Abstract
PANoptosis is a new cell death proposed by Malireddi et al in 2019, which is characterized by pyroptosis, apoptosis and necroptosis, but cannot be explained by any of them alone. The interaction between pyroptosis, apoptosis and necroptosis is involved in PANoptosis. In this review, from the perspective of PANoptosis, we focus on the relationship between pyroptosis, apoptosis and necroptosis, the key molecules in the process of PANoptosis and the formation of PANoptosome, as well as the role of PANoptosis in diseases. We aim to understand the mechanism of PANoptosis and provide a basis for targeted intervention of PANoptosis-related molecules to treat human diseases.
Keywords: PANoptosis, pyroptosis, apoptosis, necroptosis
Introduction
Regulated cell death plays an important role in host defense. Pyroptosis, apoptosis and necroptosis are three types of regulated cell death. Recent studies have found that necroptosis, apoptosis and pyroptosis are superimposed among each other, thus a concept of total cell death called PANoptosis (P, pyroptosis; A, apoptosis; N, necroptosis) has been proposed.1 Studies have reported that PANoptosis is involved in many diseases, including infectious diseases and tumor diseases.2,3 Therefore, it is important to understand the mechanism of PANoptosis and provide treatment for human diseases. PANoptosis is regulated by a cascade of upstream receptor and molecular signals, which assemble into polymeric complexes known as PANoptosome.4 PANoptosis is a new cell death proposed by Malireddi et al1 in 2019, which is characterized by necroptosis, apoptosis and pyroptosis, but cannot be explained by any of them alone.5,6 In the following text, we provide a brief review of pyroptosis, apoptosis, and necroptosis to help understand the fundamental mechanism of PANoptosis.
Pyroptosis involves classical signaling pathway mediated by cysteinyl aspartate specific proteinase-1 (caspase-1) and non-classical signaling pathway mediated by caspase-4, 5, 11 (human caspase-11 is a murine ortholog of caspase-4/5).7 Activation of caspase-1 is mediated by inflammasome, which binds to caspase-1 and cleaves caspase-1 precursor into catalytically active caspase-1. Caspase-1 activates downstream molecules, forming pores in cell envelope and destroys the osmotic pressure balance, resulting in ion imbalance on the envelope, leading to cell swelling, lysis and death.8 In addition, activated caspase-1 can also cleave interleukin-1β (IL-1β) and IL-18 precursor, promoting IL-1β and IL-18 release, mediating pyroptosis.9 The non-classical pyroptosis inflammasome pathway is also known as the non-caspase-1-dependent cell death, which is mainly mediated by caspase-4, 5, 11. After activation of caspase-4, 5, 11, the cleavage of gasdermin D (GSDMD) causes pyroptosis, or the Pannexin-1 channel is opened under stimulation, the release of adenosine triphosphate (ATP) leads to ion imbalance, and then the pyroptosis process is initiated.10
There are three ways of apoptosis: endogenous apoptosis, exogenous apoptosis and endoplasmic reticulum stress. Endogenous apoptosis pathway is also known as mitochondrial apoptosis pathway. After stimulation, the outer membrane of mitochondria is permeated, which promotes the release of cytochrome C (cyto-C). Cyto-C activates caspase-9, and then, caspase-3, 6, 7 are activated, leading to cell apoptosis.11 The initiation of mitochondrial apoptosis is regulated by B-cell lymphoma-2 (Bcl-2) family proteins.12 Exogenous apoptosis pathway is mainly mediated by the interaction of receptors and ligands to transmit apoptosis signals. Receptors bind to caspase-8 precursor through their Fas-associating protein with a novel death domain (FADD) or tumor necrosis factor (TNF) receptor 1 associated via death domain (TRADD), and then activate caspase-8, which further activates caspase-3, 6, 7 to induce apoptosis.13,14 Endoplasmic reticulum stress apoptosis pathway is a relatively new apoptosis regulatory pathway. When the body experiences endoplasmic reticulum stress due to various factors, the system will respond to endoplasmic reticulum stress through unfolded protein response. The unfolded protein response is activated by three endoplasmic reticulum stress integrated proteins: inositol-requiring enzyme 1 (IRE1), protein kinase R-like endoplasmic reticulum kinase (PERK) and activating transcription factor 6 (ATF6).15 The interaction between IRE1 and adaptor protein TNF receptor associated factor 2 (TRAF2) leads to the activation of Jun n-terminal kinase (JNK), which promotes the expression of pro-apoptosis protein Bcl-2 associated death promoter (Bad), then inducing cell apoptosis.16 PERK promotes the phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) and activates the translation of ATF4, which can promote C/EBP-homologous protein (CHOP) expression, an endoplasmic reticulum stress protein.17 CHOP promotes apoptosis by upregulating pro-apoptosis proteins Bcl-2 associated X protein (Bax) and Bcl-2 antagonist/killer (Bak).
Necroptosis is regulated by receptor-interacting serine/threonine-protein kinase 1 (RIP1), RIP3 and mixed lineage kinase domain-like (MLKL). RIP1 and RIP3 combine to form a complex called necrosome, which can induce RIP3 auto phosphorylation. And then RIP3 phosphorylates MLKL protein, induces the formation of MLKL oligomers and translocation to cell membrane, resulting in rupture and eventually cell destruction.18
In this review, from the perspective of PANoptosis, we focus on the relationship between necroptosis, apoptosis and pyroptosis, the key molecules in the process of PANoptosis and the formation of PANoptosome, as well as the role of PANoptosis in diseases. Studies have reported that PANoptosis is involved in many diseases, including infectious diseases and tumor diseases.2,3 We aim to understand the mechanism of PANoptosis and provide a basis for targeted PANoptosis to treat human diseases.
