Abstract
Purpose:
There is a need to understand physicians’ diagnostic uncertainty in the initial management of microbial keratitis (MK). This study aimed to understand cornea specialists’ diagnostic uncertainty by establishing risk thresholds for treatment of MK that could be used to inform a decision curve analysis for prediction modeling.
Methods:
A cross-sectional survey of cornea specialists with at least two years clinical experience was conducted. Clinicians provided the percentage risk at which they would always or never treat MK types (bacterial, fungal, herpetic, and amoebic) based on initial ulcer sizes and locations (<2mm2 central, <2mm2 peripheral, and >8mm2 central).
Results:
72 of 99 ophthalmologists participated who were 50% female with an average of 14.7 (standard deviation=10.1) years of experience, 60% in academic practices, and 38% outside the United States (U.S.). Clinicians reported they would “never” and “always” treat a <2mm2 central MK infection if the median risk was 0% and 20% for bacterial (interquartile range, IQR=0–5 and 5–50), 4.5% and 27.5% for herpetic (IQR=0–10 and 10–50), 5% and 50% for fungal (IQR=0–10 and 20–75), and 5% and 50.5% for amoebic (IQR=0–20 and 32–80), respectively. Mixed-effects models showed lower thresholds to treat larger and central infections (p<0.001, respectively), and thresholds to always treat differed between MK types for U.S. (p<0.001) but not international clinicians.
Conclusion:
Risk thresholds to treat differed by practice location, and MK types, location, and size. Researchers can use these thresholds to understand when a clinician is uncertain and to create decision support tools to guide clinicians’ treatment decisions.
Keywords: microbial keratitis, corneal ulcer treatment, diagnostic uncertainty, Decision Curve Analysis, Deep Learning
Introduction
Clinicians are often faced with uncertainty when making diagnostic decisions. A few causes of diagnostic uncertainty include lack of knowledge about a disease, unclear clinical histories, misinterpretation of findings, and limitations of diagnostic testing, to name a few causes.1 Diagnostic uncertainty impacts the wellness of patients, effectiveness of clinicians, and other aspects of healthcare including utilization, cost, and medical errors.2 For eye conditions, delays in diagnosis can contribute to worse vision loss.3 Understanding and quantifying diagnostic uncertainty for specific conditions help to identify areas for improvement regarding high-risk conditions and develop solutions to address the uncertainties.2
Diagnostic uncertainty exists for microbial keratitis (MK) as identifying the underlying causative organism can prove difficult due to overlapping clinical presentations of differing organisms.4 Cultures obtained from scrapings are the diagnostic gold standard, but only identify 50% of the causative MK organisms.5–6 Cultures take days and there are no current in-office point-of-care tests. This uncertainty in identification of the causative organism drives suboptimal care for patients as treatments are often broadly targeted which can increase unnecessary side effects and costs for both the patient and health care system. Timely and accurate diagnosis of the causative organism has the potential to improve outcomes for patients with MK.7 Machine-learning prediction algorithms for MK organism type are being explored.8,9 However, the performance of these algorithms have not been evaluated with respect to the decision that clinicians face. For example, in a case of a corneal ulcer for a contact lens-wearing patient in the United States (U.S.), clinicians will have a low threshold to always treat with an antibacterial agent. In that case, the clinician would have minimal need for a prediction algorithm to recommend antibacterial treatment. Similarly, a U.S. clinician may never treat a small peripheral lesion with an antifungal agent, so, again, there is no need for an algorithm to aid the clinician. Thus, prediction models are most helpful in cases when the clinician is uncertain if they should treat or not treat. Defining uncertainty quantitatively allows researchers to evaluate both performance and value of a diagnostic prediction tool.
Decision Curve Analysis (DCA) is a methodology that provides a framework to assess the clinical usefulness of prediction models by considering the range of risk thresholds for treatment without explicitly assigning costs and benefits to all possible outcomes (i.e., true positives, true negatives, false positives, and false positives) that traditional decision analysis requires.10–12 DCA can be useful when assessing the range of uncertainty of treatment risk to provide a net benefit to clinicians from evaluations of real-world performances of prediction algorithms.10,13–15 DCA has been effectively used in other disciplines of medicine such as bladder cancer16, low birth weight17, and in the eye condition myopia.18 Lin and colleagues utilized DCA for evaluating strategies to screen for myopia in school aged children. Children completed three tests, and their data was used to determine both the accuracy and the net benefits of screening strategies.18 To our knowledge, DCA has not been used in microbial keratitis research to date but has been used for eye conditions such as cataracts and myopia.18–19 Creating the clinical context to inform ophthalmic prediction modeling will be critical if algorithms are going to be implemented. Thus, the goal of this study is to establish clinicians’ risk thresholds for a specific management decision: when to treat or not treat MK with a specific medication class.
