In the past 5 years, work focused on seizures in neural antibody‐associated disease has led to dramatic advancements in this field. One such advancement has been the development of predictive scores like the Antibody Prevalence in Epilepsy and Encephalopathy (APE2) score, which help determine which patients with unexplained seizures would benefit from neural antibody testing to diagnose an immune etiology, or “autoimmune epilepsy.” 1 , 2 , 3 , 4 , 5 Another advancement has been the proposal by the International League Against Epilepsy Autoimmunity and Inflammation Taskforce to replace the term “autoimmune epilepsy” with “acute symptomatic seizures secondary to autoimmune encephalitis” and “autoimmune‐associated epilepsy,” which better reflect whether an enduring predisposition to seizures (i.e., epilepsy) is present. 6 In an effort to further advance this field, it has recently been highlighted that a predictive score would be of particular value if it were able to effectively identify neural antibody‐associated disease among patients with seizures who do not have features suspicious for neural antibody positivity, such as broader symptoms of encephalitis. 7 A 2021 systematic review of neural antibody frequency in epilepsy used the term “autoimmune‐associated epilepsy without encephalitis” to describe this patient population. 8 Although well intentioned, this term has the potential to generate misunderstanding regarding the relationship between autoimmune‐associated epilepsy and encephalitis, as well as the degree of overlap between broader symptoms of encephalitis and other features suspicious for neural antibody positivity, which we aim to clarify herein.
1. THE INTRODUCTION OF NEW TERMS: “ACUTE SYMPTOMATIC SEIZURES SECONDARY TO AUTOIMMUNE ENCEPHALITIS” AND “AUTOIMMUNE (ENCEPHALITIS)‐ASSOCIATED EPILEPSY”
For patients with seizures caused by inflammation of the brain that resolve following immunotherapy, the term “acute symptomatic seizures secondary to autoimmune encephalitis” has been proposed. 6 In these patients, inflammation is both directly responsible for seizures and responsive to treatment, indicating a reversible provoking factor that supports use of the term “acute symptomatic seizures” rather than “epilepsy.” The classic example of acute symptomatic seizures secondary to autoimmune encephalitis is seizures mediated by neural antibodies against extracellular targets (e.g., anti‐N‐methyl‐D‐aspartate receptor, anti‐leucine‐rich glioma‐inactivated 1 [LGI1]) that typically respond well to immunotherapy, particularly when promptly diagnosed and treated. 9 , 10 , 11 Meanwhile, for patients with seizures caused by inflammation of the brain that persist despite an adequate trial of immunotherapy, the term “autoimmune‐associated epilepsy” has been proposed. 6 In these patients, inflammation may be either directly or indirectly responsible for seizures; it could be directly causative but poorly responsive to immunotherapy as may be seen with cytotoxic T‐cell‐mediated pathology, indirectly causative through secondary processes such as structural injury, or a combination of both. Regardless of whether the mechanism is direct or indirect, the effect of inflammation in these patients is irreversible and results in an enduring predisposition to seizures that is compatible with the traditional definition of epilepsy. 12 As discussed later on, the modified term “autoimmune encephalitis‐associated epilepsy” may be helpful in making explicit that epilepsy is linked to inflammation of the brain (i.e., encephalitis) in all cases. The classic example of autoimmune encephalitis‐associated epilepsy is seizures associated with neural antibodies against intracellular targets (e.g. anti‐glutamic acid decarboxylase 65, most high‐risk paraneoplastic antibodies) that typically respond poorly to immunotherapy, which may reflect cytotoxic T‐cell‐mediated pathology and/or subsequent structural injury. 13 , 14 , 15 , 16 , 17 , 18 Of note, although the focus of this commentary is seizures in neural antibody‐associated disease, the terms described above can also be applied to seizures in other neuroinflammatory diseases. As an example, in a patient with Rasmussen encephalitis (a disease that has not clearly been shown to associate with neural antibodies of clinical significance, but in which cytotoxic T cells seem to play a prominent role), the persistence of focal motor seizures despite immunotherapy may appropriately be referred to as autoimmune encephalitis‐associated epilepsy. 19 , 20 , 21
2. THE EVOLUTION OF PREDICTIVE SCORES, AND THE SEARCH FOR PATIENTS WHO HAVE SEIZURES “WITHOUT SUSPICION OF ENCEPHALITIS” THAT WOULD BENEFIT FROM NEURAL ANTIBODY TESTING
