Abstract
Cervical cancer continues to affect women in the United States and throughout the world despite an effective vaccine against human papillomavirus and cancer screening programs. For the women who develop cervical cancer, surgery, radiation, and chemotherapy have been the mainstays of treatment for years. Recently, novel therapeutics have been developed that offer new treatment opportunities for women living with advanced and/or recurrent disease. Immunotherapy has become an important tool against cervical cancer with the approval of pembrolizumab in the second line for advanced or recurrent disease. Checkpoint inhibitors have recently been approved in the front line for advanced and/or recurrent disease in combination with chemotherapy, and they are being studied in the front line in combination with chemoradiation. Antibody–drug conjugates—specifically tisotumab vedotin (TV)—also have recently received Food and Drug Administration (FDA) approval, and TV is currently being studied in combination with checkpoint inhibitors and with carboplatin. Tumor‐infiltrating lymphocytes have been studied in early‐phase trials and have shown promise in small patient series. Despite these new therapies, there continue to be racial, ethnic, and socioeconomic inequities with respect to access to care, access to and participation in clinical trials, and survival in the United States as well as globally. New FDA guidance requires researchers to work to reduce disparities by including women of more diverse backgrounds in clinical trials. Finally, as progress continues to be made in the treatment of established disease, prevention through vaccination and screening remains paramount.
Plain language summary
The treatment of cervical cancer remains a significant problem in the United States and especially worldwide.
Although early cases can be cured, cervical cancer that has spread remains difficult to treat.
The past few years have seen significant advances in new therapies and combinations of therapies for women with advanced or recurrent disease.
Although this is excellent news for these women, cervical cancer is a preventable disease through screening with Papanicolaou smears and vaccination with the human papillomavirus vaccine.
By improving access to and acceptance of screening and vaccination, we can eradicate cervical cancer in the United States and the world.
Keywords: antibody–drug conjugate, cervical cancer, disparities, immunotherapy, tumor‐infiltrating lymphocytes
Short abstract
The past few years have seen US Food and Drug Administration approval of novel therapies and combinations for advanced and/or recurrent cervical cancer that are providing new hope for women with this disease. All cervical cancer can and must be eradicated by improving access to and acceptance of screening modalities and vaccination and by addressing disparities related to race, ethnicity, and socioeconomic status.
INTRODUCTION
Despite successful screening modalities and the approval of an effective vaccine, cervical cancer will take the lives of an estimated 4280 women in the United States in 2022. 1 Cervical cancer is also the fourth most common cancer among women globally, with an estimated 604,000 new cases and 342,000 deaths in 2020 2 (Figure 1). The majority of cases globally occur in middle and lower income countries. 3 Moreover, although new diagnoses of cervical cancer in the United States decreased by more than 50% from 1975 to 2010, the decrease in the incidence of cervical cancer in the United States slowed in the 2000s and plateaued during the 2012–2017 period 4 ; this underscores the need for targeted efforts not just to increase human papillomavirus vaccination and effective screening but also to identify novel therapies for those women already affected by disease. Finally, cervical cancer is a disease that disproportionately affects women of color and low socioeconomic status 5 ; it is these women that will most benefit from novel treatment opportunities.
FIGURE 1.
