Abstract
Aims
This study aims to evaluate the stability of C‐peptide over time and to compare fasting C‐peptide and C‐peptide response after mixed‐meal tolerance test (MMTT) at T90 or T120 with C‐peptide area under the curve (AUC) in long‐standing type 1 diabetes.
Methods
We included 607 type 1 diabetes individuals with diabetes duration >5 years. C‐peptide concentrations (ultrasensitive assay) were collected in the fasting state, and in a subpopulation after MMTT (T0, just prior to, T30‐T60‐T90‐T120, 30–120 min after ingestion of mixed‐meal) (n = 168). Fasting C‐peptide concentrations (in n = 535) at Year 0 and Year 1 were compared. The clinical determinants associated with residual C‐peptide secretion and the correspondence of C‐peptide at MMTT T90 / T120 and total AUC were assessed.
Results
A total of 153 participants (25%) had detectable fasting serum C‐peptide (i.e ≥ 3.8 pmol/L). Fasting C‐peptide was significantly lower at Year 1 (p < 0.001, effect size = −0.16). Participants with higher fasting C‐peptide had a higher age at diagnosis and shorter disease duration and were less frequently insulin pump users. Overall, 109 of 168 (65%) participants had both non‐detectable fasting and post‐meal serum C‐peptide concentrations. The T90 and T120 C‐peptide values at MMTT were concordant with total AUC. In 17 (10%) individuals, C‐peptide was only detectable at MMTT and not in the fasting state.
Conclusions
Stimulated C‐peptide was detectable in an additional 10% of individuals compared with fasting in individuals with >5 years of diabetes duration. T90 and T120 MMTT measurements showed good concordance with the MMTT total AUC. Overall, there was a decrease of C‐peptide at 1‐year follow‐up.
Keywords: C‐peptide, fasting, insulin‐secreting cells, reproducibility of results, type 1 diabetes
Novelty statement.
What is already known?
Residual and low C‐peptide levels correlate to a lower incidence of complications and fewer episodes of severe hypoglycaemia.
What this study has found?
In long‐standing diabetes, measuring C‐peptide using mixed‐meal tolerance test (MMTT) identified an additional 10% of individuals with detectable C‐peptide than measuring C‐peptide in the fasting state.
An MMTT C‐peptide value at T90 corresponds well with MMTT C‐peptide total response.
What are the implications of the study?
In one of the first large studies with MMTT and fasting ultra‐sensitive C‐peptide measurements in long‐standing diabetes, T90 C‐peptide of MMTT can be used as a proxy for determining beta cell function in individuals with established type 1 diabetes.
1. INTRODUCTION
Type 1 diabetes is an auto‐immune disease characterized by insulin deficiency and the presence of islet cell autoantibodies. It is well established now that not all beta cells are destroyed by this mechanism and that many individuals with type 1 diabetes are still secreting insulin as measured by C‐peptide release in blood and urine. 5 Moreover, early studies showed a relation between C‐peptide reserve and fewer complications and severe hypoglycemia. 1 , 2 Using highly sensitive assays, 3 , 4 it became clear that in some type 1 diabetes individuals, even decades after diagnosis, residual insulin secretion persists. The presence or absence of residual insulin secretion is a biomarker of heterogeneity in type 1 diabetes disease (course). In around 30% of individuals with type 1 diabetes C‐peptide is detectable. 6 , 7 , 8 Having detectable C‐peptide, even at low concentrations, is associated with favourable clinical outcomes and fewer diabetes‐related microvascular complications. 9 , 10 , 11 , 12
C‐peptide can be evaluated by a single measurement in the fasting state, random, or after a standardized stimulus, for instance with a glucagon stimulation test (GST) or with a mixed‐meal tolerance test (MMTT). In the context of clinical trials where relatively high C‐peptide levels can be expected‐ both GST and MMTT are being used and well evidenced. 13 , 14 , 15 , 16 In a head‐to‐head comparison of these two tests in 77 individuals with type 1 diabetes, the reproducibility of the MMTT was slightly better and the MMTT caused fewer side‐effects. 13 The reproducibility of the C‐peptide response after MMTT, calculated as the area under the curve (AUC), was assessed over the course of several weeks, and proved to be very high. 13 However, the MMTT takes 2.5 h to perform, and requires five consecutive measurements of C‐peptide, which poses a considerable burden for the participant, and makes the test laborious and expensive. An easier and cheaper way to reliably assess residual C‐peptide secretion is, therefore, required, for example, a different test or a simplified MMTT. Moreover, measuring C‐peptide in individuals with longer duration type 1 diabetes requires a lower limit of detection (LOD) than measuring C‐peptide in newly‐diagnosed type 1 diabetes individuals. In the ‘Biomarkers of heterogeneity in type 1 diabetes’ project, we attempt to stage type 1 diabetes individuals based on heterogeneity in type 1 diabetes. In the current study, we focus on residual insulin secretion.
