Abstract
Objective
To investigate the clinical efficacy of intensity-modulated radiotherapy (IMRT) combined with transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC) patients with extrahepatic oligometastasis and the prognosis of patients receiving this treatment.
Patients and Methods
Twenty-one HCC patients with extrahepatic oligometastasis were retrospectively analyzed; seven patients received IMRT only, and 14 received IMRT plus TACE. TACE treatment was administered before IMRT (50 mg epirubicin, oxaliplatin 100 mg, and mitomycin 10 mg). The short-term efficacy of this treatment and patient prognosis were evaluated.
Results
Complete response (CR) and partial response (PR) in the intrahepatic region were achieved in three and 14 patients, respectively. The objective response rate (ORR) approached 81%. CR and PR were achieved in six and 10 patients with extrahepatic metastases, respectively, for an ORR of 100%. Pain was completely relieved in all patients with bone metastases. The median overall survival (OS) and progression-free survival (PFS) were 21 months and 9.1 months, respectively. The 1-year PFS rate was 43%, and the 1-, 2-, 3-, and 4-year OS rates were 83%, 35%, 9%, and 4%, respectively. Univariate analysis showed that the prognostic factors for patient survival included Child–Pugh class, vascular thrombus, Karnofsky performance status (KPS), radiotherapy dose, ascites, combination therapy, and pattern of progression. Multivariate analysis showed that vascular thrombus, combination therapy, and pattern of failure were prognostic factors for PFS, and the KPS was the only prognostic factor for OS. No grade 3–4 adverse reactions were observed.
Conclusion
IMRT combined with TACE is safe and feasible without major toxicities for the treatment of advanced HCC patients with extrahepatic oligometastasis and results in excellent objective efficacy and a potential survival benefit. The KPS is the only predictive factor for OS. This approach is expected to be a useful palliative option for selected HCC patients with extrahepatic metastases.
Keywords: hepatocellular carcinoma, metastasis, radiotherapy, transcatheter arterial chemoembolization, prognosis
Introduction
Liver cancer was the sixth most commonly diagnosed cancer and the third leading cause of cancer-related death worldwide in 2020, and eastern Asia has a high incidence.1 Surgical resection is the preferred treatment for early-stage liver cancer, whereas ablation with radiofrequency ablation (RFA) and stereotactic ablative radiotherapy (SABR) remains the primary intervention for nonsurgical early-stage liver cancer.2,3 Unfortunately, patients are often in an advanced stage and may even have extrahepatic metastasis at diagnosis. Radiotherapy appears to be beneficial for patients with extrahepatic metastases.4,5 Transcatheter arterial chemoembolization (TACE) is commonly used to treat patients with unresectable hepatocellular carcinoma,6 and external radiotherapy can improve the efficacy against foci with poor lipiodol deposition after TACE. On the other hand, TACE also contributes to eliminating residual lesions after radiotherapy. Compared to TACE alone, radiotherapy combined with TACE has been shown to improve the prognosis of hepatocellular carcinoma (HCC) patients.7,8 Whether the efficacy of radiotherapy plus TACE is superior to that of radiotherapy alone is unclear. In the current study, we investigated the efficacy of this combined treatment in patients with extrahepatic oligometastatic HCC and the prognosis of patients receiving this treatment. Specifically, administration of IMRT with or without TACE for treating primary liver foci and IMRT for treating extrahepatic metastatic lesions was assessed.
Patients and Methods
Patient Selection and General Information
The inclusion criteria were as follows: stage IVb HCC diagnosed by pathology or imaging based on the guidelines of the seventh version of the American Joint Committee on Cancer guidelines (AJCC 7th edition). The clinical diagnostic criteria were based on the expert consensus on standardization of the management of primary HCC developed by the Chinese Society of Liver Cancer and the Chinese Anti-Cancer Association in 2009.9 According to the patient’s symptoms and signs combined with computed tomography (CT) or magnetic resonance imaging (MRI), patients had to have one of the following metastatic sites: 1) pulmonary space-occupying lesions on imaging, numbering ≤3; 2) space-occupying lesions in the adrenal gland; 3) mediastinal/supraclavicular lymph node metastases; 4) bone metastases; and 5) brain metastases (≤3).