The Interaction Between Pyroptosis, Apoptosis and Necroptosis
For a long time, it has been considered that cell death pathways operate in parallel, without overlap. However, it is clear that pyroptosis, apoptosis and necroptosis are closely linked and can regulate each other.19,20 In the rat sepsis associated encephalopathy model, treatment of apoptosis inhibitor could suppress pyroptosis and activate necroptosis. Pyroptosis inhibitor could suppress pyroptosis and inhibit apoptosis, but activate necroptosis.21 However, both apoptosis and pyroptosis were activated when necroptosis was inhibited.21 The differences between pyroptosis, apoptosis and necroptosis, including the mechanisms of membrane damage and subcellular localization, have been widely reported and no more repeat. In the following, we focused on the relationship and regulation among pyroptosis, apoptosis and necroptosis.
One of the first identified bridges between different cell death types is caspase-8, which is an important regulator in necroptosis, apoptosis and pyroptosis.22 Caspases can be divided into inflammatory caspases (caspase-1, 4, 5 and 11) and apoptosis caspases (caspase-3, 6, 7, 8, 9 and −10).23,24 However, caspase-3 and caspase-8 were found to mediate pyroptosis.22,25 Caspase-8 is considered to be a molecular switch of cell death, which can promote apoptosis, pyroptosis or necroptosis, respectively.26,27
GSDMD is also an important molecular regulating pyroptosis, apoptosis and necroptosis. Activation of caspase-3 and caspase-7 inactivates GSDMD by processing GSDMD to produce p20 fragment, which is a form of inactivated GSDMD.28 In the absence of GSDMD, caspase-1 fails to trigger pyroptosis and instead redirects cell fate to apoptosis pathway.29 Studies on macrophage apoptosis have reported that in the absence of GSDMD, caspase-1 activates caspase-3 and caspase-7 to induce apoptosis; conversely, during apoptosis, caspase-3 and caspase-7 specifically prevented pyroptosis by processing GSDMD, indicating a close interaction between pyroptosis and apoptosis.28
RIP3 protein is reported to be the key molecule to regulate cell apoptosis or cell necroptosis. If RIP3 expression is high, cells will go to the necroptosis pathway, while RIP3 expression is low, cells will go to the apoptosis pathway.30 RIP3 can also promote IL-1β release mediated by NLRP3-caspase-1 axis.31 These findings indicate that RIP3 regulates the balance of pyroptosis, apoptosis and necroptosis.
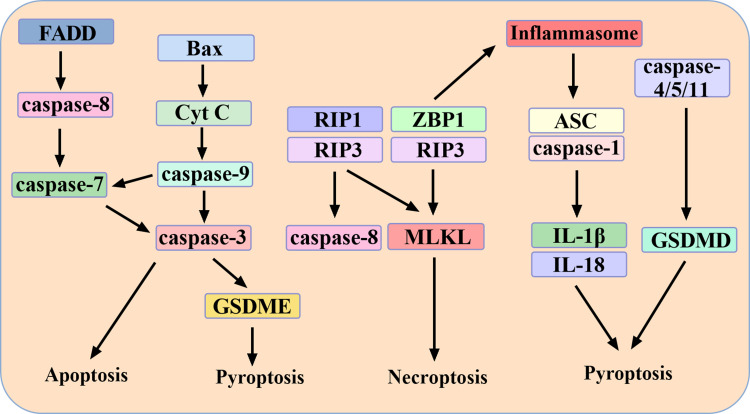
MLKL, an important molecule in necroptosis, has also been reported to activate NLRP3 inflammasome.31 MLKL inhibitor necrosulfonamide can suppress GSDMD, a key protein of pyroptosis.32 These suggest a cross between pyroptosis and necroptosis. In addition, loss of nod-like receptor (NLR) family CARD domain containing 4 (NLRC4) inflammasome resulted in enhanced MLKL phosphorylation and necroptosis. During P. aeruginosa challenge, in the absence of NLRC4, alternative cell-death molecules such as RIP1 and MLKL were activated, whereas activation of caspase-1, 3, 7, 8 was reduced.33 Therefore, there are inseparable interaction relationships among necroptosis, apoptosis and pyroptosis. The relationship among necroptosis, apoptosis and pyroptosis is summarized in Figure 1.
Figure 1.
The interaction among pyroptosis, apoptosis and necroptosis. FADD mediates the exogenous apoptosis pathway. Bax mediates the endogenous apoptosis pathway. Caspase-7, caspase-9 and caspase-3 are the hubs of the two pathways. Caspase-3 can also mediate pyroptosis through GSDME. RIP1, RIP3 and ZBP1 mediate necroptosis. Caspase-8 is the hub of apoptosis and necroptosis. ZBP1 can also activate inflammasome, which can activate caspase-1/4/5 and induce pyroptosis.
