Abstract
Vaccines for tetanus prevention have rapidly progressed, and the number of outbreaks, especially the incidence of tetanus in developed countries, has decreased dramatically. However, the mortality rate associated with severe tetanus remains high. Tetanus eradication is difficult owing to the widespread presence of the spores of tetanus bacteria in the environment, but tetanus can be prevented by acquired immunity from vaccines. Older people, intravenous drug users, and migrants are at a high risk of tetanus in developed countries owing to the lack of booster vaccination programs. Natural disasters, especially floods, often cause an increase in the prevalence of tetanus because of the associated injuries. Precautions should be taken to combat the threat of a new tetanus outbreak due to floods in urban areas owing to global warming. In particular, Japan is facing a high risk of urban flooding‐induced tetanus, despite its status as a developed country. This review aims to highlight the data on the epidemiology, causes, treatment, and prevention of tetanus and problems associated with tetanus countermeasures during future floods.
Keywords: Disaster medicine, flooding, infection control, tetanus, urban flood
Tetanus prevention is largely dependent on adequate vaccination, but it might also be influenced by climate change and not necessarily the level of development of a country. In the future, it will be necessary for not only developing countries but also developed countries that are susceptible to climate change to be on guard against urban flooding–induced tetanus.
INTRODUCTION
Tetanus is a rare but fatal condition. 1 Increased attention should be paid to the risk of a tetanus outbreak in the community, particularly during natural disasters. Previous studies have reported the occurrence of tetanus outbreaks during natural disasters. However, most of the studies focused on earthquakes and tsunamis. 2 , 3 , 4 The occurrence of flooding due to typhoons and torrential rains has increased worldwide. 5 There are concerns about urban flooding causing disease outbreaks. 6 Tetanus prevention is largely dependent on adequate vaccination, but it might also be influenced by climate change and not necessarily the level of development of a country. In the future, it will be necessary for not only developing countries but also developed countries that are susceptible to climate change to be on guard against urban flooding–induced tetanus. Herein, we wish to report the epidemiology, causes, treatment, and prevention of tetanus and anticipated problems regarding tetanus countermeasures in future floods. For this narrative review, the PubMed (National Center for Biotechnology Information, National Institutes of Health; Bethesda, Maryland, USA) database was searched for articles published from 2010 to 2022, using the keywords “tetanus”, “disaster”, “flooding disaster”, “prevention and control”, and “urban flood.” In addition, the World Health Organization (WHO) website and government databases such as the Centers for Disease Control and Prevention (CDC) website were searched for articles in English. We selected articles published in the last 10 years but did not exclude commonly referenced and highly regarded older publications.
REVIEW
Epidemiology
Vaccines against tetanus have rapidly progressed, and the number of outbreaks has decreased dramatically. However, many cases occur in countries where vaccination has not shown much progress (e.g., the 3350 non‐neonatal tetanus cases reported in 2014 in Uganda or the 139 tetanus cases that occurred following the Yogyakarta earthquake in 2010). Assessing the true burden of tetanus occurrence globally is difficult because most cases are reported in developing countries, where surveillance systems for tetanus are insufficient and vaccine programs are still immature. 1 Approximately 80% of deaths due to tetanus have been reported to occur in south Asia and Africa. 7 According to the WHO data published in 2019, 14,745,000 cases of tetanus were reported worldwide. 8 Approximately 1,000,000 people contract the disease each year, and more than 200,000 people die from it. 9 Tetanus is most likely to be fatal in persons aged ≥60 years and those who are unvaccinated. 10 Severe tetanus has a high fatality rate. 9
In developed countries, the occurrence of tetanus is rare, and most cases include those of older people aged ≥60 years. 11 , 12 An increasing occurrence of tetanus has been reported among patients with diabetes. 1 Intravenous drug users are known to be at a high risk of contracting tetanus and may also contract it via contaminated heroin. 13 The herd immunity, especially that afforded by vaccines against tetanus, tends to decline over time. 14 Migrants who have not received the vaccine despite living in developed countries might become the new high‐risk group in such countries. 15 In developing countries, without intensive care and appropriate ventilatory support, deaths from severe tetanus exceed 50% of all severe tetanus. A mortality rate of 10% is considered an acceptable goal in developed countries. 16 The mortality rate of severe tetanus in Japan has been reported to be 6.8%, 17 which is an acceptable rate of morality for a developed country; however, the number of incidences is higher than that in other developed countries (27 cases per year in the United Kingdom, five cases in France). According to Nakajima et al. 17 who reported the incidence using a National Database in Japan, there were 499 tetanus cases between 2010 and 2016; the median age of the patient group was 74 years, probably due to low immunity in older individuals.