In this study, using an ultrasensitive assay, we assess the additional value of the MMTT C‐peptide total AUC compared with a single fasting C‐peptide measurement, C‐peptide at T90 and T120 of MMTT in a population of type 1 diabetes individuals after the honeymoon phase (diabetes duration >5 years). In addition, we repeated C‐peptide measurements after 1 year to evaluate the changes in fasting and meal‐stimulated residual insulin secretion over time.
2. METHODS
2.1. Study design and population
The study population consisted of individuals with a clinical diagnosis of type 1 diabetes, age 16 years and older with a disease duration of >5 years. A clinical diagnosis of type 1 diabetes means that the diagnoses was made by a medical specialist and was based on the guidelines of diagnosing type 1 diabetes that were applicable during the time period at which diagnosis occurred. They participated between 2016 and 2019 in the ‘Biomarkers of heterogeneity in type 1 diabetes’ project. This is a longitudinal study in which a biobank is established through clinical and metabolic phenotyping of individuals with established type 1 diabetes. The aim is to improve disease staging and to identify biomarkers, that reveal the risk and early development of damage and complications. Blood and urine samples were collected at baseline (Year 0), and repeated measurements, including fasting C‐peptide, took place approximately 1 year later (Year 1). Patient characteristics were extracted from the electronic patient management systems of the participating centers. The Biomarker Study is a collaboration between Diabeter, the University Medical Center Groningen (UMCG), Haaglanden Medical Center and Ikazia hospital, The Netherlands (Clinicaltrials.gov/NCT04977635). The project was approved by the Medical Ethics Review Board of the UMCG (METC 2015/493).
Figure S1 shows the flow diagram of participants. A total of 611 participants were recruited, provided written informed consent and were followed prospectively. Of these, we recruited 168 participants for additional metabolic testing comprising two MMTTs, one at Year 0 and one at Year 1. At study entrance participants were asked if they were interested in taking part in this additional sub‐study that was focussed on residual insulin secretion requiring them to visit the hospital/clinic in the fasting state for two additional visits for a mixed‐meal test. Participants were invited for this sub‐study without foreknowledge of their C‐peptide status. In total, 607 individuals had fasting C‐peptide measured at Year 0 and in 535 of them C‐peptide was measured again at Year 1. In addition, 168 individuals underwent an MMTT at Year 0 and in 102 of these individuals, the MMTT was repeated at Year 1.
2.2. MMTT
MMTTs were performed according to a protocol previously described. 13 Before the start of the MMTT fasting blood glucose was tested, the MMTT was only performed when the capillary glucose value was between 3.3 and 12.0 mmol/L. Blood was collected through an intravenously‐inserted line, just prior to (T0) and at 30, 60, 90 and 120 min (T30, T60, T90, T120, respectively) after ingestion of the mixed‐meal (T0). The participants received 6 ml/kg of the liquid mixed‐meal (Resource® protein, Nestlé) with a maximum of 360 ml, which in total contains 450 kcal (50 g carbohydrate, 13 g fat and 34 g protein). After clotting and centrifugation, serum samples were frozen at −80°C until analysis.
2.3. Glucose and C‐peptide measurement
Glucose was measured with a hexokinase method. C‐peptide was measured by the IRMA (Beckman Coulter, cat. no. IM3639, distributed by IMMUNOTECH s.r.o., Prague, Czech Republic). 4 The limit of quantification of this ultrasensitive assay was 3.8 pmol/L, and interassay coefficient of variation (c.v.) was 9.1% at 6.5 pmol/L.
2.4. Statistical analyses
Descriptive data were summarized as median and interquartile range [IQR], and n (%) for ordinal/categorical data. Results were illustrated using scatterplots or boxplots and paired data were connected by lines. Where appropriate, the axes of plots were on a 10log scale to improve visualisation of the results. Clinical determinants of both fasting C‐peptide and C‐peptide AUC were assessed using Tobit regression analyses. Variables that were associated with the outcome univariately with a p < 0.1 were selected for the multivariable analysis.
The ranking of the Year 0 and Year 1 fasting C‐peptide concentrations were compared with the nonparametric Wilcoxon‐signed‐rank test for paired samples. The effect size was calculated as the standardized Z‐score and indicates the shift in the ranks of C‐peptide concentrations over time. The same test was used to compare MMTT C‐peptide production (AUC) in Year 0 and Year. Total AUC was calculated using five MMTT measurements of C‐peptide (pmol/L) over the total test duration of 120 minutes using the trapezoidal rule. In addition, the timepoint at which the C‐peptide concentration was at its peak (peak C‐peptide) was assessed. A C‐peptide response at MMTT was categorised as a C‐peptide value at 30, 60, 90 or 120 min (T30‐120) after ingestion of mixed‐meal shortly after T0, being higher than the C‐peptide concentration at T0.