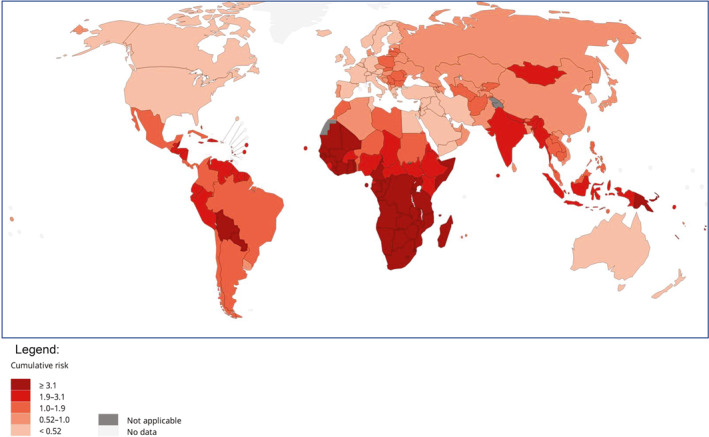
Estimated cumulative risk of mortality in 2020, cervix uteri, all ages. The global estimated cumulative risk of mortality from cervical cancer is shown by country for 2020. The data have been estimated from the World Health Organization. Reprinted from the International Agency for Research on Cancer. 26
Fortunately for patients with cervical cancer, the past few years have seen the development of new therapies that offer novel treatment opportunities and renewed hope for those women already living with the disease. Until recently, since the publication of Gynecology Oncology Group (GOG) Study 240 in 2014, which established the standard of care for advanced and/or recurrent disease as platinum chemotherapy plus paclitaxel plus bevacizumab, 6 there have been few to no new options for women with cervical cancer. Now, with exploration into the roles of checkpoint inhibitors and antibody–drug conjugates (ADCs), among other approaches, there are new treatment options for all stages of disease.
IMMUNOTHERAPY (Table 1)
TABLE 1.
Selected recent immunotherapy trials
| Name | NCT ID | Phase | Population | Patients, No. | Treatment | Study timeline | Overall response rate | Median duration of response | Median PFS | Overall survival | Safety | Median follow‐up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Keynote 158 4 | 2628067 | International, open‐label, multicohort, phase 2 | Advanced cervical cancer with disease progression during or after first‐line chemotherapy | 98 | Pembrolizumab | Resulted and published | 12.2% (95% CI, 6.5%–20.4%) | NR | 2.1 months (95% CI, 2.0–2.2 months) | 9.4 months (95% CI, 7.7–13.1 months) | 12.2% with grade 3 or 4 | 10.2 months |
| EMPOWER‐Cervical 1/GOG‐3016/ENGOT‐cx9 5 | 3257267 | Open‐label, multicenter, phase 3 | Disease progression after first‐line platinum‐containing chemotherapy | 608 | Cemiplimab | Resulted and published | 16.4% (95% CI, 12.5%–21.1%) with cemiplimab versus 6.3% (95% CI, 3.8%–9.6%) with chemotherapy | 16.4 months (95% CI, 12.4 months to NR) with cemiplimab versus 6.9 months (95% CI, 5.1–7.7 months) with chemotherapy | 2.8 months (95% CI, 2.6–3.9 months) with cemiplimab versus 2.9 months (95% CI, 2.7–3.4 months) with chemotherapy | Overall population: 12.0 months (95% CI, 10.3–13.5 months) with cemiplimab versus 8.5 months (95% CI, 7.5–9.6 months) with chemotherapy. | Fewer grade 3 or higher adverse events with cemiplimab than chemotherapy: 45.0% versus 53.4% | 18.2 months (range, 6.0–38.2 months) |
| Keynote 826 6 | 3635567 | International, phase 3 | Persistent, recurrent, or metastatic adenocarcinoma or adenosquamous carcinoma; no systemic treatment aside from CRT | 617 | Pembrolizumab | Resulted and published | 68.1% with pembrolizumab versus 50.2% with placebo for PDL score > 1 | 18 months with pembrolizumab versus 10.4 months with placebo | 10.4 months (95% CI 9.7–12.3 months) with pembrolizumab versus 8.2 months (95% CI 6.3–8.5 months) for PDL score > 1 | Not reached in pembrolizumab group | Serious adverse events: 49.8% with pembrolizumab versus 42.4% with placebo | 22 months (range, 15.1–29.4 months) |
| BEATcc ENGOT‐Cx10/GEICO 68‐C/GOG‐3030/JGOG 1084 | 3556839 | Randomized, open‐label, phase 3 | Metastatic/recurrent | Goal is 404 | Atezolizumab | Completed accrual | ||||||
| CALLA trial 7 | 3830866 | Global, randomized, phase 3 | Upfront with LACC | Goal is 770 | Durvalumab | Interim analysis completed, presentation anticipated in September 2022 | ||||||
