Abstract
Information on prolonging the storage duration of cold semen with acceptable fertility in roosters is limited. This study aimed to determine the efficiency of solid storage with the addition of various concentrations of serine to the Thai native rooster (Pradu Hang Dum) semen extender on semen quality and fertility potential during storage at 5°C for up to 120 h. Pooled semen was diluted with a base extender and a gelatin extender containing 0, 2, 4, and 6 mM serine, then stored at 5°C for 120 h. In Experiment 1, the semen quality and malondialdehyde (MDA) concentrations were assessed at 0, 24, 72, and 120 h after storage. In Experiment 2, fertility potential in terms of fertility and hatchability rates was determined using the most effective solid-storage semen from Experiment 1. Sperm quality decreased with increasing storage time (P < 0.05). The lowest semen quality was observed in the control group since T24 of storage compared with the other groups (P < 0.05). Progressive motility, viability, and mitochondrial function were higher (P < 0.05) in the extender supplemented with gelatin and serine groups than those in the gelatin alone group at T72 and T120. In the extender supplemented with gelatin and serine groups, the highest semen quality was observed in the gelatin with 4 mM serine groups. The differences among extenders supplemented with serine were insignificant (P > 0.05), and the lowest MDA was observed in the gelatin with 4 mM serine groups. The fertility and hatchability rates in gelatin with 4 mM serine at T24 were comparable to those in fresh semen (83.87 and 86.12% vs. 86.66 and 88.3%; P > 0.05). Those of T72 were significantly better than those of the control at the same hour of storage (64.08 and 71.61% vs. 52.38 and 64.48%), while those of T120 were not different among groups. In summary, a semen extender as a solid medium supplemented with 4 mM serine successfully preserved the rooster semen for a long duration up to 72 h of storage time.
Key words: long-term storage, cooling storage, rooster semen, gelatin
INTRODUCTION
Artificial insemination (AI) contributes to increased chicken production by allowing the wider use of genetically superior roosters with high production performance. Liquid storage of semen with preservative extenders is a common method used to reduce sperm metabolism and effectively maintain sperm quality over a long period (Tabatabaei and Aghaei, 2012). Sperm storage in vitro retains sperm fertilizing capacity for up to 24 h, which is not as efficient as the oviductal storage system; meanwhile, an in vivo system could retain fertilizing ability for several weeks (Hocking, 2009). This might be because only sperm with normal morphology and motility can enter the sperm storage region, where sperm integrity is maintained for an extended period of future fertilization (Bakst et al., 1994). To achieve the best fertility in chickens, the AI procedure requires superior quality semen that should be inseminated close to the sperm storage tubules in females.
Temperature and diluent qualities (pH, osmolarity control, and energy supply) for in vitro spermatozoa storage are the most crucial criteria for the success of AI (Sarkar, 2020). In addition, preserving sperm in a liquid state at low temperatures of 2°C to 5°C is used to decrease sperm metabolism and its viability for a longer duration (Fattah et al., 2017). The sedimentation of sperm cells during liquid storage decreases the pH and increases toxic metabolic products in the sedimented regions (Nagy et al., 2002). To prevent sedimentation, the use of gelatin supplemented in semen extenders can improve liquid semen storage in many species, such as rabbits (Nagy et al., 2002; López-Gatius et al., 2005), sheep (Yániz et al., 2005), and goats (Salvador et al., 2006). Gelatin has properties similar to collagen hydrolysate, a large molecule that does not enter the spermatozoa cell. The viscosity of the medium is increased and motility is diminished, resulting in a semisolid state during storage at temperatures below 20°C (Resseguie et al., 1981); thus, sedimentation is prevented. Besides, the solid extender can extend the storage duration. This is interesting whether we can prolong the storage duration with acceptable fertility. To the best of our knowledge, supplementation of gelatin in semen extenders during cooled storage has never been reported in roosters.
However, this method is insufficient to ensure long-term sperm survival because, in roosters, the sperm membrane is composed of a high concentration of polyunsaturated fatty acids (PUFAs) (Cerolini et al., 2006; Mussa et al., 2021), which readily undergo lipid peroxidation (LPO) in the presence of reactive oxygen species (ROS) during storage (Cerolini et al., 2006), resulting in motility loss, DNA sperm damage, mitochondrial function deterioration, adenosine triphosphate production, reduction, and consequently, reduced fertility (Sangani et al., 2017; Masoudi et al., 2019). Improving antioxidant-supplemented and enriching cooling storage procedures is necessary to maintain maximum sperm quality during preservation.
Serine is traditionally considered a nonessential amino acid that provides crucial biological activities, ranging from protein synthesis to cell signaling, the latter primarily through post-translational modification by phosphorylation (Metcalf et al., 2008; Hunter, 2012). Serine is a precursor of glycine and cysteine, which can be used to synthesize glutathione to reduce oxidative stress and increase the activity of antioxidant enzymes. (Zhou et al., 2017a, 2018). In our previous study, a serine supplement at approximately 4 mM, which was used as an antioxidant, positively affected sperm quality and fertilization after chilled storage for 24 h (Chankitisakul et al., 2022). Theoretically, the amount of reactive oxygen species gradually increases as sperm are exposed to cooling stress during cold storage (Waberski et al., 2011). It was speculated that the serine supplementation concentration increased when the storage duration increased.
This study aimed to investigate the efficiency of solid storage with the addition of various concentrations of serine to the rooster semen extender (2, 4, and 6 mM) during storage at 5°C for up to 120 h. The quality of the rooster sperm in each treatment was evaluated in terms of sperm motility, sperm viability, and functional mitochondria. The malondialdehyde (MDA) concentration was measured as an index of lipid peroxidation in semen samples. In addition, we examined the effect of insemination on the most effective solid-stored semen.
