Abstract
Nutritional interventions often rely on subjective assessments of energy intake (EI), but these are susceptible to measurement error. To introduce an accelerometer-based intake-balance method for assessing EI using data from a time-restricted eating (TRE) trial. 19 participants with overweight/obesity (25–63 years old; 16 females) completed a 12-week intervention (NCT03129581) in a control group (unrestricted feeding; n=8) or TRE group (n=11). At the start and end of the intervention, body composition was assessed by dual-energy X-ray absorptiometry (DXA), and daily energy expenditure (EE) was assessed for two weeks via wrist-worn accelerometer. EI was back-calculated as the sum of net energy storage (from DXA) and EE (from accelerometer). Accelerometer-derived EI estimates were compared against estimates from the body weight planner of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Mean EI for the control group declined by 33 and 104 kcal/day for the accelerometer and NIDDK methods, respectively (both p ≥ 0.38), versus 300 and 351 kcal/day, respectively, for the TRE group (both p < 0.01). At follow-up, the accelerometer and NIDDK methods showed excellent group-level agreement (mean bias of −71 kcal/day across arms; standard error of estimate 252 kcal/day) but high variability at the individual level (limits of agreement from −577 to +436 kcal/day). The accelerometer-based intake-balance method showed plausible sensitivity to change, and EI estimates were biologically and behaviorally plausible. The method may be a viable alternative to self-report EI measures. Future studies should assess criterion validity using doubly labeled water.
Keywords: Energy balance, weight loss, accelerometry, interventions, overweight/obesity
Introduction
Caloric restriction is essential for weight loss in humans, but many barriers prevent individuals from adhering to a low-energy diet (e.g., cost, frustration, and lack of support(1)). Interventions focused on intentional caloric restriction only produce desired weight loss in 30%–50% of participants(2, 3). Thus, there is growing interest in alternative behavioral approaches that can potentially yield better results. Time-restricted eating (TRE) is a promising example that focuses on restriction of meal timing rather than calories. Prior studies have shown that TRE (ad libitum intake during an 8–10 hour window each day, followed by 14–16 hours of fasting) aids weight loss by reducing eating occasions by 22%(4) and daily energy intake (EI) by ~8%–20%(5, 6).
As with other areas of nutrition research, assessment of EI is a key component of TRE research. Prior studies have used a range of techniques, from seven-day food diaries(5) to retrospective estimations based on photo and text diaries(6). These methods can be highly subjective, which is a common limitation when measuring EI(7–9), sometimes entailing >30% error(10–12). Therefore, there is a need to investigate more accurate methods for assessing EI in TRE research.
One such promising method is the ‘intake-balance’ or ‘expenditure/balance’ method(13–16). This method infers EI from highly accurate measurements of net energy storage (ES) and energy expenditure (EE). Specifically, since the net ES (i.e., change in body composition over time) is defined as EI minus EE, it is possible to rearrange the equation and infer EI by summing the measured values of EE and net ES(16). Typically, EE is assessed via doubly labeled water, and net ES is assessed via dual X-ray absorptiometry (DXA). However, the use of doubly labeled water limits this approach, due its cost-prohibitive, labor-intensive, and highly technical nature. Thus, the standard intake-balance method has limited scalability for widespread use.
To improve the scalability of the intake-balance method, doubly labeled water could potentially be replaced with a surrogate EE measure, particularly an accelerometry-based method(17, 18). Although some measurement error would result from this change, the degree of error would potentially be lower than the errors observed with self-reported EI(19–21). Thus, it is important to investigate the utility of accelerometer-based intake balance methods, which has not been done in the setting of a TRE intervention, nor with open-source and research-grade accelerometry solutions that may also benefit other areas of nutrition research. Therefore, the purpose of this paper is to provide proof-of-concept for an accelerometer-based intake-balance method.
Experimental Methods
Participants and Ethical Approval
This is a secondary analysis of data from a prior study, for which full methods have been presented elsewhere(4). Participants were adults (age 18–65 years) who were overweight or obese at baseline. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Institutional Review Board of the University of Minnesota on March 21, 2017 (Project identification code number: 1701M06001). Use of the myCircadianClock app (Salk Institute, La Jolla, CA, USA) was approved by the Institutional Review Board at the Salk Institute for Biological Studies (Project identification code number: 15–0003). Written informed consent was obtained from all subjects/patients. The study is registered on ClinicalTrials.gov (#NCT03129581).
Study Design/Intervention
The intervention duration was 12 weeks with two-week assessments beforehand (Pre) and during the final two intervention weeks (Post). All potential participants first underwent a screening procedure in which they were asked to document their food intake (i.e., meal timing and food type) for ≥ 1 week using a smartphone application (myCircadianClock). Those who had a daily eating window ≥ 14 hours were enrolled and randomized into one of two intervention arms, namely unrestricted eating (control) or TRE. The participants in the control group were instructed to continue their usual eating habits while tracking all meal timing and food types via the myCircadianClock application. The participants in the TRE group self-selected a daily 8-hour eating window, which they were asked to keep consistent throughout the 12-week intervention. During the window, ad libitum food intake was permitted. Outside the window, participants were instructed to limit their oral intake to medications and water.
Procedures/Measures
For the Pre and Post assessments, each participant had their anthropometric variables and study endpoints measured, along with wearing an accelerometer (ActiGraph GT9X Link, ActiGraph LLC, Pensacola, FL, USA) for two weeks.
Anthropometric Variables and Study Endpoints.
Body composition was assessed using a GE Lunar iDXA system (GE Healthcare, Chicago, IL, USA) and analyzed by the enCore™ software (Version 16.2). The resulting variables were gross ES, fat mass (FM), fat-free mass (FFM), and total mass (i.e., the sum of FM and FFM). Automated quality assurance checks were performed at the start of each day the system was operated. Full body scans were performed for all participants, and symmetrical estimations were applied if a portion of the participant’s body fell outside the 198×66 cm scanning area. The radiation dose was 3–6 μGy per scan. Participants fasted for at least 8 hours before each DXA scan.