Pathogen and pathogenesis, diagnosis, and treatment
Pathogen and pathogenesis
Tetanus is caused by Clostridium tetani. C. tetani is an anaerobic Gram‐positive and spore‐forming bacteria. The spores of tetanus bacteria are widespread in the environment; they are found mainly in soil, and the intestines and feces of some farm animals such as horses, cattle, and chickens. Spores are also present in the saliva of infected patients and in contaminated heroin. The spores are extremely resistant to heat and disinfectants. They can survive autoclaving at 249.8°F (121°C) for 10–15 min. The spores are also relatively resistant to phenol and other chemical agents. In the presence of anaerobic conditions, the spores germinate. Tetanus is caused by two exotoxins: tetanospasmin and tetanolysin. Both are secreted under anaerobic conditions found in necrotic or infected tissue. Tetanospasmin is a neurotoxin that causes muscle spasms in patients with tetanus. 10 Tetanolysin is thought to be capable of damaging otherwise viable tissue surrounding the infected area and optimizing the conditions for bacterial multiplication. 18 Tetanospasmin inhibits the release of γ‐amino‐butyric acid and glycine in the central nervous system. 16 Once tetanospasmin is activated, the heavy chain of tetanospasmin travels in a retrograde fashion into the central nervous system. 19 , 20 , 21 Tetanospasmin cleaves to synaptobrevin (a vesicle‐associated membrane protein) after reaching the spinal cord and brainstem. It inhibits glycine release and γ‐amino‐butyric acid, both of which are skeletal muscle inhibitors. Clinical muscle spasm is caused by this mechanism. 21 , 22 , 23 , 24 , 25
The spores usually enter the body through broken skin caused by injuries. Wounds contaminated with dirt, feces, or saliva; puncture wounds (caused by an object such as a nail or needle); burns; crash injuries; and injuries causing tissue necrosis are common sources of tetanus infection. C. tetani can also infect a person through breaks in the skin caused by surgical procedures, insect bites, dental infections, intravenous drug use, and intramuscular injections. In most cases, the period of symptom onset (incubation period) is approximately 8 days (range 1–21 days). The shorter the incubation period, the more severe the disease and the higher the mortality. The distance of the wound from the central nervous system is also associated with the duration of the incubation period. 10
Tetanus is clinically classified as generalized, neonatal, local, and cephalic (Table 1). 10 Generalized tetanus is the most common form (>80% of reported cases). The first sign is spasm of the muscles of the jaw, or “lockjaw,” followed by stiffness of the neck, difficulty in swallowing, rigidity of abdominal muscles, and reflex spasms. Reflex spasms can occur with minimal external stimuli and increase in frequency with disease progression. Autonomic disturbances are seen in severe cases with other extra‐muscular symptoms including fever, sweating, hypertension, and tachycardia. On the other hand, hypotension, bradycardia, and asystole may arise from increased vagal tone and activity. 26 , 27 “Autonomic storm” occurs with marked cardiovascular instability. Severe hypertension and tachycardia may occur alternately with profound hypotension, bradycardia, or recurrent cardiac arrest. 28 , 29
Table 1.
Clinical classifications of tetanus
| Classification | Description |
|---|---|
| Generalized |
The most common type (more than 80%). Trismus or lockjaw, followed by stiffness of the neck, difficulty in swallowing, rigidity of abdominal muscles, and dysautonomia such as high temperature, sweating, abnormal blood pressure, and episodic rapid heart rate. Spasm continues for 3–4 weeks. It takes months to recover completely. |
| Neonatal | Neonatal tetanus is a form of generalized tetanus that occurs in newborn infants without protective immunity because of lack of mother's immunity. It usually occurs through infection of the unhealed umbilical stump |
| Localized | Contraction of muscles are limited to the sight of injury. |
| Cephalic |
Involvement of the cranial nerves, especially in the facial area. Occasionally occurring with otitis media or following head injury. |
Produced table refer to “Pink Book”, CDC.
Source: “Pink Book”, Centers for Disease Control and Prevention (US). 10
Diagnosis
Tetanus must be diagnosed clinically, but there are very few confirmatory tests available at present. Wound cultures frequently yield negative results for C. tetani. C. tetani is recovered from the wound in only 30% of cases and can be isolated from patients who do not have tetanus. 30 Tetanus is a vaccine‐preventable disease; acquired immunoglobulin G antibodies after vaccination afford immunity against tetanus. Measuring antibody concentrations using ELISA (cut‐off antibody concentrations for protection: 0.1–0.2 IU/mL) 1 or rapid immunoassay [using the Tetanus Quick Stick (TQS); Nephrotek Laboratory, Rungis, France] may be useful in emergency departments. 31 , 32 Tetanus in the presence of protective concentrations of antibody is rare, and therefore a diagnosis of tetanus should be considered unlikely in individuals with serum antibody titers of more than 0.1 IU/mL, as tested by ELISA. 1 However, conventional tetanus antibody assays by ELISA are laboratory based and challenging to implement in clinical settings, such as the emergency room. Thus, the usefulness of the rapid immune assay in clinical settings, such as the emergency room, has been recommended. 31 , 32
The Ablett classification system score (Table 2) is the most widely used system for defining tetanus severity. 33 The Ablett classification has been used in intensive care units (ICUs) outside the United States to identify the severity of tetanus and prevent airway and respiratory complications, which are the most common causes of death. The classification can be used as a guide to identify patients with upper airway obstruction of Grade II or higher severity, who may benefit from early tracheostomy, or difficult respiratory management. 34
Table 2.