| MK‐3475‐A18/Keynote‐A18/ENGOT‐cx11/GOG‐3047 | 4221945 | Randomized, phase 3 | Upfront with LACC | Goal is 980 | Pembrolizumab | Recruiting, accrual completion anticipated in third quarter of 2022 | ||||||
| NRG‐GY017 8 | 3738228 | Phase 1 | Before and/or with standard‐of‐care chemoradiotherapy | Goal is 40 | Atezolizumab | Presented at SGO in March 2022, awaiting publication |
Abbreviations: BEATcc, BEv ATezo cervic; CI, confidence interval; CRT, chemoradiation; ENGOT, European Network of Gyneacological Oncological Trial Groups; GEICO, Grupo Espanol de Investigacion en Cancer de Ovario; GOG, Gynecology Oncology Group; JGOG, Japanese Gynecologic Oncology Group; LACC, locally advanced cervical cancer; NCT, National Clinical Trial; NR, not reached; PDL, programmed death ligand; PFS, progression‐free survival; SGO, Society of Gynecologic Oncology.
The 2018 Food and Drug Administration (FDA) approval of the PD‐1 inhibitor pembrolizumab for cervical cancer with disease progression during or after first‐line chemotherapy opened the door for the rapid development of immunotherapy in cervical cancer, with the overall response rate (ORR) of 14.3% for PD‐L1–positive (combined positive score ≥ 1) tumors surpassing the historical response rate of most available traditional chemotherapy agents. 7 The relative superiority of immunotherapy with respect to traditional chemotherapy in this patient population was later confirmed in the EMPOWER‐Cervical 1/GOG‐3016/ENGOT‐cx9 trial, an open‐label, multicenter, phase 3 study of cemiplimab, a PD‐1 inhibitor, versus the investigator’s choice of chemotherapy. 8 In the overall trial population (including all patients, regardless of their tumor PD‐L1 status), the median overall survival was longer in the cemiplimab group than the chemotherapy group (12.0 vs. 8.5 months; hazard ratio for death, 0.69; 95% confidence interval [CI], 0.56–0.84; two‐sided p < .001). 8 For the first time in years, patients with recurrent or metastatic disease who have already received one line of systemic chemotherapy now have an option for treatment that is superior to traditional chemotherapy and has a safety profile and infusion schedule that allow for an acceptable quality of life. 9
The interest in immunotherapy for this disease has led to further exploration of its use in the frontline setting: in combination with chemotherapy for patients with recurrent and/or metastatic disease and in combination with chemoradiation (CRT) for patients with locally advanced disease. The results of Keynote 826, a multicenter, randomized, double‐blind, placebo‐controlled trial, led to FDA approval of pembrolizumab in combination with chemotherapy, with or without bevacizumab, for patients with persistent, recurrent, or metastatic cervical cancer whose tumors expressed PD‐L1 (combined positive score ≥ 1). 10 It is worth noting, however, that in Keynote 826, there were patients for whom the subset analysis suggested less benefit from a four‐drug regimen in this setting (see Table 2); notably, only the PD‐L1 expression level was prespecified as an end point. The study also did not address the potential for sequencing immunotherapy rather than using it in the front line. A similar but unblinded trial in the same patient population, using platinum chemotherapy plus paclitaxel with bevacizumab and atezolizumab, a PD‐L1 inhibitor (BEATcc ENGOT‐Cx10/GEICO 68‐C/GOG‐3030/JGOG 1084), recently completed accrual but has not yet reported results (NCT03556839).
TABLE 2.