Accelerometer.
Wrist accelerometry was used to quantify EE at the Pre and Post assessments. Each participant wore the GT9X on the non-dominant wrist. The devices were initialized to sample at 30 Hz with the Bluetooth and inertial measurement unit features disabled, and with idle sleep mode enabled. This configuration allowed a single battery charge to last the full 14 days. For the Pre assessment, GT9X data were collected for two weeks ending just before randomization (i.e., the start of Week 1). For the Post assessment, GT9X data were collected from the start of Week 11 to the end of Week 12 (end of study). On both occasions, participants were asked to wear the monitors continuously to the greatest extent possible.
Data Processing
Accelerometer data were read into R using the AGread package(22). Two broad tasks were performed that each used a different data format: First, EE was calculated from raw acceleration data (in gravitational units, 30 Hz resolution); and second, non-wear and sleep periods were determined from filtered and aggregated data (activity counts, minute-by-minute resolution). Activity counts are a proprietary unit of cumulative acceleration calculated at regular intervals(23), in this case every minute (i.e., counts·min−1).
Calculating EE.
For each sample, the Euclidian norm minus one (ENMO) was calculated from the individual axes , with negative values rounded to 0. The output was then averaged each second, converted to milli-gravitational units (i.e., multiplied by 1000), and used to calculate oxygen consumption (VO2). The Hildebrand non-linear method was used (Eq. 1), as described by Ellingson et al.(24). The method includes a floor value of 3.0 ml/kg/min to account for the lack of intercept in the model. It was selected instead of its linear counterpart(25) because it outperformed the latter method in the validation study by Ellingson et al.(24), yielding mean estimates within 0.05–0.23 metabolic equivalents (0.2–0.8 ml/kg/min) of indirect calorimetry for sedentary and light intensity behaviors, and within 0.8–2.4 metabolic equivalents (2.8–8.4 ml/kg/min) for moderate and vigorous intensity behaviors. For the present analysis, VO2 values were converted to kcal/kg/min assuming a respiratory quotient of 0.85 (4.862 kcal/L O2)(26). Finally, the data were reduced to minute-by-minute resolution by averaging the values each minute.
| (Eq. 1) |
Non-Wear and Sleep Classification.
The minute-by-minute activity count data were first analyzed using the non-wear detection algorithm of Choi et al.(27, 28) to verify compliance with the wear protocol, as discussed later. After applying the non-wear algorithm, the wear time periods were analyzed to identify sleep using the algorithm of Tracy et al.(29, 30). The prior steps resulted in each minute being labeled as either awake, asleep, or non-wear. These labels (derived from activity counts) were then merged with the EE estimates (derived from raw acceleration data) to obtain a complete set of minute-by-minute accelerometer data. For non-wear and sleep periods, a basal EE value was imputed based on the Schofield equations(31). The original prediction units (MJ/day) were converted to kcal assuming a thermochemical kilocalorie (239.006 kcal/MJ).
Cleaning and Aggregation of EE Data.
Cleaning procedures involved discarding data from days with < 22 h of wear time, then excluding participants if they had < 4 d remaining at either time point. These steps ensured the aggregation procedures would draw from sufficiently compliant data. For each participant, aggregation involved calculating mean daily EE (kcal/day) from each valid day during the two weeks before randomization (EEpre) and during Weeks 11–12 (EEpost).
Calculating ES, Energy Balance, and EI.
Based on the DXA measurements of FM and FFM (both in kg), gross ES was calculated using Eq. 2 for baseline (ESpre) and Weeks 11–12 (ESpost)(17, 18). Daily net ES was calculated using Eq. 3 before determining EI. For the baseline assessment, individuals were assumed weight stable, and thus accelerometer data were used to determine EI (i.e., EIpre = EEpre). For the follow up assessment, EI was calculated as the sum of EE and net ES (i.e., EIpost = EEpost + net ES).
| (Eq. 2) |
| (Eq. 3) |
Comparison Measure of EI.
Alternative EI predictions were obtained using the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Body Weight Planner(32). This was done through the online interface (https://www.niddk.nih.gov/bwp) in expert mode with advanced controls activated. Specifically, the following variables were inputted for each participant: sex, age, height, baseline body mass, baseline resting EE (from Schofield’s equations; see(31)), baseline physical activity level (total EE divided by resting EE), baseline body fat percentage (assessed by DXA), “goal weight” (i.e., body mass at the end of the intervention), number of days between assessments, and percentage change in physical activity level from baseline to the end of the intervention (based on accelerometer data). The system produced a baseline caloric intake (i.e., EIpre) commensurate with maintaining the original weight, as well as a daily caloric intake (i.e., EIpost) commensurate with losing the observed amount of weight in the observed amount of time. The purpose of including the NIDDK estimates was to allow comparison of the accelerometer-based method against an established method that uses similar information. The key difference between the two methods is that the NIDDK method is primarily for individualized and prospective use, while the accelerometer-based intake-balance method will allow scalable batch processing in retrospective analyses.
Analysis
Statistical Tests.
Paired T-tests were used to compare Pre and Post energy balance values (ES, EE, and EI) within each group. To assess agreement between the accelerometer-based intake-balance method and the NIDDK method, we used tests of statistical equivalence (± 100 kcal/day tolerance) for each group and timepoint(33). Additional analyses were conducted to test agreement for the Post assessment, where individuals were not assumed to be weight stable. These included regression-based and Bland-Altman analyses to examine individual-level error and systematic bias(34, 35). For the regression model, the key performance metrics were intercept and slope with 95% confidence intervals (CIs), as well as standard error of the estimate (SEE). Perfect agreement would be represented by an intercept of 0 and a slope of 1 (i.e., following the line of identity). Regression coefficients were tested statistically using the equivalence methods suggested by Dixon et al.(33), namely by centering both variables on the mean of the accelerometer-based intake-balance method, and by using specific equivalence zones for the intercept (±10% of the intake-balance mean) and slope (0.9 to 1.1). To account for the number of statistical tests, all p-values were adjusted using the false discovery rate correction(36).