Materials and Methods
The University of Michigan Institutional Review Board reviewed and exempted this study. Survey development included multiple iterative reviews with the research team, including cornea specialists, and underwent internal pilot testing with cornea specialists at University of Michigan. The final survey included questions about the percentage risk thresholds to always or never treat initial MK according to different scenarios. Survey scenarios varied by MK types (bacterial, fungal, herpetic, and acanthamoeba), and sizes and locations (<2mm2 central, <2mm2 peripheral and >8mm2 central). For example, “In a case of a <2mm2 central corneal ulcer, if I believed that the patient had a _xx_% risk of bacterial keratitis, I would always treat with antibacterial medications.”
The main outcome measure was the percentage risk threshold determined by the clinicians. Demographic factors of the clinicians were self-reported and included age, gender, race, ethnicity, years of experience, practice type, and practice location. Clinicians were included if they completed cornea training (including fellowship, as applicable to their nation) and had practiced for at least two years. They were intentionally sampled across gender. The survey data were collected and managed using the REDCap electronic data capture tools (Vanderbilt University, Nashville, TN) between March and April 2022. Purposive sampling was conducted by generating a list of cornea specialists to be contacted for potential participation. Emails sent to potential participants included an explanation of the study and a survey participation code. Participants were contacted up to three times via email.
Statistical Analysis:
Demographic and survey responses of the ophthalmologists were summarized with descriptive statistics, including means, standard deviations (SD), medians, interquartile ranges (IQR)s, frequencies, and percentages. Threshold differences between MK types were investigated with Kruskal-Wallis tests followed by Holm-adjusted Dunn tests for post-hoc pairwise comparisons. Linear mixed effects regression models were used to investigate factors associated with percentage risk to always or never treat. Fixed effects included gender, years of experience, practice type, MK location and size, and interaction between practice location and MK type. Random effects included ophthalmologists as random intercepts and MK type as random slopes. Repeated risk threshold measures within a respondent were modeled with an “unstructured” covariance structure. R version 4.1.1 (R Core Team; Vienna, Australia) was used for statistical analysis.
Results
The survey response rate was 72.7% (n=72 of 99 clinicians). One response was excluded from analysis due to inverse responses for treat and never treat risk thresholds, implying a misunderstanding of the question stem. Participants were on average 44.8 years (SD=9.9) with an average of 14.7 (SD=10.1) years of experience and 50% female, 49% Asian, 45% White, 3% Black, and 6.2% Hispanic. There were 60% (n=43) of respondents who practiced in an academic setting. There were 38% of respondents (n=27) from international locations. International locations included 14 countries and included: Argentina, Armenia, Australia, Egypt, Ethiopia, India, Israel, Malaysia, Mexico, Nepal, Netherlands, Singapore, Thailand, and the United Kingdom. Further demographics of study participants are displayed in Table 1.
Table 1.
Demographics and survey responses of Ophthalmologists
| Continuous Variable | Mean (SD) | Min, Max | Median (IQR) |
|---|---|---|---|
| Age (years) | 44.8 (9.9) | 31.0, 77.0 | 42.0 (37.0, 51.0) |
| Experience (years) | 14.7 (10.1) | 2.0, 51.0 | 11.0 (7.8, 19.0) |
| Categorical Variable | Frequency (%) | ||
| Gender | |||
| Female | 34 (50.0) | ||
| Male | 34 (50.0) | ||
| Race | |||
| Asian | 33 (49.3) | ||
| White | 30 (44.8) | ||
| Black/African American | 2 (3.0) | ||
| Other | 2 (3.0) | ||
| Ethnicity | |||
| Hispanic | 4 (6.2) | ||
| Non-Hispanic | 61 (93.8) | ||
| Practice Type | |||
| Academic | 43 (59.7) | ||
| Private | 11 (15.3) | ||
| Hybrid | 15 (20.8) | ||
| Other | 3 (4.2) | ||
| Location | |||
| USA | 44 (62.0) | ||
| International | 27 (38.0) | ||
SD, Standard Deviation; IQR, Interquartile Range; USA, the United States of America
Clinicians reported they would never and always treat an initial <2mm2 central infection with specific medication types if the median risk were 0% and 20% for bacterial (IQR=0–5 and 5–50), 4.5% and 27.5% for herpetic (IQR=0–10 and 10–50), 5% and 50% for fungal (IQR=0–10 and 20–75), and 5% and 50.55% for amoebic (IQR=0–20 and 32–80), respectively. Reported risk thresholds were significantly lower for bacterial infections compared to fungal (never, p=0.006; always, p<0.001), amoebic (never, p=0.003; always, p<0.001), and herpetic infections (never, p=0.046), and for herpetic compared to amoebic infections (always, p=0.001). Further results for <2mm2 peripheral and >8mm2 central infection scenarios are displayed in Table 2. All post-hoc pairwise comparisons are provided in Supplemental Table 1.
