Summary
Background
Plasmacytoid dendritic cells (pDCs) sense viral and bacterial products through Toll-like receptor (TLR)-7 and -9 and translate this sensing into Interferon-α (IFN-α) production and T-cell activation. The understanding of the mechanisms involved in pDCs stimulation may contribute to HIV-cure immunotherapeutic strategies. The objective of the present study was to characterize the immunomodulatory effects of TLR agonist stimulations in several HIV-1 disease progression phenotypes and in non HIV-1 infected donors.
Methods
pDCs, CD4 and CD8 T-cells were isolated from 450 ml of whole blood from non HIV-1 infected donors, immune responders (IR), immune non responders (INR), viremic (VIR) and elite controller (EC) participants. pDCs were stimulated overnight with AT-2, CpG-A, CpG-C and GS-9620 or no stimuli. After that, pDCs were co-cultured with autologous CD4 or CD8 T-cells and with/without HIV-1 (Gag peptide pool) or SEB (Staphylococcal Enterotoxin B). Cytokine array, gene expression and deep immunophenotyping were assayed.
Findings
pDCs showed an increase of activation markers levels, interferon related genes, HIV-1 restriction factors and cytokines levels after TLR stimulation in the different HIV-disease progression phenotypes. This pDC activation was prominent with CpG-C and GS-9620 and induced an increase of HIV-specific T-cell response even in VIR and INR comparable with EC. This HIV-1 specific T-cell response was associated with the upregulation of HIV-1 restriction factors and IFN-α production by pDC.
Interpretation
These results shed light on the mechanisms associated with TLR-specific pDCs stimulation associated with the induction of a T-cell mediated antiviral response which is essential for HIV-1 eradication strategies.
Funding
This work was supported by Gilead fellowship program, the Instituto de Salud Carlos III (Fondo Europeo de Desarrollo Regional, FEDER, “a way to make Europe”) and the Red Temática de Investigación Cooperativa en SIDA and by the Spanish National Research Council (CSIC).
Keywords: Plasmacytoid dendritic cells, TLR agonists, HIV-Infection, HIV-1 restriction factors and immunotherapy
Research in context.
Evidence before this study
pDCs are considered a link between innate and adaptive immunity, inducing and maintaining antigen-specific T-cell responses and contributing to the control and eventually to the immune activation and disease progression in HIV-infection scenario. On the other hand, TLR agonists have been recently used for immunomodulatory strategies demonstrating an improvement of pDCs functionality and antiviral adaptive responses. However, the cellular and molecular mechanisms by which TLR stimulation can modulate HIV-1 replication are still largely unclear.
Added value of this study
pDCs, after TLR agonist stimulation, showed an activated phenotype determined by an increased MFI of HLA-DR, CCR7 and CD86 expression. Interferon related genes; restriction factors and cytokines levels were also increased. This pDCs activation induces an increase of HIV-specific T-cell response that was especially prominent with CpG-C and GS-9620. pDCs cytokines production and restriction factors were associated with HIV-specific T-cell response. These results, demonstrate an increased activation phenotype of pDCs that favor an effective antigen presentation to CD4 and CD8 T-cell.
Implications of all the available evidence
To elicit an antiviral response is essential for current HIV-1 eradication strategies. The understanding of the mechanisms involved in pDC stimulation may contribute to immunotherapeutic strategies aiming to decrease HIV reservoir. In this sense, the current study provides a better understanding of how TLR-specific pDCs stimulation induce antiviral T-cell response.
Introduction
Antiretroviral therapy (ART) for the treatment of Human Immunodeficiency Virus (HIV)-1 infection has dramatically improved survival.1,2 Although ART is highly effective at suppressing HIV-1 replication, viral reservoirs persist despite treatment and lead to rapid viral rebound when ART is interrupted.3 The latent HIV-1 reservoir is predominantly found in long-lived memory CD4 T-cells in which the provirus is transcriptionally silent and undetected by the host immune system.4 Great efforts have been made to understand how to harness adaptive immune response to ultimately eliminate HIV-infected cells; however, less attention has been placed to the link of innate immune function to mount an effective adaptive immune response. The innate immune system constitutes a key part of the early defence and control of established HIV-1 infection. Important players of innate immune response are the plasmacytoid dendritic cells (pDCs). These cells represent less than 1% of the peripheral blood mononuclear cells (PBMCs) and are considered a link between innate and adaptive immunity. pDCs are the main type I Interferon (IFN-α, -β, -ε, -ω and -κ) producers by Toll-like receptor (TLR) −7 and −9 stimulation, conferring a high antiviral capacity.5 These intracellular TLRs recognize viral and microbial nucleic acids and rapidly induce an immune response characterized by the production of acute-phase cytokines and antiviral factors.6 The IFN-I production activates antiviral host restriction factors7,8 and involves the activation of other cell types such as natural killer cells (NK) and myeloid dendritic cells (mDCs).9 pDCs have been associated with the spontaneous control of HIV-1 infection through IFN-α production and HIV-infected CD4 T-cell apoptosis induction.10,11 In addition, we also previously reported that after TLR-7 and -9 stimulation, hepatitis C virus (HCV) infectivity was reduced through pDCs’ IFN-α production.12 TLR-7 and -9 stimulated pDCs exhibited a mature phenotype, increasing the antigen presentation and lymph node homing-related markers.12 Therefore, a better understanding of how TLR-specific pDC stimulation induce and antiviral T-cell response is essential for current HIV-1 eradication strategies.
TLR-7 and -9 agonists have been recently used for immunomodulatory strategies demonstrating an improvement of pDCs functionality and antiviral adaptive responses. Buitendijk et al. observed that agonists for TLR-3, TLR-7, TLR-8 and TLR-9 all inhibited HIV-1 infection and induced IFN-α/interferon-stimulated genes (ISGs) expression in PBMCs in vitro13. GS-9620 is a potent, orally active TLR-7 agonist with selectivity for induction of IFN-α over proinflammatory cytokines.14 It has been reported that oral administration of an analog of GS-9620 in simian immunodeficiency virus (SIV)-infected rhesus macaques under ART induced transient plasma viremia, reduced the viral DNA content in lymphoid tissues, and established lower viral set points after ART cessation.15 Additionally, in the simian-human immunodeficiency virus (SHIV)-infected rhesus macaques model, the co-administration of GS-9620 and the potent neutralizing antibody PGT121 delayed viral rebound following discontinuation of ART and induced durable virus control in 55% of the animals.16 In human, a recent study shows that GS-9620 could contribute to a modest delay in viral rebound in ART-treated HIV-1 subjects who undergo ART interruption.17
The cellular and molecular mechanisms by which TLR stimulation can modulate HIV-1 replication are still largely unclear. To achieve this goal, we have designed a co-culture system of pDCs (previously stimulated through TLR-7 and -9 agonists) with autologous CD4 or CD8 T-cells from people living with HIV-1 (PLWH) with diverse HIV-1 disease progression phenotypes. Our aim was to investigate how TLR-7 and -9 agonists could serve as a potential booster of pDCs activity and consequently enhance virus specific T-cell response.
Methods
Study subjects
The study was performed at Clinic Unit of Infectious Diseases, Microbiology and Parasitology at Virgen del Rocío University Hospital (Seville, Spain). PLWH were recruited from the Clinic Unit of Infectious Diseases HIV-infected patients’ cohort and classified according to the HIV-1 disease progression phenotype in four groups: 1) HIV-1 Elite Controllers (EC), were defined as PLWH with undetectable viral loads (<40 HIV-1 RNA copies/ml) in the absence of any ART during at least last year; 2) Viremic patients (VIR), were defined as PLWH naïve for any ART with high viral loads above 104 HIV-1 RNA copies/ml; 3) Immune Responders (IR), were defined as PLWH on ART with undetectable viral load (<40 HIV-1 RNA copies/ml) at least during the last six months and with CD4 T-cells counts above 350 CD4 T cell/mm3; 4) Immune Non Responders (INR), were defined as PLWH on ART with undetectable viral loads at least during the last six months who fail to achieve 350 CD4 T-cell/mm3 during at least two years on ART. All the participants recruited were white European (Table 1). Every PLWH donated 450 ml of peripheral blood. The same amount of whole blood was obtained from a control group (Ctrl, n = 21), seronegative for HIV and HCV, obtained at the Regional Center for Blood Transfusion and Tissue Bank Sevilla- Huelva (Seville, Spain).
Table 1.
Characteristics of the studied participants.
| Ctrl (n = 21) | PLWH (n = 23) |
p valuea (Ctrl vs PLWH) | ||||
|---|---|---|---|---|---|---|
| HIV-1 VIR (n = 7) | HIV-1 IR (n = 6) | HIV-1 INR (n = 6) | HIV-1 EC (n = 4) | |||
| Male sex, n (%) | 15 (71.42) | 7 (100) | 5 (83.3) | 6 (100) | 3 (75) | 0.880 |
| Age, (years) | 42 [35–51] | 36 [32–45] | 50 [41–62] | 56 [49–60] | 48 [37–57] | 0.697 |
| CD4 Nadir counts, (cells/μl) | NA | 376 [273–448] | 162 [75–310] | 42 [36–86] | 615 [459–697] | NA |
| CD4+ T-cell counts, (cells/μl) | NA | 354 [299–456] | 724 [536–1022] | 277 [157–322] | 738 [611–940] | NA |
| CD8+ T-cell counts, (cells/μl) | NA | 773 [527–1148] | 592 [376–1011] | 711 [423–1561] | 543 [477–585] | NA |
| CD4+/CD8+ T-cell ratio | NA | 0.5 [0.33–0.71] | 1.38 [0.91–1.41] | 0.36 [0.18–0.51] | 1.3 [1.22–1.73] | NA |
| Time since diagnosis, (years) | NA | 0 [0–0] | 14.5 [8.5–16.75] | 18 [14.25–29.50] | 16 [6.25–31] | NA |
| Time on ART (years) | NA | NA | 13.5 [8.75–19] | 14.5 [10.5–22.75] | NA | NA |
| HIV-1 RNA (Log10 copies/ml) | NA | 4.80 [3.73–4.96] | 1.60 [1.60–1.60] | 1.60 [1.60–1.60] | 1.61 [1.60–1.93] | NA |
| Anti-HCV n (%) | NA | 0 (0) | 0 (0) | 3 (50) | 2 (50) | NA |
| HCV RNA (Log10 copies/ml) | NA | NA | NA | 0 | 0 | NA |
| Time from HCV treatment (years)b | NA | NA | NA | 11 [9.5–12.5] | 20 | NA |
Values are given as No. (%) for categorical variables or median (interquartile ranges [IQR]) for continuous variables. Abbreviation: NA, not applicable; HCV, hepatitis C virus; HIV-1, human immunodeficiency virus; IQR, interquartile range; Ctrl, Control group; VIR, Viremic; IR, Immune Responders; INR, Immune Non Responders and EC, HIV Elite Controllers.
