Abstract
Background
The application of allergen immunotherapy (AIT) in combination with biological agents has been found to increase both the safety and efficacy of the desensitisation procedure in patients with food and insect venom allergy. The aim of our study was to compare the effectiveness of AIT in patients with house dust mite (HDM)-driven asthma treated with and without omalizumab.
Materials and methods
The study was a placebo-controlled, three-armed, randomised, parallel-group, multicentre trial that included 52 patients with HDM-driven asthma. Only patients with monosensitisation to HDM were included. The study compared three patterns of therapy: omalizumab alone, HDM subcutaneous immunotherapy (SCIT-HDM)+ omalizumab, and SCIT alone. The main outcomes were evaluate the Asthma Control Questionnaire (ACQ) score, number of asthma exacerbations and reduction in the daily dose of inhaled steroids during 12 months of observation.
Results
All variants of therapy led to significantly improved ACQ scores and reduction of asthma exacerbations in all study groups after 12 months of treatment. A statistically significant reduction in the daily doses of inhaled corticosteroids in the group with omalizumab alone (650±150 µg versus 500±50 µg for p=0.003) or SCIT-HDM+omalizumab (550±250 µg versus 375±75 µg for p=0.001) was observed, favouring the latter group.
Conclusion
The efficacy of AIT for HDM-driven asthma is significantly increased by the combination of allergen vaccine with omalizumab.
Short abstract
A combination of AIT to HDM and omalizumab is more effective in reducing symptoms and reducing the daily dose of ICS than using omalizumab alone or AIT alone in patients with HDM-driven asthma https://bit.ly/3W6Ff61
Introduction
Allergen immunotherapy (AIT) is the only one causative mode of treatment for allergic rhinitis and select types of allergic bronchial asthma. AIT allows asthma control, reduction of daily doses of anti-asthmatic drugs and improvement of quality of life. However, the use of AIT in allergic asthma raised various controversies, resulting from the lack of appropriate controlled studies [1]. Currently, house dust mite (HDM)-driven asthma associated with allergic rhinitis is an important recommendation for HDM-AIT [2, 3]. Some prospective studies of AIT with HDM extract have suggested that three years of immunotherapy produces long-term remission of symptoms for further years after discontinuation [2, 3].
Few studies have evaluated the effectiveness of AIT in combination with other proven treatments in patients with allergic asthma, for example, with the use of biological therapy [4].
Omalizumab is an anti-IgE monoclonal antibody currently approved for the treatment of asthma and chronic urticaria. This biological treatment is confirmed for moderate-to-severe persistent allergic asthma management. It binds circulating IgE on the Fcε3 domain and prevents IgE from binding to the high-affinity receptor (FcεRI) on basophils and mast cells. At therapeutic levels, it has also been shown to competitively dissociate cell-bound IgE from its receptor on the cell surface in a dose-dependent manner [5]. Another potential mechanism that has been proposed to reduce the risk of anaphylaxis is the direct neutralisation of allergens in the bloodstream by omalizumab–IgE complexes, acting as blocking antibodies and sweeping away the allergen molecules entering the bloodstream before they can reach mast cells and basophils [6].
Most current studies involve using a biological drug to increase the safety of AIT, especially in the treatment of food allergies, to avoid the risk of anaphylaxis [5, 6].
However, adding omalizumab to AIT may improve the therapy's safety and enhance its effectiveness. There are still few observations on this topic, especially in patients with HDM-driven asthma.
This study compared the effectiveness of the combined treatment of injection AIT to house dust mites (HDM subcutaneous immunotherapy (SCIT-HDM)) and omalizumab with SCIT-HDM alone or omalizumab alone.
Materials and methods
Study design
This study was a placebo-controlled, three-armed, randomised, parallel-group, multicentre trial including 52 patients with HDM-driven asthma. It was registered at ClinicalTrials.gov with number NCT03209245.