The exclusion criteria were as follows: intrahepatic metastasis; previous systemic therapy; multiple extrahepatic metastases (metastases in >2 organs or >3 foci within solitary organs); and interrupting treatment.
Ethics and Consent
The study protocol complied with the Declaration of Helsinki guidelines and was approved by the ethics committee of Hainan Cancer Hospital, Affiliated Hospital of Hainan Medical University, Haikou, China. Each patient provided informed consent.
Treatment Methods
Precise Radiotherapy
Simulated positioning: The patient was in the supine position and was fixed with a vacuum pad. A 64-slice enhanced CT scan was performed under calm breathing, with a thickness of 5 mm. A precise planning system was used for planning design. An Elekta 1591 linear accelerator was used for 6-MV X–irradiation.
Determination of the target areas: The gross tumor volume (GTV) was the tumor range determined by CT or MRI images. The planning target volume (PTV) of HCC was 1–1.5 cm outside the GTV. The PTV of the metastatic tumor was 0.5–1 cm outside the GTV. The target volume was modified according to the anatomical structure. The isodose lines of 95% and above covered the PTV. The treatment regimens were comprehensively evaluated using the dose-volume histogram and the isodose line.
Radiotherapy fraction (f) and dose regimen: liver lesion PTV (Dt 44–66 Gy, 22–33 f, 2 Gy/f; or 36–45 Gy, 12–15 f, 3 Gy/f), lung metastasis PTV (Dt 54–66 Gy, 27–33 f, 2 Gy/f), bone metastasis PTV (Dt 40–55 Gy, 20–25 f, 2 Gy/f), and adrenal metastasis PTV (Dt 50–56 Gy, 20–25 f, 2 Gy/f). Radiotherapy was administered 5 days per week with a daily fraction.
Hepatic TACE
Digital subtraction angiography (DSA) was performed to visualize the lesions, and TACE was performed on HCC lesions with an abundant blood supply. Lipiodols and drugs (oxaliplatin 100 mg, epirubicin 60 mg, mitomycin 10 mg) were infused, and TACE was repeated every 21 days for a total of 1–2 cycles.
Other Treatments
For patients with hepatitis B, radiotherapy or TACE was performed when HBV-DNA was reduced to ≤ 103 by antiviral therapy, and the treatment was maintained for 1 year. Splenic embolization was given to patients with hypersplenism whose cytopenia was ≥ II°.
Follow-Up
Blood count, liver and kidney function, HBV-DNA, α-fetoprotein (AFP), CT or MRI were examined during treatment and follow-up, and the last follow-up time was June 2015. The causes of patient death or disease progression were classified as intrahepatic progression, extrahepatic progression, and other causes. The overall survival (OS) and progression-free survival (PFS) were calculated as the time from diagnosis to death or last follow-up.
Acute Reactions and Criteria for Efficacy Evaluation
Acute Reactions
Acute radiation injury was determined according to the criteria of the Radiation Therapy Oncology Group (RTOG): bone marrow suppression, gastrointestinal reactions, and radiation-induced liver and lung injury.
Efficacy Evaluation Criteria
Short-term efficacy evaluation was performed according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1). Abdominal CT or MRI scans were performed 2–3 months after radiotherapy, followed by reexamination every 2–3 months.
Statistical Methods
Statistical analysis was performed with SPSS 23.0. The Kaplan–Meier method was used to calculate OS and PFS. Multivariate analysis was performed using Cox risk regression. Due to the small sample size, factors with p≤0.05 in univariate analysis were taken as covariates.
Results
Follow-Up and Treatment
Twenty-one patients with oligometastatic liver cancer between April 2010 and December 2014 were analyzed; there were 19 males and 2 females, with a median age of 56 years (Table 1). The median follow-up time was 23 months (3–55 months), and the follow-up rate was 100%. The baseline characteristics of the patients are shown in Table 1. There were 14 (67%), 5 (24%) and 2 (9%) patients with Child–Pugh classifications of class A, B, and C, respectively. Fifteen and 6 patients received IMRT plus TACE or IMRT alone, respectively.
