Accurate measurement of arterial carbon dioxide partial pressure (PaCO2) is critical in emergency medicine as a marker of sufficient alveolar ventilation [1], and in human physiology to understand respiratory and cerebrovascular regulation [2]. The current gold-standard technique to measure PaCO2 is through arterial catheterisation and analysis of blood gases, although arterialised capillary blood (e.g. earlobe or fingertip) is often used to alleviate the invasiveness and risk associated with arterial sampling [3].
Short abstract
End-tidal CO2 tension provides an accurate estimation of PaCO2 in healthy awake individuals over an extensive range of CO2 pressures induced by 17 environmental conditions combining different O2, CO2 and barometric pressures https://bit.ly/3YuKPAY
To the Editor:
Accurate measurement of arterial carbon dioxide partial pressure (PaCO2) is critical in emergency medicine as a marker of sufficient alveolar ventilation [1], and in human physiology to understand respiratory and cerebrovascular regulation [2]. The current gold-standard technique to measure PaCO2 is through arterial catheterisation and analysis of blood gases, although arterialised capillary blood (e.g. earlobe or fingertip) is often used to alleviate the invasiveness and risk associated with arterial sampling [3]. In either case, direct measurement of blood gases does not allow for continuous data recording. Several pioneering works [4, 5] and recent studies [3, 6, 7] attempted to investigate noninvasive surrogates of PaCO2 with greater temporal resolution. End-tidal carbon dioxide partial pressure (PETCO2) represents a noninvasive measurement of alveolar ventilation, and is typically considered an adequate substitute for PaCO2 in healthy adults [7]. However, PETCO2 and PaCO2 values may differ with discrepancies ranging between 1.8 and 4.9 mmHg in awake or anaesthetised healthy individuals [8–10]. We aimed to investigate the PETCO2–PaCO2 relationship over a wide range of environmental conditions (combining different oxygen (O2), carbon dioxide (CO2) and barometric pressures (PB)), to induce a large range of CO2 pressure variations in resting healthy individuals.
17 healthy males (mean±sd age 21±2 years, body mass index 22.8±1.8 kg·m−2) volunteered and gave written informed consent to participate in this study. All participants were not taking any medication and were free from any cardiorespiratory and haematological diseases. Data on normal lung function and diffusion capacity of the lung for carbon monoxide of our participants are available elsewhere [11]. The experimental protocol was pre-registered at ClinicalTrials.gov (NCT04739904), approved by both the University of Ljubljana, Faculty of Sport ethics committee (8/2020–316) and the Aosta Hospital ethical committee (06/05/2021.0038781.I), and performed according to the Declaration of Helsinki.
Participants were instructed to abstain from exercise (>12 h), alcohol and caffeine (>24 h), and avoid heavy meals (>4 h) before testing. Participants were tested under the following 17 environmental conditions while comfortably seated in a quiet and thermoneutral room: 1) normobaric normoxia (NNx); 2) normobaric normoxic hypercapnia (NNx+3%CO2); 3) hypobaric hypoxia (HHx); 4) hypobaric normoxia (HNx); 5) hypobaric normoxic hypercapnia (HNx+3%CO2); 6) hypobaric hypoxic isocapnia (with PETCO2 clamped at NNx value (HHx+clamp)); 7) normobaric hypoxia (NHx); 8) normobaric hypoxic hypercapnia (NHx+3%CO2); 9) normobaric hypoxic isocapnia (NHx+clamp); 10) normobaric hyperoxic (97%O2) hypercapnia (3%CO2; NHx+3%CO2); 11) normobaric hyperoxic (94%O2) hypercapnia (6%CO2; NHx+6%CO2); 12) hypobaric hyperoxic (97%O2) hypercapnia (3%CO2; HHx+3%CO2); 13) hypobaric hyperoxic (94%O2) hypercapnia (6%CO2; HHx+6%CO2); and 14–17) normobaric and hypobaric hypocapnia (i.e. voluntary hyperventilation). Normobaric conditions were performed near sea level (295 m; PB=737±2 mmHg), while hypobaric measurements were carried out at high altitude (3375 m; PB =503±3 mmHg). NNx+CO2 and NHx+CO2 were induced by switching the inspired gas from ambient air to 3%CO2 (in 20.93%O2, balance N2). In HNx and HNx+CO2, participants breathed supplemental O2 (inspiratory oxygen fraction (FIO2) 32%, with 0.03%CO2, balance N2 and with 3%CO2, balance N2, respectively) to induce the same FIO2 as in NNx. During conditions 6 and 9, end-tidal clamping was performed using a modified version of a breathing system detailed elsewhere [12]. Briefly, the system delivered high-flow, low-resistance inspired gas with a fixed FIO2 and a varying inspiratory carbon dioxide fraction. The inspiratory end-point of this system included an open-ended reservoir where room air was mixed with 100%CO2 compressed gas. The 8-L custom-made reservoir was connected, via a plastic flexible tube, to a two-way nonrebreathing valve (2700 series; Hans Rudolph, Kansas City, MO, USA) attached to a low dead-space face mask (Hans Rudolph mask; 7400 oronasal series; dead space 73 mL). In the normobaric and hypobaric hyperventilation stages, participants were instructed to increase their frequency and/or depth of breathing to reduce their PETCO2 by the same magnitude as the increase observed during the corresponding hyperoxic hypercapnic condition. Each condition lasted 4 min. PETCO2 was continuously monitored by a calibrated metabolic cart (Ergocard Professional; Medisoft, Sorinnes, Belgium) and the 30-s average at the end of each stage was recorded. Arterialised capillary blood was collected from the earlobe during the last 30 s of each stage, and analysed for PaCO2 using an arterial blood gas analyser (ABL-90 FLEX; Radiometer, Copenhagen, Denmark).
After having checked for normality by Shapiro–Wilk test, linear regression and correlation analyses between PETCO2 and PaCO2 were performed by the least-squares residual method (Prism v.6.0; GraphPad Software, La Jolla, CA, USA). Residual plot analysis was used to determine the linear fitting of our data. Moreover, a linear regression of all the individual residuals versus the average CO2 response was tested to determine that there was not a systematic difference throughout the range of values. Bland–Altman analysis calculating the difference versus the mean was used to compare paired readings of PETCO2 and PaCO2; 95% confidence intervals were also calculated. All p-values were two-tailed, and statistical significance was defined a priori at p<0.05.
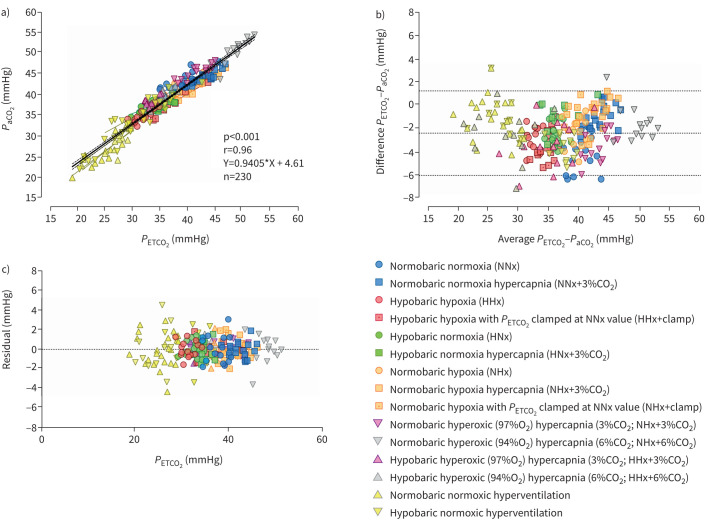
PETCO2 and PaCO2 showed a strong to very strong correlation for each of the 17 environmental conditions, separately (r>0.60, p<0.044), as well as when all conditions were pooled (figure 1a). Bland–Altman analysis indicated that, when all conditions were pooled, the bias of the PETCO2 was −2.43 mmHg (95% CI −6.08–1.23 mmHg; figure 1b). Taken separately, the bias ranged from −3.99 (95% CI −7.13– −0.85 mmHg) in NNx to −0.18 (95% CI −1.84–1.47 mmHg) in NHx+3%CO2. The linear regression analysis of the residuals showed that there was not a systematic difference throughout the range of values (slope= −0.331, p=0.798; figure 1c).