The significance level was set at p < 0.05 (two‐sided). Missing data were ignored, except for missing C‐peptide data from the MMTT, which were imputed. T120 was imputed censored (<3.8 pmol/L) if all preceding C‐peptide measurements were censored (<3.8 pmol/L), T60‐90 were imputed by taking the mean of the two flanking C‐peptide measurements. In this way, the imputed value does not influence the AUC calculation. Analyses were performed with R version 4.1 and Integrated Development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com/ 17 using the ‘rstatix’ package for statistical analyses. 18
3. RESULTS
3.1. Baseline characteristics and fasting serum C‐peptide
Table 1 shows the baseline characteristics of the participants. Of the total cohort of 607 participants, median [IQR] age was 31.6 [23.1–52.3], median age at diagnosis was 12.2 [8.0–20.4] and diabetes duration was 18.5 [11.8–29.9] years. Of these, 59% were women and 61% were insulin pump users, the other participants used multiple daily insulin injections. In total, 153 participants (25%) had detectable fasting C‐peptide ≥3.8 pmol/L. Overall, fasting C‐peptide was lower with longer duration of diabetes (Figure S2, Table S1). Participants with higher fasting C‐peptide also had a significantly higher age at diagnosis, shorter disease duration and were more frequently insulin pump users (Table S1).
TABLE 1.
Baseline characteristics of the study participants
| All participants (n = 607) | Participants with MMTT at Year 0 (n = 168) | |
|---|---|---|
| Women, n (%) | 359 (59) | 98 (58) |
| Age, years | 31.6 [23.1, 52.3] | 26.1 [21.5, 47.1] |
| Age at diagnosis, years | 12.2 [7.90, 20.2] | 11.5 [7.9, 16.7] |
| Diabetes duration, years | 18.5 [11.8, 29.9] | 17.5 [10.3, 27.8] |
| Body mass index (BMI), kg/m2 | 25.1 [23.0, 27.7] | 25.1 [23.1, 27.4] |
| Total insulin dose, U/day | 50 [40, 65] | 52 [42, 64] |
| Pump users, n (%) | 366 (61) | 113 (68) |
| Detectable fasting serum C‐peptide, n (%) | 153 (25) | 42 (25) |
| Fasting serum C‐peptide, pmol/L | 0.0 [0.0, 3.9] | 0.00 [0.00, 0.98] |
| Detectable C‐peptide* after MMTT, n (%) | N.A. | 56 (33) |
Notes: Data are presented as median and interquartile range [IQR], or n (%). Number of missing values in all participants: BMI – 42, Daily insulin dose – 19, Pump use (or MDI) – 7, all other covariates no missing values. Number of missing values in MMTT subgroup: BMI – 5, Daily insulin dose – 4, Pump use (or MDI) – 1, all other covariates no missing values.
Abbreviation: NA, not applicable.
Detectable C‐peptide at least at one timepoint after mixed‐meal tolerance test (MMTT).
3.2. Fasting serum C‐peptide stability over 1 year
Repeated fasting C‐peptide measurements were available in 535 of 607 participants, 118 participants (22.1%) had fasting C‐peptide ≥3.8 pmol/L 1 year later. A total of 417 participants had an undetectable C‐peptide at Year 1, of these 26 (4.9%) had a detectable C‐peptide 1 year earlier (Table 2). Figure 1 shows the variability of fasting C‐peptide over time in the individuals with repeated measurements and detectable fasting C‐peptide at either Year 0 or Year 1 (n = 144). In total, 70% (n = 101) of the individuals had a lower fasting C‐peptide at Year 1 compared with Year 0. Overall, fasting C‐peptide levels were significantly lower at Year 1 (paired Wilcoxon‐signed‐rank p < 0.001, effect size = −0.16).
TABLE 2.
Fasting serum C‐peptide at Year 0 and Year 1
| Year 1 | ||||||
|---|---|---|---|---|---|---|
| <3.8 pmol/L | ≥3.8 pmol/L decreased | ≥3.8 pmol/L increased | Not measured | Total | ||
| Year 0 | <3.8 pmol/L | 391 | N.A. | 9 | 54 | 454 |
| ≥3.8 pmol/L | 26 | 75 | 34 | 18 | 153 | |
| 607 | ||||||
FIGURE 1.
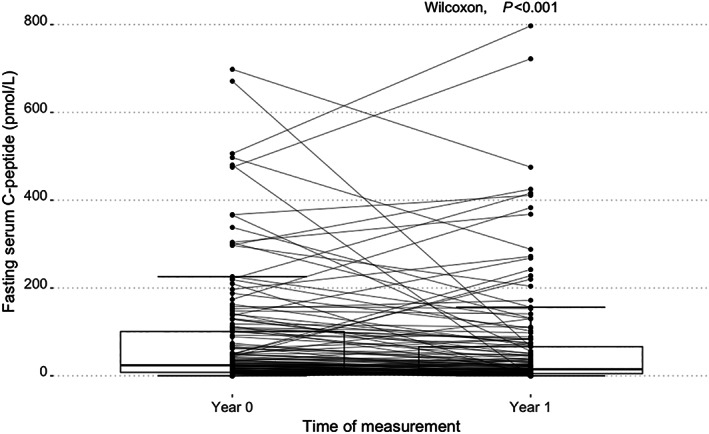
Boxplots of fasting serum C‐peptide of Year 0 and Year 1 of participants with detectable C‐peptide (n = 144). The measurements at Year 0 and Year 1 from individuals with both measurements available are connected by lines.