Ablett classification of severity of tetanus
| Grade | Severity | Symptoms |
|---|---|---|
| I | Mild | Mild trismus; general spasticity; no respiratory distress; no spasms; no dysphasia |
| II | Moderate | Moderate trismus; rigidity, short spasms, mild dysphagia, moderate respiratory distress, respiratory rate greater than 30 breaths/min. |
| III | Severe | Severe trismus, generalized rigidity, reflex prolonged spasms; severe dysphagia, apneic spells; increased respiratory greater than 40 breaths/min, heart rate greater than 120 beats/min. |
| IV | Very severe | Grade III autonomic disturbances. The cardiovascular system; severe hypertension and tachycardia alteration |
Management
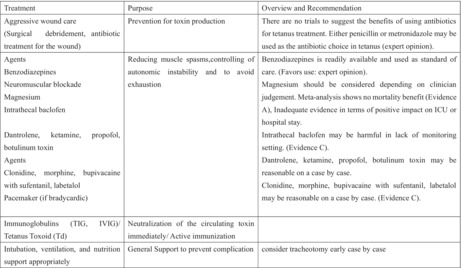
There are four basic patient management strategies: aggressive wound care including surgical debridement and antibiotic treatment for the wound, reducing muscle spasms and controlling autonomic instability, neutralizing the circulating toxin with human tetanus immunoglobulin, and general support to prevent complications. Airway and breathing complications are the most concerning complications of tetanus due to laryngospasm. 21 Aspiration pneumonia is also a common complication of tetanus in the late phase, as evidenced in 50%–70% of autopsied cases. 10 Tracheostomy is preferable to endotracheal intubation, and it reduces the risk of tracheal stenosis after prolonged mechanical ventilation. 1 Heavy sedative and paralytic condition using by much sadative drugs or neuromuscular blockade by muscle relaxants supproted by ventilation are used for patients with severe tetanus in routine practice, and many kinds of drugs have been used so far for tetanus management; however, establishing evidence‐based management by conducting clinical trials is difficult for ethical reasons. Tetanus is less incident but more severe than other diseases and occurs in different medical settings. Rodrigo et al. 35 reviewed the drugs used for treating tetanus in terms of action mechanism and recommendations. The key management strategy for tetanus is shown in Table 3.
Table 3.
Management of tetanus
| Treatment | Purpose | Overview and recommendation |
|---|---|---|
| Aggressive wound care (surgical debridement, antibiotic treatment for the wound) | Prevention for toxin production | There are no trials to suggest the benefits of using antibiotics for tetanus treatment. Either penicillin or metronidazole may be used as the antibiotic choice in tetanus (expert opinion). |
|
Agents Benzodiazepines Neuromuscular blockade Magnesium Intrathecal baclofen Dantrolene, ketamine, propofol, botulinum toxin Agents Clonidine, morphine, bupivacaine with sufentanil, labetalol Pacemaker (if bradycardic) |
Reducing muscle spasms, controlling of autonomic instability and to avoid exhaustion |
Benzodiazepines is readily available and used as standard of care (Favors use: expert opinion). Magnesium should be considered depending on clinician judgment. Meta‐analysis shows no mortality benefit (Evidence A), inadequate evidence in terms of positive impact on intensive care unit or hospital stay. Intrathecal baclofen may be harmful in lack of monitoring setting (Evidence C). Dantrolene, ketamine, propofol, botulinum toxin may be reasonable on a case by case. Clonidine, morphine, bupivacaine with sufentanil, labetalol may be reasonable on a case by case (Evidence C). |
| Immunoglobulins (TIG, IVIG)/tetanus toxoid (Td) | Neutralization of the circulating toxin immediately/Active immunization | |
| Intubation, ventilation, and nutrition support appropriately | General support to prevent complication | Consider tracheotomy early case by case |
Produced this table refer to Rodrigo et al. 35
IVIG, intravenous immunoglobulin; TIG, tetanus immune globulin.
