Abstract
Long-term dyspnea and exercise intolerance are common clinical problems after acute pulmonary embolism. Unfortunately, no single test can distinguish among the range of potential pathologic outcomes after pulmonary embolism. We illustrate a stepwise approach to post-pulmonary embolism evaluation that uses a hierarchic series of clinically validated diagnostic tests. The algorithm is represented by the acronym SEARCH, which stands for Symptom screening, Exercise testing, Arterial perfusion, Resting echocardiography, Confirmatory chest imaging, and Hemodynamics measured by right heart catheterization. We illustrate the algorithm with a patient whom we saw in our pulmonary embolism follow-up clinic. Patients are asked at least 6 months after pulmonary embolism whether they have returned to their baseline level of respiratory comfort and exercise tolerance. Patients with dyspnea and exercise intolerance undergo noninvasive cardiopulmonary exercise testing to identify elevated ventilatory dead space ratios, decreased stroke volume augmentation with exercise, and other physiologic abnormalities during exertion. Ventilation-perfusion scanning is performed on those patients with exercise-related physiologic findings to confirm the presence of residual pulmonary arterial obstruction or to suggest alternative diagnoses. Resting echocardiography may provide evidence of pulmonary hypertension; confirmatory imaging with pulmonary angiography or CT angiography may disclose findings characteristic of chronic pulmonary artery obstruction. Finally, right heart catheterization is performed to confirm chronic thromboembolic pulmonary hypertension; if resting pulmonary hemodynamics are normal, then invasive cardiopulmonary exercise testing may disclose exercise-induced defects.
Key words: dyspnea, exercise intolerance, pulmonary embolism
Long term-sequelae are common after acute pulmonary embolism (PE), even apart from the risk of recurrence. In fact, it has been shown that nearly one-half of patients experience chronic dyspnea and exercise impairment, even in the absence of recurrent PE.1 A single PE episode can be as life-altering as myocardial infarction by permanently worsening activity tolerance and quality of life.2, 3, 4 Although chronic conditions after PE are used rarely as endpoints in randomized clinical trials of acute PE treatment, they have enormous implications for patient care. In this article, we highlight the importance of structured follow up and describe an algorithm that we developed within the University of California Alliance on Pulmonary Embolism for identifying, distinguishing, and managing the range of possible post-PE outcomes that may occur.
There is a wide range of potential outcomes after PE. Persistent exercise intolerance, which is seen in approximately one-half of patients, is often due to deconditioning or anxiety, even when cardiopulmonary function is normal.5 Cardiopulmonary conditions aside from, and possibly predating, PE may also be responsible for dyspnea.
Residual pulmonary vascular occlusion (RPVO) after PE itself, however, may also lead to dyspnea and reduced exercise capacity by undermining efficient alveolar ventilation or by limiting the ability to increase stroke volume, especially during exertion. In cases of more extensive obstruction, the result may be overt chronic thromboembolic pulmonary hypertension (CTEPH).
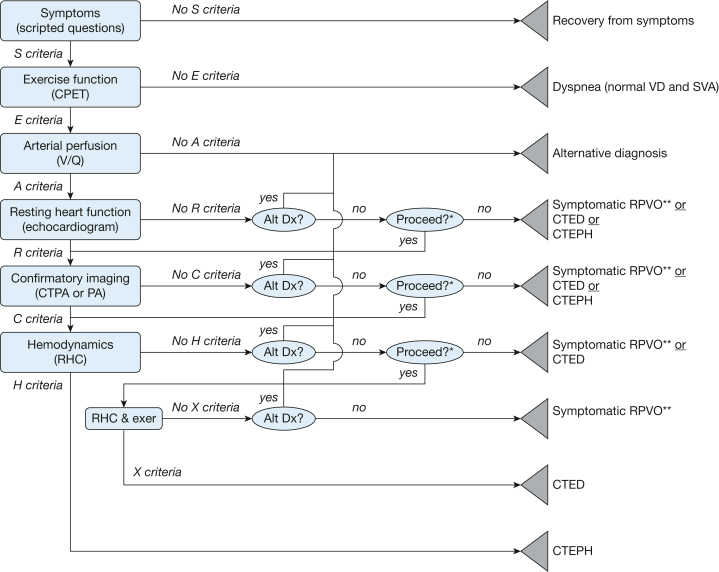
Although a battery of advanced diagnostic tests could distinguish each of those conditions, the yield of individual tests is low enough that routine testing of all patients after PE typically is not performed.6 On the other hand, having a structured, stepwise approach to follow-up testing after acute PE will enable timely diagnosis of post-PE sequelae. Several proposed algorithms for follow-up testing after acute PE have been published.7,8 Here we propose one approach through which the test results at each step inform the performance of the subsequent steps. The order of the tests in our algorithm uses the acronym SEARCH: Symptom screen, Exercise function, Arterial perfusion, Resting heart function, Confirmatory imaging, and Hemodynamics). The diagnostic tests illustrated in Figure 1 increase in complexity and specificity at each step of the SEARCH algorithm, which allows efficient and accurate categorization of each patient.
Figure 1.
SEARCH (symptom screen, exercise function, arterial perfusion, resting heart function, confirmatory imaging, and hemodynamics) algorithm decision tree.