Key Molecules During PANoptosis and the Formation of PANoptosome
Z-DNA-Binding Protein 1 (ZBP1)
ZBP1 is a nucleic acid innate immune sensor that regulates host defense response.34 Activation of ZBP1 triggers necroptosis, apoptosis and pyroptosis (PANoptosis) through activating RIP3, caspase-8 and NLR thermal protein domain associated protein 3 (NLRP3).35,36 ZBP1 is considered to be a key component of PANoptosome. PANoptosome is mainly composed of receptors (ZBP1, inflammasome), adapters (apoptosis-associated speck-like protein containing a CARD (ASC) and FADD) and catalytic effectors (caspase-1, RIP3, RIP1, and caspase-8).5 ZBP1 and its Zα2 domain are required for the activation of NLRP3 and PANoptosis. Loss of ZBP1 and Zα2 domains leads to decreased activation of NLRP3 and pyroptosis, decreased cleavage of caspase-3, caspase-8 and caspase-7 (apoptosis), and decreased MLKL phosphorylation (necroptosis), which means decreased PANoptosis. These results indicate that ZBP1 and its Zα2 domain play a key role in PANoptosis. Moreover, the Zα2 domain of ZBP1 is required to promote the interaction between ZBP1 and RIP3. In the absence of Zα2 domain, the interaction between RIP3 and ZBP1 disappeared and PANoptosis was blocked.37,38 Caspase-6 is a factor regulating the interaction between RIP3 and ZBP1, and caspase-6 promotes the interaction between RIP3 and ZBP1 by binding to RIP3.39,40 The role of ZBP1 and its Zα2 domain during influenza A virus and β-coronavirus infection has also been described. The Zα2 domain of ZBP1 can regulate influenza-induced PANoptosis, and deficiency of Zα2 domain abrogated influenza A virus-induced activation of PANoptosis. Moreover, loss of Zα2 domain rescued mice from death caused by ZBP1-driven inflammation.35 Besides, ZBP1 is upregulated in severe patients with corona virus disease-19 (COVID-19) during β-coronavirus infection. ZBP1 drives cytokine storm and death in mice infected with β-coronavirus, and its Zα2 domain is essential for β-coronavirus-induced cell death (PANoptosis). Further studies demonstrated that ZBP1-mediated activation of RIP3-dependent regulated cell death was responsible for β-coronavirus-induced cell death. ZBP1-dependent inflammatory cell death, regulated cell death, and cytokine storm can disrupt the efficacy of interferon (IFN) treatment.41
RIP1
RIP1 regulation on PANoptosis is required for cell death and inflammatory response.42,43 Loss of RIP1 can activate RIP3-regulated PANoptosis through caspase-8 and FADD, leading to cell death.44,45 In disease, deletion of RIP1 abolished Yersinia-induced inflammasome activation/pyroptosis and apoptosis, but enhanced necroptosis. Yersinia induced the assembly of PANoptosome complex (RIP1, caspase-8, FADD, NLRP3, ASC, RIP3), which could regulate all three pathways of PANoptosis.2 Transforming growth factor-β (TGF-β)-activated kinase 1 (TAK1) was discovered to be the regulatory switch of PANoptosome. Under the condition of TAK1 deficiency, RIP1 recruited NLRP3 and ASC to form a cell death complex that activates inflammasome, caspase-8 and GSDMD to drive pyroptosis and apoptosis.1,46 Further studies found that the lack of TAK1 could also induce the activation of RIP1 non-dependent PANoptosis pathway, which was mainly mediated by RIP3-MLKL. Inactivation of TAK1 led to myelodysplasia and severe sepsis-like syndrome driven by the RIP3‑caspase-8 signaling axis, without triggering high expression of RIP1.47 RIP1 is also involved in the FADD/caspase-8 axis driving TNF-α and IFN-γ-induced PANoptosis. However, knockdown of RIP3 and caspase-8 or RIP3 and FADD protected cells from death.48 Therefore, RIP1 plays a key role in PANoptosis and host defense.
Inflammasome
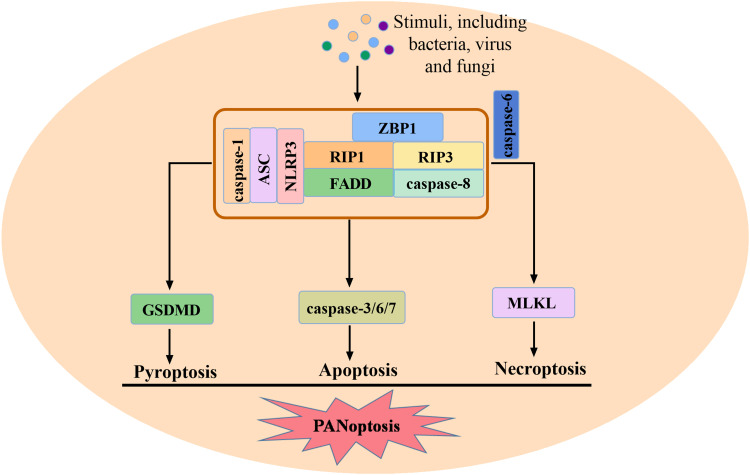
Inflammasome is one of the important components of PANoptosome, and the activation of inflammasome is crucial for pyroptosis and PANoptosis.49 There are five identified inflammasome sensors, including NLRP1, NLRP3, absent in melanoma 2 (AIM2), NLRC4 and Pyrin. The last four have been found to be involved in PANoptosis. NLRP3 activation and GSDMD-mediated pyroptosis play key roles in apoptosis and necroptosis. After NLRP3 inflammasome activation, inflammatory cells die through PANoptosis. Knockdown of NLRP3 or GSDMD led to a decrease in cell death at an early stage, but the incidence of inflammatory cell death increased substantially over time, which was mediated by caspase-8 and RIP3, suggesting that in the absence of NLRP3 or pyroptosis, increased cell death is dependent on caspase-8 and RIP3.50 After P. aeruginosa infection, in the absence of NLRC4, alternative cell death molecules such as RIP1 and MLKL are activated, while the activation of caspase-1, 3, 7, 8 is reduced. This suggests the important effect of the NLRC4 in PANoptosis.33 However, recent studies have reported that caspase-1 cleavage, IL-1β and IL-18 release, and cell death induced by herpes simplex virus 1 (HSV1) infection are AIM2-dependent, not NLRP3 and NLRC4 dependent. AIM2 was found to regulate pyrin and ZBP1 to drive PANoptosome formation, PANoptosis, and provide protection against HSV1 infection. The composition of this multiprotein complex mediated by AIM2 includes AIM2, ZBP1, RIP1, RIP3, caspase-1, ASC, caspase-8, pyrin and FADD.51 The process of PANoptosis is summarized in Figure 2.
Figure 2.