MATERIALS AND METHODS
The Institutional Animal Care and Use Committee authorized the study based on the Ethics of Animal Experimentation of the National Research Council of Thailand (record no. IACUC-KKU-133/64). All animals were housed in the Network Center for Animal Breeding and Omics Research (Khon Kean University, Khon Kean, Thailand). Unless otherwise specified, all the chemicals used in this study were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO).
Animals and Management
Twenty Thai Native roosters (Pradu Hang Dum; 10–12 mo of age) using standard management practices, which included individual cages (60 × 45 × 45 cm), an open environment house system, water ad libitum, and a twice-a-day feeding regimen (approximately 130 g of commercial breeder feed for male chickens). Roosters were routinely collected twice a week for AI. One hundred twenty-six Thai Native chicken hens (Pradu Hang Dum; 35–39 wk of age) with egg production ≥60% were used for the fertility test. The hens were housed individually, fed approximately 110 g of layer feed per day (Charoensin et al., 2021), and provided water ad libitum.
Rooster Sperm Preparation and Semen Dilution
Semen samples were collected using the dorsal abdominal massage method, as described by Burrows and Quinn (1936). The individual ejaculate was collected in a 1.5 mL microtube with 0.1 mL IGGKPh diluent, which was composed of 0.14 g potassium citrate, H2O, 1.40 g sodium glutamate, 0.21 g sodium dihydrogen phosphate, 0.98 g sodium hydrogen phosphate, 0.9 g glucose, and 0.9 g inositol in 100 mL of deionized water; pH was 6.95, and osmotic pressure was 380 (mOsm/kg) (Voronina et al., 1986). For gelatin extender preparation, IGGKPh with 1.5% added gelatin was dissolved in a water bath at 37°C. After dissolution, the diluent and gelatin were homogeneous, packed into 50 mL test tubes, sealed, and stored at 25°C before use. To control for semen quality and quantity, semen was collected by the same person under the same conditions. Considerable care was taken to avoid contamination of sperm with feces, urates, and clear fluid, all of which could decrease sperm quality. Within 20 min of collection, sperm samples were transferred to the laboratory, and the volume, concentration, and motility were determined. The criteria for a standard quality of sperm were as follows: 0.2 to 0.6 mL of volume, sperm concentration ≥3 × 109 spermatozoa/mL, and motility ≥80% were pooled to eliminate individual differences and divided into 5 aliquots according to the experimental design.
Experimental Design
Experiment 1: Effects of Solid Storage With the Addition of Various Concentrations of Serine to the Rooster Semen Extender (2, 4, and 6 mM) on Sperm Quality During Storage at 5°C for Up To 120 h. Based on preliminary results and previous reports in rabbits, sheep, goats, and pigs (Nagy et al., 2002; López-Gatius et al., 2005; Yániz et al., 2005; Salvador et al., 2006), the concentration of gelatin was 1.5% (w/v). The pooled semen was extended into 5 different diluents: control, gelatin, gelatin with 2 mM serine, gelatin with 4 mM serine, and gelatin with 6 mM serine to a final concentration of 150 to 250 × 106 spermatozoa/mL. Then, diluted semen was placed in a rack, cooled from 25°C to 5°C for 60 min, and maintained in a refrigerator at 5°C. Total motility, progressive motility, viability, spermatozoa mitochondrial membrane potential, and lipid peroxidation levels were evaluated at 0 (after the cooling process), 24, 72, and 120 h of storage. The experiment was repeated 8 times. The most effective solid-stored semen was used in the fertility test in Experiment 2.
Experiment 2: Effects of the Solid Storage With Serine Supplementation in Semen Extender During Storage at 5°C for Up To 120 h on Fertility Potential. The most effective solid storage semen from Experiment 1 was used to determine the fertility. Solid storage semen supplemented with or without serine was diluted with sperm at a final concentration of 150 × 106 sperm/dose. It was used for AI after storage for 24, 72, and 120 h. Insemination after semen collection and dilution with the extender supplemented without gelatin and serine served as a control. The treatments were divided into 10 groups. Eggs were collected for 7 d during d 2 to 8 after insemination, and the fertility rate of candling eggs was determined on d 7 of incubation. The hatchability rates were recorded.
Sperm Quality Evaluation
Sperm Motility
Total motility (MOT) and progressive motility (PMOT) were assessed using a computer-assisted semen analysis (CASA) system (Hamilton Thorne Biosciences, version 10 HIM-IVOS, Beverly, MA); this system was set up as described by our previous study (Chuaychu-noo et al., 2021) using the following settings: frames per second, 60 Hz; minimum contrast, 25; minimum cell size, 4 pixel. A sperm was defined as nonmotile at an average path velocity of less than 5 μm/s, and sperm was considered progressively motile when the average path velocity was greater than 20 μm/s and the straightness index was 80. Each semen sample was diluted at 1:10 (v/v) as required for each extender, and then 3 μL of diluted semen was placed into a prewarmed chamber slide 38°C. At least 5 fields containing a minimum of 300 sperm were evaluated for each sample. The percentages of MOT and PMOT were recorded.
