Abstract
The objective of this study is to quantify the impact of the COVID-19 pandemic on attention deficit hyperactivity disorder (ADHD) medication consumption globally and nationally using pharmaceutical sales data from 2014 to 2021 across 47 countries and regions. A seasonal autoregressive integrated moving average model (SARIMA) was applied to the time series until the end of 2019 at country level and used for the prediction of the ADHD medication consumption in 2020 and 2021. The deviations from the actual to the forecasted sales, which simulate the development without the emergence of COVID-19, yield estimates for the pandemic's impact. In 36 of the 47 countries and regions, the actual sales in 2020 were lower than predicted, with an average relative drop of 6.2% in defined daily doses (DDD) per 1000 inhabitants per day at country-level. In 2021, most countries recorded actually higher ADHD medication use than predicted at the end of 2019. On average, the consumption increased per country by 1.60%. The deviations strongly correlate with the stringency of anti-pandemic government policies. The findings suggest that the pandemic led to a substantially lower consumption of ADHD medication in 2020. However, in 2021 the pandemic had an accelerating effect as the increasing consumption trends are more pronounced than before the pandemic.
Keywords: ADHD, COVID-19 pandemic, Defined daily dose, Oxford stringency index, Pharmacoepidemiology, Time series forecasting
1. Introduction
Attention deficit hyperactivity disorder (ADHD) is a common childhood neurodevelopmental disorder, characterised by impairing levels of inattention and/or hyperactivity-impulsivity (American Psychiatric Association, 2013). Up to 65% of individuals diagnosed in childhood continue to demonstrate ADHD symptoms in adulthood (Faraone et al., 2006). The worldwide prevalence is estimated as 5–7% in children and adolescents (Polanczyk et al., 2014; Thomas et al., 2015), and approximately 2.5% in adults (Simon et al., 2009; Song et al., 2021). Although epidemiological studies suggests that the prevalence of ADHD is similar between different countries (Polanczyk et al., 2014), there is significant variation in the rate of diagnosis depending on the geographical location (Polanczyk et al., 2007; Raman et al., 2018). No single risk factor is necessary or sufficient to cause ADHD. In most cases ADHD arises from several genetic and environmental risk factors that each have a small individual effect and act together to increase susceptibility. The multifactorial causation of ADHD is consistent with the heterogeneity of the disorder, which is shown by its extensive psychiatric co-morbidity, its multiple domains of neurocognitive impairment and the wide range of structural and functional brain anomalies associated with it (Faraone et al., 2015; Mortimer et al., 2020; Pujol-Gualdo et al., 2021). ADHD is frequently comorbid with other mental health disorders including anxiety and substance abuse (Jensen and Steinhausen, 2015; Wilens and Spencer, 2010), and associated with impairments of social, academic and occupational functioning (American Psychiatric Association, 2013; Shaw et al., 2012). ADHD severity is a risk factor for nicotine use as well as for gaming (Schoenmacker et al., 2020).
ADHD treatment recommendations vary across countries, but usually include behavior therapy and medication (Faraone et al., 2021). Most international guidelines recommend a stepwise approach to manage ADHD in school-aged children, beginning with psychoeducation and behavioural interventions including parent training, then moving on to pharmacological intervention (NICE, 2019; Thapar and Cooper, 2016). In preschool-aged children, the first-line treatment is behavioural therapy alone (NICE, 2019). Available guidelines for ADHD treatment in adulthood recommend pharmacological treatment as the first-line therapy (Bolea-Alamañac et al., 2014; Kooij et al., 2019; NICE, 2019). Stimulants (methylphenidate, lisdexamfetamine or dexamfetamine) are the most frequently prescribed medications, followed by non-stimulants (atomoxetine or extended release formulations of guanfacine, clonidine and viloxazine) as second-line pharmaceutical treatment in children and adolescents (Bolea-Alamañac et al., 2014; Kooij et al., 2019; Pliszka et al., 2007; Thapar and Cooper, 2016; Wolraich et al., 2019). Despite largely similar management guidelines, ADHD medication use differs greatly across countries (Bachmann et al., 2017; Hinshaw et al., 2011; Setyawan et al., 2018). However, increasing recognition of ADHD as well as increasing medication use have been observed during the last decades across all age groups in numerous countries (Bachmann et al., 2017; Burcu et al., 2016; Karlstad et al., 2016; Man et al., 2017; Raman et al., 2018;), which gave rise to concerns about inappropriate prescription of ADHD medications, especially in children (Zito and Burcu, 2017), and potential overdiagnosis.
In December 2019, the novel coronavirus was first identified from an outbreak in China. Within several weeks, it spread worldwide and caused the World Health Organization (WHO) to declare it as a pandemic in March 2020 (WHO, 2020a). Preventive measures to slow the spread of the disease have been implemented nearly worldwide. Besides vaccinations, containment measures included face masks, good respiratory hygiene, quarantines and social distancing (ECDC, 2022). As vaccinations were not available before December 2020, non-pharmaceutical measures were essential to slow down the infection rate (Anderson et al., 2020). The near-global governmental social distancing recommendations and regulations such as staying at home and keeping distance from others (WHO, 2020b), led to lockdowns, business restrictions and school closures. These interventions caused not only a large global recession (IMF, 2022), but also impacted mental health by increasing anxiety, depression and post-traumatic stress disorder symptoms (Luo et al., 2020; Santomauro et al., 2021). Children, adolescents and students were particularly vulnerable for the negative effects due to risk factors such as familial conflicts, decreased physical activity and loneliness (Manchia et al., 2022). Recent studies suggest a negative impact of the pandemic and containment measures on individuals with ADHD in form of worsened ADHD symptoms (Behrmann et al., 2022; Sciberras et al., 2022; Shah et al., 2021). To our knowledge, no studies to date have investigated global and country-level patterns of ADHD medication use since the COVID-19 pandemic outbreak. The relation between psychotropic drugs and COVID-19 is complex; while the antidepressant Fluvoxamine reduces the risk of mortality for COVID-19, antipsychotics are associated with an increased risk of severe COVID-19 and mortality (Fico et al., 2022). However, the meta-analysis did not consider the effect of central nervous system (CNS) stimulants on COVID-19.
This study analysed the ADHD medication use in 47 countries and regions from 2014 to 2021. We set out to capture any changes in ADHD medication consumption in 2020 and 2021 due to the pandemic mitigation measures. We also identified countries with the highest and lowest losses in the ADHD market compared to the predicted sales in 2020 and 2021 under usual, non-pandemic conditions.
2. Experimental procedures
2.1. Data sources
We used quarterly data obtained from IQVIA Multinational Integrated Data Analysis System (IQVIA MIDAS®). IQVIA MIDAS data combine country-level data, healthcare expertise and therapeutic knowledge in 90+ countries to deliver data in globally standardized forms to facilitate multi-country analyses, a leading source of insight into international market dynamics relating to the distribution and use of medicines.
The database includes the sales of generic and brand products and does not contain individual-level data. Thus, institutional review board approval was not required. This study is based on sales volume data of ADHD medications from Quarter 1, 2014 to Quarter 4, 2021 of 45 countries and regions (Table 2) including information about the number of sold standard units, the strength, the active substances and the sales value of each drug. Data of one further country and one further region (Canada and Hong Kong) are available from Quarter 4, 2016 to Quarter 4, 2021.
Table 2.