Mann Whitney U test and Chi-square test were used to compare age and sex between PLWH as a whole and Control Group (Ctrl), respectively.
The three INR were treated with direct antiviral agents for HCV, one of the EC was treated for HCV 20 years before the present study and the other one spontaneously cleared HCV.
Laboratory measurements
Absolute CD4 and CD8 T-cell counts were measured by using Flow-Check™ Fluorospheres (Cat: 6605359), FC500 flow cytometer (Beckman–Coulter, Brea, CA). The plasma HIV-1 RNA concentration was measured using quantitative PCR (Cobas AmpliPrep/Cobas TaqMan HIV-1 test; Roche Molecular Systems) (detection limit: 40 HIV-1 RNA copies/mL). HCV-RNA (hepatitis C virus) was assayed on sera samples using an available PCR procedure kit (COBAS Amplicor, Roche Diagnosis) with detection limit of 10IU/ml. HCV exposure (measured by testing for the presence of anti-HCV) was detected using an HCV-specific ELISA (Siemens Healthcare Diagnosis). All procedures were performed according to the manufacturer's protocol.
Assay of soluble biomarkers
Serum and plasma samples were collected in serum separation tubes and in EDTA tubes and stored at −20 °C until subsequent analysis of the following biomarkers: high-sensitivity C-reactive protein (hsCRP), β2-microglobulin (β2-M) and D-dimer. The levels of hsCRP and β2M were determined by an immunoturbidimetric serum assay using a Cobas 701 analyzer (Roche Diagnostics). D-dimer levels were measured by an automated latex-enhanced immunoassay (HemosIL D-dimer HS 500; Instrumentation Laboratory). All the assays were performed following the manufacturers’ instructions.
Isolation of CD4, CD8 T-Cells and pDCs from blood
In vitro experiments were performed in PBMCs freshly isolated by ficoll density gradient centrifugation from 450 ml of whole blood samples (Lymphoprep Cat: 07801, StemCell Technologies). Primary CD4, CD8 T-cells and pDCs were negatively isolated (purity of >90%) from whole blood using EasySep Human CD4 (Cat: 17952), EasySep Human CD8 T-cell Isolation kits (Cat: 19663) and EasySep Human Plasmacytoid DC enrichment kit (Cat: 19062) (StemCellm, Technologies), respectively. The minimum amount of required sample (5 × 107 to 1 × 108 PBMCs) was used to isolate CD4+ and CD8+ T-cells. All the remaining PBMCs were used to isolate pDCs, according to the manufacturer's instructions.
pDC stimulation and co-culture
2 × 105 isolated pDCs were cultured with R10 media (RPMI 1640 supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin-l-glutamine) overnight (ON) at 37 °C and 5% CO2 in the presence or absence of the following agonists: TLR-9 class C CpG-ODN M362 (Cat: tlrl-m362, InvivoGen) (CpG-C, 1 μM), TLR-9 class A CpG-ODN 2216 (Cat: tlrl-2216, InvivoGen) (CpG-A, 1 μM), TLR-7 inactivated HIV with Aldrithiol-2 (AT-2, 20 ng/ml) and TLR-7 agonist GS-9620 (Cat: 19628, Cayman Chemical Company) (10 ng/ml). AT-2 virus preparation was kindly provided by Dr. Jeffrey D. Lifson, National Cancer Institute, Frederick, MD. pDCs stimulation was performed in duplicate, one duplicate was used for subsequent co-culture and the other for gene expression analyses in pellet and cytokine quantification in supernatants. Isolated CD4 and CD8 T-cells remained in R10 media ON at 4 °C, during the pDCs stimulations. Afterwards, stimulated and unstimulated pDCs were co-cultured with autologous CD4 or CD8 T-cells at a ratio of 1:20, T-cells were added directly to the pDC culture without replacing the supernatant, therefore, all the soluble factors induced after ON TLR incubation were present in the co-culture. The cells were cultured in the presence of anti-CD107a (BV605), 1 μg/ml of anti-CD28, 1 μg/ml anti-CD49d (BD Biosciences), 10 μg/ml of brefeldin A (BFA) (Sigma Chemical Company) and 0.7 μg/ml of monensin (BD Biosciences) and stimulated in the absence or presence of 2 μg/ml HIV (Gag)-specific peptide pool stimulation (in the case of PLWH, NIH AIDS Research and Referenced Reagent Program https://www.hivreagentprogram.org/) and in the absence or presence of staphylococcal enterotoxin B (SEB, in the case of Ctrl) during 6 h at 37 °C and 5% CO2. SEB or HIV-specific T cells were defined as the frequency of cells expressing intracellular cytokines and/or degranulation markers after stimulation and the corresponding background subtraction of the condition without SEB o Gag stimuli, respectively, as previously reported.18,19
pDCs gene expression analyses
After ON incubation, pDCs were removed from each well, pelleted and stored at −80 °C. Pellets were thawed and lysed with Real Time ready Cell Lysis Kit (Cat: 06366821001, Roche). cDNA synthesis was performed immediately after the lysis with Transcriptor First Strand cDNA Synthesis Kit (Cat: 04379012001, Roche) according to the manufacturer's instructions. Specific primers for qPCR were designed to measure the expression of IFN-α, IRF3, IRF7, IFNL3, TLR-7, TLR-9, TRIM5α, Tumor Necrosis Factor-α (TNF-α), BST2, HDAC6, APOBEC3G, SAMHD1 and TRAIL genes (Supplementary Table S1). qPCRs were performed on a Roche Light Cycler 480. Values were normalized by the housekeeping gene β-actin and relative quantification analysis was performed by delta–delta CT method (2−ΔΔct) taking the unstimulated condition as the reference.
Cytokines quantification
Supernatants from ON incubation of pDCs were collected and stored at −80 °C. Tumor necrosis factor-α (TNF-α), Interferon-γ (IFN-γ), Interleukine-6 (IL-6), Interferon gamma-induced protein 10 (IP-10), Macrophage Inflammatory Protein-1α (MIP-1α) and MIP-1β were measured by MILLIPLEX HCYTA-60 K Human Cyto-Panel A according to the manufacturer's instructions (Cat: 900020, Merck Millipore). The Interferon-α (IFN-α) levels in the supernatants were assessed by an IFN-α Multi-Subtype ELISA kit (Cat: 41110, PBL Interferon Source) according to the manufacturer's instructions.
Immunophenotyping and intracellular cytokine staining
For peripheral dendritic cell (DC) quantification and characterization, ex vivo PBMCs immediately isolated from fresh blood were washed with PBS and incubated with LIVE/DEAD Fixable Aqua Dead Cell Stain (Cat: L34957, Life Technologies) and extracellular anti-human lineage cocktail 2 (LIN-2) (CD3, CD14, CD19, CD20, CD56) FITC, anti-CD86 BV421, anti-CCR7 BV786, anti-PDL1 PECF594, anti-CD141 PE-Cy7, anti-CD11c BV650, anti-HLA-DR BV711, anti-CD123 AF700, anti-CD1c APC-Cy7, anti-β7 integrin (APC), anti-CD4 PerCP-Cy5.5 and anti-CD16 BV605 (Supplementary Table S2). Cells were then washed and permeabilized using Fixation/Permeabilization FoxP3 Kit (Cat: 00-5521-00, eBioscience) according to the manufacturer's instructions. Cells were stained intracellularly with anti-IDO PE and then washed and fixed in PBS containing 4% paraformaldehyde (PFA).
Ex vivo pDCs immediately isolated from PBMCs and stimulated isolated pDCs were washed with PBS and incubated with LIVE/DEAD Fixable Aqua Dead Cell Stain (Cat: L34957Life Technologies) and extracellularly with anti-CD81 FITC, anti-CCR7 BV786, anti-HLA-DR BV711, anti-β7 intregrin APC, anti-CD4 BV605, anti-CD86 BV650, anti-CD5 APC-Cy7, anti-CD2 PeCy5.5 and anti-TIM3 PECF594. pDCs were then washed and permeabilized and fixed using a Cytofix/Cytoperm Kit (Cat: 554714, BD Biosciences) according to the manufacturer's instructions. pDCs were stained intracellularly with anti-IDO PE, anti-TNF-α PE-Cy7, anti-TLR-7 AF700 and anti-TLR-9405 and then washed and fixed in PBS containing 4% PFA (Supplementary Table S2).
Ex vivo CD4, CD8 T-cells immediately isolated from PBMCs and co-culture pDCs-CD4 and pDCs-CD8 T-cells were washed with PBS and incubated with LIVE/DEAD Fixable Aqua Dead Cell Stain (Cat: L34957, Life Technologies) and extracellularly with antibodies anti-CD45RA FITC, anti-TIGIT PerCP-Cy5.5, anti-β7 integrin BV711, anti-LAG3 BV605, anti-CD123 BV510, anti-PD1 BV786, anti-CD27 APC-Cy7 and anti-TIM3 PeCF594. Cells were then washed and permeabilized and fixed using a Cytofix/Cytoperm Kit (Cat: 554714, BD Biosciences) according to the manufacturer's instructions. Cells were then stained intracellularly with anti-IL-2 BV421, anti-TNF-α AF700, anti–IFN–γ PCy7 and anti- IL-17a PE (only for CD4 T-cells)/anti-perforin PE (only for CD8 T cells) and then washed and fixed in PBS containing 4% PFA (Supplementary Table S2).