Numerous predictive scores have been developed to help select which patients with unexplained seizures would benefit from neural antibody testing to diagnose an autoimmune cause. However, such scores contain items that overlap substantially with preexisting diagnostic criteria for autoimmune encephalitis, raising questions surrounding their additive clinical utility. 22 , 23 Intuitively, a predictive score that would be of most use to the clinician is one that could help identify which patients without features suspicious for neural antibody positivity would benefit from testing. This was the aim of the Antibodies Contributing to Focal Epilepsy Signs and Symptoms (ACES) score, which was intended to be developed in a cohort of patients who had focal seizures of unknown etiology “without suspicion of encephalitis.” 7 However, virtually all patients enrolled in this study who were ultimately diagnosed with neural antibody‐associated disease had features that were, in retrospect, suspicious for neural antibody positivity. In addition to broader symptoms of encephalitis (e.g., cognitive symptoms, behavioral changes), other suspicious features included peripheral nervous system manifestations (e.g., peripheral nerve hyperexcitability) and characteristic seizure semiologies (e.g., faciobrachial dystonic seizures). 7 , 24 , 25 , 26 , 27 , 28 , 29 Due to lack of recognition of these features being suspicious for neural antibody‐associated disease at time of patient enrollment in this study, the clinical utility of predictive scores remained unclear. Another recent study comparing use of predictive scores to only performing neural antibody testing in patients with seizures of unknown etiology who had features that were “obviously” indicative of possible neural antibody positivity found that predictive scores conferred no diagnostic advantage, adding to uncertainty surrounding the role of currently available scores in clinical practice. 30
3. THE EMERGENCE OF THE TERM “AUTOIMMUNE‐ASSOCIATED EPILEPSY WITHOUT ENCEPHALITIS” AND ITS POTENTIAL TO GENERATE MISUNDERSTANDING
In 2021, a systematic review sought to determine neural antibody positivity rates across studies of patients such as those intended to be recruited in the ACES study (i.e., those with seizures of unknown etiology who did not have features suspicious for neural antibody positivity recognized). 8 These patients, who may not be captured by predictive scores that preceded ACES such as APE2, were referred to as possibly having “autoimmune‐associated epilepsy without encephalitis.” 8 This term would seem to refer to patients with neural antibody‐associated disease who have seizures without disease features, such as broader symptoms of encephalitis, that are suspicious for neural antibody positivity. However, it has the potential to be problematic for several reasons. First, as discussed earlier, inflammation of the brain (i.e., encephalitis) is responsible, either directly or indirectly, for the development of autoimmune‐associated epilepsy in all cases, rendering the term “autoimmune‐associated epilepsy without encephalitis” somewhat counterintuitive. This potential for misunderstanding may be avoided by use of the term “autoimmune encephalitis‐associated epilepsy,” which emphasizes that encephalitis and the development of epilepsy are inextricably linked in this condition. Second, use of the phrase “without encephalitis” to refer to patients with neural antibody‐associated disease who have seizures without features suspicious for neural antibody positivity does not fully encompass current knowledge in this field. This is because other disease features that are suspicious for neural antibody positivity, separate from those indicative of a broader encephalitis, have been uncovered in recent years. A prime example of this is characteristic seizure semiologies such as faciobrachial dystonic seizures, which may occur in patients with anti‐LGI1‐mediated seizures before the development of broader symptoms of encephalitis. 31 Although such patients may present with faciobrachial dystonic seizures in isolation, this seizure semiology is virtually pathognomonic for neural antibody positivity and thus renders the need for testing immediately apparent. Third, patients with antibody‐mediated seizures who present early in their disease course, prior to developing broader symptoms of encephalitis, may promptly respond to immunotherapy, indicating acute symptomatic seizures; the term “autoimmune‐associated epilepsy without encephalitis” does not capture these patients, and the term “acute symptomatic seizures secondary to autoimmune encephalitis without encephalitis” would seem paradoxical, highlighting the need for clarification.