Actual and expected annual ADHD medicine consumption in 2020 and 2021, absolute and relative difference per country in DDD per 1000 inhabitants per day.
| 2020 |
2021 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Consumption |
Difference |
Consumption |
Difference |
|||||
| actual | expected | absolute | relative | actual | expected | absolute | relative | |
| Global | 3.69 | 3.82 | −0.13 | −3.31% | 3.97 | 3.95 | 0.02 | 0.37% |
| Argentina | 0.13 | 0.19 | −0.06 | −31.95% | 0.17 | 0.20 | −0.03 | −14.69% |
| Australia | 10.23 | 10.03 | 0.20 | 1.99% | 12.62 | 11.39 | 1.23 | 10.78% |
| Austria | 1.06 | 1.10 | −0.04 | −3.59% | 1.24 | 1.21 | 0.03 | 2.97% |
| Belgium | 3.57 | 4.02 | −0.45 | −11.05% | 3.93 | 4.32 | −0.39 | −8.99% |
| Brazil | 0.87 | 1.01 | −0.14 | −13.68% | 1.16 | 1.11 | 0.05 | 5.01% |
| Bulgaria | 0.01 | 0.01 | −0.00 | −9.34% | 0.01 | 0.01 | 0.00 | 0.79% |
| Canada | 19.14 | 19.81 | −0.67 | −3.41% | 21.45 | 21.20 | 0.25 | 1.17% |
| China | 0.02 | 0.02 | −0.00 | −21.88% | 0.03 | 0.03 | 0.00 | 2.77% |
| Colombia | 0.01 | 0.00 | 0.01 | 108.51% | 0.01 | 0.00 | 0.01 | 353.11% |
| Croatia | 0.03 | 0.04 | −0.01 | −7.53% | 0.05 | 0.04 | 0.01 | 3.62% |
| Czech Republic | 0.79 | 0.88 | −0.09 | −10.67% | 0.86 | 0.95 | −0.09 | −9.45% |
| Denmark | 13.43 | 13.46 | −0.03 | −0.16% | 15.53 | 14.73 | 0.80 | 5.42% |
| Estonia | 1.00 | 0.97 | 0.03 | 2.47% | 1.24 | 1.30 | −0.06 | −4.33% |
| Finland | 6.69 | 6.60 | 0.09 | 1.41% | 8.61 | 7.74 | 0.87 | 11.21% |
| France | 0.85 | 0.90 | −0.05 | −6.34% | 1.01 | 0.98 | 0.03 | 2.8% |
| Germany | 3.25 | 3.31 | −0.06 | −1.69% | 3.46 | 3.51 | −0.05 | −1.34% |
| Greece | 0.13 | 0.12 | 0.01 | 5.12% | 0.16 | 0.13 | 0.03 | 24.33% |
| Hong Kong | 2.23 | 2.53 | −0.30 | −11.64% | 2.67 | 2.74 | −0.07 | −2.55% |
| Hungary | 0.21 | 0.22 | −0.01 | −7.98% | 0.25 | 0.25 | −0.00 | −0.16% |
| Indonesia | 0.01 | 0.01 | −0.00 | −12.88% | 0.01 | 0.01 | 0.00 | 4.76% |
| Ireland | 1.81 | 1.83 | −0.02 | −0.58% | 2.10 | 2.02 | 0.08 | 4.08% |
| Italy | 0.07 | 0.09 | −0.02 | −14.88% | 0.09 | 0.10 | −0.01 | −10.18% |
| Japan | 1.69 | 1.74 | −0.05 | −2.61% | 1.94 | 1.93 | 0.01 | 0.47% |
| Jordan | 0.03 | 0.03 | −0.00 | −10.7% | 0.04 | 0.04 | 0.01 | 18.13% |
| Korea | 1.04 | 1.06 | −0.02 | −2.17% | 1.31 | 1.15 | 0.16 | 14.49% |
| Latvia | 0.16 | 0.13 | 0.03 | 24.16% | 0.16 | 0.14 | 0.02 | 14.47% |
| Lithuania | 0.10 | 0.10 | −0.00 | −6.34% | 0.12 | 0.12 | 0.00 | 5.96% |
| Luxembourg | 2.17 | 2.26 | −0.11 | −4.28% | 2.32 | 2.19 | 0.13 | 5.83% |
| Malaysia | 0.02 | 0.02 | 0.00 | 12.61% | 0.03 | 0.02 | 0.01 | 36.27% |
| Mexico | 0.41 | 0.44 | −0.03 | −6.35% | 0.39 | 0.42 | −0.03 | −6.45% |
| Netherlands | 9.73 | 9.26 | 0.47 | 5.04% | 11.40 | 9.71 | 1.69 | 17.35% |
| Norway | 14.38 | 14.84 | −0.46 | −3.09% | 17.05 | 16.72 | 0.33 | 1.96% |
| Philippines | 0.01 | 0.01 | −0.00 | −31.43% | 0.01 | 0.01 | −0.00 | −24.98% |
| Poland | 0.32 | 0.35 | −0.03 | −8.28% | 0.41 | 0.38 | 0.03 | 8.4% |
| Portugal | 2.30 | 2.77 | −0.47 | −17.27% | 2.73 | 2.93 | −0.20 | −6.9% |
| Romania | 0.13 | 0.18 | −0.05 | −27.17% | 0.14 | 0.20 | −0.06 | −31.83% |
| Singapore | 0.50 | 0.50 | −0.00 | −0.73% | 0.52 | 0.53 | −0.01 | −1.95% |
| Slovakia | 0.18 | 0.20 | −0.02 | −10.24% | 0.18 | 0.21 | −0.03 | −14.09% |
| Slovenia | 0.53 | 0.61 | −0.08 | −13.26% | 0.62 | 0.64 | −0.02 | −3.13% |
| South Africa | 1.59 | 2.03 | −0.44 | −21.87% | 1.60 | 2.14 | −0.54 | −25.49% |
| Spain | 3.26 | 3.47 | −0.21 | −6.12% | 3.66 | 3.63 | 0.03 | 0.87% |
| Sweden | 18.53 | 18.12 | 0.41 | 2.30% | 20.17 | 19.38 | 0.80 | 4.12% |
| Switzerland | 6.17 | 6.11 | 0.06 | 1.06% | 7.01 | 6.37 | 0.64 | 10.11% |
| Taiwan | 1.29 | 1.20 | 0.09 | 6.85% | 1.45 | 1.28 | 0.17 | 13.31% |
| Turkey | 1.12 | 1.36 | −0.24 | −17.68% | 1.23 | 1.46 | −0.23 | −15.84% |
| UK | 2.83 | 2.92 | −0.09 | −2.83% | 3.23 | 3.11 | 0.12 | 3.76% |
| US | 30.52 | 31.34 | −0.82 | −2.64% | 32.09 | 31.99 | 0.10 | 0.33% |
| ∅ (winsorized) | −6.20% | 1.60% | ||||||
DDD = defined daily dose. Source: IQVIA MIDAS Quarterly Sales data Q1 2014–Q4 2021.
Population estimates of each country and region were obtained from the UN World Population Prospects 2019 report (UN, 2019). The daily stringency index for each country (Ritchie et al., 2020) is a composite measure of government's responses to the COVID-19 pandemic published by the Oxford COVID-19 Government Response Tracker (University of Oxford). It is based on nine response indicators including school closures, workplace closures, and travel bans, rescaled to a value from 0 to 100 (100 = strictest). Definitions of the defined daily dose (DDD), which is the assumed average maintenance dose per day for a drug used for its main indication in adults (WHO, 2022) were obtained by the WHO for each substance.
2.2. Data analysis
First, we calculated the DDD per 1000 inhabitants per day, the sold standard units (one tablet or capsule for oral solid forms, one teaspoon (5 ml) for syrup forms, one ampoule or vial for injectable forms) per person as well as the total sales value in Euro per year using the country-level sales volume data. The measure ‘DDD per 1000 inhabitants per day’ was calculated as following:
DDD per 1000 inhabitants per day = sum(1000 * ((standard.units * strength)/DDD)/population)/#days,
where standard.units are the number of sold standard units of each medication, strength is the dose of its active substance, DDD is the defined daily dose depending on the substance, population is the population estimate of the country at the time of sales and #days is the number of days in the time period. This measure is useful as it is standardized not only by time but also by population size. In contrast, the sum of sold standard units per person in a time period only takes the population size into account, while the total sales value simply accumulates the sales values of all sold medications in a time period.