3.3. C‐peptide at fasting and after the mixed‐meal tolerance test
Of the 168 participants who underwent a baseline MMTT, 109 (65%) had both non‐detectable fasting and post‐meal C‐peptide concentrations, while 17 (10%) had non‐detectable fasting C‐peptide combined with an increase of C‐peptide to detectable concentrations during the MMTT (Table S2). Thus, 42 (25%) participants of the MMTT Year 0 group had detectable fasting C‐peptide. In five of these individuals, there was no increase of the C‐peptide at MMTT compared with the fasting C‐peptide concentration. In these six individuals, fasting C‐peptide concentration was near the detection limit (3.9–5.5 pmol/L).
The time course of the C‐peptide concentration in all 54 participants who showed an increase of C‐peptide during the first MMTT are depicted in Figure 2. The majority of them had a peak C‐peptide during MMTT at T120 (n = 26) and T90 (n = 20). One individual had similar C‐peptide levels at T90 and T120. Seven participants had the highest C‐peptide concentration measured at T30 or T60. Two individuals did not show a response to the MMTT and had a similar C‐peptide concentration during all timepoints of the MMTT, and not exceeding the concentration at serum C‐peptide measured at T0. A higher total AUC, T90 or T120 C‐peptide was associated with a higher fasting C‐peptide (Figure S3). Participants with higher C‐peptide response at MMTT had a shorter diabetes duration and higher age at diagnosis, a lower BMI and were more frequently insulin pump users (Table S3).
FIGURE 2.
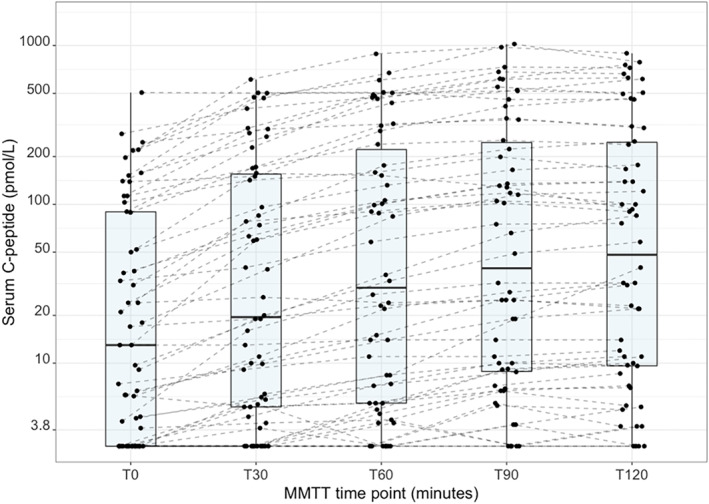
C‐peptide response at mixed‐meal tolerance test at Year 0 (n = 54). In this subpopulation of individuals with C‐peptide response to a mixed‐meal (n = 54) median [IQR] age was 25.1 [21.6, 48.2] years and diabetes duration was 10.2 [7.7, 22.1] years; 56% were women. C‐peptide was undetectable in n = 15 T0, n = 10 T30, n = 7 T60, n = 5 T90, n = 4 T120.
3.4. Mixed‐meal tolerance test C‐peptide stability over 1 year
Figure 3 shows the C‐peptide response during the first and the second MMTT in those with detectable C‐peptide. In total, 57 individuals showed no C‐peptide response in either test, eight had a detectable C‐peptide response during the first test but no response during the second test, while 35 had a C‐peptide response in both tests (Table 3). Two individuals had no response during the first MMTT but did have a response at the second test. There was a significant overall decrease of C‐peptide AUC after 1 year in the group with detectable C‐peptide response (n = 57, paired Wilcoxon‐signed‐rank p < 0.001, effect size = −0.35). Figure 4a,b display the concordance of C‐peptide measurements at T90 and T120 with MMTT total AUC.
FIGURE 3.