The process of PANoptosis and the formation of PANoptosome. After stimulated, ZBP1, RIP1, RIP3, caspase-1, ASC, NLRP3, FADD and caspase-8 form PANoptosome, mediating PANoptosis. Caspase-6 is a factor regulating the interaction between RIP3 and ZBP1.
PANoptosis and Diseases
PANoptosis in Infectious Diseases
The role of PANoptosis in infectious diseases has been reported in several articles, including bacterial, viral and fungal infections.52–55 It has been reported that Yersinia can induce assembly of the PANoptosome complex, and RIP1 knockdown can eliminate Yersinia-induced PANoptosis.2 Furthermore, macrophages lacking the molecules required for PANoptosis were resistant to cell death induced by B. thailandensis. In contrast to wild-type or pyroptosis deficient macrophages, PANoptosis deficient macrophages failed to restrict cell–cell fusion induced by B. thailandensis.56 In addition, when enterococcus faecalis infected macrophage, expression of caspase-1, GSDMD, caspase-3, MLKL, RIP3, NLRP3, IL-1β, IL-18 and caspase-3 and p-MIKL were upregulated in macrophage, which induced different degrees of cell death.57 In sepsis associated encephalopathy rat model, PANoptosis occured in rat cortical nerve cells. The upstream mechanism may be that toll-like receptor 9 (TLR9) activates PANoptosis through mitogen-activated protein kinase (MAPK) signaling pathway. Inhibition of TLR9 resulted in suppression of MAPK pathway, which induced suppression of PANoptosis, improved survival rate in sepsis associated encephalopathy rats.21
In addition to bacterial infections, PANoptosis is also an effective defense against viral infections.58,59 HSV1 induced caspase-1 cleavage in an AIM2-dependent manner, driving the formation of PANoptosome, causing PANoptosis, and providing protection against HSV-1 infection.51 When the host was infected with influenza A virus, ZBP1 could mediate the activation of NLRP3 inflammasome, and combine with RIP3 to induce PANoptosis.35 The role of PANoptosis in coronaviruses is also of wide interest to researchers.60,61 SARS-CoV-2 virus can cause multiple organ failure, and the failure symptoms may be related to cytokine storm. The strong release of cytokines during COVID-19 may be related to PANoptosis.62 During coronavirus infection, ZBP1 is upregulated in critical patients with COVID-19. ZBP1 drives cell death and mice death in β-coronavirus infected mice, and the Zα2 domain of ZBP1 is essential for β-coronavirus induced cell death. ZBP1-dependent cell death can disrupt the efficacy of IFN treatment. Therefore, the combination strategy of blocking ZBP1 and providing IFN therapy may be beneficial to the prognosis of patients.41 In addition, murine coronavirus mouse hepatitis virus (MHV) infection can activate NLRP3 and PANoptosis.50 Together, these data suggest that PANoptosis plays a key role in virus-induced cell damage.
Studies of PANoptosis in fungal infections have also been reported.63 Candida albicans and Aspergillus fumigatus have been reported to activate caspase-1, GSDMD, caspase-8, 3, 7, phosphorylate MLKL, and knock down PANoptosis-related molecules (such as caspase-8, RIP3, caspase-1) can reduce PANoptosis caused by Candida albicans and Aspergillus fumigatus.37 Besides, P. aeruginosa has been reported to induce PANoptosis in murine macrophage. Only the combined deletion of caspase-1, 11, 8 and RIP3 protected mice macrophage from cell death.33
PANoptosis in Non-Infectious Diseases
The role of PANoptosis in tumors, especially colorectal cancer, has been reported.3,64–66 According to the results of gene analysis, the expression of PANoptosis-related genes was different between colon cancer samples and normal samples. PANoptosis-related genes were mutated, among which NLRP3 had the highest mutation frequency, and ZBP1, AIM2, NLRP3 and GSDMD had the highest copy number variation. NLRP3, caspase-7, RIP1 and RIP3 were down-regulated in tumor samples. Molecular clustering and prognostic features based on PANoptosis can predict the survival and immune status of colon cancer patients.4 Multivariate Cox analysis showed that PANoptosis-related lncRNA SNHG7 was significantly associated with the prognosis of colon adenocarcinoma. Subsequent analysis showed that its expression was related to lymph node metastasis and tumor stage. In addition, susceptibility analysis showed that lncRNA SNHG7 was involved in the drug resistance of colon adenocarcinoma.67 Since the occurrence of PANoptosis is reduced in colorectal cancer, activation of PANoptosis may be a strategy to kill cancer cells. Interferon regulatory factor 1 can prevent colorectal cancer by regulating PANoptosis.68 Besides, the synergistic effect of cysteine desulfurase nitrogen fixation 1 (NFS1) deletion and oxaliplatin induced PANoptosis by increasing intracellular reactive oxygen species (ROS), which improved the antitumor efficacy of platinum-based chemotherapy in colorectal cancer treatment.69 It was also found that the combination of TNF-α and IFN-γ stimulation in multiple colon cancer cell lines could cause the activation of PANoptosis, and the inhibition of Janus kinase (JAK) signaling pathway significantly reduced the cell death caused by TNF-α and IFN-γ, indicating that the cell death induced by TNF-α and IFN-γ is an important mechanism to inhibit tumor growth and kill cancer cells.70 The role of PANoptosis in other tumors has also been reported. Most PANoptosis genes are considered as favorable genes for the prognosis of gastric cancer, and the expression level of PANoptosis genes is positively correlated with the survival ability of patients, that is, the lower expression level, the worse survival ability.71 In addition, the activation of pyroptosis, apoptosis and necroptosis (PANoptosis) promotes cancer cell death, thus preventing adrenocortical carcinoma and melanoma development.72,73
In addition to tumors, PANoptosis also occurs in the process of acute respiratory distress syndrome (ARDS) and acute lung injury, which is manifested by the formation of ASC-caspase-8-RIP3 complex accompanied by caspase-3, GSDMD, phosphorylated MLKL, ZBP1 increased.74,75 A study showed that genes associated with PANoptosis were differentially expressed in mice middle cerebral artery ischemia-reperfusion injury and control brain tissue samples.76 The occurrence of PANoptosis has also been found in cerebral ischemia or ischemia-reperfusion injury,77 indicating that PANoptosis plays an important role in ischemia-reperfusion injury.78,79 In addition, an in vivo and in vitro experiment found that PANoptosis occurred in retinal neurons under ischemia-reperfusion injury from morphological characteristics to gene level, specifically manifested in significantly increased cleaved caspase-3, NLRP3, cleaved caspase-1 (p20), GSDMD, phosphorylated RIP3 and MLKL, and high expression of pro-apoptotic protein BAX and proinflammatory cytokines IL-1β (p17), IL-18 (p22). However, the expression of these molecules decreased after inhibition of GSDMD or administration of necroptosis inhibitors. When the three pathways of death were intervened simultaneously, the cell survival rate was significantly improved compared with intervention with one death alone.80 PANoptosis is also involved in retinal ganglion cell (RGC) death and diabetic retinopathy caused by acute ocular hypertension. Melatonin and Dickkopf-1 can inhibit cell death by inhibiting PANoptosis.81,82 These studies show that PANoptosis is widely present in the pathological process of various diseases. A deeper understanding of the pathogenesis of PANoptosis will not only provide preliminary experimental clues for the future study of this novel regulatory cell death, but also develop a promising therapeutic target for the treatment of the disease.