Flow cytometry was performed on an LSR-II Fortessa Cytometer (BD Immunocytometry Systems) and analysis was performed using FlowJo, version 9.2.
Statistical analysis
Continuous variables were expressed as medians and interquartile ranges (IQRs), and categorical variables were expressed as numbers and percentages. Non-parametric tests were performed after Kolmogorov–Smirnov test for assessing non-normal distribution. Friedman and Kruskal–Wallis tests including Dunn's multiple comparisons test correction were applied. Correlations between variables were assessed using Spearman's rank test. All p values < 0.05 were considered significant. Statistical analysis was performed using Statistical Package for the Social Sciences software (SPSS 22.0; SPSS, Chicago, IL, USA). Cytokine and immune check point molecule combinations were constructed using Pestle version 1.6.2 and Spice version 6 (provided by M. Roederer, NIH, Bethesda, MD) and quantified with the polyfunctionality index algorithm (Pindex) employing the 0.1.2 beta version of “FunkyCells Boolean Dataminer” software, provided by Martin Larson (INSERM U1135, Paris, France).
Ethics statement
All participants gave written informed consent prior to blood sampling. The study was approved by the Ethics Committee of the Virgen del Rocio University Hospital (Internal Code: 0628-M1-21, Study Code: PI19/01127).
Role of funders
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, of writing of the report.
Results
Characteristics of the studied subjects and experimental conditions
Twenty-three PLWH (seven VIR, six INR, six IR and four EC), and twenty-one Ctrl as reference group were enrolled in the study. Clinical, demographic and immunovirological participants’ characteristics are summarized in Table 1 pDCs, CD8 and CD4 T-cells were freshly isolated from these subjects (gating strategy is shown in Supplementary Fig. S1). pDCs were stimulated ON with AT-2, CpG-A, CpG-C, GS-9620 or leaved unstimulated. Afterwards, pDCs were co-cultured for 6 h with autologous CD4 or CD8 T-cells and with/without HIV-specific peptides (Gag peptide pool) or staphylococcal enterotoxin B (SEB) (see study design in Fig. 1).
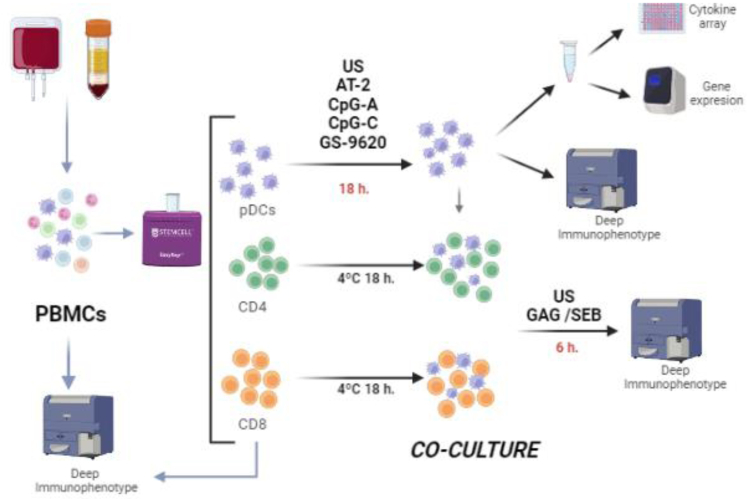
Fig. 1.
Experimental workflow. pDCs, CD4 and CD8 T-cells were freshly isolated from 450 ml of peripheral blood. pDCs were stimulated with AT-2, CpG-A, CpG-C, GS-9620 or leaved unstimulated overnight (US). A cytokine array and gene expression was assayed in the supernatant and cells respectively. Afterwards, pDCs were co-cultured for 6 h with autologous CD4 or CD8 T-cells and with/without HIV-specific peptides (Gag peptide pool) or SEB in the case of control group. PBMCs and isolated pDCs, CD4 and CD8 T-cells were deep immunophenotyped before pDCs stimulations and co-culture. Figure created with Biorender.com.
Increased soluble inflammatory biomarkers and T-cell exhaustion levels in PLWH are associated with pDC activation
Firstly, we characterized by multiparametric flow cytometry the surface phenotype of freshly isolated DCs (pDCs and mDCs). INR's and VIR's DCs presented increased CCR7 levels with respect to Ctrl (Fig. 2a). Inflammatory soluble markers such as the coagulation biomarker D-dimer and B2-M also presented higher concentration in PLWH than Ctrl (Fig. 2b). A positive correlation between pDCs activation phenotype and inflammatory soluble markers were observed (Fig. 2c). It is of note that the pDCs CCR7+ levels were positively correlated with the levels of D-Dimer, B2-M and hsCRP (rho = 0.399, p = 0.035; rho = 0.467, p = 0.012 and rho = 0.415, p = 0.028; respectively).
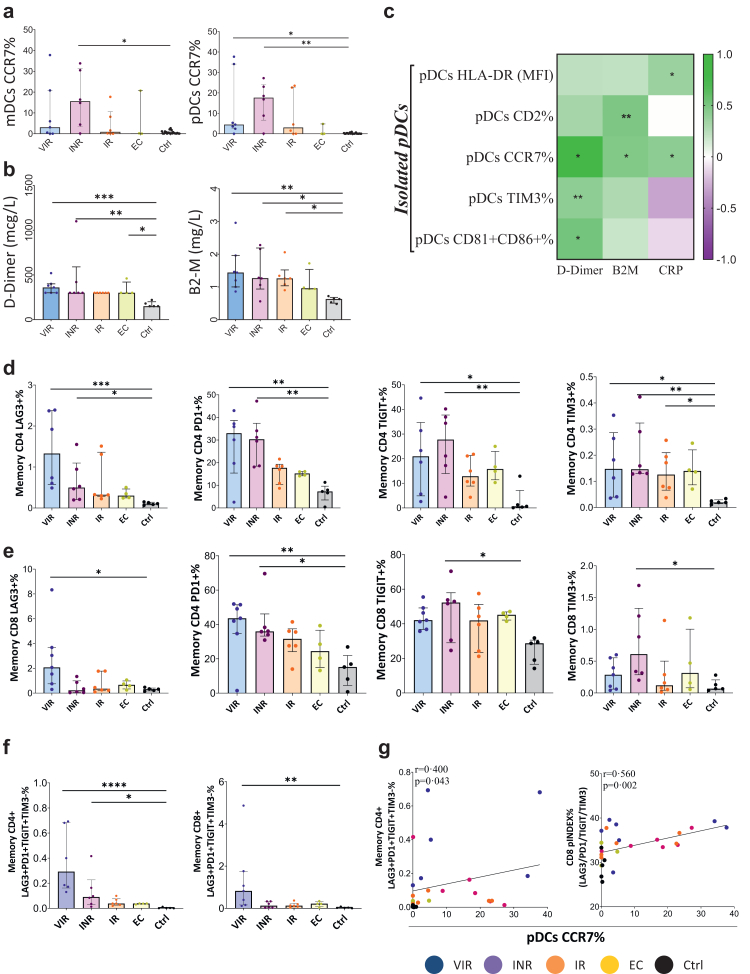
Fig. 2.
DC activation, inflammatory soluble markers and T-cell exhaustion phenotype are increased in PLWH. (a) Percentage of mDCs and pDCs CCR7 expression among PLWH and Ctrl. (b) Levels of soluble markers of inflammation and coagulation in PLWH and Ctrl. (c) Correlation matrix representing negative (purple shading) and positive (green shading) association between the percentage of isolated pDCs activation markers before pDCs stimulation and inflammatory soluble biomarkers. (d and e) Percentage of isolated memory CD4 and CD8 T-cell, before co-culture, expressing the exhaustion markers: LAG3, PD1, TIGIT and TIM3. (f) Representative combination of simultaneous exhaustion markers expression within memory CD4 and CD8 T-cells. (g) Positive correlation between combination of exhaustion markers within memory CD4 and CD8 T-cell exhaustion polyfunctionality index, and the percentage of isolated pDCs CCR7 expression before stimulation. Combinations of exhaustion markers were constructed using Pestle version 1.6.2 and Spice version 6 and quantified with the exhaustion polyfunctionality index algorithm (Pindex) employing the “FunkyCells Boolean Dataminer” software. The Kruskal–Wallis test including Dunn's multiple comparisons test correction were used to compare the parameters between groups (VIR, INR, IR, EC and Ctrl). Spearman p correlation coefficient test was use for correlations. Statistical significant values are shown as: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. VIR, Viremic; INR, Immune Non Responders; IR, Immune Responders; EC, HIV Elite Controllers and Ctrl, Control group.
We also quantified the levels of the exhaustion markers LAG3, PD1, TIGIT and TIM3 in freshly isolated memory CD4 and CD8 T-cells (Fig. 2 d-e). PLWH presented more elevated individual levels of these markers in both CD4 and CD8 T-cells compared with Ctrl. Moreover, INR and VIR stand out as the groups with the most elevated exhaustion markers compared to Ctrl. The “multiple exhausted phenotype” (simultaneous expressions of three or more of the analyzed exhaustion markers) was also calculated and represented by pie charts. EC and IR exhibited a lower multiple exhausted phenotype especially in CD4 T-cells with respect to Ctrl (Supplementary Fig. S2). Additionally, the combination of exhaustion markers LAG3+PD1+TIGIT + TIM3-in memory CD4 and CD8 T-cells was significantly increased in PLWH (Fig. 2f). Furthermore, LAG3+PD1+TIGIT + TIM3-memory CD4 T-cell levels and CD8 T-cells expressing all the analyzed exhaustion markers simultaneously, calculated with the polyfunctional index, correlated with pDCs CCR7+ levels (rho = 0.400, p = 0.043 and rho = 0.560, p = 0.002 respectively) (Fig. 2g). Overall, these results show that pDC activation phenotype was associated to a higher T-cell exhaustion phenotype.