The SEARCH algorithm grades potential outcomes after pulmonary embolism into distinct, nonoverlapping diagnostic categories. Rectangles represent criteria-driven nodes that reflect dichotomous objective test results. The ovals represent subjective clinical decision nodes. The triangles represent endpoint nodes that reflect the specific differential diagnoses warranted from the clinical data. Alt Dx = alternative diagnosis; CPET = cardiopulmonary exercise testing; CTED = chronic thromboembolic disease (pulmonary hypertension with exercise); CTEPH = chronic thromboembolic pulmonary hypertension; CTPA = CT pulmonary arteriogram; exer = exercise; PA = pulmonary arteriogram; SVA = stroke volume augmentation during exercise; symptomatic RPVO = symptomatic residual pulmonary vascular occlusion; VD = physiologic dead space ventilation; X Criteria = pattern of findings during exercise right heart catheterization.
∗The choice to proceed will depend on the clinical importance of distinguishing among the remaining diagnostic possibilities (eg, symptomatic residual pulmonary vascular occlusion vs chronic thromboembolic disease (pulmonary hypertension with exercise vs chronic thromboembolic pulmonary hypertension) in the patient being evaluated. ∗∗Symptomatic residual pulmonary vascular occlusion is subtyped as chronic thromboembolic disease with increased physiologic dead space ventilation, chronic thromboembolic disease with decreased stroke volume augmentation, chronic thromboembolic disease with increased physiologic dead space ventilation and decreased stroke volume augmentation, or chronic thromboembolic disease with unspecified physiologic effect. S, E, A, R, C, and H criteria represent prespecified test results, as described in the text.
Case Example
The patient is a 34-year-old man who presented for routine follow up 1 year after being diagnosed with acute PE. He endorsed having recovered symptomatically from a PE 6 years previously that he experienced when working as a bus driver. He had been treated with warfarin for 6 months. During the recent episode, there was no clinical evidence of right ventricular strain at the time of PE diagnosis.
S (Symptom) Criteria
Regardless of the stability or instability of the original presentation of PE, our routine is to ask patients whether they have resolved their symptoms 6 months afterwards. Although patients may not bring up residual symptoms spontaneously at this point, more than one-half of those who are asked directly will endorse the presence of chronic dyspnea, most of which is new or worsened since their PE.1 We screen for symptoms after provoked PE and after unprovoked PE, because the frequency of chronic problems is comparable between the two groups.9 Our symptom screen emphasizes dyspnea and exercise limitation, which dominate the symptoms of those patients with impaired quality of life after PE.4
Three to 6 months after PE is an optimal time to screen for symptoms because the resolution of acute defects in lung perfusion may continue for this amount of time.10 Waiting longer to screen for symptoms is not necessary, because dyspnea and exercise intolerance typically improve only modestly throughout the rest of the first year.11
In the interest of brevity, we ask simply if a patient’s tolerance of exertion has recovered fully to the level that existed prior to the PE. We ask what types of activities are limited by dyspnea and how those limitations compare with the status shortly before the PE-associated symptoms began. We focus our questions on dyspnea and exercise tolerance, which are more likely to be specific for the physiologic consequences of residual pulmonary perfusion defects.12 It is possible that clinically validated dyspnea scales, such as the modified Medical Research Council (mMRC) score,13 could help patients compare their current symptoms to their pre-PE state, but such scores have not yet been validated for this purpose.
On direct questioning, the patient endorsed dyspnea on exertion. His exercise tolerance had improved since the acute PE but was not back to his typical level prior to the PE. Specifically, he endorsed dyspnea when playing with his daughter, whereas the same type of activity was well tolerated before his PE. As per the SEARCH algorithm, he was referred for cardiopulmonary exercise testing (CPET).