Table 2.
Ophthalmologists’ responses to “At what perceived risk of bacterial/fungal/herpetic/amoebic keratitis would you never or always treat with antibacterial/antifungal/antiviral/steroid medications?”
| “Never” Treat | “Always” Treat | |||
|---|---|---|---|---|
| MK Scenarios | Median (IQR), Mean (SD) | P-value * | Median (IQR), Mean (SD) | P-value * |
| <2mm2 central | ||||
| Bacterial | 0.0 (0, 5), 4.5 (8.8) | 0.002a,b,c | 20.0 (5, 50), 29.1 (27.8) | <0.001a,b,f |
| Fungal | 5.0 (0, 10), 9.9 (13.9) | 50.0 (20, 75), 48.4 (29.9) | ||
| Herpetic | 4.5 (0, 10), 9.0 (12.8) | 27.5 (10, 50), 35.6 (27.5) | ||
| Amoebic | 5.0 (0, 20), 12.1 (15.9) | 50.5 (32, 80), 55.0 (30.1) | ||
| <2mm2 peripheral | ||||
| Bacterial | 1.5 (0, 10), 6.3 (9.1) | 0.126 | 25.0 (10, 56), 35.4 (28.2) | <0.001a,b,c,f |
| Fungal | 8.5 (0, 16), 11.3 (14.5) | 50.0 (20, 79), 53.5 (31.7) | ||
| Herpetic | 5.0 (0, 16), 10.6 (14.1) | 40.0 (19, 70), 41.2 (28.7) | ||
| Amoebic | 5.0 (0, 20), 14.2 (20.1) | 60.0 (30, 84), 58.2 (30.7) | ||
| >8mm2 central | ||||
| Bacterial | 0.0 (0, 1), 3.4 (8.3) | <0.001a,b,c | 10.0 (5, 26), 23.0 (29.3) | <0.001a,b,c,f |
| Fungal | 3.0 (0, 10), 9.0 (13.7) | 30.0 (10, 63), 40.0 (29.6) | ||
| Herpetic | 1.0 (0, 10), 7.7 (12.3) | 20.0 (10, 50), 30.2 (25.9) | ||
| Amoebic | 5.0 (0, 20), 10.7 (14.4) | 50.0 (24, 75), 49.0 (29.9) | ||
Kruskal-Wallis test
Holm-adjusted Dunn’s tests for post-hoc pairwise comparison showed significant risk threshold differences for:
Bacterial versus Fungal
Bacterial versus Herpetic
Bacterial versus Amoebic
Fungal versus Herpetic
Fungal versus Amoebic
Herpetic versus Amoebic
SD, Standard Deviation; IQR, Interquartile Range
Figure 1 displays the uncertainty range between which clinicians would always and never treat by MK types and practice location. U.S. clinicians reported the greatest median range of uncertainty (when to never or always treat) for acanthamoeba (53%), followed by fungal (40%), herpetic (13.5%), and bacterial (10%) infections. International clinicians reported the greatest uncertainty range for acanthamoeba (46%), followed by fungal (17%) and herpetic (17%), and bacterial (5%) infections.