TLR agonist stimulation induces a pDCs activated phenotype
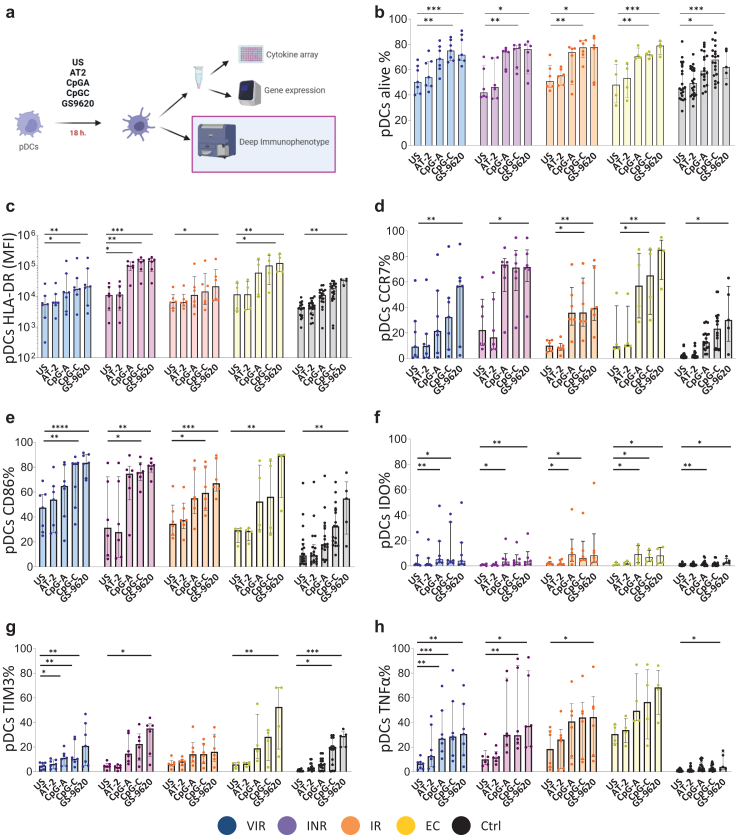
In order to characterize the pDCs modulation through TLR agonists (AT-2, CpG-A, CpG-C and GS-9620) we characterize the surface phenotype measuring by multiparametric flow cytometry the percentage of cell expressing of different markers denoting: antigen presentation (CD81, CD86), activation (TIM3 and HLA-DR) and tolerance (IDO), homing (CCR7 and β7 integrin), innate intracellular sensing markers (TLR7 and TLR9) and intracellular cytokine production (TNF-α) (Fig. 3a and Supplementary Figs. S3 and S4). Before stimulation, participants with detectable viremia, VIR, we observed positive correlations between viral load and the percentage of pDCs expressing β7 integrin, CCR7 and CD86 (Supplementary Fig. S3d). After stimulation, TLR agonists increased the percentage of pDCs alive in culture; without differences between groups (Fig. 3b). Median fluorescence intensity (MFI) of HLA-DR levels increased with the different stimuli. This increase was especially striking with CpG-C and GS-9620 agonists in INR and EC groups (Fig. 3c). The main statistical differences between groups were observed in INR with respect to Ctrl (Supplementary Fig. S4). Regarding the homing marker CCR7, the activation marker CD86, the tolerogenic marker IDO and the immune checkpoint molecule TIM3, were also increased in all study groups after TLR stimulation with CpG-A and specially with CpG-C and GS-9620 (Fig. 3d, e, f and g). Finally, TNF-α production, after stimulation with TLR agonist, in PLWH increased to a greater extent than the Ctrl (Fig. 3h). In general, all markers reached their highest levels with CpG-C and GS-9620 stimuli and the majority of the differences between studies groups were with Ctrl.
Fig. 3.
pDCs activated phenotype after TLR agonist stimulation. (a) Experimental workflow: Deep immunophenotyping of stimulated pDCs. (b–h) pDCs surface phenotype after AT-2, CpG-A, CpG-C and GS-9620 stimulation. Friedman test including Dunn's multiple comparisons test correction was applied. Statistical significant values are shown as: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ns. VIR, Viremic; INR, Immune Non Responders; IR, Immune Responders; EC, HIV Elite Controllers; Ctrl, Control group and ns, non-significant.
TLR agonist stimulation increase IFN-α, interferon regulatory genes and restriction factors levels on pDCs
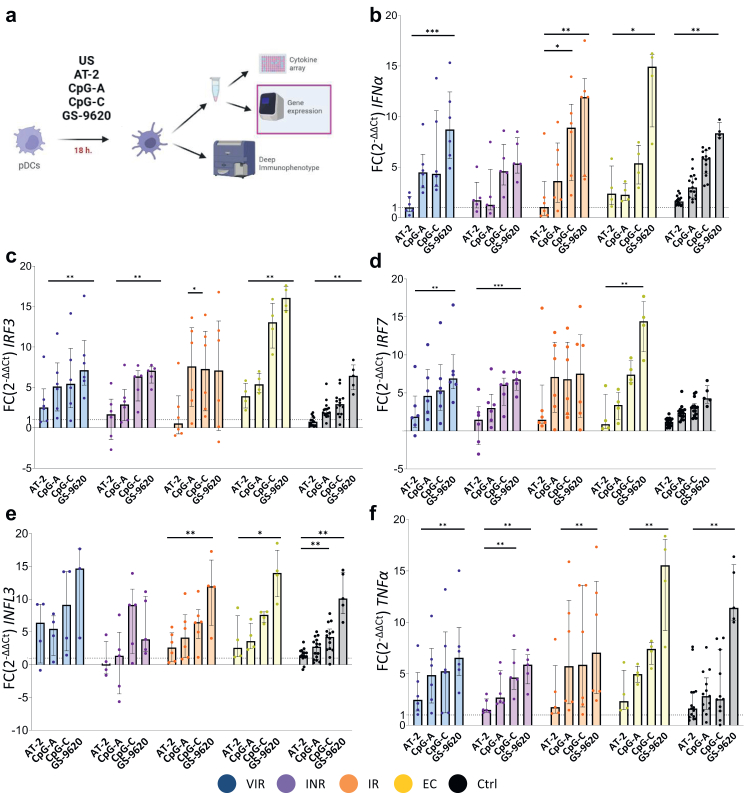
The gene expression profiles of in vitro activated pDCs were also determined (Fig. 4a). The expression of IFN-α, interferon related genes (IRF3, IRF7 and IFNL3), TLR-7, TLR-9, TNF-α, restriction factors (TRIM5α, BST2, HDAC6, APOBEC3G and SAMHD1) and TNF-related apoptosis-inducing ligand (TRAIL) genes were assayed by qPCR (Fig. 4 and Supplementary Figs. S5 and S6). There was generally an increase of transcription of IFNα, IRF3, IRF7, IFNL3 and TNF-α genes with the different stimulus (Fig. 4b–f). Maximum levels of expression were observed with GS-9620 stimuli. Surprisingly, no statistical differences were obtained in IFN-α, IFNL3 and TNFα expression genes between the study groups (Supplementary Fig. S6). Even VIR and INR reached levels of IFN-α, IFNL3 and TNFα expression similar to those found in EC. Interestingly, positive correlations were observed between the transcription levels of TLR9, IFN-α, IRF7 and IRF3 and the restriction factor BST2 with the different stimuli (Supplementary Fig. S7).
Fig. 4.
pDCs gene expression profile after TLR stimulations. (a) Experimental workflow: pDCs gene expression analyses of previously stimulated pDCs. (b–f) Fold change expression of IFNα, IRF3, IRF7, IFNL3 and TNFα of the different stimuli related to the unstimulated condition. Friedman test including Dunn's multiple comparisons test correction was applied. Statistical significant values are shown as: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. VIR, Viremic; INR, Immune Non Responders; IR, Immune Responders; EC, HIV Elite Controllers; Ctrl, Control group and FC, Fold Change.
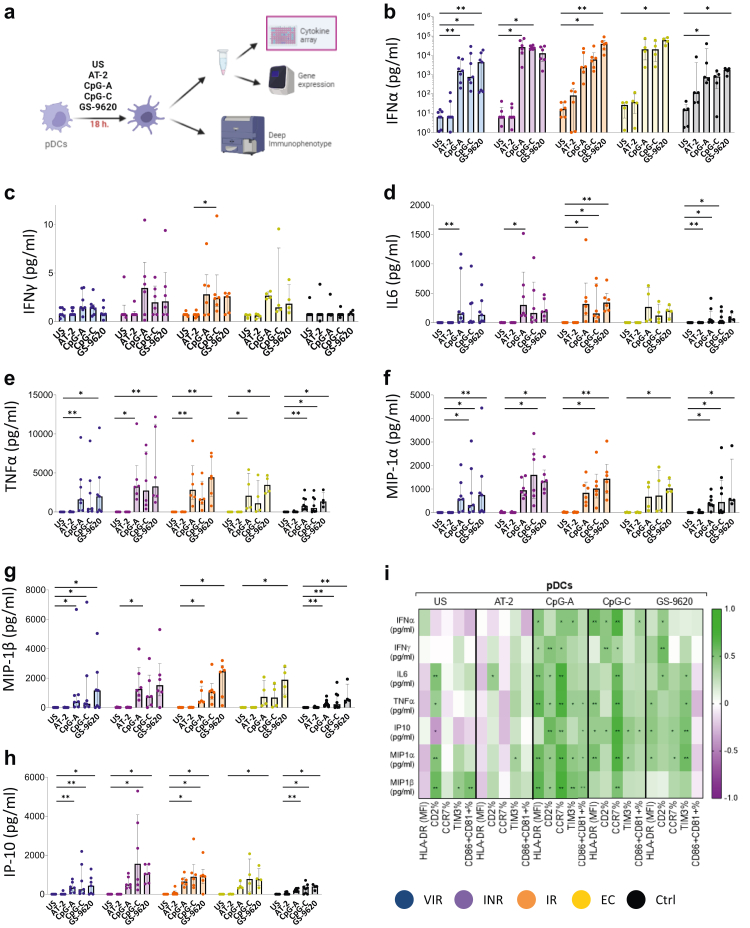
Cytokine production by pDCs is higher after GS-9620 stimulation
We next analyzed the production of cytokines by pDCs in culture supernatants (Fig. 5a). We observed a significant increase of IFN-α production on pDCs after TLR stimulation (Fig. 5b). The main differences between groups were observed after CpG-C and GS-9620 stimulation (Supplementary Fig. S8). We observed lower levels of IFN-α production in Ctrl after CpG-C stimulation in relation with EC, IR and INR subjects (Fig. 5b and Supplementary Fig. S8). These differences consistently appear in EC and IR also after GS-9620 stimulation (Supplementary Fig. S8). The main increase was observed in EC after pDCs stimulation with GS-9620 molecule (Fig. 5b). There were no differences of IFN-α production between Ctrl and VIR after CpG-A, CpG-C and GS-9620 stimulation (Supplementary Fig. S8). On the other hand, significant increases of IFN-γ production were only observed on IR, and also differences with Ctrl were observed only with IR after CpG-C stimulation (Fig. 5c). IL-6 and TNF-α are needed for the properly pDCs maturation.20 After TLR agonist stimulation an increase of their production were observed in all study groups (Fig. 5d and e). MIP-1α and MIP-1β production were also increased after TLR agonist stimulation, the main production was observed after GS-9620 stimulation (Fig. 5f and g). Finally, interferon gamma-induced protein 10 (IP-10) production, which is secreted by pDCs in order to recruit T-cells,21 was also increased after TLR agonist stimulation. Interestingly, INR pDCs secreted the highest levels of IP-10 after CpG-C stimulation (Fig. 5h).