E (Exercise Test) Criteria
In patients with dyspnea and exercise intolerance after PE, we perform CPET to look for physiologic defects in ventilatory dead space proportion (VD/VT) and stroke volume augmentation in response to exercise. Measurement of specific defects related to pulmonary perfusion and right ventricular function are helpful at this point in the workup because dyspnea may be related to a coexisting conditions (eg, advanced age, obesity, anemia, active malignancy, and cardiopulmonary comorbidities) that may not manifest similar pathophysiologic findings.2 For example, anxiety and depression are present in approximately one-third of patients after PE, but they do not correlate with measurable limitations in exercise function.4
Although the peak oxygen consumption (VO2_peak) achieved during CPET is a useful predictor of left ventricular failure outcomes, it has not proven specific for defects related to lung perfusion or right ventricular function. Low VO2_peak is relatively common, even 12 months after PE and is associated with a variety of factors that include male sex, advanced age, increased BMI, and smoking.14 Low VO2_peak does not correspond to the extent of residual perfusion defects,14 ventilatory inefficiency,14,15 or right ventricular dysfunction.15 The commonly cited threshold of 80% of the predicted VO2_peak is also insensitive for residual pulmonary artery obstruction, because nearly all patients with chronic thromboembolic disease (CTED) and more than one-half of patients with CTEPH can exceed that threshold during invasive CPET.16
Two specific CPET findings suggest pathophysiologic defects caused by incomplete resolution after PE: (1) increased VD/VT and (2) decreased stroke volume augmentation during exercise. VD/VT is typically 0.3 at rest and decreases to 0.2 by the time anaerobic threshold occurs during exercise. Substantially increased VD/VT values during exercise correspond to scintigraphically measured persistent pulmonary artery obstruction after acute PE.5 Chronic thromboembolic disease and CTEPH, which represent the extremes of unresolved acute PE, are characterized by increased VD/VT.16
VD/VT can be calculated directly from the Bohr equation
where exhaled Pco2 (PEco2) is measured directly and arterial Pco2 (Paco2) is estimated from the transcutaneous Pco2 (Ptcco2).17,18 If neither Paco2 nor Ptcco2 are measured during CPET, elevated VE/VCO2 may be used to detect excess VD/VT according to the alveolar gas equation
rearranged to
as long as Paco2 is assumed to be normal.19 That assumption, however, can lead to imprecision if the Paco2 is different than the assumed normal value.20,21
During exercise, stroke volume augmentation by the time of anaerobic threshold (SVAAT) is approximately 140% of the stroke volume at rest.22 Decreased stroke volume response to exercise corresponds to cardiac dysfunction in a number of clinical conditions.23 SVAAT can be estimated noninvasively from the VO2/heart rate (O2·pulse) at rest (O2·pulserest) and at anaerobic threshold (O2·pulseAT) with the method described by Fernandes et al5 and Stringer et al22 with the formula
SVAAT = (O2·pulseAT/O2·pulserest) x 0.55.
Decreased SVAAT corresponds to persistent pulmonary artery obstruction after acute pulmonary embolism.5
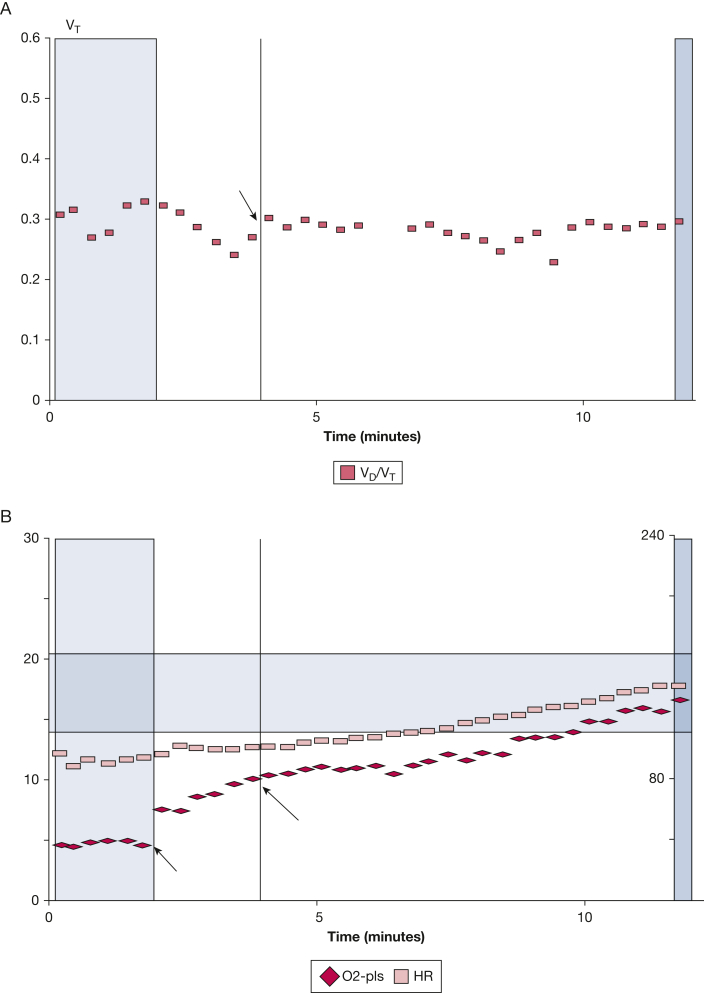
Figure 2A shows that the patient’s CPET disclosed a normal VD/VT at rest but an elevated VD/VT at anaerobic threshold (0.29; predicted = 0.20) and at peak exercise (0.30; predicted = 0.19). His SVAAT estimate, shown in Figure 2B, was decreased (102%; predicted 140%). Because his symptoms corresponded to physiologic exercise defects, the SEARCH strategy dictated referral for scanning to evaluate his pulmonary artery perfusion.
Figure 2.
A-B, Elevated ventilatory dead space ratio and decreased stroke volume augmentation at anaerobic threshold and during cardiopulmonary exercise testing. Noninvasive cardiopulmonary exercise testing disclosed physiologic defects that corresponded to the patient’s dyspnea and exercise tolerance. A, Ventilatory dead space ratio (squares) at anaerobic threshold (arrow) is elevated (0.29; predicted = 0.20) and remains elevated for the remainder of exercise. B, Stroke volume augmentation at anaerobic threshold, which is estimated by the ratio of the rate of oxygen consumption/heart rate (diamonds) at anaerobic threshold (9.7 mL oxygen/beat; long arrow) to the rate of oxygen consumption/heart rate at rest (5.2 mL oxygen/beat; short arrow) multiplied by 0.55, is decreased (102%; predicted 140%). HR = heart rate; O2-Pls = rate of oxygen consumption/heart rate; VD/VT = dead space proportion of tidal volume; VT = tidal volume.