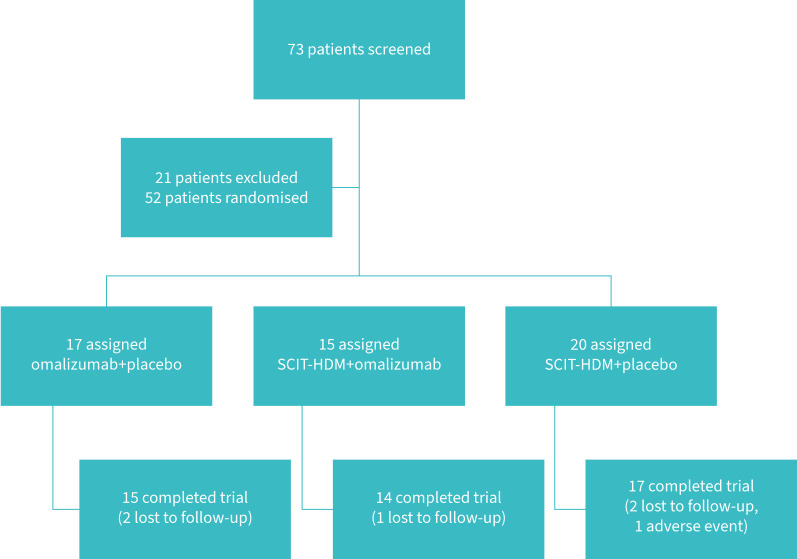
The study compared three therapies: omalizumab alone, SCIT-HDM+omalizumab and SCIT alone. The flowchart is presented in figure 1. The recruitment period was 12 months.
FIGURE 1.
Flowchart showing the process of inclusion and retention of patients in the study. House dust mite subcutaneous immunotherapy (SCIT-HDM).
Patients
The inclusion criteria were as follows: >18 years old; a total IgE between 30 and 700 IU·mL−1; mild or moderate asthma with perennial symptoms confirmed as HDM-driven asthma based on medical history and positive nasal provocation tests, controlled or partial controlled asthma; forced expiratory volume in 1 s (FEV1) >75% at baseline; positive monovalent skin prick test (SPT) results for Dermatophagoides pteronyssinus and D. farinae, and negative SPT for other inhalant allergens (grass pollen, hazel, birch, alder, Alternaria, Cladosporium, mugwort, dog and cat dander); and positive allergen-specific IgE results for D. pteronyssinus and D. farinae.
The exclusion criteria were as follows: uncontrolled asthma, other serious diseases or chronic unstable diseases contraindicating AIT and omalizumab treatment.
Short-acting β-agonist (SABA), long-acting β-agonist inhaled corticosteroids (ICS), anti-allergy drugs and glucocorticoid nasal drops were permitted during study observation.
The local bioethics committees approved the Medical University of Silesia's study in Poland (KNW-1-133/N/9/K). All patients signed a consent form.
Asthma diagnosis
Asthma was diagnosed based on the Global Initiative for Asthma (GINA) criteria. The treatment, daily symptoms, and episodes of asthma exacerbation and coincidences with asthma symptoms and HDM natural exposure (heating season) were analysed. Controlled asthma was confirmed by the Asthma Control Questionnaire (ACQ). A score of 0.0–0.75 is classified as well-controlled asthma, 0.75–1.5 as a “grey zone” and >1.5 as poorly controlled.
All patients had a positive reversibility test, defined as an increase of ≥12% and 200 mLin FEV1 within 10 to 40 min following four inhalations of salbutamol. FEV1 was also measured at the start and for 12 months during the study. Before this procedure, all patients abstained from receiving β-2 agonists for >8 h.
A full medical examination, including rhinolaryngological assessment, was performed.
Interventions
All patients were randomly assigned to the following groups: Group A: omalizumab with placebo; Group B: SCIT-HDM and omalizumab; and Group C: SCIT-HDM with placebo.
AIT
Participants from Groups B and C received SCIT-HDM (Purethal mites, Hal Allergy or placebo). This vaccine contained major allergen equivalents of 14.0 μg·mL−1 for Group 1 and 20.0 μg·mL−1 for Group 2 as measured by ELISA in the extract before modification and adsorption of aluminium hydroxide. The vaccine was administered as a perennial therapy using the following regimen: 1 dose 0.1 mL; 2 doses 0.2 mL; 3 doses 0.5 mL every week, and 0.5 mLevery 4 weeks for 12 months. The mean cumulative dose was 490 500 bioequivalent allergy units (BAU) administered to each patient undergoing active treatment for 12 months of the study.
Omalizumab
Participants from Group A and Group B received omalizumab (Novartis, Europharm Limited, Dublin, Ireland) or placebo as a double dummy 4 weeks before SCIT 150 mg (one vial) and a continued dose of 150 mg every 4 weeks for 12 months of SCIT.
Outcome parameters
The primary outcomes were the reduction in asthma exacerbations per year and the evaluation of the ACQ, FEV1 changes and the possible reduction in daily doses of inhaled steroids during the 12-month observation.