Data Loss and Statistical Power.
20 of 22 participants were retained through the full intervention(4). One participant did not meet the valid data requirements for this analysis (i.e., lacked ≥ 4 days with ≥ 22 h of wear time at both the Pre and Post assessments), and thus the analytic sample included 19 participants (n = 8 control; n = 11 TRE). The sample size in each group allowed detection of an effect size (d) of 1.4, with α = 0.05 and β = 0.80(37).
Results
Participant characteristics are shown in Table 1. Hereafter, summary statistics are given as mean ± SD. The time between the Pre and Post visits was 94 ± 7 days (control group) and 96 ± 6 days (TRE group).
Table 1.
Participant characteristics and sample descriptives. Values are mean (SD) except where otherwise noted. Accelerometer-derived variables are grand averages across participants.
| Control (n = 8)a | TRE (n = 11)b | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Body Mass (kg) | 103.6 (26.8) | 102.7 (25.8) | 94.0 (21.6) | 90.9 (21.3) |
| Fat Mass (kg) | 48.8 (19.7) | 48.1 (19.4) | 41.1 (16.8) | 39.4 (16.4) |
| Fat-free Mass (kg) | 54.9 (9.3) | 54.6 (8.4) | 52.9 (10.3) | 51.5 (10.3) |
| BMI (kg/m2) | 35.1 (7.7) | 35.0 (7.6) | 33.2 (7.1) | 32.3 (7.2) |
| Weight Status (n) | ||||
| Healthy Weight (BMI 18.5–24.9) | 0 | 0 | 0 | 1 |
| Overweight (BMI 25–29.9) | 2 | 3 | 5 | 5 |
| Class 1 Obese (BMI 30–34.9) | 3 | 2 | 2 | 2 |
| Class 2 Obese (BMI 35–39.9) | 1 | 1 | 2 | 1 |
| Class 3 Obese (BMI ≥ 40) | 2 | 2 | 2 | 2 |
| Valid Accelerometer Days (n) | 9.0 (2.5) | 9.8 (3.1) | 9.0 (2.7) | 10.3 (3.7) |
| Non-wear Time (min/day) | 6.6 (9.4) | 3.7 (7.3) | 5.0 (10.5) | 12.4 (19.7) |
| Sleep Time (min/day) | 495.6 (61.9) | 477.4 (29.1) | 500.0 (89.1) | 518.2 (77.1) |
| Resting Energy Expenditure (kcal/day) c | 1,801.3 (321.6) | 1,794.7 (318.7) | 1,686.1 (275.0) | 1,656.5 (265.1) |
TRE, time restricted eating; BMI, body mass index; kcal, kilocalories.
44.0 ± 13.0 years old (87.5% females)
46.8 ± 12.4 years old (81.8% females)
Estimated from Schofield method (age stratified equations with height and body mass as predictors)
Changes in Energy Balance
Table 2 shows summary statistics for energy balance variables, and individual values are plotted in Figure 1. Mean ES decreased from Pre to Post in both groups, by a small amount in the control group (6.8 megacalories; p = 0.39) and a more substantial amount in the TRE group (16.8 megacalories; p = 0.01). Mean relative EE changed by only ± 0.2 kcal/kg/day in either group (p = 0.85–0.93), but individual trends were variable (Figure 1B). Thus, the small mean changes were attributable to cancellation, with some participants increasing their relative EE and others decreasing it. For the accelerometer-based intake balance method, mean EI decreased slightly in the control group (33 kcal/day; p = 0.85), while it decreased more considerably for the TRE group (300 kcal/day; p = 0.01). Similarly, the NIDDK method showed a decrease of 104 kcal/day for the control group (p = 0.38), versus 351 kcal/day for the TRE group (p < 0.001).
Table 2.
Energy balance values, presented as mean (SD).
| Control (n = 8) | TRE (n = 11) | |||||
|---|---|---|---|---|---|---|
| Prea | Post | Δ | Prea | Post | Δ | |
| ES (megacalories) | 519.1 (193.2) | 512.3 (190.5) | −6.8 (14.2) | 444.0 (162.2) | 427.1 (158.4) | −16.8 (13.2) |
| Relative EE (kcal/kg/day) | 32.8 (5.2) | 32.9 (3.2) | 0.2 (4.8) | 31.0 (3.7) | 30.8 (3.8) | −0.2 (2.3) |
| Absolute EE (kcal/day) | 3,321 (606) | 3,360 (776) | 39 (390) | 2,871 (521) | 2,747 (445) | −125 (169) |
| EI (kcal/day) – accelerometer | 3,321 (606) | 3,288 (790) | −33 (365) | 2,871 (521) | 2,571 (397) | −300 (219) |
| EI (kcal/day) – NIDDK | 3,343 (601) | 3,239 (654) | −104 (203) | 2,836 (504) | 2,485 (433) | −351 (114) |
TRE, time restricted eating; ES, energy storage; EE, energy expenditure; EI, energy intake; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases (body weight planner).
Individuals assumed weight stable (i.e., accelerometer EI = EE).
Figure 1.