Funding Statement
This work was supported by the National Natural Science Foundation of China (82270627).
Summary
So far, a cell can die in many ways, but what does it depend on which way it dies? In our opinion, the stimulant, the cell state, and the expression of the cell death gene may be the three factors that the cell chooses to die. Different stimuli can activate different signaling pathways in the cell, which in turn can activate different death pathways. The state of the cell is related to how quickly the mode of death occurs. When the cell is in a state of emergency, it may preferentially activate the death mode with a faster time, such as pyroptosis. Finally, the choice of cell death is related to the death genes expressed by the cells. That is, if a cell tends to express pyroptosis-related genes under the same stimulation, then the cell death may be pyroptosis. If a cell tends to express genes involved in apoptosis, then the cell may die by apoptosis. Therefore, the occurrence of PANoptosis may be the result of the comprehensive action of cells under various factors. Pyroptosis, apoptosis and necroptosis affect each other and regulate each other. In this review, we described the relationship between necroptosis, apoptosis and pyroptosis, the key molecules in the process of PANoptosis and the formation of PANoptosome, as well as the role of PANoptosis in diseases. These findings provide new ideas for treating diseases. In treating some diseases, a single therapeutic target may not be sufficient to block the progression of the disease. Treatments that target key molecules or block multiple pathways at the same time may become effective ways to treat diseases. Further study on the occurrence mechanism of PANoptosis and suppression of the three pathways of PANoptosis is expected to be an effective means for the treatment of diseases.
Abbreviations
Caspase-1, cysteinyl aspartate specific proteinase-1; IL-1β, interleukin-1β; GSDMD, gasdermin D; ATP, adenosine triphosphate; cyto-C, cytochrome C; Bcl-2, B-cell lymphoma-2; FADD, Fas-associating protein with a novel death domain; TNF, tumor necrosis factor; TRADD, TNF receptor 1 associated via death domain; IRE1, inositol-requiring enzyme 1; PERK, protein kinase R-like endoplasmic reticulum kinase; ATF6, activating transcription factor 6; TRAF2, TNF receptor associated factor 2; JNK, Jun n-terminal kinase; Bad, Bcl-2 associated death promoter; eIF2α, eukaryotic translation initiation factor 2α; CHOP, C/EBP-homologous protein; Bax, Bcl-2 associated X protein; Bak, Bcl-2 antagonist/killer; RIP1, receptor-interacting serine/threonine-protein kinase 1; MLKL, mixed lineage kinase domain-like; NLRC4, nod-like receptor (NLR) family CARD domain containing 4; ZBP1, Z-DNA-binding protein 1; NLRP3, NLR thermal protein domain associated protein 3; ASC, apoptosis-associated speck-like protein contain a CARD; COVID-19, corona virus disease-19; IFN, interferon; TGF-β, transforming growth factor-β; TAK1, TGF-β-activated kinase 1; AIM2, absent in melanoma 2; HSV1, herpes simplex virus 1; TLR9, toll-like receptor 9; MAPK, mitogen-activated protein kinase; MHV, mouse hepatitis virus; NFS1, nitrogen fixation 1; ROS, reactive oxygen species; JAK, Janus kinase; ARDS, acute respiratory distress syndrome; RGC, retinal ganglion cell.