Fig. 5.
TLR agonists upregulate cytokines production by pDCs. (a) Experimental workflow: pDCs cytokine production of previously stimulated pDCs. (b–h) Cytokine levels of IFNα, IFNγ, IL6, TNFα, MIP-1α, MIP-1β and IP-10. (i) Correlation matrix representing negative (purple shading) and positive (green shading) association between the percentage of pDCs activation markers expression and cytokine production in all the studied groups. Friedman test including Dunn's multiple comparisons test correction was applied. Spearman p correlation coefficient test was used. Statistical significant values are shown as: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. VIR, Viremic; INR, Immune Non Responders; IR, Immune Responders; EC, HIV Elite Controllers and Ctrl, Control group.
The increase of the pDCs activation markers as CD2, CCR7, TIM3, CD86 and CD81 after CpG-A, CpG-C and GS-9620 stimulation positive correlated with the cytokine and chemokine production in all studied groups (Fig. 5i). Overall, these results suggest that TLR agonists could act as a potent booster of the natural pDCs antimicrobial profile.
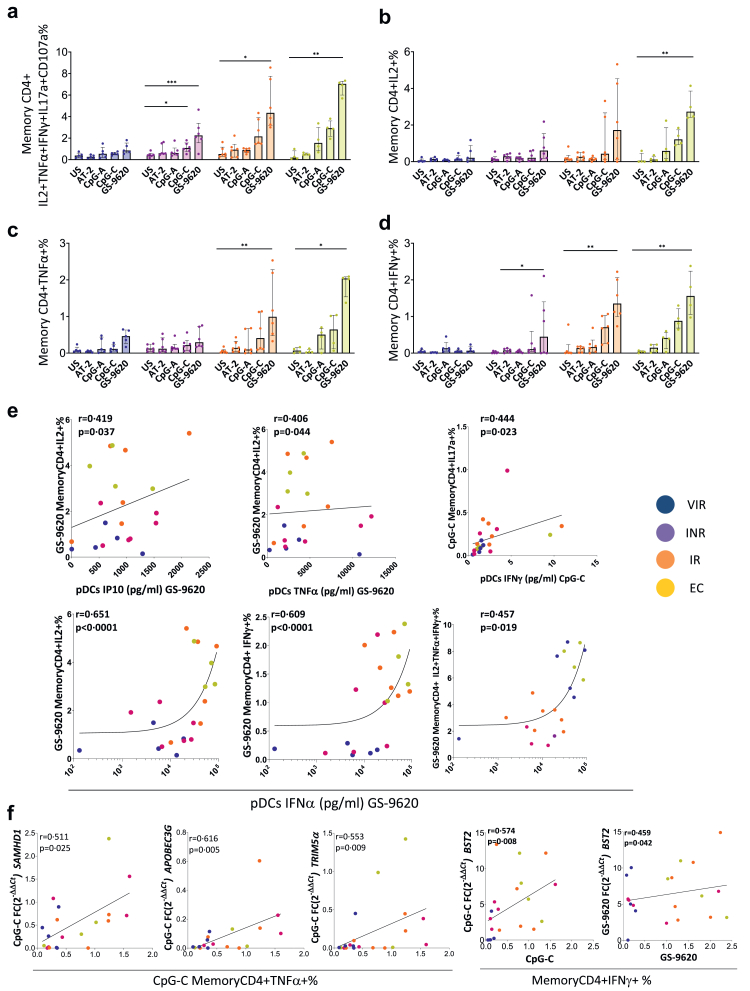
pDCs stimulated with TLR agonists induce an increase in HIV-specific T-cell response
In order to investigate how TLR-7 and -9 agonists could serve as a booster of pDCs activity enabling antiviral T-cell function, we analyzed the memory CD4 and CD8 T-cell HIV-specific responses, defined as the frequency of cells with detectable intracellular cytokine production (IL-2, TNF-α and IFN-γ) together with the degranulation marker CD107a. In the case of memory CD4 T-cells, IL-17a was also included and the cytotoxic marker perforin in the case of memory CD8 T-cells. Previously stimulated pDCs (AT-2, CpG-A, CpG-C and GS-9620) were co-cultured with CD4 or CD8 T-cells and with or without Gag peptides pool (Fig. 6, Fig. 7 and Supplementary Figs. S9–S11). Ctrl cocultures were stimulated with SEB also showing an increase in the intracellular cytokine production (Supplementary Fig. S9a). In all the cases the Gag-specific response is the result of the background subtraction of the condition without Gag stimulation (Supplementary Fig. S9b and c) to the one with stimulus. In the absence of Gag stimulus, no increase in individual or combined cytokine production was observed for CD4+ or CD8+ T-cells (Supplementary Fig. S9b and c).
Fig. 6.
TLR agonist induces an increase in HIV-specific memory CD4+ T-cell response. (a) Sum of IL2, TNFα, IFNγ, IL17a and CD107a intracellular memory CD4 T-cell production in response to HIV (Gag)-specific peptide pool minus the condition without Gag-stimulation. (b–d) Single cytokine intracellular memory CD4 T-cell production in response to HIV (Gag)-specific peptide pool minus the condition without Gag-stimulation. (e) Correlations between cytokine production of previously stimulated pDCs with GS9620 and single cytokine intracellular memory CD4 T-cell production in response to HIV (Gag)-specific peptide pool after co-culture minus the condition without Gag-stimulation. (f) Correlations between fold change expression of BST2, SAMHD1, APOBEC3G and TRIM5α of previously stimulated pDCs with respect to the unstimulated condition and single cytokine intracellular CD4 memory T-cell production in response to HIV (Gag)-specific peptide pool after co-culture minus the condition without Gag-stimulation. Friedman test including Dunn's multiple comparisons test correction was applied. Spearman p correlation coefficient test was used. Statistically significant values are shown as: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. VIR, Viremic; INR, Immune Non Responders; IR, Immune Responders and EC, HIV Elite Controllers.
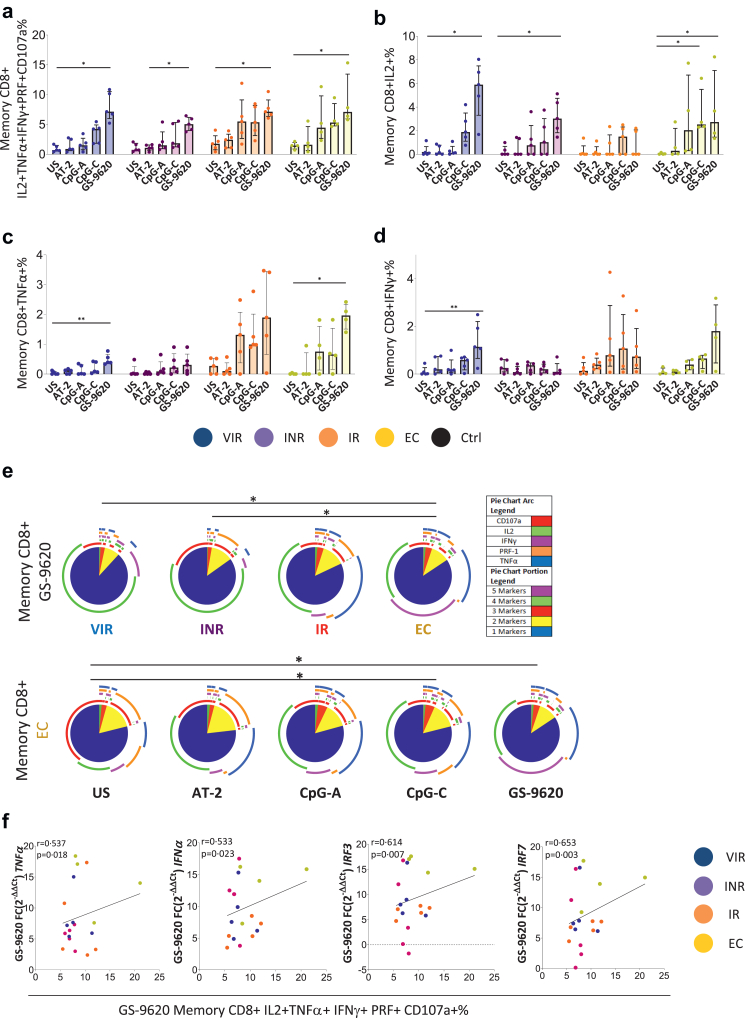
Fig. 7.