Figure 1.
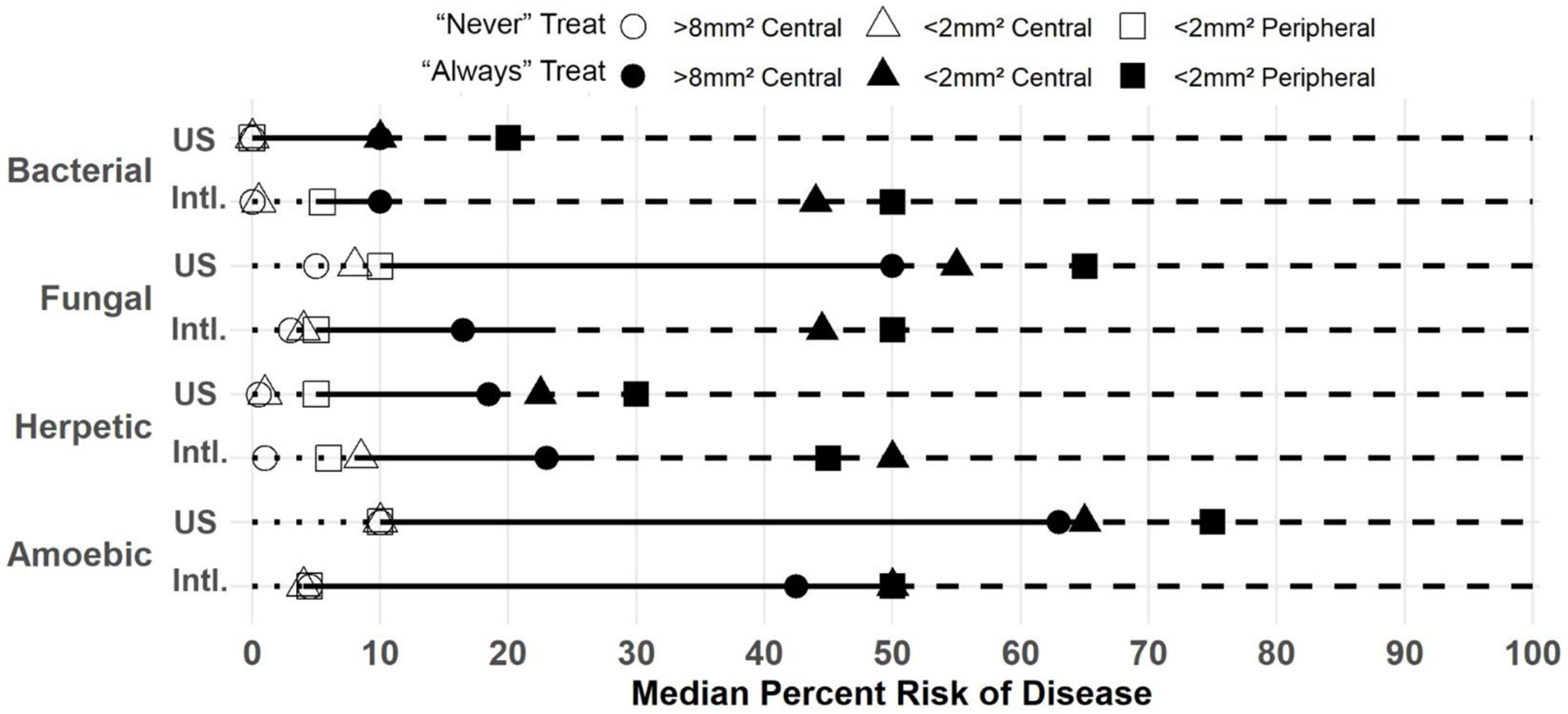
Line plot displaying the median reported percentage risk of microbial keratitis (MK) at which a clinician would never or always treat with corresponding medications, stratified by MK type and the ophthalmologist’s practice location (US, United States; Intl, International).
The results from linear mixed effects models showed risk responses for always treat differed significantly by size, location, and organism type for U.S. clinicians only (Table 3). Specifically, the threshold to treat >8mm2 central ulcers was lower by 6.8 percentage points compared to <2mm2 central ulcers (p<0.001), and by 12.1 percentage points compared to <2mm2 peripheral ulcers (p<0.001). The risk threshold to treat <2mm2 central MK was lower by 5.2 percentage points compared to <2mm2 peripheral ulcers (p<0.001). U.S. respondents’ threshold to treat bacterial infections was lower by 26.2 percentage points compared to fungal (p<0.001) and by 37.3 percentage points compared to amoebic (p<0.001). Similarly, threshold to never treat >8mm2 central was lower by 1.2 percentage points compared to <2mm2 central (p=0.04) and by 2.9 percentage points compared to <2mm2 peripheral ulcers (p<0.001); threshold of <2mm2 central was lower than <2mm2 peripheral ulcers by 1.6 percentage points (p=0.01). U.S. respondents reported bacterial infection median risk was lower than fungal by 8.4 percentage points (p=0.001) and amoebic by 10.9 percentage points (p<0.001). Risk was not associated with years of experience, gender, or practice type. Supplemental Figure 1 displays estimates of threshold for MK scenario comparisons by practice locations.
Table 3.
Results of linear mixed effects models estimating the effect of clinician characteristics and microbial keratitis (MK) scenarios on the reported risk threshold to always or never treat with targeted medication.
