Abstract
Around 10–15% of COVID-19 patients affected by the Delta and the Omicron variants exhibit acute respiratory insufficiency and require intensive care unit admission to receive advanced respiratory support. However, the current ventilation methods display several limitations, including lung injury, dysphagia, respiratory muscle atrophy, and hemorrhage. Furthermore, most of the ventilatory techniques currently offered require highly trained professionals and oxygen cylinders, which may attain short supply owing to the high demand and misuse. Therefore, the search for new alternatives for oxygen therapeutics has become extremely important for maintaining gas exchange in patients affected by COVID-19. This review highlights and suggest new alternatives based on micro and nanostructures capable of supplying oxygen and/or enabling hematosis during moderate or acute COVID-19 cases.
Keywords: COVID-19, Microbubbles, Nanobubbles, Nanotechnology, Oxygen therapy, Hypoxia
Graphical abstract
1. Introduction
The COVID-19 pandemic has revealed severe deficiencies in the treatment of hypoxia, that is, inadequate tissue oxygenation, often as a consequence of hypoxemia [1]. Hypoxemia is defined as a reduction in arterial oxygen content or a reduction in hemoglobin saturation (SaO2 < 90%), which is considered severe when SaO2 is <85% [2]. In particular, SARS-CoV-2 infection is associated with hypoxemic hypoxia, characterized by reduced arterial O2 content [3] – caused by several factors that reduce or hinder O2 alveolar diffusion to arterial blood, such as diminished endothelial permeability, tendency to form microthrombi, and the presence of capillary shunts resulting from chaotic neoangiogenesis triggered by the inflammatory process [4].
The initial stages of COVID-19 are associated with the period described as “happy” or “silent” hypoxia, which consist of a significant reduction in arterial O2 content with the lung parenchyma still relatively preserved [5]. Nonetheless, many patients do not present with respiratory discomfort or dyspnea [6]. With the progression of the disease, however, the patients begin to report a “heavy chest” feeling, characterized by extensive pulmonary involvement. The histopathological findings in advanced stages include pneumocyte desquamation, diffuse alveolar damage, and hyaline membrane formation, preserving important similarities with acute respiratory distress syndrome (ARDS) [7,8].
The direct pathway of SARS-CoV-2 respiratory damage occurs through the binding of the virus spike glycoprotein to angiotensin-converting enzyme 2 (ACE2), predominantly in the lower respiratory tract cells [7]. Systemic damage, in turn, is caused by the release of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), which, once recognized by resident macrophages in the lungs, trigger an extensively described cytokine storm, in which IL-6 plays a prominent role [9]. In addition, this interleukin has an important function in the vascular endothelium, negatively regulating cell adhesion molecules (E-cadherins) and increasing the expression of endothelial growth factors, whose main consequence is the formation of edema and hypoalbuminemia. This results in a vicious cycle of inflammation that progressively hinders alveolar gas exchange [10,11]. This process favors thrombotic events and the formation of pulmonary microemboli, which are also limiting factors for pulmonary perfusion and oxygenation in COVID-19 [12,13]. In a comparative study between COVID-19 and H1N1 patients, the expression of IL-6 and TNF-α was significantly higher in SARS-CoV-2 [10]; the expression of IL-6 in COVID-19 was between 2% and 6%, while in H1N1 patients, this value was less than 3%. TNF-α expression in H1N1 was <5%, although in COVID-19 it varies from 5 to 25% [14].
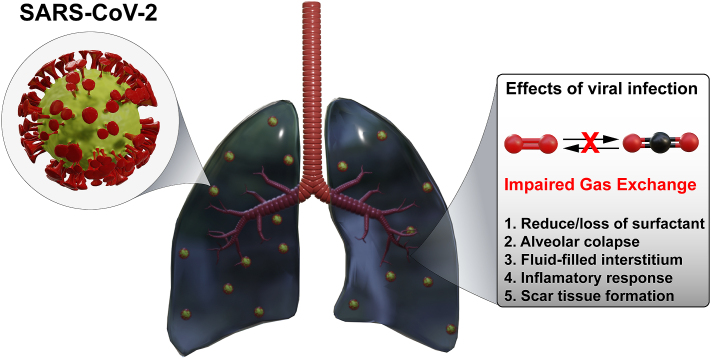
The exacerbated inflammatory response caused by the invasion of SARS-CoV-2, endothelial dysfunction, accumulation of fibropurulent exudates in the vicinity of alveoli and pulmonary capillaries, as well as the deficit between ventilation and perfusion generated by difficulty in oxygen diffusion are evident in the context of hypoxemia, and consequently, the hypoxia associated with COVID-19 [15] (Fig. 1). ARDS is the most common clinical manifestation in these patients and requires oxygen therapy, usually treated with invasive mechanical ventilation (IMV). Around 10–15% of COVID-19 patients affected by the Delta and the Omicron variants exhibit acute respiratory insufficiency (ACI) and require intensive care unit (ICU) admission to receive advanced respiratory support [[16], [17], [18]].
Fig. 1.
Schematic representation of the main mechanisms involved with the impairment of gas exchanged induced by SARS-CoV-2 infection.
However, despite its beneficial potential, IMV might also cause major health problems, such as the development of ventilator-associated pneumonia (VAP), dysphagia, ventilator-induced lung injury (VILI), hemorrhages, and kidney damage [15,19]. Therefore, new oxygen therapies are required to perform gas exchange in mild-to-severely affected COVID-19 patients to prevent and/or increase the effectiveness of IMV. In this review, we summarize the classical oxygen therapies and highlight several potential micro and nanotechnological strategies to be employed as oxygen therapy alternatives in COVID-19 patients.
2. Methodology
This is a non-systematic review of research articles included in PubMed, MedLine, Science Direct, and Google Scholar databases during the period 2015–2022.
3. Classical oxygen therapies
3.1. Non-invasive ventilation
Non-invasive ventilation (NIV) includes masks, helmets, modified snorkel masks, and a high-flow nasal cannula (HFNC). Oxygen therapy consists in the application of ventilatory support without the need for invasive methods, such as the orotracheal intubation technique (OIT) [20]. There are two main types of NIV: (i) continuous positive airway pressure (CPAP), which is most commonly used in COVID-19 patients, and (ii) bilevel positive airway pressure (BiPAP) that is mainly required in chronic obstructive pulmonary diseases (COPD) as well as in COVID-19 patients [21,22]. NIV is a common treatment for COVID-19 hypoxemia and is used to normalize SPO2 values when the patient has no immediate requirement of OIT [23]. According to Bertania et al. [24] when NIV is chosen, a decrease of more than 50% in the risk of late OIT is observed. Furthermore, NIV should not be used in patients who start treatment with OIT and require reintubation because there is an increase in the death rate, especially due to VILI [25].
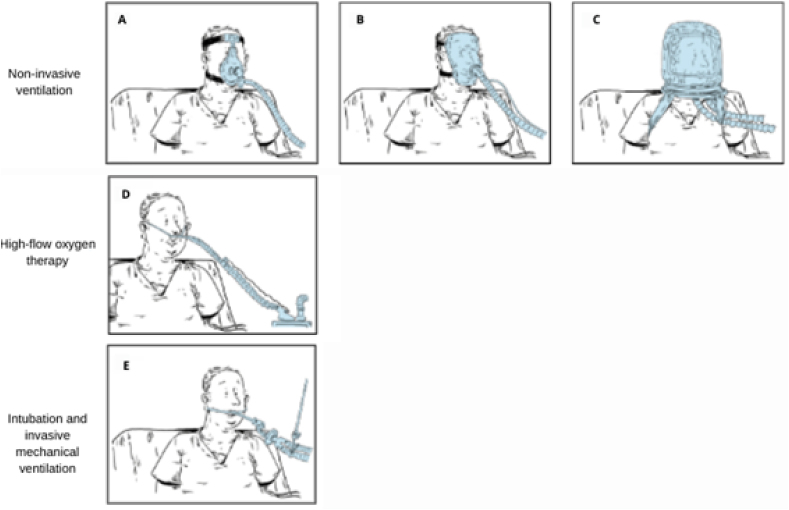
The use of masks (Fig. 2A and B) exhibits important benefits, including fast pH stabilization [26], maintenance of adequate alveolar ventilation [27] and improvement in 60-day in-hospital mortality rates of COVID-19 patients compared to those who initially underwent invasive mechanical ventilation (IMV) support [28]. Another alternative, the helmet, is among the most used NIV strategies and has the advantage of covering the patient's head, and providing adequate oxygen support [29] as shown in Fig. 2C. In addition, helmets decrease the levels of aerosol scattering [30] and display more physiologic benefit compared to high-flow nasal oxygen among patients with more severe oxygenation impairment and intense inspiratory effort [31]. Moreover, it provides a better level of positive end-expiratory pressure (PEEP) when compared to face masks and lower levels of pressure support, which contributes to the reduction of transpulmonary pressure and potential for VILI, since high volumes of pulmonary pressure may cause barotraumas [32]. However, NIV strategies, such as masks or helmets, can cause claustrophobia [33] and increase the anatomical lung dead space [27]. Finally, the helmet is more expensive than other types of NIVs [16].
Fig. 2.