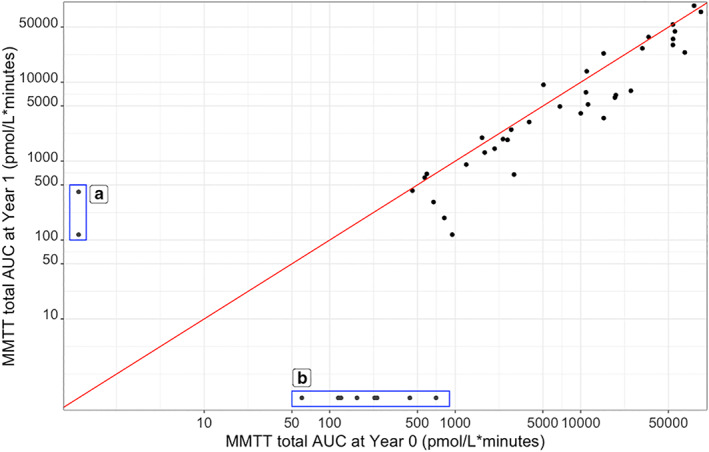
C‐peptide AUC during the mixed‐meal tolerance test (MMTT) at Year 0 and Year 1 (n = 45) of those with detectable C‐peptide at MMTT. (a) Undetectable C‐peptide at first MMTT and detectable response at second MMTT (n = 2). (b) C‐peptide response at first MMTT and undetectable C‐peptide at second MMTT (n = 8). The diagonal red line represents MMTT total AUC at Year 0 = MMTT total AUC at Year 1.
TABLE 3.
C‐peptide during MMTT at Year 0 and Year 1
| Year 1 | ||||
|---|---|---|---|---|
| Undetectable | Detectable | Total | ||
| Year 0 | Undetectable | 57 | 2 | 59 |
| Detectable | 8 | 35 | 43 | |
| 102 | ||||
FIGURE 4.
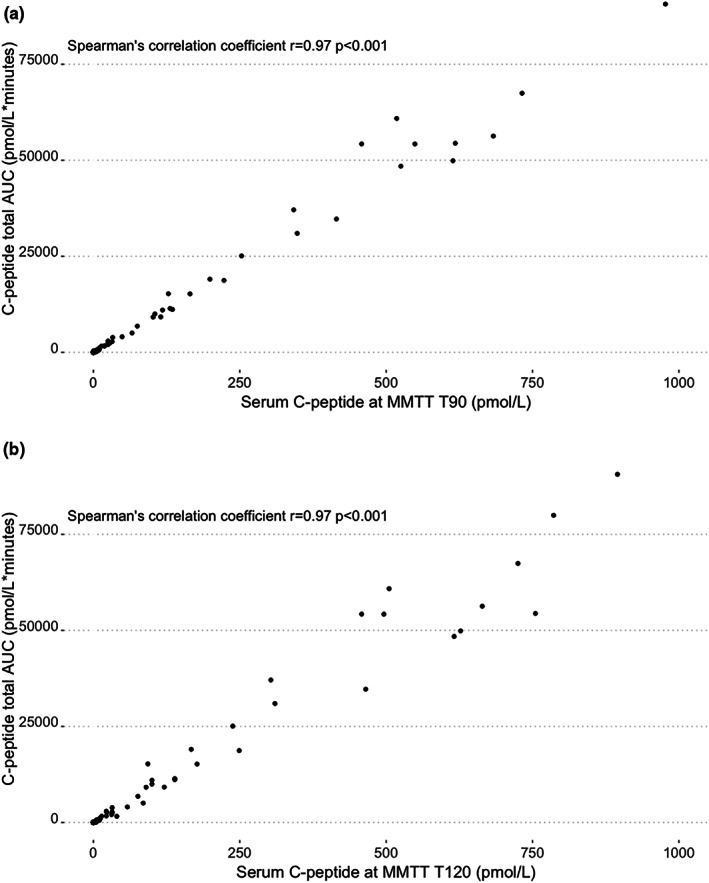
Relationship between C‐peptide total AUC and serum C‐peptide at T90 or T120 during MMTT in n = 56 with a C‐peptide response at MMTT (a) C‐peptide at MMTT T90 and total AUC n = 5 with undetectable C‐peptide at T90 (b) C‐peptide at MMTT T120 and total AUC n = 4 with undetectable C‐peptide at T120.
3.5. Plasma glucose response to mixed‐meal tolerance test
Mean increase of plasma glucose at MMTT was 12.2 (±3.8) mmol/L. The mean increase of glucose was significantly lower with increased C‐peptide response (B = −0.006, SE = 0.002, p = 0.007). In those who switched from having a C‐peptide response at Year 0 MMTT to no response at Year 1 (n = 8), six had a higher glucose peak at MMTT at Year 0 compared with Year 1. Vice versa, two participants went from having no C‐peptide response to the MMTT at Year 0 to having a response at Year 1, one had a higher glucose peak at the MMTT at Year 1.
4. DISCUSSION
In this study, we examined residual C‐peptide secretion in a population of individuals with long‐duration type 1 diabetes (median duration 18.5 [11.8, 29.9] years), and using an ultrasensitive C‐peptide assay, we report that one‐third of individuals with long‐standing type 1 diabetes exhibits some degree of residual insulin secretion. Measuring C‐peptide using MMTT identified an additional 10% of individuals with detectable C‐peptide than measuring C‐peptide only in the fasting state. Both the 90 and 120 min timepoint of the MMTT showed very good concordance with the MMTT total AUC. Thus, measuring C‐peptide at T90 of the MMTT provides a simplified approach to identify and accurately quantify residual insulin secretion compared to performing a complete MMTT with five C‐peptide measurements. Finally, the repeated MMTT 1 year later suggested both a gradual decrease of residual insulin secretion over time as well as some potentiation of C‐peptide with higher plasma glucose concentrations.
