Abstract
Patient: Male, newborn
Final Diagnosis: Glutathione synthetase deficiency
Symptoms: Hemolytic anemia • metabolic acidosis
Clinical Procedure: —
Specialty: Metabolic Disorders and Diabetics • Pediatrics and Neonatology
Objective:
Rare disease
Background:
Glutathione synthetase deficiency (GSD) is a rare autosomal recessive disorder caused by glutathione synthetase (GSS) gene variants that occur in 1 in 1 million individuals. The severe form of GSD is characterized by hemolytic anemia, metabolic acidosis with 5-oxoprolinuria, progressive neurological symptoms, and recurrent bacterial infections. This case report presents a male Japanese infant with severe hemolytic anemia and metabolic acidosis at birth caused by GSD, who developed progressive neurological symptoms on follow-up.
Case Report:
A Japanese male term infant developed severe hemolytic anemia and metabolic acidosis in the early neonatal period. We suspected GSD based on his symptoms and a high 5-oxoproline urine concentration. We began correcting his metabolic acidosis and administering vitamins C and E supplements. The patient required blood transfusion twice during the acute phase for hemolytic anemia. After age 1 month, he maintained good control of metabolic acidosis and hemolytic anemia. A definitive diagnosis of GSD was made based on high concentrations of 5-oxoproline in urine, low concentrations of glutathione and GSS activity in erythrocytes, and genetic testing. Several episodes of febrile convulsions were started at age 11 months, but none occurred after 2 years. At the last follow-up at age 25 months, metabolic acidosis and hemolytic anemia were well controlled, but he had mild neurodevelopmental delay.
Conclusions:
This case report shows that GSD can present with severe hemolytic anemia and metabolic acidosis at birth, and manifest with subsequent neurological impairment despite early diagnosis and treatment. Therefore, a careful long-term follow-up that includes neurological evaluation is essential for patients with GSD.
Keywords: Acidosis; Anemia, Hemolytic; Glutathione Synthetase Deficiency; Jaundice, Neonatal; Metabolic Diseases
Background
Glutathione synthetase deficiency (GSD) is a rare autosomal recessive disorder caused by mutations in the glutathione synthetase (GSS) gene [MIM 266130], which is located on chromosome 20q11.2 and consists of 13 exons [1]. This gene encodes GSS, which is the second enzyme in the glutathione (GSH) biosynthesis pathway. GSH is important for various biological functions, including protection of cells from oxidative damage by free radicals, detoxification of xenobiotics, and membrane transport [2].
Patients with GSD present with hemolytic anemia alone or with metabolic acidosis, neurological symptoms, and recurrent bacterial infections. To date, more than 80 cases in 50 families have been reported worldwide [2–4]. A classification based on the severity of clinical symptoms has been proposed: mild, moderate, and severe GSD [5]. Patients with mild GSD have hemolytic anemia as their only clinical symptom. Those with moderate GSD typically show the symptoms in the neonatal period, including hemolytic anemia, metabolic acidosis, and 5-oxoprolinuria. Those with severe GSD develop progressive neurological symptoms and, occasionally, recurrent bacterial infections, probably due to defective granulocyte function [5]. Ristoff et al reported that 31% (5/16) of patients with severe GSD died before the age of 1 year [5]. In addition, Ameur et al reported a patient with GSD who died from severe bacterial infection and metabolic crisis at age 15 months [6]. The severe form of GSD is a life-threatening disease in infants.
The diagnosis of GSD is made by elevated urinary 5-oxypro-line by gas chromatography-mass spectrometry, decreased GSS activity on red blood cells, and sequencing analysis of the GSS gene [2].
Currently, no fundamental treatments exist for GSD; therefore, prognosis depends on the quality of supportive care. Metabolic acidosis can be corrected by administering sodium bicarbonate or citric acid, and the dose should be increased to reduce the risk of acute exacerbation, especially in patients with acute infections. Reduced GSH concentrations increase the vulnerability of various tissues to oxidative stress; thus, supplementation with vitamins C (100 mg/kg/day) and E (10 mg/kg/day) is recommended to compensate for the loss of free radical scavenging and to improve the neurological prognosis [7,8]. In fact, Atwal et al reported a case of GSD with good intellectual development after 19 years of continuous vitamin therapy [8]. Vitamin E improves granulocyte function and normalizes microtubule assembly in phagocytosis [5,9].
This case report presents a male Japanese infant with severe hemolytic anemia and metabolic acidosis at birth due to GSD, who developed progressive neurological symptoms on follow-up.