Oxygen Therapies - Non-invasive ventilation provides ventilator support without an endotracheal tube, using an oronasal (A) mask or modified snorkel (total face) (B) mask or a helmet (C). High Flow Nasal Cannula through Prongs or Nasal Cannulas (D) delivers accurate FiO2, PEEP and tidal volume at a flow equal to or greater than the patient's inspiratory flow demand. Nasal oxygen is delivered at a flow rate of up to 60 L/min. It is heated to the body's need and saturated to full moisture. Invasive mechanical ventilation (E) uses a tracheal tube inserted into the trachea under general anesthesia. The tube is then secured to the face or neck and connected to a ventilator. In patients with hypoxemic acute respiratory failure, such as COVID-19 patients, intubation and invasive mechanical ventilation are used after failure of non-invasive methods. Adapted from Ref. [34].
The high-flow nasal cannula (HFNC) consists of a canalization system that must be placed in the nostrils to allow O2 delivery (Fig. 2D). The HFNC common components include a flow generator capable of delivering varied rates of oxygen (up to 60 L/min), an air mixer capable of boosting (from 21% to 100%), fraction of inspired oxygen (FiO2) and an air humidifier and heater (from 31 °C to 37 °C) [23,35]. More information about the equipment and the application methodology can be found at [36]. Owing to the high flow rate, HFNC reduces airway resistance and increases the ventilation oxygenation potential of the lung tissue [37]. In addition, warm and humidified air tends to improve mucociliary function and expectoration [38]. Furthermore, this technique can increase FiO2 due to the higher partial pressure of O2, which implies lower levels of PEEP, improving oxygenation, and discharging the anatomical dead space due to CO2 elimination [27,38]. All of these properties contribute to a reduction in the patient's respiratory effort [39], although without it does not reduce 28-day mortality compared with standard oxygen therapy [40,41]. All these settings, such as FiO2, PEEP, tidal volume, humidification, and heating are controlled, which guarantees a high safety level [42]. Finally, a high-flow nasal cannula is more comfortable and less obstructive, allowing the patient to communicate, swallow, and allow simultaneous orotracheal intubation during the period of endotracheal apnea and intubation [33,38].
The study by Artigas et al. [43] with 122 COVID-19 patients revealed a shorter ICU stay when HFNC was used. Therefore, HFNC is an important alternative to hypoxia therapy and should be adapted only in patients who do not require immediate OTI or those that do not have hypercapnia but still have SpO2 between 90 and 94% [44,45]. A meta-analysis by Rochwerg et al. [46] concluded that the use of HFNC decreased the need for OIT by 4.4%, thus decreasing the risk of VILI, and consequently reducing patient mortality by approximately 20% [43,47]. However, a high-flow nasal cannula was associated with higher ICU mortality rates when used for longer periods, because it may cause muscle fatigue and cardiac dysfunction. Therefore, if subsequent OIT is required, it may also cause less successful extubation, as the muscles will be fatigued [48]. Nevertheless, HFNC causes exhaled air dispersion even when well fitted to the patient, which may facilitate transmission and, consequently, has limited use [49,50].
3.2. Invasive mechanical ventilation (IMV)
The IMV consists of an oxygen ventilator connected to the patient by an endotracheal tube adapted through the mouth until it reaches the trachea (Fig. 2E). This ventilator can deliver oxygen following the FiO2, PEEP, and pO2 settings established by the clinician according to the patient's need [51]. The ventilator conditions used in COVID-19 patients are set to generally deliver levels from 30 L/min to 60 L/min of O2, which is more than the common NIVs (∼15 L/min), while PEEP and FiO2 are set to maintain a pO2 of 55–80 mmHg and a tidal volume of 6 mL/kg. Additionally, the IMV also ensures a PEEP rate that prevents alveoli from collapsing [52].
IMV is used to maintain respiratory and acid-base regulation for NIV non-responder patients according to an escalation criterion [53,54]. Several institutions follow a stepwise increase for hypoxic patients with COVID-19 pneumonia: most procedures initiate with conventional oxygen therapy (COT) via Venturi masks (starting at SpO2 <92% to target SpO2 of 92–96%). If the SpO2 values decrease ≥2% or if the respiratory rate is > 30, the patient should be escalated to HFNC and so on. For IMV, the criteria include respiratory arrest, respiratory pause with unconsciousness, severe hemodynamic instability, PaO2:FiO2 ratio <100, among others [53]. However, IMV is considered a difficult procedure and extubating is relatively complex [55], especially when used for long periods (>18 h) because it might cause mild atrophy of breathing and swallowing muscles [52]. As in orotracheal intubation, there is no effort required from the breathing muscles, and a loss of mucosal sensitivity of the larynx is frequently observed [56]. This factor favors the probability of contracting ventilatory pneumonia due to possible microaspiration of secretions from the oropharynx or gastric contents, once the defense against invaders, particularly the absence of the cough reflex, is affected [57].
Moreover, IMV may cause or worsen lung injury due to complications arising from a ventilator-induced lung injury [58], such as (i) volutrauma caused by overdistension, and random expansion of alveoli due to high transpulmonary pressures or high volumes [59]; (ii) atelectrauma resulting from cyclical opening and closing of the distal airways [60]; and (iii) biotrauma determined by the inflammatory process resulting from harmful ventilatory strategies adopted, such as the IMV and the COVID-19 cytokine storm [61,62]. In addition, it can lead to general pulmonary distension above the accepted parameters and asynchronous ventilation [63], which can be extremely harmful to the patient.
4. Artificial oxygen delivery systems
Artificial oxygen delivery systems are molecules and/or structures that are capable of carrying and exchanging gases into biological tissues under hypoxic conditions. Such systems display a high affinity for O2 and can be released locally according to the partial pressure of gas in the tissue. Some formulations may also remove high CO2 concentrations originated from cellular metabolism [64]. Such systems were developed to provide the oxygen required by cells in extreme situations, such as trauma, thromboembolism, and pulmonary emphysema [65]. The most recent experimental or preclinical examples and those with the greatest translational potential for COVID-19 cases are discussed in this section.
4.1. Hemoglobin-based oxygen carrying systems
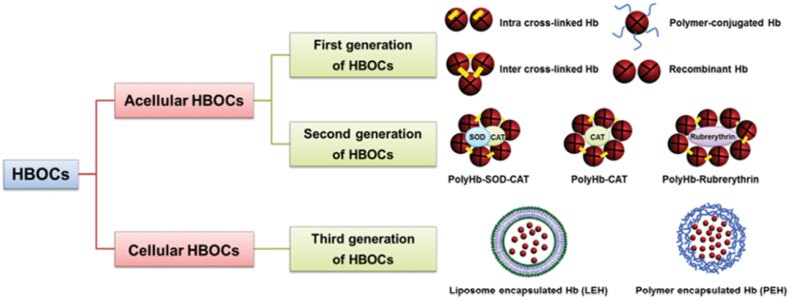
Hemoglobin-based oxygen transport systems (HBOCs) are semi-synthetic structures that utilize the natural hemoglobin (Hb) molecule as an oxygen transport component (Fig. 2). Within these systems, HBOCs are classified as acellular, divided into first-generation transporters – Hb molecules conjugated with each other and/or other materials [64] – and second-generation transporters – formed by conjugated and cross-linked materials of polymers and enzymes (e.g., superoxide dismutase and catalase) [64]. During the synthesis process, the Hb is usually derived from human, bovine or recombinant sources [64,66]. The third generation (or cellular transporters) are characterized by Hb molecules encapsulated into nanostructures, such as liposomes or polymeric nanocapsules that protect the oxygen carrier molecule against leakage or rapid in vivo degradation [66]. This classification is illustrated in Fig. 3 and some examples are further discussed bellow.
Fig. 3.
Schematic representation of HBOCs classification. 1st generation transporters, characterized as Hb molecules conjugated with each other and/or other materials. 2nd generation transporters, formed by materials conjugated and cross-linked materials of polymers and enzymes. 3rd generation transporters, which are characterized by Hb molecules encapsulated in nanostructures, such as liposomes or polymeric nanocapsules. Adapted from Ref. [66].
The 1st HBOCs generation displayed great progress in delivering oxygen to the hypoxic tissues, as they aim to prevent the dissociation of hemoglobin tetramers into dimers and exhibit antioxidant properties, thereby protecting the heme group from reactive oxygen species to prevent the formation of methemoglobin. However, side effects (e.g., transient hypertension, elevation of pancreatic and hepatic enzymes, and neurotoxicity) [67] occurred in various cases [64]. The 1st HBOCs generation are chemically modified via intra- and intermolecular cross-linking processes. For example, HemAssist®, generated by the acylation of two alpha subunits of Hb with bis-(3,5-dibromosalicyl)-fumarate, forms an intramolecular cross-linked material [67,68]. This process allows Hb to maintain its oxygen-carrying properties and minimizes the dissociation effect of tetramers into dimers and monomers, which are eliminated by glomerular filtration [68]. The main objective of the 1st HBOCs generation is to deliver oxygen in extreme situations, in which the patient's red blood cells are not able to supply the necessary amount of gas (e.g., ischemic cerebral vascular accidents) [68,69]. In the case of HemAssist®, the system is generated from washed, lysed, filtered, and deoxygenated Hb, which is subsequently crosslinked and reoxygenated [68]. Unfortunately, HemAssist® showed a >70% increase in mortality rates in human patients compared to the use of saline, which also provoked transient hypertension, organ damage, microvascular dysfunction, and neurotoxicity [69]. Therefore, the study involving this particular formulation was interrupted in 2007 [64]. Despite the failure in human trials, the in vitro results revealed that the derived systems were effective in increasing O2 levels and performing gas exchange, thereby opening new perspectives for the study of HBOCs that may be applicable in diseases such as COVID-19.