A (Arterial Perfusion) Criteria
is accepted as an extremely sensitive test for excluding CTEPH and CTED. A normal scan effectively excludes perfusion defects as the cause of dyspnea as opposed to CT angiography that can be diagnostic for proximal disease but may miss distal defects.24 In addition to incomplete PE resolution, after PE, patients are prone to other cardiopulmonary derangements, which provides a rationale for doing a scan early on in the work up so that alternative diagnoses can be pursued if the scan suggests that RPVO is not present.25 RPVO is associated with respiratory symptoms,26 hypoxemia,27,28 gas exchange deficits,10,29,30 and exercise intolerance.12,31, 32, 33 Approximately one-sixth of patients with RPVO may experience CTED or CTEPH.12,34
In some cases, single-photon emission CT (SPECT)- may be preferred over planar . In the acute setting, SPECT- produces fewer nondiagnostic test results than planar , which removes one of the major limitations of planer testing.35, 36, 37 SPECT- has a high degree of agreement with contrast to enhance the pulmonary arteries (CTPA) in patients suspected of having acute PE.36 Notably, SPECT is superior to planar for the quantification of vascular obstruction in CTEPH as well.38,39
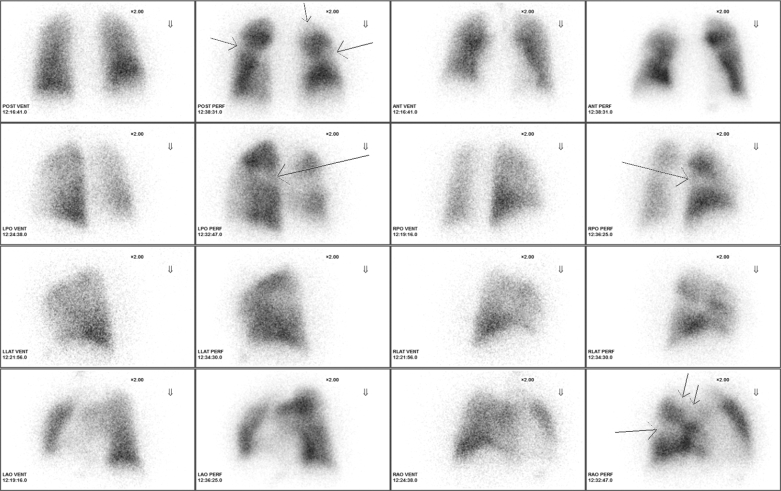
The patient’s SPECT- (Fig 3) disclosed pleural-based segmental perfusion defects in the superior segment of the left lower lobe, the superior segment of the right lower lobe, and the anterior and the apical segments of the right upper lobe. There were no matching ventilation defects. In accordance with the SEARCH algorithm, he was referred for resting echocardiography.
Figure 3.
SPECT perfusion scan. Pleural-based segmental perfusion defects (arrows) are present in the superior segment of the left lower lobe, the superior segment of the right lower lobe, and the anterior and the apical segments of the right upper lobe. There were no matching ventilation defects. ANT = anterior view; LAO = left anterior oblique; LLAT = left lateral; LPO = left posterior oblique; PERF = perfusion scan; POST = posterior view; RAO = right anterior oblique; RLAT = right lateral; RPO = right posterior oblique; SPECT = single-phton emission CT; VENT = ventilation scan.
R (Resting Echocardiogram) Criteria
For symptomatic patients with exercise deficits and unmatched perfusion defects, echocardiography is performed to evaluate for right ventricular dysfunction and pulmonary hypertension.40 The yield of echocardiography at rest is low among post-PE populations without those findings.6 We recommend the echocardiographic criteria published by the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology and the European Respiratory Society.41 The reader is referred to the European Society of Cardiology and the European Respiratory Society Guidelines for an detailed description of those criteria. The echocardiogram may also suggest alternative diagnoses (eg, left ventricular or valvular heart disease) to explain the patient’s presentation and findings.
The patient’s echocardiogram disclosed normal right ventricular size, normal global right ventricular systolic function, and right ventricular systolic pressure estimated at 26 mm Hg. Despite normal resting echocardiographic parameters, the symptoms, abnormal exercise study, and abnormal scan guided us to proceed along SEARCH algorithm to confirmatory imaging.
C (Confirmatory Imaging) Criteria
Confirmatory imaging is performed typically with CT scanning with timing of IV CTPA to disclose unresolved PE and the development of chronic intravascular scars.42 CTPA is less sensitive than scanning for the persistence of pulmonary artery obstruction after PE.43,44 It can, however, disclose characteristics that are typical of CTEPH or CTED. Characteristic CT scan findings of chronic disease coincidentally also follow a mnemonic characterized by the word “SEARCH”: smaller than normal caliber arteries contain filling defects, eccentric and web-like filling defects, anastomoses of bronchial arteries, right-sided heart enlargement, contracted lung regions, and heterogeneous lung parenchyma.45 The CT scan may also disclose pulmonary vascular obstruction caused by alternative diagnoses, such as tumors of the mediastinum and lung, fibrosing mediastinitis, or arteritis.