Highlights from Keynote 826: Pembrolizumab for persistent, recurrent, or metastatic cervical cancer 10
| Subgroup | No. of patients | Overall survival—24‐month estimate of patients alive | Median PFS | Hazard ratio for disease progression or death |
|---|---|---|---|---|
| Intention to treat | 617 (308 in the pembrolizumab group and 309 in the placebo group) | 50.4% (95% CI, 43.8%–56.6%) of patients in the pembrolizumab group versus 40.4% (95% CI, 34.0%–46.6%) of patients in the placebo group | 10.4 months (95% CI, 9.1–12.1 months) in the pembrolizumab group versus 8.2 months (95% CI, 6.4–8.4 months) in the placebo group | 0.65 (95% CI, 0.53–0.79) |
| PD‐L1 CPS ≥ 1 a | 548 (273 in the pembrolizumab group and 275 in the placebo group) | 53.0% (95% CI, 46.0%–59.4%) of patients in the pembrolizumab group versus 41.7% (95% CI, 34.9%–48.2%) of patients in the placebo group | 10.4 months (95% CI, 9.7–12.3 months) in the pembrolizumab group versus 8.2 months (95% CI, 6.3–8.5 months) in the placebo group | 0.62 (95% CI, 0.50–0.77) |
| PD‐L1 CPS ≥ 10 a | 317 (158 in the pembrolizumab group and 159 in the placebo group) | 54.4% (95% CI, 45.5%–62.4%) of patients in the pembrolizumab group versus 44.6% (95% CI, 36.3%–52.5%) of patients in the placebo group | 10.4 months (95% CI, 8.9–15.1 months) in the pembrolizumab group versus 8.1 months (95% CI, 6.2–8.8 months) in the placebo group | 0.58 (95% CI, 0.44–0.77) |
| PD‐L1 CPS < 1 a | 69 (35 in the pembrolizumab group and 34 in the placebo group) | Not specified | Not specified | 0.94 (95% CI, 0.52–1.70) |
Abbreviations: CI, confidence interval; CPS, combined positive score; PDL, programmed death ligand; PFS, progression‐free survival.
The PD‐L1 expression level subgrouping was prespecified.
In the upfront setting in locally advanced cervical cancer (LACC), immunotherapy has been combined with CRT to improve treatment outcomes for these women. The Study of Durvalumab with Chemoradiation for Women with Locally Advanced Cervical Cancer (CALLA) trial, a randomized, multicenter, double‐blind, placebo‐controlled, phase 3 study, was designed to determine the efficacy and safety of durvalumab plus CRT versus CRT alone in patients with LACC (NCT03830866). In March 2022, the sponsor shared that the primary end point of improving progression‐free survival (PFS) with the combination over CRT alone was not met 11 ; the study was presented at the International Gynecologic Cancer Society meeting on September 29, 2022, without definitive data expanding on the negative PFS end point. The MK‐3475‐A18/Keynote‐A18/ENGOT‐cx11/GOG‐3047 trial is a study similar to CALLA, in this case using pembrolizumab, that addresses a slightly different population of women with LACC and anticipates accrual completion in the fourth quarter of 2022 (NCT04221945).
Lessons learned from these two trials are anticipated to inform future immunotherapy studies, particularly with respect to the sequencing and combination of immunotherapy with radiation. Studies such as the phase 1 study NRG‐GY017, a study of atezolizumab before or during CRT in patients with node‐positive disease that was presented at the Society of Gynecologic Oncology meeting in 2022, 12 will also help us to better understand the biology of combining and/or sequencing immunotherapy and CRT. It is also worth noting that if immunotherapy moves into the front line, alternative therapies for those women who experience disease progression or recurrence must be considered. Combinations of immune checkpoint inhibitors, such as the combination of balstilimab, a PD1 inhibitor, and zalifrelimab, a CTLA‐4 inhibitor, may offer options in this setting, 13 and further investigation is ongoing.