Asthma exacerbation was defined as deterioration in asthma symptoms resulting in hospitalisation or emergency room treatment or the need for oral steroids for ≥3 days (as judged by the investigator).
A secondary goal was the reduction in clinical symptoms and the reduction in asthma medications after 12 months of treatment compared to the start of each study arm. Patients completed a diary every day between the screening visit and their last visits during the trial. The total asthma symptom score (TASS) and total medication score (TMS) were analysed. The TASS is a four-point symptom scale for asthma that analyses cough, wheezing, breathlessness and dyspnoea. The TMS was based on summed points: 1 point for ICS, 2 points for β-agonists and 3 points for systemic steroids. Daily symptoms and medication scores were monitored, after which mean monthly scores were calculated.
We also analysed global evaluation of treatment effectiveness (GETE) changes after 1 year of treatment. The GETE is a five-point scale assessment of asthma symptom control, which ranges from excellent to good, moderate or poor effectiveness to worsening in response to asthma treatment.
Statistics
Analyses were performed using Statistica 8.12 (SoftPol, Cracow, Poland). The primary analysis was conducted in the ITT population comparing treatments with ANOVA with parametric models of treatment (scores, asthma control test (ACT)). Treatment groups were also compared with the nonparametric Wilcoxon test. GETE was analysed by the Chi-squared test. All analyses of efficacy were performed with intention to treat (ITT) and per protocol (PP) populations. p<0.05 was considered statistically significant.
Results
Patients
52 patients were randomised and given therapy (ITT), and 46 completed 12 months of therapy in a randomly selected treatment arm. The characteristics of the patients are presented in table 1.
TABLE 1.
Characteristics of the study groups after randomisation
| Group A | Group B | Group C | |
| Patients n | 17 | 15 | 20 |
| Age years, mean±sd | 23.6±8.5 | 26.1±11.9 | 22.3±7.4 |
| Female % | 45 | 50 | 40 |
| Controlled asthma n | 9 | 7 | 12 |
| Partially controlled asthma n | 8 | 8 | 8 |
| Asthma therapy n | |||
| SABA+ICS | 9 | 8 | 10 |
| LABA+ICS | 8 | 7 | 10 |
| ICS daily dose of budesonide µg, mean±sd | 650±150 | 550±250 | 600±200 |
| Time since diagnosis of asthma years, mean±sd | 6±4.7 | 5.2±3.5 | 7.2±2.4 |
| Allergic rhinitis n | 11 | 10 | 12 |
SABA: short-acting β-agonist; LABA: long-acting β-agonist; ICS: inhaled glucocorticosteroids.
Efficacy
15 patients after therapy with omalizumab (Group A), 14 patients after SCIT-HDM and omalizumab therapy (Group B) and 17 after SCIT-HDM (Group C) completed the study.
During 1 year of study, asthma exacerbations were significantly reduced in all study groups, with no significant differences between them (table 2).
TABLE 2.
Results of TASS and TMS in the analysed groups (intention to treat population)
| Group A | Group B | Group C | |||||||
| Baseline | 12 months | MD (p) | Baseline | 12 months | MD (p) | Baseline | 12 months | MD (p) | |
| ACQ, mean±sd | 0.99±0.21 | 0.68±0.23 | 0.3±0.03 | 1.12±0.04 | 0.52±0.35 | 0.6±0.02 | 0.93±0.34 | 0.71±0.31 | 0.22±0.03 |
| Asthma exacerbation, PP±sd | 1.8±0.7 | 0.9±0.2 | 0.87±0.01 | 2.1±0.7 | 0.7±0.3 | 1.4±0.01 | 2.1±1.2 | 1.1±0.7 | 0.9±0.04 |
| TASS, mean±sd | 3.01±0.71 | 1.35±0.56 | 1.66±0.01 | 2.98±0.78 | 1.18±0.71 | 1.8±0.01 | 2.91±0.64 | 1.39±0.85 | 1.53±0.03 |
| TMS, mean±sd | 2.12±0.39 | 1.27±0.56 | 0.86±0.01 | 2.24±0.75 | 0.79±0.28# | 1.45±0.02 | 2.16±0.22 | 1.21±0.51# | 0.94±0.01 |
MD: mean differences; TASS: total asthma symptom score; TMS: total medication score; PP: patient per year; ACQ: Asthma Control Questionnaire. #: TMS score was significantly decreased during therapy for p<0.05.