A 2nd HBOCs generation, designed as HemoPure®, is an example of a chemically modified O2 carrier, which is manufactured from bovine-derived hemoglobin-201, polymerized via cross-linking with glutaraldehyde, and stored in lactic acid solution [70]. The structure of the system is similar to that of human Hb, and its diameter is smaller than that of red blood cells (∼7 μm), thus facilitating its diffusion along the microvasculature [71]. As this product promotes O2 diffusion and supply by convection, HemoPure® increases gas transfer between red blood cells and tissues, and offers an alternative for local and systemic oxygenation – 1 g of HemoPure® is equivalent to 3 g of human Hb [72]. HemoPure® has been used in the treatment of ischemic and hypoxic diseases, such as myocardial infarction, hemolysis, cardiopulmonary bypass, and organ transplantation [[73], [74], [75], [76]]. However, its use may cause adverse effects, such as systemic vasoconstriction and arterial hypertension, in addition to decreased blood flow, increased pro-inflammatory mediators, and platelet inactivation due to the high affinity of HemoPure® for nitric oxide, thereby, decreasing its circulating levels. Owing to its side effects, the Food and Drug Administration (FDA) does not recommend its commercialization [64,70].
The 3rd HBOC generation consists of systems encapsulated via micro- and nanotechnology. These systems stabilize the Hb within the mesh of transport materials, such as phospholipids and poly (ethylene glycol)-b-poly (lactide) (PEG-PLA) [65,77]. These micro- and nanostructures encapsulate Hb and display erythrocyte-like oxygen dissociation curves, in addition to maintaining the activity of enzymes, such as 2,3-diphosphoglycerate, carbonic anhydrase, and catalase that may be incorporated into the structure [78]. Polymeric structures offer greater physical resistance and enhanced blood vessel permeability and may be produced in organic solvents that maintain Hb bioactivity [79,80]. As a result, both systems present great prospects for use in COVID-19 patients.
4.2. Hemoglobin derived from marine annelids
Recently, Hb derived from Arenicola marina and Nereis virens, two polychaete annelids (or “sea worms”), have drawn attention for their high O2 transport ability. The two Hb obtained from giant polychaete annelids are under investigation to be employed as endogenous oxygen carriers, products designed as HEMOXCell® and Hemo2Life®. Both of these structures are examples of 2nd generation of HBCOs, and have already display oxygenation capacity and biosafety, as demonstrated in preclinical studies [73,81]. Although both exhibit translational perspectives, neither was yet evaluated in COVID-19 patients.
The Hemo2Life® (M101 protein – produced by collecting blood samples from the ventral vessel of the A. marina [82].) has a molecular weight of ∼3600 kDa and exhibits structural and functional properties essential for a good oxygen transporter. It is a hexagonal bilayer Hb composed of 156 globins and 44 non-globin binding chains that are capable of transporting up to 156 molecules of O2 when saturated [80]. However, because of the high molecular weight, their leakage to the endothelium and glomerular capillaries is reduced, a factor that increases the material's performance compared with traditional HBOCs [83]. Moreover, Hemo2Life® displays intrinsic superoxide dismutase-like activity (based on its copper and zinc center) and might reduce reactive oxygen species (ROS) formation, thereby decreasing the probability of cytokine storms induced by COVID-19 [73,83].
Analysis of the Hemo2Life® biodistribution revealed that, with just one intravenous injection, this functional Hb rapidly diffuses throughout the body of mice (∼15 min), including poorly vascularized areas [84]. This is an important advantage compared to previous generations of HBOCs, with a molecule size about 250 smaller than an erythrocyte, allowing its diffusion in all areas of microcirculation, in addition to a wide temperature range of activity (from 4 to 37 °C) [82,85,86]. In addition, the molecule may be detected in the plasma for up to 6 h after injection and is eliminated after 96 h [82]. Most importantly, it is a safe substance for use in vivo, as the animals did not exhibit external clinical signs or changes in heart rate, blood pressure, or microvascular vasoconstriction [84]. Therefore, owing to its biocompatibility, in vivo functionality, bioavailability, and previous use in lung preservation solutions, this molecule has great potential and could be tested in COVID-19 cases to provide better tissue oxygenation. The use of Hemo2Life® is expected to improve the transport of gases with an increase in survival, lower O2 requirement, and in prevention of orotracheal intubation.
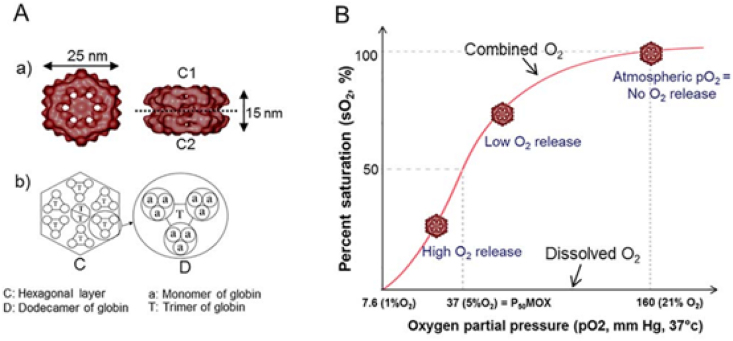
A distinct system designed, HEMOXCell® (Fig. 4A) also has great potential for oxygen therapy in patients with COVID-19. HEMOXCell® is a hemoglobin derivative obtained from the marine annelid N. virens, with a diameter also ∼250 times smaller than that of a red blood cell [79,84]. The molecule consists of 198 polypeptide chains, with 156 globin chains and 42 oxygen-binding chains [83,87]. The molecule's proposed structure and O2 saturation curve are shown in Fig. 4B.
Fig. 4.
Structural representation of HEMOXCell® (A) with oxygen binding characteristics (B). Adapted from Ref. [79].
HEMOXCell® captures oxygen and releases it via simple diffusion along the gas partial pressure gradient, depending on cellular requirements [79]. The report carried out by Le Pape et al. [74] highlights the use of HEMOXCell® with a positive effect on the growth of mesenchymal stem cells due to a constant concentration of dissolved O2 throughout the culture medium [74,79]. According to the study, 25 mg/mL dissolved HEMOXCell® was able to increase the cell growth by 25% during a 14-day experiment, thereby confirming its oxygen-carrying ability and positive effects on cell metabolism [83]. As HEMOXCell® is an O2 transporter derived from polychaete annelids, its use could have beneficial effects in the treatment of patients with COVID-19. Furthermore, cytotoxicity studies have shown that it has no immunogenic effects; it does not cause fever, hypertension, vasoconstriction, or kidney damage [74,83]. However, more studies are needed to assess the effectiveness of O2 delivery by HEMOXCell® in hypoxic environments caused by COVID-19.
5. Micro and nanotechnology for extracorporeal oxygenation
Both microtechnology and nanotechnology are cutting-edge alternatives for therapeutic and diagnostic use in a wide variety of pathologies [[88], [89], [90]]. They are also employed to transport and deliver drugs, and other molecules such as oxygen and nitric oxide, allowing an increase in pO2 [91,92]. The following section is intended to present and highlight the therapeutic results promoted by experimental or preclinical systems, which are potentially capable of carrying molecules that the body needs [93].
5.1. Oxygen microcarriers
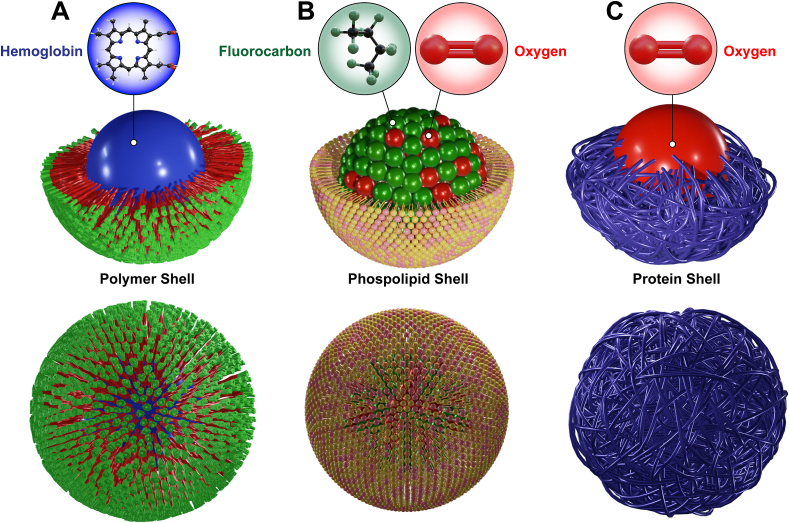
Oxygen microbubbles (OMBs) are micrometric structures composed of a gaseous O2 core, stabilized, and surrounded by a thin layer that can be formed by a wide variety of biomaterials, such as phospholipids, polymers or proteins [88,90,91,94,95] (Fig. 5A, B and C).