ADCS
Since the FDA approval of the first ADC for acute myeloid leukemia in 2000, the development of ADCs has been rapidly evolving for solid tumors, particularly in the setting of cervical cancer. In 2021, the ADC tisotumab vedotin (TV) received accelerated approval for recurrent or metastatic cervical cancer. 14 This approval was granted on the basis of the remarkable 24% ORR (95% CI, 15.9%–33.3%) and the median duration of response of 8.3 months (95% CI, 4.2 months to not reached) seen in innovaTV 204, an open‐label, multicenter, single‐arm, phase 2 trial of 101 patients with cervical cancer who had received no more than two prior lines of therapy in the recurrent or metastatic setting. 15 Similar to other ADCs, TV is associated with a unique spectrum of toxicities; these include ocular complications, which are adverse events of special interest. Preventative measures must be used when this drug is being administered, and suggested side effect mitigation is included in the package insert.
Interest in improving the efficacy of TV in cervical cancer continues, and preliminary results of the combinations of TV with carboplatin and pembrolizumab have been reported. The carboplatin combination (up to one prior line of chemotherapy; ORR, 55%) and the pembrolizumab combination (two to three prior lines; ORR, 35%) were presented at the European Society of Medical Oncology meeting in 2021. 16 Most recently, the interim results of a phase 2 trial in which patients were treated with TV and pembrolizumab if they had not received prior systemic therapy (aside from CRT) were reported at the American Society of Clinical Oncology (ASCO) in 2022. 17 The ORR was 41% (95% CI, 24%–59%) with a median PFS of 5.3 months.
TUMOR‐INFILTRATING LYMPHOCYTES
There has long been an interest in the use of tumor‐infiltrating lymphocytes (TILs) in cervical cancer because of the viral nature of this disease. In 2019, the results of a phase 2 study of adoptive cell transfer using autologous TILs for the treatment of recurrent, metastatic, or persistent cervical cancer was reported at ASCO with an ORR of 44.4% and a complete response rate of 11.1% in 27 women with one or more prior therapies. 18 The median duration of response at the time of presentation was not reached. As a result of this study, the FDA granted the breakthrough therapy designation to the technology known as LN‐145 for advanced cervical cancer, a designation designed to help to advance TIL research. Despite these exciting preliminary results, this treatment is not currently available for most women and can be accessed only via clinical trial participation.
The combination of LN‐144, another autologous adoptive cell therapy that uses TILs, and pembrolizumab led to promising ORRs, including some complete responses for patients with immune checkpoint inhibitor–naive cervical cancer, melanoma, and head and neck cancer, according to the results of a phase 2 trial presented at the 2021 annual meeting of the Society for Immunotherapy of Cancer. 19 In cohort 3 of the C‐145‐04 trial (NCT03108495), the ORR for 14 patients with persistent, recurrent, or metastatic cervical cancer was 57.1%; this included one complete response. 19 Although these TIL studies in cervical cancer are small, they are certainly hypothesis‐generating, and further study with TILs in cervical cancer is warranted on the basis of these early results.
NEGATIVE TRIALS
Despite the advances in treatment already discussed, there have been negative study results this year. One example is CALLA (described previously). The Outback trial (Australia New Zealand Gynaecological Oncology Group 0902, Radiation Therapy Oncology Group 1174, and NRG 0274), a randomized phase 3 trial of adjuvant chemotherapy following CRT as the primary treatment for LACC versus CRT alone, also reported negative results at ASCO in 2021, with no improvement in overall survival or PFS seen with the additional cycles of chemotherapy. 20
ACKNOWLEDGMENT OF DISPARITIES AND IMPROVEMENT IN TRIAL ACCESS
Despite increasing treatment options, effective vaccines, and strong screening programs, cervical cancer remains a racially and socioeconomically disparate disease. Women diagnosed with cervical cancer disproportionately are Black and Hispanic and come from backgrounds of low socioeconomic status. The mortality rates for women from these populations are significantly higher than the population rates 21 (Figure 2).
FIGURE 2.