Sperm Viability
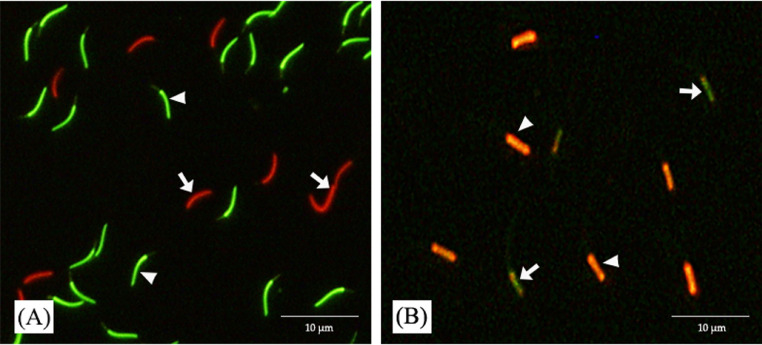
Dual fluorescent staining using SYBR-14 and propidium iodide kits (Live/dead sperm viability kit L7011; Invitrogen, Thermo Fisher Scientific, Waltham, MA) was used to analyze the sperm viability (Chankitisakul et al., 2022). Briefly, 300 μL of diluted semen was mixed with 5 μL of SYBR-14 solution and then incubated at 25°C for 10 min. After incubation, the sample was added to 5 μL propidium iodide and was incubated at 25°C for 10 min. A 10% formaldehyde solution fixed the spermatozoa cells. Viability was assessed under a fluorescence microscope IX71 (Olympus, Tokyo, Japan) at 400× magnification by counting at least 200 spermatozoa for intact plasma membrane stained bright green with SYBR-14 (live) and the damaged plasma membrane stained red with propidium iodide (dead) as shown in Figure 1A. Sperm viability was expressed as the percentage of live sperms with intact plasma membranes.
Figure 1.
Fluorescent staining of rooster sperm with SYBR-14 and propidium iodide (A): sperm with intact plasma membrane stained bright green (arrowheads), sperm with damaged plasma membrane stained red (arrow). Staining with JC-1 (B): greater or lesser mitochondrial functions are characterized by red-orange (arrowheads) and green (arrows) fluorescence, respectively.
Mitochondrial Function
Sperm mitochondrial function was evaluated by fluorescent staining using 5, 5′, 6, 6′-tetrachloro-1, 1′, 3, 3′,-tetraethylbenzimidazolylcarbocyanine iodide (JC-1), according to Rui et al. (2017) with minor modifications. Briefly, 300 µL of each semen sample was stained with 0.5 μL of JC-1 (153 μM in DMSO) and incubated at 25°C for 10 min. All procedures were conducted in a dark room. Then, 3 to 5 µL mixtures were loaded onto a glass slide, covered with a cover slip, and immediately evaluated under a fluorescent microscope with a micromanipulator (Olympus IX71, Japan) at 1,000× magnification by counting at least 200 spermatozoa, which were then categorized as having a greater mitochondrial function as stained bright red-orange of JC-1 and lesser mitochondrial function as green fluorescence as shown in Figure 1B.
Malondialdehyde Concentrations
The thiobarbituric acid reaction was used to assess MDA concentrations in diluted sperm as an indicator of lipid peroxidation, according to our previous study (Chuaychu-noo et al., 2021). Briefly, 0.30 mL of the diluted semen (250 × 106 sperm/mL) was mixed with 0.25 mL of ferrous sulfate (0.2 mM) and 0.25 mL of ascorbic acid (1 mM), and the samples were incubated in a water bath at 37°C for 60 min. After incubation, the sample was added 1 mL of trichloroacetic acid [15% (w/v)] and 1 mL of thiobarbituric acid [0.375% (w/v)] before boiling in a water bath at 100°C for 10 min, and the samples were cooling down to 4°C to stop the reaction. Finally, the precipitate was pelleted by centrifugation at 800 × g for 10 min at 4°C. The supernatants (2 mL) were analyzed using a spectrophotometer (UV-1200, Shimadzu, Japan) at 532 nm. All MDA concentrations were expressed as nmol/mL.
Fertility
A single intravaginal insemination hen tested the fertilizing ability of chilled sperm in Experiment 2 with 0.10 mL (150 × 106 spermatozoa/dose) of chilled semen from each group. One hundred and eighty Thai native hens were randomly assigned to 10 groups (18 hens per group). The insemination was performed between 03.00 and 05.00 pm. Each treatment group's eggs were collected on d 2 to 8 after insemination, marked, and stored on paper trays at a temperature of 22°C to 25°C and relative humidity of 70 to 85% until incubation. Prior to incubation, eggs were randomly placed on setting trays. The fertility rate (total number of fertile eggs/total number of incubated eggs × 100) was determined by candling on d 7 following the start of incubation. The hatchability rate (total number of hatching eggs/total number of fertile eggs) was determined by the hatching of fertile eggs approximately 21 d after the start of incubation.
Statistical Analysis
The study was conducted at the experimental farm of Khon Kean University using a split-plot design in a completely randomized design (CRD) with 2 factors and 8 replications. In Experiment 1, the first factor was treatment, which included 5 treatments: control, gelatin, gelatin with 2 mM serine, gelatin with 4 mM serine, and gelatin with 6 mM serine. The second factor was the storage time of the semen extender, which was 4 times as follows: 0 (after the cooling process), 24, 72, and 120 h of storage (T0, T24, T72, and T120), respectively. In Experiment 2, the first factor was treatment, which was 4 treatments as follows: fresh semen, control, gelatin, and gelatin with 4 mM serine. The second factor was the insemination time after semen storage, which was 4 times that in Experiment 1.
Before using the data for statistical analysis, the Proc UNIVARIATE procedure using SAS v.9.0 software was used to examine the data distribution, including assessing normality and checking data outliers (±3SD was defined as an outlier). After the editing process, in Experiments 1 and 2, data were analyzed using the general linear model (GLM) procedure of SAS (SAS Institute, Inc., Cary, NC). All results are expressed as mean values ± SEM. The treatment, storage time of semen extender, and their interaction mean values for each parameter were compared using Duncan's new multiple range tests (P < 0.05).