Case Report
A Japanese male infant was born at 38 weeks and 0 days of gestational age to nonconsanguineous Japanese parents with unremarkable medical history. The patient’s father and older brother had recurrent febrile convulsions in infancy. Fetal growth restriction was noted at 24 weeks of gestation. He was born via induced delivery because the reversal of fetal umbilical artery was noted at 38 weeks of gestation. Apgar scores were 10 and 10 at 1 and 5 min, respectively. At birth, the weight was 2174 g (−2.2 standard deviation [SD]), length was 44.5 cm (−1.7 SD), and head circumference was 32.0 cm (−0.7 SD), without marked abnormal physical presentation. Umbilical cord blood gas analyses showed the following: pH, 7.249 (normal range 7.15–7.38); pCO2, 45.0 (normal range 32–68) mmHg; HCO3–, 19.7 (normal range 15.4–26.8) mmol/L; and hemoglobin, 10.4 (normal range 12.5–15.6 at 38 weeks of gestation by fetal blood sampling) g/dL. He was orally fed on breast milk and developed neonatal jaundice at 2 days of age. However, he gradually developed difficulty in breathing and oral feeding. At 5 days of age, venous blood gas analysis and blood tests revealed anemia (hemoglobin, 8.3 [normal range 15.3–18.7] g/dL) and severe metabolic acidosis without lactic acidosis (pH, 6.84 [normal range 7.35–7.45]; HCO3–, 2.9 [normal range 24–26] mmol/L.; base excess, −29.5 [normal range±2] mmol/L; lactate, 11.7 mg/dL [normal range <20] mg/dL; total ketone bodies, 1,275 [normal range <130] μmol/L). Respiratory distress worsened, and mechanical ventilation was required for 2 days.
He was diagnosed with hemolytic anemia based on the elevated concentration of indirect bilirubin (24.1 mg/dL) and the presence of schistocytes in the peripheral blood smear. He was subsequently administered intravenous sodium bicarbonate and erythrocyte transfusion. Urine organic acid analysis revealed a high 5-oxoproline concentration in the urine (5668 [normal range, 27.1–79.3] µg/mg Cr); therefore, GSD was suspected, and he was treated with a vitamin cocktail containing vitamins C and E. The initial improvement in metabolic acidosis worsened the next day and the patient required daily intravenous sodium bicarbonate. Similarly, the hemoglobin concentration initially increased followed by a gradual decline. After the acute phase, he could feed on breast milk while on regular oral treatment with sodium bicarbonate, vitamin C (100 mg/kg/day), and vitamin E (10 mg/kg/day) (Figure 1).
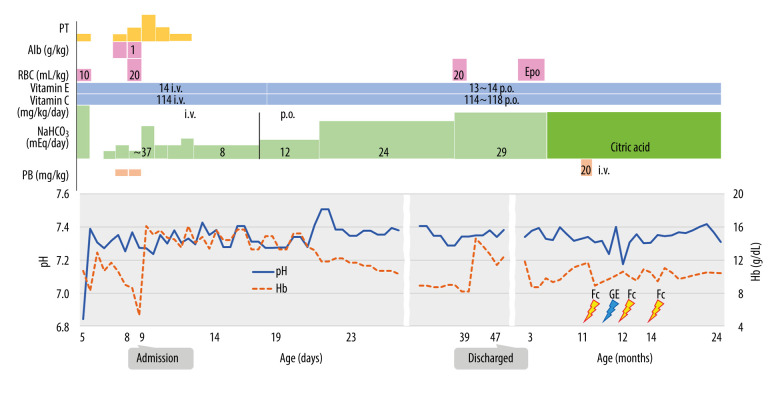
Figure 1.
Clinical course of the patient, including changes in hemoglobin (Hb) concentrations and administered treatments. Administration of vitamins C and E was started at 5 days of age, and metabolic acidosis was corrected with sodium bicarbonate injection depending on the degree of worsening acidosis at first presentation and with regular sodium bicarbonate injection after 14 days of age. The oral route was used to start administering sodium bicarbonate when the patient was 19 days of age. After discharge, several episodes of febrile convulsions were observed from age 11 months, but since the age of 2 years no seizures have recurred. Hb – hemoglobin; Fc – febrile convulsions; GE – gastroenteritis; NaHCO3 – sodium bicarbonate; PB – Phenobarbital; PT – phototherapyl Alb – albumin; RBC – red blood cell transfusion; Epo – erythropoietin; i.v. – intravenous injection; p.o. – per os. This original figure was created by Satoshi Ekuni.