Table 1.
Analysis of Baseline Characteristics of Patients
| Patient Characteristics | n | Median PFS | P | Median OS | P | |
|---|---|---|---|---|---|---|
| Age | ≤50 years old | 6 | 6 | 0.654 | 21 | 0.721 |
| >50 years old | 15 | 13 | 21 | |||
| Sex | Male | 19 | 10 | 0.396 | 20 | 0.488 |
| Female | 2 | 5 | 15 | |||
| Child-Pugh class | A | 14 | 12 | 0.012 | 21 | 0.003 |
| B | 5 | 9 | 8 | |||
| C | 2 | 2 | - | |||
| Tumor thrombus | Yes | 5 | 3 | 0.003 | 24 | 0.598 |
| None | 16 | 12 | 21 | |||
| KPS | >70 | 11 | 13 | 0.028 | 28 | 0.001 |
| 60–70 | 10 | 3 | 13 | |||
| Liver cirrhosis | Yes | 14 | 8 | 0.153 | 20 | 0.2 |
| No | 7 | 12 | 24 | |||
| Combination therapy | Yes | 14 | 10 | 0.298 | 24 | 0.038 |
| No | 7 | 6 | 20 | |||
| Metastatic site | Lung | 10 | 6 | 0.149 | 13 | 0.194 |
| Mediastinal/supraclavicular | 3 | 6 | - | |||
| Brain | 1 | 17 | - | |||
| Adrenal gland | 2 | 3 | - | |||
| Bone | 5 | 13 | 8 | |||
| Radiotherapy dose | ≤52Gy | 8 | 6 | 0.007 | 13 | 0.038 |
| >52Gy | 13 | 12 | 21 | |||
| Failure mode | Intrahepatic | 9 | 9 | 0.533 | 14 | 0.032 |
| Extrahepatic | 9 | 10 | 28 | |||
| Ascites | Yes | 7 | 3 | 0.001 | 13 | 0.001 |
| None | 14 | 12 | 24 | |||
Abbreviations: PFS, progression-free survival; OS, overall survival; KPS, Karnofsky performance status; CIK, cytokine-induced killer.
Evaluation of Short-Term Efficacy
Complete response (CR) was obtained in three patients with intrahepatic lesions, partial response (PR) was reached in 14 patients, and the ORR was 81%. Three patients had stable disease (SD), and one patient exhibited recurrence within 6 months after treatment. For extrahepatic metastases, CR and PR were achieved in six and 10 patients, respectively, and the ORR was 100%. Pain was relieved in all five patients with bone metastases.
Failure Mode and Survival Score
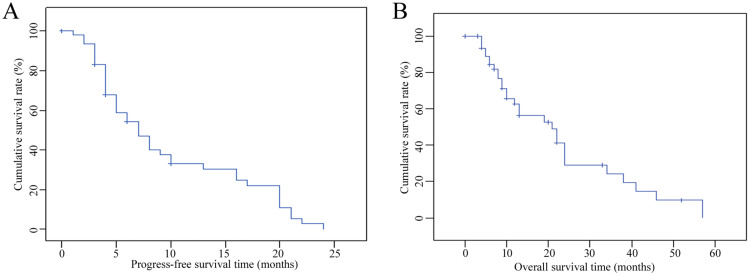
At the end of follow-up, 18 patients had died, and three patients had survived. The main cause of death was intrahepatic progression. There were nine patients with intrahepatic metastasis (50%), among which one patient developed diffuse intrahepatic metastasis. Nine patients were found to have extrahepatic metastasis (50%), and the deaths of the other two patients were not related to metastasis. The median PFS time was 9 months, the median OS was 21 months, the 1-year PFS rate was 43%, and the 1-, 2-, 3-, and 4-year OS rates were 83%, 35%, 9%, and 4%, respectively (Figures 1a and b).