Fig. 5.
Schematic illustration of the shell (lipids, polymers and proteins) and core composed of hemoglobin (A), O2 (B) and perfluorocarbon (C) employed to formulate micro or nanocarriers to enhance pO2 in experimental studies.
Microbubbles are routinely used in various treatments and diagnostic procedures, such as in the therapy of cardiovascular diseases such as thrombosis [96], ultrasound diagnosis [97] and treatment of several tumors [[98], [99], [100]]. Nonetheless, the impact of OMBs on hypoxic locals has been previously elucidated by various reports. For example, Feshitan et al. [95] showed successful oxygenation of rats submitted to acute pulmonary trauma, promoting their survival for more than 2 h through intraperitoneal administration of OMBs (diameter 3–5 μm) containing 0.88 mg mL−1 of oxygen [101]. The animals that did not receive the treatment presented a rapid decay of vital parameters, culminating in a survival time approximately 7 times lower [95]. Moreover, the observed tissue oxygenation was greater than that expected by other carriers, such as hemoglobin itself, as it is able to carry a higher concentration between 50 and 100% of O2. In addition, it does not display limiting factors, such as the finite solubility of perfluorocarbons in the blood or oxygen transport via hemoglobin [95,102]. The results of this in vivo study highlighted the OMBs ability to diffuse into capillaries adjacent to the peritoneal mesothelium and provide systemic oxygenation, a fundamental feature since such micrometric structures are not able to transit through the pulmonary microcirculation.
Another important study, carried out by Kheir et al. [93] revealed the efficiency of ∼5 μm OMBs to provide O2 in hypoxemic rabbits via intravenous (IV) injection. The estimated O2 release from OMBs was approximately 4 mL/kg min, which promoted a ∼50% increase in SpO2 and pO2 and also enabled the maintenance of vital parameters, such as blood pressure and heart rate. As evidenced by Raymond et al. [102], a major limitation of OMBs is the coalescence phenomenon during infusion, which may cause cytotoxicity, embolism and thrombosis. Although the efficiency of lipid-stabilized OMBs has been demonstrated, many studies and structural refinements are required before large scale clinical studies especially due to the effect of possible coalescence, Ostwald ripening, and Laplace overpressure during storage [103], as most are lipid-base carriers.
In a recent study carried out with colons of swine models purposely affected by severe hypoxia due to smoke inhalation, OMBs were infused at a rate of 500 mL/min for a total dose of 75–100 mL/kg. After 2 h, most organs displayed statistically higher O2 levels. Furthermore, the same study demonstrated arterial CO2 decay in the animals that received OMBs [104,105]. The microbubbles presented an O2 diffusion capacity of ∼2.4 x 10−6 cm2/s [106], while the CO2 removal permeability was ∼20x greater than of oxygen [107]. It is worth noting that the organs of swine are similar to those of humans [108], thereby encouraging future and improved investigations. However, the study followed the hypoxic condition for about just 3 h, while in SARS-CoV-2 cases, the hypoxic condition may last days or even weeks [101,102].
Recently, Raymond et al. [102] suggested the introduction of other polymers to carry oxygen to tissues with greater accuracy, safety and fewer negative consequences, such as PLGA, PLA or PLA-PEG. They also demonstrated that when stored under ambient conditions, such formulations are more stable and reduce the effects of Ostwald ripening as well as Laplace overpressure during a period of up to 2 months [102]. In fact, PLGA is currently considered a highly effective synthetic polymer for the development of nanoparticle delivery vaccines owing to its biodegradability, and biocompatibility [109,110]. Therefore, OMBs are promising for maintaining SpO2 at normal levels for short time intervals and for their effectiveness in large-scale applications, as they have reduced cost compared to hemoglobin-derived materials [89,103]. Additionally, although intraperitoneal administration has been shown to be more viable than IV, further studies are still required to investigate its efficacy in clinical use due to differences in peritoneal extension and vascularization between humans, rodents and porcine [111], especially in cases of COVID-19 thrombosis.
5.2. Oxygen nanocarriers
Oxygen nanobubbles (ONBs) are structures composed of a gaseous core of oxygen and, such as OMBs, are stabilized by a thin biomaterial layer [112,113]. However, ONBs exhibit a diameter between 50 and 400 nm [[112], [113], [114]]. These nanostructures may be viable alternatives for hypoxemic hypoxia mitigation in COVID-19 cases because of their small size, which facilitates their transposition through the microcirculation [115]. Such nanodevices may be easily prepared in loco by simply mixing the polymeric component previously synthesized at the nanometric scale with purified O2 and then infused into the patient's bloodstream.
The use of ONBs was recently described by Muhammed et al. [112], who evaluated O2 transport capacity and cytotoxic effects through in vitro studies. In the experiment, the authors developed ONBs with stabilized O2 by a thin phospholipid layer, which was administered at different concentrations to tumor breast cancer cells (MDA-MB-231). Previously, these cells were subjected to 6–8 h of incubation in hypoxic chambers and were treated with ONBs for 30 min. Ultimately, anti-HIF-1α (hypoxia inducible factor 1α) antibodies conjugated to fluorescein isothiocyanate (FITC) were used as hypoxic indicators [113,116]. The authors verified that a 10% ONBs solution is sufficient to mitigate hypoxia [112]. A similar effect was observed by Matsuki et al. [99] that recommended the use of nanobubbles for various diseases and clinical emergencies, especially to increase pO2 in patients with severe acute hypoxia, proving to be even more effective than OMBs.
Recently, Owen et al. [111] elucidated the efficiency of nanobubbles in reducing tumor hypoxia using an oxygen solution stabilized in ONBs. In this experiment, 100 μL of this solution was orally administered to mice with xenografts from human pancreatic tumors. Oral administration was chosen to overcome the risks of intravenous infusion [95] and oxygen delivery was primarily determined by measuring HIF-1α expression. It was verified that the mice that received the treatment showed a 75% decrease in the transcriptional expression of HIF-1α and a 25% decrease in the translational expression compared to the control group [111,117]. Similarly, Khan et al. [118] showed the effectiveness of lipid shell nanobubbles to increase cell viability in 20% with reduced HIF-1α levels. The use of polysaccharides as stabilizing agent for O2 delivery was also explored in hypoxic tumor models. Song et al. [119] verified the effect of dextran nanobubbles with ∼300 nm in diameter: after the intravenous administration, the tumor area pO2 increased gradually, causing a 6-fold pO2 increment that lasted for more than 2 h. The in vivo results demonstrated that nanobubbles could effectively increase the intratumoral pO2 up to ∼30 mmHg. As a result, it is possible that a system initially proposed to overcome hypoxic tumor resistance might be translated to increase oxygen tissue perfusion in COVID-19 patients by O2 diffusion during its traffic through the bloodstream.
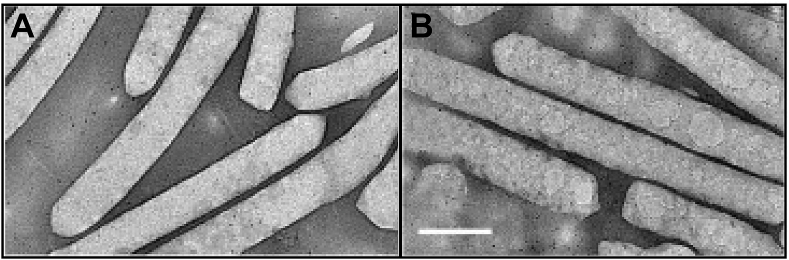
Likewise, distinct system architectures were developed to release oxygen in the hypoxic tumor environment. However, the report of Song et al. [120], describes the employment of naturally occurring nanovesicles containing oxygen produced by cyanobacteria or archaea as an interesting alternative for tumor therapeutics (Fig. 6A and B). These 200–400 nm gas vesicles are protein structures produced to control the buoyancy and, hence, offer access to optimal light and nutrient conditions in water mediums. Due to the shell stability reduced Laplace pressure, these systems are more stable than phospholipid nanobubbles. Nonetheless, to reduce gas loss before application, the authors coated the nanovesicles with a lipid layer and observed that the system was able to achieve an oxygen concentration of severe hypoxic solution in 10-fold after ∼5 min, increasing the viability o human hepatoma cells. The in vivo results revealed that the nanovesicles attain tumor masses after 15 min of tail-vein injection and provide a significant increment of oxy-hemoglobin locally, which hinders the mass growth. Therefore, similar systems may be able to maintain organ viability for short time intervals even during acute hypoxic conditions – this suggestion is supported by in vivo and ex vivo imaging assays that reveals full body distribution by the nanovesicles.
Fig. 6.
Transmission electron microscopy images of nanovesicles containing oxygen produced by the algae Anabaena flos-aquae before (A) and after the coating process (B). Adapted from Ref. [120].