He was discharged at 47 days of age. Since his last red blood cell transfusion at 39 days of age, he has had chronic anemia (7.8–9.6 g/dL) due to GSD but has not had hemolytic attacks requiring red blood cell transfusion. Although subcutaneous erythropoietin administration was initiated on day 66, it had no apparent effect and was terminated on day 105.
He had difficulty taking sodium bicarbonate orally; therefore, we switched to citric acid at 2 months of age.
A subsequent GSH assay in erythrocytes revealed decreased concentrations of GSH (10 [normal range, 62.4–98.9] mg/dL red blood cells) and GSS activity (0.18 [normal range, 0.40–0.78] U/g hemoglobin). Target capture sequencing analysis for congenital hemolytic anemia-related genes, including the 68 genes, was performed [10,11]. Written informed consent for genetic studies was obtained from the parents and the report was approved by the Human Ethics Committee of Tokyo Women’s Medical University. Novel compound heterozygous variants of the GSS gene were detected, NM_000463 c. 430G>A (p. Glu144Lys) from the mother and c. 805G>A (p. Gly269Ser) from the father. These findings confirmed the diagnosis of GSD.
At 11 months, he was admitted for clustered and prolonged febrile convulsions. Diazepam and fosphenytoin sodium hydrate had no effect on the convulsions; however, convulsions did not occur after phenobarbital administration (20 mg/kg, single injection). Electroencephalography revealed normal findings. Brain magnetic resonance imaging (MRI) showed mild ventricular enlargement and corpus callosum hypoplasia. He was discharged 3 days later.
The patient was subsequently hospitalized several times for febrile convulsions, viral infection, and asthma. Nevertheless, he had only 2 episodes of metabolic acidosis: gastroenteritis and viral pneumonia at 1 year of age. His metabolic acidosis was usually well controlled. However, he subsequently experienced recurrent febrile convulsions again, and oral administration of valproate sodium was initiated. After age 2 years, he has been stable without recurring seizures.
Developmental delays became apparent during infancy. He could sit without support at 9 months, stand while holding on to something at 12 months, began to walk at 20 months, and spoke a few words at 24 months. The Kyoto Scale of Psychological Development test [12] was performed at 19 months. His developmental quotient was 63, indicating mild developmental delay. Brain MRI at 25 months showed dilated ventricular and mild brain atrophy (Figure 2).
Figure 2.
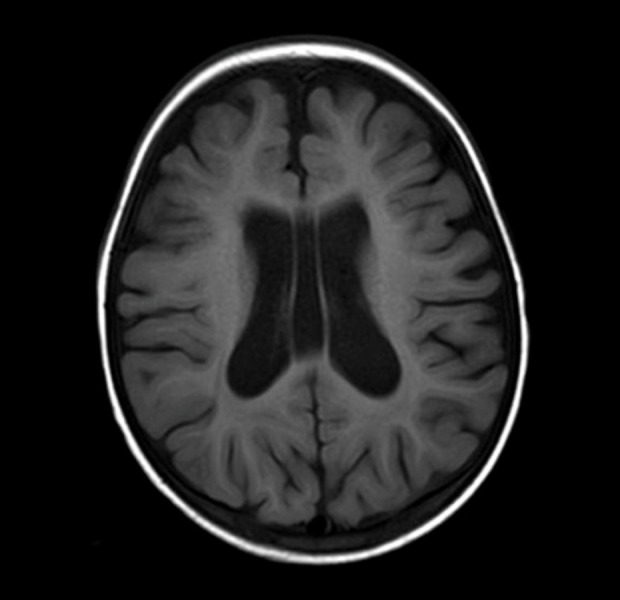
Brain magnetic resonance imaging (MRI) at age 25 months. Brain MRI shows dilated ventricular and mild brain atrophy. This figure is an original image.
Discussion
In this case, we suspected GSD relatively early and started acidosis correction and vitamin administration; however, mild developmental delay in infancy was observed at follow-up. Since neurological symptoms may present even after infancy, ongoing long-term medical care and support are required.