Prevention
As there is no herd immunity against tetanus, and immunity is not conferred even after being afflicted by the illness, vaccination is essential. The risk of tetanus should be assessed according to wound condition and immune status. All wounds should be cleaned, and the suspicion of tetanus should not be ruled out in patients with wounds contaminated by dirt. Depending on the history of a patient receiving prior doses of tetanus toxoid–containing vaccines (TT), age‐appropriate tetanus toxoid–containing vaccination is needed. Tetanus immunoglobulin should be administered to high‐risk patients as soon as possible because it cannot neutralize toxins that are already bound to the nerves (Table 4). 36
Table 4.
Guide to tetanus prophylaxis in routine wound management
| History of TT † | Clean or minor wound TT † (DT, DTap, Tdap, or Td) | Clean wound TIG ‡ | All other wounds § TT † (DT, DTap, Tdap, or Td) | All other wounds TIG ‡ |
|---|---|---|---|---|
| Unknown or <3 | Yes | No | Yes | Yes |
| ≧3 | No ¶ | No | No †† | No |
Reproduced table from “Pink Book”, CDC.
Source: “Pink Book”, Centers for Disease Control and Prevention (US). 10
DT, diphtheria and tetanus toxoid–containing vaccine; DTaP, tetanus toxoid combined with diphtheria and acellular pertussis vaccine; Td, tetanus and diphtheria toxoid–containing vaccine (Td contains reduced amounts of diphtheria toxoid compared with DT); Tdap, Tdap contains the same pertussis components, but a reduced quantity of some pertussis antigens and diphtheria toxoid; TT, tetanus toxoid–containing vaccine; D.
TIG, tetanus immune globulin. People with HIV infection or severe immunodeficiency who have contaminated wounds (including minor wounds) should receive TIG, regardless of the history of tetanus immunizations.
All other wounds are, but not limited to, wounds contaminated by dirt, feces, soil, or saliva; puncture wounds; avulsions; and wounds resulting from missiles, crushing, burns, and frostbite.
Yes, if ≥10 years since the last tetanus toxoid–containing vaccine dose.
Yes, if ≥5 years since the last tetanus toxoid–containing vaccine dose.
Preparation for tetanus mitigation during flood disasters
Flooding risk
Recently, flooding has been the most common type of disaster reported globally, responsible for almost half of all victims of natural disasters. 6 The risk of floods is considered to be increasing worldwide due to torrential rains brought about by climate change, which is a result of global warming caused by the release of greenhouse gases. The risk is also accelerated by socioeconomic factors, 5 such as population growth, increased development along coasts and riverbanks, land subsidence due to increased number of settlements along coastal areas, and over‐extraction of groundwater. According to a report by the World Resources Institute, the number of people affected by floods is expected to double by 2030, 37 and people living in urban areas are also at risk of experiencing flood disasters in the future. One reason is that three‐quarters of large cities are located on waterfronts, and large populations cluster along water sources. Furthermore, the urban population is expected to reach 5 billion in 2030. 6
Vaccination
Natural disasters themselves do not cause epidemics of infectious diseases. Tetanus is caused by physical trauma during disasters, and not the natural disaster itself. 10 Vaccination programs for eradicating tetanus have greatly reduced the incidence of the illness. 38 However, outbreaks may still occur if there is an increase in the frequency of floods, lack of medical resources, delay in treatment interventions, and low vaccination rates.
Although there are limited reports on the topic, an increase in tetanus outbreaks after earthquakes has been reported previously. At the time of the Great East Japan Earthquake, 10 cases of tetanus were reported 39 ; at the time of the 2004 Indonesian tsunami, 106 cases were reported in 1 month; and in Haiti in 2010, 34 tetanus had spread at a high rate. In particular, there have been many reports of infections in areas where tetanus vaccination coverage is low. 34 It is believed that the tetanus bacillus that lives in the soil is brought out by flood water, thus increasing people's susceptibility to the infection. Immunity acquisition is a problem in countries with low vaccination rates. Prevention is important for tetanus because of its high fatality rate. On a global scale, tetanus vaccination rates are decreasing due to the coronavirus disease 2019 (COVID‐19) pandemic. 40
Urban floods
The construction of asphalt pavements due to unplanned urbanization and pumping of groundwater worsen the water retention capacity of groundwater, resulting in a land environment that is always wet, with water seeping out. This, in turn, exposes the area to the risk of flooding. Increased storm frequency can also push the limits of cities and result in inland flooding. There are also concerns related to unplanned urbanization. 6 , 41
Green infrastructure
Infrastructure development, such as levees, is not the only type of disaster mitigation plan that deserves consideration. Green infrastructure such as mangroves, reefs, and sand dunes also function as natural buffers against coastal storms, and protecting such natural infrastructure will also help prevent flooding and reduce greenhouse gas emissions. 42 One method of mitigating urban disasters caused by global warming is the sponge city concept. 6 Vegetation should be increased in cities for disaster mitigation, and because this would require unpaved areas of land, individuals could be residing in closer proximity to soil in cities than before. Urban flooding results in increased rates of infectious diseases 43 and proliferation of water‐borne pathogens. 44 , 45 , 46 The differences in the proliferation and degree of activation of each water‐borne pathogen in a flood event in a paved environment have been demonstrated in papers. 47 Further, several studies have reported on the emergence of Clostridium spores in soils in Nigeria and Zambia after flooding. 48 , 49 Huang et al. 50 used PCR and conducted a surveillance study on changes in Clostridium spp. in the soil in Taiwan after flooding. However, the effect of various urban developments on spore content and activation in soil in the context of disaster mitigation during flood events remains unclear. 44