This concept may be applied to preserve oxygen supply to several tissues, including the eyes as recently reported by Fayyaz et al. [121]. The authors aim to treat cases of central retinal artery occlusion, a pathology that obstructs the retinal artery and prevent oxygen supply to the organ, which may result in irreversible loss of sight if not treated within 24–36 h. To mitigate the hypoxic conditions, the authors developed oxygen nanobubbles of ∼120 nm in diameter composed by a complex shell mainly formed by dextran capable to release ∼3.5 nL/min of O2. The in vivo results revealed that the system was able to maintain an almost typical pO2 concentration in vitreous, inner retina, outer retina and choroid, whereas the hypoxic eye presented lower pO2 levels in all structures. Thereby, the nanosystems meet the oxygen consumption requirement in rats and humans to rescue the inner retina and other structures from blindness. Accordingly, similar strategies may be envisioned to preserve and rescue several organs from hypoxic conditions.
Finally, the study of Bhandari et al. [122] provide an insight about the molecular mechanisms triggered by nanobubbles in hypoxic conditions as verified in tumor models. The group synthesized oxygen nanobubbles (∼100 nm) using a carboxymethyl cellulose shell stabilized with aluminum chloride. The in vivo results revealed an O2 increase greater than 140% within the hypoxic tumor microenvironment (MB49 tumors) after injection that was able to supply the hypoxic regions with O2 for at least 5 days. The therapy reduced the tumor vascularization cross-section and revealed an altered epigenetic profile for the animals submitted to the nanobubbles. The authors screened 22 tumor suppressor genes and noticed that 11 experienced a significant decrease in promoter methylation, thereby indicating that the nanobubble treatment has the potential to reshape the cancerous landscape of DNA methylation to a relatively normal status. Therefore, it is possible to suggest that the O2 delivery mediated by nanostructures in COVID-19 might be crucial not only to preserve the organ in short time intervals, but also to avoid specific cellular regulatory damages induced by hypoxic state – which may be related to long COVID-19 cases.
5.3. Perfluorocarbon nanocarriers
Perfluorocarbons (PFCs) are organic compounds that display high gaseous dissolution capacity, which allows the entrapment of O2, CO2, N2, and NO within stable CF3 pockets formed by adjacent PFC molecules [123]. PFCs may be incorporated into nanostructures via emulsification and many exhibit higher O2 solubility than water (∼20-fold higher) (Fig. 7A and B) [124]. Several studies reported the use of PFCs as blood substitutes for O2 and CO2 transport [125,126]. The experimental or preclinical studies described below demonstrate the potential to increase O2 levels in patients affected by COVID-19 in future translational studies.
Fig. 7.
Comparative representation of the gaseous dissolution capacity between PFC (A) and water (B). PFCs are able dissolve ∼20× more O2 compared to water. Adapted from Ref. [124].
The first experiment performed with PFCs to increase the supply of O2 was conducted in 1966 by Clark et al. [127] using a fluorobutyltetrahydrofuran fluid (FX-80). They demonstrated that mice were able to survive under anesthesia and submerged in O2-saturated liquid for up to 4 h. In this sense, it is worth mentioning that the only characteristic that implies the dissolution of O2 carried by the PFCs is the difference in the partial pressure of O2 between the PFC molecule and the tissue [128].
Since the first study by Clark et al., many other research groups have attempted to find the correct formulation for O2 transport by PFCs, such as Fluosol [129], Perftoran [130], Oxyfluor, and Oxygent [131]. However, most system had their development interrupted, either due to instability [132], organ retention [130], complement activation [133] or induction of flu-like symptoms [131]. Among them, Fluosol is the only compound approved by the FDA that was, however, withdrawn from the market due to difficulties encountered in its storage and use [134].
Nonetheless, recently developed nanosystems may offer a significant change in the effectiveness of PFC-containing formulations to exchange gases in COVID-19 patients. For example, pulmonary administration of perfluorooctylbromide nanoemulsion (encapsulated in PLGA-PEG nanocapsules, with an O2 accumulation capacity of 12 mL/dL) via aerosols achieved efficient O2 delivery in animals with acute lung injury and normalized pO2 levels 4 h after inhalation [135,136]. Although these materials have been studied in the lung injured rabbits, they may also exhibit the ability to perform gas exchange in COVID-19 patients because of their nanometric size (diameter of ∼200 nm). These structures may diffuse from the affected alveoli to the bloodstream to provide O2 for deoxyhemoglobin.
Another interesting study entrapped PFCs inside the membrane of red blood cells, generating nanosystems ∼150 nm in diameter with a total amount of 2 mg L−1 of dissolved O2 (4-fold higher than water) [137]. The system was able to preserve the viability of the neuroblastoma cell line, Neuro2a, for more than 18 h cultured under hypoxic conditions, hindering the expression of H1F1α and maintaining cell morphology as well as growth. The in vivo experiment revealed that the nanoformulation was able to revert a hemorrhagic shock model attributed to both the O2 delivery ability and the physicochemical characteristics induced by the cell membrane. Therefore, such alternatives have the potential to increase pO2 levels in COVID-19 cases because of the nanometric scale, as well as maintain biocompatibility for exhibiting the possibility of being prepared from the patient's own red blood cells.
The use of a nanosystem also allows transdermal oxygen therapy. This potential was reported by Magnetto et al. [138], that entrapped PFCs inside polymeric capsules and verified their performance in providing O2 in vitro and in vivo with or without ultrasound to enhance the skin permeation. This approach may be employed to provide O2 in critically ill COVID-19 patients locally or to increase nanosystem uptake by vital organs via focused ultrasound.
In general, it was possible to observe, by several studies presented, that the oxygenation therapy based on PFC nanoemulsions in the fight against hypoxia and tissue preservation is potentially viable, due to its solubility, affinity, stability, carrying capacity, among other characteristics. In addition, it is worth remembering its multifunctionality, not necessarily for the delivery of O2 but also for other molecules. However, nanostructures containing PFCs have limitations that prevent their clinical use. For example, as O2 nanobubbles, PFC nanoparticles tend to coalesce and generate micrometric or even millimeter structures to reduce Laplace pressure, which can cause microemboli to form [132]. This and other limitations (e.g., opsonization and clearance by lipoproteins) are under intense investigation to produce stable and safe formulations capable of providing O2 to critically ill patients, such as those affected by COVID-19.
6. Conclusion
COVID-19 highlighted several limitations exhibited by the current oxygen therapy techniques. In this review, we selected and discussed the most common and highly specific novel technological strategies that might mitigate, overcome or reduce undesirable impacts caused by ventilation and/or intubation methods. Apart from conventional techniques, the systems with greatest potential to improve O2 levels and perform gas exchange range from chemically modified hemoglobin to nanostructures containing perfluorocarbons enriched with O2. Although all display important features that may enhance O2 levels, most still require further elucidation in actual COVID-19 animal models and patients. Based on the discussed system's potential and the current scenario of COVID-19 pandemic, we expect the appearance of new alternatives able to perform hematosis in the forthcoming years.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors acknowledge the Pontifical Catholic University of Paraná (PUC-PR), Federal University of Paraná (UFPR), National Council for Teaching and Research (CNPq), and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We also thank Prof. Geraldo Picheth for invaluable advices throughout the research.