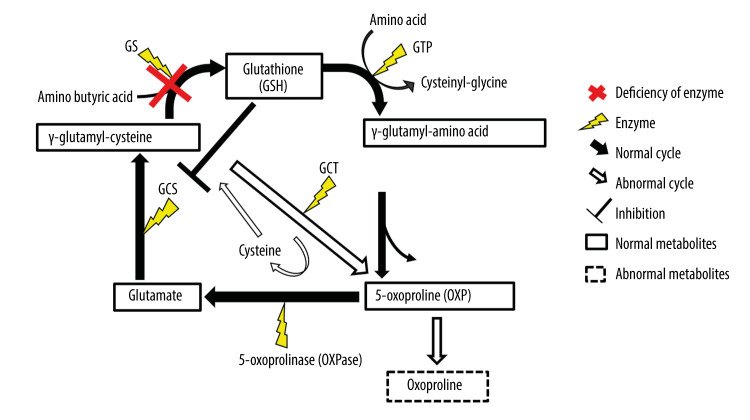
GSD is an inherited metabolic disease with reduced GSS activity and low concentrations of GSH; it follows an autosomal recessive inheritance, and is caused by a variety of genetic alterations in the GSS gene [1,13]. GSH is present in erythrocytes and nucleated cells in various tissues and acts as a free radical scavenger and a strong intracellular reductant. GSH is synthesized via the γ-glutamyl cycle by the consecutive action of GS and other enzymes (Figure 3) [2]. γ-glutamylcysteine synthetase (GCS) activity is regulated through feedback inhibition by GSH. Deficiency of GS and the low concentrations of GSH increase GCS activity and intracellular accumulation of γglutamylcysteine, which is converted to 5-oxoproline and cysteine by γ-glutamyl cyclotransferase, resulting in abnormally high concentrations of 5-oxoproline in blood and urine [2]. As it is a highly acidic compound, it causes metabolic acidosis.
Figure 3.
γ-Glutamyl cycle. Glutathione (GSH) is synthesized from glutamine and cysteine by γ-glutamylcysteine synthetase (GS) and glutathione synthetase (GSS) during the γ-glutamyl cycle. In patients with glutathione synthetase deficiency (GSD), the reduction in GS activity leads to the intracellular accumulation of γ-glutamylcysteine, which is converted to 5-oxoproline (OXP) and cysteine, with the resultant increase in 5-OXP concentration in blood and urine. This figure was created by Satoshi Ekuni, based on data from Ristoff E, Larsson A. Inborn errors in the metabolism of glutathione. Orphanet J Rare Dis 2007; 2: 16.
Additionally, the decrease in GSH concentration in erythrocytes leads to oxidation and degeneration of the erythrocyte membrane, leading to hemolysis, which causes hemolytic anemia and jaundice. A decline in hemolysis in adulthood can be explained by a decrease in lipid peroxidation in the erythrocyte membrane or an increase in hydrogen peroxide detoxification with increasing age [14]. In patients with GSD, neurological symptoms are considered to result from several factors, including exposure to oxidative stress induced by the accumulating free radicals, as well as altered concentrations of GSH, which is also an important neuromodulator and neurotransmitter in the central nervous system [9]. Additionally, kernicterus caused as a result of hemolysis can cause neurological symptoms. The present case showed no signs or symptoms of athetosis, and brain MRI did not show any abnormalities in the basal ganglia, indicating that the mild developmental delay observed in the present case was not associated with severe jaundice in the neonatal period. Based on the clinical course, EEG findings, and family history, we have determined that the patient has febrile seizures, but there is a high possibility that the seizures may progress to epilepsy, so the patient will be monitored carefully.
Patients with GSD reportedly present with increased susceptibility to infection derived from defective granulocyte function [5,15]. Njålsson et al reported that mortality in the neonatal period was attributable to infection or electrolyte abnormalities in 25% of the patients with GSD [15]. In addition, Ameur et al reported a case of GSD that resulted in death at age 15 months from bacterial septic shock and severe metabolic acidosis [6]. In our case, the patient was hospitalized several times for viral infections, but his general condition was not severe. In patients with GSD, it is important to take early action during infection.
Some reviews in patients with GSD have stated that it is best to avoid administration of drugs that can induce hemolytic crises in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency, such as phenobarbital and acetylsalicylic acid [5,16], but phenobarbital is not a contraindicated drug for G6PD deficiency [17]. Also in our case, high-dose administration of phenobarbital did not cause any adverse effects. Single-dose phenobarbital may be used without hemolytic crises in patients with GSD.