Risk of tetanus in Japan
Tetanus has a low incidence in developed countries, and it has become a topic of little interest to clinicians. 12 As a result, early diagnosis of the illness has become difficult. Furthermore, regarding vaccination issues, Japan had no booster vaccination program before 1967; therefore, lack of appropriate immunity acquisition is a major risk factor in Japan. 51
Japan is located at the eastern end of monsoon‐prone Asia, which is one of the zones with the heaviest rainfall, with the country experiencing an average annual rainfall of 1,718 mm, approximately two times the world average (880 mm). Furthermore, precipitation in Japan fluctuates widely from season to season, with it being concentrated in the rainy season and typhoon season. For example, the average monthly rainfall in Tokyo is 208.5 mm in September, which is the wettest month, and 39.6 mm in December, which is the driest month, and the difference between them is a factor of five. 52
According to the Global Climate Risk Index 2021 published by Germanwatch in Germany, Japan ranks fourth, among 180 countries, in terms of the risk of climate change. 53 The Climate Risk Index is an index that analyzes the damage caused by natural disasters that actually occurred in a given fiscal year and captures the scale of future risks; the world's largest disaster database that is provided by Germany is used to assess the damage caused by extreme weather events such as droughts and floods in terms of both the number of deaths and economic impact. The majority of the damage on a global scale was due to the Cyclone Idai, which mainly hit Mozambique, Zimbabwe, and Malawi, and the damage caused by Hurricane Dorian, which hit the Bahamas. Developing countries generally tend to be more vulnerable to environmental changes, but Japan was the only developed country to make it to the top 10. Thinking back, Japan has faced torrential rains recently, such as the 2018 Japan floods, 2019 Typhoon Faxai, and 2020 Kyushu floods. For Japan, the magnitude of the economic loss is the main factor behind the judgment of high risk, and Japan's flood damage is a representative example of the severe impact of climate change on developed countries despite the small number of related deaths. Owing to the low incidence of tetanus and decreased tetanus vigilance in nondisaster periods, high rate of climate change, and lack of a booster vaccination program for those born before 1967, Japan may have the “perfect conditions” for a flooding‐induced tetanus outbreak despite its status as a developed country.
Prevention and treatment for tetanus in Japan in times of disaster
For avoiding tetanus outbreak in Japan, we would like to add comments to inform readers about specific measures regarding prevention and treatment.
Raise awareness of vaccination before the disaster (especially for the elderly)
We believe that it is necessary to educate the elderly about tetanus vaccination. The tetanus toxoid vaccine (TT) was introduced in 1952, and routine immunization against the diphtheria, pertussis, and tetanus combined vaccine under the Immunization Law began in 1968; however, the number of cases and deaths in Japan have decreased considerably since 1967. This indicates that the majority of individuals born before 1967 are at risk of not having received tetanus antitoxin. The incidence of tetanus and mortality is reportedly higher among the elderly in Japan. 17 Antibody titers are low in the elderly, which could be due to inadequate vaccination before 1968. 51 Thus, tetanus vaccination should be administered with the expectation of a booster effect.
Prevention of trauma
Tetanus is considered a trauma‐related infection. It is important to encourage volunteer staff and citizens in the affected area to wear gloves when cleaning up after a flood or other water‐related disaster to prevent traumatic injuries.
Rapid diagnosis
Early diagnosis and therapeutic intervention are essential; as cases are rarely encountered in developed countries, it is important to be able to recall and diagnose tetanus in the event of a disaster. As mentioned earlier, this is a trauma‐related infection, but there are cases where the trauma is not identified or the wound site is not clear; thus, it is necessary to consider the risk of tetanus when treating patients.
Disaster preparedness for tetanus treatment
As medical resources would be scarce in some areas during a disaster, TT and tetanus immune globulin (TIG) stockpiling and supply may be required to have treatment in place (Table 4). Considering the effective use of resources during times of disaster, the risk identification shown in Table 4 may be challenging to apply, because vaccination history may be difficult to ascertain during a disaster, and the risk of tetanus with unspecified trauma might be present. Savioli et al. 54 reported that many individuals are not aware of their vaccination history; this is especially the case among the elderly who are reported to be less aware of their vaccination history. Thus, the elderly is a target group for whom TQS could be useful in identifying tetanus morbidity risk according to their study. They also mentioned the usefulness of TQS considering vaccination risk and cost‐effectiveness to avoid unnecessary TT and TIG.