We analysed the global and national ADHD medicine consumption trends in the pre-pandemic period (2014 to 2019) and during the pandemic (2020 to 2021) by calculating absolute and percentage changes. Additionally, time trend coefficients were estimated by linear regression models using consumption data in DDD per 1000 inhabitants per day up to 2019 including an intercept and time of sales as regressors. To quantify the impact of the COVID-19 pandemic, we forecasted the sales volume of the years 2020 and 2021 based on the national consumption trends until 2019. The impact was assessed by fitting a SARIMA model (Brockwell and Davis, 2016) and comparing its predictions to the actual sales volume in all three measures (DDD per 1000 inhabitants, standard units per person and sales value in Euro). As the US market accounts for a huge share of the entire ADHD medication market, we not only calculated the difference globally in DDD per 1000 inhabitants per day, which basically corresponds to an average weighted by the countries’ population size, but also unweighted means of the relative differences per country as measure of the country-level loss. This reflects much better the worldwide picture. Winsorised averages were used to avoid strong distortions by outliers. Countries with very high and very low losses during the pandemic period were identified. Further, we examined the association between the COVID-19 stringency index and the difference of the actual and the predicted medication use under non-pandemic conditions by calculating Spearman's correlation coefficient as it is more robust to outliers than Pearson. Last, substance-specific analyses were done. Some of the substances included in the data set are also approved for other medications than ADHD treatment. However, in this study only sales data of medications specifically indicated for the treatment of ADHD (ATC class N06B) were evaluated. We used R (R Core Team, 2022), version 4.1.3, for data analysis.
3. Results
3.1. Consumption of ADHD medicines in 47 countries and regions before COVID19 pandemic
In the pre-pandemic period, we found a significant steady increase (p < 0.001) in the global annual consumption of ADHD medicines (Table 1 ). The rise of consumed DDD per 1000 inhabitants per day from 3.08 in 2014 to 3.67 in 2019 corresponds to a relative change of 19.0% and a relative average increase of 3.09% annually. From 2019 to 2020 the consumption level nearly stagnated with a percentage growth of only 0.69%. However, from 2020 to 2021 we found a relative increase by 7.47% far above average. See Table S5 in the Supplement for global annual trends of the consumption in standard units and sales value.
Table 1.
Global annual ADHD medicine consumption and percentage change in 47 countries and regions, 2014–21. DDD = defined daily dose. Source: IQVIA MIDAS Quarterly Sales data Q1 2014–Q4 2021.
| 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | |
|---|---|---|---|---|---|---|---|---|
| Consumption in DDD per 1000 inhabitants per day | 3.08 | 3.27 | 3.34 | 3.52 | 3.57 | 3.67 | 3.69 | 3.97 |
| Relative change | 6.17% | 2.08% | 5.30% | 1.57% | 2.65% | 0.69% | 7.47% |
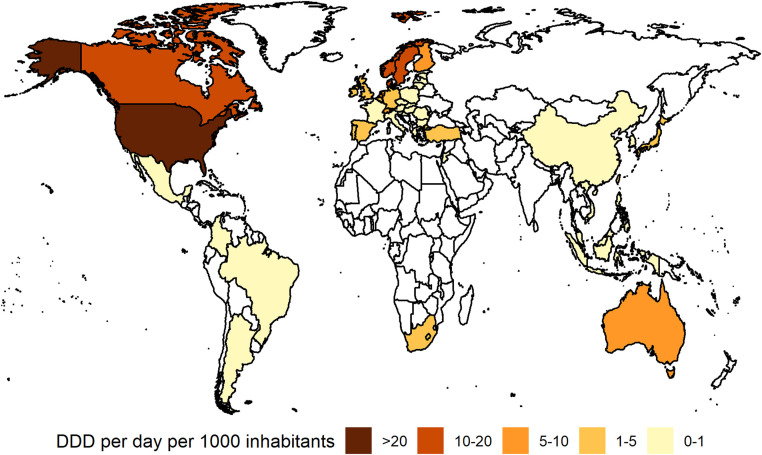
Considerable national variation was evident in the consumption of ADHD medicines during the pre-pandemic study period, ranging from 0.004 DDD per 1000 inhabitants per day in Colombia to 30.54 DDD per 1000 inhabitants per day in the US in 2019 (Fig. 1 ). Generally, the highest levels of ADHD medication use were recorded in North American countries (Canada: 18.1 DDD per 1000 inhabitants per day), in Scandinavia (Sweden: 16.8, Norway: 13.3, Denmark: 12.4 DDD per 1000 inhabitants per day), the Netherlands (8.8 DDD per 1000 inhabitants per day) and Australia (8.7 DDD per 1000 inhabitants per day). In 26 countries, mainly from Asia, East Europe and South America, the ADHD medication consumption level was very low (< 1 in DDD per 1000 inhabitants per day). The lowest usage levels besides Colombia were recorded in Bulgaria and the Philippines (both 0.009 DDD per 1000 inhabitants per day), Indonesia (0.013 DDD per 1000 inhabitants per day), China (0.016 DDD per 1000 inhabitants per day) and Malaysia (0.019 DDD per 1000 inhabitants per day).
Fig. 1.
Consumption of ADHD medicines in 47 countries and regions, 2019. White area = no data available. DDD = defined daily dose. Source: IQVIA MIDAS Quarterly Sales data Q1 2014–Q4 2021.
However, the trends in ADHD medicine consumption were comparable across most countries. An increase in ADHD medication use was found for all countries except Luxembourg, Romania and Colombia with average relative annual decreases of 3.22%, 2.61% resp. 11.1% in DDD per 1000 inhabitants per day from 2014 to 2019 (Table S1). The highest absolute changes from 2014 to 2019 in DDD per 1000 inhabitants per day were seen in the Scandinavian countries (Sweden +6.20, Norway +4.90, Denmark +4.2, Finland +3.2) as well as Australia (+3.7). The highest annual relative increase in DDD per 1000 inhabitants per day was recorded in Jordan with 67.27%, Lithuania with 58.01% and Latvia with 48.78%. Time trend estimates by linear regression models using quarterly data up to 2019 were significant at the 5% level in all countries and regions with exception of Argentina, Korea, Portugal, Romania and Spain. The time trend coefficients were estimated positive in all countries except Colombia, Luxembourg, Romania and Slovakia.
Besides similar time trends, the same seasonal pattern was observable in many countries and regions. Due to drug holidays which are mainly during the summer holiday season, medication use often drops in Quarter 3 (see Fig. S1 in the Supplement). This pattern was seen in 20 countries (Austria, Belgium, Canada, Czech Republic, Estonia, France, Germany, Greece, Hungary, Ireland, Italy, Luxembourg, Netherlands, Poland, Portugal, Slovenia, South Africa, Spain, Switzerland, Turkey) as well as in Australia and Argentina, where the drop in sales is observable in Quarter 1.