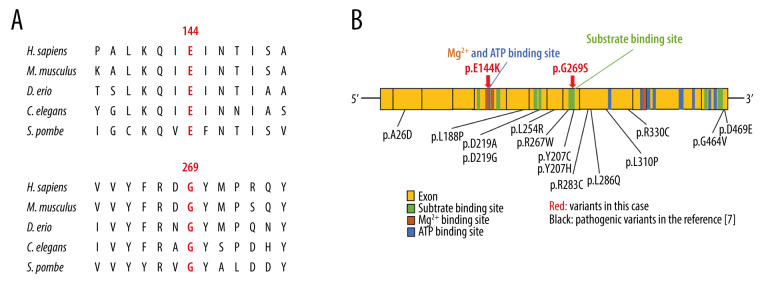
The current patient harbored novel compound heterozygous variants (c. 430G>A: p. Glu144Lys, c. 805G>A: p. Gly269Ser) inherited from his parents. These residues are highly conserved between species (Figure 4A) [18]. The Glu144 residue is in magnesium binding site and ATP binding site, and the Gly269 residue is in substrate biding site (Figure 4B) [19]. Several pathogenic variants (p. Arg267Trp, p. Tyr270Cys, and p. Tyr270His) were previously reported in the vicinity of the Gly269 residue [7]. These variants (p. Glu144Lys and p. Gly269Ser) were predicted to be disease-causing by several major in silico analyses (Table 1). These results suggest that these variants might be pathogenic and may lead to protein damage.
Figure 4.
(A) Amino acid conservation among species in the GSS. The Glu144 and Gly269 residues was highly conservation of among species in GSS. (B) A scheme of the predicted binding sites in the human glutathione synthetase cDNA and missense variants. GSS is composed of 13 exons. The variants in this case are shown in red arrow. Fourteen pathogenic missense variants in GSS [7] are indicated in black. The Glu144 residue is in a magnesium binding site and ATP binding site. The p.G269S in this case is in the vicinity of the pathogenic variants. This figure was created from data provided in previous studies [7,18,19].
Table 1.
Results of in silico predictive algorithms for variants in the present case. Multiple lines of computational analysis showed supportive data of deleterious effects on the gene or gene product.
| Gene | Position (GRCh37) | Substitution | Amino acid change | Inherited | dbSNP | SIFT | PolyPhen-2 | CADD | M-CAP |
|---|---|---|---|---|---|---|---|---|---|
| GSS | Chr20; 33530352 | 430G>A | Glu144Lys | Mother | rs140328832 | 0 (deleterious) | 0.998 (probably damaging) | 32 | 0.312 (possibly pathogenic) |
| GSS | chr20; 33523408 | 805G>A | Gly269Ser | Father | 0 (deleterious) | 0.991 (probably damaging) | 32 | 0.237 (possibly pathogenic) |
GSS – glutathione synthetase; CADD – Combined Annotation Dependent Depletion score is a tool for scoring the deleteriousness of single nucleotide variants in the human genome; M-CAP – Mendelian Clinically Applicable Pathogenicity score is the pathogenicity classifier for rare missense variants in the human genome.
In a study of 16 severely affected patients with metabolic acidosis, hemolytic anemia, and neurological symptoms, 7 of them died, 5 of which died before the age of 1 year [5]. Hence, the severe form of GSD is a life-threatening disease in infants. Ristoff et al indicated that early diagnosis, acidosis correction, and early supplementation with vitamins C and E were the most important determinants of favorable long-term clinical outcomes in patients with GSD [5].
In the current case, the treatment was initiated at 5 days of age and the presumptive diagnosis of GSD was reached based on high urine concentrations of 5-oxoproline determined with urine organic acid analysis conducted at 11 days of age. He was diagnosed and started treatment relatively early, which saved his life, but he has developmental delay with a severe form of GSD. Even when metabolic acidosis and hemolytic anemia are well controlled, neurologic findings, such as seizures and developmental delays, may present after infancy.
However, other clinical courses may also occur. Atwal et al reported a case of severe GSD treated with long-term vitamin therapy and acidosis correction with follow-up until 19 years. The GSD initially manifested with developmental delay in early childhood, but the patient later had normal intellectual development [8]. The neurodevelopmental course may be variable, and we believe that it is important to continue vitamin therapy and correction of metabolic acidosis with long-term follow-up.
Conclusions
As shown in this case report, GSD can present with severe hemolytic anemia and metabolic acidosis at birth and manifest with subsequent neurological impairment in infancy. Early diagnosis, starting treatment as soon as possible and continuing medication, and long-term follow-up that includes neurological evaluation are essential for patient with GSD.