RESULTS
Experiment 1: Effects of Solid Storage With the Addition of Various Concentrations of Serine to the Rooster Semen Extender (2, 4, and 6 mM) on Sperm Quality During Storage at 5°C for Up To 120 h
The interaction effect between treatment and storage time was significant for PMOT, viability, and mitochondrial function (P < 0.05) but not for MOT and MDA (P > 0.05; Table 1). Sperm quality (MOT, PMOT, viability, and mitochondrial function) continued to decrease with increasing storage time (P < 0.05), but the MDA concentration increased with increasing storage time (P < 0.05). Therefore, to compare the differences among treatments, they were compared within the same storage time.
Table 1.
Percentage of total motility (MOT), progressive motility (PMOT), viability, mitochondrial function, and MDA concentration of semen extender supplemented with gelatin and serine during storage at T0, T24, T72, and T120 h at 5°C.
| Time to storage (h) | Treatment |
SEM |
P values |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Gelatin | Gelatin with 2 mM serine | Gelatin with 4 mM serine | Gelatin with 6 mM serine | Treatment1 | Time to storage2 | Interaction | ||
| MOT | |||||||||
| T0 | 89.90a,w | 89.6a,w | 91.86a,w | 91.43a,w | 90.94a,w | 0.66 | <0.0001 | <0.0001 | 0.2745 |
| T24 | 80.78c,x | 81.11bc,x | 84.25ab,x | 85.07a,x | 81.96a,x | 0.67 | |||
| T72 | 62.59c,y | 66.23b,y | 69.66a,y | 70.07a,y | 69.06a,y | 0.68 | |||
| T120 | 45.14b,z | 52.11ab,z | 54.18a,z | 55.68a,z | 50.61ab,z | 1.19 | |||
| PMOT | |||||||||
| T0 | 74.99a,w | 72.52a,w | 72.28a,w | 73.37a,w | 73.24a,w | 3.88 | <0.0001 | <0.0001 | <0.0001 |
| T24 | 62.43b,x | 63.89ab,x | 65.04bc,w | 67.65a,w | 67.02a,w | 4.07 | |||
| T72 | 43.96c,y | 46.72bc,y | 49.70ab,y | 52.66a,y | 47.92b,y | 3.00 | |||
| T120 | 19.60c,z | 29.01b,z | 38.46a,z | 40.48a,z | 38.25a,z | 4.15 | |||
| Viability | |||||||||
| T0 | 87.04a,w | 89.27a,w | 89.06a,w | 88.33a,w | 89.96a,w | 0.60 | <0.0001 | <0.0001 | <0.0001 |
| T24 | 79.66c,x | 82.90b,x | 84.77ab,x | 86.13a,x | 85.98ab,x | 0.73 | |||
| T72 | 67.90b,y | 70.07b,y | 74.76a,y | 75.83a,y | 74.08a,y | 0.66 | |||
| T120 | 53.60c,z | 63.85b,z | 69.82a,z | 70.38a,z | 68.51a,z | 1.26 | |||
| Mitochondrial function | |||||||||
| T0 | 87.69a,w | 86.88a,w | 87.30a,w | 87.18a,w | 88.22a,w | 0.47 | <0.0001 | 0.0031 | <0.0001 |
| T24 | 77.85b,x | 80.91ab,x | 82.62a,x | 83.69a,x | 82.99a,x | 0.57 | |||
| T72 | 66.64c,y | 70.29b,y | 74.82a,y | 75.36a,y | 73.50ab,y | 0.69 | |||
| T120 | 48.63d,z | 62.48c,z | 68.34b,z | 72.06a,z | 68.20b,z | 1.42 | |||
| MDA (250 × 106 spz/nmol/mL) | |||||||||
| T0 | 0.62a,w | 0.59a,w | 0.60a,w | 0.58a,w | 0.58a,w | 0.02 | <0.0001 | <0.0001 | 0.5022 |
| T24 | 2.50a,x | 2.36ab,x | 2.12ab,x | 1.93b,x | 1.96b,x | 0.09 | |||
| T72 | 4.15a,y | 4.27a,y | 3.57b,y | 3.28b,y | 3.49b,y | 0.12 | |||
| T120 | 6.24a,z | 6.03ab,z | 5.77abc,z | 5.34c,z | 5.69bc,z | 0.14 | |||
Abbreviation: SEM, standard error of the means.
For treatment effects, means within a row with superscript letters a, b, c, and d indicate significant differences (P < 0.05).
For time-to-storage effects, means within a column with superscripts w, x, y, and z indicate significant differences (P < 0.05).
At T0, semen quality and MDA concentration did not differ among the groups (P > 0.05). However, the lowest semen quality was observed in the control group at T24 of storage compared with the other groups (P < 0.05). PMOT, viability, and mitochondrial function were higher (P < 0.05) in the extender supplemented with gelatin and serine groups than those in the gelatin alone group at T72 and T120. However, among extenders supplemented with gelatin and serine groups, the highest semen quality was observed in the gelatin with 4 mM serine groups.
The MDA concentration results were based on the semen quality. The differences among extenders supplemented with serine were not statistically significant (P > 0.05), and the lowest MDA was observed in the gelatin with 4 mM serine groups.
Therefore, an extender supplemented with gelatin and 4 mM serine was used to determine the fertility in Experiment 2.
Experiment 2: Effects of the Solid Storage With Serine Supplementation in Semen Extender During Storage at 5°C for Up To 120 h on Fertility Potential
The fertility and hatchability rates of fresh rooster semen after semen collection were 86.66 and 88.30%, respectively. The levels of significance of the influence of treatments and days of storage on fertility and hatchability rates are shown in Table 2. The interaction effect between treatment and storage duration was significant for these parameters (P < 0.05). The percentage of fertility and hatchability was reduced when semen was used with increased storage time (P < 0.05).