3.2. Substance-specific analyses
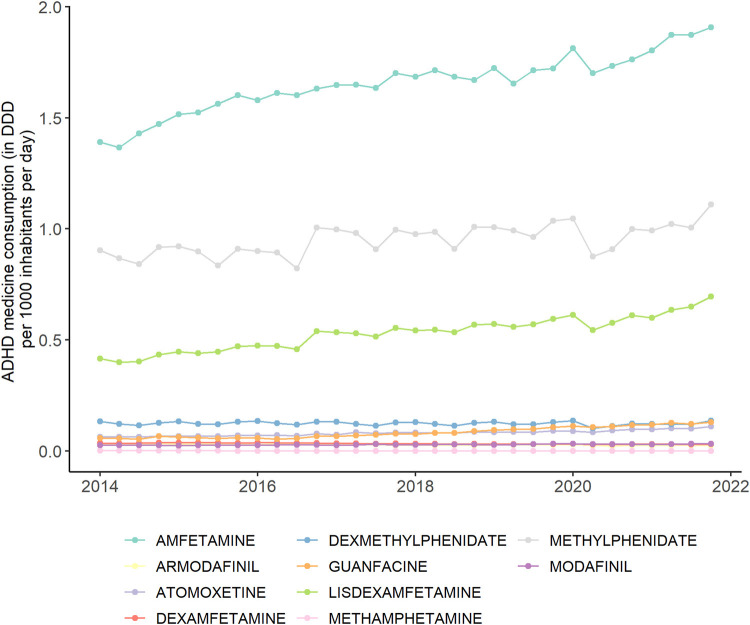
Ten different active substances in the ADHD medication were recorded in the considered 47 countries and regions: amfetamine, armodafinil, atomoxetine, dexamfetamine, dexmethylphenidate, guanfacine, lisdexamfetamine, methamphetamine, methylphenidate and modafinil. Overall, the most common substances were amfetamine, methylphenidate and lisdexamfetamine (Figs. 2 , S2, and Table S2 in the Supplement), which together account for approximately 90% of the total ADHD medication use. While amfetamine and methylphenidate have particularly high usage levels in the US, methylphenidate is clearly the most common in other countries and regions. The usage levels of the remaining eight substances were substantially lower. The share of each substance on the global consumption of ADHD medicine remained quite steady over the study period. Only lisdexamfetamine seems to have a slight increasing trend while methylphenidate's share seems to slightly decrease.
Fig. 2.
Quarterly global consumption of ADHD medicine by substance, 2014–2021. DDD = defined daily dose. Source: IQVIA MIDAS Quarterly Sales data Q1 2014–Q4 2021.
Analogous to the global upward trend of ADHD medicine consumption, most substances also show increasing usage levels (Fig. 2). We observed an increase in consumed DDD per 1000 inhabitants per day for seven of the ten substances, while declines were recorded for armodafinil, dexamfetamine and methamphetamine. Changes over time were significant (p < 0.001) for all substances except dexmethylphenidate (p = 0.37).
3.3. Consumption of ADHD medicines in 47 countries and regions during/after COVID19 pandemic
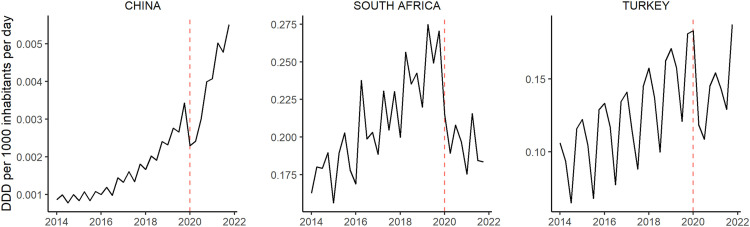
Starting in Quarter 2 of 2020, at the onset of the COVID-19 pandemic, almost all countries saw a plunge in the use of ADHD medications of varying length and severity (Fig. 3 ). In China, where the pandemic occurred a few months before it spread globally, we observed a sharp drop of sales in the first quarter. The annual percentage increase in global ADHD medication consumption was much smaller from 2019 to 2020 than in all previous years (Table 1). However, the relative increases from 2020 to 2021 in turn were remarkably high. Similar observations were made at the country-level in most countries and regions (Table S1). Comparing the trends per substance (Fig. 2), we observed a slight drop in the second quarter of 2020 as well, especially for amfetamine, methylphenidate, lisdexamfetamine and dexmethylphenidate.
Fig. 3.
Quarterly ADHD consumption in three countries before (2014–2019) and during the COVID-19 pandemic (2020–2021). Outbreak of the pandemic is marked as dashed red line in 2020, Quarter 1. DDD = defined daily dose. Source: IQVIA MIDAS Quarterly Sales data Q1 2014–Q4 2021.
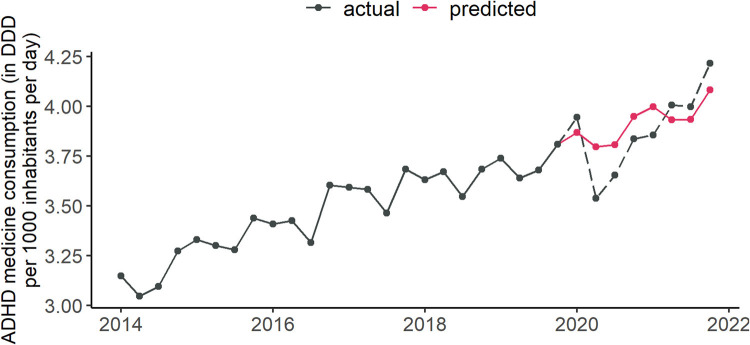
To quantify the impact of the COVID-19 pandemic on ADHD medication use, we calculated the difference between the actual sales in 2020–2021 and the predicted sales under normal, non-pandemic conditions for each country. Using the previous year sales volume of 2019 as reference values for the predicted sales in 2020 would not take into account the largely significant national trends. Therefore, a seasonal autoregressive integrated moving average (SARIMA) model of order (1, 1, 1) × (1, 1, 1)4 was fitted using the Maximum-Likelihood method for each country based on their quarterly sales during 2014 Q1 to 2019 Q4. The model estimates provided reliable forecasts for 2020 and 2021, projecting previous trends in the medication consumption under the assumption of constant conditions, i.e., no emergence of a worldwide pandemic and far-reaching mitigation measures. Fig. 4 shows the actual quarterly sales (DDD per 1000 inhabitants per day) form 2014 to 2019, the SARIMA forecast based on this data for 2020–2021, and the actual sales in 2020 and 2021. The reliability of SARIMA forecasts as a prediction method was shown using retrospective analyses in the pre-pandemic period. When predicting similarly the eight quarters of 2018 and 2019 by fitting a SARIMA model to data from 2014 to 2017, we found high forecast accuracy for all target values, likewise when predicting 2019 only based on the respective past. For details see Table S3 and Fig. S3 in the Supplement.
Fig. 4.
Predicted and actual global quarterly consumption of ADHD medication. DDD = defined daily dose. Source: IQVIA MIDAS Quarterly Sales data Q1 2014–Q4 2021.