4. A CLARIFICATION OF TERMS: “ACUTE SYMPTOMATIC SEIZURES SECONDARY TO AUTOIMMUNE ENCEPHALITIS” AND “AUTOIMMUNE ENCEPHALITIS‐ASSOCIATED EPILEPSY,” AS WELL AS “SEIZURES WITH OR WITHOUT FEATURES SUSPICIOUS FOR NEURAL ANTIBODY POSITIVITY”
“Acute symptomatic seizures secondary to autoimmune encephalitis” and “autoimmune encephalitis‐associated epilepsy,” as well as “seizures with or without features suspicious for neural antibody positivity,” reflect distinct yet overlapping terms that can be used to describe seizures in neural antibody‐associated disease. This is depicted via Venn diagram in Figure 1. Importantly, the most appropriate terminology for a given patient can change over time. As an example, a patient may present with acute symptomatic seizures secondary to autoimmune encephalitis that resolve after immunotherapy, followed by disease relapse causing structural injury and autoimmune encephalitis‐associated epilepsy. A patient may also present with seizures in the absence of features suspicious for neural antibody positivity, but go on to develop suspicious features later in their disease course. Furthermore, it should be acknowledged that distinguishing between features suspicious for neural antibody positivity and those common among patients with epilepsy (e.g., cognitive or psychiatric symptoms) can be exceptionally challenging, and the ability to determine whether a disease feature is suspicious for neural antibody positivity may improve as more sophisticated clinical tools to help make these distinctions are developed. 30 , 32 Despite this dynamic aspect to terminology, its accurate use at the time of enrolling patients with seizures in studies of neural antibody testing and prevalence is critical to determining applicability of findings to patients encountered in clinical practice.
FIGURE 1.
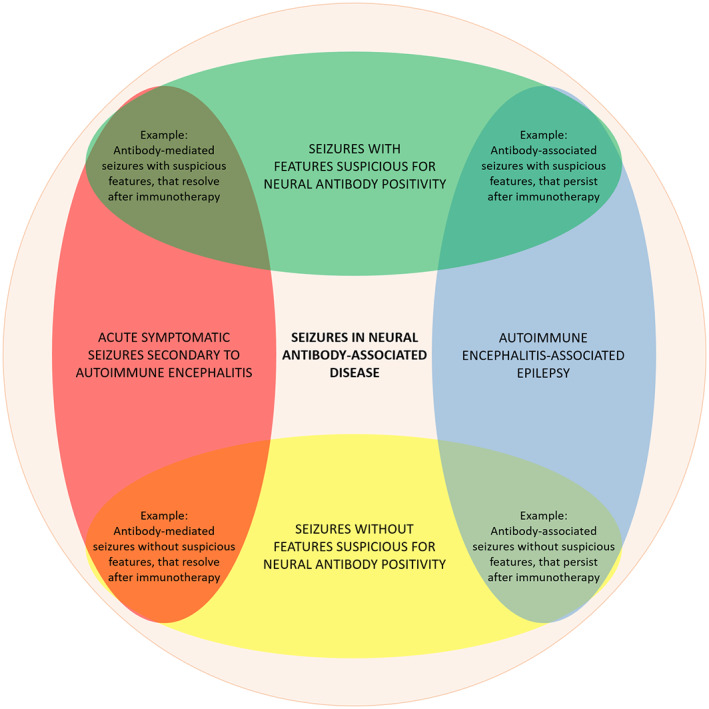
Venn diagram depicting the overlap between “acute symptomatic seizures secondary to autoimmune encephalitis,” “autoimmune encephalitis‐associated epilepsy,” and “seizures with or without features suspicious for neural antibody positivity.”
5. CONCLUSIONS
As our knowledge of seizures in neural antibody‐associated disease advances, precise use of terminology becomes essential for this knowledge to be effectively translated to clinical research and patient care. The terms “acute symptomatic seizures secondary to autoimmune encephalitis” and “autoimmune encephalitis‐associated epilepsy,” as well as “seizures with or without features suspicious for neural antibody positivity,” aid in describing the various patient disease states that may be encountered. These states are dynamic, and so the most appropriate terminology for a given patient may change as their clinical course evolves, or as breakthroughs in the field of autoimmune neurology are made (e.g., the identification of new therapeutics for cytotoxic T‐cell‐mediated pathology, or new features suspicious for neural antibody positivity). As scientific discoveries related to seizures in neural antibody‐associated disease continue, placing emphasis on accurateness of terminology ensures that its use helps rather than hinders progress in this field.