FIGURE 1.
Pooled correlation and linear regression analyses of a) end-tidal carbon dioxide tension (PETCO2) versus arterial carbon dioxide tension (PaCO2) measured during 17 experimental conditions and b) Bland–Altman plot of the actual difference versus the mean of PETCO2 and PaCO2; c) the residuals plot. In b) dotted lines represent the bias and the 95% confidence intervals. CO2: carbon dioxide; O2: oxygen.
PETCO2 represents an attractive, noninvasive alternative for PaCO2 measurement. We observed a strong correlation between PETCO2 and PaCO2 over a wide range of inspired CO2 partial pressures in young healthy adults. Previous studies investigating the PETCO2–PaCO2 relationship in both healthy and mechanically ventilated individuals concluded that PETCO2 may [8, 9] or may not [13, 14] represent a surrogate of PaCO2 in different population and/or experimental settings. There are several conditions where PETCO2 does not accurately reflect PaCO2, such as exercise, ageing and body position, as well as in patients with lung diseases [7]. Moreover, respiration and dead space undoubtedly influence both PETCO2 and PaCO2, although recent work reported a moderate-to-strong correlation between PETCO2 and PaCO2 across a wide range of dead space to tidal volume ratios [15]. However, in this study we only focused on understanding the influence of different environmental conditions (i.e. hypobaric versus normobaric, normocapnic versus hypercapnic, normoxia versus hypoxia) on the PETCO2–PaCO2 relationship in healthy individuals, and demonstrated that the PETCO2–PaCO2 relationship remains valid across numerous environmental conditions combining different levels of O2, CO2 and barometric pressures.
In conclusion, these novel findings suggest that PETCO2 measurement provides an accurate estimation of PaCO2 in healthy awake individuals over an extensive range of CO2 pressures induced by various environmental conditions combining different O2, CO2 and barometric pressures. Therefore, our results support the use of PETCO2 as an alternative to invasive monitoring and/or repeated arterial blood gas analyses in applied environmental physiology research. However, the present findings can be only used to draw conclusions in healthy adults, since the PETCO2–PaCO2 relationship does not persist in patients with alveolar ventilation/perfusion abnormalities [7], leading to a significant underestimation of PaCO2 [6]. Nonetheless, in healthy individuals, PETCO2 can be easily measured breath-by-breath or continuously, which is particularly useful in a variety of experimental and applied contexts.
Acknowledgements
The authors thank all the volunteers who enthusiastically participated in this research project, the team at Torino Hut (Italy) where the high-altitude data collection was carried out, and SkyWay Mont Blanc (Entrèves, Italy) who supported the transport of the equipment and the participants to Torino Hut.
Provenance: Submitted article, peer reviewed.
The experimental protocol for this study was pre-registered at ClinicalTrials.gov with identifier number NCT04739904.
Conflict of interest: No conflicts of interest, financial or otherwise, are declared by the authors.
Support statement: This work was funded by the Swiss National Science Foundation (SNSF grant number 320030L_192073) and the Slovenian Research Agency (ARRS grant number N5-0152).