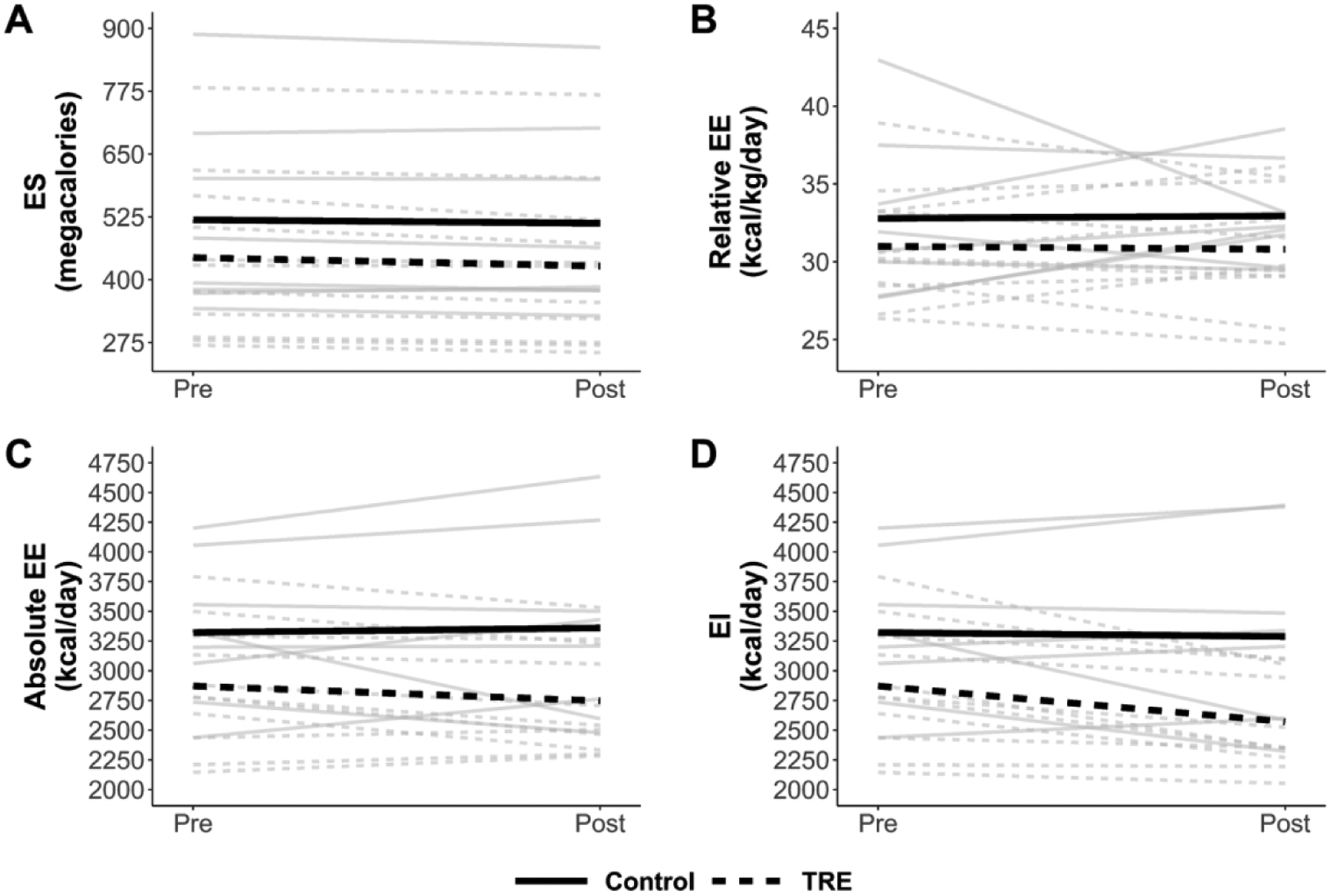
Spaghetti plot of changes in energy storage (ES; panel A), relative energy expenditure (EE; panel B), absolute EE (panel C), and energy intake (EI; panel D). Gray lines are individual participants, and heavy black lines are group means. Solid lines represent the control group while dashed lines represent the time restricted eating (TRE) group.
Agreement of Accelerometer and NIDDK Methods
The accelerometer and NIDDK methods showed strong agreement for EIpre in both the control group (mean separation of 22 ± 48 kcal/day; equivalence p = 0.01) and the TRE group (mean separation of 36 ± 54 kcal/day; equivalence p = 0.01). At the Post assessment, the accelerometer and NIDDK methods remained similar, but there was greater variability (separations of 49 ± 333 kcal/day in the control group, and 86 ± 205 kcal/day in the TRE group; equivalence p = 0.56 and 0.57, respectively). The same was true for Pre-to-Post changes in EI (separations of 71 ± 294 kcal/day in the control group, and 51 ± 177 kcal/day in the TRE group; equivalence p = 0.57 and 0.38, respectively).
Figure 2 shows individual-level data for EI predictions at the Post assessment. There, the accelerometer and NIDDK methods were related with a regression intercept of −71 kcal/day (95% CI = [−193, 51]; equivalence p = 0.01) and slope of close to one (B = 0.88; 95% CI = [0.69, 1.06]; equivalence p = 0.76). The model had SEE of 252 kcal/day. Bland-Altman analysis showed a small mean bias (−71 kcal/day, consistent with the regression model intercept) but wide limits of agreement spanning a range of 1013 kcal/day (i.e., [−577, 436]). There was negligible evidence of systematic error, with the trendline having slope of −0.05 and explaining < 2% of variance.
Figure 2.