AUTHOR CONTRIBUTIONS
Adrian Budhram: Conceptualization, writing–original draft. Jorge G. Burneo: Conceptualization, writing–review & editing.
ACKNOWLEDGMENTS
A.B. holds the London Health Sciences Centre and London Health Sciences Foundation Chair in Neural Antibody Testing for Neuro‐Inflammatory Diseases, and receives support from the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. J.G.B. holds the Jack Cowin Endowed Chair in Epilepsy Research at Western University.
Budhram A, Burneo JG. Acute symptomatic seizures, epilepsy, and autoimmune encephalitis: Clarifying terminology in neural antibody‐associated disease. Epilepsia. 2023;64:306–310. 10.1111/epi.17478
The authors have not published, posted, or submitted any related manuscripts from the same study.
REFERENCES
- 1. Dubey D, Alqallaf A, Hays R, Freeman M, Chen K, Ding K, et al. Neurological autoantibody prevalence in epilepsy of unknown etiology. JAMA Neurol. 2017;74(4):397–402. [DOI] [PubMed] [Google Scholar]
- 2. Dubey D, Singh J, Britton JW, Pittock SJ, Flanagan EP, Lennon VA, et al. Predictive models in the diagnosis and treatment of autoimmune epilepsy. Epilepsia. 2017;58(7):1181–9. [DOI] [PubMed] [Google Scholar]
- 3. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dubey D, Kothapalli N, McKeon A, Flanagan EP, Lennon VA, Klein CJ, et al. Predictors of neural‐specific autoantibodies and immunotherapy response in patients with cognitive dysfunction. J Neuroimmunol. 2018;323:62–72. [DOI] [PubMed] [Google Scholar]
- 5. Dubey D, Pittock SJ, McKeon A. Antibody prevalence in epilepsy and encephalopathy score: increased specificity and applicability. Epilepsia. 2019;60(2):367–9. [DOI] [PubMed] [Google Scholar]
- 6. Steriade C, Britton J, Dale RC, Gadoth A, Irani SR, Linnoila J, et al. Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune‐associated epilepsy: conceptual definitions. Epilepsia. 2020;61(7):1341–51. [DOI] [PubMed] [Google Scholar]
- 7. de Bruijn M, Bastiaansen AEM, Mojzisova H, van Sonderen A, Thijs RD, Majoie MJM, et al. Antibodies contributing to focal epilepsy signs and symptoms score. Ann Neurol. 2021;89(4):698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steriade C, Gillinder L, Rickett K, Hartel G, Higdon L, Britton J, et al. Discerning the role of autoimmunity and autoantibodies in epilepsy: a review. JAMA Neurol. 2021;78:1383–90. [DOI] [PubMed] [Google Scholar]
- 9. de Bruijn MAAM, van Sonderen A, van Coevorden‐Hameete MH, Bastiaansen AEM, Schreurs MWJ, Rouhl RPW, et al. Evaluation of seizure treatment in anti‐LGI1, anti‐NMDAR, and anti‐GABABR encephalitis. Neurology. 2019;92(19):e2185–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shen CH, Fang GL, Yang F, Cai MT, Zheng Y, Fang W, et al. Seizures and risk of epilepsy in anti‐NMDAR, anti‐LGI1, and anti‐GABA(B) R encephalitis. Ann Clin Transl Neurol. 2020;7(8):1392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu X, Guo K, Lin J, Gong X, Li A, Zhou D, et al. Long‐term seizure outcomes in patients with autoimmune encephalitis: a prospective observational registry study update. Epilepsia. 2022;63(7):1812–21. [DOI] [PubMed] [Google Scholar]
- 12. Fisher RS, van Emde BW, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the international league against epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46(4):470–2. [DOI] [PubMed] [Google Scholar]
- 13. Bien CG, Vincent A, Barnett MH, Becker AJ, Blümcke I, Graus F, et al. Immunopathology of autoantibody‐associated encephalitides: clues for pathogenesis. Brain. 2012;135(Pt 5):1622–38. [DOI] [PubMed] [Google Scholar]