TLR agonist induces an increase in HIV-specific CD8 memory response. (a) Sum of IL2, TNFα, IFNγ, Perforin and CD107a production in response to HIV (Gag)-specific peptide pool minus the condition without Gag-stimulation. (b–d) Single cytokine intracellular memory CD8 T-cell production in response to HIV (Gag)-specific peptide pool minus the condition without Gag-stimulation. (e) HIV-specific CD8 T-cell polyfunctionality pie charts. Permutation test, following the Spice version 6 software was used to assess differences between pie charts. (f) Positive correlations between the pDCs fold change of TNFα, IFNα, IRF3 and IRF7 expression after GS9620 stimulation with respect to the unstimulated condition and the HIV-specific memory CD8 T-cell response. Friedman test including Dunn's multiple comparisons test correction was applied. Spearman p correlation coefficient test was used. Statistically significant values are shown as: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. VIR, Viremic; INR, Immune Non Responders; IR, Immune Responders and EC, HIV Elite Controllers.
Gag-specific CD4 T-cell response
TLR stimulation of pDCs induced HIV-specific CD4 T-cell response through intracellular CD4 T-cell production of IL-2, TNF-α and IFN-γ. The highest levels of response were observed in IR and EC with previously GS-9620 stimulated pDCs (Fig. 6a–d). A cumulative HIV-1 specific T-cell measurement was calculated as the sum of IL-2, TNF-α, IFN-γ, IL17-a and CD107a production. Although the increase in this cumulative measurement was particularly strong in IR and EC, even in INR, responses were increased after co-cultured with previously GS-9620 stimulated pDCs (Fig. 6a and d). Interestingly, this increase in HIV-specific memory CD4 T-cells was positively associated with pDCs IP10, TNF-α, IFN-γ and IFN-α production after CpG-C and GS-9620 stimulation (Figura 6e). In addition, the memory CD4 T-cell intracellular production of IFN-γ and TNF-α were also correlated with the increase of pDCs restriction factors transcription BST2, SAMHD1, APOBEC3G and TRIM5α (Fig. 6f).
Gag-specific CD8 T-cell response
An increase in HIV-1 specific memory CD8 T-cell response mediated by pDCs was observed in all PLWH groups after TLR stimulation, especially with GS-9620. This was true for individual cytokine production and for the sum of the cytokines, the degranulation marker CD107a and the production of perforin (Fig. 7a–d). We also analyzed the characterization of simultaneous multiple cytokine expression, also termed polyfunctionality (Fig. 7e). EC showed higher polyfunctionality distribution than INR and VIR after co-cultured with GS-9620 previously stimulated pDCs. There were no differences between EC and IR (Fig. 7e, top panel). Indeed, EC memory CD8 T-cells showed the highest polyfunctional distribution after co-cultured with CpG-C and GS-9620 previously stimulated pDCs (Fig. 7e, bottom panel). Interestingly, the HIV-specific memory CD8 T-cell response cumulative measurement (IL-2+TNF-α+IFN-γ+PRF + CD107a+) was positively associated with the increase of TNF-α, IFN-α, IRF3 and IRF7 pDCs expression after GS-9620 stimulation (Fig. 7f).
The sum of three, four and five cytokines produced by memory CD4 and CD8 T-cells after the co-culture with CpG-C previously stimulated pDCs were positively associated, showing EC and IR the highest levels of response (Supplementary Fig. S11a). Interestingly, memory CD8 IL-2+TNF-α+IFN-γ+ production was inversely associated with the expression of the exhaustion markers PD1 and TIM3 after co-cultured with GS-9620 (Supplementary Fig. S11b). Indeed, after the co-culture, we observed normalization in memory CD4 PD1% in IR and EC compared with Ctrl (Supplementary Fig. S11c).
Discussion
The results presented herein showed that pDCs stimulation through different TLR agonists increases pDCs activation marker levels, interferon related genes, restriction factor transcription and upregulated cytokines levels. All markers reached their highest levels with CpG-C and GS-9620 stimuli. Even PLWH with uncontrolled and detectable viremia and with immunodiscordant response on ART reached similar pDCs activation levels to those found in EC. Moreover, we observed an increase in the HIV specific memory CD4 and CD8 T-cell response mediated by pDCs in all PLWH groups after TLR stimulation, even in those on ART.
HIV infection is characterized by high levels of inflammatory biomarkers and T-cell exhaustion even on people on ART.22,23 It is known that soluble markers of inflammation and coagulation are associated with a higher risk of non-AIDS-related morbidity and mortality, especially in INR.24, 25, 26 It is crucial to find immune correlates associated with this heightened immune activation. pDCs have been associated with HIV control but also with HIV-disease progression.11,27,28 In this study, we found association of the comprehensive pDCs phenotype ex vivo with biomarkers of inflammation and T-cell exhaustion. First, we observed that elevated D-Dimer, B2-M, and hsCRP levels in PLWH were associated with the ex vivo levels of CCR7, CD2, CD86, CD81 and HLA-DR (MFI) on pDCs, showing INR the highest levels and EC the lower within PLWH. Ex vivo INR and VIR shared a more activated and exhausted phenotype. Regarding T-cell exhaustion, we found a direct correlation of CCR7+ pDC levels with the combined expression of T-cell exhaustion markers in CD4 and CD8 T-cell. T-cell exhaustion is due to constant antigen stimulation resulting in chronic cellular dysfunction and finally leading to a dysfunctional state that can include: impaired proliferation of T-cells, reduced cytokine secretion, in addition, and importantly, exhaustion phenotype seems to contribute to HIV reservoir in CD4 T-cells.29, 30, 31, 32, 33 The expression of the exhaustion markers PD1, LAG3 and TIM3 on CD4 and CD8 T-cells prior to ART was previously identified as a preferential niche for the establishment reservoir for HIV-1.29 The co-expressing phenotypes of these exhaustion markers have been shown to exhibit stronger correlations with HIV viral load and lower expression of cytokines against HIV-1.23 Indeed, these immune checkpoint molecules were identified as a strong predictor of time to viral rebound after treatment interruption in the SPARTAC study.31 TIM3 CD4 and CD8 T-cells have been shown to be elevated in subjects with acute HIV-1 infection but not in PLWH with viral control, as compared with non-infected individuals, TIM3 could be effectively reduced by ART.33,34 Indeed, in our cohort we only observed differences in TIM3 CD8 T-cell levels between INR and Ctrl. In this study, the simultaneous expression of LAG3, PD1 and TIGIT, but not TIM3, were positively associated with the levels of homing marker CCR7 on pDCs ex vivo. These results suggest the involvement of pDCs activation phenotype in T-cell exhaustion: HIV-1 can directly induce partial maturation of pDCs and CCR7 up-regulation inducing their migration to secondary lymphoid organs,35 hence the immunomodulation of pDCs through TLR agonists may have influence in T-cell exhaustion and reservoir establishment. In this sense, importantly, after co-culture we observed the normalization of PD1 levels in CD4 T-cell after TLRs stimulation in the different HIV-disease progression phenotypes, especially in IR and EC.
Emerging preclinical and clinical studies strongly indicate that TLR agonists have great potential as immune boosting and priming agents in HIV-1 cure research. The primary obstacle to curing HIV-1 is the presence of a latent viral reservoir in immune cells. TLR agonists are demonstrated to be very interesting in this context because of their potential effects as latency reverting agents.36 Some of these TLR agonists are already being used in clinical trials on PLWH such as the TLR-7, GS-9620 and the TLR-9, agonist MGN1703.17,37, 38, 39 Previously, it has also been observed that administrating the potent neutralizing antibody PGT121 together with GS-9620 during early ART delayed viral rebound following discontinuation of ART in simian-human immunodeficiency virus (SHIV)-infected rhesus macaques. These combinations induced complete elimination of the viral reservoir in some animals and durable virus control in others.16 However, the molecular mechanisms behind the immunomodulatory effect of these compounds in relation to pDCs are mostly unknown. The results presented herein shed light on the underling mechanisms of pDCs activation through different TLR agonists. Indeed, we have previously described that after TLR stimulation, pDCs showed upregulation of some key molecules such as HLA-DR, CCR7, CD40 and CD86 associated to HCV infection control.12,40 The cellular and molecular mechanisms of the synergistic effect of TLR agonists and broadly neutralizing antibodies combination needs further investigation. In the present study, PLWH pDCs reached highest levels of activation markers, and higher TNF-α and IFN-α production than Ctrl after TLRs agonist stimulation. Importantly this was independent of the HIV infection phenotype, most of the markers in people on ART, including INR, and even VIR, reached the same levels of activation as in EC. This result highlights that harnessing pDCs function through TLR stimulation in non-controllers help to reach a similar pDC phenotype of HIV-1 spontaneous control. These increases were especially remarkable with CpG-C and GS-9620. Besides the expected increment in IFN-α production, it was important to note the production of TNF-α. TNF-α secreted in an autocrine and paracrine manner is able to activate HIV-1 latently infected cells with the potential of reducing the reservoir.41 We also observed an increase of interferon related genes transcription. This is important because this activation seems to revert the HIV-1 induced deficits in IFN-α production through the IRF7 pathway in pDCs.42 In addition, the upregulation of interferon related genes is positively correlated with the restriction factor BST2. It is known that BST2/Tetherine strongly inhibit the release of HIV impeding cell-to-cell HIV-1 transmission.43, 44, 45 Secreting type I IFN allows pDCs to activate both innate and adaptive immune responses through induction of NK cell migration, macrophage and dendritic cell maturation, T-cell response, antigen presentation and differentiation of antibody-producing plasma cells.46,47 In addition, the upregulation of MHC and T-cell costimulatory molecules (e.g., HLA-DR and CD86) enables mature pDCs to play a direct role in antigen presentation and T-cell activation.48 However, it is known that prolonged type I IFN exposure will damage haematopoiesis, leading to lymphopenia49 and increased risk of autoimmunity.50 Indeed, Baenzinger S. et al. showed that sustained TLR7 stimulation in mice may results in immune activation and disruption of the lymphoid system.51 In this context, BST2 may also contribute to a negative feedback mechanism controlling IFN-I overproduction by pDCs after viral infection and/or sustained inflammatory response.52,53 Our results showed that TLR agonists achieve effective pDCs activation and activate a negative feedback loop mechanism of control by increasing BST2 transcription with the additional antiviral activity.
