMed] [Google Scholar]
- 30.Goebel A, Krock E, Gentry C, Israel MR, Jurczak A, Urbina CM, et al. Passive transfer of fibromyalgia symptoms from patients to mice. J Clin Invest. 2021;131(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tékus V, Hajna Z, Borbély É, Markovics A, Bagoly T, Szolcsányi J, et al. A CRPS-IgG-transfer-trauma model reproducing inflammatory and positive sensory signs associated with complex regional pain syndrome. Pain. 2014;155(2):299–308. [DOI] [PubMed] [Google Scholar]
- 32.Ornitz DM. FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays. 2000;22(2):108–12. [DOI] [PubMed] [Google Scholar]
- 33.Eckenstein FP. Fibroblast growth factors in the nervous system. J Neurobiol. 1994;25(11):1467–80. [DOI] [PubMed] [Google Scholar]
- 34.Latko M, Czyrek A, Porebska N, Kucinska M, Otlewski J, Zakrzewska M, et al. Cross-Talk between Fibroblast Growth Factor Receptors and Other Cell Surface Proteins. Cells. 2019;8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mason I Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nat Rev Neurosci. 2007;8(8):583–96. [DOI] [PubMed] [Google Scholar]
- 36.Kreuger J, Salmivirta M, Sturiale L, Giménez-Gallego G, Lindahl U. Sequence analysis of heparan sulfate epitopes with graded affinities for fibroblast growth factors 1 and 2. J Biol Chem. 2001;276(33):30744–52. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 2008;130(4):635–53. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi Y Heparan sulfate proteoglycans in the nervous system: their diverse roles in neurogenesis, axon guidance, and synaptogenesis. Semin Cell Dev Biol. 2001;12(2):99–106. [DOI] [PubMed] [Google Scholar]
- 39.Davis JB, Stroobant P. Platelet-derived growth factors and fibroblast growth factors are mitogens for rat Schwann cells. J Cell Biol. 1990;110(4):1353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida K, Gage FH. Cooperative regulation of nerve growth factor synthesis and secretion in fibroblasts and astrocytes by fibroblast growth factor and other cytokines. Brain Res. 1992;569(1):14–25. [DOI] [PubMed] [Google Scholar]
- 41.Jungnickel J, Gransalke K, Timmer M, Grothe C. Fibroblast growth factor receptor 3 signaling regulates injury-related effects in the peripheral nervous system. Mol Cell Neurosci. 2004;25(1):21–9. [DOI] [PubMed] [Google Scholar]
- 42.Oellig C, Pirvola U, Taylor L, Elde R, Hokfelt T, Pettersson RF. Acidic FGF and FGF receptors are specifically expressed in neurons of developing and adult rat dorsal root ganglia. Eur J Neurosci. 1995;7(5):863–74. [DOI] [PubMed] [Google Scholar]
- 43.Bourque PR, Chardon JW, Massie R. Autoimmune peripheral neuropathies. Clin Chim Acta. 2015;449:37–42. [DOI] [PubMed] [Google Scholar]
- 44.Tholance Y, Moritz CP, Rosier C, Ferraud K, Lassabliere F, Reynaud-Federspiel E, et al. Clinical characterisation of sensory neuropathy with anti-FGFR3 autoantibodies. J Neurol Neurosurg Psychiatry. 2020;91(1):49–57. [DOI] [PubMed] [Google Scholar]
- 45.Liew MS, Zhang M, Kim E, Akpek EK. Prevalence and predictors of Sjogren’s syndrome in a prospective cohort of patients with aqueous-deficient dry eye. Br J Ophthalmol. 2012;96(12):1498–503. [DOI] [PubMed] [Google Scholar]
- 46.Gwathmey KG. Sensory neuronopathies. Muscle Nerve. 2016;53(1):8–19. [DOI] [PubMed] [Google Scholar]