Author Contributions
All authors have read and agreed to the published version of the manuscript. Conceptualization, Shi C; writing—original draft preparation, Shi C; writing—review and editing, Cao P, Wang YK, Zhang Q and Zhang D; visualization and supervision, Wang Y, Wang L and Gong Z. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Malireddi RKS, Kesavardhana S, Kanneganti TD. ZBP1 and TAK1: master regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis). Front Cell Infect Microbiol. 2019;9:406. doi: 10.3389/fcimb.2019.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malireddi RKS, Kesavardhana S, Karki R, Kancharana B, Burton AR, Kanneganti TD. RIPK1 distinctly regulates Yersinia-induced inflammatory cell death, PANoptosis. Immunohorizons. 2020;4(12):789–796. doi: 10.4049/immunohorizons.2000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Hong M, Li Y, Chen D, Wu Y, Hu Y. Programmed cell death tunes tumor immunity. Front Immunol. 2022;13:847345. doi: 10.3389/fimmu.2022.847345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Sun R, Chan S, et al. PANoptosis-based molecular clustering and prognostic signature predicts patient survival and immune landscape in colon cancer. Front Genet. 2022;13:955355. doi: 10.3389/fgene.2022.955355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samir P, Malireddi RKS, Kanneganti TD. The PANoptosome: a deadly protein complex driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front Cell Infect Microbiol. 2020;10:238. doi: 10.3389/fcimb.2020.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christgen S, Tweedell RE, Kanneganti TD. Programming inflammatory cell death for therapy. Pharmacol Ther. 2022;232:108010. doi: 10.1016/j.pharmthera.2021.108010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Jiang M, Qi L, et al. Pyroptosis, a new bridge to tumor immunity. Cancer Sci. 2021;112(10):3979–3994. doi: 10.1111/cas.15059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang CC, Li CG, Wang YF, et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis. 2019;24(3–4):312–325. doi: 10.1007/s10495-019-01515-1 [DOI] [PubMed] [Google Scholar]
- 9.Tsuchiya K. Inflammasome-associated cell death: pyroptosis, apoptosis, and physiological implications. Microbiol Immunol. 2020;64(4):252–269. doi: 10.1111/1348-0421.12771 [DOI] [PubMed] [Google Scholar]
- 10.Shi J, Zhao Y, Wang Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. doi: 10.1038/nature13683 [DOI] [PubMed] [Google Scholar]
- 11.Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20(3):175–193. doi: 10.1038/s41580-018-0089-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pena-Blanco A, Garcia-Saez AJ. Bax, Bak and beyond - mitochondrial performance in apoptosis. FEBS J. 2018;285(3):416–431. doi: 10.1111/febs.14186 [DOI] [PubMed] [Google Scholar]
- 13.Cavalcante GC, Schaan AP, Cabral GF, et al. A cell’s fate: an overview of the molecular biology and genetics of apoptosis. Int J Mol Sci. 2019;20(17):4133. doi: 10.3390/ijms20174133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Re DP, Amgalan D, Linkermann A, Liu Q, Kitsis RN. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol Rev. 2019;99(4):1765–1817. doi: 10.1152/physrev.00022.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groenendyk J, Sreenivasaiah PK, Kim DH, Agellon LB, Michalak M. Biology of endoplasmic reticulum stress in the heart. Circ Res. 2010;107(10):1185–1197. doi: 10.1161/CIRCRESAHA.110.227033 [DOI] [PubMed] [Google Scholar]
- 16.Li B, Yi P, Zhang B, et al. Differences in endoplasmic reticulum stress signalling kinetics determine cell survival outcome through activation of MKP-1. Cell Signal. 2011;23(1):35–45. doi: 10.1016/j.cellsig.2010.07.019 [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Liu L, Naik I, Braunstein Z, Zhong J, Ren B. Transcription factor C/EBP homologous protein in health and diseases. Front Immunol. 2017;8:1612. doi: 10.3389/fimmu.2017.01612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J, Jitkaew S, Cai Z, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109(14):5322–5327. doi: 10.1073/pnas.1200012109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Kanneganti TD. From pyroptosis, apoptosis and necroptosis to PANoptosis: a mechanistic compendium of programmed cell death pathways. Comput Struct Biotechnol J. 2021;19:4641–4657. doi: 10.1016/j.csbj.2021.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gullett JM, Tweedell RE, Kanneganti TD. It’s all in the PAN: crosstalk, plasticity, redundancies, switches, and interconnectedness encompassed by PANoptosis underlying the totality of cell death-associated biological effects. Cells. 2022;11(9):1495. doi: 10.3390/cells11091495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou R, Ying J, Qiu X, et al. A new cell death program regulated by toll-like receptor 9 through p38 mitogen-activated protein kinase signaling pathway in a neonatal rat model with sepsis associated encephalopathy. Chin Med J. 2022;135(12):1474–1485. doi: 10.1097/CM9.0000000000002010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarhan J, Liu BC, Muendlein HI, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 2018;115(46):E10888–E10897. doi: 10.1073/pnas.1809548115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kesavardhana S, Malireddi RKS, Kanneganti TD. Caspases in cell death, inflammation, and pyroptosis. Annu Rev Immunol. 2020;38:567–595. doi: 10.1146/annurev-immunol-073119-095439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Husain M. Identifying caspases and their motifs that cleave proteins during Influenza A virus infection. J Vis Exp. 2022;185. doi: 10.3791/64189 [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi: 10.1038/nature22393 [DOI] [PubMed] [Google Scholar]
- 26.Fritsch M, Gunther SD, Schwarzer R, et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575(7784):683–687. doi: 10.1038/s41586-019-1770-6 [DOI] [PubMed] [Google Scholar]
- 27.Jiang M, Qi L, Li L, Wu Y, Song D, Li Y. Caspase-8: a key protein of cross-talk signal way in “PANoptosis” in cancer. Int J Cancer. 2021;149(7):1408–1420. doi: 10.1002/ijc.33698 [DOI] [PubMed] [Google Scholar]