Classically known for their IFN-α producing abilities, pDCs act also as antigen-presenting cells (APC).54,55 pDCs can process and present endogenous and exogenous antigens on MHC I and II molecules and induce antigen-specific activation of both CD8 and CD4 T cells.56, 57, 58, 59, 60 It was previously published that the administration of the TLR-9 agonist MGN1703 to PLWH on ART enhances pDCs activation markers and T cell activation38 and in vitro the use of this agonist enhanced innate immune activation.61 We extended our analysis to explore the impact of pDCs stimulated through AT-2, CpGA, CpGC and GS9620 on HIV-specific T-cell response. Our results showed that pDCs stimulated with TLR agonists induce an increase in HIV-specific T-cell response. No increase in cytokine production by T-cells after co-culture was observed without Gag stimulation with different TLR agonist compared to unstimulated condition, confirming the antigen-specific nature of this response. Our findings, in combination with other studies62,63 showed the important role of human pDCs as professional APCs able to effectively induce the T-cell response, because of their capacity to efficiently present antigens.64 In contrast, Ram et al. previously showed a lack of enhancement of T cell-specific responses by GS9620 in vivo.17,64 Although, the highest levels of T-cell response were observed in IR and EC, even INR and VIR (with probably the largest reservoir) responses were increased after co-cultures with GS9620 previously stimulated pDCs. Interestingly, BST2, SAMHD1, APOBEC3G and TRIM5α pDCs transcription induced by CpG-C and GS-9620 were positive correlated with IFN-γ and TNF-α memory CD4 T-cell intracellular production. BST2, APOBEC3G and Trim5α attack HIV-1 directly exerting an antiviral activity.65, 66, 67 Furthermore, SAMHD1 interferes with HIV-1 reverse transcription by reducing the intracellular desoxyribonucleoside triphosphate (dNTP) pool.68 Nevertheless, despite it is known that the HIV is able to elude all the restriction factor of human cells, the expression profile of host restriction factors can play an important role in determining HIV-1 reservoir size.69 Indeed, it was previously observed a significant decrease in the frequency of cells harbouring intact HIV virus in PLWH on ART treated with Vesatolimod (Gilead GS-9620 molecule) that was associated with a modest delayed viral rebound during analytic treatment interruption.17 Our results show an increase in HIV-specific CD8 T-cell response in PLWH that was positively associated with the increase with the increase of TNF-α, IFN-α, IRF3 and IRF7 pDCs transcription after GS-9620 stimulation.
Even showing some limitations, our study may represent the basis of molecular mechanisms for the present and future implementation of these immunomodulatory therapies in humans. First, the study design does not include other dendritic cell populations. Although the major targets of HIV-1 infection are CD4 T-cells, pDCs represent a crucial dendritic cell subset in HIV-1 infection as the main IFN-α producer, the expression of restriction factors and its role as APC.70 A second limitation is the low number of participants included in each study group. The complexity of the experiments and the low representation of pDCs in peripheral blood (<1%) that involves the extraction of 450 ml of blood sample has limited the number of participants included. This fact, has also contributed to the recruitment of all the PLWH from a single center. Nevertheless, all participants have been carefully selected including a variety of clinical phenotypes of HIV-1 disease progression and significant results have been obtained.
This study provides extensive new phenotypic and functional data by which TLR agonists act to boost pDCs and induce HIV-specific T-cell responses. Collectively, these findings suggest that TLR agonists might have an important role to play in HIV-1 cure strategies. This study and recent findings support that to further enhance the anti-HIV-1 effect of CpG-C and GS-9620, a combination of immune therapeutic strategies with potent LRAs and/or additional immune-based interventions should be developed.
Contributors
All authors reviewed and approved the submitted version of the manuscript. ERM, LTD and BDM designed the experiments. MRJL, CGC, IG, MICS, AIAR and RR performed the experiments. LLC, AGV, NE, CRO and MLV recruited the participants and provided PLWH blood samples. MRJL and ERM analysed, interpreted the data and wrote the paper. JV, SB, MREIB and APG reviewed and contributed to paper discussion. ERM and MRJL have verified the underlying data. ERM, conceived the idea, coordinate the project and acquired funding for the study.
Data sharing statement
Due to the sensitivity of the data, individual participant data will not be made available. Data generated by this study is available upon request to the corresponding author.
Declaration of interests
The authors declare that no conflicts of interest exist.
Acknowledgements
This study would not have been possible without the collaboration of all the patients, medical and nursing staff, and data managers who have taken part in this project. We would like to thank to Esperanza Muñoz-Muela for the critical reading of this manuscript. This work was supported by Gilead fellowship program (GLD17-00299), the Instituto de Salud Carlos III (ISCIII) and co-financed by the European Union, Fondos FEDER, “a way to make Europe” (research contracts CP19/00159 to AGV, FI17/00186 to MRJL, FI19/00083 to CGC, and research projects PI16/01684, PI19/01127 and PI22/01796) and the Red Temática de Investigación Cooperativa en SIDA (RD16/0025/0020 and RD16/0025/0026 to E.R.M.), which is included in the Acción Estratégica en Salud, Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica, 2008 to 2011 and 2013 to 2016, Instituto de Salud Carlos III. ERM was granted by the Spanish National Research Council (CSIC).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104549.
Appendix A. Supplementary data
References
- 1.Wada N., Jacobson L.P., Cohen M., French A., Phair J., Muñoz A. Cause-specific mortality among HIV-infected individuals, by CD4+ cell count at HAART initiation, compared with HIV-uninfected individuals. AIDS. 2014;28:257–265. doi: 10.1097/QAD.0000000000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palella F.J., Jr., Delaney K.M., Moorman A.C., et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Finzi D., Hermankova M., Pierson T., et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/SCIENCE.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 4.Chun T.W., Carruth L., Finzi D., et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 5.Siegal F.P., Kadowaki N., Shodell M., et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/SCIENCE.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 6.Bam R.A., Hansen D., Irrinki A., et al. TLR7 agonist GS-9620 is a potent inhibitor of acute HIV-1 infection in human peripheral blood mononuclear cells. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.01369-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jakobsen M.R., Bak R.O., Andersen A., et al. IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proc Natl Acad Sci U S A. 2013;110:E4571–E4580. doi: 10.1073/PNAS.1311669110/-/DCSUPPLEMENTAL/PNAS.201311669SI.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotter D., Sauter D., Kirchhoff F. Emerging role of the host restriction factor tetherin in viral immune sensing. J Mol Biol. 2013;425:4956–4964. doi: 10.1016/J.JMB.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald-Bocarsly P., Feng D. The role of type I interferon production by dendritic cells in host defense. Biochimie. 2007;89:843–855. doi: 10.1016/J.BIOCHI.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barblu L., MacHmach K., Gras C., et al. Plasmacytoid dendritic cells (pDCs) from HIV controllers produce interferon-α and differentiate into functional killer pDCs under HIV activation. J Infect Dis. 2012;206:790–801. doi: 10.1093/INFDIS/JIS384. [DOI] [PubMed] [Google Scholar]
- 11.Machmach K., Leal M., Gras C., et al. Plasmacytoid dendritic cells reduce HIV production in elite controllers. J Virol. 2012;12:4245–4252. doi: 10.1128/JVI.07114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominguez-Molina B., Machmach K., Perales C., et al. Toll-like receptor 7 (TLR-7) and TLR-9 agonists improve hepatitis C virus replication and infectivity inhibition by plasmacytoid dendritic cells. J Virol. 2018;92 doi: 10.1128/JVI.01219-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buitendijk M., Eszterhas S.K., Howell A.L. Gardiquimod: a Toll-like receptor-7 agonist that inhibits HIV type 1 infection of human macrophages and activated T cells. AIDS Res Hum Retroviruses. 2013;29:907–918. doi: 10.1089/AID.2012.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fosdick A., Zheng J., Pflanz S., et al. Pharmacokinetic and pharmacodynamic properties of GS-9620, a novel toll-like receptor 7 agonist, demonstrate interferon-stimulated gene induction without detectable serum interferon at low oral doses. J Pharmacol Exp Ther. 2014;348:96–105. doi: 10.1124/JPET.113.207878. [DOI] [PubMed] [Google Scholar]
- 15.Lim S.Y., Osuna C.E., Hraber P.T., et al. TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci Transl Med. 2018;10:eaao4521. doi: 10.1126/SCITRANSLMED.AAO4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borducchi E.N., Liu J., Nkolola J.P., et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature. 2018;563:360–364. doi: 10.1038/s41586-018-0600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SenGupta D., Brinson C., DeJesus E., et al. The TLR7 agonist vesatolimod induced a modest delay in viral rebound in HIV controllers after cessation of antiretroviral therapy. Sci Transl Med. 2021;13:1–15. doi: 10.1126/scitranslmed.abg3071. [DOI] [PubMed] [Google Scholar]