| “Never” Treat | “Always” Treat | ||||||
|---|---|---|---|---|---|---|---|
| Term | Contrast | Effect | 95% CI | Holm-adjusted P-value | Effect | 95% CI | Holm-adjusted P-value |
| Experience (Years) | - | 0.1 | −0.01, 0.3 | 0.072 | −0.2 | −0.7, 0.3 | 0.46 |
| Gender | Male vs Female | −0.6 | −4.0, 2.9 | 0.754 | −7.1 | −18.5, 4.3 | 0.216 |
| MK Size & Location | <2mm2 Central vs >8mm2 Central | 1.2 | −0.2, 2.6 | 0.043 | 6.8 | 4.5, 9.2 | <0.001 |
| <2mm2 Peripheral vs >8mm2 Central | 2.9 | 1.4, 4.3 | <0.001 | 12.1 | 9.7, 14.4 | <0.001 | |
| <2mm2 Peripheral vs <2mm2 Central | 1.6 | 0.2, 3.1 | 0.012 | 5.2 | 2.8, 7.6 | <0.001 | |
| MK Type x Practice Location | Comparison between MK Type in US | ||||||
| F vs B | 8.4 | 2.1, 14.7 | 0.001 | 26.2 | 10.9, 41.5 | <0.001 | |
| H vs B | 5.4 | −0.3, 11.1 | 0.078 | 9.3 | −1.1, 19.7 | 0.097 | |
| H vs F | −3.0 | −8.4, 2.5 | 1 | −16.9 | −31.0, −2.8 | 0.005 | |
| A vs B | 10.9 | 3.3, 18.4 | <0.001 | 37.3 | 22.1, 52.6 | <0.001 | |
| A vs F | 2.5 | −3.5, 8.5 | 1 | 11.1 | 2.1, 20.2 | 0.004 | |
| A vs H | 5.4 | −0.7, 11.6 | 0.131 | 28.0 | 14.4, 41.6 | <0.001 | |
| Comparison between MK Type for Intl. | |||||||
| F vs B | 1.8 | −5.9, 9.4 | 1 | 4.6 | −14.0, 23.2 | 1 | |
| H vs B | 3.6 | −3.3, 10.6 | 1 | 2.8 | −9.8, 15.5 | 1 | |
| H vs F | 1.9 | −4.7, 8.5 | 1 | −1.7 | −18.9, 15.4 | 1 | |
| A vs B | 4.2 | −4.9, 13.4 | 1 | 7.2 | −11.4, 25.7 | 1 | |
| A vs F | 2.5 | −4.8, 9.7 | 1 | 2.6 | −8.4, 13.6 | 1 | |
| A vs H | 0.6 | −6.9, 8.1 | 1 | 4.4 | −12.2, 20.9 | 1 | |
| Comparison of Intl. vs US for each MK Type | |||||||
| B | 2.5 | −4.2, 9.2 | 1 | 20.5 | −3.5, 44.5 | 0.133 | |
| F | −4.1 | −15.3, 7.1 | 1 | −1.1 | −27.1, 24.9 | 1 | |
| H | 0.7 | −9.9, 11.3 | 1 | 14.0 | −10.3, 38.4 | 0.978 | |
| A | −4.1 | −17.7, 9.5 | 1 | −9.7 | −35.1, 15.8 | 1 | |
| Remaining Comparison | |||||||
| B Intl. vs F US | −5.9 | −14.5, 2.8 | 0.736 | −5.7 | −30.2, 18.8 | 1 | |
| B Intl. vs H US | −2.9 | −11.3, 5.5 | 1 | 11.2 | −12.8, 35.2 | 1 | |
| B Intl. vs A US | −8.4 | −18.3, 1.6 | 0.198 | −16.8 | −41.1, 7.5 | 0.483 | |
| F Intl. vs B US | 4.3 | −5.3, 13.8 | 1 | 25.1 | 0.2, 50.0 | 0.037 | |
| F Intl. vs H US | −1.1 | −12.0, 9.7 | 1 | 15.8 | −9.3, 40.9 | 0.735 | |
| F Intl. vs A US | −6.6 | −18.7, 5.5 | 1 | −12.3 | −37.9, 13.4 | 1 | |
| H Intl. vs B US | 6.1 | −3.0, 15.3 | 0.736 | 23.3 | −0.7, 47.4 | 0.05 | |
| H Intl. vs F US | −2.2 | −13.0, 8.5 | 1 | −2.9 | −27.6, 21.9 | 1 | |
| H Intl. vs A US | −4.7 | −16.5, 7.1 | 1 | −14.0 | −38.6, 10.6 | 0.983 | |
| A Intl. vs B US | 6.7 | −4.5, 18.0 | 1 | 27.7 | 3.1, 52.3 | 0.011 | |
| A Intl. vs F US | −1.6 | −14.2, 11.0 | 1 | 1.5 | −24.1, 27.0 | 1 | |
| A Intl. vs H US | 1.3 | −11.1, 13.7 | 1 | 18.4 | −6.4, 43.2 | 0.343 | |
| Practice Type | Private vs Academic | −2.8 | −9.4, 3.8 | 0.49 | 2.2 | −19.3, 23.6 | 1 |
| Hybrid vs Academic | −5.7 | −12.7, 1.3 | 0.119 | −15.9 | −38.7, 7.0 | 0.377 | |
| Other vs Academic | 8.7 | −3.0, 20.5 | 0.14 | 3.6 | −34.6, 41.8 | 1 | |
| Hybrid vs Private | −2.9 | −11.0, 5.2 | 0.49 | −18.0 | −44.4, 8.4 | 0.377 | |
| Other vs Private | 11.6 | −1.0, 24.2 | 0.075 | 1.4 | −39.6, 42.5 | 1 | |
| Other vs Hybrid | 14.5 | 2.3, 26.6 | 0.012 | 19.5 | −20.3, 59.2 | 0.746 | |
B=Bacterial; F=Fungal; H= Herpetic; A=Acanthamoeba; Intl=International; US = United States; CI, Confidence Interval
Discussion
Cornea specialists had the lowest threshold to always treat bacterial keratitis stating 20%, 25% and 10% (IQR=5–50, 10–56, and 5–26) for each scenario (<2mm2 central, <2mm2 peripheral, and >8mm2 central), followed by herpetic with 27.5%, 40%, and 20% (IQR=20–75, 20–79, and 10–63), fungal with 50%, 50% and 30% (IQR=10–50, 19–70, and 10–50), and acanthamoeba with 50.5%, 60% and 50% (IQR= 32–80, 30–84, and 24–75). Not surprisingly, clinicians had a lower risk threshold for large central ulcers compared to small peripheral and small central. Uncertainty was also found to vary by presumed risk of organism type and clinical parameters. Organisms risk assessments differed as well, where it was observed that risk thresholds were significantly lower for bacterial infections, specifically for >8mm2 central “always” and “never” treat. This was also observed for the “never” treat <2mm2 central scenario, while it was only significant for amoebic and fungal in the <2mm2 central scenario and <2mm2 peripheral scenario “always” treat scenarios. Differences were observed amongst uncertainty ranges for ophthalmologists practicing in the U.S. and internationally, specifically acanthamoeba had the greatest range of uncertainty (53% vs. 46%), followed by fungal (40% vs. 17%), herpetic (13.5% vs. 17%), and bacterial (10% vs. 5%) MK types. In addition, ophthalmologists practicing internationally had a greater range of uncertainty as compared to those practicing in the U.S.