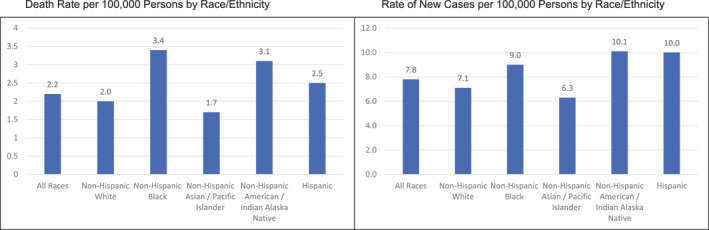
Rate of new cervical cancers in the United States, 2015–2019, by race and ethnicity. Reprinted from the National Cancer Institute. 27
Diversity in clinical trial accrual, including global site participation, is critical to the generalizability of results to all individuals. Many of even the recent trials described previously do not include data regarding race and ethnicity or suffer from low accruals of representative, racially and ethnically diverse populations of patients. The recognition of barriers to clinical research participation, including social determinants of health, a lack of community engagement, and financial toxicity, will ideally lead to innovative strategies for overcoming these barriers and improving the diversity of clinical trial accrual, particularly for cervical cancer. 22 , 23
Efforts are currently underway to improve the diversity of patient accrual in clinical trials; these efforts are not unilateral but include our national clinical trial organizations as well as the pharmaceutical industry. In April 2022, the FDA issued a new draft guidance to industry for developing plans to enroll more participants from underrepresented racial and ethnic populations in the United States into clinical trials. 24 This guidance is already being implemented at multiple levels, including an important focus on the role of provider bias. 25
CONCLUSION
Although the advances in cervical cancer that began in 1999 with the National Cancer Institute (NCI) alert urging CRT for invasive cervical cancer have brought us far, more effort is needed. Thanks to the advances of the past year, we now have novel, effective, and approved options to offer patients with recurrent disease, including immunotherapy and ADCs. Importantly, many of these studies are being conducted internationally, and this allows them to report results more quickly. These collaborations are critical to developing new and generalizable therapies across the world, including low‐ and middle‐income countries, for all patients with cervical cancer.
However, although new treatments offer important improvements in survival and treatment, the prevention of cervical cancer remains the best option for preventing disease and death as well as the substantial morbidity of modern treatment. We have an effective vaccine that is underused in the United States and around the world; addressing the multiple and differing barriers to implementation is critical in the coming years. In the United States and other developed countries where we have access to a vaccine but low uptake, we can learn from those countries that have been successful with effective community engagement and establishment of trust. Improving screening options and making them available to those women who currently are unable to participate in screening for whatever reason are also critical. Together, we can and must fully, innovatively, and equitably commit to the eradication of cervical cancer.
AUTHOR CONTRIBUTIONS
Sarah E. Podwika: Conceptualization, formal analysis, writing–original draft, and writing–review and editing. Linda R. Duska: Conceptualization, formal analysis, supervision, writing–original draft, and writing–review and editing.
CONFLICTS OF INTEREST
Linda R. Duska reports service on a data safety monitoring board for Inovio and on a scientific advisory board for Regeneron as well as funding to her university for clinical trials from Syndax, Tesaro/GSK, Merck, Mersana, OncoQuest, Morab, PLX, Corcept, Seattle Genetics, Harpoon, Ludwig, Ellipses, K‐Group Beta (ZN‐c3 studies), Constellation, Astellas, Arch Oncology, Pfizer, Lycera, and Seagen. The other author made no disclosures.