Figure 1.
(a) PFS-vs.-time curves of patients. (b) OS-vs.-time curves of patients.
Analysis of Prognostic Factors
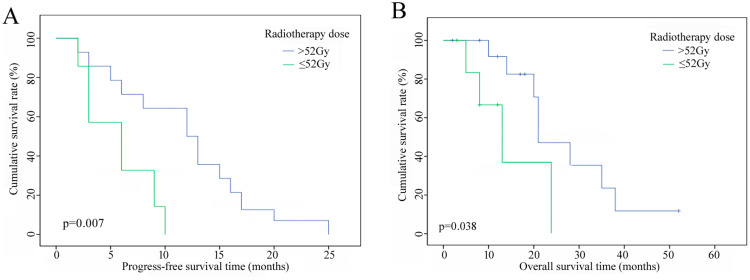
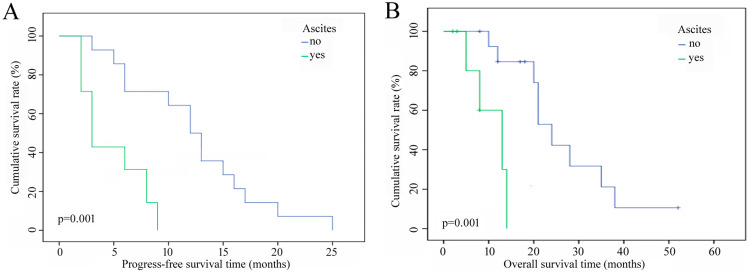
Univariate analysis showed that Child–Pugh class, portal vein tumor thrombus (PVTT), KPS score, radiotherapy dose (Figures 2a and b), and ascites (Figures 3a and b) were factors that affected the PFS and OS of patients. Combination therapy and intrahepatic or extrahepatic progression were also related to OS. The median OS of patients receiving IMRT plus TACE was better than that of patients who underwent IMRT alone (24 vs 20 months), and the median OS of patients with intrahepatic metastases (14 months) was significantly lower than that of patients with extrahepatic metastases (28 months). Multivariate analysis showed that PVTT, combination therapy, and pattern of progression were prognostic factors for PFS. The only prognostic factor for OS was the KPS score; on the other hand, radiotherapy dose was a potential influencing factor (p=0.059) (Table 2).
Figure 2.
(a) PFS curves of patients with different doses of radiotherapy. (b) OS curves of patients with different doses of radiotherapy.
Figure 3.
(a) PFS curves of patients with vs without ascites. (b) OS curves of patients with vs without ascites.
Table 2.
Multivariate Survival Analysis of Patients
| Patient Condition | n | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| PFS | OS | |||||
| RR | p | RR | p | |||
| Child–Pugh class | A | 14 | 1.72 | 0.511 | 3.229 | 0.072 |
| B | 5 | |||||
| C | 2 | |||||
| Tumor thrombus | Yes | 5 | 94.9 | 0 | 0.03 | 0.863 |
| No | 16 | |||||
| KPS score | >70 | 11 | 11.567 | 0.093 | 8.8 | 0.003 |
| 60–70 | 10 | |||||
| Treatment | Radiotherapy + TACE | 14 | 9.14 | 0.048 | 3.092 | 0.079 |
| Radiotherapy | 7 | |||||
| Radiotherapy dose | ≤52 Gy | 8 | 0.825 | 0.83 | 3.572 | 0.059 |
| >52 Gy | 13 | |||||
| Failure model | Intrahepatic | 9 | 0.111 | 0.009 | 1.953 | 0.162 |
| Extrahepatic | 9 | |||||
| Ascites | Yes | 7 | 25.9 | 0.019 | 21.32 | 0.007 |
| No | 14 | |||||
Note: P < 0.05 was considered statistically significant.