Reported studies often take a random C‐peptide measurement to estimate the presence of residual insulin secretion in people with recent‐onset type 1 diabetes, potentially because of the convenience of a single simple blood draw and the fact that participants need not travel while being fasted. 9 , 19 , 20 With a random C‐peptide measurement, both the time since the last meal as well the content of this meal may vary considerably among individuals. In the current study, we used a standardized mixed meal as a challenge for C‐peptide secretion. We observed that most participants with detectable fasting C‐peptide showed an increase of C‐peptide at MMTT. In addition, we identified 17 individuals with a C‐peptide increase in whom fasting C‐peptide was not detectable. Moreover, the peak C‐peptide concentrations after MMTT were observed after 90 and/or 120 min. This suggests that the optimum between using a simple single C‐peptide measurement and an optimal quantitative estimate of residual insulin secretion can be achieved by measuring C‐peptide at T90 or T120 of an MMTT. Measuring C‐peptide at T90 has some advantages as it shortens the test duration and reduces the period of hyperglycaemia after the meal. The finding that T90 C‐peptide corresponds very well with MMTT AUC has been reported in the literature previously by Besser et al. (2013). 21 However, they studied young individuals with type 1 diabetes (age < 18 years) and short diabetes duration, varying between 3 months and 6 years.
By taking a single timepoint of C‐peptide measurement at MMTT, some information regarding the acute versus continuous stimulation of beta‐cells is not captured. However, in type 1 diabetes individuals MMTT AUC is used as the gold standard for illustrating the total beta cell secretory capacity for insulin. When a single measurement at T90 is used as a proxy for AUC, the peak C‐peptide level of 25 individuals at 120 minutes is not captured. The mean difference between the C‐peptide at T90 and T120 in individuals who reached their peak C‐peptide at T120 is 16 pmol/L (data not shown). Thus, the T90 C‐peptide concentration can be used as an excellent proxy for MMTT AUC, but if peak C‐peptide is the desired outcome, T90 is a slight underestimation for some individuals.
Since C‐peptide is excreted through urine, it can be argued that urine analysis of C‐peptide can achieve similar results in measuring residual insulin secretion. Urine can be easily collected by a person at home, with the urine sample later delivered in the hospital or sent by mail. Previous studies have demonstrated that urinary C‐peptide corresponds well with serum C‐peptide concentrations. 8 , 22 , 23 , 24 In a future study, we plan to investigate if urinary C‐peptide measurements can also be used as a proxy for residual insulin secretion in a population with long‐standing (>5 years) type 1 diabetes. It should be noted that a reduction of renal function may influence urinary C‐peptide excretion, but also elevate circulating serum C‐peptide concentrations, so both may not adequately reflect true residual insulin secretion. In the present study, four participants had an eGFR <45 ml/min and the highest fasting C‐peptide concentration in these individuals was 84 pmol/L. These individuals did not participate in the MMTT. C‐peptide is mostly cleared by the kidneys; however, to the best of our knowledge, there are no studies that have researched the exact quantitative effect of reduced kidney function on C‐peptide concentration in type 1 diabetes individuals. As a result of impaired kidney function, there may be an overestimation of an individual's beta cell secretory capacity; however, this overestimation can only occur if there was any residual C‐peptide secretion to begin with.
In the evaluation of residual insulin secretion, it is pivotal to use a C‐peptide assay which can reliably measure small amounts of C‐peptide, for instance <10 pmol/L. In a considerable number of individuals with type 1 diabetes residual C‐peptide cannot be detected with a standard assay (with LOD around 30 pmol/L) while the ultrasensitive assay does detect the presence of low levels of C‐peptide. 3 Earlier, we reported on the characteristics of the IRMA C‐peptide assay, used in the current study, which has a lower LOD of 3.8 pmol/L, with an acceptable coefficient of variation when concentrations are below 20 pmol/L4. We are currently evaluating the use of this assay for urinary C‐peptide measurements as well.
Our analyses showed that the number of insulin pump users was significantly lower in the high C‐peptide categories. Potentially individuals with a higher C‐peptide concentration have less difficulty achieving adequate glycaemic regulation and are, therefore, less likely to be prescribed an insulin pump. 25 A few participants showed large intra‐individual differences in C‐peptide measurements at Year 0 and Year 1, which may not be fully explained by test‐to‐test variation. Since the majority of participants who had detectable C‐peptide at either the first or the second MMTT had a higher glucose peak value at the MMTT when C‐peptide was detectable. This phenomenon may be explained by glucose potentiation. 26 Conversely, in the total population there was a negative association between glucose increase and C‐peptide increase at MMTT. This could be explained by a functional effect of residual insulin secretion on the glucose rise to a meal. Similar results were found in another study; however, only in individuals with a considerable C‐peptide response >200 pmol/L27. Our study is underpowered to draw definite conclusions about the complex interplay between glucose and the C‐peptide response.