This survey focused on scenarios of various MK types. Eye clinicians’ ability to predict the organism causing MK infections has wide range of accuracy.20–22 Dalmon and colleagues reported that cornea specialists examining fungal and bacterial ulcers were able to accurately predict gram stain 46% of the time, while only identifying the genus and species 25% and 10% of the time, respectively.22 An additional study including 421 ophthalmologists by Xu and colleagues found that ophthalmologists classifying infectious keratitis had a 49.7% ± 11.5% (range: 20.00%–86.67%) of diagnostic accuracy.20 Clinicians have been able to identify acanthamoeba infections (positive predictive value of 89%, 95% CI: 52% to 100%) by observing a ring infiltrate on examination.23
In this study, fungal and acanthamoeba MK types had the greatest risk thresholds for initial treatment. This could possibly be because acanthamoeba and fungal infections are treated with very toxic medications as compared to bacterial infections. In addition to the medications being very toxic they are needed for prolonged periods of time.24 This study also observed that international clinicians have differed from U.S. clinicians potentially due to different barriers to care and supported by previous research suggesting differences in practice patterns by region.25 Peeler and colleagues found that ophthalmologists from Nepal recommended that Nepal patients with anterior segment disease come back sooner than the recommendations by ophthalmologists in the U.S.25 Internationally, areas such as Nepal and India, may have a higher percentage of fungal or atypical corneal ulcers. Because of these additional exposures, ophthalmologists practicing in these countries may have more comfort in treating and recognizing these infections, thus they may have a lower risk threshold for initial treatment of these MK types.
Current advancements in the identification of organisms causing MK include the use of machine-learning prediction.26–28 Evaluating the performance of an algorithm with real-world context is possible using DCA. The findings from this research demonstrate that between identified risk thresholds lies uncertainty where a diagnostic tool could be beneficial to inform patient treatment decisions. Other future research could explore the reasons for the clinicians assigned specific risk thresholds. They could also explore the patient and decision-making factors to understand why risk thresholds differ among clinicians.
Limitations of this study include utilizing purposive sampling which was chosen to optimize diversity in responses and increase response rate but may cause selection bias due to its non-random nature. Participants may have understood the survey questions differently, as one participant “reversed” their responses assigning a higher threshold to never treat instead of a higher threshold to always treat. Not all countries were represented equally as recruitment could not be universally implemented. Lastly, specific patient demographics for each clinician were not known, potentially affecting the risk threshold choices by the clinicians.
In summary, these findings suggest that ophthalmologists’ risk thresholds to always or never treat MK infections vary by organism types, size, and location. Between these risk thresholds to always and never treat MK infections is the range of diagnostic uncertainty. There is still a need for clinical judgment, but this diagnostic uncertainty range is where a decision curve analysis could be beneficial for showing value of prediction modeling and area of future exploration.
Supplementary Material
Supplemental Figure 1. Comparison of estimated marginal mean effect of risk threshold between US and international respondents.
Supplemental Table 1. Holm-adjusted Dunn Test Pairwise Comparisons for at which a clinician would never or always treat with corresponding medications, for all MK scenarios.