Toxic Side Effects
During the treatment, 16 patients had mild anorexia, nausea, and other discomfort, and three patients had mild chest tightness and shortness of breath. These side effects were all relieved after medical treatment. There were no grade III–IV reactions in this study and no discontinuation of treatment due to toxic side effects. All patients who received TACE developed fever (38.5°C) within 3 to 5 days after treatment and had abnormal transaminase levels within 1 week. They all recovered after medical treatment.
Discussion
The incidence of extrahepatic metastasis for patients with HCC was approximately 13.5–41%,10 and the median OS of most patients was less than 6 months.10 The continuously updated and optimized management of HCC has substantially improved the prognosis over the past decade.11 Local intervention, including surgery, radiotherapy, especially stereotactic body radiotherapy (SBRT) or SABR, and RFA, has been shown to improve the prognosis of HCC patients with extrahepatic metastases.12–16 In this study, IMRT combined with or without TACE was used to treat HCC patients with extrahepatic oligometastasis. The short-term ORR was 81% for intrahepatic lesions and 100% for extrahepatic metastases, and the pain remission rate for bone metastasis was 100%. The results were similar to a Korean study in which helical tomotherapy was used to treat advanced HCC patients with metastasis. It was reported that 45.2% of patients with intrahepatic metastases exhibited objective remission after radiotherapy, approximately 67% of patients with extrahepatic metastases exhibited objective remission, and six patients (31.3%) exhibited CR.17 In addition, in the current study, the median PFS and OS were 9 and 21 months, respectively. The PFS rate at 12 months was 43%, and the OS rates at 12, 24, 36, and 48 months were 83%, 35%, 9%, and 4%, respectively. In general, the ORR and prognosis of the above Korean study were significantly lower than those in the current study, which was related to baseline features. The Korean study included patients with intrahepatic metastasis and multiple metastases, and only 21.4% (9/42) of patients had extrahepatic metastasis without intrahepatic foci; moreover, 21.4% of patients had two or more organ metastases, 78.8% of patients had intrahepatic lesions and extrahepatic metastases, and the incidence of PVTT was approximately 72.7%.17 The controllability of intrahepatic lesions is an important prognostic factor in patients with advanced HCC and extrahepatic metastasis,18 and PVTT is an adverse prognostic factor in the international staging systems (BCLC, AJCC).19 Radiotherapy is effective and safe in HCC with PVTT.20–25 Furthermore, the radiotherapy dose is tightly related to prognosis in patients with HCC,15,26–28 and a biologically effective dose (BED) ≥ 72 Gy is significantly related to PFS and OS.28 A high BED for patients with HCC bone metastases is associated with prolonged OS,15,26 and a high BED results in pain relief and excellent local control, which improves the KPS score and prognosis.15,28
Surgical resection was applied in HCC patients with extrahepatic metastasis, and it was demonstrated that the 1- and 3-year postoperative OS rates of HCC patients with lung metastasis were 24% and 7%, respectively; the median OS of patients with lung, bone, and brain metastases was 26.5–32 months, 10 months, and 6.1 months, respectively.29 Heterogeneity was found between the surgery and radiotherapy groups in the current study and was associated with multiple factors. First, there was selection bias, as patients who are not suitable for surgery naturally have a poor prognosis.29 Second, there was heterogeneity at baseline, such as in stage, intrahepatic lesions, and PVTT. Finally, there are various metastasis target organs, most patients undergo surgery for lung metastases, and the prognosis of patients with pulmonary metastasis resection is better than that of patients with metastases to other sites.12 Additionally, the findings of the current study should be interpreted in view of the following limitations: retrospective analysis can influence the reliability, and the small sample size and delayed timeliness can produce various biases.
Nevertheless, in recent decades, targeted therapy and immune checkpoint inhibitors have been extensively administered to patients with advanced HCC,11 and local control combined treatment provides a future for patients with advanced HCC.20,30–32
Conclusions
In summary, this study suggests that precision radiotherapy combined with TACE for the treatment of advanced HCC patients with extrahepatic oligometastasis yields a good objective remission rate and survival benefit and is an ideal palliative treatment option. The limitations of this study were the small sample size, retrospective nature of the study, and selection bias. Prospective clinical trials are necessary to provide more reliable evidence for improving the value of radiotherapy in the treatment of advanced HCC.