4.1. Strengths and weaknesses
We have measured serum C‐peptide in the fasting state and after MMTT in a large group of individuals with varying diabetes duration. In addition, this is the first study investigating MMTTs in type 1 diabetes individuals using an ultra‐sensitive C‐peptide assay.
Repeating the MMTT in a subset of participants 1 year later allowed us to investigate changes in C‐peptide response over time. The limitations of our study are the fact that we did not measure other hormones related to food intake and stimulation of beta‐cells such as incretin and GLP1. Overall, our data suggest that there may be a functional effect of C‐peptide to the total glucose rise to MMTT and in the low levels there may be some effect of glucose potentiation on the beta cell response, future studies are warranted to confirm these findings.
4.2. Conclusion
Measuring C‐peptide after MMTT identified an additional 10% of individuals with detectable C‐peptide than measuring C‐peptide in the fasting state, while T90 and T120 minutes timepoints after MMTT showed good concordance with the MMTT total AUC. From a practical point of view, we propose to uniformly measure serum C‐peptide concentration 90 min after mixed‐meal ingestion for the estimation of residual insulin secretion in individuals with a longer diabetes duration. Future research should focus on the potentiation effect of glucose and additional determinants that are associated with the stimulation of C‐peptide secretion after a meal.
FUNDING INFORMATION
This study was supported by the Juvenile Diabetes Research Foundation (JDRF), grant no.
3‐SRA‐2014‐291‐M‐R, and the Dutch Diabetes Research Foundation, project 2015.16.1856, for which we are very grateful.
CONFLICT OF INTEREST
D.M., P.D., M.M.C. deV‐V., E.B. and H.J.A. are employed at Diabeter Netherlands, an independent clinic, which was acquired by Medtronic. The research presented here was independently performed and there are no conflicts of interest.
Supporting information
Appendix S1.
ACKNOWLEDGMENTS
The authors would like to thank all participants and acknowledge the work of laboratory technicians at the participating hospitals and the IJsselland Hospital, as well as Dr. M.L. Brugts (Ikazia Hospital, Rotterdam, The Netherlands). C.E.V. is supported by the MD‐PhD program of the University of Groningen and the UMCG.
Vollenbrock CE, Mul D, Dekker P, et al. Fasting and meal‐stimulated serum C‐peptide in long‐standing type 1 diabetes mellitus. Diabet Med. 2023;40:e15012. doi: 10.1111/dme.15012
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request. All data requests will be subject to relevant GDPR and ethics considerations.
REFERENCES
- 1. Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic ß‐cell turnover after 50 years of diabetes: Joslin medalist study. Diabetes. 2010;59(11):2846‐2853. doi: 10.2337/DB10-0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steffes MW, Sibley S, Jackson M, Thomas W. β‐Cell function and the development of diabetes‐related complications in the diabetes control and complications trial. Diabetes Care. 2003;26(3):832‐836. doi: 10.2337/DIACARE.26.3.832 [DOI] [PubMed] [Google Scholar]
- 3. Wang L, Lovejoy NF, Faustman DL. Persistence of prolonged C‐peptide production in type 1 diabetes as measured with an ultrasensitive C‐peptide assay. Diabetes Care. 2012;35(3):465‐470. doi: 10.2337/dc11-1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Leur K, Vollenbrock C, Dekker P, et al. How low is really low? Comparison of two C‐peptide assays to establish residual C‐peptide production in type 1 diabetes. Diabet Med. 2022;39:(5):e14785. doi: 10.1111/dme.14785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steiner DF, Cunningham D, Spigelman L, Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967;157(3789):697‐700. doi: 10.1126/science.157.3789.697 [DOI] [PubMed] [Google Scholar]
- 6. Kuhtreiber WM, Washer SLL, Hsu E, et al. Low levels of C‐peptide have clinical significance for established type 1 diabetes. Diabet Med. 2015;32(10):1346‐1353. doi: 10.1111/dme.12850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luppi P, Drain P. C‐peptide antioxidant adaptive pathways in β cells and diabetes. J Intern Med. 2017;281(1):7‐24. doi: 10.1111/joim.12522 [DOI] [PubMed] [Google Scholar]
- 8. Jones AG, Hattersley AT. The clinical utility of C‐peptide measurement in the care of patients with diabetes. Diabet Med. 2013;30(7):803‐817. doi: 10.1111/dme.12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeyam A, Colhoun H, McGurnaghan S, et al. Clinical impact of residual C‐peptide secretion in type 1 diabetes on Glycemia and microvascular complications. Diabetes Care. 2021;44(2):390‐398. doi: 10.2337/DC20-0567 [DOI] [PubMed] [Google Scholar]