REFERENCES
- 1. Cancer Stat Facts: cervical cancer. National Cancer Institute. Accessed July 21, 2022. https://seer.cancer.gov/statfacts/html/cervix.html
- 2. Cervical cancer. World Health Organization. February 22, 2022. Accessed July 21, 2022. https://www.who.int/news‐room/fact‐sheets/detail/cervical‐cancer [Google Scholar]
- 3. Hull R, Mbele M, Makhafola T, et al. Cervical cancer in low and middle‐income countries. Oncol Lett. 2020;20(3):2058‐2074. doi: 10.3892/ol.2020.11754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deshmukh AA, Suk R, Shiels MS, et al. Incidence trends and burden of human papillomavirus–associated cancers among women in the United States, 2001–2017. J Natl Cancer Inst. 2021;113:792‐796. doi: 10.1093/jnci/djaa128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoo W, Kim S, Huh WK, et al. Recent trends in racial and regional disparities in cervical cancer incidence and mortality in United States. PLoS One. 2017;12(2):e0172548. doi: 10.1371/journal.pone.0172548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tewari KS, Sill MW, Long HJ, III , et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734‐743. doi: 10.1056/nejmoa1309748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chung HC, Ros W, Delord JP, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE‐158 study. J Clin Oncol. 2019;37(17):1470‐1478. doi: 10.1200/jco.18.01265 [DOI] [PubMed] [Google Scholar]
- 8. Tewari KS, Monk BJ, Vergote I, et al. Survival with cemiplimab in recurrent cervical cancer. N Engl J Med. 2022;386(6):544‐555. doi: 10.1056/nejmoa2112187 [DOI] [PubMed] [Google Scholar]
- 9. Oaknin A, Monk BJ, Vergote I, et al. EMPOWER CERVICAL‐1: effects of cemiplimab versus chemotherapy on patient‐reported quality of life, functioning and symptoms among women with recurrent cervical cancer. Eur J Cancer. 2022;174:299‐309. doi: 10.1016/j.ejca.2022.03.016 [DOI] [PubMed] [Google Scholar]
- 10. Colombo N, Dubot C, Lorusso D, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385(20):1856‐1867. doi: 10.1056/nejmoa2112435 [DOI] [PubMed] [Google Scholar]
- 11. Update on CALLA phase III trial of concurrent use of Imfinzi and chemoradiotherapy in locally advanced cervical cancer. AstraZeneca. March 24, 2022. Accessed July 21, 2022. https://www.astrazeneca.com/media‐centre/press‐releases/2022/update‐on‐calla‐phase‐iii‐trial‐for‐imfinzi.html [Google Scholar]
- 12. Mayadev J, Zamarin D, Deng W, et al. Safety and immunogenicity of anti PD‐L1 (atezolizumab) given as an immune primer or concurrently with extended field chemoradiotherapy for node positive locally advanced cervical cancer: an NRG Oncology trial. Paper presented at: Annual Meeting on Women’s Cancer for the Society of Gynecologic Oncology; March 18–21, 2022; Phoenix, AZ. [Google Scholar]
- 13. O’Malley DM, Neffa M, Monk BJ, et al. Dual PD‐1 and CTLA‐4 checkpoint blockade using balstilimab and zalifrelimab combination as second‐line treatment for advanced cervical cancer: an open‐label phase II study. J Clin Oncol. 2022;40(7):762‐771. doi: 10.1200/jco.21.02067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. FDA grants accelerated approval to tisotumab vedotin‐tftv for recurrent or metastatic cervical cancer. US Food and Drug Administration. September 21, 2021. Accessed July 21, 2022. https://www.fda.gov/drugs/resources‐information‐approved‐drugs/fda‐grants‐accelerated‐approval‐tisotumab‐vedotin‐tftv‐recurrent‐or‐metastatic‐cervical‐cancer [Google Scholar]