Table 2.
Effects of different extenders with different times to storage on the percentages of fertility and hatchability after a single dose of artificial insemination.
| Time to storage (h) | Treatment |
SEM |
P values |
|||||
|---|---|---|---|---|---|---|---|---|
| Fresh semen | Control | Gelatin | Gelatin with 4 mM serine | Treatment1 | Time to storage2 | Interaction | ||
| Fertility rate (%) | ||||||||
| T0 | 86.66 | – | – | – | 3.54 | <0.0001 | <0.0001 | 0.0078 |
| T24 | – | 53.33c,x | 66.55b,x | 83.87a,x | 4.68 | |||
| T72 | – | 52.38b,y | 57.64ab,y | 64.08a,y | 5.58 | |||
| T120 | – | 43.76b,z | 46.13b,z | 50.76a,z | 3.71 | |||
| Hatchability rate (%) | ||||||||
| T0 | 88.30 | – | – | – | 0.99 | 0.0208 | <0.0001 | 0.0072 |
| T24 | – | 76.11c,x | 79.53ab,x | 86.12a,x | 3.83 | |||
| T72 | – | 64.48b,y | 66.39ab,y | 71.61a,y | 2.72 | |||
| T120 | – | 40.27a,z | 41.48a,z | 44.10a,z | 5.70 | |||
For treatment effects, means within a row with superscript letters a, b, and c indicate significant differences (P < 0.05).
For time-to-storage effects, means within a column with superscript x, y, and z indicate significant differences (P < 0.05).
At T24 and T72, the highest fertility and hatchability were observed in the extender supplemented with gelatin and 4 mM serine. In contrast, the lowest fertility and hatchability were observed in the control groups (P < 0.05). Interestingly, those of the most effective group (83.87 vs. 86.12) showed a minor decrease compared with fresh semen at T0 (86.66 vs. 88.30). However, the percentages of fertility and hatchability were not significantly different among the 3 groups at T120 (P > 0.05). Figure 2 shows the day-old chick of Pradu Hang Dum native chicken after hatching.
Figure 2.
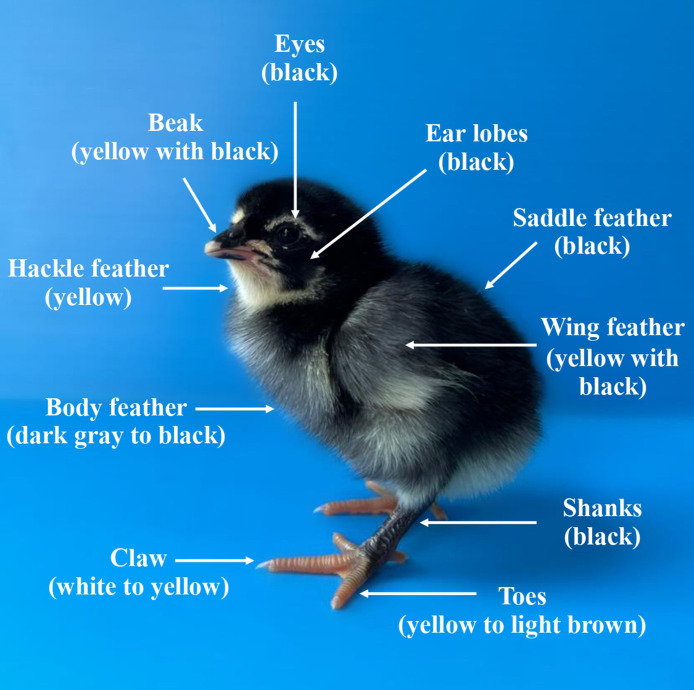
The day-old chick of Pradu Hang Dum native chicken after hatching. Overall, the ideal traits were strong, with the downy feather covering most of the body being black except for the hackle and the end of the wing feather being yellow—mostly black eyes, black ear lobes, yellow with black beaks, and black shanks. The yellow to light brown toes and claws should be white to yellow.
DISCUSSION
Several studies have attempted to improve the long-term cold storage procedures for poultry (Fattah et al., 2017; Bui et al., 2021; Masoudi et al., 2022). However, the fertility capacity was unsatisfactory when semen was stored for 24 h or longer. Our results showed that the semen quality deteriorated as the storage period increased. Adding gelatin to the semen extender improved rooster quality during storage at 5°C compared to the extender without gelatin. In addition, serine supplementation in the gelatin-supplemented extender could enhance the quality after storage at 5°C for up to 120 h, with the greatest values in the 4 mM serine group. Although fertility decreased as storage time increased, fertility capacity was still higher and was considered for up to 72 h when semen was stored in a solid with the serine supplement.
Rooster semen must be diluted immediately after semen collection to maintain sperm viability; otherwise, the sperm would die because of water evaporation from the seminal plasma (Lake and Stewart, 1978). Generally, rooster-diluted semen is recommended to store at low temperatures of 2°C to 5°C, which is appealing for practical reasons to slow its metabolism without seriously decreasing semen quality for up to 24 h (Slanina et al., 2015). Previous studies have demonstrated that adding gelatin to the mammalian semen extender is beneficial for preserving medium homogeneity (Santos et al., 2015), protecting spermatozoa from cold shock (Corcini et al., 2011), increasing the viscosity of dilute semen in such a way that it becomes temporarily solidified (Yániz et al., 2005), and reducing sperm metabolic demand (Yániz et al., 2005), resulting in improved quality of rabbit sperm while preserving longer for up to 5 d. In the present study, adding gelatin to the semen extender improved all sperm characteristics in Thai native roosters compared to a nongelatin extender for up to 120 h (Table 1). A semisolid state occurs during storage of the rooster semen in a gelatin-supplemented extender at 5°C. Thus, the sperm cells, microorganisms, and other particles deposited at the bottom as sediment, which mainly increase toxic metabolic products in the sedimented regions (Nagy et al., 2002), might be prevented, resulting in extended semen quality then.