During the12 months of therapy, a statistically significant reduction in the daily doses of ICS in Groups A and B was observed. Details are presented in figure 2. Daily ICS dose reduction was considerably more significant in Group B. Monthly SABA dose reductions were observed in all groups.
ACQ scores were significantly improved in all study groups, and there were no significant changes between groups after 12 months of therapy (table 2).
Significant reductions in TASS and TMS during 12 months of treatment were observed in all analysed groups (table 2). The TASS was decreased in favour of SCIT+omalizumab. These data are shown in figure 3.
FIGURE 3.
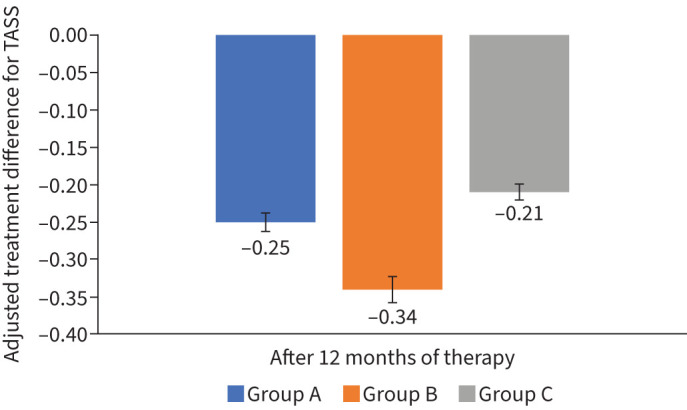
Changes in the total asthma symptoms score (TASS) after therapy as adjusted treatment differences in the per protocol population. A significant change in Group B for p=0.029 (Wilcoxon test).
FIGURE 2.
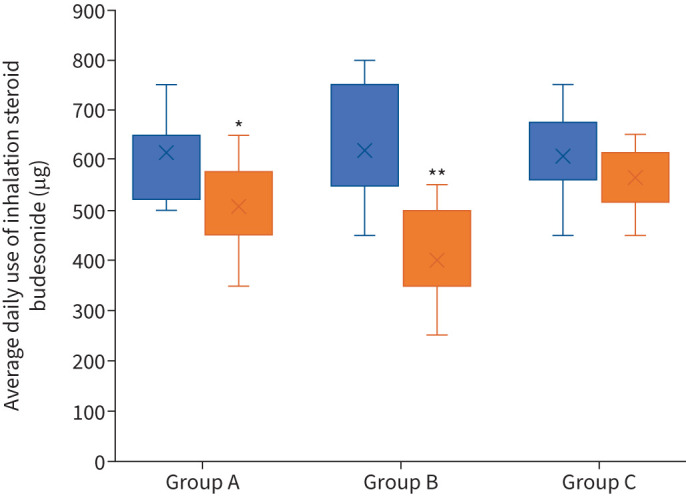
Changes in daily doses of inhalation steroid budesonide after 12 months of observation. Group A: omalizumab+placebo; Group B: SCIT-HDM+omalizumab; Group C: SCIT-HDM+placebo. *: significant decrease (Chi-square test) for p=0.029; **: significant decrease for p=0.015.
When we used ANOVA with logarithmically transformed data, we also observed significant differences between the three treatments in both populations after 12 months of therapy in favour of Group B. TMS decreased significantly more after 12 months of treatment in favour of Group B. Details are presented in table 2.
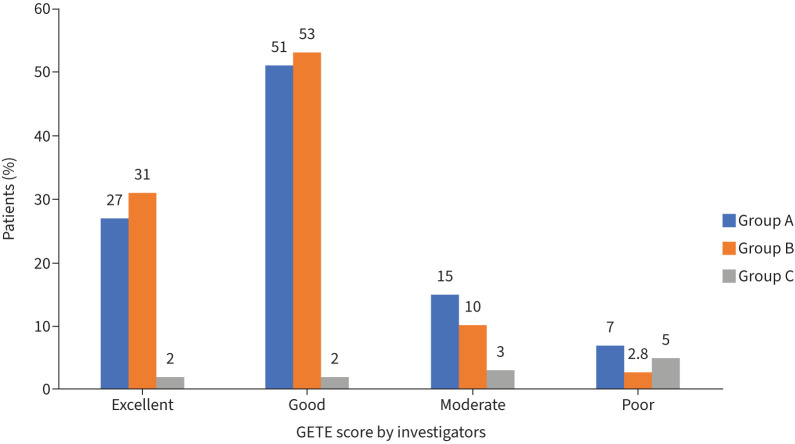
The positive effect of all therapies on the GETE score was observed by the investigator, and there were no significant differences between groups (figure 4).