Thus, publishing guidelines in times of disaster that allow consideration of administering TT and TIG doses to more suitable victims after identifying risk identification using rapid antibody kits (TQS) could be useful. In fact, some foreign countries have already incorporated rapid tetanus immune antibody testing kits into their prophylaxis protocols. 55
As the reason for low tetanus mortality in developed countries is the availability of ICU‐based multidisciplinary care, early planning for medical evacuation to areas where ICUs are available may be necessary. Since medical transport after the patient has become severely ill is highly risky, we believe that an appropriate strategy is warranted to enable early medical transport that can provide ICU‐based treatment for patients with tetanus at an early stage.
Further reduction in mortality may require improvements in surveillance content that could investigate the relationship between treatment administered to patients with severe tetanus and the outcome for establishing appropriate treatment for patients with severe tetanus.
CONCLUSION
Eradication of tetanus is considered impossible because of the abundance of tetanus spores in the environment. Even people who live in urban cities are exposed to abundant tetanus spores due to new threats such as urban flooding caused by global warming, a problem that we have not experienced so far. Precautions should be taken against the possibility of a new tetanus outbreak due to flooding in urban areas.
DISCLOSURE
Approval of the Research Protocol with Approval No. and Committee Name: This paper comprises brief literature presented as a narrative review. No formal research ethics approval was required.
Informed Consent: Not applicable.
Registry and Registration No. of the Study/Trial: Not applicable.
Animal Studies: Not applicable.
Conflict of Interest: None declared.
ACKNOWLEDGEMENTS
Not applicable.
REFERENCES
- 1. Yen LM, Thwaites CL. Tetanus. Lancet 2019; 393: 1657–68. [DOI] [PubMed] [Google Scholar]
- 2. Jeremijenko A, McLaws ML, Kosasih H. A tsunami related tetanus epidemic in Aceh, Indonesia. Asia Pac. J. Public Health 2007; 19: 40–4. [DOI] [PubMed] [Google Scholar]
- 3. Sutiono AB, Qiantori A, Suwa H, Ohta T. Characteristic tetanus infection in disaster‐affected areas: case study of the Yogyakarta earthquakes in Indonesia. BMC Res. Notes 2009; 2: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morita T, Tsubokura M, Tanimoto T, Nemoto T, Kanazawa Y. A need for tetanus vaccination before restoration activities in Fukushima, Japan. Disaster Med. Public Health Prep. 2014; 8: 467–8. [DOI] [PubMed] [Google Scholar]
- 5. Guzman O, Jiang H. Global increase in tropical cyclone rain rate. Nat. Commun. 2021; 12: 5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaines JM. Flooding: Water potential. Nature 2016; 531: S54–5. [DOI] [PubMed] [Google Scholar]
- 7. Kyu HH, Mumford JE, Stanaway JD et al. Mortality from tetanus between 1990 and 2015: findings from the Global Burden of Disease Study 2015. BMC Public Health 2017; 17: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . Immunization data portal. [cited 12 April 2023]. Available from: https://immunizationdata.who.int/.
- 9. Ergonul O, Egeli D, Kahyaoglu B, Bahar M, Etienne M, Bleck T. An unexpected tetanus case. Lancet Infect. Dis. 2016; 16: 746–52. [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention (US) . Tetanus. Epidemiology and prevention of vaccine‐preventable diseases (2021). [cited 15 Dec 2022]. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/tetanus.pdf.