The rooster sperm membrane contains a high concentration of PUFAs, which readily undergo LPO in the presence of ROS (Cerolini et al., 2006). Dead sperm, leukocytes, and ambient or molecular oxygen can produce ROS. According to Aitken et al. (1995), hydrogen peroxide (H2O2) is the most toxic ROS in sperm and can damage sperm morphology, decrease sperm ability, and reduce fertilizer capacity (Sangani et al., 2017; Masoudi et al., 2019). Rooster sperm have a natural cellular antioxidant system to prevent cell membrane damage from ROS, comprising antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px); soluble antioxidants such as vitamins A, C, and E; and phospholipases, proteases, and transferases that repair or remove damaged molecules caused by cellular oxidative stresses (Braque et al., 2003). However, the natural antioxidants thoroughly diminished after semen collection, while rooster sperm were exposed to chilling injuries, leading to membrane damage throughout cold storage. This may result from an imbalance between ROS generation and inadequate ability of the cellular antioxidant system during in vitro preservation (Reiter et al., 2003). Serine has antioxidant characteristics associated with the reduction of oxidative reactions by enhancing glutathione concentration and controlling the expression of glutathione synthesis-related genes (Zhou et al., 2017a,b), improving the activity of glutathione peroxidase (Wang et al., 2016), increasing glutathione content (Sim et al., 2015), and reducing the quantity of MDA, while increasing the levels of superoxide dismutase, GSH-Px, and catalase (Zhou et al., 2018). Recently, Chankitisakul et al. (2022) showed that an NCAB extender supplemented with 4 mM serine positively affects sperm quality and fertilization after chilled storage for 24 h. However, the main purpose of this study was to extend the duration of cold semen storage. Theoretically, the amount of ROS gradually increases as sperm are exposed to cooling stress during cold storage (Waberski et al., 2011), resulting in an increase in ROS and, subsequently, a decline in sperm motility and viability. Therefore, we hypothesized that the concentration of serine supplementation in the extender might be increased to allow for reduced lipid peroxidation while increasing the storage time (Table 1). Hence, the levels of serine were determined during cold storage for 120 h in Experiment 1. The results revealed that gelatin supplementation alone was insufficient to maintain sperm quality compared with gelatin supplemented with serine. Sperm quality was not different among gelatin-supplemented extenders with different levels of serine, except for a minor increase in mitochondrial function in gelatin with 4 mM serine at T120. Therefore, the latter group was selected for the fertility test in Experiment 2.
In addition to greater sperm quality, fertility potential is also important for reproduction success. Little information is available on the use of cold storage semen after 24 h, especially on fertility capacity. To the best of our knowledge, the present study is the first to present fertility and hatchability using cold semen stored for up to 120 h. The fertility and hatchability rates (Table 2) of this semen diluent stored at T24 were highly satisfying, as they were comparable to inseminating with fresh semen after collection (83.87 and 86.12% vs. 86.66 and 88.3%), while those of T72 were significantly better than the control at the same hour of storage (64.08 and 71.61% vs. 52.38 and 64.48%). This result was superior compared to previous studies that supplemented only creatine as an antioxidant to diluent with a maximum fertility rate in Vietnamese native chickens at 48 h after storage of approximately 60% (Bui et al., 2021) while Penfold et al. (2001) demonstrated a fertility rate of approximately 20% in northern pintail (Anas acuta) after inseminating with storage semen at 72 h. Therefore, it can be inferred that gelatin supplemented with serine acts as a protective component to preserve sperm quality and subsequently aids sperm transport in the hen's reproductive tract to the fertilization site.
The fertility and hatchability of gelatin with serine at T120 tended to be higher than those of the control group, but the differences were not significant (50.76 vs. 43.76; P > 0.05). Although the sperm quality of gelatin with serine at T120 was better than that of the other groups, these qualities were similar to those of the control group at T72, especially progressive motility with lower at approximately 40% in both groups. Sperm motility is reported to be the main factor in the success of sperm reaching sperm storage tubules (SSTs) (Donoghue and Wishart, 2000; Kheawkanha et al., 2021). In this case, it can be inferred that lower fertility develops when the quality of stored semen, at least in our study with progressive motility, is less than 50%. This is speculated to be due to the intrinsic ability of sperm to reach and be stored in the SSTs (Donoghue and Wishart, 2000).
CONCLUSIONS
In conclusion, this is the first report on rooster semen and the development of a semen extender as a solid storage medium supplemented with serine at 5°C. The semen quality in gelatin with 4 mM serine could be sustained for up to 120 h of storage time. Moreover, the fertility potential of insemination with rooster semen preserved for 24 h is satisfactory and comparable to that of fresh semen. However, the T72 values were still acceptable.
ACKNOWLEDGMENTS
This study was financially supported by Thailand Research Fund (TRF) under The Royal Golden Jubilee Ph.D. Program (RGJ Ph.D.) (PHD/0191/2558). The authors would like to thank all staff from the Network Center for Animal Breeding and Omics Research (NCAB, Khon Kean University, Thailand) for their assistance with the animals used in this study.
DISCLOSURES
This manuscript has not been published or submitted for publication elsewhere. The content does not impose any conflict, and all the authors agree with the manuscript's content.