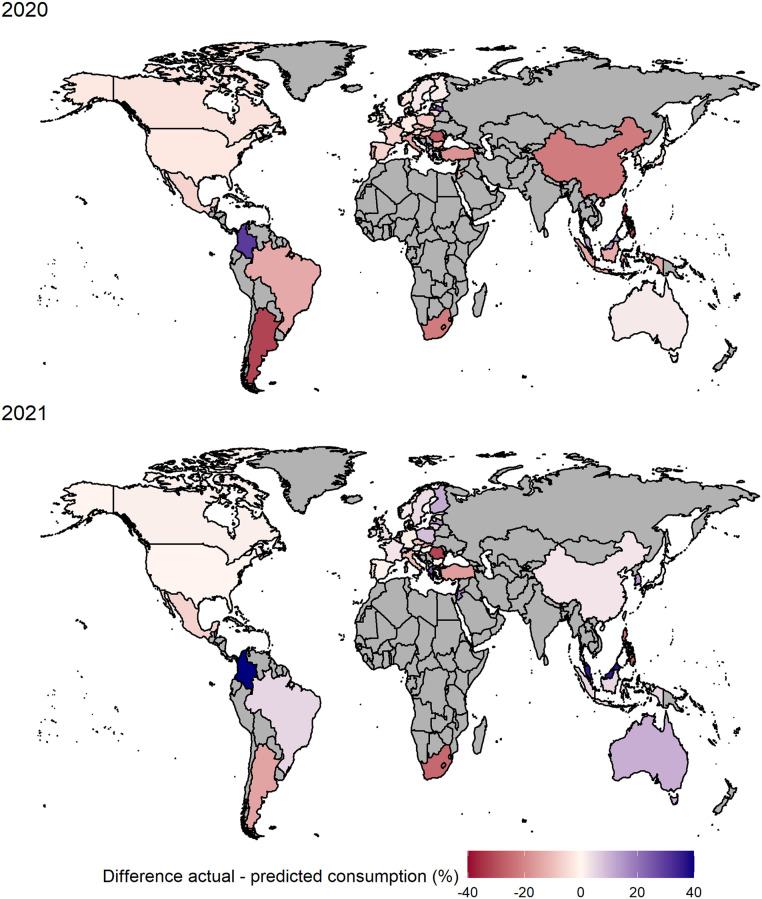
To calculate the predicted ADHD medication use in 2020 and 2021 under the assumption of unchanged conditions, we fitted a SARIMA model using the quarterly sales volume data from 2014 to 2019 and predicted the following eight quarters for each of the 47 countries and regions separately. The resulting predicted ADHD medicine consumption in DDD per 1000 inhabitants per day as well as their absolute and relative difference to the actual consumption per country are found in Table 2 . In 2020, the first year of the pandemic, we found lower actual consumption than predicted in 36 of the 47 countries and regions. The unweighted 95%-winsorised mean yields an average relative loss of 6.2% per country. Highest relative losses were recorded in Argentina (−32%), Philippines (−31%) and Romania (−27%). The greatest absolute losses were seen in Canada (−0.67 DDD per 1000 inhabitants per day), Portugal (−0.48 DDD per 1000 inhabitants per day) and Norway (−0.46 DDD per 1000 inhabitants per day). Only few countries recorded significantly greater consumption in 2020 than predicted: Netherlands and Greece (both +5%), Taiwan (+7%), Malaysia (+13%), Latvia (+24%) and Colombia (+109%). Colombia is a severe outlier in several ways: Firstly, the consumption pattern was steadily decreasing until 2019, in strong contrast to the worldwide trend. Secondly, Colombia recorded the lowest level of ADHD medicine consumption in our study. Thus, this extremely high percentage difference has little relevance. The largest absolute gains compared to the predicted consumption were found in the Netherlands (+0.47 DDD per 1000 inhabitants per day), Sweden (+0.42 DDD per 1000 inhabitants per day) and Australia (+0.2 DDD per 1000 inhabitants per day). Generally, we found stronger impact of COVID-19 in form of greater relative deviations between actual and predicted ADHD medication use in countries with a low consumption level. In regions with high usage levels like North America, Scandinavia and Australia, the losses in 2020 were fairly low (Fig. 5 ). Globally, the annual ADHD medication use in 2020 was 3.69 DDD per 1000 inhabitants per day and thus 0.13 lower than the prediction of 3.82, corresponding to a relative loss of 3.31% in 2020.
Fig. 5.
Percentage difference of actual and predicted ADHD medication consumption in 47 countries and regions in DDD per 1000 inhabitants per day in 2020 and 2021. Gray area = no data available. DDD = defined daily dose. Source: IQVIA MIDAS Quarterly Sales data Q1 2014–Q4 2021.
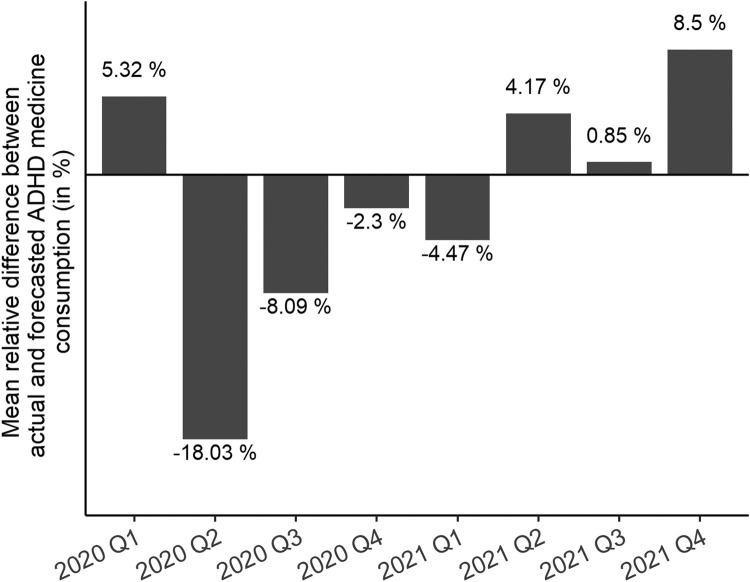
In 2021, the inhibiting effect of the pandemic on the ADHD medicine consumption ceased: The unweighted, winsorised mean yields a positive deviation of 1.60% per country compared to expectations. Only 17 of the 46 countries and regions showed lower actual medication use than predicted, with the highest relative losses in Romania (−32%), South Africa and the Philippines (both −25%) and highest absolute losses in South Africa (−0.55 DDD per 1000 inhabitants per day), Belgium (−0.39 DDD per 1000 inhabitants per day) and Turkey (−0.23 DDD per 1000 inhabitants per day). In most countries, actual ADHD medicine consumption in 2021 exceeded predicted consumption. The greatest relative positive deviations were found in Malaysia (36%), Greece (24%) and Jordan (18%), the greatest absolute excesses in the Netherlands (+1.68 DDD per 1000 inhabitants per day), Australia (+1.23 DDD per 1000 inhabitants per day) and Finland (+0.87 DDD per 1000 inhabitants per day). The again extreme percentage difference in Colombia (+353%) can be considered as outlier for the above reasons. Globally, the actual ADHD medication consumption of 3.97 DDD per 1000 inhabitants per day exceeds the forecasted consumption by 0.02 DDD per 1000 inhabitants per day corresponding to a percentage difference of about 0.37%. Averaging the winsorised relative differences across all countries per quarter (Fig. 6 ) revealed that consumption started to drop in Quarter 2 of 2020, where the loss is also the greatest with approximately 18% on average. In the following three quarters the average loss becomes smaller until the second quarter of 2021, where the actual consumption starts to exceed the predictions. In Quarter 4 of 2021 the actual medication use was on average 8.5% greater than predicted.
Fig. 6.
Mean relative difference between actual and predicted ADHD medicine consumption (in DDD per 1000 inhabitants per day) per quarter, 2020–2021. DDD = defined daily dose. Source: IQVIA MIDAS Quarterly Sales data Q1 2014–Q4 2021.