- 11. Filia A, Bella A, von Hunolstein C et al. Tetanus in Italy 2001–2010: a continuing threat in older adults. Vaccine 2014; 32: 639–44. [DOI] [PubMed] [Google Scholar]
- 12. Chaudhry R, Dhawan B, Mohanty S, Dey AB. Tetanus in the elderly: a forgotten illness. Lancet 2001; 357: 1805. [DOI] [PubMed] [Google Scholar]
- 13. Kimura AC, Higa JI, Levin RM, Simpson G, Vargas Y, Vugia DJ. Outbreak of necrotizing fasciitis due to Clostridium sordellii among black‐tar heroin users. Clin. Infect. Dis. 2004; 38: e87–91. [DOI] [PubMed] [Google Scholar]
- 14. Weinberger B. Adult vaccination against tetanus and diphtheria: the European perspective. Clin. Exp. Immunol. 2017; 187: 93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rechel B, Mladovsky P, Ingleby D, Mackenbach JP, McKee M. Migration and health in an increasingly diverse Europe. Lancet 2013; 381: 1235–45. [DOI] [PubMed] [Google Scholar]
- 16. Cook TM, Protheroe RT, Handel JM. Tetanus: a review of the literature. Br. J. Anaesth. 2001; 87: 477–87. [DOI] [PubMed] [Google Scholar]
- 17. Nakajima M, Aso S, Matsui H, Fushimi K, Yasunaga H. Clinical features and outcomes of tetanus: analysis using a national inpatient database in Japan. J. Crit. Care 2018; 44: 388–91. [DOI] [PubMed] [Google Scholar]
- 18. Pinder M. Controversies in the management of severe tetanus. Intensive Care Med. 1997; 14: 129–43. [Google Scholar]
- 19. Greene CE. Infectious Diseases of the Dog and Cat, 4th edn. Philadelphia: Elsevier/Saunders, 2012. [Google Scholar]
- 20. Edlich RF. Management and prevention of tetanus. J. Long Term Eff. Med. Implants 2003; 13: 139–54. [PubMed] [Google Scholar]
- 21. Farrar JJ, Yen LM, Cook T et al. Tetanus. J. Neurol. Neurosurg. Psychiatry 2000; 69: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Finkelstein P, Teisch L, Allen CJ, Ruiz G. Tetanus: a potential public health threat in times of disaster. Prehosp. Disaster Med. 2017; 32: 339–42. [DOI] [PubMed] [Google Scholar]
- 23. Linnenbrink T, McMichael M. Tetanus: pathophysiology, clinical signs, diagnosis, and update on new treatment modalities. J. Vet. Emerg. Crit. Care 2006; 16: 199–207. [Google Scholar]
- 24. González‐Forero D, Morcuende S, Alvarez FJ, de la Cruz RR, Pastor AM. Transynaptic effects of tetanus neurotoxin in the oculomotor system. Brain 2005; 128 (Pt 9): 2175–88. [DOI] [PubMed] [Google Scholar]
- 25. Ahnert‐Hilger G, Bigalke H. Molecular aspects of tetanus and botulinum neurotoxin poisoning. Prog. Neurobiol. 1995; 46: 83–96. [DOI] [PubMed] [Google Scholar]
- 26. Edmondson RS, Flowers MW. Intensive care in tetanus: management, complications and mortality in 100 cases. BMJ 1979; 1: 1401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Udwadia FE, Sunavala JD, Jain MC, D'Costa R, Jain PK, Lall A, et al. Haemodynamic studies during the management of severe tetanus. Q J Med. 1992; 83: 449–60. [PubMed] [Google Scholar]
- 28. Kerr JH, Corbett JL, Prys‐Roberts C, Crampton‐Smith A, Spalding JMK. Involvement of the sympathetic nervous systemin tetanus. Studies on 82 patients. Lancet 1968; 2: 236–41. [DOI] [PubMed] [Google Scholar]
- 29. Tsueda K, Oliver PB, Richter RW. Cardiovascular manifestations of tetanus. Anesthesiology 1974; 40: 588–92. [DOI] [PubMed] [Google Scholar]
- 30. Kefer MP. Tetanus. Am. J. Emerg. Med. 1992; 10: 445–8. [DOI] [PubMed] [Google Scholar]
- 31. Stubbe M, Mortelmans LJ, Desruelles D et al. Improving tetanus prophylaxis in the emergency department: a prospective, double blind cost effectiveness study. Emerg. Med. J. 2007; 24: 648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martín‐Casquero T, Ruescas‐Escolano E, Tuells J. Use of the tetanus quick stick (TQS) test in the emergency services. Med. Clin. (Barc) 2019; 153: 394–401. [DOI] [PubMed] [Google Scholar]
- 33. Ablett JJL. Analysis and main experiences in 82 patients treated in the Leeds Tetanus Unit. In: Ellis M (ed). Symposium on Tetanus in Great Britain. Boston Spa: National Lending Library, 1967; 1–10. [Google Scholar]
- 34. Afshar M, Raju M, Ansell D, Bleck TP. Narrative review: tetanus‐a health threat after natural disasters in developing countries. Ann. Intern. Med. 2011; 154: 329–35. [DOI] [PubMed] [Google Scholar]
- 35. Rodrigo C, Fernando D, Rajapakse S. Pharmacological management of tetanus: an evidence‐based review. Crit. Care 2014; 18: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. CDC . CDC's age‐appropriate tetanus vaccination recommendation. [cited 15 Dec 2022]. Available from: https://www.cdc.gov/tetanus/images/tetanus‐vacc‐media.jpg.
- 37. Kuzuma S, Luo T. The number of people affected by floods will double between 2010 and 2030. [cited 15 Dec 2022]. Available from: https://www.wri.org/insights/number‐people‐affected‐floods‐will‐double‐between‐2010‐and‐2030.