REFERENCES
- Aitken R.J, Paterson M., Fisher H., Buckingham D.W., Duin M.V. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J. Cell Sci. 1995;108:2017–2025. doi: 10.1242/jcs.108.5.2017. [DOI] [PubMed] [Google Scholar]
- Bakst M.R., Wishart G., Brillard J.P. Oviducal sperm selection, transport, and storage in poultry. Poult. Sci. Rev. 1994;5:117–143. [Google Scholar]
- Breque C., Surai P., Brilard J.P. Roles of antioxidants on prolonged storage of avian spermatozoa in vivo and in vitro. Mol. Reprod. Dev. 2003;63:314–323. doi: 10.1002/mrd.10347. [DOI] [PubMed] [Google Scholar]
- Bui H.Y.T., Dang H.L., Phuong N.T.H. Effects of creatine on the quality and fertility of chicken semen during liquid storage. J. ISSAAS. 2021;27:27–37. [Google Scholar]
- Burrows W.H., Quinn J.P. The collection of spermatozoa from the domestic fowl and turkey. Poult. Sci. 1936;16:19–24. [Google Scholar]
- Cerolini S., Zaniboni L., Maldjian A., Gliozzi T. Effect of docosahexaenoic acid anda-tocopherol enrichment in chicken sperm on semen quality, sperm lipid composition and susceptibility to peroxidation. Theriogenology. 2006;66:877e86. doi: 10.1016/j.theriogenology.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Chankitisakul V., Boonkum W., Kaewkanha T., Pimprasert M., Ratchamak R., Authaida S., Thananurak P. Fertilizing ability and survivability of rooster sperm diluted with a novel semen extender supplemented with serine for practical use on small holder farms. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoensin S., Laopaiboon B., Boonkum W., Phetcharaburanin J., Villareal M.O., Isoda H., Duangjinda M. Thai native chicken as a potential functional meat source rich in anserine, anserine/carnosine, and antioxidant substances. Animals. 2021;11:902. doi: 10.3390/ani11030902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuaychu-Noo N., Thananurak P., Boonkum W., Vongpralub T., Chankitisakul V. Effect of organic selenium dietary supplementation on quality and fertility of cryopreserved chicken sperm. Cryobiology. 2021;98:57–62. doi: 10.1016/j.cryobiol.2020.12.008. [DOI] [PubMed] [Google Scholar]
- Corcini C.D., Moreira F., Pigozzo R., Varela-Junior A.S., Torres N.U., Lucia-Junior T. Semen quality and reproductive performance after artificial insemination with boar sperm stored in a gelatin-supplemented extender. Lives Sci. 2011;138:289–292. [Google Scholar]
- Donoghue A.M., Wishart G.J. Storage of poultry semen. Anim. Reprod. Sci. 2000;62:213–232. doi: 10.1016/s0378-4320(00)00160-3. [DOI] [PubMed] [Google Scholar]
- Fattah A., Sharafi M., Masoudi R., Shahverdi A., Esmaeili V. L-carnitine is a survival factor for chilled storage of rooster semen for a long time. Cryobiology. 2017;74:13–18. doi: 10.1016/j.cryobiol.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Hocking P.M. In: Pages 151–171 in Semen Quality and Semen Storage. Hocking P.M., editor. CABI; Wallingford, Oxfordshire, UK: 2009. Biology of breeding poultry. [Google Scholar]
- Hunter T. Why nature chose phosphate to modify protein. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:2513–2516. doi: 10.1098/rstb.2012.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheawkanha T., Boonkum W., Vongpralub T., Chankitisakul V. Characterization of oviduct lining, with emphasis on the sperm storage tubule region (uterovaginal junction), correlated with fertility in mature and old Thai native hens. Animals. 2021;11:3446. doi: 10.3390/ani11123446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake P.E., Stewart J.M. Preservation offowl semen in liquid nitrogen – an improved method. Br. Poult. Sci. 1978;19:187–194. doi: 10.1080/00071667808416462. [DOI] [PubMed] [Google Scholar]
- López-Gatius F., Sances G., Sancho M., Yániz J., Santolaria P., Gutiérrez R., Núñez M., Núñez J., Soler C. Effect of solid storage at 15°C on the subsequent motility and fertility of rabbit semen. Theriogenology. 2005;64:252–260. doi: 10.1016/j.theriogenology.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Masoudi R., Esmaeilkhanian S., Sharafi M., Abdollahi Z., Jafari V., Hatefi A., Zarei F., Asadzadeh N., Sadeghipanah A., Barfourooshi H.J., Banabazi M.H. Cysteamine enhances quality and fertility potential of rooster semen in cooled storage. Theriogenology. 2022;177:29–33. doi: 10.1016/j.theriogenology.2021.09.023. [DOI] [PubMed] [Google Scholar]
- Masoudi R., Sharafi M., Shahneh A.Z., Khodaei-Motlagh M. Effects of reduced glutathione on the quality of rooster sperm during cryopreservation. Theriogenology. 2019;128:149–155. doi: 10.1016/j.theriogenology.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Metcalf E.S., Dideon B.A., Blehr R., Schlimagen T., Bartend W., Varner D.D., Teague S.R., Hausman M.S. Effect of DMSO and L-Ergothioneine on post-thaw semen parameters in stallions: preliminary results. Anim. Reprod. Sci. 2008;107:332–333. [Google Scholar]