FIGURE 4.
Global evaluation of treatment effectiveness (GETE) score presentation after 12 months of therapy performed by investigators. Group A: omalizumab+placebo; Group B: SCIT-HDM+omalizumab; Group C: SCIT-HDM+placebo.
Safety
During all observations, only one mild systemic reaction was observed during the second month of treatment after the SCIT dose was maintained in patients from Group C. This reaction did not require medical intervention, and the AIT continued as planned. In Group B, slightly fewer lower local reactions were observed compared to Group C (without omalizumab): 13 (8%) injections versus 18 (11%) injections (p=0.072).
Discussion
AIT is a disease modulator and results in symptom relief in allergy symptoms, including asthma. On the other hand, biological treatments are now available for asthma, such as omalizumab and many others [4]. The combination of omalizumab and AIT appears to be a promising approach to reduce adverse events and increase the efficacy of AIT itself [5, 6, 7]. In the present study, the combination of omalizumab+AIT was the most effective treatment among the others proposed in patients with HDM-driven asthma. In these patients, the most significant reduction in asthmatic exacerbations and daily dose of ICS was observed, which shows the benefit of such therapy. However, the ICS daily doses did not decrease in the AIT-only group. The presented treatment method for mild and moderate asthma goes beyond the current indications for the use of omalizumab. Increasing the dose of inhaled steroids in the studied patients might improve asthma. However, most of the examined patients had temporarily increased doses of inhaled steroids in the past. This did not result in fewer asthma exacerbations, especially in the autumn and winter. The authors decided to present a different method of treatment, which, might give a more long-term treatment effect and greater safety. Further follow-up of the patients is ongoing. A critical assessment of this method of treatment revealed its significantly higher costs. Perhaps a long-term evaluation of the treatment's effectiveness will reduce the overall cost of treating this disease in the future. This requires further research.
It the present study, omalizumab was used in a fixed dose and was not adjusted to body weight. In some cases, this dose of omalizumab was less than expected for the weight. Worse overall results were observed in the group treated with omalizumab alone. On the other hand, combining AIT with a fixed dose of omalizumab may favourably reduce the dose of the biological drug and thus affect the costs of treatment in some patients.
Kopp et al. [8, 9], in two studies, proved that omalizumab in combination with SCIT in patients with seasonal allergic rhinitis and/or asthma reduces symptoms more than SCIT alone. The GETE results fared favourably, where most doctors and patients rated the treatment effect as excellent or very good. Similar to the present study, better asthma control was obtained, as expressed in the ACQ questionnaire. However, in the Kopp et al. observations mentioned above, patients with seasonal allergies and intense symptoms were analysed over relatively short periods, which differs from the present study, where clinical symptoms were perennial, often with lower intensity and were associated with allergies to mites [9].
Few studies have evaluated omalizumab combination therapy and AIT in dust mite allergies [10, 11]. One studied children and was mainly focused on the safety of the treatment and, to a lesser extent, analysed its effectiveness [10]. Stelmach et al. [10] conducted a real-life observational study of the clinical experience of maintenance treatment with omalizumab in children and adolescents with allergies to mites or moulds and with severe asthma who did not tolerate AIT prior to omalizumab initiation. Most of these patients successfully continued AIT.
The authors of this study emphasised the increased safety of AIT after adding omalizumab; however, there was less information and no comparison of the effectiveness of the combination of omalizumab+AIT and AIT alone [10]. In this study, when starting AIT, slightly more local reactions were observed in the group without omalizumab than in the group with it. However, in both analysed subgroups, there was no systemic response other than a single first-degree one-off response in the SCIT group.
Massanari et al. [11] proved that in adult patients with allergies to various allergens, including dust mites, treatment with omalizumab before AIT significantly reduced asthma symptoms and β-agonists. This finding is consistent with the observations in our trial; however, we additionally showed reductions in the daily dose of inhaled steroids, especially in the group with combination therapy.
Most other studies concerned patients with seasonal allergies, which increased the chances of noticing a quick improvement after adding omalizumab [12, 13].
According to Abdel-Gadir and colleagues’ [14] opinion, AIT supplemented by omalizumab promotes allergen desensitisation through an initial-dependent step that acutely depletes allergen-reactive T-cells, followed by an increase in allergen T-cell activity due to the reversal of their Th2 cell-like programme. Improved cell function may be a key to this mechanism.