References
- 1.Serebrovska Z.O., et al. Hypoxia, HIF-1α, and COVID-19: from pathogenic factors to potential therapeutic targets. Acta Pharmacol. Sin. 2020;41(12):1539–1546. doi: 10.1038/s41401-020-00554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Østergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021;9(3) doi: 10.14814/phy2.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navas-Blanco J.R., Dudaryk R. Management of respiratory distress syndrome due to COVID-19 infection. BMC Anesthesiol. 2020;20(1):177. doi: 10.1186/s12871-020-01095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackermann M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhont S., et al. The pathophysiology of 'happy' hypoxemia in COVID-19. Respir. Res. 2020;21(1):198. doi: 10.1186/s12931-020-01462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gattinoni L., et al. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020;201(10):1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batah S.S., Fabro A.T. Pulmonary pathology of ARDS in COVID-19: a pathological review for clinicians. Respir. Med. 2021;176 doi: 10.1016/j.rmed.2020.106239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y., Wang L., Ben S. Meta-analysis of chest CT features of patients with COVID-19 pneumonia. J. Med. Virol. 2021;93(1):241–249. doi: 10.1002/jmv.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J.S., et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316–329. doi: 10.7150/thno.49713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagashima S., et al. Endothelial dysfunction and thrombosis in patients with COVID-19-brief report. Arterioscler. Thromb. Vasc. Biol. 2020;40(10):2404–2407. doi: 10.1161/ATVBAHA.120.314860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito G.N., et al. COVID‐19 pathophysiology and ultrasound imaging: a multiorgan review. J. Clin. Ultrasound. 2022;50(3):326–338. doi: 10.1002/jcu.23160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abou-Ismail M.Y., et al. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb. Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lax S.F., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome : results from a prospective, single-center, clinicopathologic case series. Ann. Intern. Med. 2020;173(5):350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer K., et al. SARS-CoV-2 spike protein induces paracrine senescence and leukocyte adhesion in endothelial cells. J. Virol. 2021;95(17):e0079421. doi: 10.1128/JVI.00794-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gadotti A.C., et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020;289 doi: 10.1016/j.virusres.2020.198171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alhazzani W., et al. Springer Berlin Heidelberg; 2020. Surviving Sepsis Campaign : Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Beltran W.F., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185(3):457–466.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modes M.E., et al. Clinical characteristics and outcomes among adults hospitalized with laboratory-confirmed SARS-CoV-2 infection during periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant predominance – one hospital, California, july 15-september 23, 2021, and december 21, 2021-january 27, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71(6):217–223. doi: 10.15585/mmwr.mm7106e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos L.L.d., Magro M.C.d.S. Escola Paulista de Enfermagem. Vol. 28. Universidade Federal de São Paulo; 2015. Ventilação mecânica e a lesão renal aguda em pacientes na unidade de terapia intensiva. [Google Scholar]
- 20.Ferreira S., et al. Revista Portuguesa de Pneumologia. 2009. Non-invasive ventilation; pp. 655–667. [DOI] [PubMed] [Google Scholar]
- 21.Whittle J.S., et al. Respiratory support for adult patients with COVID‐19. J. Am. Coll. Emerg. Physicians Open. 2020;1(2):95–101. doi: 10.1002/emp2.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter C., Aedy H., Notter J. COVID-19 disease: NonInvasive Ventilation and high frequency nasal oxygenation. Clin. Integr. Care. 2020;1 [Google Scholar]
- 23.Carvalho C.R.R.d., et al. 2021. Orientações Sobre a Otimização do Uso de Oxigênio e Suporte Ventilatório em Pacientes Graves com COVID-19. [Google Scholar]
- 24.Bertaina M., et al. Non-invasive ventilation for SARS-CoV-2 acute respiratory failure: a subanalysis from the HOPE COVID-19 registry. Emerg. Med. J. 2021;38(5):359–365. doi: 10.1136/emermed-2020-210411. [DOI] [PubMed] [Google Scholar]
- 25.Eden A. Noninvasive positive-pressure ventilation for respiratory failure after extubation. Surv. Anesthesiol. 2005;49(4):187–188. [Google Scholar]
- 26.Keenan S.P., et al. Which Patients with Acute Exacerbation of Chronic Obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation. Ann. Intern. Med. 2003;138:861–870. doi: 10.7326/0003-4819-138-11-200306030-00007. [DOI] [PubMed] [Google Scholar]
- 27.Miguel-Montanes R., et al. Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit. Care Med. 2015;43(3):574–583. doi: 10.1097/CCM.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 28.Kurtz P., et al. Evolving changes in mortality of 13,301 critically ill adult patients with COVID-19 over 8 months. Intensive Care Med. 2021;47(5):538–548. doi: 10.1007/s00134-021-06388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ing R.J., et al. Role of helmet-delivered noninvasive pressure support ventilation in COVID-19 patients. J. Cardiothorac. Vasc. Anesth. 2020;34(10):2575–2579. doi: 10.1053/j.jvca.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hui D.S., et al. Chest; 2015. Exhaled Air Dispersion during Noninvasive Ventilation via Helmets and a Total Facemask. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grieco D.L., et al. Phenotypes of patients with COVID-19 who have a positive clinical response to helmet noninvasive ventilation. Am. J. Respir. Crit. Care Med. 2022;205(3):360–364. doi: 10.1164/rccm.202105-1212LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz R., Heller D. StatPearls; 2021. Barotrauma and Mechanical Ventilation. [PubMed] [Google Scholar]
- 33.Manikant Lodaya S.R.S. High - flow nasal cannula: can it be a saviour in the present COVID - 19 pandemic? Air Water Pollut. Rep. 2020;3(2):57–59. [Google Scholar]
- 34.Azoulay E., et al. Acute respiratory failure in immunocompromised adults. Lancet Respir. Med. 2019;7(2):173–186. doi: 10.1016/S2213-2600(18)30345-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma S., et al. 2021. High Flow Nasal Cannula. [PubMed] [Google Scholar]
- 36.Guia M., et al. High-flow nasal oxygen therapy in acute hypoxemic respiratory failure: concise review on technology and initial methodology. Turk. Thorac. J. 2021;22(6):494–500. doi: 10.5152/TurkThoracJ.2021.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir. Care. 2016;61(4):529–541. doi: 10.4187/respcare.04577. [DOI] [PubMed] [Google Scholar]
- 38.Chaudhuri D., et al. Moderate certainty evidence suggests the use of high-flow nasal cannula does not decrease hypoxia when compared with conventional oxygen therapy in the peri-intubation period: results of a systematic review and meta-analysis. Crit. Care Med. 2020;48(4):571–578. doi: 10.1097/CCM.0000000000004217. [DOI] [PubMed] [Google Scholar]
- 39.Möller W., et al. Nasal high flow clears anatomical dead space in upper airway models. J. Appl. Physiol. 2015;118(12):1525–1532. doi: 10.1152/japplphysiol.00934.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grieco D.L., et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA. 2021;325(17):1731–1743. doi: 10.1001/jama.2021.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frat J.P., et al. Effect of high-flow nasal cannula oxygen vs standard oxygen therapy on mortality in patients with respiratory failure due to COVID-19: the SOHO-COVID randomized clinical trial. JAMA. 2022;328(12):1212–1222. doi: 10.1001/jama.2022.15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segovia B., et al. Combination therapy in patients with acute respiratory failure: high-flow nasal cannula and non-invasive mechanical ventilation. Arch. Bronconeumol. 2019;55(3):166–167. doi: 10.1016/j.arbres.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Artigas M., et al. High - flow nasal oxygen in patients with COVID - 19 - associated acute respiratory failure. Crit. Care. 2021:1–10. doi: 10.1186/s13054-021-03469-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J., Fink J.B., Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur. Respir. J. 2020;55(5) doi: 10.1183/13993003.00892-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calligaro G.L., et al. The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: a multi-centre prospective observational study. EClinicalMedicine. 2020;28:100570. doi: 10.1016/j.eclinm.2020.100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rochwerg B., et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure : a systematic review and meta - analysis. Intensive Care Med. 2019;45:563–572. doi: 10.1007/s00134-019-05590-5. [DOI] [PubMed] [Google Scholar]
- 47.Frat J. A Randomised Study (FLORALI Study) Clinical Trials; 2011. Clinical effect of the association of noninvasive ventilation and high flow nasal oxygen therapy in resuscitation of patients with acute lung injury. [Google Scholar]