Conventional (planar) pulmonary angiography is an alternative means of confirming the diagnosis of CTEPH or CTED and of assessing the accessibility of the lesions for potential interventions, such as pulmonary thromboendarterectomy or balloon pulmonary angioplasty. In a conventional pulmonary angiogram, contrast is ejected from a catheter in the right pulmonary artery and cine digital subtraction angiography is obtained with anterior-posterior and lateral views; the images of the contralateral side are then obtained in a similar fashion. Although important for the determination of operability of CTEPH and CTED, the conventional pulmonary angiogram is somewhat limited for the assessment of other causes of dyspnea. It also requires experience in performance and interpretation that may necessitate referral to a center that specializes in CTEPH and CTED.
As was the case for echocardiography, the absence of diagnostic findings on CTPA does not exclude the presence of significant disease among symptomatic patients with physiologic dysfunction and persistent perfusion defects after PE. The absence of abnormal findings on CTPA does not prevent us from proceeding along the SEARCH workup algorithm.
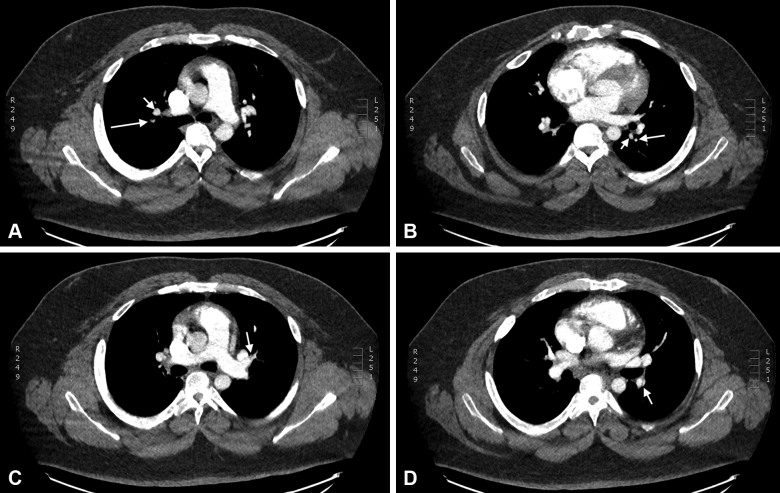
CTPA on the patient (Fig 4) disclosed several instances of smaller-than-normal pulmonary artery caliber. There was local narrowing of the apical and anterior segmental pulmonary arteries of the right upper lobe. Marked diffuse narrowing of the lateral segmental branch of the right middle lobe was also noted, and the superior segment pulmonary artery of the left lower lobe appeared attenuated. Eccentric filling defects were also present in the superior lingular pulmonary artery. Multiple web-like filling defects were noted in the bilateral basilar trunk pulmonary arteries. Anastomoses of the bronchial arteries were not present. The right ventricle was mildly enlarged. Heterogeneous lung parenchyma (“mosaicism”) was not present. Following the SEARCH strategy, we next performed direct hemodynamic measurements.
Figure 4.
A-D, Confirmatory chest imaging. Contrast to enhance the pulmonary arteries disclosed multiple regions smaller-then-normal pulmonary artery caliber. A, Apical segment of the right upper lobe. The pulmonary artery (long arrow) is smaller in caliber than the corresponding vein (short arrow). B, Superior segment of the left lower lobe. The pulmonary artery (long arrow) is smaller in caliber than the corresponding vein (short arrow). C, Superior branch of the lingular pulmonary artery. An eccentric filling defect (arrow) is apparent on the anterior wall of the artery. D, Descending branch of the left pulmonary artery. A web-like filling defect (arrow) is present on the lateral aspect of the artery.
H (Hemodynamic Measurement) Criteria
Right heart catheterization (RHC) performed while the patient is at rest will disclose whether the patient has experienced the development of pulmonary hypertension, defined as a mean pulmonary artery pressure (mPAP) of > 20 mm Hg in the presence of a pulmonary arterial wedge pressure no > 15 mm Hg and, in precapillary PH, a pulmonary vascular resistance (PVR) of at least 3 Wood units. If the resting RHC criteria for pulmonary hypertension are met, then, given the symptoms, exercise data, and perfusion scan, the patient is diagnosed with CTEPH.
If the resting RHC does not disclose pulmonary hypertension, the patient’s post-PE dyspnea may yet reflect pulmonary hypertension that is manifested only during exercise, which is termed CTED. Hemodynamics may be measured by RHC either during simple exercise, in which the patient is prompted to do a moderate amount of exertion, or during invasive CPET with measurement of exhaled gases. Combining RHC with CPET allows VO2 measurement for the direct Fick method of determining cardiac output. VO2 also provides a standardized measurement of the level of the patient’s exertion. Finally, the invasive CPET can confirm the physiologic defect(s) identified during noninvasive CPET: SVA confirmation via simultaneous cardiac output measurement and VD/VT confirmation with the use of arterial catheterization to facilitate repeated Paco2 measurement. Either method, however, may detect exercise-induced hemodynamic changes that are characteristic of CTED. Specifically, if the mPAP increases excessively during exercise as the cardiac output goes up, as assessed by the equation
then the patient is considered to have exercise-induced pulmonary hypertension and, if other criteria outlined earlier are present, to have CTED.46 Aside from the ΔmPAP/Δcardiac output (CO) slope, other signs of an abnormal pulmonary vascular response to exercise include increase in PVR47 and decrease in pulmonary arterial compliance48 as exertion increases. Together, we refer to this pattern of findings during exercise RHC as the “X criteria.” If neither CTED nor CTEPH is present, the patient may yet be dyspneic from inadequate gas exchange if CPET disclosed elevated VD/VT during exercise.