- 28.Taabazuing CY, Okondo MC, Bachovchin DA. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol. 2017;24(4):507–514 e504. doi: 10.1016/j.chembiol.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, Alnemri ES. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat Commun. 2019;10(1):1689. doi: 10.1038/s41467-019-09397-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang DW, Shao J, Lin J, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. doi: 10.1126/science.1172308 [DOI] [PubMed] [Google Scholar]
- 31.Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 2019;26(1):99–114. doi: 10.1038/s41418-018-0212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rathkey JK, Zhao J, Liu Z, et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol. 2018;3(26). doi: 10.1126/sciimmunol.aat2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundaram B, Karki R, Kanneganti TD. NLRC4 deficiency leads to enhanced phosphorylation of MLKL and necroptosis. Immunohorizons. 2022;6(3):243–252. doi: 10.4049/immunohorizons.2100118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hao Y, Yang B, Yang J, et al. ZBP1: a powerful innate immune sensor and double-edged sword in host immunity. Int J Mol Sci. 2022;23(18):10224. doi: 10.3390/ijms231810224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kesavardhana S, Malireddi RKS, Burton AR, et al. The Zalpha2 domain of ZBP1 is a molecular switch regulating influenza-induced PANoptosis and perinatal lethality during development. J Biol Chem. 2020;295(24):8325–8330. doi: 10.1074/jbc.RA120.013752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng M, Kanneganti TD. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol Rev. 2020;297(1):26–38. doi: 10.1111/imr.12909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banoth B, Tuladhar S, Karki R, et al. ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis). J Biol Chem. 2020;295(52):18276–18283. doi: 10.1074/jbc.RA120.015924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karki R, Sundaram B, Sharma BR, et al. ADAR1 restricts ZBP1-mediated immune response and PANoptosis to promote tumorigenesis. Cell Rep. 2021;37(3):109858. doi: 10.1016/j.celrep.2021.109858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng M, Karki R, Vogel P, Kanneganti TD. Caspase-6 is a key regulator of innate immunity, inflammasome activation, and host defense. Cell. 2020;181(3):674–687 e613. doi: 10.1016/j.cell.2020.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng M, Kanneganti TD. Newly identified function of Caspase-6 in ZBP1-mediated innate immune responses, NLRP3 Inflammasome Activation, PANoptosis, and host defense. J Cell Immunol. 2020;2(6):341–347. doi: 10.33696/immunology.2.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karki R, Lee S, Mall R, et al. ZBP1-dependent inflammatory cell death, PANoptosis, and cytokine storm disrupt IFN therapeutic efficacy during coronavirus infection. Sci Immunol. 2022;7(74):eabo6294. doi: 10.1126/sciimmunol.abo6294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao P, Sun J, Wu Z, et al. A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature. 2020;577(7788):109–114. doi: 10.1038/s41586-019-1830-y [DOI] [PubMed] [Google Scholar]
- 43.Lalaoui N, Boyden SE, Oda H, et al. Mutations that prevent caspase cleavage of RIPK1 cause autoinflammatory disease. Nature. 2020;577(7788):103–108. doi: 10.1038/s41586-019-1828-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471(7338):373–376. doi: 10.1038/nature09878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dillon CP, Weinlich R, Rodriguez DA, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157(5):1189–1202. doi: 10.1016/j.cell.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samir P, Kanneganti TD. DDX3X sits at the crossroads of liquid-liquid and prionoid phase transitions arbitrating life and death cell fate decisions in stressed cells. DNA Cell Biol. 2020;39(7):1091–1095. doi: 10.1089/dna.2020.5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malireddi RKS, Gurung P, Kesavardhana S, et al. Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity-independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J Exp Med. 2020;217(3). doi: 10.1084/jem.20191644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karki R, Sharma BR, Tuladhar S, et al. Synergism of TNF-alpha and IFN-gamma triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184(1):149–168 e117. doi: 10.1016/j.cell.2020.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sundaram B, Kanneganti TD. Advances in understanding activation and function of the NLRC4 Inflammasome. Int J Mol Sci. 2021;22(3):1048. doi: 10.3390/ijms22031048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng M, Williams EP, Malireddi RKS, et al. Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection. J Biol Chem. 2020;295(41):14040–14052. doi: 10.1074/jbc.RA120.015036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S, Karki R, Wang Y, Nguyen LN, Kalathur RC, Kanneganti TD. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature. 2021;597(7876):415–419. doi: 10.1038/s41586-021-03875-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Place DE, Lee S, Kanneganti TD. PANoptosis in microbial infection. Curr Opin Microbiol. 2021;59:42–49. doi: 10.1016/j.mib.2020.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang W, Deng Z, Dai X, Zhao W. PANoptosis: a new insight into oral infectious diseases. Front Immunol. 2021;12:789610. doi: 10.3389/fimmu.2021.789610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X, Zhang W, Yi W, et al. Pathway of cell death and its role in virus infection. Viral Immunol. 2022;35(7):444–456. doi: 10.1089/vim.2022.0010 [DOI] [PubMed] [Google Scholar]