- 48.Kang B.J., et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41(4):623–632. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 49.Cheung J.C., et al. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir. Med. 2020;8(4):e19. doi: 10.1016/S2213-2600(20)30084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J., Fink J.B., Ehrmann S. High-flow nasal cannula for COVID-19 patients: risk of bio-aerosol dispersion. Eur. Respir. J. 2020;56(4) doi: 10.1183/13993003.03136-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tobin M., Manthous C. Mechanical ventilation. Am. J. Respir. Crit. Care Med. 2017;196(2):3–4. doi: 10.1164/rccm.1962P3. [DOI] [PubMed] [Google Scholar]
- 52.Walter J.M., Corbridge T.C., Singer B.D. Invasive mechanical ventilation. South. Med. J. 2018;111(12):746–753. doi: 10.14423/SMJ.0000000000000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winck J.C., Scala R. Non-invasive respiratory support paths in hospitalized patients with COVID-19: proposal of an algorithm. Pulmonology. 2021;27(4):305–312. doi: 10.1016/j.pulmoe.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carrier F.M., et al. Effect of epidural analgesia in patients with traumatic rib fractures: a systematic review and meta-analysis of randomized controlled trials. Can. J. Anesth. 2009;56(3):230–242. doi: 10.1007/s12630-009-9052-7. [DOI] [PubMed] [Google Scholar]
- 55.Kurtz P., et al. Evolving changes in mortality of 13 , 301 critically ill adult patients with COVID - 19 over 8 months. Intensive Care Med. 2021;47(5):538–548. doi: 10.1007/s00134-021-06388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duncan S., et al. Interventions for oropharyngeal dysphagia in acute and critical care: a systematic review and meta-analysis. Intensive Care Med. 2020;46(7):1326–1338. doi: 10.1007/s00134-020-06126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalil A.C., et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin. Infect. Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beitler J.R., Malhotra A., Thompson B.T. Ventilator-induced lung injury. Clin. Chest Med. 2016;37(4):633–646. doi: 10.1016/j.ccm.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gattinoni L., et al. What has computed tomography taught us about the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2001;164(9):1701–1711. doi: 10.1164/ajrccm.164.9.2103121. [DOI] [PubMed] [Google Scholar]
- 60.Dos Santos C.C., Slutsky A.S. The contribution of biophysical lung injury to the development of biotrauma. Annu. Rev. Physiol. 2006;68:585–618. doi: 10.1146/annurev.physiol.68.072304.113443. [DOI] [PubMed] [Google Scholar]
- 61.Tremblay L., et al. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J. Clin. Invest. 1997;99(5):944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tremblay L.N., Slutsky A.S. Ventilator-induced lung injury from barotrauma to biotrauma. Proc. Assoc. Am. Phys. 1998;110(6):482–488. [PubMed] [Google Scholar]
- 63.Yoshida T., et al. Patient self-inflicted lung injury and positive end-expiratory pressure for safe spontaneous breathing. Curr. Opin. Crit. Care. 2020;26(1):59–65. doi: 10.1097/MCC.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 64.Sen Gupta A. Hemoglobin-based oxygen carriers: current state-of-the-art and novel molecules. Shock. 2019;52(1S Suppl 1):70–83. doi: 10.1097/SHK.0000000000001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferenz K.B., Steinbicker A.U. Artificial oxygen carriers-past, present, and future-a review of the most innovative and clinically relevant concepts. J. Pharmacol. Exp. Therapeut. 2019;369(2):300–310. doi: 10.1124/jpet.118.254664. [DOI] [PubMed] [Google Scholar]
- 66.Jia Y., Duan L., Li J. Hemoglobin-based nanoarchitectonic assemblies as oxygen carriers. Adv. Mater. 2016;28(6):1312–1318. doi: 10.1002/adma.201502581. [DOI] [PubMed] [Google Scholar]
- 67.Alayash A.I. Blood substitutes: why haven't we been more successful? Trends Biotechnol. 2014;32(4):177–185. doi: 10.1016/j.tibtech.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stowell C.P., et al. Progress in the development of RBC substitutes. Transfusion. 2001;41(2):287–299. doi: 10.1046/j.1537-2995.2001.41020287.x. [DOI] [PubMed] [Google Scholar]
- 69.Chen J.Y., Scerbo M., Kramer G. A review of blood substitutes: examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics. 2009;64(8):803–813. doi: 10.1590/S1807-59322009000800016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loscalzo J. Nitric oxide binding and the adverse effects of cell-free hemoglobins: what makes us different from earthworms. J. Lab. Clin. Med. 1997;129(6):580–583. doi: 10.1016/s0022-2143(97)90191-8. [DOI] [PubMed] [Google Scholar]
- 71.Spahn D.R., Kocian R. Artificial O2 carriers: status in 2005. Curr. Pharmaceut. Des. 2005;11(31):4099–4114. doi: 10.2174/138161205774913354. [DOI] [PubMed] [Google Scholar]
- 72.Dubé G.P., Pitman A.N., Mackenzie C.F. Relative efficacies of HBOC-201 and polyheme to increase oxygen transport compared to blood and crystalloids. Shock. 2019;52(1S Suppl 1):100–107. doi: 10.1097/SHK.0000000000001058. [DOI] [PubMed] [Google Scholar]
- 73.Rousselot M., Le Guen D., Zal F. Novel dissociation mechanism of a polychaetous annelid extracellular haemoglobin. FEBS J. 2006;273(7):1582–1596. doi: 10.1111/j.1742-4658.2006.05151.x. [DOI] [PubMed] [Google Scholar]
- 74.Le Pape F., et al. HEMOXCell, a new oxygen carrier useable as an additive for mesenchymal stem cell culture in platelet lysate-supplemented media. Artif. Organs. 2017;41(4):359–371. doi: 10.1111/aor.12892. [DOI] [PubMed] [Google Scholar]
- 75.Hosgood S.A., Nicholson M.L. The role of perfluorocarbon in organ preservation. Transplantation. 2010;89(10):1169–1175. doi: 10.1097/TP.0b013e3181da6064. [DOI] [PubMed] [Google Scholar]
- 76.Chaves R.C.F., et al. Extracorporeal membrane oxygenation: a literature review. Rev Bras Ter Intensiva. 2019;31(3):410–424. doi: 10.5935/0103-507X.20190063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang T.M. Pharmaceutical and therapeutic applications of artificial cells including microencapsulation. Eur. J. Pharm. Biopharm. 1998;45(1):3–8. doi: 10.1016/S0939-6411(97)00117-3. [DOI] [PubMed] [Google Scholar]
- 78.Hunt C.A., et al. Synthesis and evaluation of a prototypal artificial red cell. Science. 1985;230(4730):1165–1168. doi: 10.1126/science.4071041. [DOI] [PubMed] [Google Scholar]
- 79.Le Pape F., et al. Advancement in recombinant protein production using a marine oxygen carrier to enhance oxygen transfer in a CHO-S cell line. Artif. Cells, Nanomed. Biotechnol. 2015;43(3):186–195. doi: 10.3109/21691401.2015.1029632. [DOI] [PubMed] [Google Scholar]
- 80.Zal F., et al. Quaternary structure of the extracellular haemoglobin of the lugworm Arenicola marina: a multi-angle-laser-light-scattering and electrospray-ionisation-mass-spectrometry analysis. Eur. J. Biochem. 1997;243(1–2):85–92. doi: 10.1111/j.1432-1033.1997.85_1a.x. [DOI] [PubMed] [Google Scholar]
- 81.Przybelski R.J., et al. Phase I study of the safety and pharmacologic effects of diaspirin cross-linked hemoglobin solution. Crit. Care Med. 1996;24(12):1993–2000. doi: 10.1097/00003246-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 82.Lupon E., et al. Combating hypoxemia in COVID-19 patients with a natural oxygen carrier, HEMO(2)Life® (M101) Med. Hypotheses. 2021;146 doi: 10.1016/j.mehy.2020.110421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rousselot M., et al. Arenicola marina extracellular hemoglobin: a new promising blood substitute. Biotechnol. J. 2006;1(3):333–345. doi: 10.1002/biot.200500049. [DOI] [PubMed] [Google Scholar]
- 84.Le Gall T., et al. In vivo biodistribution and oxygenation potential of a new generation of oxygen carrier. J. Biotechnol. 2014;187:1–9. doi: 10.1016/j.jbiotec.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 85.Toulmond A., Tchernigovtzeff C. Ventilation and respiratory gas exchanges of the lugworm Arenicola marina (L.) as functions of ambient PO2 (20-700 torr) Respir. Physiol. 1984;57(3):349–363. doi: 10.1016/0034-5687(84)90083-5. [DOI] [PubMed] [Google Scholar]
- 86.Toulmond A. Blood oxygen transport and metabolism of the confined lugworm Arenicola marina (L.) J. Exp. Biol. 1975;63(3):647–660. doi: 10.1242/jeb.63.3.647. [DOI] [PubMed] [Google Scholar]
- 87.Weber R.E., Vinogradov S.N. Nonvertebrate hemoglobins: functions and molecular adaptations. Physiol. Rev. 2001;81(2):569–628. doi: 10.1152/physrev.2001.81.2.569. [DOI] [PubMed] [Google Scholar]
- 88.Eisenbrey J.R., et al. Development of an ultrasound sensitive oxygen carrier for oxygen delivery to hypoxic tissue. Int. J. Pharm. 2015;478(1):361–367. doi: 10.1016/j.ijpharm.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoon Y.I., et al. Ultrasound-mediated gene and drug delivery using a microbubble-liposome particle system. Theranostics. 2014;4(11):1133–1144. doi: 10.7150/thno.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abou-Saleh R.H., et al. Horizon: microfluidic platform for the production of therapeutic microbubbles and nanobubbles. Rev. Sci. Instrum. 2021;92(7):74105. doi: 10.1063/5.0040213. [DOI] [PubMed] [Google Scholar]
- 91.Black K.J., et al. Hemodynamic effects of lipid-based oxygen microbubbles via rapid intravenous injection in rodents. Pharm. Res. 2017;34(10):2156–2162. doi: 10.1007/s11095-017-2222-3. [DOI] [PubMed] [Google Scholar]
- 92.Picheth G.F., et al. S-nitrosothiol-terminated Pluronic F127: influence of microstructure on nitric oxide release. J. Colloid Interface Sci. 2020;576:457–467. doi: 10.1016/j.jcis.2020.05.049. [DOI] [PubMed] [Google Scholar]