The patient underwent RHC, which disclosed no evidence of pulmonary hypertension at rest. His mPAP was 19 mm Hg; his pulmonary artery occlusion pressure was 12 mm Hg; his CO was 10.3 L/min, and his cardiac index was 3.78. His PVR was 0.7 Wood units. CTEPH was excluded based on these results.
Exercise RHC demonstrated an increase in mPAP to 61 mm Hg; pulmonary artery occlusion pressure increased only to 18 mm Hg. His CO was 20.8 L/min, and his cardiac index was 7.61, yielding a PVR of 2 Wood units. The slope of ΔmPAP/ΔCO between rest and exercise was 4, which is consistent with CTED. Invasive CPET confirmed decreased SVAAT (135 mL/116 mL = 116%; predicted 140%) and elevated VD/VT (0.3) during exercise.
The patient was referred for pulmonary endarterectomy to treat his CTED that was complicated by decreased SVAAT and elevated VD/VT. He tolerated the procedure well. On follow-up visits, his condition had improved markedly. During his last visit, 3 years after his procedure, he continued to be free of dyspnea at rest and during exertion.
Summary
A systematic approach to follow-up testing after acute PE is critical. The use of an algorithm that individualizes the degree of testing, such as the SEARCH algorithm outlined here, will facilitate early complete and efficient categorization of patients. Although the patient underwent all the SEARCH diagnostic elements, the stepwise approach will also identify patients in whom more invasive testing for residua of PE is not necessary, such as those who have no residual symptoms, normal exercise parameters, or normal lung perfusion. Conversely, RPVO with elevated VD/VT, decreased SVAAT, or both may limit exercise tolerance and respiratory comfort even in the absence CTEPH and CTED.
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: T. A. M., T .M. F., and R. N. C. disclose research support from Inari Medical.
References
- 1.Klok F.A., Tijmensen J.E., Haeck M.L., van Kralingen K.W., Huisman M.V. Persistent dyspnea complaints at long-term follow-up after an episode of acute pulmonary embolism: results of a questionnaire. Eur J Intern Med. 2008;19(8):625–629. doi: 10.1016/j.ejim.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Klok F.A., van Kralingen K.W., van Dijk A.P., et al. Quality of life in long-term survivors of acute pulmonary embolism. Chest. 2010;138(6):1432–1440. doi: 10.1378/chest.09-2482. [DOI] [PubMed] [Google Scholar]
- 3.van Es J., den Exter P.L., Kaptein A.A., et al. Quality of life after pulmonary embolism as assessed with SF-36 and PEmb-QoL. Thromb Res. 2013;132(5):500–505. doi: 10.1016/j.thromres.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Tavoly M., Utne K.K., Jelsness-Jorgensen L.P., et al. Health-related quality of life after pulmonary embolism: a cross-sectional study. BMJ Open. 2016;6(11) doi: 10.1136/bmjopen-2016-013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes T.M., Alotaibi M., Strozza D.M., et al. Dyspnea postpulmonary embolism from physiological dead space proportion and stroke volume defects during exercise. Chest. 2020;157(4):936–944. doi: 10.1016/j.chest.2019.10.047. [DOI] [PubMed] [Google Scholar]
- 6.Klok F.A., van Kralingen K.W., van Dijk A.P., Heyning F.H., Vliegen H.W., Huisman M.V. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica. 2010;95(6):970–975. doi: 10.3324/haematol.2009.018960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivera-Lebron B., McDaniel M., Ahrar K., et al. Diagnosis, treatment and follow up of acute pulmonary embolism: consensus practice from the PERT Consortium. Clin Appl Thromb Hemost. 2019;25 doi: 10.1177/1076029619853037. 1076029619853037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konstantinides S.V., Meyer G., Becattini C., et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur Respir J. 2019;54(3) doi: 10.1183/13993003.01647-2019. [DOI] [PubMed] [Google Scholar]
- 9.Klok F.A., Zondag W., van Kralingen K.W., et al. Patient outcomes after acute pulmonary embolism: a pooled survival analysis of different adverse events. Am J Respir Crit Care Med. 2010;181(5):501–506. doi: 10.1164/rccm.200907-1141OC. [DOI] [PubMed] [Google Scholar]
- 10.Prediletto R., Paoletti P., Fornai E., et al. Natural course of treated pulmonary embolism: evaluation by perfusion lung scintigraphy, gas exchange, and chest roentgenogram. Chest. 1990;97(3):554–561. doi: 10.1378/chest.97.3.554. [DOI] [PubMed] [Google Scholar]