- Mussa N.J., Ratchamak R., Ratsiri T., Vongpralub T., Boonkum W., Semaming Y., Chankitisakul V. Lipid profile of sperm cells in Thai native and commercial roosters and its impact on cryopreserved semen quality. Trop. Anim. Health Prod. 2021;53:321. doi: 10.1007/s11250-021-02664-9. [DOI] [PubMed] [Google Scholar]
- Nagy S.Z., Sinkovics G.Y., Kovác A. Viability and acrosome integrity of rabbit spermatozoa processed in a gelatin supplemented extender. Anim. Reprod. Sci. 2002;70:283–286. doi: 10.1016/s0378-4320(01)00189-0. [DOI] [PubMed] [Google Scholar]
- Penfold L., Harnal V., Lynch W., Bird D., Derricson S., Wildt E. Characterization of northern pintail (Anas acuta) ejaculate and the effect of sperm preservation on fertility. Reproduction. 2001;121:267–275. doi: 10.1530/rep.0.1210267. [DOI] [PubMed] [Google Scholar]
- Reiter R.J., Tan D.X., Mayo J.C., Sainz R.M., Leon J., Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003;50:1129–1146. [PubMed] [Google Scholar]
- Resseguie W.D., Hughes B.L., Jones J.E., Thurston R.J. An evaluation of gelatin as a diluent component for storage of chicken semen. Poult. Sci. 1981;60:469–476. doi: 10.3382/ps.0600469. [DOI] [PubMed] [Google Scholar]
- Rui B.R., Angrimani D., Losano J., Bicudo L.C., Nichi M., Pereira R. Validation of simple and cost-effective stains to assess acrosomal status, DNA damage and mitochondrial activity in rooster spermatozoa. Anim. Reprod. Sci. 2017;187:133–140. doi: 10.1016/j.anireprosci.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Salvador I., Yániz J., Viudes de Castro M.P., Gómez E.A., Silvestre M.A. Effect of solid storage on caprine semen conservation at 5°C. Theriogenology. 2006;66:974–981. doi: 10.1016/j.theriogenology.2006.02.042. [DOI] [PubMed] [Google Scholar]
- Sangani A.K., Masoudi A.A., Torshizi R.V. Association of mitochondrial function and sperm progressivity in slow-and fast-growing roosters. Poult. Sci. 2017;96:211–219. doi: 10.3382/ps/pew273. [DOI] [PubMed] [Google Scholar]
- Santos F.C.C., Corcini C.D., Costa V.G.G., Gheller S.M.M., Nogueira C.E.W., da Rosa Curcio B., Varela A.S., Jr Effect of solid medium during cooled storage on stallion sperm parameters. Cryo-Lett. 2015;36:313–317. [PubMed] [Google Scholar]
- Sarkar P.K. Motility, viability and fertilizing ability of avian sperm stored under in vitro conditions. Rev. Agric. Sci. 2020;8:15–27. [Google Scholar]
- Sim W.C., Yin H.Q., Choi H.S., Choi Y.J., Kwak H.C., Kim S.K., Lee B.H. L-serine supplementation attenuates alcoholic fatty liver by enhancing homocysteine metabolism in mice and rats. J. Nutr. 2015;145:260–267. doi: 10.3945/jn.114.199711. [DOI] [PubMed] [Google Scholar]
- Slanina T., Petrovičová L., Miškeje M., Kňížat L., Mirda J., Lukáč N., Trandžík J., Petrovičová I., Massányi P. The effect of diluent, temperature and age on turkey spermatozoa motility in vitro. J. Appl. Anim. Res. 2015;43:131–136. [Google Scholar]
- Tabatabaei S., Aghaei A. Effect of L-carnitine on sperm quality during liquid storage of chicken semen. Comp. Clin. Pathol. 2012;21:711–717. [Google Scholar]
- Voronina M.S., Komarova V., Moskalenko L.I. Effect of different diluents and cock sperm cryopreservation methods on results of artificial insemination. Sbornik Trudov VNIRGJ Leningred. 1986:71–79. [Google Scholar]
- Waberski D., Henning H., Petrunkina A.M. Assessment of storage effects in liquid preserved boar semen. Reprod. Domest. Anim. 2011;46:45–48. doi: 10.1111/j.1439-0531.2011.01836.x. [DOI] [PubMed] [Google Scholar]
- Wang Q., Sun L.C., Liu Y.Q., Lu J.X., Han F., Huang Z.W. The synergistic effect of serine with seleno compounds on the expression of SelP and GPx in HepG2 cells. Biol. Trace. Elem. Res. 2016;173:291–296. doi: 10.1007/s12011-016-0665-8. [DOI] [PubMed] [Google Scholar]
- Yániz J., Martí J.L., Silvestre M.A., Folch J., Santolaria P., Alabart J.L., López-Gatius F. Effects of solid storage of sheep spermatozoa at 15°C on their survival and penetrating capacity. Theriogenology. 2005;64:1844–1851. doi: 10.1016/j.theriogenology.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Zhou X., He L., Zuo S., Zhang Y., Wan D., Long C., Huang P., Wu X., Wu C., Liu G., Yin Y. Serine prevented high-fat diet induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes. Biochem. Biophys. Acta. 2017;1867:488–498. doi: 10.1016/j.bbadis.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Zhou X., Hu L., Wu C., Zhang Y., Wu X., Yin Y. Serine alleviates oxidative stress via supporting glutathione synthesis and methionine cycle in mice. Mol. Nutr. Food Res. 2017;61:1–13. doi: 10.1002/mnfr.201700262. [DOI] [PubMed] [Google Scholar]
- Zhou X., Zhang Y., Wu X., Wan D., Yin Y. Effects of dietary serine supplementation on intestinal integrity, inflammation and oxidative status in early-weaned piglets. Cell Physiol. Biochem. 2018;48:993–1002. doi: 10.1159/000491967. [DOI] [PubMed] [Google Scholar]