- 38. WHO . Tetanus reported cases and incidence. [cited 15 Dec 2022]. Available from: https://immunizationdata.who.int/pages/incidence/TTETANUS.html?CODE=Global&DISEASE=TTETANUS&YEAR=.
- 39. The National Institute of Infectious Diseases . Tetanus related to the Great East Japan Earthquake (Outbreaks in Japan and the three affected prefectures from 2006 to 2011) [cited 15 Dec 2022]. Available from: https://www.niid.go.jp/niid/ja/tetanis‐m/730‐idsc/2937‐idwrs‐1244.html.
- 40. WHO . Immunization and vaccine‐preventable communicable diseases. [cited 15 Dec 2022]. Available from: https://www.who.int/data/gho/data/themes/immunization.
- 41. The Lancet Global Health . Disaster prevention should be equal. Lancet Glob. Health 2017; 5: e1047. [DOI] [PubMed] [Google Scholar]
- 42. Nieuwenhuijsen MJ. Green Infrastructure and Health. Annu. Rev. Public Health 2021; 42: 317–28. [DOI] [PubMed] [Google Scholar]
- 43. Mulder AC, Pijnacker R, de Man H et al. “Sickenin' in the rain” – increased risk of gastrointestinal and respiratory infections after urban pluvial flooding in a population‐based cross‐sectional study in The Netherlands. BMC Infect. Dis. 2019; 19: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. ten Veldhuis JA, Clemens FH, Sterk G, Berends BR. Microbial risks associated with exposure to pathogens in contaminated urban flood water. Water Res. 2010; 44: 2910–8. [DOI] [PubMed] [Google Scholar]
- 45. Scoullos IM, Lopez Vazquez CM, van de Vossenberg J, Brdjanovic D. Die‐off of E. coli as fecal indicator organism on different surfaces after urban floods. J. Environ. Manage. 2019; 250: 109516. [DOI] [PubMed] [Google Scholar]
- 46. de Man H, van den Berg HH, Leenen EJ et al. Quantitative assessment of infection risk from exposure to waterborne pathogens in urban floodwater. Water Res. 2014; 48: 90–9. [DOI] [PubMed] [Google Scholar]
- 47. Scoullos IM, Adhikari S, Lopez Vazquez CM, van de Vossenberg J, Brdjanovic D. Inactivation of indicator organisms on different surfaces after urban floods. Sci. Total Environ. 2020; 704: 135456. [DOI] [PubMed] [Google Scholar]
- 48. Bagadi HO. The relationship between the annual rainfall and outbreaks of blackquarter of cattle in northern Nigeria. Tropl. Anim. Health Prod. 1978; 10: 124–6. [DOI] [PubMed] [Google Scholar]
- 49. Kadohira M, Samui LK. A study of risk factors associated with suspected blackquarter outbreaks in traditionally managed cattle in Lusaka province, Zambia. J. Vet. Epidemiol. 2001; 1: 43–8. [Google Scholar]
- 50. Huang SW, Chan JP, Shia WY, Shyu CL, Tung KC, Wang CY. The utilization of a commercial soil nucleic acid extraction kit and PCR for the detection of Clostridium tetanus and Clostridium chauvoei on farms after flooding in Taiwan. J. Vet. Med. Sci. 2013; 75: 489–95. [DOI] [PubMed] [Google Scholar]
- 51. Nakano T. Japanese vaccinations and practices, with particular attention to polio and pertussis. Travel Med. Infect. Dis. 2011; 9: 169–75. [DOI] [PubMed] [Google Scholar]
- 52. Website, Japan Ministry of Land, Infrastructure, Transport and Tourism . Japan's precipitation is twice the world average and precipitation in Japan is highly variable from season to season, concentrated in the rainy season and typhoon season [cited 15 Dec 2022]. Available from: https://www.mlit.go.jp/river/pamphlet_jirei/bousai/saigai/kiroku/suigai/suigai_3‐1‐1.html.
- 53. Germanwatch . Global climate risk index 2021. [cited 15 Dec 2022]. Available from: https://www.germanwatch.org/en/19777.
- 54. Savioli G, Ceresa IF, Giordano M et al. The reliability of anamnestic data in the management of Clostridium tetani infection in elderly. Front. Med. (Lausanne) 2021; 8: 684594. 10.3389/fmed.2021.684594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Elkharrat D, Espinoza P, De la Coussaye J, Potel G, Pourriat JL et al. Inclusion of a rapid test in the current Health Ministry Guidelines with the purpose of improving anti‐tetanus prophylaxis prescribed to wounded patients presenting at French Emergency Departments. Med. Mal. Infect. 2005; 35: 323–8. [DOI] [PubMed] [Google Scholar]