- 11.Kahn S.R., Akaberi A., Granton J.T., et al. Quality of life, dyspnea, and functional exercise capacity following a first episode of pulmonary embolism: results of the ELOPE Cohort study. Am J Med. 2017;130(8):990. doi: 10.1016/j.amjmed.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez O., Helley D., Couchon S., et al. Perfusion defects after pulmonary embolism: risk factors and clinical significance. J Thromb Haemost. 2010;8(6):1248–1255. doi: 10.1111/j.1538-7836.2010.03844.x. [DOI] [PubMed] [Google Scholar]
- 13.Bestall J.C., Paul E.A., Garrod R., Garnham R., Jones P.W., Wedzicha J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahn S.R., Hirsch A.M., Akaberi A., et al. Functional and exercise limitations after a first episode of pulmonary embolism: results of the ELOPE Prospective Cohort study. Chest. 2017;151(5):1058–1068. doi: 10.1016/j.chest.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Albaghdadi M.S., Dudzinski D.M., Giordano N., et al. Cardiopulmonary exercise testing in patients following massive and submassive pulmonary embolism. J Am Heart Assoc. 2018;7(5):e006841. doi: 10.1161/JAHA.117.006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCabe C., Deboeck G., Harvey I., et al. Inefficient exercise gas exchange identifies pulmonary hypertension in chronic thromboembolic obstruction following pulmonary embolism. Thromb Tes. 2013;132(6):659–665. doi: 10.1016/j.thromres.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Stege G., van den Elshout F.J., Heijdra Y.F., van de Ven M.J., Dekhuijzen P.N., Vos P.J. Accuracy of transcutaneous carbon dioxide tension measurements during cardiopulmonary exercise testing. Respiration. 2009;78(2):147–153. doi: 10.1159/000187631. [DOI] [PubMed] [Google Scholar]
- 18.Cao M., Stringer W.W., Corey S., et al. Transcutaneous PCO2 for exercise gas exchange efficiency in chronic obstructive pulmonary disease. COPD. 2021;18(1):16–25. doi: 10.1080/15412555.2020.1858403. [DOI] [PubMed] [Google Scholar]
- 19.Sue D.Y. Excess ventilation during exercise and prognosis in chronic heart failure. Am J Respir Crit Care Med. 2011;183(10):1302–1310. doi: 10.1164/rccm.201006-0965CI. [DOI] [PubMed] [Google Scholar]
- 20.Roman M.A., Casaburi J.D., Porszasz J., Casaburi R. Noninvasive assessment of normality of VD/VT in clinical cardiopulmonary exercise testing utilizing incremental cycle ergometry. Eur J Appl Physiol. 2013;113(1):33–40. doi: 10.1007/s00421-012-2407-8. [DOI] [PubMed] [Google Scholar]
- 21.Morris T.A., Porszasz J., Stringer W.W. Response. Chest. 2020;158(4):1781–1782. doi: 10.1016/j.chest.2020.07.037. [DOI] [PubMed] [Google Scholar]
- 22.Stringer W.W., Hansen J.E., Wasserman K. Cardiac output estimated noninvasively from oxygen uptake during exercise. J Appl Physiol. 1997;82(3):908–912. doi: 10.1152/jappl.1997.82.3.908. [DOI] [PubMed] [Google Scholar]
- 23.Rowell L.B. Oxford University Press; New York: 1993. Human cardiovascular control. [Google Scholar]
- 24.Delcroix M., Torbicki A., Gopalan D., et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J. 2021;57(6):2002828. doi: 10.1183/13993003.02828-2020. [DOI] [PubMed] [Google Scholar]
- 25.Klok F.A., van Kralingen K.W., van Dijk A.P., Heyning F.H., Vliegen H.W., Huisman M.V. Prevalence and potential determinants of exertional dyspnea after acute pulmonary embolism. Respir Med. 2010;104(11):1744–1749. doi: 10.1016/j.rmed.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Phear D. Pulmonary embolism: a study of late prognosis. Lancet. 1960;276(7155):832–835. doi: 10.1016/s0140-6736(60)91903-6. [DOI] [PubMed] [Google Scholar]
- 27.Paraskos J.A., Adelstein S.J., Smith R.E., et al. Late prognosis of acute pulmonary embolism. N Engl J Med. 1973;289(2):55–58. doi: 10.1056/NEJM197307122890201. [DOI] [PubMed] [Google Scholar]
- 28.Donnamaria V., Palla A., Petruzzelli S., Carrozzi L., Pugliesi O., Giuntini C. Early and late follow-up of pulmonary embolism. Respiration. 1993;60(1):15–20. doi: 10.1159/000196167. [DOI] [PubMed] [Google Scholar]
- 29.Bass H., Banas J.S., Jr., Dalen J.E. Pulmonary function studies: aid to diagnosis of pulmonary embolism. Arch Intern Med. 1970;126(2):266–268. doi: 10.1001/archinte.126.2.266. [DOI] [PubMed] [Google Scholar]
- 30.Sharma G.V., Burleson V.A., Sasahara A.A. Effect of thrombolytic therapy on pulmonary-capillary blood volume in patients with pulmonary embolism. N Engl J Med. 1980;303(15):842–845. doi: 10.1056/NEJM198010093031502. [DOI] [PubMed] [Google Scholar]