Acknowledgments
The authors thank the patient and his family. We also thank Takako Aoki and Hiromi Ogura for the technical supports. The authors would like to thank Enago (www.enago.jp) for the English language review.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Webb GC, Vaska VL, Gali RR, et al. The gene encoding human glutathione synthetase (GSS) maps to the long arm of chromosome 20 at band 11.2. Genomics. 1995;30(3):617–19. doi: 10.1006/geno.1995.1287. [DOI] [PubMed] [Google Scholar]
- 2.Ristoff E, Larsson A. Inborn errors in the metabolism of glutathione. Orphanet J Rare Dis. 2007;2:16. doi: 10.1186/1750-1172-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyori H, Hirono A, Kobayashi N, et al. Glutathione synthetase deficiency. Rinsho Ketsueki. 1996;37:329–34. [PubMed] [Google Scholar]
- 4.Xia H, Ye J, Wang L, et al. A case of severe glutathione synthetase deficiency with novel GSS mutations. Braz J Med Biol Res. 2018;51:e6853. doi: 10.1590/1414-431X20176853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ristoff E, Mayatepek E, Larsson A. Long-term clinical outcome in patients with glutathione synthetase deficiency. J Pediatr. 2001;139:79–84. doi: 10.1067/mpd.2001.114480. [DOI] [PubMed] [Google Scholar]
- 6.Ben Ameur S, Aloulou H, Nasrallah F, et al. Hemolytic anemia and metabolic acidosis: Think about glutathione synthetase deficiency. Fetal Pediatr Pathol. 2015;34:18–20. doi: 10.3109/15513815.2014.947543. [DOI] [PubMed] [Google Scholar]
- 7.Njålsson R, Ristoff E, Carlsson K, et al. Genotype, enzyme activity, glutathione level, and clinical phenotype in patients with glutathione synthetase deficiency. Hum Genet. 2005;116:384–89. doi: 10.1007/s00439-005-1255-6. [DOI] [PubMed] [Google Scholar]
- 8.Atwal PS, Medina CR, Burrage LC, Sutton VR. Nineteen-year follow-up of a patient with severe glutathione synthetase deficiency. J Hum Genet. 2016;61:669–72. doi: 10.1038/jhg.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janáky R, Ogita K, Pasqualotto BA, et al. Glutathione and signal transduction in the mammalian CNS. J Neurochem. 1999;73:889–902. doi: 10.1046/j.1471-4159.1999.0730889.x. [DOI] [PubMed] [Google Scholar]
- 10.Kanno H, Ogura H. [Diagnosis of congenital hemolytic anemia by comprehensive gene analysis: significance and limitations.] Rinsho Ketsueki. 2021;62:472–79. doi: 10.11406/rinketsu.62.472. [in Japanese] [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto KS, Utshigisawa T, Kanno H, et al. Clinical and genetic diagnosis of thirteen Japanese patients with hereditary spherocytosis. H Hum Genome Var. 2022;9:1. doi: 10.1038/s41439-021-00179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoki S, Hashimoto K, Ikeda N, et al. Comparison of the Kyoto scale of psychological Development 2001 with the parent-rated Kinder Infant Development Scale (KIDS) Brain Dev. 2016;38:481–90. doi: 10.1016/j.braindev.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Guney Varal I, Dogan P, et al. Glutathione synthetase deficiency: A novel mutation with femur agenesis. Fetal Pediatr Pathol. 2020;39:38–44. doi: 10.1080/15513815.2019.1627627. [DOI] [PubMed] [Google Scholar]
- 14.Younkin S, Oski FA, Barness LA. Mechanism of the hydrogen peroxide hemolysis test and its reversal with phenols. Am J Clin Nutr. 1971;24:7–13. doi: 10.1093/ajcn/24.1.7. [DOI] [PubMed] [Google Scholar]
- 15.Njålsson R. Glutathione synthetase deficiency. Cell Mol Life Sci. 2005;62:1938–45. doi: 10.1007/s00018-005-5163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsson A, Anderson ME. Glutathione synthetase deficiency and other disorders of the gamma-glutamyl cycle. In: Scriver CF, Andrea B, Sly WB, Valle DL, editors. The metabolic and molecular bases of inherited disease. New York: McGraw Hill Education; 2001. pp. 2205–16. [Google Scholar]
- 17.Beulter E. G6PD Deficiency. Blood. 1994;84:3613–36. [PubMed] [Google Scholar]
- 18.National library of Medicine HomoloGene. accessed date 2/17/2023 https://www.ncbi.nlm.nih.gov/homologene?cmd=Retrieve&dopt=MultipleAlignment&list_uids=148. [DOI] [PubMed]
- 19.Uniprot consortium accessed date 8/26/2022. https://www.uniprot.org/uniprot/P48637.