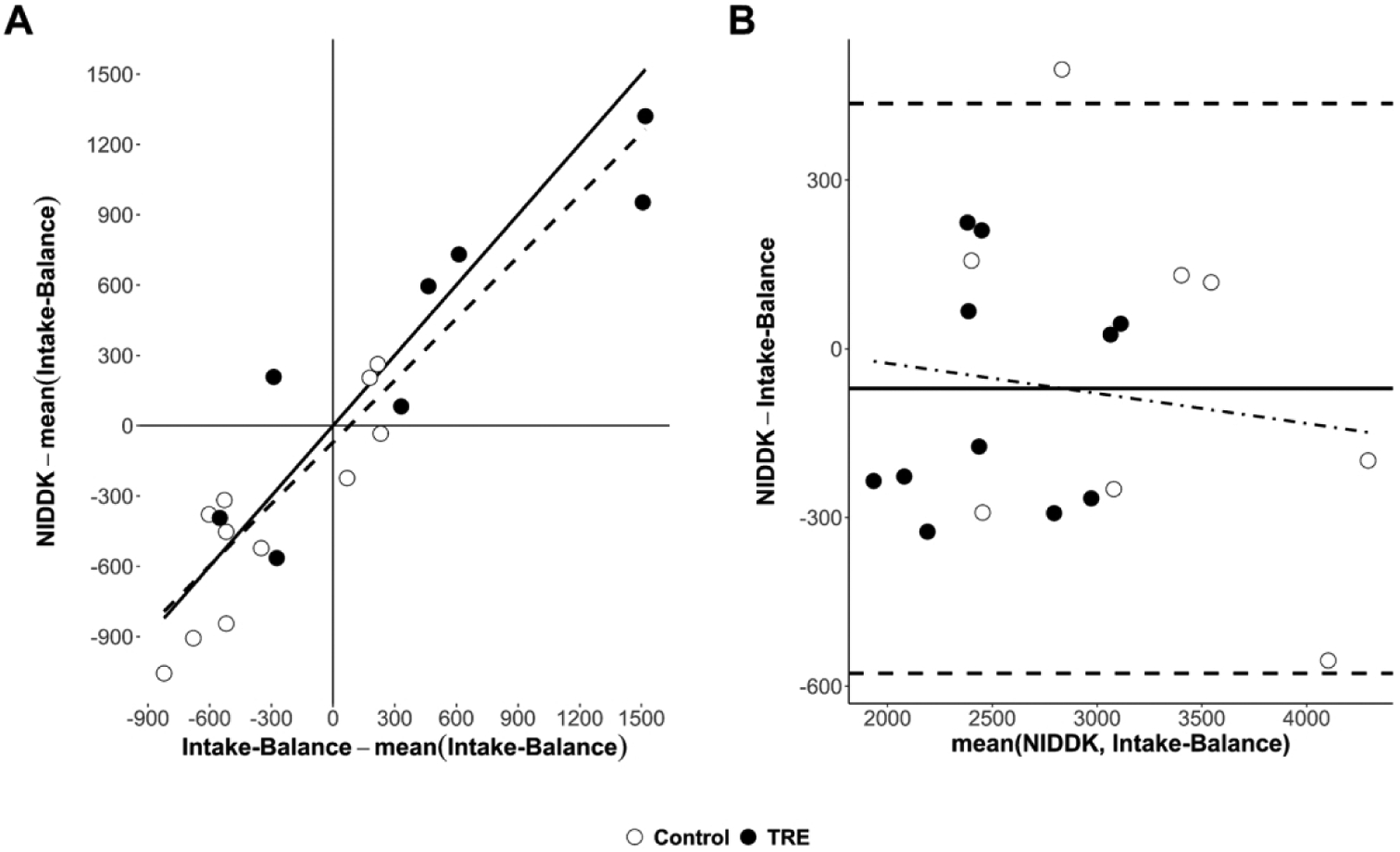
Comparison of estimated energy intake (kcal/day) between the accelerometer-based intake-balance method and the NIDDK bodyweight planner. Values are from the Post assessment where, unlike the Pre assessment, individuals were not assumed to be weight stable. A) Scatterplot showing line of identity (solid) and line of best fit (dashed, from least-squares regression), where both variables are centered on the mean of the accelerometer-based intake-balance method to ensure a non-extrapolated intercept with a null-hypothesized value of 0; B) Bland-Altman plot showing limits of agreement (horizontal dashed lines), mean bias (solid horizontal line), and systematic bias (dot-dashed trendline from least squares regression) - Note: NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; TRE, time restricted eating.
Discussion
In this study, we provided proof-of-concept for an accelerometer-based intake-balance method. This was done in the setting of a TRE intervention, but the method may have utility in other settings as well. Although we did not have criterion values against which we could compare our estimates, the findings nevertheless suggest the accelerometer-based technique can detect enough meaningful EI signal to warrant further study and application. In particular, we observed comparable EI reductions for the TRE group when using the accelerometer-based method (9.9% ± 6.4%) and the NIDDK method (12.3% ± 2.9%). Furthermore, the accelerometer-based estimates were comparable with prior studies showing TRE produces EI reductions of 8%–20%(5, 6).
The accelerometer-based intake-balance method is a promising alternative to self-reported EI, which many have recommended abandoning for estimation of true EI(9, 38, 39). A further advantage is that it can be refined over time as innovation continues in the fields of body composition assessment and accelerometry(40). Many current innovations in accelerometry use open-source tools to streamline usage and increase accessibility for end-users(41). In keeping with the latter trend, we have provided sample code and commentary to facilitate using our method (see paulhibbing.com/TREaccel).
To our knowledge, this is the first study to present an open-source, accelerometer-based intake-balance method in the setting of a TRE intervention. Shook et al.(17) were among the first to use a general device-based approach, including a comparison of their predictions against values derived from doubly labeled water. They showed outstanding utility of the SenseWear Armband, but the latter device was closed-source and has now been discontinued for several years(42). Today, ActiGraph devices are among the most commonly used in research(43), with an abundance of ongoing work being devoted to improving their utility for EE assessment(44). Thus, our use of an ActiGraph device represents a logical starting place for developing an open-source accelerometer-based method. Consumer devices have may also have utility in this space(45–47), although concerns still exist, many relating to the proprietary nature of the underlying algorithms(48). Overall, our method provides a starting point from which future studies can begin refining the use of accelerometers for determining EI.