3.4. Correlation between the median stringency index and ADHD medicine consumption
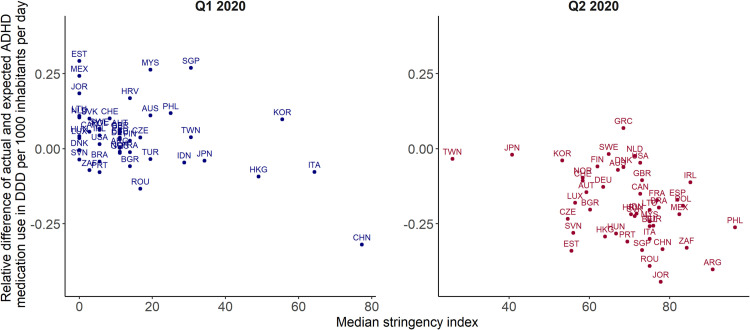
We found negative associations between the median stringency index and the relative difference between the actual and the predicted sales volume of each country. We calculated Spearman's correlation coefficient per year and overall and tested for significance (Table S4 in the Supplement). This yielded a significant overall correlation estimate of −0.34 (p < 0.001). In 2020 the association is particularly strong achieving a correlation of −0.51 (p < 0.001), in 2021 it reduced to −0.22 (p = 0.003). Fig. 7 illustrates the negative correlation in Quarter 1 and 2 of 2020: the greater the stringency index, i.e., the stricter the anti-pandemic regulations, the greater the relative decrease in ADHD medication use compared to the forecasts. We found particularly strong correlation between the mean quarterly school closure index and the ADHD medicine use with an overall estimate of −0.39 (p < 0.001). In 2020, the Spearman correlation coefficient is even −0.55 (p < 0.001), in 2021 it reduces to −0.25 (p < 0.001).
Fig. 7.
Correlation between the median stringency index and the relative difference of actual and predicted ADHD medicine consumption in Quarter 1 (Q1) and Quarter 2 (Q2) of 2020. DDD = defined daily dose. Source: IQVIA MIDAS Quarterly Sales data Q1 2014–Q4 2021.
4. Discussion
This study reports ADHD medication sales volumes for 47 countries and regions. We compared global trends and noted a mean annual consumption increase of 3.09% in DDD per 1000 inhabitants per day in the pre-pandemic period from 2014 to 2019. Consumption declines were only recorded in three countries. The comparable trends across most countries are similar to previously published research (Bachmann et al., 2017; Man et al., 2017). The observation of strong variation in the prevalence of ADHD medication use with markedly higher usage levels in North America and North European countries is in line with previous studies (Bachmann et al., 2017; Raman et al., 2018). Consistent with treatment guidelines, the most common substances were amfetamine, methylphenidate and lisdexamfetamine.
In the second quarter of 2020, when measures aimed at preventing the spread of the Coronavirus were taken, we observed a drop in medication consumption in most countries disrupting the previous trends. In 2020, 36 of the 47 countries and regions recorded lower consumption in DDD per 1000 inhabitants per day than predicted. On average, consumption was 6.20% below the predictions at country-level with particularly high losses in the second quarter. In 2021, the impact of the pandemic seems to be largely diminished. Only seventeen countries recorded fewer actual sales than predicted. On average, ADHD medication consumption exceeded expectations by 1.60% per country. The positive deviations from the predictions were highest in the fourth quarter of 2021. Generally, the impact of the pandemic was higher in regions with a low consumption level. In North American and Scandinavian countries, which have the highest levels of ADHD medication usage, the losses in 2020 were fairly low. Globally, the actual annual consumption of 3.69 DDD per 1000 inhabitants per day was 0.13 below the prediction, corresponding to a relative loss of 3.31% in 2020. In 2021, the actual annual consumption of 3.97 DDD per 1000 inhabitants per day exceeds the forecast by 0.37%.
The losses during the studied pandemic period strongly negatively correlated with the stringency index, especially in 2020, indicating that greater preventive measures led to lower consumption of medications for ADHD. This might be for several reasons: firstly, mitigation measures included stay at home recommendations leading to fewer health care visits and thus to fewer prescriptions. Additionally, a decline of healthcare workers due to quarantine regulations and terminations caused a shortage of skilled professionals (Gohar et al., 2020), as well as supply chain problems, impeding the access to ADHD prescriptions. Secondly, the consumption drops in 2020 might also be explained by parental decision to reduce ADHD medication dosages during school closures, analogous to drug holidays (Segenreich, 2022). This idea is supported by the strong correlation of school closures and consumption losses in 2020. Decreased medication intake could diminish or cancel out the positive impact of methylphenidate on hyperactivity and inattention as its effects are limited to the days on which the patient takes the medication (Tamminga et al., 2021).
In 2021, when containment measures were scaled back, the consumption of ADHD medications returned to and even exceeded the level one would have expected if the pre-pandemic trends had continued without disruptive events. Possible causes are a backlog from patients who had not received sufficient treatment in 2020 or the general increase of mental health issues, including the worsening of ADHD symptoms (Panda et al., 2021), which might lead to greater medication demand. Recent meta-analyses also showed that higher levels of social withdrawal, which occurred as consequence of the COVID19 containment measures, decreased the likelihood of symptom remission in patients with major psychiatric disorders (Olivia et al., 2022).
We found no structural changes in the distribution of active substances in 2020 or 2021 compared to the previous years, only a slight drop in the second quarter of 2020 for most substances.
Other studies which aimed to examine the medication use during the COVID19 pandemic also found a decline in psychotropic drug use in Canadian children and adolescents in 2020 (Leong et al., 2022), and an increase in rates of psychotropic treatment with hypnotics, antidepressants and psychostimulants for Danish youths with and without psychiatric disorder in March 2020 to June 2022 (Bliddal et al., 2022). Both suggested similar potential underlying causes as we conjectured. Kuitunen (2022) found an increase in the prevalence of psychostimulants users in Finnish pediatric children already in the third and fourth quarter of 2020, while we found that the consumptions were lower than predicted. However, the study only compared the consumption of 2020 to 2019 without taking the pre-pandemic growing trends into account.
We conducted analogous analyses based on standard units and sales value in Euro as consumption measure. The qualitative conclusions are the same. Details are given in Tables S5, S6 and S7 in the Supplement. The sales value from ADHD medication sales decreased throughout 2014 to 2019 annually by 1.06% on average (see Table S5 in the Supplement). This is caused by a decline in the United States, whose sales volume accounts for approximately 80% of the global sales. The growth of the US ADHD market was mainly driven by low-cost generic drugs, which leads to descending sales values. Outside the US, we recorded a sharp increase in the annual sales value of 120% in the pre-pandemic period. In 2020 annual sales value suffered a loss of 10.09% per country on average compared with the predictions (Table S7 in the Supplement). Globally, it was 0.004% lower than predicted. In 2021 the global sales value was 6.48% greater than predicted, however, the unweighted, winsorised mean per country still revealed a loss of 1.99% per country.
To our knowledge, this study is the first to quantify the effect of COVID-19 pandemic induced restrictions on the use of ADHD medications. The use of international pharmaceutical sales data enabled an analysis of global trends and changes in the ADHD medication consumption before and during the pandemic. However, the sales data do not contain individual-level treatment data which are needed to measure trends by age, gender or appropriateness of prescribing. For this reason, we cannot draw any conclusions regarding overuse, underuse or misuse. In the past there has been clear evidence that ADHD medications are also used off-label (Faraone et al., 2020; Sinita and Coghill, 2014). As this study only evaluates sales data of ADHD medications, the prevalence of ADHD was not taken into account. However, medication consumption measured as active substance quantity per day standardized by population size serves as suitable proxy.
Further research is warranted to describe the impact of the pandemic restrictions on ADHD prevalence and medication trends in children and adults, as well as the consequences for mental health, well-being and functioning in the academic, social and/or workplace domains, after the social distancing measures. Moreover, prescribers and health care systems should develop procedures that can maintain patients on medications prescribed for ADHD during pandemics and other events that disrupt access to typical care pathways.
Contributors
S.G. drafted the manuscript, conducted the literature search, made the figures and carried out the data preparation and analysis. D.V. conceptualised the study. D.V. and R.F. advised on statistical methodology and reviewed the analysis. R.A. acquired the data and the funding. All authors reviewed the results and critically revised the manuscript for important intellectual content. S.G., D.V., and R.F. have directly accessed and verified the underlying data.