- 10. Lam A, Dayan C, Herold KC. A little help from residual β cells has long‐lasting clinical benefits. J Clin Invest. 2021;131(3):e143683. doi: 10.1172/JCI143683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gubitosi‐Klug RA, Braffett BH, Hitt S, et al. Residual β cell function in long‐term type 1 diabetes associates with reduced incidence of hypoglycemia. J Clin Invest. 2021;131(3):e143011. doi: 10.1172/JCI143011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wellens MJ, Vollenbrock CE, Dekker P, et al. Residual C‐peptide secretion and hypoglycemia awareness in people with type 1 diabetes. BMJ Open Diabetes Res Care. 2021;9(1):e002288. doi: 10.1136/BMJDRC-2021-002288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenbaum CJ, Thomas MP, Mcgee PF, et al. Mixed‐meal tolerance test versus glucagon stimulation test for the assessment of β‐cell function in therapeutic trials in type 1 diabetes. Diabetes Care. 2008;31(10):1966‐1971. doi: 10.2337/dc07-2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gjessing HJ, Damsgaard EM, Matzen LE, Faber OK, Frøland A. The beta‐cell response to glucagon and mixed meal stimulation in non‐insulin dependent diabetes. Scand J Clin Lab Invest. 1988;48(8):771‐777. doi: 10.3109/00365518809088759 [DOI] [PubMed] [Google Scholar]
- 15. Marena S, Montegrosso G, De Michieli F, Pisu E, Pagano G. Comparison of the metabolic effects of mixed meal and standard oral glucose tolerance test on glucose, insulin and C‐peptide response in healthy, impaired glucose tolerance, mild and severe non‐insulin‐dependent diabetic subjects. Acta Diabetol. 1992;29(1):29‐33. doi: 10.1007/BF00572826 [DOI] [PubMed] [Google Scholar]
- 16. Forbes S, Lam A, Koh A, et al. Comparison of metabolic responses to the mixed meal tolerance test vs the oral glucose tolerance test after successful clinical islet transplantation. Clin Transplant. 2018;32(8):e13301. doi: 10.1111/ctr.13301 [DOI] [PubMed] [Google Scholar]
- 17. R Studio Team . RStudio: Integrated Development Environment for R. 2019. http://www.rstudio.com/
- 18. Kassambara A. rstatix: Pipe‐Friendly Framework for Basic Statistical Tests. 2021. https://cran.r‐project.org/package=rstatix
- 19. Hope S, Knight B, Shields B, Hattersley A, McDonald T, Jones A. Random non‐fasting C‐peptide: bringing robust assessment of endogenous insulin secretion to the clinic. Diabet Med. 2016;33(11):1554‐1558. doi: 10.1111/DME.13142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davis AK, DuBose SN, Haller MJ, et al. Prevalence of detectable C‐peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care. 2015;38(3):476‐481. doi: 10.2337/DC14-1952 [DOI] [PubMed] [Google Scholar]
- 21. Besser REJ, Shields BM, Casas R, Hattersley AT, Ludvigsson J. Lessons from the mixed‐meal tolerance test: use of 90‐minute and fasting C‐peptide in pediatric diabetes. Diabetes Care. 2013;36(2):195‐201. doi: 10.2337/DC12-0836/-/DC1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Besser REJ, Ludvigsson J, Jones AG, et al. Urine C‐peptide creatinine ratio is a noninvasive alternative to the mixed‐meal tolerance test in children and adults with type 1 diabetes. Diabetes Care. 2011;34(3):607‐609. doi: 10.2337/DC10-2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu W, Huang X, Zhang X, et al. Urinary C‐peptide creatinine ratio as a non‐invasive tool for identifying latent autoimmune diabetes in adults (LADA). Diabetes, Metab Syndr Obes Targets Ther. 2019;12:2531‐2537. doi: 10.2147/DMSO.S229675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDonald TJ, Knight BA, Shields BM, Bowman P, Salzmann MB, Hattersley AT. Stability and reproducibility of a single‐sample urinary C‐peptide/creatinine ratio and its correlation with 24‐h urinary C‐peptide. Clin Chem. 2009;55(11):2035‐2039. doi: 10.1373/CLINCHEM.2009.129312 [DOI] [PubMed] [Google Scholar]
- 25. Rickels MR, Evans‐Molina C, Bahnson HT, et al. High residual C‐peptide likely contributes to glycemic control in type 1 diabetes. J Clin Invest. 2020;130(4):1850‐1862. doi: 10.1172/JCI134057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Henquin JC. The dual control of insulin secretion by glucose involves triggering and amplifying pathways in β‐cells. Diabetes Res Clin Pract. 2011;93(Suppl 1):S27‐S31. doi: 10.1016/S0168-8227(11)70010-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
Data are available upon reasonable request. All data requests will be subject to relevant GDPR and ethics considerations.