Acknowledgements:
The personnel who conducted this study was supported in part by a gift by Ms. Susan Lane.
Conflict of Interest and Source of Funding:
Funding for this research was provided by the National Eye Institute (R01EY031033, M.A.W), (P30 EY005722, S.F.), and a Research to Prevent Blindness Career Advancement Award (M.A.W). For the remaining authors no disclosures were declared. The funding support played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Corneal Ulcer Study Group:
Guillermo Amescua, Masih Ahmed, Zaina Al-Mohtaseb, Diana Alvarez-Melloni, Sejal Amin, Menen Ayalew, Ashwin Balasubramanian, Winston Chamberlain, Matilda Chan, Elsie Chan, Meenu Chaudhary, Thomas Chia, James Chodosh, Josephine Christy, John Clements, John Dart, Mohammad Dastjerdi, Matthew Denny, Mohamed Elghobaier, Chris Estopinal, Preethika Gandhi, Miles F. Greenwald, Nikhil Gokhale, Natalie Hernandez, Anna Hovakimyan, Frank Hwang, David Hwang, Tomas Jaeschke, Vishal Jhanji, Faris Karas, Carol Karp, Lakshmi Kattana, Jeremy Keenan, Sumitra Khandelwal, Tyson Kim, Ellen Koo, Aaleya Koreishi, Jennifer Li, Tom Lietman, Marian Macsai, Jaime Martinez, Jod Mehta, Michael Mimouni, Adam Moss, Afshan Nanji, Nathan Nataneli, Jennifer Nussbaumer, Vasudha Panday, Stephen Pflugfelder, Sayali Pradhan, N. Venkatesh Prajna, Naveen Rao, Travis Redd, Satya Reddy, Alfonso Sabater, Julie Schallhorn, Gerami Seitzman, Ruti Sella, Neha Shaik, Sankalp Singh Sharma, Nakul Shekhawat, David Spokes, Alan Sugar, Audrey Talley Rostov, Napaporn Tananuvat, Chulaluck Tangmonkongvoragul, Rahul Tonk, Sonal Tuli, Phit Upaphong, Suvitha Vairavan, Bart Van Dooren, Manoj Vasudevan, Angela Verkade, Meraf Wolle, Maria Woodward, Rachel Wozniak, Choong YeanYaw.
Data Availability:
Dr Woodward had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The dataset generated and analyzed during the current study are not available.
References
- 1.Santhosh L, Chou CL, Connor DM. Diagnostic uncertainty: from education to communication. Diagnosis (Berl) 2019;6(2):121–126. [DOI] [PubMed] [Google Scholar]
- 2.Bhise V, Rajan SS, Sittig DF, Morgan RO, Chaudhary P, Singh H. Defining and Measuring Diagnostic Uncertainty in Medicine: A Systematic Review. J Gen Intern Med 2018;33(1):103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahraman-Koytak P, Bruce BB, Peragallo JH, Newman NJ, Biousse V. Diagnostic Errors in Initial Misdiagnosis of Optic Nerve Sheath Meningiomas. JAMA Neurol 2019;76(3):326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen V, Lee GA. Management of microbial keratitis in general practice. Aust J Gen Pract 2019;48(8):516–519. [DOI] [PubMed] [Google Scholar]
- 5.Ung L, Bispo PJM, Shanbhag SS, Gilmore MS, Chodosh J. The persistent dilemma of microbial keratitis: Global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol 2019;64(3):255–271. doi: 10.1016/j.survophthal.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somerville TF, Herbert R, Neal T, Horsburgh M, Kaye SB. An Evaluation of a Simplified Impression Membrane Sampling Method for the Diagnosis of Microbial Keratitis. J Clin Med 2021;10(23):5671. Published 2021 Nov 30. doi: 10.3390/jcm10235671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austin A, Lietman T, Rose-Nussbaumer J. Update on the Management of Infectious Keratitis. Ophthalmology 2017;124(11):1678–1689. doi: 10.1016/j.ophtha.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo MT, Hsu BWY, Yin YK, et al. A deep learning approach in diagnosing fungal keratitis based on corneal photographs. Sci Rep 2020;10(1):14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh AK, Thammasudjarit R, Jongkhajornpong P, Attia J, Thakkinstian A. Deep Learning for Discrimination Between Fungal Keratitis and Bacterial Keratitis: DeepKeratitis. Cornea 2022;41(5):616–622. doi: 10.1097/ico.0000000000002830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vickers AJ, van Calster B, Steyerberg EW. A simple, step-by-step guide to interpreting decision curve analysis. Diagn Progn Res 2019;3:18. Published 2019 Oct 4. doi: 10.1186/s41512-019-0064-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006. Nov;26(6):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgerald M, Saville BR, Lewis RJ. Decision curve analysis. JAMA: the journal of the American Medical Association 2015. p. 409–410. [DOI] [PubMed] [Google Scholar]