- 15. Coleman RL, Lorusso D, Gennigens C, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG‐3023/ENGOT‐cx6): a multicentre, open‐label, single‐arm, phase 2 study. Lancet Oncol. 2021;22(5):609‐619. doi: 10.1016/s1470-2045(21)00056-5 [DOI] [PubMed] [Google Scholar]
- 16. Vergote I, Monk BJ, O’Cearbhaill RE, et al. Tisotumab vedotin (TV) + carboplatin (Carbo) in first‐line (1L) or + pembrolizumab (Pembro) in previously treated (2L/3L) recurrent or metastatic cervical cancer (r/mCC): interim results of ENGOT‐Cx8/GOG‐3024/innovaTV 205 study. Ann Oncol. 2021;32(suppl 5):S726‐S727. doi: 10.1016/j.annonc.2021.08.1166 [DOI] [Google Scholar]
- 17. Lorusso D, Vergote I, O’Cearbhaill RE, et al. Tisotumab vedotin (TV) + pembrolizumab (pembro) in first‐line (1L) recurrent or metastatic cervical cancer (r/mCC): interim results of ENGOT Cx8/GOG 3024/innovaTV 205. J Clin Oncol. 2022;16(suppl):5507. doi: 10.1200/jco.2022.40.16_suppl.5507 [DOI] [Google Scholar]
- 18. Jazaeri AA, Zsiros E, Amaria RN, et al. Safety and efficacy of adoptive cell transfer using autologous tumor infiltrating lymphocytes (LN‐145) for treatment of recurrent, metastatic, or persistent cervical carcinoma. J Clin Oncol. 2019;15(suppl):2538. doi: 10.1200/jco.2019.37.15_suppl.2538 [DOI] [Google Scholar]
- 19. O’Malley D, Lee S, Psyrri A, et al. Phase 2 efficacy and safety of autologous tumor‐infiltrating lymphocyte (TIL) cell therapy in combination with pembrolizumab in immune checkpoint inhibitor–naïve patients with advanced cancers. J Immunother Cancer. 2021;9(suppl 2):492. doi: 10.1136/jitc-2021-sitc2021.492 [DOI] [Google Scholar]
- 20. Mileshkin LR, Moore KN, Barnes E, et al. Adjuvant chemotherapy following chemoradiation as primary treatment for locally advanced cervical cancer compared to chemoradiation alone: the randomized phase III OUTBACK trial (ANZGOG 0902, RTOG 1174, NRG 0274). J Clin Oncol. 2021;39(18)(suppl):LBA3. doi: 10.1200/jco.2021.39.15_suppl.lba3 [DOI] [Google Scholar]
- 21. Yu L, Sabatino SA, White MC. Rural–urban and racial/ethnic disparities in invasive cervical cancer incidence in the United States, 2010–2014. Prev Chronic Dis. 2019;16:E70. doi: 10.5888/pcd16.180447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aviki EM, Manning‐Geist BL, Sokolowski SS, et al. Risk factors for financial toxicity in patients with gynecologic cancer. Am J Obstet Gynecol. 2022;226(6):817.e1‐817.e9. doi: 10.1016/j.ajog.2021.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aviki EM, Thom B, Braxton K, et al. Patient‐reported benefit from proposed interventions to reduce financial toxicity during cancer treatment. Support Care Cancer. 2022;30(3):2713‐2721. doi: 10.1007/s00520-021-06697-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. FDA takes important steps to increase racial and ethnic diversity in clinical trials. US Food and Drug Administration. April 13, 2022. Accessed July 21, 2022. https://www.fda.gov/news‐events/press‐announcements/fda‐takes‐important‐steps‐increase‐racial‐and‐ethnic‐diversity‐clinical‐trials [Google Scholar]
- 25. Niranjan SJ, Martin MY, Fouad MN, et al. Bias and stereotyping among research and clinical professionals: perspectives on minority recruitment for oncology clinical trials. Cancer. 2020;126(9):1958‐1968. doi: 10.1002/cncr.32755 [DOI] [PubMed] [Google Scholar]
- 26. Estimated cumulative risk of mortality in 2020, cervix uteri, females, ages 0–74. International Agency for Research on Cancer. Accessed July 31, 2022. https://gis.cdc.gov/Cancer/USCS/#/AtAGlance [Google Scholar]
- 27. Cancer statistics at a glance. Centers for Disease Control and Prevention. Accessed July 31, 2022. https://gis.cdc.gov/Cancer/USCS/%23/AtAGlance/ [Google Scholar]