- 14. Flanagan EP. Paraneoplastic disorders of the nervous system. Continuum (Minneap Minn). 2020;26(6):1602–28. [DOI] [PubMed] [Google Scholar]
- 15. Joubert B, Belbezier A, Haesebaert J, Rheims S, Ducray F, Picard G, et al. Long‐term outcomes in temporal lobe epilepsy with glutamate decarboxylase antibodies. J Neurol. 2020;267(7):2083–9. [DOI] [PubMed] [Google Scholar]
- 16. Budhram A, Sechi E, Flanagan EP, Dubey D, Zekeridou A, Shah SS, et al. Clinical spectrum of high‐titre GAD65 antibodies. J Neurol Neurosurg Psychiatry. 2021;92(6):645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graus F, Vogrig A, Muñiz‐Castrillo S, Antoine JG, Desestret V, Dubey D, et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tröscher AR, Mair KM, de Juan LV, Köck U, Steinmaurer A, Baier H, et al. Temporal lobe epilepsy with GAD antibodies: neurons killed by T cells not by complement membrane attack complex. Brain. 2022. 10.1093/brain/awac404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bien CG, Bauer J, Deckwerth TL, Wiendl H, Deckert M, Wiestler OD, et al. Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in Rasmussen's encephalitis. Ann Neurol. 2002;51(3):311–8. [DOI] [PubMed] [Google Scholar]
- 20. Varadkar S, Bien CG, Kruse CA, Jensen FE, Bauer J, Pardo CA, et al. Rasmussen's encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol. 2014;13(2):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spatola M, Dalmau J. Seizures and risk of epilepsy in autoimmune and other inflammatory encephalitis. Curr Opin Neurol. 2017;30(3):345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Geis C, Planagumà J, Carreño M, Graus F, Dalmau J. Autoimmune seizures and epilepsy. J Clin Invest. 2019;129(3):926–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Irani SR, Michell AW, Lang B, Pettingill P, Waters P, Johnson MR, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69(5):892–900. [DOI] [PubMed] [Google Scholar]
- 25. van Sonderen A, Ariño H, Petit‐Pedrol M, Leypoldt F, Körtvélyessy P, Wandinger KP, et al. The clinical spectrum of Caspr2 antibody‐associated disease. Neurology. 2016;87(5):521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gadoth A, Pittock SJ, Dubey D, McKeon A, Britton JW, Schmeling JE, et al. Expanded phenotypes and outcomes among 256 LGI1/CASPR2‐IgG‐positive patients. Ann Neurol. 2017;82(1):79–92. [DOI] [PubMed] [Google Scholar]
- 27. Smith KM, Zalewski NL, Budhram A, Britton JW, So E, Cascino GD, et al. Musicogenic epilepsy: expanding the spectrum of glutamic acid decarboxylase 65 neurological autoimmunity. Epilepsia. 2021;62(5):e76–81. [DOI] [PubMed] [Google Scholar]
- 28. Garrido Sanabria ER, Zahid A, Britton J, Kraus GJ, López‐Chiriboga AS, Zekeridou A, et al. CASPR2‐IgG‐associated autoimmune seizures. Epilepsia. 2022;63(3):709–22. [DOI] [PubMed] [Google Scholar]
- 29. Kaaden T, Madlener M, Angstwurm K, Bien CG, Bogarin Y, Doppler K, et al. Seizure semiology in antibody‐associated autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm. 2022;9(6):e200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang YC, Nouri MN, Mirsattari S, Burneo JG, Budhram A. “obvious” indications for neural antibody testing in epilepsy or seizures: the ONES checklist. Epilepsia. 2022;63(7):1658–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thompson J, Bi M, Murchison AG, Makuch M, Bien CG, Chu K, et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain. 2018;141(2):348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steriade C. The search for autoimmune‐associated epilepsy continues‐are we getting closer to our target? Epilepsy Curr. 2021;21(4):255–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