Acknowledgments
This research was supported by grants from the Science Foundation of Hainan Cancer Hospital (grant #2022BS02).
Funding Statement
There is no funding to report.
Ethical Considerations
This study was approved by the Ethics Committee of Hainan Cancer Hospital.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
All authors have declared no conflicts of interest and have not accepted any improper positions or financial incentives for this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2021;71:209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Rajyaguru DJ, Borgert AJ, Smith AL, et al. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: analysis of the national cancer database. J Clin Oncol. 2018;36(6):600–608. doi: 10.1200/JCO.2017.75.3228 [DOI] [PubMed] [Google Scholar]
- 3.Kim N, Cheng J, Jung I, et al. Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. J Hepatol. 2020;73(1):121–129. doi: 10.1016/j.jhep.2020.03.005 [DOI] [PubMed] [Google Scholar]
- 4.He J, Zeng Z-C, Tang Z-Y, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer. 2009;115(12):2710–2720. doi: 10.1002/cncr.24300 [DOI] [PubMed] [Google Scholar]
- 5.Zhou L-Y, Zeng Z-C, Fan J, et al. Radiotherapy treatment of adrenal gland metastases from hepatocellular carcinoma: clinical features and prognostic factors. BMC Cancer. 2014;14:878. doi: 10.1186/1471-2407-14-878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raoul J-L, Forner A, Bolondi L, Cheung TT, Kloeckner R, De Baere T. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36. doi: 10.1016/j.ctrv.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 7.Zeng Z-C, Tang Z-Y, Fan J, et al. A comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinoma. Cancer J. 2004;10(5):307–316. doi: 10.1097/00130404-200409000-00008 [DOI] [PubMed] [Google Scholar]
- 8.Huo YR, Eslick GD. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2015;1(6):756–765. doi: 10.1001/jamaoncol.2015.2189 [DOI] [PubMed] [Google Scholar]
- 9.Binghui Y, Wenming C, Xiaojun Z, et al. 原发性肝癌规范化诊治的专家共识 [Expert consensus on standardized diagnosis and treatment of primary liver cancer]. 内科理论与实践. 2009;4(04):353–360. [Google Scholar]
- 10.Uka K, Aikata H, Takaki S, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13(3):414–420. doi: 10.3748/wjg.v13.i3.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–1917. doi: 10.1016/S0140-6736(03)14964-1 [DOI] [PubMed] [Google Scholar]
- 12.Zhan H, Zhao X, Lu Z, Yao Y, Zhang X. Correlation and survival analysis of distant metastasis site and prognosis in patients with hepatocellular carcinoma. Front Oncol. 2021;11:652768. doi: 10.3389/fonc.2021.652768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kai M, Jie W. 肝癌合并肝外转移的外科治疗现状 [Present treatment situation of hepatocellular carcinoma with extrahepatic metastasis]. 中华外科杂志 [Zhonghua Wai Ke Za Zhi]. 2019;57(06):466–470. [DOI] [PubMed] [Google Scholar]
- 14.Yoon JH, Goo YJ, Lim C-J, et al. Features of extrahepatic metastasis after radiofrequency ablation for hepatocellular carcinoma. World J Gastroenterol. 2020;26(32):4833–4845. doi: 10.3748/wjg.v26.i32.4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung I-H, Yoon SM, Kwak J, et al. High-dose radiotherapy is associated with better local control of bone metastasis from hepatocellular carcinoma. Oncotarget. 2017;8(9):15182–15192. doi: 10.18632/oncotarget.14858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutiontov SI, Pitroda SP, Weichselbaum RR. Oligometastasis: past, Present, future. Int J Radiat Oncol Biol Phys. 2020;108(3):530–538. doi: 10.1016/j.ijrobp.2020.02.019 [DOI] [PubMed] [Google Scholar]