The present study is the first to compare treatment with omalizumab alone without AIT in patients allergic to mites. The greater effectiveness of AIT+omalizumab therapy than AIT alone or omalizumab alone emphasises the value of such a combined treatment. Moreover, this is the first study to evaluate a homogeneous group of patients with HDM-driven asthma after treatment.
This study had some limitations, such as relatively small subgroups of patients due to the restrictive inclusion criteria (asthma and mite monosensitisation) and no long-term evaluation, but the study is ongoing. Another limitation of the study is the lack of an assessment of in vitro biomarkers, apart from the review of allergen-specific IgE, which did not change significantly during the year of treatment (unpublished data).
Conclusion
A combination of AIT to HDM and omalizumab was more effective in reducing symptoms and reducing the daily dose of ICS than omalizumab alone or AIT alone in patients with HDM-driven asthma. Further studies are necessary.
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: None declared.
Support statement: This study was supported by Śląski Uniwersytet Medyczny. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Pfaar O, Agache I, de Blay F, et al. . Perspectives in allergen immunotherapy: 2019 and beyond. Allergy 2019; 74 Suppl 108, 3–25. doi: 10.1111/all.14077.31872476 [DOI] [PubMed] [Google Scholar]
- 2.Dhami S, Kakourou A, Asamoah F, et al. . Allergen immunotherapy for allergic asthma: a systemic review and meta-analysis. Allergy 2017; 72912: 1825–1848. doi: 10.1111/all.13208 [DOI] [PubMed] [Google Scholar]
- 3.Agache I, Lau S, Akdis CA, et al. . EAACI guidelines on allergen immunotherapy: house dust mite-driven allergic asthma. Allergy 2019; 74: 855–873. doi: 10.1111/all.13749 [DOI] [PubMed] [Google Scholar]
- 4.Pfutzner W, Schuppe M. Use of biologics in allergen immunotherapy. Allergol Select 2021; 5: 108–118. doi: 10.5414/ALX02206E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lombardi C, Canonica GW, Passalacqua G. Allergen immunotherapy as add-on to biologic agents. Curr Opin 2018; 18: 502–508. doi: 10.1097/ACI.0000000000000479 [DOI] [PubMed] [Google Scholar]
- 6.Dantzer JA, Wood RA. Update on omalizumab in allergen immunotherapy. Curr Opin 2021; 21: 559–568. doi: 10.1097/ACI.0000000000000781 [DOI] [PubMed] [Google Scholar]
- 7.Malipiero G, Melone G, Puggioni F, et al. . Allergen immunotherapy and biologics in respiratory allergy: friends or foes? Curr Opin Allergy Clin Immunol 2021; 21: 16–23. doi: 10.1097/ACI.0000000000000707 [DOI] [PubMed] [Google Scholar]
- 8.Kopp MV, Hamelmann E, Bendiks M, et al. . Transient impact of omalizumab in pollen allergic patients undergoing specific immunotherapy. Pediatr Allergy Immunol 2013; 24: 427–433. doi: 10.1111/pai.12098 [DOI] [PubMed] [Google Scholar]
- 9.Kopp MV, Hamelmann E, Zielen S, et al. . Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clin Exp 2008: 39: 271–279. doi: 10.1111/j.1365-2222.2008.03121.x [DOI] [PubMed] [Google Scholar]
- 10.Stelmach I, Majak P, Jerzynska J,et al. . Children with severe asthma can start allergen immunotherapy after controlling asthma with omalizumab: a case series from Poland. Arch Med Sci 2015; 11: 901–904. doi: 10.5114/aoms.2015.48546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massanari M, Nelson H, Casale T,et al. . Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol 2010; 125: 383–389. doi: 10.1016/j.jaci.2009.11.022 [DOI] [PubMed] [Google Scholar]
- 12.Kuehr J, Brauburger J, Zielen S,et al. . Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J Allergy Clin Immunol 2002; 109: 274–280. doi: 10.1067/mai.2002.121949 [DOI] [PubMed] [Google Scholar]
- 13.Casale TB, Busse WW, Kine JN, et al. . Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed induced seasonal allergic rhinitis. J Allergy Clin Immunol 2006; 117: 134–140. doi: 10.1016/j.jaci.2005.09.036 [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Gadir A, Schneider L, Caini A, et al. . Oral immunotherapy with omalizumab reverses the Th2 cell-like programme of regulatory T cells and restores their function. Clin Exp Allergy 2018; 48: 825–836. doi: 10.1111/cea.13161 [DOI] [PMC free article] [PubMed] [Google Scholar]