Strengths and Weaknesses Compared to the NIDDK Body Weight Planner
In addition to providing proof-of-concept for the accelerometer-based method, our analysis compared the accelerometer-based intake-balance method to the existing NIDDK Body Weight Planner method. While the planner is primarily intended for prospective use, data can also be entered retrospectively to infer caloric intake over a particular period (e.g., the duration of an intervention). As discussed below, the NIDDK method may be advantageous to use in some settings while the accelerometer-based method is advantageous to use in others.
Accessibility is a major strength of the NIDDK method. This is true in both a literal sense (the method is freely available without needing to purchase an accelerometer or related software) and an abstract sense (the online interface is easy to navigate). Furthermore, the NIDDK method is based on a model that accounts for adaptations to weight loss over time, making it a highly useful tool for both weight loss and weight maintenance. These advantages make the NIDDK method especially useful in clinical and consumer settings. A limitation of the method is that the web interface currently requires manual data entry. This creates a logistical barrier for research at scale, and also increases the risk of data entry error. Furthermore, the method requires that users provide information about their physical activity level, which must either be measured independently or self-reported through a two-item submodule. These characteristics may make the NIDDK method less advantageous for use in research than for clinical and commercial use.
The accelerometer-based method’s strengths and weaknesses broadly complement the NIDDK method. As noted previously, a major strength of the accelerometer-based method is its open-source setup and potential for ongoing refinement. Furthermore, the ability to automate the accelerometer-based method for batch processing enhances its scalability and consequent utility for research. That is, the accelerometer-based method can reduce burden on participants and researchers alike by eliminating the need to complete and score self-report instruments or similar tools such as the NIDDK method. Automation would also enhance quality control by reducing the risk of data entry errors. While these are certainly strengths of the accelerometer-based method in research settings, they may not be as applicable in commercial and clinical settings. This is due to both the cost barrier of obtaining an ActiGraph device, and the procedural barrier of processing the data in R (even with the sample code mentioned earlier). Furthermore, the accelerometer-based method is designed primarily for retrospective use and does not account for adaptations to weight loss like the NIDDK method. Thus, the accelerometer-based method should be considered primarily a tool for research, with a need for ongoing investigation in terms of its long-term utility for studies on weight maintenance and adaptations to weight loss.
Assumptions and Implications
While the intake-balance method finds its theoretical basis in the First Law of Thermodynamics(49), some additional assumptions were necessary to implement the method in the form described above. The key assumptions were that 1) participants were weight stable at baseline, and 2) there was linear change in ES from Pre to Post (see Eq. 3). These assumptions made it possible to infer daily net ES for each two-week measurement period, despite having only one DXA scan at each time point. For the Post assessment, a third, minor assumption accompanied the previous two, namely that the daily net ES values (derived from change throughout the intervention) and the mean daily EE values (derived from valid days in the final two weeks) were comparable enough to support calculating EI.
The prior assumptions have implications for interpreting the present results and designing future studies. For the present results, the assumed linear change in ES implies that a constant energy balance was maintained throughout the intervention (i.e., that EI and EE maintained a consistent subtractive relationship). While this does not require that EI and EE were constant from day to day, it does require that they were offset by a consistent amount to keep net ES stable. In practice, the latter assumption was able to withstand minor day-to-day deviations, provided they canceled out over the course of the intervention. Nevertheless, it is important to consider this characteristic of the method when interpreting the results.
In terms of study design, it should be noted that future study protocols could incorporate mid-trial assessments of ES and EE to facilitate different (e.g., non-linear) approaches to predicting EI. This would be an especially promising use for accelerometry, since a similar approach with doubly labeled water would face many feasibility barriers. Future studies could also perform two DXA scans at each time point, which would ensure exact concurrence of EE and net ES measurements. This would sidestep the assumption of linear change in ES, but it could also be too short of a measurement window for DXA to detect meaningful changes(50, 51).
Further implications for interpretation and design may arise when considering the duration of the intervention. A longer intervention would result in greater separation between the Pre and Post assessments, potentially amplifying the impact of an assumed linear change in ES. A longer intervention could also elicit metabolic adaptations that are modeled in the NIDDK method, but not the current version of the accelerometer-based method. Refined versions of the accelerometer-based method could be developed to address this, but more research and development are needed to attain this. In the meantime, results must be interpreted with careful attention to the unique design features of each study.
Strengths and Limitations of This Study
The present study had strengths and limitations. Its main strength was the presentation of an innovative accelerometer-based intake-balance method applicable to a widely used, wrist-worn activity monitor (GT9X). Participants were also exceptionally compliant with wearing the device, which was another strength. The main limitations were the small sample size and lack of data from criterion measures or self-report methods. Additionally, estimates of agreement may have been inflated when comparing the accelerometer-based and NIDDK methods, as there was partial overlap of the information used in each approach. This issue is discussed in more detail in the supplementary material. Overall, there is a clear need for more research to test the criterion validity of this accelerometer-based intake-balance approach. However, our study provides proof-of-concept and preliminary evidence to suggest the method is a feasible and scalable option with great potential to enhance ongoing work. Future studies should directly compare the method against values obtained from self-reported EI as well as objective measures such as doubly labeled water.
Conclusions
The accelerometer-based intake-balance method showed promising utility when applied to data from a TRE intervention. This strong proof-of-concept calls for ongoing refinement and validation of the method. Such efforts have the potential to increase the quality and consistency of EI measurements, while also reducing their burden on participants and researchers.
Supplementary Material
Financial Support
This work was support by the Healthy Foods Healthy Lives program (L.C., 17SFR-2YR50LC), Robert Wood Johnson Foundation (S.P., Pioneer award 76014), Wu Tsai Human Performance Alliance and the Joe and Clara Tsai Foundation (gift to SP), and the National Institutes of Health (NIH National Center for Advancing Translational Sciences, UL1TR002494). The funders had no role in the design, analysis, or writing of this article.