- 13.Van Calster B, Wynants L, Verbeek JFM, Verbakel JY, Christodoulou E, Vickers AJ, Roobol MJ, Steyerberg EW. Reporting and Interpreting Decision Curve Analysis: A Guide for Investigators. Eur Urol 2018. Dec;74(6):796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, Okoro C, Raskolnikov D, Parnes HL, Linehan WM, Merino MJ, Simon RM, Choyke PL, Wood BJ, Pinto PA. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015. Jan 27;313(4):390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vickers AJ, Van Calster B, Steyerberg EW. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ 2016. Jan 25;352:i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shariat SF, Savage C, Chromecki TF, et al. Assessing the clinical benefit of nuclear matrix protein 22 in the surveillance of patients with nonmuscle-invasive bladder cancer and negative cytology: a decision-curve analysis. Cancer 2011;117(13):2892–2897. doi: 10.1002/cncr.25903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rejali M, Mansourian M, Babaei Z, Eshrati B. Prediction of Low Birth Weight Delivery by Maternal Status and Its Validation: Decision Curve Analysis. Int J Prev Med 2017;8:53. Published 2017 Jul 25. doi: 10.4103/ijpvm.IJPVM_146_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin S, Ma Y, He X, Zhu J, Zou H. Using Decision Curve Analysis to Evaluate Common Strategies for Myopia Screening in School-Aged Children. Ophthalmic Epidemiol 2019;26(4):286–294. doi: 10.1080/09286586.2019.1616774 [DOI] [PubMed] [Google Scholar]
- 19.Sande SZ, Li J, D’Agostino R, Yin Wong T, Cheng CY. Statistical inference for decision curve analysis, with applications to cataract diagnosis. Stat Med 2020;39(22):2980–3002. doi: 10.1002/sim.8588 [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Kong M, Xie W, Duan R, Fang Z, Lin Y, Zhu Q, Tang S, Wu F, Yao Y-F. Deep Sequential Feature Learning in Clinical Image Classification of Infectious Keratitis. Proc Est Acad Sci Eng 2021. Jul 1;7(7):1002–1010. [Google Scholar]
- 21.Redd TK, Prajna NV, Srinivasan M, Lalitha P, Krishnan T, Rajaraman R, Venugopal A, Lujan B, Acharya N, Seitzman GD, Rose-Nussbaumer J, Lietman TM, Campbell JP, Keenan JD, Corneal Ulcer Image Interpretation Study Group. Expert Performance in Visual Differentiation of Bacterial and Fungal Keratitis. Ophthalmology [Internet] 2021. Oct 6; Available from: 10.1016/j.ophtha.2021.09.019 [DOI] [Google Scholar]
- 22.Dalmon C, Porco TC, Lietman TM, et al. The clinical differentiation of bacterial and fungal keratitis: a photographic survey. Invest Ophthalmol Vis Sci 2012;53(4):1787–1791. Published 2012 Apr 2. doi: 10.1167/iovs.11-8478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahlgren MA, Lingappan A, Wilhelmus KR. The clinical diagnosis of microbial keratitis. Am J Ophthalmol 2007;143(6):940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrd LB, Martin N. Corneal Ulcer. [Updated 2021 Aug 11]. In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2022. Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539689/ [Google Scholar]
- 25.Peeler CE, Dhakhwa K, Mian SI, et al. Telemedicine for corneal disease in rural Nepal. J Telemed Telecare 2014;20(5):263–266. doi: 10.1177/1357633X14537769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ting DSJ, Foo VH, Yang LWY, Sia JT, Ang M, Lin H, et al. Artificial intelligence for anterior segment diseases: Emerging applications in ophthalmology. Br J Ophthalmol 2021. Feb;105(2):158–68. [DOI] [PubMed] [Google Scholar]
- 27.Redd TK, Prajna NV, Srinivasan M, Lalitha P, Krishnan T, Rajaraman R, et al. Image-Based Differentiation of Bacterial and Fungal Keratitis Using Deep Convolutional Neural Networks. Ophthalmology Science 2022. Jun 1;2(2):100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh AK, Thammasudjarit R, Jongkhajornpong P, Attia J, Thakkinstian A. Deep Learning for Discrimination Between Fungal Keratitis and Bacterial Keratitis: DeepKeratitis. Cornea [Internet] 2021. Sep 29; Available from: 10.1097/ICO.0000000000002830 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Comparison of estimated marginal mean effect of risk threshold between US and international respondents.
Supplemental Table 1. Holm-adjusted Dunn Test Pairwise Comparisons for at which a clinician would never or always treat with corresponding medications, for all MK scenarios.
Data Availability Statement
Dr Woodward had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The dataset generated and analyzed during the current study are not available.