- 17.Jang JW, Kay CS, You CR, et al. Simultaneous multitarget irradiation using helical tomotherapy for advanced hepatocellular carcinoma with multiple extrahepatic metastases. Int J Radiat Oncol Biol Phys. 2009;74(2):412–418. doi: 10.1016/j.ijrobp.2008.08.034 [DOI] [PubMed] [Google Scholar]
- 18.Uchino K, Tateishi R, Shiina S, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117(19):4475–4483. doi: 10.1002/cncr.25960 [DOI] [PubMed] [Google Scholar]
- 19.Zhang X-P, Gao Y-Z, Chen Z-H, et al. An eastern hepatobiliary surgery hospital/portal vein tumor thrombus scoring system as an aid to decision making on hepatectomy for hepatocellular carcinoma patients with portal vein tumor thrombus: a multicenter study. Hepatology. 2019;69(5):2076–2090. doi: 10.1002/hep.30490 [DOI] [PubMed] [Google Scholar]
- 20.Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9(6):682–720. doi: 10.1159/000509424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu J, Zhang X-P, Zhong B-Y, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol. 2019;4(9):721–730. doi: 10.1016/S2468-1253(19)30178-5 [DOI] [PubMed] [Google Scholar]
- 22.Wei X, Jiang Y, Zhang X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol. 2019;37(24):2141–2151. doi: 10.1200/JCO.18.02184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallemeier CL, Apisarnthanarax S, Dawson LA. BCLC 2022 update: important advances, but missing external beam radiotherapy. J Hepatol. 2022;76(5):1237–1239. doi: 10.1016/j.jhep.2021.12.029 [DOI] [PubMed] [Google Scholar]
- 24.Jihye C, Jinsil S. Application of radiotherapeutic strategies in the BCLC-defined stages of hepatocellular carcinoma. Liver Cancer. 2012;1(3–4):216–225. doi: 10.1159/000343836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rim CH, Cheng J, Huang W-Y, et al. An evaluation of hepatocellular carcinoma practice guidelines from a radiation oncology perspective. Radiother Oncol. 2020;148:73–81. doi: 10.1016/j.radonc.2020.03.027 [DOI] [PubMed] [Google Scholar]
- 26.Choi Y, Kim J, Lee I, Seong J. Dose escalation using helical tomotherapy improves local control in spine metastases from primary hepatic malignancies. Liver Int. 2014;34(3):462–468. doi: 10.1111/liv.12260 [DOI] [PubMed] [Google Scholar]
- 27.Kim N, Cheng J, Huang W-Y, et al. Dose-response relationship in stereotactic body radiation therapy for hepatocellular carcinoma: a pooled analysis of an Asian liver radiation therapy group study. Int J Radiat Oncol Biol Phys. 2021;109(2):464–473. doi: 10.1016/j.ijrobp.2020.09.038 [DOI] [PubMed] [Google Scholar]
- 28.Byun HK, Kim HJ, Im YR, Kim DY, Han K-H, Seong J. Dose escalation by intensity modulated radiotherapy in liver-directed concurrent chemoradiotherapy for locally advanced BCLC stage C hepatocellular carcinoma. Radiother Oncol. 2019;133:1–8. doi: 10.1016/j.radonc.2018.12.025 [DOI] [PubMed] [Google Scholar]
- 29.Kuo T-M, Chang K-M, Cheng T-I, Kao K-J. Clinical factors predicting better survival outcome for pulmonary metastasectomy of hepatocellular carcinoma. Liver Cancer. 2017;6(4):297–306. doi: 10.1159/000477134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Association for the Study of the Liver, European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 31.Vogel A, Cervantes A, Chau I, et al.; ESMO Guidelines Committee. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl4):iv238–iv255. doi: 10.1093/annonc/mdy308 [DOI] [PubMed] [Google Scholar]
- 32.Galun D, Mijac D, Filipovic A, Bogdanovic A, Zivanovic M, Masulovic D. Precision medicine for hepatocellular carcinoma: clinical perspective. J Pers Med. 2022;12(2):149. doi: 10.3390/jpm12020149 [DOI] [PMC free article] [PubMed] [Google Scholar]