- 31.Helmers R.A., Zavala D.C. Serial exercise testing in pulmonary embolism. Chest. 1988;94(3):517–520. doi: 10.1378/chest.94.3.517. [DOI] [PubMed] [Google Scholar]
- 32.Pesavento R., Filippi L., Palla A., et al. Impact of residual pulmonary obstruction on the long-term outcome of patients with pulmonary embolism. Eur Respir J. 2017;49(5):1601980. doi: 10.1183/13993003.01980-2016. [DOI] [PubMed] [Google Scholar]
- 33.Wan T., Rodger M., Zeng W., et al. Residual pulmonary embolism as a predictor for recurrence after a first unprovoked episode: results from the REVERSE cohort study. Thromb Res. 2018;162:104–109. doi: 10.1016/j.thromres.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 34.Pengo V., Lensing A.W., Prins M.H., et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257–2264. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 35.Leblanc M., Leveillee F., Turcotte E. Prospective evaluation of the negative predictive value of V/Q SPECT using 99mTc-Technegas. Nucl Med Commun. 2007;28(8):667–672. doi: 10.1097/MNM.0b013e32827a8e99. [DOI] [PubMed] [Google Scholar]
- 36.Bajc M., Olsson B., Palmer J., Jonson B. Ventilation/perfusion SPECT for diagnostics of pulmonary embolism in clinical practice. J intern med. 2008;264(4):379–387. doi: 10.1111/j.1365-2796.2008.01980.x. [DOI] [PubMed] [Google Scholar]
- 37.Lemb M., Pohlabeln H. Pulmonary thromboembolism: a retrospective study on the examination of 991 patients by ventilation/perfusion SPECT using Technegas. Nuklearmedizin. 2001;40(6):179–186. [PubMed] [Google Scholar]
- 38.Soler X., Hoh C.K., Test V.J., Kerr K.M., Marsh J.J., Morris T.A. Single photon emission computed tomography in chronic thromboembolic pulmonary hypertension. Respirology. 2011;16(1):131–137. doi: 10.1111/j.1440-1843.2010.01867.x. [DOI] [PubMed] [Google Scholar]
- 39.Soler X., Kerr K.M., Marsh J.J., et al. Pilot study comparing SPECT perfusion scintigraphy with CT pulmonary angiography in chronic thromboembolic pulmonary hypertension. Respirology. 2012;17(1):180–184. doi: 10.1111/j.1440-1843.2011.02061.x. [DOI] [PubMed] [Google Scholar]
- 40.Ribeiro A., Lindmarker P., Johnsson H., Juhlin-Dannfelt A., Jorfeldt L. Pulmonary embolism: one-year follow-up with echocardiography doppler and five-year survival analysis. Circulation. 1999;99(10):1325–1330. doi: 10.1161/01.cir.99.10.1325. [DOI] [PubMed] [Google Scholar]
- 41.Galiè N., Humbert M., Vachiery J.L., et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46(4):903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 42.Remy-Jardin M., Louvegny S., Remy J., et al. Acute central thromboembolic disease: posttherapeutic follow-up with spiral CT angiography. Radiology. 1997;203(1):173–180. doi: 10.1148/radiology.203.1.9122389. [DOI] [PubMed] [Google Scholar]
- 43.Cosmi B., Nijkeuter M., Valentino M., Huisman M.V., Barozzi L., Palareti G. Residual emboli on lung perfusion scan or multidetector computed tomography after a first episode of acute pulmonary embolism. Intern Emerg Med. 2011;6(6):521–528. doi: 10.1007/s11739-011-0577-8. [DOI] [PubMed] [Google Scholar]
- 44.van Es J., Douma R.A., Kamphuisen P.W., et al. Clot resolution after 3 weeks of anticoagulant treatment for pulmonary embolism: comparison of computed tomography and perfusion scintigraphy. J Thromb Haemost. 2013;11(4):679–685. doi: 10.1111/jth.12150. [DOI] [PubMed] [Google Scholar]
- 45.Grosse A., Grosse C., Lang I.M. Distinguishing chronic thromboembolic pulmonary hypertension from other causes of pulmonary hypertension using CT. AJR Am J Roentgenol. 2017;209(6):1228–1238. doi: 10.2214/AJR.17.17871. [DOI] [PubMed] [Google Scholar]
- 46.Kim N.H., Delcroix M., Jais X., et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;53(1):339–351. doi: 10.1183/13993003.01915-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovacs G., Olschewski A., Berghold A., Olschewski H. Pulmonary vascular resistances during exercise in normal subjects: a systematic review. Eur Respir J. 2012;39(2):319–328. doi: 10.1183/09031936.00008611. [DOI] [PubMed] [Google Scholar]
- 48.Slife D.M., Latham R.D., Sipkema P., Westerhof N. Pulmonary arterial compliance at rest and exercise in normal humans. Am J Physiol. 1990;258(6 Pt 2):H1823–H1828. doi: 10.1152/ajpheart.1990.258.6.H1823. [DOI] [PubMed] [Google Scholar]