- 93.Kheir J.N., et al. Bulk manufacture of concentrated oxygen gas-filled microparticles for intravenous oxygen delivery. Adv Healthc Mater. 2013;2(8):1131–1141. doi: 10.1002/adhm.201200350. [DOI] [PubMed] [Google Scholar]
- 94.Weiss C., et al. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 2020;14(6):6383–6406. doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- 95.Feshitan J.A., et al. Systemic oxygen delivery by peritoneal perfusion of oxygen microbubbles. Biomaterials. 2014;35(9):2600–2606. doi: 10.1016/j.biomaterials.2013.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang X., et al. Thrombus-targeted theranostic microbubbles: a new technology towards concurrent rapid ultrasound diagnosis and bleeding-free fibrinolytic treatment of thrombosis. Theranostics. 2016;6(5):726–738. doi: 10.7150/thno.14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Picheth G.F., et al. Chitosan-coated microvesicles: effect of polysaccharide-phospholipid affinity on decafluorobutane dissolution. Carbohydr. Polym. 2016;153:169–175. doi: 10.1016/j.carbpol.2016.07.099. [DOI] [PubMed] [Google Scholar]
- 98.de Saint Victor M., et al. Properties, characteristics and applications of microbubbles for sonothrombolysis. Expet Opin. Drug Deliv. 2014;11(2):187–209. doi: 10.1517/17425247.2014.868434. [DOI] [PubMed] [Google Scholar]
- 99.Matsuki N., et al. Oxygen supersaturated fluid using fine micro/nanobubbles. Int. J. Nanomed. 2014;9:4495–4505. doi: 10.2147/IJN.S68840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Akbar A., et al. Artificial intelligence and guidance of medicine in the bubble. Cell Biosci. 2021;11(1):108. doi: 10.1186/s13578-021-00623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kheir J.N., et al. Oxygen gas-filled microparticles provide intravenous oxygen delivery. Sci. Transl. Med. 2012;4(140):140ra88. doi: 10.1126/scitranslmed.3003679. [DOI] [PubMed] [Google Scholar]
- 102.Seekell R.P., et al. Oxygen delivery using engineered microparticles. Proc. Natl. Acad. Sci. U. S. A. 2016;113(44):12380–12385. doi: 10.1073/pnas.1608438113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kheir J.N., et al. Oxygen gas-filled microparticles provide intravenous oxygen delivery. Sci. Transl. Med. 2012;4(140):140ra88. doi: 10.1126/scitranslmed.3003679. [DOI] [PubMed] [Google Scholar]
- 104.Mountford P.A., et al. Colonic oxygen microbubbles augment systemic oxygenation and CO2 removal in a porcine smoke inhalation model of severe hypoxia. bioRxiv. 2021 doi: 10.1186/s40635-023-00517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kwan J.J., Borden M.A. Lipid monolayer dilatational mechanics during microbubble gas exchange. Soft Matter. 2012;8(17):4756–4766. [Google Scholar]
- 106.Valentin J.E., et al. Oxygen diffusivity of biologic and synthetic scaffold materials for tissue engineering. J. Biomed. Mater. Res. 2009;91(4):1010–1017. doi: 10.1002/jbm.a.32328. [DOI] [PubMed] [Google Scholar]
- 107.Wright C.I. The diffusion of carbon dioxide in tissues. J. Gen. Physiol. 1934;17(5):657–676. doi: 10.1085/jgp.17.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Graves J. Australian Academy of Science; 2017. The Similarities between Humans and Pigs. [Google Scholar]
- 109.Danhier F., et al. PLGA-based nanoparticles: an overview of biomedical applications. J. Contr. Release. 2012;161(2):505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 110.Zuo J., et al. Archives of Medical Research; 2015. WITHDRAWN: PLGA-Der P1 Vaccine Inhibited Tumor Growth in a Murine Model of Lung Cancer. [DOI] [PubMed] [Google Scholar]
- 111.Owen J., et al. Reducing tumour hypoxia via oral administration of oxygen nanobubbles. PLoS One. 2016;11(12):e0168088. doi: 10.1371/journal.pone.0168088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khan M.S., et al. Engineering oxygen nanobubbles for the effective reversal of hypoxia. Artif. Cells, Nanomed. Biotechnol. 2018;46(sup3):S318–s327. doi: 10.1080/21691401.2018.1492420. [DOI] [PubMed] [Google Scholar]
- 113.Chauhan G., et al. Nanotechnology for COVID-19: therapeutics and vaccine research. ACS Nano. 2020;14(7):7760–7782. doi: 10.1021/acsnano.0c04006. [DOI] [PubMed] [Google Scholar]
- 114.Afshari R., et al. Review of oxygenation with nanobubbles: possible treatment for hypoxic COVID-19 patients. ACS Appl. Nano Mater. 2021;4(11):11386–11412. doi: 10.1021/acsanm.1c01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Picheth G.F., et al. Ligand-mediated nanomedicines against breast cancer: a review. Nanomedicine. 2022;17(9):645–664. doi: 10.2217/nnm-2021-0473. [DOI] [PubMed] [Google Scholar]
- 116.Wigerup C., Påhlman S., Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol. Ther. 2016;164:152–169. doi: 10.1016/j.pharmthera.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 117.Yarin Y.M., et al. Argon protects hypoxia-, cisplatin- and gentamycin-exposed hair cells in the newborn rat's organ of Corti. Hear. Res. 2005;201(1):1–9. doi: 10.1016/j.heares.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 118.Khan M.S., et al. Engineering oxygen nanobubbles for the effective reversal of hypoxia. Artif. Cell Nanomed. Biotechnol. 2018;46(sup3):S318–S327. doi: 10.1080/21691401.2018.1492420. [DOI] [PubMed] [Google Scholar]
- 119.Song R., et al. pH-responsive oxygen nanobubbles for spontaneous oxygen delivery in hypoxic tumors. Langmuir. 2019;35(31):10166–10172. doi: 10.1021/acs.langmuir.8b03650. [DOI] [PubMed] [Google Scholar]
- 120.Song L., et al. Biogenic nanobubbles for effective oxygen delivery and enhanced photodynamic therapy of cancer. Acta Biomater. 2020;108:313–325. doi: 10.1016/j.actbio.2020.03.034. [DOI] [PubMed] [Google Scholar]
- 121.Fayyaz M., et al. Dextran-based oxygen nanobubbles for treating inner retinal hypoxia. ACS Appl. Nano Mater. 2021;4(7):6583–6593. [Google Scholar]
- 122.Bhandari P.N., et al. Oxygen nanobubbles revert hypoxia by methylation programming. Sci. Rep. 2017;7(1):1–14. doi: 10.1038/s41598-017-08988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Riess J.G. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif. Cells Blood Substit. Immobil. Biotechno. 2005;33(1):47–63. doi: 10.1081/bio-200046659. [DOI] [PubMed] [Google Scholar]
- 124.Jägers J., Wrobeln A., Ferenz K.B. Perfluorocarbon-based oxygen carriers: from physics to physiology. Pflueg. Arch. Eur. J. Physiol. 2021;473(2):139–150. doi: 10.1007/s00424-020-02482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rapoport N. Drug-loaded perfluorocarbon nanodroplets for ultrasound-mediated drug delivery. Adv. Exp. Med. Biol. 2016;880:221–241. doi: 10.1007/978-3-319-22536-4_13. [DOI] [PubMed] [Google Scholar]
- 126.Torres L.N., Spiess B.D., Torres Filho I.P. Effects of perfluorocarbon emulsions on microvascular blood flow and oxygen transport in a model of severe arterial gas embolism. J. Surg. Res. 2014;187(1):324–333. doi: 10.1016/j.jss.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 127.Leland C., Clark J., Gollan F. Survival of mammals breathing organic liquids equilibrated with oxygen at atmospheric pressure. Science. 1966;152(3730):1755–1756. doi: 10.1126/science.152.3730.1755. [DOI] [PubMed] [Google Scholar]
- 128.Farris A.L., Rindone A.N., Grayson W.L. Oxygen delivering biomaterials for tissue engineering. J. Mater. Chem. B. 2016;4(20):3422–3432. doi: 10.1039/C5TB02635K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lowe K.C. Perfluorochemicals in vascular medicine. Vascular Medicine Review. 1994;vmr-5(1):15–32. [Google Scholar]
- 130.Castro C.I., Briceno J.C. Perfluorocarbon-based oxygen carriers: review of products and trials. Artif. Organs. 2010;34(8):622–634. doi: 10.1111/j.1525-1594.2009.00944.x. [DOI] [PubMed] [Google Scholar]
- 131.Flaim S.F. Pharmacokinetics and side effects of perfluorocarbon-based blood substitutes. Artif. Cells Blood Substit. Immobil. Biotechno. 1994;22(4):1043–1054. doi: 10.3109/10731199409138801. [DOI] [PubMed] [Google Scholar]
- 132.Krafft M.P., Riess J.G. Therapeutic oxygen delivery by perfluorocarbon-based colloids. Adv. Colloid Interface Sci. 2021;294 doi: 10.1016/j.cis.2021.102407. [DOI] [PubMed] [Google Scholar]
- 133.Vercellotti G.M., et al. Activation of plasma complement by perfluorocarbon artificial blood: probable mechanism of adverse pulmonary reactions in treated patients and rationale for corticosteroids prophylaxis. Blood. 1982;59(6):1299–1304. [PubMed] [Google Scholar]
- 134.Spence R.K., et al. Perfluorocarbons as blood substitutes: the early years. Experience with Fluosol DA-20% in the 1980s. Artif. Cells Blood Substit. Immobil. Biotechno. 1994;22(4):955–963. doi: 10.3109/10731199409138794. [DOI] [PubMed] [Google Scholar]
- 135.Wang J., et al. High-performance reoxygenation from PLGA-PEG/PFOB emulsions: a feedback relationship between ROS and HIF-1α. Int. J. Nanomed. 2018;13:3027–3038. doi: 10.2147/IJN.S155509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yao Y., et al. Perfluorocarbon-encapsulated PLGA-PEG emulsions as enhancement agents for highly efficient reoxygenation to cell and organism. ACS Appl. Mater. Interfaces. 2015;7(33):18369–18378. doi: 10.1021/acsami.5b04226. [DOI] [PubMed] [Google Scholar]
- 137.Zhuang J., et al. Biomimetic nanoemulsions for oxygen delivery in vivo. Adv. Mater. 2018;30(49):e1804693. doi: 10.1002/adma.201804693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Magnetto C., et al. Ultrasound-activated decafluoropentane-cored and chitosan-shelled nanodroplets for oxygen delivery to hypoxic cutaneous tissues. RSC Adv. 2014;4(72):38433–38441. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.