- 18.Ferrando-Martínez S., Casazza J.P., Leal M., et al. Differential gag-specific polyfunctional T cell maturation patterns in HIV-1 elite controllers. J Virol. 2012;86:3667–3674. doi: 10.1128/JVI.07034-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pernas M., Tarancón-Diez L., Rodríguez-Gallego E., et al. Factors leading to the loss of natural elite control of HIV-1 infection. J Virol. 2018;92:e01805–e01817. doi: 10.1128/JVI.01805-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 21.Lei J., Yin X., Shang H., Jiang Y. IP-10 is highly involved in HIV infection. Cytokine. 2019;115:97–103. doi: 10.1016/J.CYTO.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Sereti I., Krebs S.J., Phanuphak N., et al. Persistent, albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis. 2017;64:124–131. doi: 10.1093/CID/CIW683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y., Xue J. Expression profile and biological role of immune checkpoints in disease progression of HIV/SIV infection. Viruses. 2022;14:1–28. doi: 10.3390/v14030581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenorio A.R., Zheng Y., Bosch R.J., et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210:1248–1259. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boulware D.R., Hullsiek K.H., Puronen C.E., et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203:1637–1646. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho J.E., Scherzer R., Hecht F.M., et al. The association of CD4+ T-cell count on cardiovascular risk in treated HIV disease. AIDS. 2012;26:1115–1120. doi: 10.1097/QAD.0B013E328352CE54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwa S., Kannanganat S., Nigam P., et al. Plasmacytoid dendritic cells are recruited to the colorectum and contribute to immune activation during pathogenic SIV infection in rhesus macaques. Blood. 2012;118:2763–2773. doi: 10.1182/blood-2011-02-339515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbeuval J.P., Nilsson J., Boasso A., et al. Differential expression of IFN-α and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci U S A. 2006;103:7000. doi: 10.1073/PNAS.0600363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fromentin R., Bakeman W., Lawani M.B., et al. CD4+ T cells expressing PD-1, TIGIT and LAG-3 contribute to HIV persistence during ART. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pardons M., Baxter A.E., Massanella M., et al. Single-cell characterization and quantification of translation-competent viral reservoirs in treated and untreated HIV infection. PLoS Pathog. 2019;15(1–28) doi: 10.1371/JOURNAL.PPAT.1007619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurst J., Hoffmann M., Pace M., et al. Immunological biomarkers predict HIV-1 viral rebound after treatment interruption. Nat Commun. 2015;6(1–9):8495. doi: 10.1038/NCOMMS9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wherry E.J. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 33.Jones R.B., Ndhlovu L.C., Barbour J.D., et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/JEM.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kassu A., Marcus R.A., D'Souza M.B., et al. Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. J Immunol. 2010;185:3007–3018. doi: 10.4049/JIMMUNOL.1000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fonteneau J.-F., Larsson M., Beignon A.-S., et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78:5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinsen J.T., Gunst J.D., Højen J.F., Tolstrup M., Søgaard O.S. The use of toll-like receptor agonists in HIV-1 cure strategies. Front Immunol. 2020;11:1112. doi: 10.3389/FIMMU.2020.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riddler S.A., Para M., Benson C.A., et al. Vesatolimod, a toll-like receptor 7 agonist, induces immune activation in virally suppressed adults living with human immunodeficiency virus-1. Clin Infect Dis. 2021;72:E815–E824. doi: 10.1093/cid/ciaa1534. [DOI] [PubMed] [Google Scholar]
- 38.Vibholm L., Schleimann M.H., Højen J.F., et al. Short-course toll-like receptor 9 agonist treatment impacts innate immunity and plasma viremia in individuals with human immunodeficiency virus infection. Clin Infect Dis. 2017;64:1686–1695. doi: 10.1093/cid/cix201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vibholm L.K., Konrad C.V., Schleimann M.H., et al. Effects of 24-week Toll-like receptor 9 agonist treatment in HIV type 1+ individuals. AIDS. 2019;33:1315–1325. doi: 10.1097/QAD.0000000000002213. [DOI] [PubMed] [Google Scholar]
- 40.Lubong Sabado R., O'Brien M., Subedi A., et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116:3839–3852. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlaepfer E., Speck R.F. TLR8 activates HIV from latently infected cells of myeloid-monocytic origin directly via the MAPK pathway and from latently infected CD4+ T cells indirectly via TNF-α. J Immunol. 2011;186:4314–4324. doi: 10.4049/JIMMUNOL.1003174. [DOI] [PubMed] [Google Scholar]
- 42.Dhamanage A.S., Thakar M.R., Paranjape R.S. HIV-1-Mediated suppression of IFN-α production is associated with inhibition of IRF-7 translocation and PI3K/akt pathway in plasmacytoid dendritic cells. AIDS Res Hum Retroviruses. 2019;35:40–48. doi: 10.1089/AID.2018.0136. [DOI] [PubMed] [Google Scholar]
- 43.Casartelli N., Sourisseau M., Feldmann J., Guivel-Benhassine F., Mallet A. Tetherin restricts productive HIV-1 cell-to-cell transmission. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jolly C., Booth N.J., Neil S.J.D. Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells. J Virol. 2010;84:12185–12199. doi: 10.1128/JVI.01447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bego M.G., Côté É., Aschman N., Mercier J., Weissenhorn W., Cohen É.A. Vpu exploits the cross-talk between BST2 and the ILT7 receptor to suppress anti-HIV-1 responses by plasmacytoid dendritic cells. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jego G., Palucka A.K., Blanck J.P., Chalouni C., Pascual V., Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/S1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 47.Megjugorac N.J., Young H.A., Amrute S.B., Olshalsky S.L., Fitzgerald-Bocarsly P. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J Leukoc Biol. 2004;75:504–514. doi: 10.1189/jlb.0603291. [DOI] [PubMed] [Google Scholar]
- 48.Colonna M., Trinchieri G., Liu Y.J. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/NI1141. [DOI] [PubMed] [Google Scholar]
- 49.Kamphuis E., Junt T., Waibler Z., Forster R., Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006;108:3253–3261. doi: 10.1182/BLOOD-2006-06-027599. [DOI] [PubMed] [Google Scholar]
- 50.Gota C., Calabrese L. Induction of clinical autoimmune disease by therapeutic interferon-alpha. Autoimmunity. 2003;36:511–518. doi: 10.1080/08916930310001605873. [DOI] [PubMed] [Google Scholar]
- 51.Baenziger S., Heikenwalder M., Johansen P., et al. Triggering TLR7 in mice induces immune activation and lymphoid system disruption, resembling HIV-mediated pathology. Blood. 2009;113:377–388. doi: 10.1182/BLOOD-2008-04-151712. [DOI] [PubMed] [Google Scholar]
- 52.Cao W., Bover L. Signaling and ligand interaction of ILT7: receptor-mediated regulatory mechanisms for plasmacytoid dendritic cells. Immunol Rev. 2010;234:163–176. doi: 10.1111/J.0105-2896.2009.00867.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao W., Bover L., Cho M., et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/JEM.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitchell D., Chintala S., Dey M. Plasmacytoid dendritic cell in immunity and cancer. J Neuroimmunol. 2018;322:63–73. doi: 10.1016/J.JNEUROIM.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 55.Smallwood H.S., Aquino V.H., Ferhadian D., et al. Plasmacytoid dendritic cells as cell-based therapeutics: a novel immunotherapy to treat human immunodeficiency virus infection? Front Cell Infect Microbiol. 2020;1(249):1–12. doi: 10.3389/fcimb.2020.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Villadangos J.A., Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–361. doi: 10.1016/J.IMMUNI.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 57.Isnard S., Hatton E.X., Iannetta M., Guillerme J.-B., Hosmalin A. Cell-associated HIV cross-presentation by plasmacytoid dendritic cells is potentiated by noncognate CD8 + T cell preactivation. J Immunol. 2021;207:15–22. doi: 10.4049/JIMMUNOL.2000392. [DOI] [PubMed] [Google Scholar]
- 58.Benitez-Ribas D., Adema G.J., Winkels G., et al. Plasmacytoid dendritic cells of melanoma patients present exogenous proteins to CD4+ T cells after Fc gamma RII-mediated uptake. J Exp Med. 2006;203:1629–1635. doi: 10.1084/JEM.20052364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young L.J., Wilson N.S., Schnorrer P., et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9:1244–1252. doi: 10.1038/NI.1665. [DOI] [PubMed] [Google Scholar]
- 60.Tel J., Lambeck A.J.A., Cruz L.J., Tacken P.J., de Vries I.J.M., Figdor C.G. Human plasmacytoid dendritic cells phagocytose, process, and present exogenous particulate antigen. J Immunol. 2010;184:4276–4283. doi: 10.4049/JIMMUNOL.0903286. [DOI] [PubMed] [Google Scholar]
- 61.Offersen R., Nissen S.K., Rasmussen T.A., et al. A novel toll-like receptor 9 agonist, MGN1703, enhances HIV-1 transcription and NK cell-mediated inhibition of HIV-1-Infected autologous CD4+ T cells. J Virol. 2016;90:4441–4453. doi: 10.1128/JVI.00222-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai A., Irrinki A., Kaur J., et al. Toll-like receptor 7 agonist GS-9620 induces HIV expression and HIV-specific immunity in cells from HIV-infected individuals on suppressive antiretroviral therapy. J Virol. 2017;91:0216616. doi: 10.1128/JVI.02166-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ventura J.D., Nkolola J.P., Chandrashekar A., et al. Therapeutic efficacy of an Ad26/MVA vaccine with SIV gp140 protein and vesatolimod in ART-suppressed rhesus macaques. Npj Vaccines. 2022;7:53. doi: 10.1038/s41541-022-00477-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ram R.R., Paul D., Margot N., Michael Abram, Geleziunas R., Joseph Hesselgesser C.C. Activation of HIV-specific CD8+ T-cells from HIV+ donors by vesatolimod. Antivir Ther. 2020;25:163–169. doi: 10.3851/IMP3359. [DOI] [PubMed] [Google Scholar]
- 65.Neil S.J.D., Zang T., Bieniasz P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/NATURE06553. [DOI] [PubMed] [Google Scholar]
- 66.Sheehy A.M., Gaddis N.C., Choi J.D., Malim M.H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/NATURE00939. [DOI] [PubMed] [Google Scholar]
- 67.Stremlau M., Owens C.M., Perron M.J., Kiessling M., Autissier P., Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/NATURE02343. [DOI] [PubMed] [Google Scholar]
- 68.Lahouassa H., Daddacha W., Hofmann H., et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol. 2012;13:223–228. doi: 10.1038/NI.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdel-Mohsen M., Wang C., Strain M.C., et al. Select host restriction factors are associated with HIV persistence during antiretroviral therapy. AIDS. 2015;29:411–420. doi: 10.1097/QAD.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manches O., Frleta D., Bhardwaj N. Dendritic cells in progression and pathology of HIV infection. Trends Immunol. 2014;35:114–122. doi: 10.1016/j.it.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.