Role of the funding source
The licence for the IQVIA MIDAS data was funded by MEDICE Arzneimittel Pütter GmbH & Co. KG.
Data sharing
The underlying MIDAS data were provided by IQVIA under licence. The terms of our agreement do not permit disclosure, sublicensing, or sharing of IQVIA MIDAS data. The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the following IQVIA Ltd. information service: MIDAS, 4Q2014 – 2Q2022. All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IQVIA Ltd. or any of its affiliated or subsidiary entities.
Conflict of Interest
S.G. and D.V. are employees of MEDICE Arzneimittel Pütter GmbH & Co. KG. R.F. holds grants from the German Research Foundation (Deutsche Forschungsgemeinschaft) and the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung) unrelated to this work. S.V.F. has received royalties from Elsevier, Guilford Press, Oxford University Press, holds grants from Noven, Otsuka, Shire/Takeda, Arbor, and Supernus, has received consulting fees/honoraria from Akili Interactive Labs, Arbor, Aardvark, Aveksham, Genomind, Ironshore, KemPharm/Corium, Noven, Ondosis, Rhodes, Vallon, holds stock options of Aardvark, Akili, Ironshore, Genomind, and is holder of the US Patent US20130217707 A1. T.B. has received royalties from Hogrefe, Kohlhammer, CIP Medien, Oxford University Press, has received consulting fees/honoraria from Infectopharm, Janssen Cilag, Medice, Takeda, and is on advisory boards of eyelevel, Infectopharm, Lundbeck, MEDICE, Neurim Pharmaceuticals, Oberberg GmbH, Roche, Takeda. J.B. is on advisory boards of MEDICE, Angelini, Boehringer-Ingelheim, Servier. M.D. received consulting income and research support from Lilly, MEDICE, Shire, Takeda, and eyelevel and research support from the German Research Foundation, German Ministry of Education and Research, German Ministry of Health, and Innovation Fund. He received income as head, supervisor, and lecturer of the School of Child and Adolescent Cognitive Behaviour Therapy at the University Hospital Cologne and as consultant for Child Behaviour Therapy at the National Association of Statutory Health Insurance Physicians (Kassenärztliche Bundesvereinigung). He also received royalties from treatment manuals, books and psychological tests published by Beltz, Elsevier, Enke, Guilford, Hogrefe, Huber, Kohlhammer, Schattauer, Springer, Wiley. A.R. is owner and CEO of MEDICE Arzneimittel Pütter GmbH & Co. KG.
Acknowledgements
We thank MEDICE Arzneimittel Pütter GmbH & Co. K.G. for funding this study. We further thank IQVIA for their assistance and information regarding the use of MIDAS data.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.euroneuro.2023.04.008.
Appendix. Supplementary materials
References
- American Psychiatric Association . American Psychiatric Publishing; Arlington: 2013. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. [Google Scholar]
- Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395(10228):931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann C.J., Wijlaars L.P., Kalverdijk L.J., et al. Trends in ADHD medication use in children and adolescents in five western countries, 2005–2012. Eur. Neuropsychopharmacol. 2017;27(5):484–493. doi: 10.1016/j.euroneuro.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Behrmann J.T., Blaabjerg J., Jordansen J., de López K.M.J. Systematic review: investigating the impact of COVID-19 on mental health outcomes of individuals with ADHD. J. Atten. Disord. 2022;26(7):959–975. doi: 10.1177/10870547211050945. [DOI] [PubMed] [Google Scholar]
- Bliddal M., Rasmussen L., Andersen J.H., et al. Pschotropic medication use and psychiatric disorders during the covid-19 pandemic among danish children, adolescents, and young adults. JAMA Psychiatry. 2022;80(2):176–180. doi: 10.1001/jamapsychiatry.2022.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolea-Alamañac B., Nutt D.J., Adamou M., et al. Evidence-based guidelines for the pharmacological management of attention deficit hyperactivity disorder: update on recommendations from the british association for psychopharmacology. J. Psychopharmacol. 2014;28(3):179–203. doi: 10.1177/0269881113519509. [DOI] [PubMed] [Google Scholar]
- Brockwell P.J., Davis R.A. Springer International Publishing; New York: 2016. Introduction to Time Series and Forecasting. Springer Texts in Statistics. [Google Scholar]
- Burcu M., Zito J.M., Metcalfe L., Underwood H., Safer D.J. Trends in stimulant medication use in commercially insured youths and adults, 2010-2014. JAMA Psychiatry. 2016;73(9):992–993. doi: 10.1001/jamapsychiatry.2016.1182. [DOI] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC) 2022. Prevention and Control of COVID-19.https://www.ecdc.europa.eu/en/all-topics-z/COVID-19/prevention-and-control-COVID-19 accessed May 21, 2022. [Google Scholar]
- Faraone S.V., Asherson P., Banaschewski T., et al. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers. 2015;1 doi: 10.1038/nrdp.2015.20. [DOI] [PubMed] [Google Scholar]
- Faraone S.V., Banaschewski T., Coghill D., et al. The world federation of ADHD international consensus statement: 208 evidence-based conclusions about the disorder. Neurosci. Biobehav. Rev. 2021;128:789–818. doi: 10.1016/j.neubiorev.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone S.V., Biederman J., Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol. Med. 2006;36(2):159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Faraone S.V., Rostain A.L., Montano C.B., et al. Systematic review: nonmedical use of prescription stimulants: risk factors, outcomes, and risk reduction strategies. J. Am. Acad. Child Adolesc. Psychiatry. 2020;59:100–112. doi: 10.1016/j.jaac.2019.06.012. [DOI] [PubMed] [Google Scholar]
- Fico G., Isayeva U., De Prisco M., et al. Psychotropic drug repurposing for COVID-19: a systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2022;66:30–44. doi: 10.1016/j.euroneuro.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohar B., Larivière M., Nowrouzi-Kia B. Sickness absence in healthcare workers during the COVID-19 pandemic. Occup. Med. 2020;70(5):338–342. doi: 10.1093/occmed/kqaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw S., Scheffler R., Fulton B., et al. International variation in treatment procedures for ADHD: social context and recent trends. Psychiatr. Serv. 2011;62(5):459–464. doi: 10.1176/ps.62.5.pss6205_0459. [DOI] [PubMed] [Google Scholar]
- IMF . 2020. World Economic Outlook, April 2020: The great Lockdown.https://www.imf.org/en/Publications/WEO/Issues/2020/04/14/World-Economic-Outlook-April-2020-The-Great-Lockdown-49306 (Accessed 5 June 2022) [Google Scholar]
- Jensen C.M., Steinhausen H.C. Comorbid mental disorders in children and adolescents with attention-deficit/hyperactivity disorder in a large nationwide study. Atten. Defic. Hyperact. Disord. 2015;7(19):27–38. doi: 10.1007/s12402-014-0142-1. [DOI] [PubMed] [Google Scholar]
- Karlstad O., Zoega H., Furu K., et al. Use of drugs for ADHD among adults—A multinational study among 15.8 million adults in the nordic countries. Eur. J. Clin. Pharmacol. 2016;72:1507–1514. doi: 10.1007/s00228-016-2125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij J.J.S., Bijlenga D., Salerno L., et al. Updated European Consensus Statement on diagnosis and treatment of adult ADHD. Eur. Psychiatry. 2019;56:14–34. doi: 10.1016/j.eurpsy.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Kuitunen I. Psychotropic medication use in pediatric population during COVID-19 pandemic. Acta Psychiatr. Scand. 2022;146(4):381–383. doi: 10.1111/acps.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong C., Katz L.Y., Bolton J.M., et al. Psychotropic drug use in children and adolescents before and during the COVID-19 pandemic. JAMA Pediatr. 2022;176(3):318–320. doi: 10.1001/jamapediatrics.2021.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Chua C.R., Xiong Z., Ho R.C., Ho C.S.H. A systematic review of the impact of viral respiratory epidemics on mental health: an implication on the coronavirus disease 2019 pandemic. Front. Psychiatry. 2020;11(565098) doi: 10.3389/fpsyt.2020.565098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man K.K.C., Ip P., Hsia Y., et al. ADHD drug prescribing trend is increasing among children and adolescents in Hong Kong. J. Atten. Disord. 2017;21(14):1161–1168. doi: 10.1177/1087054714536047. [DOI] [PubMed] [Google Scholar]