References
- 1.Petersson J, Glenny RW. Gas exchange and ventilation–perfusion relationships in the lung. Eur Respir J 2014; 44: 1023–1041. doi: 10.1183/09031936.00037014 [DOI] [PubMed] [Google Scholar]
- 2.Ogoh S. Interaction between the respiratory system and cerebral blood flow regulation. J Appl Physiol 2019; 127: 1197–1205. doi: 10.1152/japplphysiol.00057.2019 [DOI] [PubMed] [Google Scholar]
- 3.Huttmann SE, Windisch W, Storre JH. Techniques for the measurement and monitoring of carbon dioxide in the blood. Ann Am Thorac Soc 2014; 11: 645–652. doi: 10.1513/AnnalsATS.201311-387FR [DOI] [PubMed] [Google Scholar]
- 4.Haldane JS, Priestley JG. The regulation of the lung-ventilation. J Physiol 1905; 32: 225–266. doi: 10.1113/jphysiol.1905.sp001081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunn JF, Hill DW. Respiratory dead space and arterial to end-tidal carbon dioxide tension difference in anesthetized man. J Appl Physiol 1960; 15: 383–389. doi: 10.1152/jappl.1960.15.3.383 [DOI] [PubMed] [Google Scholar]
- 6.Lermuzeaux M, Meric H, Sauneuf B, et al. Superiority of transcutaneous CO2 over end-tidal CO2 measurement for monitoring respiratory failure in nonintubated patients: a pilot study. J Crit Care 2016; 31: 150–156. doi: 10.1016/j.jcrc.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 7.Nassar BS, Schmidt GA. Estimating arterial partial pressure of carbon dioxide in ventilated patients: how valid are surrogate measures? Ann Am Thorac Soc 2017; 14: 1005–1014. doi: 10.1513/AnnalsATS.201701-034FR [DOI] [PubMed] [Google Scholar]
- 8.Yosefy C, Hay E, Nasri Y, et al. End tidal carbon dioxide as a predictor of the arterial PCO 2 in the emergency department setting. Emerg Med J 2004; 21: 557–559. doi: 10.1136/emj.2003.005819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Razi E, Moosavi GA, Omidi K, et al. Correlation of end-tidal carbon dioxide with arterial carbon dioxide in mechanically ventilated patients. Arch Trauma Res 2012; 1: 58–62. doi: 10.5812/atr.6444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan KJ, Kissoon N, Goodwin SR. End-tidal carbon dioxide monitoring in pediatric emergencies. Pediatr Emerg Care 2005; 21: 327–332. doi: 10.1097/01.pec.0000159064.24820.bd [DOI] [PubMed] [Google Scholar]
- 11.Manferdelli G, Narang BJ, Bourdillon N, et al. Physiological responses to exercise in hypoxia in preterm adults: convective and diffusive limitations in the O2 transport. Med Sci Sports Exerc 2023; 55: 482–496. doi: 10.1249/MSS.0000000000003077 [DOI] [PubMed] [Google Scholar]
- 12.Olin JT, Dimmen AC, Subudhi AW, et al. A simple method to clamp end-tidal carbon dioxide during rest and exercise. Eur J Appl Physiol 2012; 112: 3439–3444. doi: 10.1007/s00421-012-2433-6 [DOI] [PubMed] [Google Scholar]
- 13.Doppmann P, Meuli L, Sollid SJM, et al. End-tidal to arterial carbon dioxide gradient is associated with increased mortality in patients with traumatic brain injury: a retrospective observational study. Sci Rep 2021; 11: 10391. doi: 10.1038/s41598-021-89913-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tymko MM, Ainslie PN, MacLeod DB, et al. End tidal-to-arterial CO2 and O2 gas gradients at low- and high-altitude during dynamic end-tidal forcing. Am J Physiol Regul Integr Comp Physiol 2015; 308: R895–R906. doi: 10.1152/ajpregu.00425.2014 [DOI] [PubMed] [Google Scholar]
- 15.McSwain SD, Hamel DS, Smith PB, et al. End-tidal and arterial carbon dioxide measurements correlate across all levels of physiologic dead space. Respir Care 2010; 55: 288–293. [PMC free article] [PubMed] [Google Scholar]