- 55.Nisa A, Kipper FC, Panigrahy D, Tiwari S, Kupz A, Subbian S. Different modalities of host cell death and their impact on Mycobacterium tuberculosis infection. Am J Physiol Cell Physiol. 2022;323(5):C1444–C1474. doi: 10.1152/ajpcell.00246.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Place DE, Christgen S, Tuladhar S, Vogel P, Malireddi RKS, Kanneganti TD. Hierarchical cell death program disrupts the intracellular niche required for Burkholderia thailandensis pathogenesis. mBio. 2021;12(3):e0105921. doi: 10.1128/mBio.01059-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chi D, Lin X, Meng Q, Tan J, Gong Q, Tong Z. Real-time induction of macrophage apoptosis, pyroptosis, and necroptosis by Enterococcus faecalis OG1RF and two root canal isolated strains. Front Cell Infect Microbiol. 2021;11:720147. doi: 10.3389/fcimb.2021.720147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen LN, Kanneganti TD. PANoptosis in viral infection: the missing puzzle piece in the cell death field. J Mol Biol. 2022;434(4):167249. doi: 10.1016/j.jmb.2021.167249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuriakose T, Kanneganti TD. Pyroptosis in Antiviral Immunity. Curr Top Microbiol Immunol. 2019. doi: 10.1007/82_2019_189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee S, Channappanavar R, Kanneganti TD. Coronaviruses: innate immunity, inflammasome activation, inflammatory cell death, and cytokines. Trends Immunol. 2020;41(12):1083–1099. doi: 10.1016/j.it.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yapasert R, Khaw-On P, Banjerdpongchai R. Coronavirus infection-associated cell death signaling and potential therapeutic targets. Molecules. 2021;26:24. doi: 10.3390/molecules26247459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schifanella L, Anderson J, Wieking G, et al. The defenders of the alveolus succumb in COVID-19 pneumonia to SARS-CoV-2, necroptosis, pyroptosis and panoptosis. bioRxiv. 2022. doi: 10.1101/2022.08.06.503050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Briard B, Malireddi RKS, Kanneganti TD, Chowdhary A. Role of inflammasomes/pyroptosis and PANoptosis during fungal infection. PLoS Pathog. 2021;17(3):e1009358. doi: 10.1371/journal.ppat.1009358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan B, Zheng B, Xing C, Liu J. Non-canonical programmed cell death in colon cancer. Cancers. 2022;14(14):3309. doi: 10.3390/cancers14143309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gong L, Huang D, Shi Y, Liang Z, Bu H. Regulated cell death in cancer: from pathogenesis to treatment. Chin Med. 2022. doi: 10.1097/CM9.0000000000002239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma BR, Kanneganti TD. Inflammasome signaling in colorectal cancer. Transl Res. 2022. doi: 10.1016/j.trsl.2022.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang J, Jiang S, Liang L, et al. Analysis of PANoptosis-related LncRNA-miRNA-mRNA network reveals LncRNA SNHG7 involved in chemo-resistance in colon adenocarcinoma. Front Oncol. 2022;12:888105. doi: 10.3389/fonc.2022.888105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karki R, Sharma BR, Lee E, et al. Interferon regulatory factor 1 regulates PANoptosis to prevent colorectal cancer. JCI Insight. 2020;5(12). doi: 10.1172/jci.insight.136720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin JF, Hu PS, Wang YY, et al. Phosphorylated NFS1 weakens oxaliplatin-based chemosensitivity of colorectal cancer by preventing PANoptosis. Signal Transduct Target Ther. 2022;7(1):54. doi: 10.1038/s41392-022-00889-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malireddi RKS, Karki R, Sundaram B, et al. Inflammatory cell death, PANoptosis, mediated by cytokines in diverse cancer lineages inhibits tumor growth. Immunohorizons. 2021;5(7):568–580. doi: 10.4049/immunohorizons.2100059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan H, Pan J, Li P, Gao J. Characterization of PANoptosis patterns predicts survival and immunotherapy response in gastric cancer. Clin Immunol. 2022;238:109019. doi: 10.1016/j.clim.2022.109019 [DOI] [PubMed] [Google Scholar]
- 72.Ren L, Yang Y, Li W, et al. CDK1 serves as a therapeutic target of adrenocortical carcinoma via regulating epithelial-mesenchymal transition, G2/M phase transition, and PANoptosis. J Transl Med. 2022;20(1):444. doi: 10.1186/s12967-022-03641-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song M, Xia W, Tao Z, et al. Self-assembled polymeric nanocarrier-mediated co-delivery of metformin and doxorubicin for melanoma therapy. Drug Deliv. 2021;28(1):594–606. doi: 10.1080/10717544.2021.1898703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Messaoud-Nacer Y, Culerier E, Rose S, et al. STING agonist diABZI induces PANoptosis and DNA mediated acute respiratory distress syndrome (ARDS). Cell Death Dis. 2022;13(3):269. doi: 10.1038/s41419-022-04664-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cui Y, Wang X, Lin F, et al. MiR-29a-3p improves acute lung injury by reducing alveolar epithelial cell PANoptosis. Aging Dis. 2022;13(3):899–909. doi: 10.14336/AD.2021.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shu J, Yang L, Wei W, Zhang L. Identification of programmed cell death-related gene signature and associated regulatory axis in cerebral ischemia/reperfusion injury. Front Genet. 2022;13:934154. doi: 10.3389/fgene.2022.934154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan WT, Yang YD, Hu XM, et al. Do pyroptosis, apoptosis, and necroptosis (PANoptosis) exist in cerebral ischemia? Evidence from cell and rodent studies. Neural Regen Res. 2022;17(8):1761–1768. doi: 10.4103/1673-5374.331539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gonzalez-Rodriguez P, Fernandez-Lopez A. PANoptosis: new insights in regulated cell death in ischemia/reperfusion models. Neural Regen Res. 2023;18(2):342–343. doi: 10.4103/1673-5374.343910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uysal E, Dokur M, Kucukdurmaz F, et al. Targeting the PANoptosome with 3,4-Methylenedioxy-beta-Nitrostyrene, reduces PANoptosis and protects the kidney against renal ischemia-reperfusion injury. J Invest Surg. 2022:1–12. doi: 10.1080/08941939.2022.2128117 [DOI] [PubMed] [Google Scholar]
- 80.Yan WT, Zhao WJ, Hu XM, et al. PANoptosis-like cell death in ischemia/reperfusion injury of retinal neurons. Neural Regen Res. 2023;18(2):357–363. doi: 10.4103/1673-5374.346545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye D, Xu Y, Shi Y, et al. Anti-PANoptosis is involved in neuroprotective effects of melatonin in acute ocular hypertension model. J Pineal Res. 2022;73(4):e12828. doi: 10.1111/jpi.12828 [DOI] [PubMed] [Google Scholar]
- 82.Xu X, Lan X, Fu S, et al. Dickkopf-1 exerts protective effects by inhibiting PANoptosis and retinal neovascularization in diabetic retinopathy. Biochem Biophys Res Commun. 2022;617(Pt 2):69–76. doi: 10.1016/j.bbrc.2022.05.001 [DOI] [PubMed] [Google Scholar]