Abbreviations:
- EI
energy intake
- TRE
time restricted eating
- DXA
dual-energy X-ray absorptiometry
- EE
energy expenditure
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- ES
energy storage
- ENMO
Euclidian norm minus one
- VO2
oxygen consumption
- FM
fat mass
- FFM
fat-free mass
Footnotes
Conflict of Interest
S.P. has authored a book “The Circadian Code”. All others report no conflicts of interest.
References
- 1.Vijan S, Stuart NS, Fitzgerald JT, et al. (2015) Barriers to following dietary recommendations in Type 2 diabetes. Diabet Med 22(1), 32–38. 10.1111/j.1464-5491.2004.01342.x [DOI] [PubMed] [Google Scholar]
- 2.Franz MJ, VanWormer JJ, Crain AL, et al. (2007) Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc 107(10), 1755–1767. 10.1016/j.jada.2007.07.017 [DOI] [PubMed] [Google Scholar]
- 3.LeBlanc ES, Patnode CD, Webber EM, et al. (2018) Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 320(11),1172–1191. 10.1001/jama.2018.7777 [DOI] [PubMed] [Google Scholar]
- 4.Chow LS, Manoogian ENC, Alvear A, et al. (2020) Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: A feasibility study. Obesity 28(5), 860–869. 10.1002/oby.22756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabel K, Hoddy KK, Haggerty N, et al. (2018) Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr Healthy Aging 4(4), 345–353. 10.3233/NHA-170036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkinson MJ, Manoogian ENC, Zadourian A, et al. (2020) Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab 31(1), 92–104. 10.1016/j.cmet.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkler JT. (2005) The fundamental flaw in obesity research. Obes Rev 6(3):199–202. 10.1111/j.1467-789X.2005.00186.x [DOI] [PubMed] [Google Scholar]
- 8.Archer E, Hand GA, Blair SN. (2013) Validity of U.S. nutritional surveillance: National Health and Nutrition Examination Survey caloric energy intake data, 1971–2010. PLOS ONE 8, e76632. 10.1371/journal.pone.0076632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhurandhar NV, Schoeller D, Brown AW, et al. (2015) Energy balance measurement: When something is not better than nothing. Int J Obes 39(7), 1109–1113. 10.1038/ijo.2014.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman LS, Commins JM, Moler JE, et al. (2014) Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am J Epidemiol 180(2), 172–188. 10.1093/aje/kwu116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trabulsi J, Schoeller DA (2001) Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am J Physiol-Endocrinol Metab 281(5), E891–E899. 10.1152/ajpendo.2001.281.5.E891 [DOI] [PubMed] [Google Scholar]
- 12.McClung HL, Ptomey LT, Shook RP, et al. (2018) Dietary intake and physical activity assessment: Current tools, techniques, and technologies for use in adult populations. Am J Prev Med 55(4), e93–e104. 10.1016/j.amepre.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 13.Schoeller DA (2009) The energy balance equation: Looking back and looking forward are two very different views. Nutr Rev 67(5), 249–254. 10.1111/j.1753-4887.2009.00197.x [DOI] [PubMed] [Google Scholar]
- 14.Racette SB, Das SK, Bhapkar M, et al. (2012) Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: The multicenter CALERIE study. Am J Physiol-Endocrinol Metab 302(4), E441–E448. 10.1152/ajpendo.00290.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heymsfield SB, Peterson CM, Thomas DM, et al. (2017) Establishing energy requirements for body weight maintenance: Validation of an intake-balance method. BMC Res Notes 10, 220. 10.1186/s13104-017-2546-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravelli MN, Schoeller DA (2021) An objective measure of energy intake using the principle of energy balance. Int J Obes 45(5), 725–732. 10.1038/s41366-021-00738-0 [DOI] [PubMed] [Google Scholar]
- 17.Shook RP, Hand GA, O’Connor DP, et al. (2018) Energy intake derived from an energy balance equation, validated activity monitors, and dual X-ray absorptiometry can provide acceptable caloric intake data among young adults. J Nutr 148(3), 490–496. 10.1093/jn/nxx029 [DOI] [PubMed] [Google Scholar]
- 18.Shook RP, Yeh H-W, Welk GJ, et al. (2021) Commercial devices provide estimates of energy balance with varying degrees of validity in free-living adults. J Nutr 152(2), 630–638. 10.1093/jn/nxab317 [DOI] [PubMed] [Google Scholar]
- 19.Murakami H, Kawakami R, Nakae S, et al. (2019) Accuracy of 12 wearable devices for estimating physical activity energy expenditure using a metabolic chamber and the doubly labeled water method: Validation study. JMIR MHealth UHealth 7, e13938. 10.2196/13938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ries D, Carriquiry A, Shook R (2018) Modeling energy balance while correcting for measurement error via free knot splines. PLOS ONE 13, e0201892. 10.1371/journal.pone.0201892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White T, Westgate K, Hollidge S, et al. (2019) Estimating energy expenditure from wrist and thigh accelerometry in free-living adults: A doubly labelled water study. Int J Obes 43(11), 2333–2342. 10.1038/s41366-019-0352-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hibbing PR, van Hees VT (2018) AGread: Read Data Files from ActiGraph Monitors. R package available from https://github.com/paulhibbing/AGread
- 23.Chen KY, Bassett DR (2005) The technology of accelerometry-based activity monitors: Current and future. Med Sci Sports Exerc 37(11 Suppl), S490–500. [DOI] [PubMed] [Google Scholar]
- 24.Ellingson LD, Hibbing PR, Kim Y, et al. (2017) Lab-based validation of different data processing methods for wrist-worn ActiGraph accelerometers in young adults. Physiol Meas 38, 1045–1060. 10.1088/1361-6579/aa6d00 [DOI] [PubMed] [Google Scholar]
- 25.Hildebrand M, Van Hees VT, Hansen BH, et al. (2014) Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc 46(9), 1816–1824. 10.1249/MSS.0000000000000289 [DOI] [PubMed] [Google Scholar]
- 26.Lusk G (1924) Animal calorimetry, twenty-fourth paper: Analysis of the oxidation of mixtures of carbohydrate and fat. J Biol Chem 59(1), 41–42. [Google Scholar]
- 27.Choi L, Liu Z, Matthews CE, et al. (2011) Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc 43(3), 357–364. 10.1249/MSS.0b013e3181ed61a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi L, Beck C, Liu Z, et al. (2018) PhysicalActivity: Process accelerometer data for physical activity measurement. R package available from https://cran.r-project.org/package=PhysicalActivity.