- Manchia M., Gathier A.W., Yapici-Eser H., et al. The impact of the prolonged COVID-19 pandemic on stress resilience and mental health: a critical review across waves. Eur. Neuropsychopharmacol. 2022;55:22–83. doi: 10.1016/j.euroneuro.2021.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer N., Sánchez-Mora C., Rovira P., et al. Transciptome profiling in adult attention-deficit hyperactivity disorder. Eur. Neuropsychopharmacol. 2020;41:160–166. doi: 10.1016/j.euroneuro.2020.11.005. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (NICE) Vol. 87. 2019. https://www.nice.org.uk/guidance/ng87 (Attention Deficit Hyperactivity disorder: Diagnosis and management. NICE Guideline). accessed May 10, 2022. [PubMed] [Google Scholar]
- Oliva V., Fanelli G., Kasper S., et al. Social withdrawal as a trans-diagnostic predictor of short-term remission: a meta-analysis of five clinical cohorts. Int. Clin. Psychopharmacol. 2022;37(2):38–45. doi: 10.1097/YIC.0000000000000384. [DOI] [PubMed] [Google Scholar]
- Panda P.K., Gupta J., Chowdhury S.R., et al. Behavioral impact of lockdown and quarantine measures for COVID-19 pandemic on children, adolescents and caregivers: a systematic review and meta-analysis. J. Tropic. Pedr. 2021;67(1) doi: 10.1093/tropej/fmaa122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka S., Bernet W., Bukstein O., Walter H. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46(7):894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- Polanczyk G., de Lima M.S., Horta B.L., Biederman J., Rohde L.A. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am. J. Psychiatry. 2007;164(6):942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Polanczyk G., Willcutt E., Salum G., Kieling C., Rohde L. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int. J. Epidemiol. 2014;43:434–442. doi: 10.1093/ije/dyt261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol-Gualdo N., Sánchez-Mora C., Ramos-Quiroga J.A., et al. Integrating genomics and transcriptomics: towards deciphering ADHD. Eur. Neuropsychopharmacol. 2021;44:1–13. doi: 10.1016/j.euroneuro.2021.01.002. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2022. R: A Language and Environment for Statistical Computing. R Foundation For Statistical Computing, Vienna, Austria.https://www.R-project.org/ [Google Scholar]
- Raman S., Man K., Bahmanyar S., et al. Trends in attention-deficit hyperactivity disorder medication use: a retrospective observational study using population-based databases. Lancet Psychiatr. 2018;5:824–835. doi: 10.1016/S2215-0366(18)30293-1. [DOI] [PubMed] [Google Scholar]
- Ritchie H., Mathieu E., Rodés-Guirao L., et al. 2020. Coronavirus Pandemic (COVID-19). Our World in Data.https://ourworldindata.org/coronavirus (Accessed 3 June 2022) [Google Scholar]
- Santomauro D.F., Herrera A.M.M., Shadid J., et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398(10312):1700–1712. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciberras E., Patel P., Stokes M., et al. Physical health, media use, and mental health in children and adolescents with ADHD during the COVID-19 pandemic in Australia. J. Atten. Disord. 2022;26(4):549–562. doi: 10.1177/1087054720978549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmacker G., Groenman A., Sokolova E., et al. Role of conduct problems in the relation between Attention-Deficit Hyperactivity disorder, substance use, and gaming. Eur. Neuropsychopharmacol. 2020;30:102–113. doi: 10.1016/j.euroneuro.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Segenreich D. The impact of the COVID-19 pandemic on diagnosing and treating attention deficit hyperactivity disorder: new challenges on initializing and optimizing pharmacological treatment. Front. Psychiatry. 2022;13 doi: 10.3389/fpsyt.2022.852664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setyawan J., Fridman M., Grebla R., Harpin V., Korst L.M., Quintero J. Variation in presentation, diagnosis, and management of children and adolescents with ADHD across european countries. J. Att. Disord. 2018;22(10):911–923. doi: 10.1177/1087054715597410. [DOI] [PubMed] [Google Scholar]
- Shah R., Raju V.V., Sharma A., Grover S. Impact of COVID-19 and lockdown on children with ADHD and their families - an online survey and a continuity care model. J. Nerosci. Rural Pract. 2021;12(1):71–79. doi: 10.1055/s-0040-1718645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M., Hodgkins P., Caci H., et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med. 2012;10:99. doi: 10.1186/1741-7015-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon V., Czobor P., Bálint S., Mészáros A., Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br. J. Psychiatr. 2009;194(3):204–211. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- Sinita E., Coghill D. The use of stimulant medications for non-core aspects of ADHD and in other disorders. Neuropharmacology. 2014;87:161–172. doi: 10.1016/j.neuropharm.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Song P., Zha M., Yang Q., Zhang Y., Li X., Rudan I. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. Glob. Health. 2021;11:04009. doi: 10.7189/jogh.11.04009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga H.G.H., Reneman L., Schrantee A., et al. Do effects of methylphenidate on cognitive performance last beyond treatment? A randomized placebo-controlled trial in boys and men with ADHD. Eur. Neuropsychopharmacol. 2021;46:1–13. doi: 10.1016/j.euroneuro.2021.02.002. [DOI] [PubMed] [Google Scholar]
- Thapar A., Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016;387:1240–1250. doi: 10.1016/S0140-6736(15)00238-X. [DOI] [PubMed] [Google Scholar]
- Thomas R., Sanders S., Doust J., Beller E., Glasziou P. Prevalence of attention deficit hyperactivity disorder: a systematic review and meta-analysis. Pediatric. 2015;135(4):994–1001. doi: 10.1542/peds.2014-3482. [DOI] [PubMed] [Google Scholar]
- United Nations (UN) 2019. World Population Prospects 2019.https://population.un.org/wpp/ (Accessed 26 April 2022) [Google Scholar]
- Wilens T.E., Spencer T.J. Understanding attention-deficit/hyperactivity disorder form childhood to adulthood. Postgrad. Med. 2010;122(5):97–109. doi: 10.3810/pgm.2010.09.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolraich M.L., Hagan J.F.Jr, Allan C., et al. Clinical Practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents [published correction appears in pediatrics. 2020, 145(3)] Pediatrics. 2019;144(4) doi: 10.1542/peds.2019-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) 2020. Mental Health and Psychosocial Considerations During the COVID-19 Outbreak. accessed May 1, 2022. [Google Scholar]
- World Health Organization (WHO) 2020. COVID-19.https://www.who.int/docs/default-source/coronaviruse/transcripts/who-audio-emergencies-coronavirus-press-conference-full-20mar2020.pdf?sfvrsn=1eafbff_0 (Accessed 3 June 2022) [Google Scholar]
- WHO . 2022. Collaborating Centre for Drug Statistics Methodology Oslo.https://www.whocc.no/ (Accessed 3 May 2022) [Google Scholar]
- Zito J.M., Burcu M. Stimulants and pediatric cardiovascular risk. J. Child Adolesc. Psychopharmacol. 2017;27(6):538–545. doi: 10.1089/cap.2015.0239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.