- 29.Tracy JD, Acra S, Chen KY, et al. (2018) Identifying bedrest using 24-h waist or wrist accelerometry in adults. PLOS ONE 13, e0194461. 10.1371/journal.pone.0194461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tracy JD, Xu Z, Acra S, et al. (2020) PhysActBedRest: Marks periods of “bedrest” in ActiGraph accelerometer data. R package available from https://cran.r-project.org/package=PhysActBedRest.
- 31.Schofield WN (1984) Predicting basal metabolic rate: New standards and review of previous work. Hum Nutr Clin Nutr 39C(Suppl 1), 5–41. [PubMed] [Google Scholar]
- 32.Hall KD, Sacks G, Chandramohan D, et al. (2011) Quantification of the effect of energy imbalance on bodyweight. Lancet 378(9793), 826–837. 10.1016/S0140-6736(11)60812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon PM, Saint-Maurice PF, Kim Y, et al. (2018) A Primer on the use of equivalence testing for evaluating measurement agreement. Med Sci Sports Exerc 50(4), 837–845. 10.1249/MSS.0000000000001481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bland J, Altman D (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 327(8476), 307–310. [PubMed] [Google Scholar]
- 35.Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8(2), 135–160. [DOI] [PubMed] [Google Scholar]
- 36.Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57(1), 289–300. [Google Scholar]
- 37.Faul F, Erdfelder E, Lang A-G, et al. (2007) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2), 175–191. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 38.Schoeller DA, Thomas D, Archer E, et al. (2013) Self-report-based estimates of energy intake offer an inadequate basis for scientific conclusions. Am J Clin Nutr 97(6), 1413–1415. 10.3945/ajcn.113.062125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subar AF, Freedman LS, Tooze JA, et al. (2015) Addressing current criticism regarding the value of self-report dietary data. J Nutr 145(12), 2639–2645. 10.3945/jn.115.219634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaMunion SR, Fitzhugh EC, Crouter SE. (2020) Challenges and opportunities related to the objective assessment of physical activity within United States surveillance systems. Ann Epidemiol 43, 1–10. 10.1016/j.annepidem.2020.01.011 [DOI] [PubMed] [Google Scholar]
- 41.Migueles JH, Rowlands AV, Huber F, et al. (2019) GGIR: A research community–driven open source R package for generating physical activity and sleep outcomes from multi-day raw accelerometer data. J Meas Phys Behav 2(3), 188–196. [Google Scholar]
- 42.Welk G, Kim Y, Shook RP, et al. (2017) Validation of a noninvasive, disposable activity monitor for clinical applications. J Phys Act Health 14(7), 546–551. 10.1123/jpah.2016-0003 [DOI] [PubMed] [Google Scholar]
- 43.Wijndaele K, Westgate K, Stephens SK, et al. (2015) Utilization and harmonization of adult accelerometry data: Review and expert consensus. Med Sci Sports Exerc 47(10), 2129–2139. 10.1249/MSS.0000000000000661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farrahi V, Niemelä M, Kangas M, et al. (2019) Calibration and validation of accelerometer-based activity monitors: A systematic review of machine-learning approaches. Gait Posture 68, 285–299. 10.1016/j.gaitpost.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 45.Evenson KR, Goto MM, Furberg RD (2015) Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act 12(159), 1–22. 10.1186/s12966-015-0314-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siddall AG, Powell SD, Needham-Beck SC, et al. (2019) Validity of energy expenditure estimation methods during 10 days of military training. Scand J Med Sci Sports 29(9), 1313–1321. 10.1111/sms.13488 [DOI] [PubMed] [Google Scholar]
- 47.O’Driscoll R, Turicchi J, Beaulieu K, et al. (2018) How well do activity monitors estimate energy expenditure? A systematic review and meta-analysis of the validity of current technologies. Br J Sports Med 54,332–340. 10.1136/bjsports-2018-099643 [DOI] [PubMed] [Google Scholar]
- 48.O’Driscoll R, Turicchi J, Hopkins M, et al. (2020) The validity of two widely used commercial and research-grade activity monitors, during resting, household and activity behaviours. Health Technol 10(3), 637–648. 10.1007/s12553-019-00392-7 [DOI] [Google Scholar]
- 49.de Jonge L, DeLany JP, Nguyen T, et al. (2007) Validation study of energy expenditure and intake during calorie restriction using doubly labeled water and changes in body composition. Am J Clin Nutr 85(1), 73–79. 10.1093/ajcn/85.1.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothney MP, Martin F-P, Xia Y, et al. (2012) Precision of GE Lunar iDXA for the measurement of total and regional body composition in nonobese adults. J Clin Densitom 15(4), 399–404. 10.1016/j.jocd.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 51.Dordevic A, Bonham M, Ghasem-Zadeh A, et al. (2018) Reliability of compartmental body composition measures in weight-stable adults using GE iDXA: Implications for research and practice. Nutrients 10, 1484. 10.3390/nu10101484 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


